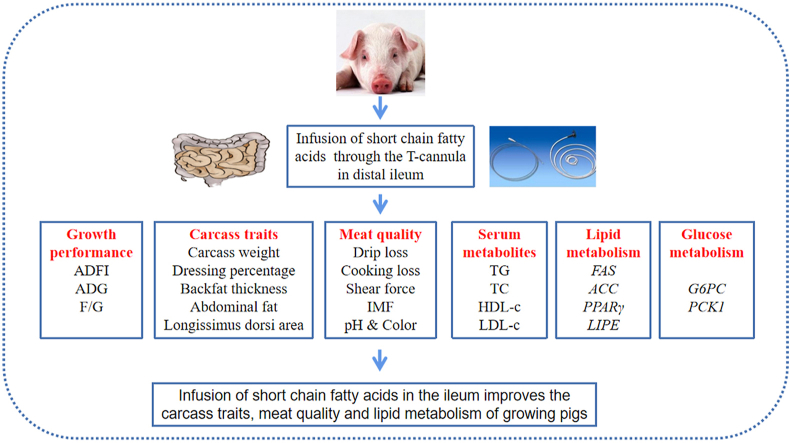
Abstract
Short chain fatty acids (SCFA) are the main products of indigestible carbohydrates undergoing bacterial fermentation in the hindgut, which are related to some physiological functions. This study was designed to investigate the effects of SCFA infusion by ileum on the carcass traits, meat quality and lipid metabolism of growing pigs. In a 28-day study, 24 growing barrows fitted with a T-cannula in distal ileum were divided into 4 treatments: 1) Control, 2) antibiotics (AB), 3) AB + 300 mL of SCFA1 solution (ABS1), 4) AB + 300 mL of SCFA2 solution (ABS2). The concentrations of acetate, propionate and butyrate in SCFA1 solution were respectively 61.84, 18.62 and 12.55 mmol/L, and in SCFA2 were respectively 40.08, 15.41 and 9.78 mmol/L. The results showed that the SCFA infusion increased the average daily feed intake and average daily gain of pigs (P < 0.05). Meanwhile, the SCFA treatments increased longissimus dorsi area (P < 0.05) and carcass weight (P = 0.058), decreased the drip loss of longissimus dorsi (P = 0.059), and reduced serum concentrations of triglyceride, total cholesterol and urea nitrogen (P < 0.05). Besides, the SCFA administration inhibited the mRNA expressions of fatty acid synthase (FAS) and acetyl-CoA carboxylase in longissimus dorsi (P < 0.05), the mRNA expression of FAS in the liver (P < 0.05), and the mRNA expression of hormone-sensitive lipase in abdominal fat (P < 0.05). Short chain fatty acid infusion also enhanced the mRNA expression of carnitine palmitoyltransferase-1α in the liver (P < 0.05), the mRNA expressions of peroxisome proliferator activated receptor gamma and lipoprotein lipase in abdominal fat (P < 0.05), and the mRNA expressions of free fatty acid receptor 2, glucose-6-phosphatase and phosphoenolpyruvate carboxykinase 1 in the colon (P < 0.05). These results suggested that SCFA administration in the ileum could improve the carcass traits and meat quality of growing pigs, which was possibly due to the fact that SCFA modulated lipid metabolism.
Keywords: Short chain fatty acid, Carcass trait, Meat quality, Lipid metabolism, Growing pig
Graphical abstract
1. Introduction
Short chain fatty acids (SCFA) are produced when indigestible carbohydrates (like dietary fiber and resistant starch) enter the large intestine and are fermented by microflora. They mainly consist of acetate, propionate and butyrate (Wong et al., 2006). Short chain fatty acids have been demonstrated to play important roles in maintaining intestinal environment, sustaining electrolyte balance, and offering energy to host cells as well as gut microflora (Canfora et al., 2015). Short chain fatty acids are not only substrates for fatty acid synthesis and gluconeogenesis, but also involve in the function and metabolism of peripheral tissues (Canfora et al., 2015). Butyrate administration enriches type I fiber in skeletal muscle via inducing peroxisome proliferator-activated receptor-γ coactivator-1 expression in rodent models (Gao et al., 2009), enhances slow-switch myofiber formation and mitochondrial biogenesis in finishing pigs (Zhang et al., 2019), and improves the meat quality of broiler chickens (Zhang et al., 2011). Besides, many studies have reported the possible effects of SCFA on adipose tissue (Yu et al., 2017). Fat deposition pattern (including backfat thickness and intramuscular fat) is supposed to be an important factor influencing meat quality (Yu et al., 2017). Thus, there are renewed interests in investigating the effects of SCFA on carcass traits and pork quality.
Short chain fatty acids also act as signal molecules to participate in glucose and energy homeostasis. Acetate was found to improve glucose tolerance in mice and propionate could lower some risk factors of cardiovascular disease and diabetes (Chambers et al., 2014; Yamashita et al., 2007). Previous studies also showed that under normal or insulin resistance conditions, propionate and butyrate exerted metabolic benefits through intestinal gluconeogenesis (Ríos-Covián et al., 2016; Devadder et al., 2014). Meanwhile, SCFA promoted beige adipogenesis and inhibited chronic inflammation in mice, which were related to the regulation of free fatty acid receptors (Lu et al., 2016).
However, the effects of SCFA administration on pork quality are rarely studied, and how they modulate the lipid metabolism of different tissues needs further investigations. Our hypothesis was that SCFA could improve the carcass traits, meat quality, and lipid metabolism of growing pigs. Therefore, this study was designed to evaluate the effects of SCFA infusion by ileum on carcass traits and meat quality in growing pigs and to investigate the potential mechanisms involved in lipid metabolism.
2. Materials and methods
2.1. Animal, management, and diet
Experimental procedure and animal care were accomplished in accordance with the guide for the care and use of laboratory animals provided by the Institutional Animal Care Advisory Committee for Sichuan Agricultural University. All of the animal protocols used in this study were approved by the Animal Care and Use Committee of Sichuan Agricultural University under permit number DKY-B20131704.
In our previous studies, we used beet pulp to formulate a high fiber diet and a low fiber diet for growing pigs and found that the concentrations of acetate, propionate, and butyrate in the colon of pigs fed by the high fiber diet were 61.84, 18.62, and 12.55 mmol/L, respectively, and those of pigs fed by the low fiber diet were 40.08, 15.41 and 9.78 mmol/L, respectively. In this study, 24 healthy barrows (Duroc × Landrace × Yorkshire, initial body weight = 30.72 kg) fitted with a T-cannula in distal ileum were randomly allocated into 4 treatments (n = 6): 1) Control, 2) antibiotics (AB), 3) AB + 300 mL of SCFA1 solution in the ileum (ABS1), 4) AB + 300 mL of SCFA2 solution in the ileum (ABS2). The concentrations of acetate, propionate and butyrate in SCFA1 solution were respectively 61.84, 18.62 and 12.55 mmol/L, and those in SCFA2 were respectively 40.08, 15.41 and 9.78 mmol/L. The pH of both solutions was adjusted to 6.1 with hydrochloric acid and sodium hydroxide. The basal diet was formulated to meet or exceed the national research council nutrient requirement recommendation for 25- to 50-kg pigs and its compositions are presented in Table 1. Water and feed were provided ad libitum and pigs were exposed to natural light throughout experiment.
Table 1.
Composition and nutrient levels of experimental diets (air-dry basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 78.20 |
| Soybean meal | 14.60 |
| Soybean oil | 1.00 |
| Fish meal | 4.50 |
| Limestone | 0.35 |
| Dicalcium phosphate | 0.27 |
| Salt | 0.25 |
| 78% Lys | 0.34 |
| dl-Met | 0.10 |
| 98.5% Thr | 0.02 |
| 98% Trp | 0.07 |
| Chloride choline | 0.05 |
| Vitamin premix1 | 0.05 |
| Mineral premix2 | 0.20 |
| Total | 100.00 |
| Calculated composition | |
| DE, MJ/kg | 3.40 |
| CP | 15.74 |
| Ca | 0.52 |
| TP | 0.50 |
| AP | 0.32 |
| Lys | 0.98 |
| Met + Cys | 0.35 |
| Thr | 0.59 |
| Trp | 0.17 |
DE = digestible energy; TP = total phosphorus; AP = available phosphorus.
The premix provides the following per kilogram of diet: vitamin A 5,512 IU, vitamin D3 2,250 IU, vitamin E 24 mg, vitamin K3 3 mg, vitamin B2 6 mg, vitamin B6 3 mg, vitamin B12 24 μg, folic acid 1.2 mg, nicotinic acid 14 mg, biotin 150 μg, d-pantothenic acid 15 mg.
The premix provides the following per kilogram diet: Fe 60 mg, Cu 4 mg, Mn 2 mg, Zn 60 mg, I 0.14 mg, Se 0.2 mg.
All of the pigs were fasted for 12 h before installing T-cannula in distal ileum. The surgical process and post-operative care were carried out according to previous methods (Dilger et al., 2004; Nyachoti et al., 2002). After pigs woke up, they were allowed a 10-d convalescent period. Then, pigs were individually housed in metabolism cages (2.5 m × 1.8 m × 0.8 m) under light, temperature and humidity control during the next 4-wk experimental period. The health status of pigs was monitored every day.
During the first 14 d, pigs in AB, ABS1 and ABS2 groups were given a combination of mixed antibiotics (2.4 mg of ampicillin, 2.4 mg of gentamicin, 2.4 mg of metronidazole, 2.4 mg of neomycin, and 1.2 mg of vancomycin per day by oral gavage) to reduce intestinal microbiota (Bruce-Keller et al., 2015; Henrik et al., 2011). After this antibiotic treatment, pigs in ABS1 and ABS2 groups were respectively given 300 mL of SCFA1 and SCFA2 solutions 3 times per day through the T-cannula in distal ileum, while pigs in Control and AB groups were given a saline solution. This treatment lasted for another 14 d. The duration of whole trail was 28 d.
The initial and final body weights of each pig were measured before feeding on d 1 and 29. The feed intake of each pig was recorded every day. Average daily body weight gain (ADG), average daily feed intake (ADFI), and the ratio of feed to gain (F:G) were calculated according to the values mentioned above.
2.2. Slaughter and sample collection
At the end of experiment, after pigs were fasted overnight, blood samples were collected by acute jugular venipuncture, centrifuged at 3,000 × g and stored at −20 °C. After pigs were weighed, pigs were electrically stunned, slaughtered, and split down through the midline according to previously described procedures (Cheng et al., 2015). Hot carcass weight of each pig was recorded and used to calculate dressing percentage. Then, the backfat thickness at the first and last ribs, and the last lumbar vertebrae were recorded, respectively. Abdominal fat was collected and weighed. Meanwhile, longissimus dorsi area was measured at the 10th rib of carcass. Longissimus dorsi was collected and stored at 4 °C for latter analysis of meat quality. Longissimus dorsi, abdominal fat, liver, caecum and colon samples were collected and stored at −80 °C for further measurements and analyses.
2.3. Meat quality
The longissimus dorsi sample was used for the measurements and analyses of meat color, pH, drip loss, cook loss, and shear force. Meat color including lightness (L∗), redness (a∗), and yellowness (b∗) were measured at 45 min and 24 h after slaughter with a chromameter (CR-300, Minolta Camera, Osaka, Japan) according to manufacturer's instructions. The pH value was also measured at 45 min and 24 h using a pH meter (pH-STAR, SFK-Technology, Denmark) according to manufacturer's instructions. Drip loss and cook loss were conducted and calculated according to previously described methods (Honikel et al., 1986). Warner-Bratzler shear force (WBS) was measured using a texture analyzer (TA.XT. Plus, Stable Micro Systems, Godalming, UK) equipped with a Warner-Bratzler shear device according to manufacturer's instructions. Finally, intramuscular fat (IMF) content was analyzed according to Association of Official Analytical Chemists (AOAC) (Hirwitz and Latimer 1995).
2.4. Biochemical analyses
The triglyceride (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), and urea nitrogen of serum were measured by commercial assay kits from Nanjing Jiancheng Biochemistry (Nanjing, China) according to manufacturer's instructions. The insulin, leptin and glucagon of serum were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits from Jiangsu Jingmei Biotechnology Co., Ltd. (Yancheng, China) according to manufacturer's instructions.
2.5. RNA isolation and reverse transcription
Total RNA was prepared from frozen longissimus dorsi, abdominal fat, liver, caecum, and colon by using Trizol Reagent (TaKaRa Biotechnology, Dalian, China) based on manufacturer's protocols. The purity and concentration of total RNA were detected with a spectrophotometer (Beckman Coulter DU800), and the ratio of OD260 to OD280 ranged from 1.8 to 2.0, which indicated a very low degree of contamination or degradation. The complementary DNA was obtained by reverse transcription using RT Reagents (TaKaRa Biotechnology, Dalian, China).
2.6. Real-time quantitative PCR
After reverse transcription, the mRNA levels of several lipid metabolism related genes were analyzed by real-time quantitative PCR using SYBR Premix Ex Taq reagents (TaKaRa Biotechnology, Dalian, China) and CFX-96 Real-Time PCR Detection System (Bio-Rad Laboratories, Richmond, CA) according to previous methods (Mao et al., 2016). All of the primers (Yan et al., 2015) used in this study were purchased from TaKaRa Biotechnology (Dalian, China), which are shown in Table 2. The tested genes included fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), peroxisome proliferator activated receptor γ (PPARγ), sterol regulatory element binding protein 1c (SREBP-1c), lipoprotein lipase (LPL), carnitine palmitoyltransferase-1α (CPT-1α), hormone-sensitive lipase (HSL), free fatty acid receptor 2 (FFAR2), free fatty acid receptor 3 (FFAR3), glucose-6-phosphatase (G6PC), phosphoenolpyruvate carboxykinase 1 (PCK1), glucagon (GCG), and peptide YY (PYY). The cycling conditions were as follows: pre-denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at annealing temperature for 30 s, and finally elongation at 72 °C for 60 s. After amplification, a melting curve analysis was conducted to verify the purity and specificity of reactions. Beta-actin was used as a reference gene, and the relative gene expression compared to β-actin was calculated based on previous methods (Livak and Schmittgen 2001). The mRNA level of each gene for Control group was set to 1.0. All of the sample analyses were repeated in triplicate simultaneously on the same PCR plate, and an average value was used for calculation mentioned above.
Table 2.
Primers lists used for real-time quantitative PCR assay.
| Gene name | Primer | Sequence 5' – 3′ |
|---|---|---|
| β-actin | Sense | TCTGGCACCACACCTTCT |
| Antisense | TGATCTGGGTCATCTTCTCAC | |
| FAS | Sense | CTACGAGGCCATTGTGGACG |
| Antisense | AGCCTATCATGCTGTAGCCC | |
| ACC | Sense | AGCAAGGTCGAGACCGAAAG |
| Antisense | TAAGACCACCGGCGGATAGA | |
| PPARγ | Sense | CGACCTGGAAAGCCCGTTAT |
| Antisense | GAGGCTTTGTCCCCACAGAT | |
| SREBP-1c | Sense | AAGCGGACGGCTCACAATG |
| Antisense | GCAAGACGGCGGATTTATTCA | |
| LPL | Sense | CACATTCACCAGAGGGTC |
| Antisense | TCATGGGAGCACTTCACG | |
| CPT-1α | Sense | GACAAGTCCTTCACCCTCATCGC |
| Antisense | GGGTTTGGTTTGCCCAGACAG | |
| HSL | Sense | GCCTTTCCTGCAGACCATCT |
| Antisense | CACTGGTGAAGAGGGAGCTG | |
| PCK1 | Sense | TCAGCACGACTCCAGCCTTCA |
| Antisense | GCTCAAGCAGTCTGGGCATTCT | |
| G6PC | Sense | AAGCCAAGCGAAGGTGTGAGC |
| Antisense | GGAACGGGAACCACTTGCTGAG | |
| PYY | Sense | AGATATGCTAATACACCGAT |
| Antisense | CCAAACCCTTCTCAGATG | |
| GCG | Sense | CAAGAGGAACAAGAATAACAT |
| Antisense | AAGAACTTACATCACTGGTA | |
| FFAR2 | Sense | CGTGTTCATCGTTCAGTA |
| Antisense | GAAGTTCTCATAGCAGGTA | |
| FFAR3 | Sense | TGGAGACCTTACGTGTTG |
| Antisense | CGAGGATGAGAAGTAGTAGAT |
FAS = fatty acid synthase; ACC = acetyl-CoA carboxylase; PPARγ = peroxisome proliferator activated receptor γ; SREBP-1c = sterol regulatory element binding protein 1c; LPL = lipoprotein lipase; CPT-1α = carnitine palmitoyltransferase-1α; HSL = hormone-sensitive lipase; PCK1 = phosphoenolpyruvate carboxy kinase 1; G6PC = glucose-6-phosphatase; PYY = peptide YY; GCG = glucagon; FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3.
2.7. Statistical analysis
Descriptive statistics was performed to evaluate whether data were normally distributed with statistical software SPSS 20.0 (Statistical Product and Service Solutions, Inc, USA). Then one-way ANOVA test was used to compare the difference of normally distributed data among groups, followed by Duncan's multiple-range test. Results were presented as means and SEM. P < 0.05 was considered statistically significant, and P < 0.1 was considered a tendency.
3. Results
3.1. Growth performance
Growth performance is one of the most concerned indicators of animal nutrition. According to Table 3, AB significantly decreased ADFI compared with Control (P = 0.015). However, ABS1 significantly increased ADFI (P = 0.015), and ABS2 significantly increased ADG compared with AB group (P = 0.036). There were no significant differences among the 4 groups regarding F:G ratio (P > 0.050).
Table 3.
Effects of SCFA on the growth performance of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| Initial BW, kg | 30.73 | 30.67 | 30.73 | 30.75 | 0.16 | 0.987 |
| Final BW, kg | 51.00b | 50.75b | 52.17ab | 53.92a | 0.71 | 0.026 |
| ADFI, kg | 1.46a | 1.42b | 1.46a | 1.45ab | 0.01 | 0.015 |
| ADG, kg | 0.72ab | 0.72b | 0.77ab | 0.83a | 0.03 | 0.036 |
| F:G ratio | 2.03 | 1.99 | 1.92 | 1.75 | 0.07 | 0.064 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; F:G ratio = the ratio of feed to gain.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
3.2. Carcass traits
Carcass traits are closely correlated with meat yield and cutability. As shown in Table 4, ABS2 tended to increase carcass weight compared with AB (P = 0.058). ABS1 and ABS2 significantly increased longissimus dorsi area compared with AB (P = 0.004), and significantly enhanced abdominal fat weight compared with Control (P = 0.020). No significant differences were observed among the 4 groups regarding dressing percentage and backfat thickness (P > 0.050).
Table 4.
Effects of SCFA on the carcass traits of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| Carcass weight, kg | 34.88 | 34.91 | 36.05 | 37.23 | 0.36 | 0.058 |
| Dressing percentage, % | 68.39 | 68.84 | 69.15 | 69.09 | 0.31 | 0.842 |
| Backfat thickness, cm | 1.35 | 1.35 | 1.49 | 1.36 | 0.04 | 0.457 |
| Abdominal fat weight, g | 169.33b | 216.00ab | 253.33a | 231.67a | 10.41 | 0.020 |
| Longissimus dorsi area, cm2 | 28.84bc | 26.75c | 31.78a | 30.27ab | 0.57 | 0.004 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a-c Within a row, means without a common superscript differ (P < 0.05).
3.3. Meat quality
As consumers’ need for high quality meat is increasing, producers pay more and more attention to safe and healthy pork production. According to Table 5, ABS1 tended to decrease the drip loss of longissimus dorsi compared with AB (P = 0.059). Besides, AB and ABS2 significantly increased the L∗45 min of longissimus dorsi compared with Control (P = 0.020). AB, ABS1, and ABS2 significantly increased the b∗45 min of longissimus dorsi compared with Control (P = 0.012). ABS1 and ABS2 significantly decreased the L∗24 h of longissimus dorsi compared with Control (P = 0.028). There were no significant differences among the 4 groups regarding cooking loss, shear force, IMF, and pH (P > 0.050).
Table 5.
Effects of SCFA on the meat quality of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| Drip loss, % | 1.76 | 1.86 | 1.31 | 1.46 | 0.09 | 0.059 |
| Cooking loss, % | 30.03 | 29.89 | 30.28 | 29.60 | 0.32 | 0.908 |
| Shear force, N | 33.28 | 32.99 | 28.20 | 30.04 | 1.50 | 0.604 |
| IMF, % | 2.36 | 2.98 | 2.61 | 2.42 | 0.12 | 0.296 |
| pH45 min | 7.03 | 6.97 | 7.06 | 6.95 | 0.05 | 0.867 |
| pH24 h | 5.36 | 5.33 | 5.42 | 5.43 | 0.02 | 0.166 |
| L∗45 min | 42.11b | 44.99a | 43.24ab | 44.52a | 0.38 | 0.020 |
| a∗45 min | 3.21 | 2.91 | 3.16 | 3.45 | 0.16 | 0.716 |
| b∗45 min | 2.60b | 3.60a | 3.47a | 4.00a | 0.16 | 0.012 |
| L∗24 h | 49.96a | 48.54ab | 45.42b | 45.78b | 0.66 | 0.028 |
| a∗24 h | 5.83 | 5.01 | 4.46 | 5.22 | 0.29 | 0.445 |
| b∗24 h | 5.80 | 5.56 | 4.42 | 4.80 | 0.30 | 0.337 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; IMF = intramuscular fat.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
3.4. Serum lipid-related metabolites and hormones
Concentrations of TG and TC circulating in the blood are important predictors of some metabolic diseases. As shown in Table 6, AB significantly increased serum TG compared with Control (P = 0.001). ABS1 and ABS2 significantly decreased serum TG (P = 0.001) and TC (P = 0.010) compared with AB group. Besides, ABS2 significantly decreased serum urea nitrogen compared with AB group and Control (P = 0.011). No significant differences were observed among the 4 groups regarding HDL-c, LDL-c, leptin, insulin and glucagon (P > 0.050).
Table 6.
Effects of SCFA on the serum lipid-related metabolites and hormones of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| TG, mmol/L | 0.42b | 0.75a | 0.44b | 0.38b | 0.04 | 0.001 |
| TC, mmol/L | 3.48a | 4.20a | 2.34b | 1.99b | 0.24 | 0.010 |
| HDL-c, mmol/L | 2.42 | 2.25 | 1.89 | 1.52 | 0.18 | 0.288 |
| LDL-c, mmol/L | 0.69 | 0.82 | 0.81 | 0.84 | 0.05 | 0.665 |
| Urea nitrogen, mmol/L | 3.07a | 2.78ab | 2.30bc | 2.12c | 0.12 | 0.011 |
| Insulin, μIU/mL | 31.83 | 30.25 | 37.22 | 33.45 | 3.46 | 0.918 |
| Glucagon, μIU/mL | 435.96 | 318.26 | 288.86 | 132.21 | 50.13 | 0.199 |
| Leptin, ng/mL | 5.83 | 5.50 | 7.01 | 6.37 | 0.35 | 0.467 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; TG = triglyceride; TC = total cholesterol; HDL-c = high density lipoprotein-cholesterol; LDL-c = low density lipoprotein-cholesterol.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a-c Within a row, means without a common superscript differ (P < 0.05).
3.5. The mRNA expressions of lipid metabolism related genes in longissimus dorsi, abdominal fat and liver
Lipid metabolism includes lipogenesis and lipolysis, and several key enzymes or transcription factors are involved in the process, like FAS, ACC, PPARγ, HSL, LPL, and CPT-1α. According to Table 7, AB significantly enhanced the mRNA expressions of FAS (P = 0.010) and ACC (P = 0.029) in longissimus dorsi compared with Control. ABS1 and ABS2 significantly down-regulated the mRNA expression of FAS in longissimus dorsi compared with AB group (P = 0.010). ABS2 significantly down-regulated the mRNA expression of ACC in longissimus dorsi compared with AB group (P = 0.029). There were no significant differences among the 4 groups regarding SREBP-1c, PPARγ, CPT-1α, LPL, and HSL mRNA expressions in longissimus dorsi (P > 0.050).
Table 7.
Effects of SCFA on the mRNA expressions for key factors related to lipid metabolism in longissimus dorsi of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| FAS | 1.00b | 1.78a | 0.97b | 0.83b | 0.12 | 0.010 |
| ACC | 1.00b | 2.12a | 1.56ab | 1.23b | 0.15 | 0.029 |
| PPARγ | 1.00 | 1.03 | 0.89 | 0.97 | 0.06 | 0.855 |
| SREBP-1c | 1.00 | 1.08 | 0.90 | 0.69 | 0.08 | 0.383 |
| HSL | 1.00 | 0.93 | 1.05 | 0.99 | 0.12 | 0.990 |
| LPL | 1.00 | 0.67 | 1.21 | 0.95 | 0.13 | 0.555 |
| CPT-1α | 1.00 | 0.69 | 1.19 | 1.31 | 0.14 | 0.463 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; FAS = fatty acid synthase; ACC = acetyl-CoA carboxylase; PPARγ = peroxisome proliferator-activated receptor-γ; SREBP-1c = sterol regulatory element binding protein 1c; HSL = hormone-sensitive lipase; LPL = lipoprotein lipase; CPT-1α = carnitine palmitoyltransferase-1α.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
As shown in Table 8, AB significantly down-regulated the mRNA expressions of PPARγ (P = 0.032) and LPL (P = 0.004) in abdominal fat compared with Control. ABS1 and ABS2 significantly enhanced the mRNA expressions of PPARγ (P = 0.032) and LPL (P = 0.004) in abdominal fat compared with AB group. AB significantly enhanced the mRNA expression of HSL (P = 0.019) in abdominal fat compared with Control. ABS1 and ABS2 significantly down-regulated the mRNA expression of HSL (P = 0.019) in abdominal fat compared with AB group. No significant differences were observed among the 4 groups regarding ACC, FAS, SREBP-1c and CPT-1α mRNA expressions in abdominal fat (P > 0.050).
Table 8.
Effects of SCFA on the mRNA expressions for key factors related to lipid metabolism in abdominal fat of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| FAS | 1.00 | 1.04 | 1.17 | 0.94 | 0.04 | 0.229 |
| ACC | 1.00 | 1.06 | 1.07 | 1.05 | 0.02 | 0.712 |
| PPARγ | 1.00a | 0.63b | 0.91a | 0.90a | 0.05 | 0.032 |
| SREBP-1c | 1.00 | 1.05 | 1.16 | 1.08 | 0.04 | 0.537 |
| HSL | 1.00b | 1.64a | 1.26b | 1.26b | 0.08 | 0.019 |
| LPL | 1.00a | 0.65b | 0.99a | 1.03a | 0.05 | 0.004 |
| CPT-1α | 1.00 | 1.10 | 1.03 | 1.08 | 0.03 | 0.612 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; FAS = fatty acid synthase; ACC = acetyl-CoA carboxylase; PPARγ = peroxisome proliferator-activated receptor-γ; SREBP-1c = sterol regulatory element binding protein 1c; HSL = hormone-sensitive lipase; LPL = lipoprotein lipase; CPT-1α = carnitine palmitoyltransferase-1α.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
According to Table 9, AB significantly enhanced the mRNA expression of FAS in the liver compared with Control (P = 0.011). ABS1 and ABS2 significantly down-regulated the mRNA expression of FAS in the liver compared with AB group (P = 0.011). AB significantly down-regulated the mRNA expression of CPT-1α in the liver compared with Control (P = 0.032). ABS1 and ABS2 significantly enhanced the mRNA expression of CPT-1α in the liver compared with AB (P = 0.032). There were no significant differences among the 4 groups regarding ACC, SREBP-1c, PPARγ, LPL and HSL mRNA expressions in the liver (P > 0.050).
Table 9.
Effects of SCFA on the mRNA expressions for key factors related to lipid metabolism in the liver of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| FAS | 1.00b | 1.70a | 1.03b | 1.10b | 0.09 | 0.011 |
| ACC | 1.00 | 0.89 | 0.93 | 1.03 | 0.05 | 0.729 |
| PPARγ | 1.00 | 1.01 | 0.91 | 1.15 | 0.05 | 0.389 |
| SREBP-1c | 1.00 | 0.87 | 0.98 | 1.10 | 0.05 | 0.531 |
| HSL | 1.00 | 1.08 | 1.07 | 1.16 | 0.05 | 0.680 |
| LPL | 1.00 | 0.84 | 0.97 | 1.15 | 0.05 | 0.222 |
| CPT-1α | 1.00a | 0.64b | 0.92a | 0.90a | 0.05 | 0.032 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; FAS = fatty acid synthase; ACC = acetyl-CoA carboxylase; PPARγ = peroxisome proliferator-activated receptor-γ; SREBP-1c = sterol regulatory element binding protein 1c; HSL = hormone-sensitive lipase; LPL = lipoprotein lipase; CPT-1α = carnitine palmitoyltransferase-1α.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
3.6. The mRNA expressions of genes in caecum and colon
FFAR2 and FFAR3 are important receptors of SCFA, both of which are widely expressed in the small intestine and hindgut. As shown in Table 10, AB significantly down-regulated the mRNA expressions of FFAR2 (P = 0.038) and FFAR3 (P = 0.041) in caecum compared with Control. ABS1 and ABS2 significantly enhanced the mRNA expressions of FFAR2 (P = 0.038) and FFAR3 (P = 0.041) in caecum compared with AB group. No significant differences were observed among the 4 groups regarding PYY, GCG, PCK1, and G6PC mRNA expressions in caecum (P > 0.050).
Table 10.
Effects of SCFA on the mRNA expressions for key factors in caecum of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| G6PC | 1.00 | 1.10 | 0.93 | 0.99 | 0.03 | 0.260 |
| PCK1 | 1.00 | 1.02 | 0.96 | 1.03 | 0.02 | 0.756 |
| GCG | 1.00 | 1.01 | 0.99 | 1.16 | 0.03 | 0.305 |
| PYY | 1.00 | 1.11 | 1.00 | 1.10 | 0.03 | 0.450 |
| FFAR2 | 1.00a | 0.68b | 1.03a | 1.08a | 0.06 | 0.038 |
| FFAR3 | 1.00a | 0.71b | 0.97a | 1.08a | 0.05 | 0.041 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; G6PC = glucose-6-phosphatase; PCK1 = phosphoenolpyruvate carboxykinase 1; GCG = glucagon; PYY = peptide YY; FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
Glucogenesis in both liver and intestine is a representative indicator of metabolic state, and it is characterized by some key enzymes like PCK1 and G6PC. According to Table 11, AB significantly down-regulated the mRNA expressions of FFAR2 (P = 0.035), PCK1 (P = 0.041), and G6PC (P = 0.012) in the colon compared with Control. ABS1 and ABS2 significantly enhanced the mRNA expressions of FFAR2 (P = 0.035) and PCK1 (P = 0.041) in the colon compared with AB group. ABS1 significantly enhanced the mRNA expression of G6PC (P = 0.012) in the colon compared with AB group. There were no significant differences among the 4 groups regarding FFAR3, PYY and GCG mRNA expressions in the colon (P > 0.050).
Table 11.
Effects of SCFA on the mRNA expressions for key factors in the colon of pigs over the experimental period.
| Item | Control | AB | ABS1 | ABS2 | SEM | P-value |
|---|---|---|---|---|---|---|
| G6PC | 1.00a | 0.67b | 0.94a | 0.85ab | 0.04 | 0.012 |
| PCK1 | 1.00a | 0.62b | 1.02a | 1.01a | 0.06 | 0.041 |
| GCG | 1.00 | 1.05 | 1.12 | 1.02 | 0.03 | 0.390 |
| PYY | 1.00 | 1.07 | 0.96 | 0.88 | 0.04 | 0.331 |
| FFAR2 | 1.00a | 0.71b | 1.01a | 1.05a | 0.05 | 0.035 |
| FFAR3 | 1.00 | 1.08 | 0.97 | 1.02 | 0.02 | 0.426 |
SCFA = short chain fatty acids; AB = antibiotics; ABS1 = AB + SCFA1; ABS2 = AB + SCFA2; G6PC = glucose-6-phosphatase; PCK1 = phosphoenolpyruvate carboxykinase 1; GCG = glucagon; PYY = peptide YY; FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3.
SCFA1, the concentrations of acetate, propionate and butyrate were 61.84, 18.62 and 12.55 mmol/L, respectively; SCFA2, the concentrations of acetate, propionate and butyrate were 40.08, 15.41 and 9.78 mmol/L, respectively.
a, b Within a row, means without a common superscript differ (P < 0.05).
4. Discussion
In order to eliminate the influences of gastrointestinal microbiota and suppress the endogenous production of SCFA in the hindgut as much as possible, we administered a high dose of combined antibiotics, which was often successfully used in rodent models (Bruce-Keller et al., 2015; Henrik et al., 2011). According to our published data, although the efficiency of these combined antibiotics was not the same as that in the study of mice, it significantly reduced total bacteria in feces around 10-fold, and decreased the concentrations of acetate, propionate, butyrate, and total SCFA in serum and/or ileum of pigs (Diao et al., 2017). The current study also found that this combined antibiotics treatment significantly down-regulated the mRNA expressions of FFAR2 and/or FFAR3 that were important receptors of SCFA in the colon and caecum of pigs. These demonstrated that the combined antibiotics administration, to some extent, successfully eliminated the effects of gut microbiota. Moreover, in our present study, infusion of SCFA enhanced the mRNA expressions of FFAR2 and FFAR3 in caecum as well as FFAR2 in the colon, which suggested that our SCFA infusion was practicable. Dietary fiber is critical for lipid metabolism (Canfora et al., 2015), and it is a typical kind of indigestible carbohydrates that can be fermented in the hindgut. After a series of bacterial processing, plenty of SCFA are produced, mainly characterized by acetate, propionate and butyrate, and formate, valerate and caproate are of relatively lower amounts, making up less than 5% of all (Wong et al., 2006). These SCFA not only act as signal molecules to impact intestinal health but also enter systematic circulation and participate in the metabolism of peripheral organs (Wong et al., 2006). In our previous studies, we used beet pulp to formulate a high fiber diet and a low fiber diet for growing pigs and found that the concentrations of acetate, propionate, and butyrate in the colon of pigs fed by the high fiber diet were 61.84, 18.62, and 12.55 mmol/L, respectively, and those of pigs fed by the low fiber diets were 40.08, 15.41 and 9.78 mmol/L, respectively. Thus, in the present study, pigs in ABS1 and ABS2 groups were infused with these 2 concentrations of SCFA through the T-cannula in distal ileum.
Several studies have reported beneficial effects of propionate or butyrate on ADFI or ADG in pigs (Real et al., 2000; Hanczakowska et al., 2014). In this study, SCFA infusion increased the ADFI and ADG of growing pigs. However, in rodents, there are often different results. Oral (rather than intravenous) butyrate decreased feed intake and inhibited orexigenic neuron activity in hypothalamus of mice (Li et al., 2017). And, SCFA attenuated obesity induced by high fat diets in mice (Lu et al., 2016). This discrepancy might be due to the differences of animal models, health situations, and SCFA administration methods.
Carcass weight and longissimus dorsi area are the important characteristics of carcass traits, and they are closely correlated with meat yield and cutability (Cameron 1990). Drip loss reflects the water-linking capacity of meat and is used to evaluate shelf life after slaughter (Fischer 2007). As the consumers’ demand for high quality meat is increasing, producers pay more and more attention to meat safety and meat flavor. According to our study, SCFA treatment increased the carcass weight and longissimus dorsi area of growing pigs and decreased the drip loss of longissimus dorsi, which was partly consistent with the studies on broilers (Zhang et al., 2011). Moreover, a study showed that sodium butyrate increased muscle contents and decreased body fat contents in mice (Gao et al., 2009). Blood urea nitrogen concentration is commonly used for the determination of protein or amino acid utilization (Eggum 1970). A high level of blood urea nitrogen demonstrated that excessive amino acids are metabolized before excretion (Eggum 1970). Our study showed that SCFA infusion could reduce the serum urea nitrogen of growing pigs, which might further illustrate the possible mechanisms for improving longissimus dorsi area. These all indicated that SCFA could be used as potential additives to improve carcass traits and meat quality, but more detailed studies concerning dosage and form are awaited.
Fat deposition in non-adipose tissues (like liver and skeletal muscle) is supposed to be ectopic fat deposition, which leads to a metabolic disorder and organ dysfunction (Shulman 2000; Snel et al., 2012). In the current study, SCFA down-regulated the mRNA expressions of FAS and ACC in longissimus dorsi, both of which acted as key enzymes to participate in de novo fatty acid synthesis (Kim 2003; Yan et al., 2015). SCFA also reduced the mRNA expression of FAS and enhanced the mRNA expression of CPT-1α in the liver. CPT-1α is involved in mitochondrial fatty acid oxidation and catalyzes the primary step (Kwangwon et al., 2011). These results suggested that SCFA could attenuate fat deposition in the liver and longissimus dorsi via regulating some lipogenesis or lipolysis related genes, thus contributing to glucose and lipid homeostasis. In addition, a previous study showed that protein oxidation was negatively related to lipid oxidation, and all of the parameters of protein metabolism were inversely correlative with visceral adipose tissue (Solini et al., 1997). Thus, according to our results, SCFA improved carcass weight and longissimus dorsi area possibly via regulating the lipid metabolism of growing pigs. Previous studies also demonstrated that acetate and propionate could attenuate intracellular lipolysis through reducing HSL phosphorylation via FFAR2 (Canfora et al., 2015). And, SCFA could stimulate PPARγ-mediated lipogenesis and be regulated by FFAR2-related mechanisms (Hong et al., 2005). Besides, extracellular lipolysis could be mediated by LPL, and propionate increased its expression in adipose tissue (Lee and Hossner, 2002). According to our findings, SCFA reduced the mRNA expression of HSL and enhanced the mRNA expressions of PPARγ and LPL in abdominal fat, which would increase TG storage in adipose tissue and decrease systemic free fatty acid circulation. These results suggested a reduced lipid overflow and ectopic fat accumulation were induced by SCFA, thus positively improved insulin sensitivity.
In the present study, SCFA treatment enhanced the mRNA expressions of PCK1 and G6PC in the colon. Phosphoenolpyruvate carboxykinase 1 catalyzes an irreversible step of gluconeogenesis, which is vital for glucose synthesis (Chou and Mansfield 2010). Glucose-6-phosphatase is involved in the hydrolysis of glucose-6-phosphate to glucose and phosphate in the final step of gluconeogenesis (Chou and Mansfield 2010). These results indicated that SCFA stimulated intestinal gluconeogenesis in growing pigs, which could further illustrate the possible mechanisms for decreasing TG and TC concentrations in serum (Ríos-Covián et al., 2016; Devadder et al., 2014).
5. Conclusion
Taken together, our results demonstrated that SCFA infusion by ileum, to some extent, improved carcass traits and meat quality via regulating lipid metabolism, which could provide some new insights into the roles of SCFA in pig breeding as feed additives to increase carcass traits and meat quality.
Author contributions
Daiwen Chen and Xiangbing Mao were responsible for the conceptualization. Anran Jiao and Hui Diao were responsible for the investigation. Bing Yu, Jun He and Jie Yu were responsible for the validation. Ping Zheng, Junqiu Luo and Yuheng Luo were responsible for the supervision. Quyuan Wang and Huifen Wang were responsible for the resources. All of the authors have read and approved the final version of this manuscript.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was financially funded by the National Natural Science Foundation of China (31672436), the earmarked fund for the China Agricultural Research System (CARS-35), Sichuan Province Science and Technology Support Project (2016NYZ0052), the National Basic Research Program of China (2013CB531406), and the National High Technology Research and Development Program of China (2014AA022209). The authors sincerely thank Shuli Liu and Xin He for their kind help during the whole study. All of the data generated or analyzed during the study are included in this article.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Xiangbing Mao, Email: acatmxb2003@163.com.
Daiwen Chen, Email: dwchen@sicau.edu.cn.
References
- Bruce-Keller A.J., Salbaum J.M., Luo M., Blanchard E., Taylor C.M., Welsh D.A., Berthoud H.-R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatr. 2015;77(7):607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N.D. Genetic and phenotypic parameters for carcass traits, meat and eating quality traits in pigs. Livest Prod Sci. 1990;26(2):119–135. [Google Scholar]
- Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E.K., MacDougall K., Preston T., Tedford C., Finlayson G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2014;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Luo J., Bing Y., Ping Z., Huang Z., Mao X., He J., Jie Y., Chen J., Chen D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Chou J.Y., Mansfield B.C. Mutations in the glucose-6-phosphatase-alpha (G6PC) gene that cause type Ia glycogen storage disease. Hum Mutat. 2010;29(7):921–930. doi: 10.1002/humu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Diao H., Jiao A., Yu B., He J., Yu J., Zheng P., Huang Z., Luo Y., Luo J., Mao X. Stimulation of intestinal growth with distal ileal infusion of short-chain fatty acid: a reevaluation in a pig model. RSC Adv. 2017;7(49):30792–30806. [Google Scholar]
- Dilger R.N., Sands J.S., Ragland D., Adeola O. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls1,2. J Anim Sci. 2004;82(3):715–724. doi: 10.2527/2004.823715x. [DOI] [PubMed] [Google Scholar]
- Eggum B.O. Blood urea measurement as a technique assessing protein quality. Br J Nutr. 1970;24(4):983–988. doi: 10.1079/bjn19700101. [DOI] [PubMed] [Google Scholar]
- Fischer K. Drip loss in pork: influencing factors and relation to further meat quality traits. J Anim Breed Genet. 2007;124(Suppl 1):12–18. doi: 10.1111/j.1439-0388.2007.00682.x. s1. [DOI] [PubMed] [Google Scholar]
- Gao Zhanguo, Yin Jun, Zhang Jin, Ward R.E., Martin R.J., Lefevre Michael, Cefalu W.T., Jianping Y. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanczakowska E., Niwin´Ska B., Grela E.R., Weglarzy K., Okon´ K. Effect of dietary glutamine, glucose and/or sodium butyrate on piglet growth, intestinal environment, subsequent fattener performance, and meat quality. Czech J Anim Sci. 2014;59(10):460–470. [Google Scholar]
- Henrik R.D., Alexander E., Anders S., Vedrana G., Lars J.F., Peter G., McCoy K.D., Macpherson A.J., Meza-Zepeda L.A., Finn-Eirik J. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirwitz W., Latimer G. Official methods of analysis of AOAC. 1995;6(11) 382-382. [Google Scholar]
- Hong Y.H., Yukihiko N., Daisuke H., Hiroaki T., Hisae M., Chizu G., Ki-Choon C., Feng D.D., Chen C., Hong-Gu L. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;12:12. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- Honikel K.O., Kim C.J., Hamm R., Roncales P. Sarcomere shortening of prerigor muscle and its influence on drip loss. Meat Sci. 1986;16(4):267–282. doi: 10.1016/0309-1740(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Kim K.H. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr. 2003;17(1):77–99. doi: 10.1146/annurev.nutr.17.1.77. [DOI] [PubMed] [Google Scholar]
- Kwangwon L., Janos K., Hoppel C.L. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem. 2011;286(29):25655–25662. doi: 10.1074/jbc.M111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Hossner K.L. Coordinate regulation of ovine adipose tissue gene expression by propionate. J Anim Sci. 2002;80(11):2840–2849. doi: 10.2527/2002.80112840x. [DOI] [PubMed] [Google Scholar]
- Li Z., Yi C.-X., Katiraei S., Kooijman S., Zhou E., Chung C.K., Gao Y., van den Heuvel J.K., Meijer O.C., Berbee J.F.P. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2017;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Y., Fan C., Li P., Lu Y., Chang X., Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. 2016;6:37589. doi: 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Gu C., Hu H., Tang J., Chen D., Yu B., He J., Yu J., Luo J., Tian G. Dietary lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0146312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyachoti C.M., McNeilage-Van de Wiele E.M., de Lange C.F.M., Gabert V.M. Evaluation of the homoarginine technique for measuring true ileal amino acid digestibilities in pigs fed a barley-canola meal-based diet. J Anim Sci. 2002;80(2):440–448. doi: 10.2527/2002.802440x. [DOI] [PubMed] [Google Scholar]
- Real D.E., Tokach M.D., Nelssen J.L., Goodband R.D., Webster M.J. Influence of calcium propionate on starter pig performance. Swine Day. 2000;10:41–43. [Google Scholar]
- Rííos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., De Los Reyes-gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.I. Cellular mechanisms of insulin resistance in humans. Exp Clin Endocrinol Diabetes. 2000;107(2):111. [Google Scholar]
- Snel M., Jonker J.T., Schoones J., Lamb H., Jazet I.M. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. International Journal of Endocrinology. 2012;2012(7):983814. doi: 10.1155/2012/983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solini A., Bonora E., Bonadonna R., Castellino P., Defronzo R.A. Protein metabolism in human obesity: relationship with glucose and lipid metabolism and with visceral adipose tissue. J Clin Endocrinol Metabol. 1997;82(8):2552. doi: 10.1210/jcem.82.8.4182. [DOI] [PubMed] [Google Scholar]
- Wong J.M.W., de Souza R., Kendall C.W.C., Emam A., Jenkins D.J.A. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Fujisawa K., Ito E., Idei S., Tsuji H. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic otsuka long-evans tokushima fatty (OLETF) rats. J Agric Chem Soc Jpn. 2007;71(5):1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- Yan H., Zheng P., Yu B., Yu J., Mao X., He J., Huang Z., Chen D. Postnatal high-fat diet enhances ectopic fat deposition in pigs with intrauterine growth retardation. Eur J Nutr. 2015;56(2):1–8. doi: 10.1007/s00394-015-1093-9. [DOI] [PubMed] [Google Scholar]
- Yu S., Ren E., Xu J., Su Y., Zhu W. Effects of early intervention with sodium butyrate on lipid metabolism-related gene expression and liver metabolite profiles in neonatal piglets. Livest Sci. 2017;195:80–86. [Google Scholar]
- Zhang W.H., Gao F., Zhu Q.F., Li C., Jiang Y., Dai S.F., Zhou G.H. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poultry Sci. 2011;90(11):2592–2599. doi: 10.3382/ps.2011-01446. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu B., Yu J., Zheng P., Huang Z., Luo Y., Luo J., Mao X., Yan H., He J., Chen D. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1α expression1. J Anim Sci. 2019;97(8):3180–3192. doi: 10.1093/jas/skz187. [DOI] [PMC free article] [PubMed] [Google Scholar]