Abstract
The present study aimed to investigate the effects of dietary pioglitazone hydrochloride (PGZ) and l-carnosine (LC) supplementation on the growth performance, meat quality, antioxidant status, and meat shelf life of yellow-feathered broiler chickens. Five hundred broiler chickens were randomly assigned into 4 experimental diets using a 2 × 2 factorial arrangement with 2 PGZ supplemental levels (0 and 15 mg/kg) and 2 LC supplemental levels (0 and 400 mg/kg) in basal diets for 28 d. The feed-to-gain ratio decreased whereas the average daily gain increased with PGZ supplementation. Greater dressing percentages, contents of intramuscular fat (IMF) in breast and thigh muscles, C18:3n-6, C18:1n-9 and monounsaturated fatty acid (MUFA) percentages of thigh muscle were observed with PGZ addition. Additionally, significant synergistic effects between PGZ and LC on the C18:1n-9 and MUFA contents were found. Supplementation with LC decreased drip loss, cooking loss and total volatile basic nitrogen, and increased the redness (a∗) value, the superoxide dismutase and glutathione peroxidase activities in thigh muscles. Moreover, the malondialdehyde content decreased when diets were supplemented with LC, and there was a synergistic effect between PGZ and LC. Additionally, the mRNA abundance of lipogenesis-related genes, such as peroxisome proliferator-activated receptor γ (PPARγ), PPARγ co-activator 1α and fatty acid-binding protein 3, increased with PGZ supplementation, and relevant antioxidation genes, such as nuclear factor erythroid-2-related factor 2 and superoxide dismutase 1, were enhanced with LC supplementation. In conclusion, the results indicated that the supplementation of PGZ and LC could improve the growth performance, antioxidant ability, IMF content, and meat shelf life of yellow-feathered broiler chickens.
Keywords: Pioglitazone hydrochloride, l-carnosine, Intramuscular fat, Antioxidant capacity, Yellow-feathered broiler chicken
1. Introduction
Chicken meat is regarded as a good choice for healthy diets due to its higher proportion of polyunsaturated fatty acids (PUFA) than that of meats from polygastric animals (Berzaghi et al., 2005). Additionally, consumers prefer to choose meats with better color and taste (Jiang et al., 2018). Intramuscular fat (IMF) is a key indicator of meat quality and has a positive impact on meat tenderness, flavor, and carcass performance (Bonny et al., 2015; Costa et al., 2012). The fatty acid profile of muscle is closely related to human health, but it is susceptible to oxidative stability and in turn causes negative effects on flavor (Wood et al., 2008). Therefore, there is an urgent need to develop a method for enhancing the IMF content and simultaneously improving the antioxidant capacity of meat. Moreover, nutritional supplementation is considered an effective way to protect poultry meat from oxidation (Engel et al., 2015).
As a high-affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ), pioglitazone hydrochloride (PGZ) can promote adipocyte differentiation (Machado et al., 2019). Early study found increased lipid accumulation in the skeletal muscle of rodents by feeding them PGZ (Todd et al., 2007). Our previous studies found that PGZ supplementation increased the IMF in the muscle of pigs, indicating that PGZ has a potential to alter marbling adipogenesis (Chen et al., 2013; Jin et al., 2018a). In addition, previous studies have indicated that PGZ can improve the proportion of the monounsaturated fatty acid (MUFA) in rodent muscle and PUFA in the muscle of pigs (Chabowski et al., 2012; Jin et al., 2018b). Moreover, we determined the residual amount of PGZ in the muscle tissue, liver and serum of chickens and found no residues, which demonstrated that PGZ has a potential as an additive.
Although PGZ can increase IMF and improve muscle juiciness, the enhanced lipids are also susceptible to oxidation. According to previous reports, lipid oxidation results in poor nutritional values and off-flavors of meat (Baron and Andersen, 2002; Faustman et al., 2010). l-Carnosine (LC) is composed of l-histidine and β-alanine and exists mainly in skeletal muscles (Bao et al., 2015). LC is a naturally present antioxidant in animals that can directly scavenge oxygen free radicals (Gariballa and Sinclair, 2000). Based on the antioxidant properties of LC, its presence in the diet could play a vital role in antioxidant defense systems.
Therefore, the purpose of this experiment was to explore whether the addition of PGZ could increase the IMF contents and whether LC could protect them from oxidation, thereby improving meat quality and extending the meat shelf life of broiler chickens.
2. Materials and methods
All experimental procedures involving animals were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of South China Agricultural University and were approved by the Animal Ethics Committee of South China Agricultural University (Guangzhou, China).
2.1. Materials
The PGZ (purity ≥ 99%) was purchased from Chengdu Yuyang High Technology Development Co., Ltd. (Chengdu, China), and LC (purity ≥ 98%) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Experimental design and dietary supplementation
Five hundred female yellow-feathered broiler chickens with a similar weight (1.63 ± 0.02 kg) that were approximately 72 d of age (1 month before slaughter) were randomly divided into 4 groups, each group with 5 replicates (with each replicate containing 25 broiler chickens). Broiler chickens were randomly assigned into 4 experimental diets using a 2 × 2 factorial arrangement with 2 PGZ supplemental levels (0 and 15 mg/kg) and 2 LC supplemental levels (0 and 400 mg/kg) in basal diets for 28 d. The basal diet (Table 1) was formulated according to the Poultry Nutrient Requirements (Ministry of Agriculture of the People’s Republic of China, 2004). The birds were housed in a room with a concrete floor covered in wheat shavings and allowed ad libitum access to the diets and water. There was a continuous lighting for 23 h and the room temperature was maintained at approximately 25 ± 2 °C. Feed intake was recorded daily to calculate the average daily feed intake (ADFI). The weight of each group of broiler chickens was weighed at 1, 15, and 29 d to calculate the average daily gain (ADG). The feed-to-gain ratio (F:G) was calculated with ADFI and ADG.
Table 1.
The ingredients and nutrient composition of basal diet (as-fed basis, %).
| Ingredients | Content | Nutritional level | Content |
|---|---|---|---|
| Corn | 71.36 | ME, MJ/kg | 12.97 |
| Soybean meal (46%) | 21.21 | Crude protein | 16.50 |
| Corn protein flour (60%) | 2.79 | Crude fiber | 1.97 |
| Soybean oil | 2.45 | Calcium | 0.80 |
| Dicalcium phosphate | 0.90 | Total phosphorus | 0.48 |
| Sodium chloride | 0.35 | Non-phytate phosphorus | 0.26 |
| l-lysine sulfate (70%) | 0.33 | Sodium | 0.16 |
| dl-methionine (98%) | 0.14 | Potassium | 0.62 |
| l-threonine (98%) | 0.04 | Lysine | 0.93 |
| Choline chloride (60%) | 0.03 | Methionine + Cystine | 0.70 |
| Premix1 | 0.40 |
ME = metabolizable energy.
Provided the following per kilogram of diet: vitamin A, 2,200 IU; vitamin D3, 410 IU; vitamin E, 25 IU; vitamin B1, 3.7 mg; niacin, 47.0 mg; Cu, 5.0 mg; Fe, 76.0 mg; Zn, 47.0 mg; Mn, 73.0 mg; Se, 190.0 μg.
2.3. Sample collection
From each replicate, 3 broiler chickens of average weight were selected (totaling 15 broiler chickens per group) after 12 h of overnight fasting. Blood were taken from the jugular vein, and serum was collected after centrifugation 1,000 × g for 15 min at 4 °C. Breast and thigh muscles were collected immediately, followed by flash-freezing in liquid nitrogen and storage at −80 °C. The remaining sample pieces were stored in a freezer at 4 °C to detect the total volatile basic nitrogen (TVB-N) of the thigh muscle at 0, 3, 5, 7, and 9 d.
2.4. Serum biochemical indices
The levels of serum urea nitrogen (SUN), total protein (TP), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), cholesterol (CHO), and triglyceride (TG) were determined using the commercial kits purchased from Jiancheng Bioengineering Institute (Nanjing, China).
2.5. Carcass performance and meat quality
After the blood and feathers were removed, the carcass weight was determined. The head, feet and all organs except the lungs and kidneys were all removed, and then the semi-eviscerated weights were determinate. After removing the lungs and kidneys, the eviscerated weights were obtained. The above indicators were then expressed as percentages of the carcass weight.
Muscle from the left body of chicken was used for the pH, color, and shear force determination. The pH was measured with a pH meter (DPH-2, ATAGO, Tokyo, Japan) and the color was detected with a colorimeter (NR10QC, Shanghai, China) at 45 min and 24 h after slaughter. Shear force was measured with a digital muscle tenderness tester (C-LM3B, Harbin, China). The determination of TVB values was followed the method described by Goulas and Kontominas (2005) and expressed as milligram of TVB-N per 100 g.
2.6. Oil red O staining
Oil red O stain solution was prepared by diluting the stock solution (0.50 g of Oil red O dry powder in 100 mL of 100% isopropyl alcohol) with distilled water at a ratio of 3:2, and let it stand for 10 min before use. The principle of ready-to-use was followed to avoid precipitation of the diluent. The frozen slice with thickness of 8 μm of thigh muscle were washed with distilled water before rinsing in 60% isopropyl alcohol for 20 s, and then placed in Oil red O staining solution for 5 min. Excess dye solution was washed away with 60% isopropyl alcohol, then rinsed the slice with distilled water for 15 s. Then the slices were covered with glycerin gelatin for detection (Sullivan et al., 2013).
2.7. Detection of antioxidant ability
The activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and the content of malondialdehyde (MDA) of the thigh muscle were measured using the commercial kits purchased from Jiancheng Bioengineering Institute (Nanjing, China).
2.8. Intramuscular fat content
Total contents of lipid in breast muscle and thigh muscle were determined using the procedure detailed by Folch et al. (1957). The total lipids were extracted in triplicate from 30-g meat samples and used for fatty acid determination.
2.9. Determination of fatty acid profile
The lipid was then methylated following the method that reported by Kramer et al. (2008). Obtained fatty acid methyl ester was analyzed using a 7890A gas chromatograph (Agilent, Madrid, Spain) equipped with a flame ionization detector, and nonadecanoic acid was used as the reference standard to identify the fatty acids (Bravo-Lamas et al., 2016).
2.10. Determination of the related mRNA abundances
Total RNA was extracted using TRIzol as previously described (Ambion, Beijing, China) (Wan et al., 2018). The synthesis of cDNA was performed using Primer Script (GenStar, Shanghai, China) and used for Real-Time PCR amplification by a T100 Thermal Cycler (GenStar, Shanghai, China). Sequences of the primers were shown in Table 2. Amplifications were performed in a 20-μL reaction volume containing 9 μL of SYBR Green Reagent (Genstar, Beijing, China), 2 μL of primer solution (100 nm), 2 μL of 3 × diluted cDNA, and 6 μL of ultrapure water. IQ5 RT-PCR device (Bio-Rad, California, USA) and 2−ΔΔCt method was used to calculate the relative mRNA abundance (Wan et al., 2018).
Table 2.
Primer sequences for real-time PCR analyses.
| Genes1 | Accession no. | Primer sequences (5′ to 3′) | Amplicon size, bp |
|---|---|---|---|
| PPARγ | NM_001001460 | F: GTGGAGATCGCCCAGGTTTG | 176 |
| R: CAGCTGCACGTGTTCCGTTA | |||
| PGC-1α | NM_001006457.1 | F: GACGTATCGCCTTCTTGCTC | 158 |
| R: CTCGATCGGGAATATGGAGA | |||
| FABP3 | NM_001030889 | F: ACCTGGAAGCTGGTGGATACGG | 140 |
| R: GTCTTCACCGTCGCCTTGTCG | |||
| NRF2 | NM_205117.1 | F: CAGAAGCTTTCCCGTTCATAGA | 120 |
| R: TGGGTGGCTGAGTTTGATTAG | |||
| SOD1 | NM_205064.1 | F: AGATGGCAGTGGGAAATGAG | 110 |
| R: ACTCAAGACAGCAGAGTAGTAATG | |||
| GPX4 | NM_001346448.1 | F: CAGTACAGGGGCTTCGTCTG | 114 |
| R: CAGCCCCTTCTCAGCGTATC | |||
| GADPH | NM_204305 | F: AGATGCAGGTGCTGAGTATG | 113 |
| R: CTGAGGGAGCTGAGATGATAAC |
PPARγ = peroxisome proliferators-activated receptor γ; PGC-1α = PPARγ coactivator 1α; FABP3 = fatty acid-binding protein 3; NRF2 = nuclear factor erythroid-2-related factor 2; SOD1 = superoxide dismutase 1; and GPX4 = glutathione peroxidase 4.
2.11. Statistical analysis
The software of SPSS (version 22.0, Chicago, IL, USA) was used to determine the levels of statistical significance (P = 0.05) using a 2 × 2 factorial treatment design. Differences among the treatments were consider significant when P < 0.05.
3. Results
3.1. Growth performance and serum biochemical indices
The growth performance of yellow-feathered broiler chickens is shown in Table 3. The supplementation of PGZ decreased the F:G ratio and increased the ADG (P = 0.037 and P = 0.043), respectively. Moreover, the F:G ratio was decreased when diets supplemented with LC (P = 0.024). As shown in Table 4, PGZ supplementation decreased the levels of serum LDL-C and CHO levels (P = 0.036 and P = 0.031), whereas the level of serum HDL-C was enhanced when diets supplemented with PGZ (P = 0.008). Dietary supplementation with LC also decreased the level of serum LDL-C (P = 0.021). There was no synergistic effect between PGZ and LC on the LDL-C level (P = 0.166).
Table 3.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the growth performance of broiler chickens (n = 5).
| Item1 | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| ADFI, g | 93.2 | 92.4 | 92.30 | 92.9 | 1.03 | 0.482 | 0.729 | 0.871 |
| ADG, g | 17.8 | 18.1 | 18.8 | 19.2 | 0.33 | 0.043 | 0.184 | 0.104 |
| F:G, g/g | 5.24 | 5.10 | 4.91 | 4.84 | 0.07 | 0.037 | 0.024 | 0.233 |
ADFI = average daily feed intake; ADG = average daily gain; F:G ratio = feed-to-gain ratio.
Table 4.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the serum biochemical indices of broiler chickens (n = 5).
| Item1 | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| SUN, mmol/L | 0.61 | 0.57 | 0.50 | 0.50 | 0.04 | 0.814 | 0.283 | 0.785 |
| TP, g/L | 45.52 | 46.20 | 46.96 | 44.44 | 0.87 | 0.487 | 0.565 | 0.892 |
| HDL-C, mmol/L | 1.85 | 1.87 | 2.54 | 2.60 | 0.05 | 0.008 | 0.752 | 0.457 |
| LDL-C, mmol/L | 0.91 | 0.65 | 0.71 | 0.60 | 0.06 | 0.036 | 0.021 | 0.166 |
| CHO, mmol/L | 3.30 | 2.99 | 2.57 | 2.42 | 0.11 | 0.031 | 0.227 | 0.543 |
| TG, mmol/L | 1.82 | 1.92 | 1.93 | 1.83 | 0.26 | 0.934 | 0.937 | 0.791 |
SUN = serum urea nitrogen; TP = total protein; HDL-C = high-density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; CHO = cholesterol; TG = triglyceride.
3.2. Carcass performance
As shown in Table 5, PGZ increased the dressing percentage and decreased abdominal fat rate (P = 0.006 and P = 0.019). Moreover, the supplementation of PGZ exhibited a tendency toward increased semi-eviscerated yield (P = 0.056).
Table 5.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the carcass performance of broiler chickens (%, n = 5).
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| Dressing percentage | 91.0 | 90.8 | 91.7 | 91.8 | 0.13 | 0.006 | 0.165 | 0.612 |
| Semi-eviscerated yield | 86.5 | 86.5 | 87.1 | 87.0 | 0.21 | 0.056 | 0.639 | 0.808 |
| Eviscerated yield | 69.8 | 69.6 | 67.0 | 69.7 | 0.19 | 0.816 | 0.708 | 0.919 |
| Breast muscle rate | 15.0 | 13.9 | 14.7 | 14.8 | 0.28 | 0.613 | 0.461 | 0.310 |
| Thigh muscle rate | 17.5 | 16.7 | 16.5 | 16.5 | 0.20 | 0.342 | 0.170 | 0.344 |
| Abdominal fat rate | 11.2 | 11.1 | 10.3 | 10.1 | 0.16 | 0.019 | 0.364 | 0.275 |
3.3. Meat quality
The supplementation of LC decreased the cooking loss and drip loss of breast muscle (P = 0.015 and P = 0.028) (Table 6). The IMF content in breast muscle was increased by the PGZ supplementation (P = 0.024).
Table 6.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the meat quality of breast muscles of broiler chickens (n = 5).
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| Shear force, N | 20.32 | 20.71 | 20.05 | 20.60 | 0.41 | 0.956 | 0.118 | 0.139 |
| Drip loss, % | 2.31 | 1.83 | 2.15 | 1.69 | 0.11 | 0.545 | 0.028 | 0.175 |
| Cooking loss, % | 19.03 | 16.62 | 18.59 | 16.76 | 0.32 | 0.960 | 0.015 | 0.178 |
| pH 45 min | 5.93 | 5.95 | 5.97 | 5.98 | 0.03 | 0.427 | 0.140 | 0.194 |
| pH 24 h | 5.78 | 5.86 | 5.77 | 5.82 | 0.25 | 0.449 | 0.327 | 0.506 |
| Color 45 min | ||||||||
| L∗ | 50.3 | 49.3 | 50.5 | 49.5 | 0.34 | 0.620 | 0.723 | 0.449 |
| a∗ | 8.69 | 8.75 | 8.67 | 8.83 | 0.20 | 0.836 | 0.714 | 0.817 |
| b∗ | 9.92 | 9.89 | 9.88 | 9.72 | 0.29 | 0.791 | 0.921 | 0.840 |
| Color 24 h | ||||||||
| L∗ | 51.5 | 49.2 | 51.0 | 49.8 | 0.39 | 0.713 | 0.214 | 0.900 |
| a∗ | 8.40 | 8.32 | 8.25 | 8.43 | 0.12 | 0.564 | 0.463 | 0.320 |
| b∗ | 8.02 | 7.76 | 7.71 | 7.67 | 0.19 | 0.540 | 0.613 | 0.545 |
| Intramuscular fat, % | 0.99 | 1.02 | 1.14 | 1.27 | 0.04 | 0.024 | 0.304 | 0.502 |
L∗=lightness; a∗=redness; b∗=yellowness.
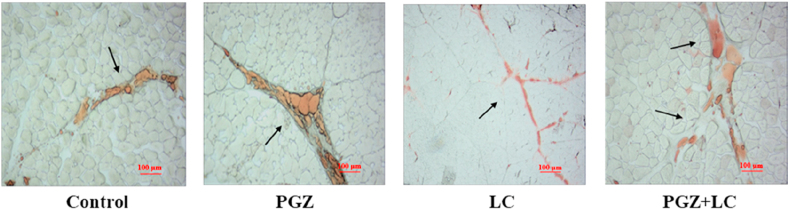
In the thigh muscle, the supplementation of LC decreased the cooking loss and drip loss (P = 0.026 and P = 0.045) (Table 7). Moreover, LC supplementation increased the pH and a∗ value at 24 h after slaughter (P = 0.031 and P = 0.033), and the IMF content was enhanced by PGZ supplementation (P = 0.005). The increased IMF content was illustrated by Oil red O staining (Fig. 1). Moreover, the IMF contents of the thigh muscles were 175.89% to 226.19% higher than those of the breast muscle in each group (Table 6, Table 7).
Table 7.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the meat quality of thigh muscles of broiler chickens (n = 5).
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| Shear force, N | 24.68 | 24.79 | 24.27 | 24.52 | 0.47 | 0.492 | 0.283 | 0.968 |
| Drip loss, % | 1.40 | 1.27 | 1.33 | 1.15 | 0.11 | 0.757 | 0.045 | 0.496 |
| Cooking loss, % | 23.25 | 21.12 | 23.85 | 20.01 | 0.79 | 0.874 | 0.026 | 0.591 |
| pH 45 min | 6.04 | 6.08 | 6.07 | 6.10 | 0.03 | 0.427 | 0.140 | 0.194 |
| pH 24 h | 5.85 | 5.91 | 5.86 | 5.93 | 0.02 | 0.966 | 0.031 | 0.167 |
| Color 45 min | ||||||||
| L∗ | 52.83 | 50.96 | 50.92 | 50.60 | 0.75 | 0.489 | 0.500 | 0.637 |
| a∗ | 12.69 | 12.60 | 12.72 | 12.68 | 0.27 | 0.582 | 0.368 | 0.412 |
| b∗ | 7.39 | 7.66 | 7.49 | 6.88 | 0.26 | 0.556 | 0.762 | 0.440 |
| Color 24 h | ||||||||
| L∗ | 52.2 | 51.3 | 51.9 | 51.4 | 0.51 | 0.911 | 0.226 | 0.429 |
| a∗ | 11.22 | 11.86 | 11.34 | 12.01 | 0.20 | 0.341 | 0.033 | 0.327 |
| b∗ | 13.1 | 13.5 | 13.7 | 12.7 | 0.29 | 0.833 | 0.437 | 0.485 |
| Intramuscular fat, % | 3.02 | 3.13 | 3.75 | 4.11 | 0.15 | 0.005 | 0.764 | 0.461 |
L∗=lightness; a∗=redness; b∗=yellowness.
Fig. 1.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) at 15 mg/kg and L-carnosine (LC) at 400 mg/kg on the Oil red O staining in the thigh muscle of broiler chickens (n = 5). The area pointed by the arrow in the figure represents intramuscular fat.
3.4. Fatty acid composition
The results of fatty acid composition in the thigh muscle of chickens are shown in Table 8. Both PGZ and LC supplementation increased the C18:1n9 and MUFA proportions of the thigh muscle (P < 0.05). Importantly, there were interactions between PGZ or LC and either C18:1n9 or MUFA (P < 0.05). Moreover, the proportion of C18:3n6 was enhanced and C22:0 was reduced by PGZ addition (P < 0.05).
Table 8.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the fatty acid compositions of thigh muscle of broiler chickens (%, n = 5).
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| C14:0 | 0.58 | 0.60 | 0.64 | 0.65 | 0.01 | 0.587 | 0.392 | 0.216 |
| C16:0 | 23.16 | 23.05 | 23.30 | 23.42 | 0.18 | 0.764 | 0.821 | 0.334 |
| C16:1 | 2.81 | 2.85 | 2.83 | 2.75 | 0.07 | 0.815 | 0.901 | 0.735 |
| C18:0 | 8.38 | 8.39 | 8.67 | 8.37 | 0.12 | 0.632 | 0.597 | 0.556 |
| C18:1n9 | 37.25 | 38.49 | 38.61 | 39.72 | 0.31 | 0.003 | 0.017 | 0.022 |
| C18:2n6 | 21.09 | 21.65 | 20.83 | 21.10 | 0.56 | 0.261 | 0.773 | 0.352 |
| C18:3n3 | 1.54 | 1.55 | 1.51 | 1.50 | 0.02 | 0.383 | 0.991 | 0.735 |
| C18:3n6 | 0.15 | 0.15 | 0.18 | 0.18 | 0.01 | 0.027 | 0.543 | 0.484 |
| C22:0 | 0.26 | 0.25 | 0.19 | 0.21 | 0.01 | 0.044 | 0.126 | 0.378 |
| C20:3n3 | 0.32 | 0.31 | 0.34 | 0.35 | 0.03 | 0.664 | 0.752 | 0.319 |
| C20:4n6 | 1.73 | 1.88 | 1.61 | 1.63 | 0.12 | 0.731 | 0.842 | 0.231 |
| SFA | 32.45 | 32.50 | 33.07 | 32.79 | 0.24 | 0.405 | 0.839 | 0.765 |
| MUFA | 40.06 | 41.34 | 41.44 | 42.47 | 0.40 | 0.001 | 0.012 | 0.015 |
| PUFA | 24.89 | 24.99 | 24.14 | 24.07 | 0.51 | 0.672 | 0.824 | 0.707 |
SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid.
3.5. Lipid antioxidant ability and TVB-N values
The antioxidant enzymes activities in the thigh muscle of chickens are shown in Table 9. The supplementation of LC resulted in higher activities of SOD and T-AOC, and lower level of MDA (P < 0.05). In addition, synergistic effects by the combined PGZ and LC supplementation were indicated by MDA levels (P = 0.018). Moreover, LC addition tended to increase GSH-Px activities (P = 0.081). As displayed in Table 10, LC supplementation decreased the TVB-N value of 4 °C refrigerated thigh muscle at 5, 7 and 9 d (P < 0.05).
Table 9.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the antioxidant abilities of thigh muscle of broiler chickens (n = 5).
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| T-AOC, U/mg protein | 0.20 | 0.42 | 0.21 | 0.33 | 0.04 | 0.541 | 0.024 | 0.472 |
| CAT, U/mg protein | 1.98 | 1.95 | 2.05 | 2.12 | 0.07 | 0.503 | 0.904 | 0.759 |
| T-SOD, U/mg protein | 40.03 | 45.71 | 41.10 | 47.30 | 1.01 | 0.660 | 0.035 | 0.695 |
| GSH-Px, U/mg protein | 11.47 | 12.54 | 11.80 | 12.80 | 0.50 | 0.468 | 0.081 | 0.293 |
| MDA, nmoL/mg protein | 5.03 | 3.92 | 4.71 | 3.81 | 0.16 | 0.497 | 0.027 | 0.018 |
T-AOC = total antioxidant capacity; CAT = catalase; GSH-Px = glutathione peroxidase; SOD = superoxide dismutase; MDA = malonaldehyde; prot = protein.
Table 10.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the total volatile basic nitrogen (TVB-N) values (mg/100 g) of the thigh muscle of broiler chickens (n = 5).
| Time1 | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| 0 d | 5.62 | 5.70 | 5.74 | 5.47 | 0.31 | 0.185 | 0.905 | 0.408 |
| 3 d | 10.23 | 10.10 | 10.58 | 10.34 | 0.42 | 0.691 | 0.941 | 0.540 |
| 5 d | 16.43 | 15.25 | 16.57 | 14.92 | 0.54 | 0.781 | 0.036 | 0.721 |
| 7 d | 22.00 | 20.28 | 21.67 | 20.11 | 0.63 | 0.846 | 0.042 | 0.108 |
| 9 d | 29.81 | 26.31 | 28.80 | 26.61 | 0.65 | 0.191 | 0.028 | 0.236 |
Time that meat was stored in the refrigerator.
3.6. mRNA abundance of lipogenesis- and antioxidation-related genes
The mRNA abundance of the lipogenesis- and antioxidation-related genes in the thigh muscle of broiler chickens are displayed in Table 11. The adjusted lipogenesis-related gene mRNA abundance of PPARγ, PPARγ co-activator 1 alpha (PGC-1α) and fatty acid-binding protein 3 (FABP3) were significantly up-regulated by the PGZ supplementation (P < 0.05). Moreover, the mRNA expression levels of genes related to antioxidation, such as nuclear factor erythroid-2-related factor 2 (NRF2) and superoxide dismutase 1 (SOD1), were greatly up-regulated by the LC supplementation (P = 0.004 and P = 0.018).
Table 11.
Effect of dietary supplementation with pioglitazone hydrochloride (PGZ) and l-carnosine (LC) on the mRNA abundances (×10−4) of lipogenesis- and antioxidation-related genes in the thigh muscle of broiler chickens (n = 5)1.
| Item | Treatment |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| PGZ, 0 mg/kg |
PGZ, 15 mg/kg |
|||||||
| LC, 0 mg/kg |
LC, 400 mg/kg |
LC, 0 mg/kg |
LC, 400 mg/kg |
PGZ | LC | PGZ × LC | ||
| PPARγ | 11.59 | 11.67 | 23.90 | 23.98 | 0.04 | 0.001 | 0.725 | 0.605 |
| FABP3 | 3.98 | 4.00 | 5.48 | 5.01 | 0.25 | 0.033 | 0.594 | 0.568 |
| PGC-1α | 9.76 | 10.21 | 14.99 | 16.78 | 0.19 | 0.025 | 0.823 | 0.502 |
| NRF2 | 5.69 | 8.62 | 5.34 | 10.67 | 0.35 | 0.769 | 0.004 | 0.357 |
| SOD1 | 17.31 | 27.03 | 18.63 | 28.88 | 0.24 | 0.219 | 0.018 | 0.561 |
| GPX4 | 2.93 | 3.37 | 3.21 | 2.99 | 0.38 | 0.647 | 0.721 | 0.604 |
PPARγ = peroxisome proliferator-activated receptor γ; FABP3 = fatty acid-binding protein 3; PGC-1α = PPAR γ coactivator 1α; NRF2 = nuclear factor erythroid-2-related factor 2; SOD1 = superoxide dismutase 1; GPX4 = glutathione peroxidase 4.
4. Discussion
Dietary supplementation with PGZ could improve adipocyte differentiation and increase lipid deposition, thus increased ADG of pigs (de Souza et al., 2001). Our results demonstrated that dietary supplementation with PGZ significantly increased the ADG, and decreased the F:G ratio of broiler chickens. These results were in accordance with previous findings by Chen et al. (2013), which might be due to the increased total fat in broiler chickens. Lee et al. (2005) found a significant increase in insulin level after LC ingestion in diabetic mice, and insulin was beneficial to the synthesis of fat and protein. In addition, Bao et al. (2015) also found that LC supplementation increased the ADFI and ADG due to the enhanced levels of serum thyroid hormones, which could promote protein synthesis and body growth (Zhan et al., 2007). Another study revealed that dietary supplementation with LC could decrease the F:G ratio of broiler chickens (Cong et al., 2017). Consistent with previous studies, in our study, the F:G ratio was decreased when diets supplemented with LC.
Our results also indicated that PGZ and LC supplementation decreased the levels of serum TC and LDL-C, and increased the level of serum HDL-C, which was beneficial for reducing serum triglycerides, indicating that lipid utilization was increased and fat metabolism was improved (Boyle et al., 2002; Chen et al., 2013).
Excessive abdominal fat content can negatively affect feed efficiency and carcass performance. The abdominal fat yield decreased with PGZ supplementation, which was more beneficial for IMF deposition (Boyle et al., 2002; Cai et al., 2011). Unlike abdominal fat, IMF is a favorable trait of great economic importance and has a positive effect on the tenderness, flavor, and juiciness of meat (Bonny et al., 2015; Gerbens et al., 2001). Jin et al. (2018b) observed a marked increase in the content of IMF in pigs when diets were supplemented with PGZ. Consistent with this result, our studies also demonstrated that IMF contents were enhanced by PGZ supplementation, which was more beneficial for improving the tenderness and flavor of the meat (Bonny et al., 2015; Costa et al., 2012).
Unsaturated fatty acids can synthesize various bioactive eicosanoid derivatives in inflammation and play a role in promoting vasodilation (Freitas et al., 2018; Vogel et al., 2000). Early studies have indicated that PGZ can improve the profile of PUFA in serum phospholipids and MUFA in rodent muscle (Veleba et al., 2015; Wu et al., 2010). Consistent with these studies, our results indicated that PGZ increased C18:1n-9 and C18:3n-6 proportions in thigh muscle. Moreover, PGZ or LC supplementation increased C18:1n-9 and MUFA levels, and there were synergistic effects between PGZ and LC. The underlying mechanism of these results might be related with the regulation of PGZ mediated lipid metabolism and that of LC as an antioxidant to prevent autoxidation in lipids (Hu et al., 2009; Machado et al., 2019). In this study, LC addition increased the values of pH and a∗ at 24 h after slaughter. These findings were in accordance with findings reported by Cong et al. (2017) and Ma et al. (2010a) when LC was added to the diets of pigs and broiler chickens, respectively. Within limits, pH is closely related to the color grading and drip loss (Zhang et al., 2009). Increases in pH24 h in this study might be due to the ability of LC to regulate intracellular hydrogen ion concentrations and promote the regulation of bicarbonate buffer system (Boldyrev et al., 2013; Vaughan-Jones et al., 2006). A previous study had demonstrated that LC addition could enhance the a∗ value of broiler muscle (Hu et al., 2009). Sánchez-Escalante et al. (2003) also found that the supplementation of LC to ground beef could increase the a∗ value of the meat. The content of metmyoglobin determines the rate of discoloration of meat, which indicated that the improvement of a∗ in thigh muscle at 24 h after slaughter in this study might be due to the increase of the myoglobin content.
Lipid oxidation accelerates the oxidation of oxymyoglobin to myoglobin, which leads to decreased sensory and nutritional qualities and may also form toxic compounds (Faustman et al., 2010; Zhang et al., 2010). Free radicals in meat will attack the subcellular membrane, causing a cytoplasmic outflow and an increasing drip loss. Unsaturated fatty acids are susceptible to free radical attack, and MDA is one of the most important end products (Kim et al., 2012). As an important antioxidant, LC can directly reduce oxidative stress in muscle of broilers (Hu et al., 2009), and its derivatives can also activate antioxidation system (Boldyrev et al., 2013), which can promote metal-chelating and scavenge oxygen free radicals (Davinelli et al., 2013; Kalaz et al., 2012). Similarly, our studies found that LC addition could strengthen antioxidant enzyme defense systems by inhibiting the accumulation of MDA and protecting SOD and GSH-Px from oxidative damage in the thigh meat of yellow-feathered broiler chickens. Moreover, PGZ has been shown to attenuate lipopolysaccharide-induced oxidative stress in neurons by activating NRF2 and heme oxygenase-1, indicating that PGZ also has the potential to improve antioxidant ability in the body (Zakaria et al., 2019). Therefore, the significant PGZ + LC interaction effect on MDA found in this study might be due to the antioxidant abilities of PGZ and LC.
As a volatile substance mainly composed of ammonia, primary ammonia and secondary ammonia, TVB-N is produced by protein decomposition during the process of spoilage of animal food. The degree of amino acid destruction in food is positively correlated with the content of TVB-N, which is an important indicator reflecting the freshness of food. It adversely affects the tyrosine and methionine components in food, leading to a reduction in the nutritional value of food and even harming human health (Cai et al., 2011; Rukchon et al., 2014). In our present study, we found that the TVB-N values decreased with the LC supplementation, which suggested that LC could prolong shelf life and improve meat quality.
To further explore the mechanism, we determined the mRNA abundance of lipogenesis- and antioxidation-relevant genes. In the present study, PGZ addition enhanced the mRNA expression of PPARγ, PGC-1α and FABP3, demonstrating an upregulated transport and accretion of fatty acids in the muscle of PGZ-fed animals. As a PPARγ agonist, PGZ has been shown to enhance the secretion of adipokines and increase adipocyte differentiation of progenitor cells (Czarnowska et al., 2016; Kishida et al., 2014). Moreover, the IMF also increased in the longissimus thoracis muscle by activating PGC-1α and FABP3 (Chen et al., 2013). As the downstream gene of PPARγ, the expression level of FABP3 influenced the level of IMF content (Cho et al., 2011). Therefore, our present study confirmed the hypothesis that the supplementation of PGZ promoted muscle fat deposition by activating the critical gene involved in fat metabolism.
The NRF2 is an important transcription factor that regulates cellular oxidative stress response and maintains intracellular redox homeostasis (Balasubramanian and Longo, 2010). Activated NRF2 induces up-regulation of a series of downstream antioxidant gene expressions, like SOD1, CAT, and GSH, thereby increasing the enzyme activities of antioxidant proteins to reduce cell damage caused by ROS and electrophiles (Loboda et al., 2016). In present study, LC addition promoted the mRNA expression of NRF2 and SOD1, and the antioxidant enzyme activity like SOD, suggesting that the antioxidant enzyme activity is regulated by the expression of related genes (Ma et al., 2010b).
5. Conclusions
Taken together, our results demonstrated that dietary supplementation with PGZ at 15 mg/kg and LC at 400 mg/kg enhanced the growth performance, IMF deposition, antioxidant capacity, and meat shelf life of yellow-feathered broiler chickens. This might be because the supplementation of PGZ and LC up-regulated the mRNA abundance of PPARγ, PGC-1α, FABP3, Nrf2 and SOD1.
Author contributions
Fan Zhang: conceptualization, methodology, writing-original draft preparation; Chenglong Jin: data curation; Xiuqi Wang: visualization, investigation; Huichao Yan: supervision; Huize Tan: software, validation; Chunqi Gao: supervision, writing-reviewing and editing.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was supported by the National Key R&D Program of China (2018YFD0500403), the Natural Science Foundation of Guangdong Province, China (2018B030315001), Guangdong Provincial Promotion Project on Preservation and Utilization of Local Breed of Livestock and Poultry, and the Technical System of Poultry Industry of Guangdong Province, China (2020KJ128).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Balasubramanian P., Longo V.D. Linking Klotho, Nrf2, MAP kinases and aging. Aging (N Y) 2010;2(10):632. doi: 10.18632/aging.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Gao C., Hao W., Ji C., Zhao L., Zhang J. Effects of dietary L-carnosine and alpha-lipoic acid on growth performance, blood thyroid hormones and lipid profiles in finishing pigs. Asian-Australas J Anim Sci. 2015;28(10):1465. doi: 10.5713/ajas.14.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C.P., Andersen H.J. Myoglobin-induced lipid oxidation. A review. J Agric Food Chem. 2002;50(14):3887–3897. doi: 10.1021/jf011394w. [DOI] [PubMed] [Google Scholar]
- Berzaghi P., Dalle Zotte A., Jansson L.M., Andrighetto I. Near-infrared reflectance spectroscopy as a method to predict chemical composition of breast meat and discriminate between different n-3 feeding sources. Poultry Sci. 2005;84(1):128–136. doi: 10.1093/ps/84.1.128. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93(4):1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Bonny S.P.F., Gardner G.E., Pethick D.W., Legrand I., Polkinghorne R.J., Hocquette J.F. Biochemical measurements of beef are a good predictor of untrained consumer sensory scores across muscles. Animal. 2015;9(1):179–190. doi: 10.1017/S1751731114002389. [DOI] [PubMed] [Google Scholar]
- Boyle P.J., King A.B., Olansky L., Marchetti A., Lau H., Magar R. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Therapeut. 2002;24(3):378–396. doi: 10.1016/s0149-2918(02)85040-8. [DOI] [PubMed] [Google Scholar]
- Bravo-Lamas L., Barron L.J., Kramer J.K., Etaio I., Aldai N. Characterization of the fatty acid composition of lamb commercially available in northern Spain: emphasis on the trans-18:1 and CLA content and profile. Meat Sci. 2016;117:108–116. doi: 10.1016/j.meatsci.2016.02.043. [DOI] [PubMed] [Google Scholar]
- Cai J., Chen Q., Wan X., Zhao J. Determination of total volatile basic nitrogen (TVB-N) content and Warner–Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011;126(3):1354–1360. [Google Scholar]
- Chabowski A., Żendzian-Piotrowska M., Nawrocki A., Gorski J. Not only accumulation, but also saturation status of intramuscular lipids is significantly affected by PPARγ activation. Acta Physiol. 2012;205(1):145–158. doi: 10.1111/j.1748-1716.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- Chen X., Feng Y., Yang W.J., Shu G., Jiang Q.Y., Wang X.Q. Effects of dietary thiazolidinedione supplementation on growth performance, intramuscular fat and related genes mRNA abundance in the longissimus dorsi muscle of finishing pigs. Asian-Australas J Anim Sci. 2013;26(7):1012. doi: 10.5713/ajas.2012.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.H., Kim M.J., Jeon G.J., Chung H.Y. Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol Biol Rep. 2011;38(3):2161–2166. doi: 10.1007/s11033-010-0344-3. [DOI] [PubMed] [Google Scholar]
- Cong J., Zhang L., Li J., Wang S., Gao F., Zhou G. Effects of dietary supplementation with carnosine on meat quality and antioxidant capacity in broiler chickens. Br Poultry Sci. 2017;58(1):69–75. doi: 10.1080/00071668.2016.1237767. [DOI] [PubMed] [Google Scholar]
- Costa P., Lemos J.P., Lopes P.A., Alfaia C.M., Costa A.S.H., Bessa R.J.B. Effect of low-and high-forage diets on meat quality and fatty acid composition of Alentejana and Barrosã beef breeds. Animal. 2012;6(7):1187–1197. doi: 10.1017/S1751731111002722. [DOI] [PubMed] [Google Scholar]
- Czarnowska E., Domal-Kwiatkowska D., Reichman-Warmusz E., Bierla J.B., Sowinska A., Ratajska A. The correlation of pparα activity and cardiomyocyte metabolism and structure in idiopathic dilated cardiomyopathy during heart failure Progression. PPAR Res. 2016:1–12. doi: 10.1155/2016/7508026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davinelli S., Di Marco R., Bracale R., Quattrone A., Zella D., Scapagnini G. Synergistic effect of L-Carnosine and EGCG in the prevention of physiological brain aging. Curr Pharmaceut Des. 2013;19(15):2722–2727. doi: 10.2174/1381612811319150007. [DOI] [PubMed] [Google Scholar]
- de Souza C.J., Eckhardt M., Gagen K., Dong M., Chen W., Laurent D. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50(8):1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- Engel E., Ratel J., Bouhlel J., Planche C., Meurillon M. Novel approaches to improving the chemical safety of the meat chain towards toxicants. Meat Sci. 2015;109:75–85. doi: 10.1016/j.meatsci.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Faustman C., Sun Q., Mancini R., Suman S.P. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 2010;86(1):86–94. doi: 10.1016/j.meatsci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Gariballa S.E., Sinclair A.J. Carnosine: physiological properties and therapeutic potential. Age Ageing. 2000;29(3):207–210. doi: 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- Gerbens F., Verburg F.J., Van Moerkerk H.T.B., Engel B., Buist W., Veerkamp J.H. Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs. J Anim Sci. 2001;79(2):347–354. doi: 10.2527/2001.792347x. [DOI] [PubMed] [Google Scholar]
- Goulas A.E., Kontominas M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93(3):511–520. [Google Scholar]
- Hu X., Hongtrakul K., Ji C., Ma Q., Guan S., Song C. Effect of carnosine on growth performance, carcass characteristics, meat quality and oxidative stability in broiler chickens. J Poult Sci. 2009;46(4):296–302. [Google Scholar]
- Jiang S., Gou Z., Li L., Lin X., Jiang Z. Growth performance, carcass traits and meat quality of yellow-feathered broilers fed graded levels of alfalfa meal with or without wheat. Anim Sci J. 2018;89(3):561–569. doi: 10.1111/asj.12968. [DOI] [PubMed] [Google Scholar]
- Jin C.L., Gao C.Q., Wang Q., Zhang Z.M., Xu Y.L., Li H.C. Effects of pioglitazone hydrochloride and vitamin E on meat quality, antioxidant status and fatty acid profiles in finishing pigs. Meat Sci. 2018;145:340–346. doi: 10.1016/j.meatsci.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Jin C.L., Wang Q., Zhang Z.M., Xu Y.L., Yan H.C., Li H.C. Dietary supplementation with pioglitazone hydrochloride and chromium methionine improves growth performance, meat quality, and antioxidant ability in finishing pigs. J Agric Food Chem. 2018;66(17):4345–4351. doi: 10.1021/acs.jafc.8b01176. [DOI] [PubMed] [Google Scholar]
- Kalaz E.B., Evran B., Develi-Is S., Vural P., Dogru-Abbasoglu S., Uysal M. Effect of carnosine on prooxidant–antioxidant balance in several tissues of rats exposed to chronic cold plus immobilization stress. J Pharmacol Sci. 2012;120(2):98–104. doi: 10.1254/jphs.12107fp. [DOI] [PubMed] [Google Scholar]
- Kim J.E., Clark R.M., Park Y., Lee J., Fernandez M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of Guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract. 2012;6(2):113–119. doi: 10.4162/nrp.2012.6.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida K., Nakagawa Y., Kobayashi H., Mazaki T., Yokoi H., Yanagi K. High serum C1q-binding adiponectin levels in male patients with acute coronary syndrome. Cardiovasc Diabetol. 2014;13(1):9. doi: 10.1186/1475-2840-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.K., Hernandez M., Cruz-Hernandez C., Kraft J., Dugan M.E. Combining results of two GC separations partly achieves determinations of all cis and trans 16:1, 18:1, 18:2, 18:3 and CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids. 2008;43:259–273. doi: 10.1007/s11745-007-3143-4. [DOI] [PubMed] [Google Scholar]
- Lee Y.T., Hsu C.C., Lin M.H., Liu K.S., Yin M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol. 2005;513(1–2):145–150. doi: 10.1016/j.ejphar.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.Y., Jiang Z.Y., Lin Y.C., Zheng C.T., Zhou G.L. Dietary supplementation with carnosine improves antioxidant capacity and meat quality of finishing pigs. J Anim Physiol Anim Nutr. 2010;94(6):e286–e295. doi: 10.1111/j.1439-0396.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- Ma X., Lin Y., Jiang Z., Zheng C., Zhou G., Yu D. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids. 2010;38(1):95–102. doi: 10.1007/s00726-008-0213-8. [DOI] [PubMed] [Google Scholar]
- Machado M.M.F., Bassani T.B., Cóppola-Segovia V., Moura E.L.R., Zanata S.M., Andreatini R. PPAR-γ agonist pioglitazone reduces microglial proliferation and NF-κB activation in the substantia nigra in the 6-hydroxydopamine model of Parkinson's disease. Pharmacol Rep. 2019;71(4):556–564. doi: 10.1016/j.pharep.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China. Feeding Standard of Chicken . China Agricultural Press; Beijing, China: 2004. Nutrient Requirements of Chinese Color-feather Chicken. Feeding Standard of Chicken. 2nd edn. (In Chinese) [Google Scholar]
- Rukchon C., Nopwinyuwong A., Trevanich S., Jinkarn T., Suppakul P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta. 2014;130:547–554. doi: 10.1016/j.talanta.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Sánchez-Escalante A., Djenane D., Torrescano G., Giménez B., Beltrán J.A., Roncalés P. Evaluation of the antioxidant ability of hydrazine-purified and untreated commercial carnosine in beef patties. Meat Sci. 2003;64(1):59–67. doi: 10.1016/s0309-1740(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Sullivan K., El-Hoss J., Quinlan K.G., Deo N., Garton F., Seto J.T. NF1 is a critical regulator of muscle development and metabolism. Hum Mol Genet. 2013;23(5):1250–1259. doi: 10.1093/hmg/ddt515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M.K., Watt M.J., Le J., Hevener A.L., Turcotte L.P. Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol-Endocrinol Metabol. 2007;292(2):E485–E493. doi: 10.1152/ajpendo.00080.2006. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R.D., Spitzer K.W., Swietach P. Spatial aspects of intracellular pH regulation in heart muscle. Prog Biophys Mol Biol. 2006;90(1–3):207–224. doi: 10.1016/j.pbiomolbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Veleba J., Kopecky J., Janovska P., Kuda O., Horakova O., Malinska H. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: improvement of lipid metabolism. Nutr Metab. 2015;12(1):52. doi: 10.1186/s12986-015-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R.A., Corretti M.C., Plotnick G.D. The postprandial effect of components of the Mediterranean diet on endothelial function. J Am Coll Cardiol. 2000;36(5):1455–1460. doi: 10.1016/s0735-1097(00)00896-2. [DOI] [PubMed] [Google Scholar]
- Wan X., Ahmad H., Zhang L., Wang Z., Wang T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J Sci Food Agric. 2018;98(10):3715–3721. doi: 10.1002/jsfa.8879. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78(4):343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wu X., Yin Y.L., Li T.J., Wang L., Ruan Z., Liu Z.Q. Dietary protein, energy and arginine affect LAT1 expression in forebrain white matter differently. Animal. 2010;4(9):1518–1521. doi: 10.1017/S1751731110000534. [DOI] [PubMed] [Google Scholar]
- Zakaria A., Rady M., Mahran L., Abou-Aisha K. Pioglitazone attenuates lipopolysaccharide-induced oxidative stress, dopaminergic neuronal loss and neurobehavioral impairment by activating Nrf2/ARE/HO-1. Neurochem Res. 2019:1–13. doi: 10.1007/s11064-019-02907-0. [DOI] [PubMed] [Google Scholar]
- Zhan X.A., Wang M., Ren H., Zhao R.Q., Li J.X., Tan Z.L. Effect of early feed restriction on metabolic programming and compensatory growth in broiler chickens. Poultry Sci. 2007;86(4):654–660. doi: 10.1093/ps/86.4.654. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yue H.Y., Zhang H.J., Xu L., Wu S.G., Yan H.J. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poultry Sci. 2009;88(10):2033–2041. doi: 10.3382/ps.2009-00128. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J Agric Food Chem. 2010;59(3):969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]