Abstract
The aim of this study was to determine the apparent total tract digestibility (ATTD) of nutrients in cottonseed meal (CSM) and soybean meal (SBM) in simple carbohydrate and more complex wheat-based diets using 2 indigestible markers and total faecal collection. Twenty-five Large White × Landrace boars (57.8 kg) were randomly allocated to either a pure wheat diet, 40% CSM or SBM in either a sugar-starch- (1:1) or wheat-based diet for 18 d. Acid-insoluble ash (AIA) and chromic oxide (Cr2O3) were included in all diets as indigestible markers. Diets were offered (1,800 g/d per pig) in 3 meals/d from d 1 to 11 and 8 meals/d from d 12 to 17. On d 9, the pigs were moved to individual metabolism cages to allow total faecal collection. On d 18, the pigs were fed hourly for 8 h. After the 8th meal, pigs were anaesthetized and digesta sampled from the terminal ileum and rectum before lethal injection. There were no differences between ATTD of nitrogen (N) determined using AIA as a marker and measured by total faecal collection. On the other hand, the ATTD of N of diets containing CSM in sugar-starch- or wheat-based diets and the pure wheat diet determined using Cr2O3 as a marker was less (−3.11%, −4.46% and −6.59%; P < 0.001) than that measured by total faecal collection. The ATTD of N determined using AIA as a marker was highly correlated with that measured using total faecal collection (P < 0.001; R2 = 0.95). Similarly, the ATTD of N determined using Cr2O3 as a marker was correlated with that measured using total faecal collection, although the correlation was not quite as strong as using AIA (P < 0.001; R2 = 0.87). Also, the slope of the regression line and the intercept were closer to unity and zero for the relationship when the ATTD of N was determined using AIA compared to Cr2O3 as an indigestible marker. The ATTD of organic and dry matter behaved similarly. These data demonstrate that the basal diet and choice of indigestible marker can substantially influence the ATTD and that the use of AIA as an indigestible marker is more suitable than Cr2O3 in digestibility studies in pigs.
Keywords: Organic matter, Pig, Wheat, Cottonseed meal, Soybean meal, Digestibility, Nitrogen
1. Introduction
The classic total collection method which quantitatively measures feed intake and ileal and/or total tract digesta output for determining digestibility of feedstuffs is laborious and time consuming. The method is particularly impracticable when there are no equipment nor conditions suitable for directly measuring the total amount of feed intake and output of ileal or faecal digesta (Zhang and Adeola, 2017).
The use of indigestible markers in digestibility studies offers the advantages of reduced time and labour (Van Keulen and Young, 1977) as well as the need for metabolism cages to facilitate total faecal collection. The inclusion of an indigestible marker is essential when the partial digesta sampling technique is used for determining apparent ileal or total tract digestibility (AID or ATTD) (Jagger et al., 1992). However, the use of metal elements as indigestible markers does present some problems. For example, inclusion of chromium (as chromic oxide, Cr2O3) in the diet at a low level may result in inaccurate digestibility measurements due to analytical errors, whereas inclusion at higher levels may depress feed intake (Jagger et al., 1992). Moreover, the use of metal elements (e.g. iron and chromium) as indigestible markers for routine digestibility studies could cause environmental issues (Van Leeuwen et al., 1996) as well as concern about the use of chromium in animal and human diets (EFSA, 2010).
Several researchers have suggested acid-insoluble ash (AIA) as an alternative neutral marker to Cr2O3 for determining digestibility of feeds in pigs (McCarthy et al., 1974; Moughan et al., 1991; Rowan et al., 1991; Van Barneveld et al., 1995; Prawirodigdo et al., 2019) whereas others maintain that Cr2O3 is more suitable as a marker than AIA (Bakker and Jongbloed, 1994; Van Leeuwen et al., 1996). Yin et al. (2000) found that the digestibility values of feed in pigs determined using Cr2O3 as a marker was lower compared with those determined using the total faecal collection method. Similarly, Prawirodigdo et al. (2019) found the use of Cr2O3 as an indigestible marker resulted in lower AID and ATTD than AIA, particularly for diets containing cottonseed meal (CSM). These authors also found that the choice of basal diet could also impact on the AID and ATTD. Thus, the aim of the present experiment was to evaluate the use of AIA and Cr2O3 as indigestible markers by comparing the digestibility of nitrogen (N), organic matter (OM) and dry matter (DM) in pigs fed CSM and soybean meal (SBM) determined with both markers with the total faecal collection method using simple (sugar-starch) and complex (cereal wheat grain) basal diets.
2. Materials and methods
2.1. Diets, animals and experimental design
The protocol used in this experiment conformed to all Animal Experimentation Ethics Committee regulations concerning the health and care of experimental animals and was approved by the Victorian Institute of Animal Science Animal Ethics Committee, Australia.
The amino acid composition of the CSM, SBM and wheat used in formulating the 5 diets used in the present study is listed in Table 1, and the total diets are provided in Table 2. The basal control diet contained 926 g of wheat/kg with wheat providing the sole source of dietary carbohydrate and protein. The other 4 diets contained 400 g/kg CSM in a sugar-starch (1:1, wt/wt) base or a wheat base and 400 g/kg SBM in both basal diets. Iron sulphate heptahydrate (FeSO4·7H2O) was added (2 g/kg) to the CSM diets to inactivate the gossypol present in CSM (Knabe et al., 1979). Chromic oxide and AIA (Celite, a diatomaceous earth produced by Celite Cooperation Lompoc, California, USA) were included in the diets (2 g/kg) as indigestible markers. The SBM, CSM and wheat contained 462, 371 and 130 g crude protein/kg, respectively.
Table 1.
Analyzed protein and amino acid profiles of the cottonseed meal, soybean meal and wheat used in the present experiment (g/kg, air-dry basis).
| Item | Cottonseed meal | Soybean meal | Wheat |
|---|---|---|---|
| Dry matter | 911 | 884 | 899 |
| Organic matter | 849 | 821 | 888 |
| Crude protein | 371 | 462 | 130 |
| Indispensable amino acids | |||
| Arginine | 38.1 | 32.8 | 6.7 |
| Histidine | 11.4 | 14.4 | 2.9 |
| Isoleucine | 12.4 | 22.9 | 4.9 |
| Leucine | 20.6 | 34.3 | 8.9 |
| Lysine | 15.9 | 28.4 | 3.5 |
| Phenylalanine | 18.9 | 25.0 | 6.9 |
| Threonine | 11.8 | 21.1 | 3.8 |
| Tyrosine | 8.9 | 17.3 | 4.5 |
| Valine | 17.5 | 23.6 | 6.1 |
| Dispensable amino acids | |||
| Alanine | 13.8 | 21.0 | 4.6 |
| Aspartic acid | 30.9 | 50.6 | 6.4 |
| Glutamic acid | 67.0 | 77.2 | 36.8 |
| Glycine | 14.6 | 19.2 | 5.3 |
| Proline | 13.4 | 24.4 | 13.3 |
| Serine | 13.4 | 26.0 | 13.3 |
Table 2.
Composition of the experimental diets (g/kg, air-dry basis).
| Item | Diets |
||||
|---|---|---|---|---|---|
| Wheat | Cottonseed meal |
Soybean meal |
|||
| Simple | Wheat | Simple | Wheat | ||
| Ingredients | |||||
| Cottonseed meal | – | 400 | 400 | ||
| Soybean meal | – | – | – | 400 | 400 |
| Wheat | 926 | – | 526 | – | 526 |
| Cane sugar (sucrose) | – | 263 | – | 263 | – |
| Starch (wheat starch) | – | 263 | – | 263 | – |
| FeSO4·7H2O | – | 2.0 | 2.0 | – | – |
| K2SO4 | 1.5 | – | – | 1.5 | 1.5 |
| Minerals + vitamins premix1 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Dicalcium phosphate | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Cr2O3 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Acid-insoluble ash (Celite) | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Tallow | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 |
| Determined analysis | |||||
| Dry matter | 895 | 917 | 897 | 906 | 887 |
| Organic matter | 856 | 871 | 829 | 859 | 819 |
| Crude protein | 120 | 149 | 216 | 185 | 247 |
| Indispensable amino acids | |||||
| Arginine | 5.4 | 13.8 | 16.4 | 12.9 | 14.6 |
| Histidine | 2.7 | 3.4 | 5.5 | 4.7 | 6.5 |
| Isoleucine | 4.7 | 4.5 | 7.1 | 9.0 | 11.3 |
| Leucine | 8.6 | 7.8 | 1.3 | 14.2 | 18.1 |
| Lysine | 6.8 | 6.0 | 7.8 | 11.4 | 12.8 |
| Phenylalanine | 6.1 | 7.6 | 10.4 | 9.7 | 12.2 |
| Threonine | 5.3 | 4.0 | 6.7 | 7.3 | 10.8 |
| Valine | 5.9 | 6.3 | 9.7 | 9.4 | 12.4 |
| Dispensable amino acids | |||||
| Alanine | 4.6 | 5.1 | 7.7 | 8.1 | 10.6 |
| Aspartic acid | 6.6 | 11.3 | 15.6 | 20.3 | 22.9 |
| Glutamic acid | 36.5 | 24.1 | 43.8 | 33.1 | 50.1 |
| Glycine | 5.3 | 5.4 | 8.3 | 7.7 | 10.2 |
| Proline | 3.3 | 4.6 | 12.3 | 9.2 | 16.5 |
| Tyrosine | 4.0 | 4.0 | 5.7 | 6.5 | 8.7 |
| Serine | 6.7 | 5.6 | 9.6 | 9.7 | 13.6 |
| Calculated analysis | |||||
| Digestible energy2, MJ/kg | 14.4 | 14.3 | 14.1 | 14.9 | 14.7 |
The minerals and vitamins premix were added to contribute the following for per kilogram of air-dried diet: retinol, 6.4 mg; cholecalciferol, 0.083 mg; α-tocopherol, 22 mg; menadione, 0.60 mg; riboflavin, 3.3 mg; nicotinic acid, 16.5 mg; pantothenic acid, 5.5 mg; pyridoxine, 1.1 mg; biotin, 0.056 mg; choline, 1,100 mg; cyanocobalamin, 0.017 mg; Fe, 88 mg; Zn, 55 mg; Mn, 22 mg; Cu, 6.6 mg; I, 0.22 mg; Se, 0.1 mg.
Estimated from ingredients.
Twenty-five Large White × Landrace entire male pigs (57.8 ± 2.3 kg, mean ± standard deviation) were individually penned and randomly assigned to 1 of the 5 experimental diets in a completely randomized design. The experimental design was a 2 × 2 × 3 factorial with an added control with the respective factors being 2 basal diets (sugar-starch vs. wheat), 2 protein sources (CSM and SBM) and 3 methods of measuring digestibility (total collection vs. Cr2O3 vs. AIA). The experimental diets were offered to pigs in a mash form for 18 d. Water was constantly available via nipple drinkers during the experimental period. The diets were introduced to the pigs over a 3-d period. Rations were offered (1,800 g/pig per d) in 3 meals/d (at 06:00, 12:00 and 18:00) from d 1 to 9 and in 8 meals/d (every 3 h) from d 9 to 17. On d 9 the pigs were moved to individual metabolism cages to allow total faecal collection. A metal tray was fitted underneath the rear part of each metabolism cage to enable total collection of voided faeces. Faeces were collected daily for a 7-d collection period, bulked in plastic bags according to each pig unique identification number and stored frozen.
Commencing at 01:00 on d 18, the pigs received their daily allocation of feed in 8 portions that were fed hourly. After the 8th meal, each pig was sedated using 5 mL of Stresnil (Janssen Pharmaceutical, Beerse, Belgium) injected intramuscularly and left undisturbed in their pens for 15 min. The sedated pigs were then anaesthetized by inhalation of isoflurane (Rhone Merieux, Australia). After each pig was anaesthetized a ventral abdominal midline incision was made, the caecum located and then a 150-cm portion of the terminal ileum and the rectum were excised to enable simultaneous collection of the ileal and faecal digesta (Prawirodigdo et al., 1998). The ileal digesta was gently expelled, collected and stored at −20 °C until analysis. Faecal digesta was also expelled, collected and stored frozen until analysis. Finally, the anaesthetized pigs were killed with a lethal injection (15 mL) of pentobarbitone sodium (Valabarb 300 mg/mL; Boehringer Ingelheim Pty. Ltd, NSW, Australia) administered directly into the vena cava. Ileal and faecal digesta from all of the pigs were freeze-dried, finely ground and analyzed for N, OM, DM, chromium and AIA contents.
2.2. Chemical analyses
Dry matter contents of wheat, CSM, SBM and experimental diets were determined by drying for 16 h in a forced-air oven at 100 °C (AOAC, 1990). Organic matter was measured in the wheat, CSM and SBM, experimental diets, and ileal and faecal digesta by combustion of the samples in a furnace chamber at 650 °C for 4 h (AOAC, 1990). Total N content of the samples was determined using a Micro Kjeldahl method (AOAC, 1990). The amino acid contents of the diet and ileal digesta were determined following acid hydrolysis in 6 mol/L hydrochloric acid. The mixture of amino acids was separated on an HPLC ion exchange (strong cation exchange) column (Waters Australia Pty. Ltd., Box Hill, Victoria, Australia) using post column derivatization with ninhydrin (Rayner, 1985).
Contents of chromium in the diets, ileal and faecal digesta were determined using a modification of the atomic absorption spectrophotometric method of Williams et al. (1962) and the chromium contents used to calculate AID and ATTD as described by Saha and Gilbreath (1993). The content of AIA in the diets and digesta was determined using a gravitation technique, modified from the technique described by McCarthy et al. (1974). Since Cr2O3 is insoluble in 4 mol/L hydrochloric acid (McCarthy et al., 1974), when AIA is simultaneously used as an inert marker with Cr2O3 in a diet, the non-Cr2O3 AIA values should be obtained by difference (McCarthy et al., 1974). Therefore, non-Cr2O3 AIA was calculated as total AIA content minus Cr2O3 content.
The Cr2O3 and AIA contents of the ileal and faecal digesta samples obtained under anaesthesia were used for estimating the AID and ATTD of N, OM and DM. The ATTD of N, OM and DM were also determined using total N, DM and OM intake and total faecal output measured by total faecal collection.
2.3. Statistical analyses
The study was a completely randomized design without blocking with treatments arranged in 2 × 2 × 3 factorial with an added control for ATTD. The ATTD data were analyzed using the restricted maximum likelihood procedure (REML) for the main and interactive effects of protein meal (CSM vs. SBM), basal diet (sugar-starch vs. wheat) and method of determination (total collection vs. Cr2O3 vs. AIA) with the pig as a random factor. Similarly, the AID data were also analyzed using REML for the main and interactive effects of protein meal (CSM vs. SBM), basal diet (sugar-starch vs. wheat) and method of determination (Cr2O3 vs. AIA) with the pig as a random factor. Where interactions occurred (P < 0.050), treatment means were separated by post hoc analysis using the least significance difference (LSD) method. The relationships between ATTD of N, OM and DM determined using either the total faecal collection method or using Cr2O3 and AIA as indigestible markers were determined using linear regression unadjusted for the base diet or protein meal and t-tests used to determine whether the intercept and regression coefficient were different than zero. To check whether the regression coefficient was different from unity the actual value for each marker and nutrient was subtracted from the value for ATTD for each nutrient. These differences were then regressed against the actual vale for each marker and nutrient to test whether the slope was different from zero. All statistical analyses were performed using GENSTAT version 18 (VSN International Ltd, Hertfordshire, UK) and P-value of <0.050 was set as the threshold for significance.
3. Results
3.1. Relationship between apparent faecal digestibility determined using the acid-insoluble ash or chromic oxide and total faecal collection methods
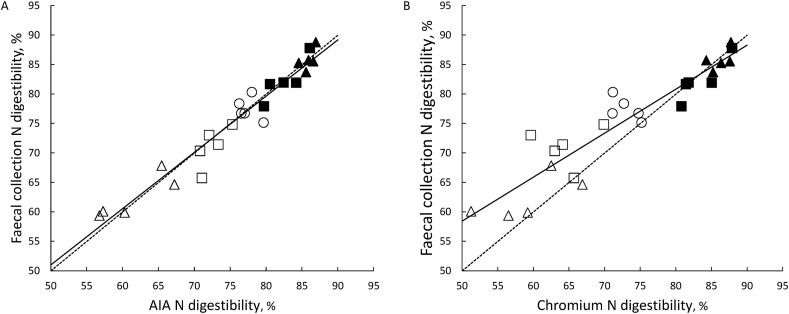
The ATTD of N in the experimental diets determined using AIA as an indigestible marker was correlated with that measured using total faecal collection (Fig. 1A, P < 0.001; R2 = 0.94). Similarly, ATTD of N determined using Cr2O3 as an indigestible marker was significantly correlated with that measured using total faecal collection, although the correlation was not quite as strong as using AIA (Fig. 1B, P < 0.001; R2 = 0.86). Also, the slope of the regression line and the intercept were not different from unity (P = 0.36) and zero (P = 0.39) for the relationship when ATTD of N was determined using AIA as an indigestible marker (Fig. 1A). On the other hand, when Cr2O3 was used as an indigestible marker, the slope of the regression line was less than unity (P < 0.001), and the intercept was greater than zero (P < 0.001) (Fig. 1B).
Fig. 1.
Relationship between apparent total tract digestibility (ATTD) of nitrogen (N) determined using either (A) acid-insoluble ash (AIA) or (B) chromic oxide (Cr2O3) as an indigestible marker and determined using total faecal collection. Data are for individual pigs (n = 5 for each diet) fed either CSM in a sugar-starch-based (Δ), CSM in a wheat-based (□), SBM in a sugar-starch-based (▲), SBM in a wheat-based (■) or a wheat (○) diet. The line of unity is represented by the dashed line while the solid line is the linear regression line. The regression equations are (A) y = 3.25 (SE 3.71) + 0.955 (SE 0.0484)x, R2 = 0.941 and (B) y = 21.1 (SE 4.56) + 0.747 (SE 0.0616)x, R2 = 0.859.
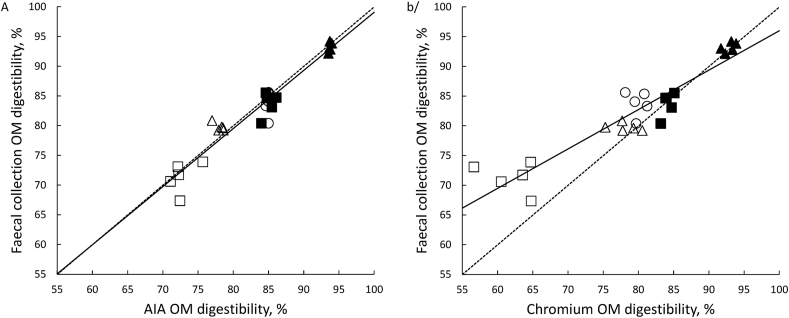
The ATTD of OM in the experimental diets determined using AIA as an indigestible marker was correlated with that measured using total faecal collection (Fig. 2A, P < 0.001; R2 = 0.93). Similarly, ATTD of OM determined using Cr2O3 as an indigestible marker was significantly correlated with that measured using total faecal collection, although the correlation was not quite as strong as using AIA (Fig. 2B, P < 0.001; R2 = 0.88). Also, the slope of the regression line and the intercept were not different from unity (P = 0.70) and zero (P = 0.78) for the relationship when ATTD of N was determined using AIA as an indigestible marker (Fig. 2A). On the other hand, when Cr2O3 was used as an indigestible marker, the slope of the regression line was less than unity (P < 0.001), and the intercept was greater than zero (P < 0.001) (Fig. 2B).
Fig. 2.
Relationship between apparent total tract digestibility (ATTD) of organic matter (OM) determined using either (A) acid-insoluble ash (AIA) or (B) chromic oxide (Cr2O3) as an indigestible marker and determined using total faecal collection. Data are for individual pigs (n = 5 for each diet) fed either CSM in a sugar-starch-based (Δ), CSM in a wheat-based (□), SBM in a sugar-starch-based (▲), SBM in a wheat-based (■) or a wheat (○) diet. The line of unity is represented by the dashed line while the solid line is the linear regression line. The regression equations are (A) y = 1.29 (SE 4.66) + 0.978 (SE 0.0561)x, R2 = 0.927 and (B) y = 29.7 (SE 4.24) + 0.663 (SE 0.0529)x, R2 = 0.867.
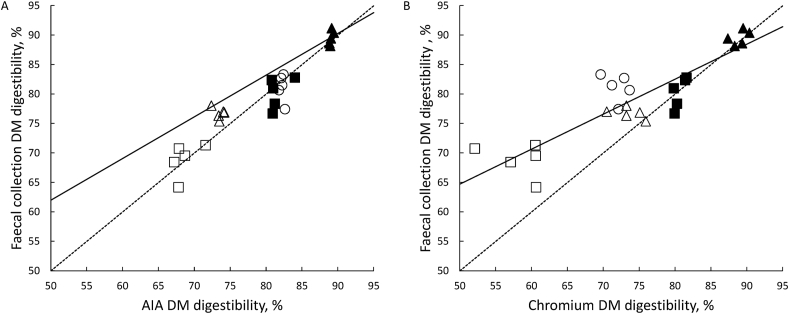
The ATTD of DM in the experimental diets determined using AIA as an indigestible marker was correlated with that measured using total faecal collection (Fig. 3A, P < 0.001; R2 = 0.88). Similarly, ATTD of DM determined using Cr2O3 as an indigestible marker was significantly correlated with that measured using total faecal collection, although the correlation was not quite as strong as using AIA (Fig. 3B, P < 0.001; R2 = 0.74). Also, the slope of the regression line and the intercept were not different from unity (P = 0.20) and zero (P = 0.19) for the relationship when ATTD of DM was determined using AIA as an indigestible marker (Fig. 3A). On the other hand, when Cr2O3 was used as an indigestible marker, the slope of the regression line was less than unity (P < 0.001), and the intercept was greater than zero (P < 0.001) (Fig. 3B).
Fig. 3.
Relationship between apparent total tract digestibility (ATTD) of dry matter (DM) determined using either (A) acid-insoluble ash (AIA) or (B) chromic oxide (Cr2O3) as an indigestible marker and determined using total faecal collection. Data are for individual pigs (n = 5 for each diet) fed either CSM in a sugar-starch-based (Δ), CSM in a wheat-based (□), SBM in a sugar-starch-based (▲), SBM in a wheat-based (■) or a wheat (○) diet. The line of unity is represented by the dashed line while the solid line is the linear regression line. The regression equations are (A) y = 7.37 (SE 5.39) + 0.910 (SE 0.0680)x, R2 = 0.881 and (B) y = 35.0 (SE 4.98) + 0.594 (SE 0.0661)x, R2 = 0.769.
3.2. Apparent faecal digestibility of nitrogen, organic matter and dry matter determined using 3 methods of determination
There were no differences between ATTD of N determined using AIA as an indigestible marker and measured by total faecal collection (Table 3). On the other hand, ATTD of N in diets containing CSM and the pure wheat diets determined using Cr2O3 as a marker was significantly less (−3.11%, −4.46% and −6.59%; P < 0.001) than that measured using total faecal collection. There were significant 2- and 3-way interactions between protein source, basal diet and method, such that ATTD of N in SBM was higher in the sugar-starch-based diet than the wheat-based diet, whereas the opposite was true for CSM, especially when determined using Cr2O3 as a marker.
Table 3.
Effect of method, protein source and basal diet on the apparent total tract digestibility (AID, %) of nitrogen, organic matter and dry matter of the experimental diets1.
| Item | Wheat | Cottonseed meal |
Soybean meal |
SED | Significance2 | ||
|---|---|---|---|---|---|---|---|
| Simple | Wheat | Simple | Wheat | ||||
| Nitrogen | |||||||
| Chromic oxide | 72.5 | 59.3 | 64.5 | 86.2 | 83.4 | 1.18 | Prot. (<0.001); BD (0.034); Prot. × BD (<0.001) |
| Acid-insoluble ash | 77.4 | 62.4 | 71.1 | 85.8 | 82.2 | Prot. × BD × M (<0.001) | |
| Total faecal collection | 77.5 | 61.4 | 72.5 | 85.9 | 82.6 | ||
| Organic matter | |||||||
| Chromic oxide | 79.9 | 78.1 | 62.1 | 92.9 | 84.2 | 1.02 | Prot. (<0.001); BD (<0.001) |
| Acid-insoluble ash | 83.8 | 79.7 | 71.3 | 93.2 | 83.7 | Prot. × BD × M (<0.001) | |
| Total faecal collection | 84.9 | 78.1 | 72.7 | 93.7 | 85.0 | ||
| Dry matter | |||||||
| Chromic oxide | 71.9 | 73.6 | 58.2 | 89.0 | 80.6 | 1.17 | Prot. (<0.001); BD (<0.001) |
| Acid-insoluble ash | 81.1 | 76.7 | 68.8 | 89.6 | 80.2 | Prot. × BD × M (<0.001) | |
| Total faecal collection | 82.2 | 73.5 | 68.6 | 89.1 | 81.6 | ||
SED = standard error of difference.
The basal diet was either a 1:1 sugar and starch mix (Simple) or pure wheat (Wheat); protein sources were either 400 g/kg cottonseed meal or soybean meal mixed with the basal diet or diet containing 926 g/kg wheat. n = 5 for each diet.
Significance of effects of pure wheat (926 g/kg) vs. other diets, protein source (Prot.), Basal diet (BD) or measurement method (M) and interactions have been included in parentheses.
There was no difference between ATTD of OM determined using AIA as an indigestible marker and that measured using total faecal collection (Table 3). However, the ATTD of OM of CSM in a wheat-based diet and ATTD of OM of pure wheat diet determined using Cr2O3 as a marker were lower (P < 0.001) than those measured using total faecal collection. The ATTD of OM of SBM was consistently higher (P < 0.001) than that of CSM when determined using AIA as a marker. The use of different basal diets significantly (P < 0.001) influenced ATTD of OM. However, both ATTD of OM of CSM and SBM decreased when pigs were offered a wheat-based diet instead of the simple sugar-starch-based diet. There was a significant interaction (P < 0.001) between protein source, basal diet and method used, such that the decrease in ATTD of OM in wheat-based diets was more pronounced when digestibility of CSM was determined using Cr2O3 as an indigestible marker.
The ATTD of DM determined using AIA as an indigestible marker and that measured using total faecal collection were similar. Nevertheless, ATTD of DM of CSM in the wheat diet and that of pure wheat diet determined using Cr2O3 were lower (P < 0.001) compared with that measured using total faecal collection. It seems that the pattern of ATTD of DM as influenced by basal diets was consistent with the value change observed for ATTD of OM. The ATTD of DM in pigs consuming CSM and SBM in simple sugar-starch-based diets was higher than that in pigs given CSM and SBM in more complex wheat-based diets (P < 0.001). There was a significant interaction (P < 0.001) between protein source, basal diet and method used, such that the decrease in ATTD of DM in wheat-based diets was more pronounced when digestibility of CSM was determined using Cr2O3 as an indigestible marker.
3.3. Apparent ileal digestibility of nitrogen, organic matter and dry matter
Generally, the AID of feed components determined using Cr2O3 as an inert marker was lower than that using AIA as an indigestible marker, likely due to the interaction of protein source × basal diet × marker (P = 0.017; P < 0.001 and P < 0.001 for AID of N, OM and DM, respectively) (Table 4).
Table 4.
Effect of marker, protein source and basal diets on the apparent ileal digestibility (AID, %) of nitrogen, organic matter and dry matter of the experimental diets1.
| Item | Chromic oxide |
Acid-insoluble ash |
SED2 | SED3 | Significance4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Cottonseed meal |
Soybean meal |
Wheat | Cottonseed meal |
Soybean meal |
||||||||
| Simple | Wheat | Simple | Wheat | Simple | Wheat | Simple | Wheat | ||||||
| Nitrogen | 69.3 | 60.7 | 67.4 | 78.4 | 74.1 | 73.7 | 66.5 | 76.7 | 80.5 | 77.9 | 1.27 | 1.12 | D (0.037); Prot. (<0.001); M (<0.001) |
| Dry matter | 62.5 | 72.9 | 54.3 | 76.8 | 61.4 | 72.2 | 77.8 | 67.4 | 79.1 | 67.7 | 1.37 | 1.21 | D (0.034); Prot. (<0.001); BD (<0.001); M (<0.001); B × M (0.007); Prot. × M (0.004) |
| Organic matter | 58.4 | 76.8 | 56.8 | 80.1 | 65.1 | 74.1 | 81.3 | 70.6 | 82.7 | 72.5 | 1.39 | 1.24 | Prot. (<0.001); BD (<0.001); M (<0.001); BD × M (<0.001) |
SED = standard error of difference.
The basal diet was either a 1:1 sugar and starch mix (simple) or pure wheat (wheat); protein sources were either 400 g/kg cottonseed meal or soybean meal mixed with the basal diet or diet containing 926 g/kg wheat. n = 5 for each diet.
When comparing different basal diet means.
When comparing the same basal diet means.
Significance of effects of pure wheat (926 g/kg) vs. other diets (D), protein source (Prot.), basal diet (BD) or indigestible marker (M) and interactions have been included in parentheses.
The AID of N differed between protein sources (P < 0.001) and there was a significant interaction (P < 0.001) between protein source and basal diet. The AID of N of CSM given to the pigs in a wheat-based diet was higher (10.2%; P < 0.001) than that in a simple base diet, and there was a reduction for the pigs given SBM diets. The AID of N was slightly lower (−2.67%; P < 0.001) when SBM was offered to the pigs in a wheat-based rather than in the sugar-starch-based diet.
In the current experiment, the AID of OM of the different protein source differed significantly (P < 0.01). The AID of OM of protein source was affected (P < 0.001) by the basal diets given with it. Both AID of OM of CSM and SBM decreased significantly (−10.7% and −10.1%, respectively; P < 0.001) when these protein meals were offered to the pigs in wheat instead of a sugar-starch-base diet. Similarly, the AID of DM of CSM and SBM were significantly lower (−10.2% and −11.4%, respectively; P < 0.001) when these meals were offered in the sugar-starch-rather than wheat-based diet.
4. Discussion
The major finding from the present study was that the ATTD determined using AIA as an indigestible marker appeared to be a more reliable value compared with the Cr2O3 technique. The ATTD of N, OM and DM determined using AIA or Cr2O3 as an indigestible marker were correlated with that determined using total faecal collection. However, the close relationship between ATTD assessed using Cr2O3 as an inert marker and from direct measurement using total faecal collection in the present study may be fortuitous. In this context, all of the coefficient of determination values obtained from regression analysis of the relationships between digestibility determined using Cr2O3 and total faecal collection were relatively low compared to the corresponding correlations between the AIA and total faecal collection methods. In part, this may be due to the low appearance of Cr2O3 in the faecal digesta which would cause a low ATTD estimates. Kozloski et al. (1998) stated that during the process of chromium measurement, after sample digestion and chromium solubilization, the use of the atomic absorption spectrometry could influence the estimates of chromium and hence Cr2O3 contents in biological samples and, consequently, the digestibility values. Yin et al. (2000) compared the use of total collection via the post-valve caecal cannula and the use of Cr2O3 and titanium oxide (TiO2) as inert markers. They demonstrated that the use of Cr2O3 marker underestimated the AID of N and DM but that AID assessed using TiO2 marker agreed well with the total collection method using post-valve caecal cannula. Furthermore, Yin et al. (2000) highlighted problems with the use of Cr2O3 as a marker for prediction of AID. Their results were in agreement with the finding of Jagger et al. (1992) that the use of TiO2 marker in digestibility studies was more suitable than Cr2O3. Using Cr2O3 as a marker, Prawirodigdo et al. (1998) compared the slaughter method, as used in the present study, with ileal cannulation and found similar estimates of AID and ATTD of N and OM between the 2 methods with similar differences between CSM and SBM as observed here.
The present study suggests that the relationships between ATTD of N, OM and DM determined using total faecal collection and either AIA or Cr2O3 are strong and essentially linear. However, the relationships for Cr2O3 changed over the range of digestibility as evidenced by the test for differences in intercepts with diet. It appears (Fig. 1, Fig. 2, Fig. 3B) that with the Cr2O3, some diets are above the line, and some diets are below the line, however, with the same test, the relationship for AIA does not change with diet. As evidenced by the joint tests of slope = 1 and intercept = 0, it can be concluded that the AIA technique is unbiased for predicting the ATTD of the total faecal collection whereas the Cr2O3 technique is substantially biased.
The results of the present experiment provided further confirmation of other studies (McCarthy et al., 1974; Van Keulen and Young, 1977; Van Leeuwen et al., 1996) that AIA is an appropriate marker for digestibility studies in pigs. However, there have been some conflicting studies. Bakker and Jongbloed (1994) suggested that Cr2O3 was a good marker for determining faecal digestibility, whereas, AIA was found to be unsuitable. However, in that study no exogenous AIA was mixed with the diet and digestibility was determined using the very low endogenous AIA present in the diet ingredients. Indeed, previous studies (McCarthy et al., 1974; Moughan et al., 1991; Van Leeuwen et al., 1996) have recommended the addition of AIA as a marker to the diet to provide an accurate quantitative analysis. Recently, Kim et al. (2020) compared AID of SBM determined using exogenous Cr2O3, TiO2 and AIA and found that the former 2 markers gave higher estimates of AID of most amino acids than AIA when pigs were fed a simple diet. Also, Brestensky et al. (2017) found that AIA and Cr2O3 provided similar estimates of AID of amino acids and N and ATTD of N in cereal-based diets containing 20% of SBM or SBM plus rapeseed meal. Therefore, it appears that the amount and type of protein meal can influence the reliability of the different markers with Cr2O3 perhaps providing biased estimates of AID and ATTD of N when using relatively high inclusion rates of less digestible protein meals such as CSM. Given that the analyses of variance results support the conclusion from the regression analyses that the use of AIA as an inert marker in digestibility study of pigs for predicting the true ATTD of N of feed is more accurate than the use of Cr2O3, particularly for CSM, the rest of the discussion will be based on findings using AIA as a marker.
The AID and ATTD values obtained in the present experiment were in agreement with the results reported by Prawirodigdo et al. (1998; 2019) who investigated the effects of method of collection and marker on AID and ATTD. In addition, the response pattern of the pigs to the effects of basal diets on the apparent faecal digestibility of feeds in our previous (Prawirodigdo et al., 2019) and present studies were also similar. The ATTD of N varied significantly depending upon the protein source. The ATTD of N determined in pigs given CSM and SBM diets were influenced by the basal diets consumed with both protein sources. The ATTD of N of CSM in a sugar-starch diet was significantly lower compared with when CSM was offered in a wheat-based diet. On the other hand, replacement of sugar-starch base with wheat base decreased the ATTD of SBM. There were significant interactions between protein source and basal diet, and between protein source, basal diet and method of determination on ATTD of N, such that ATTD of N in SBM was higher in simple base diet than the wheat-based diet, whereas the opposite was true for CSM, especially when determined using Cr2O3 as a marker.
Aswe have greater confidence in AID and ATTD determined using AIA as a marker, the following discussion about the differences between CSM and SBM and the effect of basal diet on AID and ATTD will use values obtained using this method. The present data show a generally higher AID and ATTD of N in CSM when pigs were fed a complex basal wheat diet compared to when pigs were provided a simple sugar-starch-based diet, possibly due to the different nutrient profiles of the diets. However, for diets containing SBM, the AID and ATTD of N were higher in the sugar-starch-based diet. These findings are similar to those found by Prawirodigdo et al. (2019) and other researchers who found that the AID of amino acids in CSM determined using complex cereal basal diets have generally been near the upper end of the range of normally encountered values (Batterham et al., 1990; Li et al., 2000), whereas those determined using less complex basal diets have been towards the lower end of the range (Knabe et al., 1989; Prawirodigdo et al., 1998). Ma et al. (2019) used Cr2O3 as a marker in a simple starch-based diet to determine the AID of N in 10 different CSM and the average value was 67.3% (ranged from 59.4% to 77.1%) compared to 60.7% and 66.5% determined using Cr2O3 and AIA, respectively, in the present study. Interestingly, the AID and ATTD of OM and DM were consistently higher when determined in a simple sugar-starch-based diet for both protein meals in the present study. This was particularly so for CSM when ATTD was determined using Cr2O3 as a marker as indicated by the 3-way interaction between marker, basal diet and protein meal. The reason why Cr2O3 gives such a low estimate of ATTD of OM and DM when measured in the wheat-based basal diet is most likely due to interactions between Cr2O3 and the higher fibre content of the combined CSM and wheat (Prawirodigdo et al., 2019).
The reason why many recent AID and ATTD studies have been using a cereal basal diet is to simulate the conditions under which protein meals are fed commercially (King et al., 2000; Collins et al. 2005, 2006). The likely reason for the higher AID and ATTD of N and amino acids when CSM is mixed with a wheat diet may be related to the increase in protein content of the whole diet. Some workers have found that AID of amino acids and N increased as the dietary protein content increased (Fan et al., 1994; Fan and Sauer, 1995; Langer and Fuller, 1996), possibly because of a dilution of endogenous protein losses (Power, 2000). Prawirodigdo et al. (2019) found that the higher AID of N in CSM in a wheat-based diet was associated with higher AID of proline, threonine, glycine and serine in CSM and these amino acids are high in endogenous secretions. Presumably, CSM fed in a simple sugar-starch-based diet produced a much greater proportion of endogenous protein relative to dietary protein into the small intestine, than the pigs consuming CSM in a wheat-based diet. Other investigators (Holmes et al., 1974; Sauer et al., 1977) have also found high levels of glycine and threonine in the ileal digesta of growing pigs given a protein-free diet. It is worth noting that endogenously produced mucins are rich in threonine, serine and proline (Neutra and Forstner, 1987) and so an increase in mucin production secretion would contribute to lower estimates of AID and ATTD of N. In this context, if there was any free gossypol in the CSM diets, this could also increase mucin secretion as occurs in the rat (Kuhn et al., 2002).
It has been accepted that for formulating pigs’ diets, the accuracy of the ileal digestibility measurement of feeds is very important. Consistent with the high accuracy of AIA marker for predicting the digestibility of feeds, results of the current experiment (Table 3) demonstrated that the use of Cr2O3 as a marker provided an underestimate in AID of N, OM and DM. Although, McCarthy et al. (1974) reported that the AIA method was superior to Cr2O3 as an indigestible marker in pig diets, the Cr2O3 technique has continued to be used routinely for digestibility studies.
The present study suggests that the use of AIA as an indigestible marker for determining AID with pigs is more suitable than Cr2O3. Therefore, the data obtained using AIA method are chosen to be the standard AID value for explaining the effect of basal diet on the AID in the present experiment. Replacement of sugar-starch with wheat in the basal diet increased AID of N of CSM, but reduced the AID of OM and DM. In contrast, substitution of sugar-starch with wheat in the basal diet caused reductions of AID of N, OM and DM in SBM. These results support our previous findings (Prawirodigdo et al., 2019).
5. Conclusions
These data demonstrate that the basal diet and choice of indigestible marker can substantially influence the AID and ATTD of N, OM and DM and that the use of AIA as an indigestible marker is more suitable than Cr2O3 in digestibility studies in pigs.
Author contributions
Susanto Parawirodigdo: conceptualization, investigation, methodology, data collection, data curation, formal analysis, writing – original draft; Neil Gannon: investigation, data collection, writing – review and editing; Brian Leury: conceptualization, writing – review and editing, supervision; Frank Dunshea: conceptualization, resources, formal analysis, data curation, writing – review and editing, supervision.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
The authors are grateful to Mr R.G. Nason for skilled technical assistance and Mr. M.G Kerr and Mr. C. Rayner for amino acid analyses.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AOAC . In: Official methods of analysis. 15 ed. Helrich K., editor. Association of Official Analytical Chemists; Arlington, Virginia, USA: 1990. [Google Scholar]
- Batterham E.S., Andersen L.M., Baigent D.R., Darnell R.E., Taverner M.R. A comparison of the availability and ileal digestibility of lysine in cottonseed and soya-bean meals for grower/finisher pigs. Br J Nutr. 1990;64:663–677. doi: 10.1079/bjn19900069. [DOI] [PubMed] [Google Scholar]
- Bakker G.C.M., Jongbloed A.W. The effect of housing system on apparent digestibility in pigs, using the classical and marker (chromic oxide, acid-insoluble ash) technique in relation to dietary composition. J Sci Food Agric. 1994;64:107–115. [Google Scholar]
- Brestensky M., Nitrayov S., Heger J., Patras P. Chromic oxide and acid-insoluble ash as markers in digestibility studies with growing pigs and sows. J Anim Physiol Anim Nutr. 2017;101:46–52. doi: 10.1111/jpn.12503. [DOI] [PubMed] [Google Scholar]
- Collins C.L., Dunshea F.R., Henman D.J., McCauley I., King R.H. The apparent ileal digestibility of amino acids in common vetch (Vicia sativa cv. Morava) Aust J Exp Agric. 2005;45:705–709. [Google Scholar]
- Collins C.L., Eason P.J., Dunshea F.R., Higgins T.J.V., King R.H. Starch but not protein digestibility is altered in pigs fed transgenic peas containing alpha-amylase inhibitor. J Sci Food Agric. 2006;86:1894–1899. [Google Scholar]
- European Food Standards Authority Scientific Opinion on the substantiation of health claims related to chromium and contribution to normal macronutrient metabolism (ID 260, 401, 4665, 4666, 4667), maintenance of normal blood glucose concentrations (ID 262, 4667), contribution to the maintenance or achievement of a normal body weight (ID 339, 4665, 4666), and reduction of tiredness and fatigue (ID 261) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Eur Food Standards Authority J. 2010;8(10):1732. [Google Scholar]
- Fan M.Z., Sauer W.C., Hardin R.T., Lien K.A. Determination of apparent ileal amino acid digestibility in pigs: effect of dietary amino acid level. J Anim Sci. 1994;72:2851–2859. doi: 10.2527/1994.72112851x. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Sauer W.C. Determination of apparent ileal amino acid digestibility in peas for pigs with direct, difference and regression method. Livest Prod Sci. 1995;44:61–72. doi: 10.2527/1995.7382364x. [DOI] [PubMed] [Google Scholar]
- Holmes J.H.G., Bayley H.S., Leadbeater P.A., Horney F.D. Digestion of protein in the small intestine and large intestine of the pig. Br J Nutr. 1974;32:479–489. doi: 10.1079/bjn19740102. [DOI] [PubMed] [Google Scholar]
- Jagger S., Wiseman J., Cole D.J.A., Craigon J. Evaluation of inert markers for the determination of ileal and faecal apparent digestibility values in the pig. Br J Nutr. 1992;68:729–739. doi: 10.1079/bjn19920129. [DOI] [PubMed] [Google Scholar]
- Kim B.G., Lee S.A., Park K.R., Stein H.H. At least 3 days of adaptation are required before indigestible markers (chromium, titanium, and acid insoluble ash) are stabilized in the ileal digesta of 60-kg pigs, but values for amino acid digestibility are affected by the marker. J Anim Sci. 2020:1–8. doi: 10.1093/jas/skaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R.H., Eason P.J., Kerton D.J., Dunshea F.R. Evaluation of solvent extracted canola meal for growing pigs and lactating sows. Aust J Agric Res. 2000;52:1033–1041. [Google Scholar]
- Knabe D.A., Tanksley T.D., Hebby J.H. Effect of lysine, crude fibre and free gossypol in cottonseed meal on the performance of growing pigs. J Anim Sci. 1979;49:134–142. [Google Scholar]
- Knabe D.A., LaRue D.C., Gregg E.J., Martinez G.M., Tanksley T.D. Apparent digestibility of nitrogen and amino acids in protein feedstuffs by growing pigs. J Anim Sci. 1989;67:441–458. doi: 10.2527/jas1989.672441x. [DOI] [PubMed] [Google Scholar]
- Kozloski G.V., de Moraes Flores E.M., Martins A.F. Use of chromium oxide in digestibility studies: variations of the results as a function of the measurement method. J Sci Food Agric. 1998;76:373–376. [Google Scholar]
- Kuhn G., Cermak R., Minck K., Vujicic Z., Scharrer E. Gossypol induces chloride secretion in rat proximal colon. Eur J Pharmacol. 2002;457:187–194. doi: 10.1016/s0014-2999(02)02660-2. [DOI] [PubMed] [Google Scholar]
- Langer S., Fuller M.F. The effects of excessive amounts of protein on lysine utilization in growing pigs. Br J Nutr. 1996;76:743–756. doi: 10.1079/bjn19960080. [DOI] [PubMed] [Google Scholar]
- Li D., Xu X.X., Qiao S.Y., Zheng C.T., Chen Y., Piao X.S. Growth performance of growing-finishing pigs fed diets supplemented with Chinese cottonseed meal based on amino acid digestibilities. Asian-Australas J Anim Sci. 2000;13:521–527. [Google Scholar]
- McCarthy J.F., Aherne F.X., O D.B. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can J Anim Sci. 1974;54:107–109. [Google Scholar]
- Ma X., Zhang S., Shang Q., Long S., Piao X. Determination and prediction of the apparent and standardized ileal amino acid digestibility in cottonseed meals fed to growing pigs. Anim Sci J. 2019;90:655–666. doi: 10.1111/asj.13195. [DOI] [PubMed] [Google Scholar]
- Moughan P.J., Smith W.C., Schrama J., Smits C. Chromic oxide and acid-insoluble ash as faecal markers in digestibility studies with young pigs. N Z J Agric Res. 1991;34:85–88. [Google Scholar]
- Neutra M.R., Forstner J.F. Gastrointestinal mucus:synthesis, secretion and function. In: Johnson L.R., editor. Physiology of the gastrointestinal tract. Raven Press; New York: 1987. pp. 975–1009. [Google Scholar]
- Power G.N. La Trobe University; Australia: 2000. Dietary influences on the total and individual components of endogenous protein in ileal digesta of pigs. [Google Scholar]
- Prawirodigdo S., Gannon N.J., van Barneveld R.J., Kerton D.J., Leury B.J., Dunshea F.R. Assessment of apparent ileal digestibility of amino acids and nitrogen in cottonseed and soyabean meals fed to pigs determined using ileal dissection under halothane anaesthesia or following carbon dioxide- stunning. Br J Nutr. 1998;80:183–191. doi: 10.1017/s0007114598001093. [DOI] [PubMed] [Google Scholar]
- Prawirodigdo S., Gannon N.J., Leury B.J., Dunshea F.R. Basal diet and indigestible marker influence apparent digestibility of nitrogen and amino acids of cottonseed meal and soybean meal in pig. Animal Nutrition. 2019;5:234–240. doi: 10.1016/j.aninu.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner C. Protein hydrolysis of amino feeds for amino acid content. J Agric Food Chem. 1985;33:722–725. [Google Scholar]
- Rowan A., Moughan P.J., Wilson M.N. Acid-insoluble ash as a marker compound for use in digestibility studies with humans. J Sci Food Agric. 1991;54:269–274. [Google Scholar]
- Saha D.C., Gilbreath R.L. A modified chromic oxide indicator ratio technique for accurate determination of nutrient digestibility. Can J Anim Sci. 1993;73:1001–1004. [Google Scholar]
- Sauer W.C., Stothers S.C., Parker R.J. Apparent and true availabilities of amino acids in wheat and milling by-products for growing pigs. Can J Anim Sci. 1977;57:775–784. [Google Scholar]
- Van Barneveld R.J., Baker J., Szarvas S.R., Choct M. Effect of lupin kernels on the apparent ileal digestibility of amino acids by growing pigs. In: Hennessy D.P., Cranwell P.D., editors. Manipulating pig production V. Australasian Pig Science Association; Werribee, Victoria, Australia: 1995. p. 29. [Google Scholar]
- Van Keulen J., Young B.A. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J Anim Sci. 1977;44:282–287. [Google Scholar]
- Van Leeuwen P., Veldman A., Boisen S., Deuring K., van Kempen G.J.M., Vertegen M.W.A., Schaafsma G. Apparent ileal dry matter and crude protein digestibility of ration fed to pigs and determined with the use of chromic oxide (Cr2O3) and acid-insoluble ash as digestive markers. Br J Nutr. 1996;76:551–562. doi: 10.1079/bjn19960062. [DOI] [PubMed] [Google Scholar]
- Williams C.H., David D.J., Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci. 1962;59:381–385. [Google Scholar]
- Yin Y.L., McEvoy J.D.G., Schulze H., Hennig U., S W.B., McCracken K.J. Apparent digestibility (ileal and overall) of nutrients as evaluated with PVTC-cannulated or ileo-rectal anastomised pigs fed diets containing two indigestible markers. Livest Prod Sci. 2000;62:133–141. [Google Scholar]
- Zhang F., Adeola O. Techniques for evaluating digestibility of energy, amino acids, phosphorus, and calcium in feed ingredients for pigs. Animal Nutrition. 2017;3:344–352. doi: 10.1016/j.aninu.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]