Abstract
This review aims to give an overview of the efficacy of yeast supplementation on growth performance, rumen pH, rumen microbiota, and their relationship to meat and milk quality in ruminants. The practice of feeding high grain diets to ruminants in an effort to increase growth rate and weight gain usually results in excess deposition of saturated fatty acids in animal products and increased incidence of rumen acidosis. The supplementation of yeast at the right dose and viability level could counteract the acidotic effects of these high grain diets in the rumen and positively modify the fatty acid composition of animal products. Yeast exerts its actions by competing with lactate-producing (Streptococcus bovis and Lactobacillus) bacteria for available sugar and encouraging the growth of lactate-utilising bacteria (Megasphaera elsdenii). M. elsdenii is known to convert lactate into butyrate and propionate leading to a decrease in the accumulation of lactate thereby resulting in higher rumen pH. Interestingly, this creates a conducive environment for the proliferation of vaccenic acid-producing bacteria (Butyrivibrio fibrisolvens) and ciliate protozoa, both of which have been reported to increase the ruminal concentration of trans-11 and cis-9, trans-11-conjugated linoleic acid (CLA) at a pH range between 5.6 and 6.3. The addition of yeast into the diet of ruminants has also been reported to positively modify rumen biohydrogenation pathway to synthesise more of the beneficial biohydrogenation intermediates (trans-11 and cis-9, trans-11). This implies that more dietary sources of linoleic acid, linolenic acid, and oleic acid along with beneficial biohydrogenation intermediates (cis-9, trans-11-CLA, and trans-11) would escape complete biohydrogenation in the rumen to be absorbed into milk and meat. However, further studies are required to substantiate our claim. Therefore, techniques like transcriptomics should be employed to identify the mRNA transcript expression levels of genes like stearoyl-CoA desaturase, fatty acid synthase, and elongase of very long chain fatty acids 6 in the muscle. Different strains of yeast need to be tested at different doses and viability levels on the fatty acid profile of animal products as well as its vaccenic acid and rumenic acid composition.
Keywords: Yeast, Rumen fermentation, Conjugated linoleic acid, Biohydrogenation, Milk yield, Meat quality
1. Introduction
Associated with the rapid growth in human population is the corresponding increase in the demand for livestock products in developing countries which may double by the year 2030 (FAO, 2015). This has led to an increased intensification of livestock production worldwide which necessitates the use of a high-grain diet in order to boost animal production (Lara et al., 2018). However, the use of a high grain diet to improve the performance of livestock predisposes the animals to increased fat deposition and metabolic disorders like acidosis in ruminants. As such, the use of feed additives that will improve rumen health when feeding high grain diets is necessary (Noziére et al., 2014).
Consumers in developed countries are beginning to reject meat with a high composition of saturated fatty acid which is linked to cardiovascular diseases and cancer. This has challenged animal nutritionists to find ways to modify the fatty acid content of meat to increase its acceptability (Wood et al., 2004; Webb and O'Neill, 2008; Chikwanha et al., 2018).
Probiotics, though not fully explored, have the capacity to modify the gut microbial ecology and that may have an effect on lipid metabolism which might, in turn, affect the quality of animal products. A definite mode of action for probiotics is yet to be demarcated, but a variety of pathways have been hypothesized. Probiotics have the ability to modify microbial populations in the rumen or hindgut, change its fermentation pattern, increase the flow of nutrients to the small intestine and enhance feed digestibility (Krehbiel et al., 2003). Other authors reported that the addition of probiotics to livestock diets may decrease the concentration of serum cholesterol through direct assimilation of the cholesterol molecules by the microbes (Nami et al., 2019). Therefore, the supplementation of probiotics could enhance livestock performance through the maintenance of healthy rumen, enhancing the breakdown of fibrous feeds in the rumen by improving the uptake of nutrients, thereby resulting in an increased yield of livestock products (Arowolo and He, 2018).
Yeast is a commonly used probiotic in ruminant nutrition and has been proven to be effective in restoring gut microbial balance, especially during digestive disorders (McAllister et al., 2011). It is widely used in ruminant production to improve feed efficiency, and prevent rumen acidosis through its fermentation activities, by competing with other microbes within the rumen (Fonty and Chaucheyras-Durand, 2006). Although several authors have been investigating the efficacy of including yeast into the diets of ruminants for decades, there are still a lot of research gaps that need to be filled. Many of the published literature on yeast have reported inconsistent results and its mechanism of action is not entirely understood. With the current global crusade against the use of antibiotics in livestock feeds, the use of yeast in improving rumen fermentation and growth performance has received renewed attention. However, the efficacy of yeast in improving meat and milk quality, especially their fatty acid profiles and conjugated linoleic acid (CLA) composition, have been neglected. Since yeast is capable of altering the rumen fermentation, it could affect the rumen biohydrogenation which could in turn influence the fatty acid deposited into animal products. Therefore, this review aims to give an overview of the efficacy of yeast supplementation on growth performance, rumen pH, rumen microbiota, and their relationship to meat and milk quality in ruminants.
2. Influence of dietary yeast supplementation on rumen fermentation
2.1. Effects on rumen microbial composition
Yeast is known to play a vital role in the maintenance of a healthy rumen environment through its association with lactate-utilising bacteria and it supports the proliferation of other useful microorganisms in the rumen (Newbold 1996; Yang et al., 2004). It is able to stabilise the rumen pH through fermentation activities that suppress the activities of a lactate-producing microbe and encourages the proliferation of lactate-utilising bacteria resulting in less accumulation of lactic acid in the rumen (Lesmeister et al., 2004; Guedes et al., 2008). The common lactate-utilising bacteria in the rumen of grain-fed cattle are Megasphaera elsdenii (Chaucheyras-Durand et al., 2008). The increase in the population of this bacteria has been found to be effective in decreasing the accumulation of lactate in a rumen culture (Kung and Hession, 1995) and reducing the incidence of ruminal acidosis in cattle (Robinson et al., 1992). Interestingly, yeast has been reported to stimulate the growth of this important lactate-utilising bacteria. A study conducted by Pinloche et al. (2013) observed that yeast supplementation increased the population of M. elsdenii in the rumen of cows on a high-grain diet. Similarly, yeast was found to be effective in increasing the proportion of M. elsdenii in the rumen of cows having sub-acute rumen acidosis (SARA) (Malekkhahi et al., 2016). This can be ascribed to the ability of yeast to provide nutrients that stimulate the proliferation of the bacteria (Chaucheyras et al., 1996). In another study conducted by Ogunade et al. (2019), the inclusion of yeast into the diet of steers caused an increase in the population of carbohydrate digesting bacteria (Ruminococcus albus, R. champanellensis, R. bromii, and R. obeum) and lactate-utilising bacteria (M. elsdenii, Desulfovibrio desulfuricans, and Desulfovibrio vulgaris). The rumen microbial composition of ruminants fed yeast has been summarized in Table 1.
Table 1.
Summary of the rumen microbial composition of ruminants fed yeast.
| Item | Diet | Dose of yeast | Response | Source |
|---|---|---|---|---|
| Weaner lambs | High concentrate diet | 1 mL live yeast culture (1.5×109 to 2.0 × 109 CFU/mL) per kilogram live weight | Increased ciliate protozoa; increased feed intake and growth rate; increased entodinomorphs population | Tripathi and Karim (2011) |
| Calves | Milk replacer, starter feed, and hay | Yeast at 7.5 × 108 CFU/L before weaning and 3 × 109 CFU/kg after weaning | Increased total Lactobacilli population around weaning period | Fomenky et al. (2017) |
| Holstein bull calves | Milk and starter grains (no forage) | 0.5% and 1% of yeast fermentation product on an as-fed basis in starter diets and milk | Increased Butyrivibrio and decreased Prevotella composition of the rumen fluid | Xiao et al. (2016) |
| Dry Holstein cows | F:C was 70:30 | Yeast at 3.3 g/kg of diet per d (1 × 1010 CFU/d) | Increased the relative abundance of Bacteroidales, Lachnospiracea, and Flexilinea | Bach et al. (2019) |
| Rumen-fistulated Holstein dairy cows | F:C was 40:60 | A yeast culture at 10 g/cow per d (20 × 109 CFU/cow per d) | Increased the population of Fibrobacter succinogenes during adaptation; an increased population of Megasphaera elsdenii during SARA | Malekkhahi et al. (2016) |
| Finnish Ayrshire cows | F:C was 50:50 | Live yeast at 0.5 g/cow per d (1010 CFU/cow per d) | No effects on animal performance and rumen fermentation | Bayat et al. (2015) |
| Qinchuan cattle | F:C was 55:45 | 1 and 2 g live yeast or 20 g yeast cell wall polysaccharides/cow per d | Higher digestibility of ADF and NDF; increased the population of F. succinogenes S85, Ruminococcus albus 7 and R. flavefaciens FD-1; decreased the percentage of Streptococcus bovis JB1 | Peng et al. (2020) |
| Holstein steers | F:C was 50:50 | Live yeast at 15 g/d | Increased the population of R. albus, R. champanellensis, R. bromii, R. obeum, M. elsdenii, Desulfovibrio desulfuricans, and D. vulgaris | Ogunade et al. (2019) |
F:C = forage:concentrate ratio; SARA = sub-acute rumen acidosis.
On the other hand, Zhu et al. (2017) reported a reduced proportion of lactate-utilising bacteria (M. elsdenii and Selenomonas ruminantium) whereas the population of fungi and some cellulose digesting bacteria (R. albus, R. flavefaciens, and Fibrobacter succinogenes) were observed to increase after yeast supplementation in dairy cows fed low-quality forage. Furthermore, the authors recorded a decreased population of lactic acid-producing bacteria (Streptococcus bovis). This could be due to reduced availability of fermentable sugar caused by low-quality forage. Generally, yeast is known to suppress the proliferation of lactate-producing bacteria, however, Fomenky et al. (2017) observed an increase in the total population of Lactobacilli in the rumen of weaned calves supplemented with yeast. This might be due to the age of the animals as the rumen is still developing.
The ability of yeast to stimulate the growth of fibre digesting bacteria is well established. Several authors (Wiedmeier et al., 1987; Harrison et al., 1988; Williams 1989; Mao et al., 2013) investigated the effects of yeast on rumen microbiota and reported an increase in the population of cellulolytic bacteria (F. succinogenes and R. flavefaciens). This can be attributed to yeast's ability to provide nutrients that encourage the growth of cellulolytic bacteria such as F. succinogenes, R. albus, and R. flavefaciens (Chaucheyras-Durand et al., 2008). Chiquette et al. (2012), however, reported a decrease in the population of F. succinogenes in the rumen during the SARA challenge. In another study, Bach et al. (2019) observed that the inclusion of yeast to the diets of pregnant cows increased the population of Bacteroidales, Lachnospiracea, and Flexilinea in the rumen 14 d before calving. All these bacteria are fibre-digesting because Flexilinea was reported to degrade all kinds of carbohydrates (Sun et al., 2016), and Bacteroidales and Lachnospiraceae specialise in the fermentation of cellulose (Henderson et al., 2015) and pectin (Cotta and Forster, 2006) respectively. In a recent study (Ogunade et al., 2019), the 16S rRNA gene sequencing and “liquid chromatography-mass spectrometry-based metabolomics” were used to classify rumen microbes at specie level following yeast supplementation in beef steers and the results showed that yeast increased the population of some rare species of cellulose-digesting bacteria (Rhodopseudomonas palustris and Sorangium cellulosum). These studies confirm the efficacy of yeast in supporting the growth of several species of fibre digesting bacteria.
On the other hand, Bayat et al. (2015) reported no significant effect of live yeast on the population of F. succinogenes, R. flavefaciens, Methanogenic archaea, total bacteria, protozoa, and fungi in the rumen of cows fed silage-based diets. In a study conducted by Xiao et al. (2016), the addition of yeast culture into the diets of Holstein calves improved the percentage of Butyrivibrio and reduced the relative proportion of Prevotella in the rumen. These results revealed the efficacy of the yeast in affecting the composition of rumen microbiota. The viability of the yeast has been shown to influence how the rumen microbes respond to it. Jiang et al. (2017b) compared the effects of live yeast and dead yeast on the rumen microbiota of cows and the results obtained revealed that live yeast increased the population of cellulose-digesting bacteria, whereas the dead yeast did not affect the population of cellulolytic bacteria.
In one of the few studies that investigated the efficacy of yeast on gene expression, Ogunade et al. (2019) reported an increase in the expression of genes associated with oxidative phosphorylation (ubiquinol-cytochrome c reductase cytochrome b subunit and cytochrome c oxidase subunit) which suggests the ability of yeast to scavenge oxygen within the rumen. The efficacy of yeast in removing oxygen within the rumen has earlier been reported by Rose (1987). This is very important for maintaining a healthy rumen ecosystem because the majority of the rumen microbiota are anaerobic in nature (Loesche, 1969). This could be the reason as to why yeast is able to support the growth of several species of rumen microbes.
2.2. Rumen volatile fatty acid composition
Yeast supplementation could alter the rumen microbial population, making the environment more conducive for fibre digesting bacteria, thereby causing a change in the type and proportions of individual volatile fatty acids (VFA) produced in the rumen. In a study conducted by Xiao et al. (2016), the addition of yeast to the diet of cows was effective in increasing the concentration of butyrate in the rumen. Similarly, Zhu et al. (2017) observed an increased concentration of butyrate in addition to acetate, propionate, and total VFA concentration following yeast supplementation in dairy cows. Furthermore, increased ruminal butyrate concentrations in calves (Laarman et al., 2012) and a higher proportion of propionate in the rumen of matured cattle (Harrison et al., 1988; Hinman et al., 1998; Dias et al., 2018) have been reported after yeast supplementation. This could be attributed to the ability of yeast to stimulate the growth of M. elsdenii (Chaucheyras et al., 1996; Pinloche et al., 2013; Malekkhahi et al., 2016) which has been reported to degrade lactate into propionate and butyrate (Chen et al., 2019). However, this depends on the culture pH and concentration of lactate due to their role in the regulation of genes associated with lactate metabolism. Interestingly, pH was found to be more effective than lactate concentration in influencing lactate-utilisation by M. elsdenii. Consequently, the increase in rumen pH will positively affect lactate utilisation by the bacteria during rumen acidosis (Chen et al., 2019). This suggests that increasing the rumen pH during rumen acidosis will result in higher degradation of lactate to propionate and butyrate by M. elsdenii which will help reduce the accumulation of lactate in the rumen. Since propionate is the main source of glucose supply to the ruminant animal and a major substrate for gluconeogenesis (Dijkstra et al., 2012), it will be rapidly absorbed by the rumen papillae to be used as an energy source. Butyrate is also utilized for energy production as well as rumen papillae and epithelial development (Laarman et al., 2012). In another study, Al Ibrahim et al. (2010) observed a higher proportion of ruminal acetate following yeast supplementation in dairy cows. In the same way, other studies on calves (Hučko et al., 2009) and dairy cows (Malekkhahi et al., 2016) reported an increased concentration of acetate in addition to a higher ratio of acetate to propionate after feeding yeast-based diets. The increased acetate concentration observed in these studies could be due to the positive influence of yeast on the growth of D. desulfuricans and D. vulgaris (Ogunade et al., 2019) both of which have been reported to convert lactate into acetate (Vita et al., 2015).
In another study, Opsi et al. (2012) tested the effects of yeast viability on VFA production in-vitro and observed that live yeast decreased acetate concentration but increased valerate proportions, and inactive yeast increased acetate proportions and decreased propionate concentrations.
2.3. Rumen pH
Livestock producers generally use high grain diets to encourage rapid growth which comes with serious consequences to gut health. A study conducted by Commun et al. (2009) confirmed that feeding high levels of concentrate diets to lambs resulted in lower pH and rumen acidosis. Rumen acidosis can be either acute or sub-acute. Clinical acute acidosis is the increased concentration of lactate in the rumen which leads to a decrease in rumen pH below 5.0, and sub-clinical ruminal acidosis is characterized by a pH range between 5.0 and 5.8 for a period longer than 3 h in a day (Beauchemin et al., 2000; AlZahal et al., 2007). Sub-clinical ruminal acidosis is usually caused by a high concentration of VFA rather than the accumulation of lactate (Beauchemin et al., 2003). This does not imply that lactic acid is not produced during sub-clinical ruminal acidosis, but it is usually utilized by micro-organisms faster than it is synthesized (Yang et al., 2004). Yeast is capable of either competing with S. bovis and Lactobacillus for fermentable carbohydrates or encouraging the growth of lactate-utilising bacteria which results in the low accumulation of lactate and consequently, a higher pH (Nisbet and Martin, 1990; Chaucheyras et al., 1996; Chaucheyras-Durand and Fonty 2001, 2006; Bach et al., 2007). This is because yeast is capable of utilising the available sugar within the rumen to deprive S. bovis of having access to enough glucose for its metabolic activity. Yeast has also been reported to stimulate the growth of lactate utilising bacteria (M. elsdenii) (Chaucheyras et al., 1996). Several studies have investigated the effects of yeast on rumen pH, however the response has been inconsistent. A study conducted by Dias et al. (2018) observed a higher ruminal pH in the rumen of yeast-fed cows. Similarly, yeast has been reported to decrease lactic acid concentration (Erasmus et al., 1992) and increase the ruminal pH (Bach et al., 2007; Thrune et al., 2009) in dairy cows. In a meta-analysis of several published studies, yeast was reported to increase rumen pH (Desnoyers et al., 2009). In another study, Chademana and Offer (1990) investigated the efficacy of yeast on sheep and observed a non-significant increase in rumen pH. Contrary to the above studies, some authors (Harrison et a1., 1988; Edwards et al., 1990) reported lower rumen pH in cattle supplemented with yeast. The viability of the yeast has been shown to affect its fermentation mechanism, which in turn affects the response of an animal to yeast treatment. In a study conducted by Lynch and Martin (2002), the addition of live yeast increased the culture pH, and dead yeast resulted in a decreased pH during in-vitro incubation. Similarly, Opsi et al. (2012) observed that inactivated yeast had no effects on ruminal pH, but live yeast decreased ruminal pH during an in-vitro digestibility of feed. Many other authors (Erasmus et al., 1992; Putnam et al., 1997; Miller-Webster et al., 2002; Yang et al., 2004; Xiao et al., 2016) did not observe any change in rumen pH of animals treated with yeast. The variability in the efficacy of yeast reported in the studies above could be due to the differences in basal diets given to animals, viable cell numbers in yeast, the dosage of yeast supplemented, the type of forage fed and feeding strategy (Cole et al., 1992; Piva et al., 1993; Cabrera et al., 2000; Kawas et al., 2007). The effects of yeast on rumen pH and VFA obtained from various sources are summarized in Table 2.
Table 2.
Effects of Saccharomyces cerevisiae on rumen pH and volatile fatty acids.
| Item | Diet | Dose of yeast | Response | Mean pH of treatment groups | Source |
|---|---|---|---|---|---|
| Santa Ines lambs | F:C was 40:60 | Inactive dry yeast at 4.87%, 9.73%, and 14.60% of diets | No effects on pH | 5.83 | Rufino et al. (2013) |
| Dry Holstein cows | F:C was 60:40 | Live dried yeast 3.0 g/cow per d | Increased ruminal pH | 6.26 | Křížová et al. (2011) |
| Primiparous Holstein cows | Low starch (F:C was 50:50); high starch (F:C was 40:60) |
Inactivated dry yeast at 15 g/cow per d | Increased ruminal pH | Low starch, 6.36; high starch, 6.08 |
Dias et al. (2018) |
| Malpura lambs | High starch diet (no forage) | Yeast at 9.0 × 107 CFU/kg body weight | No effects on pH | 6.59 | Bhatt et al. (2018) |
| Holstein bull calves | Milk and starter grains (no forage) | 0.5% and 1% of yeast fermentation product on an as-fed basis in starter diet and milk | Increased butyrate concentration; no effect on pH | 5.69 | Xiao et al. (2016) |
| Feedlot cattle | High grain | 10 g of E. faecium + S. cerevisiae/steer per d (6 × 108 CFU/g) | No effects on pH | 5.85 | Yang et al. (2004) |
| Multiparous Holstein cows | F:C was 60:40 | 0.5 g of active dry yeast/cow per d (1010 CFU/d) | Increase in ruminal pH; increased butyrate concentration | 6.53 | Thrune et al. (2009) |
| Holstein cows | F:C was 40:60 | 60, 120 or 180 g of yeast fermentation product/cow per d | No effects on pH | 6.34 | Zhu et al. (2017) |
| Lactating Holstein cows | High concentrate | 10 g of yeast culture/cow per d | No effects on pH; decreased lactic acid concentration | 6.0 | Erasmus et al. (1992) |
| Charolais bulls | High concentrate | 5 g of live yeast/bull or (1 × 1010 CFU/bull per d) | No effects on pH; increased acetate and butyrate concentrations; increased acetate:propionate ratio | 5.89 | Magrin et al. (2018) |
| Finnish Ayrshire cows | F:C was 50:50 | 0.5 g of live yeast/cow per d (1010 CFU/d) | No effects on pH | 6.65 | Bayat et al. (2015) |
| In vitro fermentation (Rumen fluid collected rumen-fistulated Assaf sheep) | High forage and high concentrate | 5 × 109 CFU inactivated and 109 CFU live yeast/L of medium | Live yeast decreased ruminal pH; inactivated yeast did not affect pH | High forage, 6.64; high concentrate, 6.7 | Opsi et al. (2012) |
F:C = forage:concentrate ratio.
3. Growth performance and quality of ruminant products following yeast treatment
3.1. Growth performance
Yeast is believed to improve the performance of ruminants by encouraging the growth of fibre digesting microbes. This is important when concentrate based diets are fed to ruminants (Dias et al., 2018). In addition, active yeast has the potential to stimulate the proliferation of cellulolytic bacteria and lactate-utilising bacteria resulting in increased feed intake, reduced frequency of diarrhoea, increased animal performance, and improved stability of the rumen in young ruminants at weaning (Chaucheyras et al., 1995). Galvao et al. (2005) reported improved weight gain and reduced cases of diarrhoea in calves supplemented with yeast. Similarly, the supplementation of yeast was effective in promoting increased feed intake, growth rate and rumen development in calves (Adams et al., 2008) meanwhile causing a higher weight gain in bulls on a high grain diet (Geng et al., 2016). Furthermore, improved feed intake and weight gain were recorded in ruminants fed yeast-based diets (Lesmeister et al., 2004; Tripathi and Karim, 2011; Domínguez-Vara et al., 2009). On the contrary, Sales (2011) conducted a meta-analysis and the results revealed that active dry yeast did not have any effect on growth and feed conversion ratio of sheep. Similarly, several authors (Kawas et al., 2007; Titi et al., 2008; Bayat et al., 2015) reported that the inclusion of yeast into diets of livestock (lambs, kids, and cows) did not have any effect on the growth performance of the animals.
Interestingly, the effects of the dose and strain of yeast on the performance of buffalo calves were investigated and the results revealed that the addition of a high dose of yeast (3 × 109 CFU/kg dry matter) and mixed strains had a higher effect on growth rate than the low dose (1 × 109 CFU/kg dry matter) and single strain (Malik and Bandla, 2010). In a related study, the inclusion of a higher dose of yeast (2%/kg DM) improved feed intake, weight gain, and feed efficiency of calves, whereas the inclusion of a lower dose (1% dry yeast/kg DM) has no effect on the performance of the calves (Lesmeister et al., 2004). Although the strain and dose of yeast were found to affect the performance of calves, the proximate composition of the basal diet, particularly crude protein, also has a role to play. The efficacy of yeast on a low protein diet and high protein diet were investigated in sheep and the results obtained revealed that yeast supplementation improved the weight gain of sheep that were fed low protein diets, but had no effect on the weight of animals fed high protein diets (Bonilla et al., 1992). Factors such as strain, dose, frequency, and duration of supplementation may affect the response of livestock to probiotics (Buntyn et al., 2016; Slattery et al., 2016).
3.2. Potential of yeast in improving the quality of meat and milk in ruminants
3.2.1. Effects of yeast on meat fat deposition and fatty acid profile
Limited studies have been carried out on the effects of yeast supplementation on meat quality probably due to the lack of a clear understanding of the connection between the two; however, the results obtained were promising (Table 3). The inclusion of yeast into the diet of ruminants can alter the activities of rumen microbiota through the maintenance of rumen pH in animals fed high starch diets (McAllister et al., 2011). A study by Milewski et al. (2012) revealed an increase in the proportion of cis-9, trans-11 CLA, and vitamin A content of mutton after yeast supplementation in sheep. Similarly, Milewski and Zaleska (2011) reported a higher composition of cis-9, trans-11-CLA, C14:1, C18:2, and C22:6 fatty acids in the intramuscular fat of lambs supplemented with yeast. To corroborate these findings, Liu et al. (2019) investigated the efficacy of yeast on modifying the fatty acid profile in lambs and the results obtained revealed that yeast supplementation caused an increase in the concentration of linoleic acid in the muscle by reducing its conversion to stearic acid. In another study conducted by Silva et al. (2002), the supplementation of yeast in heifers resulted in an increased concentration of polyunsaturated fatty acid and a decreased level of cholesterol in the longissimus muscle. The authors also observed a decrease in the ratio of n6:n3 fatty acids in the meat which is an indication of improved meat quality. Conversely, Maggioni et al. (2009) reported an increase in the ratio of n6:n3 fatty acids in the meat of bulls supplemented with yeast, which suggests a negative effect on meat quality. However, an increased concentration of linolenic acid was recorded by the authors. Raghebian et al. (2007) added yeast to the diets of lambs but the fatty acid profile of the meat remained unaffected. In a similar study, Rodríguez-Gaxiola et al. (2020) supplemented chromium enriched yeast to lambs but reported no effects on the fatty acid profile of the meat.
Table 3.
Meat quality parameters of ruminants supplemented with yeast.
| Item | Diet | Dose of yeast | Response | Source |
|---|---|---|---|---|
| Santa Ines lambs | F:C was 40:60 | 4.87%, 9.73%, and 14.60% inactive dry yeast of diets | The subcutaneous fat thickness decreased; meat crude protein and ash increased | Rufino et al. (2013) |
| Lambs | F:C was 60:40 | 300 μg yeast-Cr/kg of diet | Decreased serum total cholesterol and serum triglyceride levels | Zhou et al. (2013) |
| Awassi lambs and Shami goat kids | High concentrate diet | 12.6 g yeast/kg of diet | No effects on growth performance of both lambs and goat kids; increased fat content in the carcass | Titi et al. (2008) |
| Kamieniec rams | Forage and concentrate | 50 g yeast/kg of diet | Increased composition of C14:1, C18:2, C22:6, C18:2 (cis-9 trans-11) in meat; increased vitamin A content of meat; increased intramuscular fat | Milewski et al. (2012) |
| Rambouillet lambs | Forage and concentrate | 0.25 and 0.35 mg Cr-yeast or 0.3 mg Se-yeast per day | Decreased fat in the carcass; increased average daily weight gain and final body weight | Domínguez-Vara et al. (2009) |
| Male Pelibuey lambs | High grain diet | 0.12% yeast culture of diets | No effects on growth performance; no effects on marbling score and external fat score | Kawas et al. (2007) |
| Rambouillet ram lambs | Concentrates | 0.3 mg Cr enriched yeast/kg dry matter | Increase backfat thickness; higher meat pH; no effects on the fatty acid profile of meat | Rodríguez-Gaxiola et al. (2020) |
| Lambs | Forage and concentrate | Yeast culture drenched at 1 mL/kg live weight | No effects on carcass characteristics | Bhatt et al. (2018) |
| Kamieniecka lambs | Hay silage and concentrate | 50 g/kg concentrate per day | Higher protein content and lower cooking loss; improved water-holding capacity of meat; higher composition of cis-9, trans-11 CLA, C14:1, C18:2 and C22:6 fatty acids in the intramuscular fat | Milewski and Zaleska (2011) |
| Limousin-Nelore and Simmental-Nelore heifers | Corn silage and corn | Yeast at 16.35% of diets | An increased concentration of polyunsaturated fatty acid in the Longissimus muscle; a lower level of cholesterol in the Longissimus muscle; lower n6:n3 ratio in the muscle | Silva et al. (2002) |
| Crossbred young bulls (Zebu × European) | F:C was 44:56 | 15 g of yeast per animal per day | Increased linolenic acid concentration in the meat; increased n6:n3 ratio in meat | Maggioni et al. (2009) |
| Small-tailed Han lambs | Pelleted total mixed rations | 0.8 and 2.3 g of yeast/kg dietary dry matter | An increased concentration of linoleic acid concentration in the muscle; a decreased conversion of linoleic acid to stearic acid | Liu et al. (2019) |
| Iranian Zandi lambs | Forage and concentrate | 3 and 4.5 g of yeast per lamb per day | Increased meat fat; no effects on meat fatty acid composition | Raghebian et al. (2007) |
| Simmental × Luxi F1 crossbred bulls | Forage and concentrate | 0.8 g dry yeast/bull per day and 50 g yeast culture/bull per day | No effects on intramuscular fat content or cholesterol content; decreased backfat thickness; an increased concentration of free fatty acids in the blood | Geng et al. (2016) |
F:C = forage:concentrate ratio.
Other reported effects of yeast that could be linked to meat quality include the reduction of serum cholesterol and carcass fat deposition. In lambs, chromium-yeast supplementation has been effective in decreasing the level of blood cholesterol and carcass fat deposition (Zhou et al., 2013), and inactive dry yeast was found to reduce the subcutaneous fat thickness and increase the crude protein percentage of the meat (Rufino et al., 2013). Furthermore, the addition of yeast to the diets of bulls did not affect the level of intramuscular fat or cholesterol composition; however, there was a significant reduction in backfat thickness and an increase in the concentration of free fatty acids in the blood (Geng et al., 2016). Contrary to the previous studies, other authors observed a higher amount of carcass fat (Raghebian et al., 2007; Titi et al., 2008) and increased backfat thickness (Rodríguez-Gaxiola et al., 2020) following yeast supplementation in lambs. The exact reason for the conflicting response remained unclear but the proposed mechanism through which yeast influenced the fatty acid profile of meat is via the manipulation of rumen biohydrogenation (details in section 3.2.3).
3.2.2. Effects on milk quality and yield
Yeast has been used to improve milk production and quality in ruminants for more than 50 years. However, there is still a dearth of published literature on the efficacy of yeast in modifying the milk fatty acid profile and its conjugated isomers. The majority of the available literature highlights its effects on milk yield and milk fat yield as shown in Table 4.
Table 4.
Influence of yeast supplementation on milk composition and yield.
| Item | Lactation stage | Diet | Dose of yeast | Response | Source |
|---|---|---|---|---|---|
| Holstein cows | 96 ± 14 DIM | Dry ground corn grain | 56 g/cow per day | No effects on rumen pH; no effect on milk protein, lactose, and solid non-fat; no effect on individual fatty acid isomers in milk fat; increased yield of milk fat | Longuski et al. (2009) |
| Holstein cows | 284 ± 18 DIM | Concentrate and corn silage | 5.7 × 107 CFU/d (live yeast at a low level), 6.0 × 108 CFU/d (live yeast at a high level) and 6.0 × 108 CFU/d (killed yeast at a high level) | Only the low level of live yeast increased milk yield, milk fat and milk protein | Jiang et al. (2017a) |
| Holstein cows | Trial 1, 104 ± 12 DIM; trial 2, 97 ± 4.3 DIM; trial 3, 139 ± 4.5 DIM |
Grass silage and concentrate | 0.8 g/cow per day (1 × 1010 CFU/cow per day) and 4.0 g/cow per day (6 × 1010 CFU/cow per day) | No effects on milk yield, butterfat, milk protein, or lactose | Ambriz-Vilchis et al. (2017) |
| Sohagi ewes | Last 2 months of pregnancy | Concentrate and forage | 5 or 10 g of yeast per ewe per day | Increased milk yield, milk fat, protein and solids non-fats | Elaref et al. (2020) |
| Holstein cows | 116 ± 9 DIM | Concentrates and corn silage | 15 g selenium-enriched yeast per day | Increased selenium concentration; no effects on milk production | Faccenda et al. (2020) |
| Ayrshire dairy cows | 53 ± 7 DIM | Concentrate | 0.5 g live yeast per day | No effects on milk yield; no effects on milk fat, protein, and lactose; no effects on proportions of the major fatty acids in milk | Bayat et al. (2015) |
| Holstein cows | 118 to 134 DIM | Concentrate pellets | 15 and 50 g yeast culture/cow per day | Increased milk yield; increased milk fat; increased concentrations of protein, lactose, total solids and solids non-fat | Alshaikh et al. (2002) |
| Holstein cows | 21 d prepartum to 21 d postpartum | Concentrates | 57 and 227 g yeast per day | Increased milk yield; increased milk fat yield; no effects on milk protein yield, milk protein percentage, milk fat percentage | Ramsing et al. (2009) |
| Holstein cows | 14 d prepartum to 45 d postpartum | Forage and concentrates | 20 g yeast per day | Increased milk production; improved milk fat | Ayad et al. (2013) |
| Water buffalo cows | 84 ± 13 DIM | Brachiaria humidicola pasture only | 100 g yeast grown/cow per day on a media of yellow corn | No effects on milk yield; no effects on milk fat and milk protein | Ramírez et al. (2007) |
| Holstein Friesian cows | Mid-lactation | Concentrate | 2.5 g yeast/cow per day | Increased milk yield; no effects on milk composition | Maamouri et al. (2014) |
| Holstein cows | 20 ± 2 wk of lactation | Forage and concentrate | 10 g yeast/cow per day | Increased milk yield; increased yield of milk fat | Rezaeian (2003) |
| Holstein cows | 130 ± 16 DIM | Forage and concentrate | 5.4 × 1011 CFU yeast per day | No effects on milk fat yield, milk yield, milk protein concentration; no effects on milk protein yield, milk lactose concentration, and milk lactose yield | Ferreira (2019) |
| Holstein cows | 21 d prepartum to 60 d postpartum | Forage and concentrate | 4 g yeast/cow per day | Increased milk total solid; increased concentrations of milk fat; increased milk yield | Nasiri et al. (2019) |
| Holstein cows | 95 ± 12 DIM | Forage and concentrate | 35 g yeast/cow per day | No effects on milk yield, fat corrected milk, milk fat; no effects on milk protein and lactose | Al- Mallah (2018) |
| Holstein cows | 176 ± 18 DIM | Forage and concentrate | 15 g inactivated dry yeast/cow per day | Increased milk yield; increased milk fat; increased lactose concentration | Dias et al. (2018) |
| Holstein cows | 90 ± 35 DIM | 50 g yeast/cow per day | An increased concentration of 18:3 (n-3) in milk fat; increased milk yield; increased levels of methionine, tryptophan, tyrosine, phenylalanine, and taurine in the milk | Yalçın et al. (2011) | |
| Ettawa crossed breed goats | Late lactation (4.6 ± 0.55 months postpartum) | Forage and concentrate | 0.5% or 5 g yeast/goat per day | An increased concentration of long-chain fatty acids and medium-chain fatty acids in the milk; decreased concentration of short-chain fatty acids and unsaturated fatty acids in the milk; decreased n6:n3 ratio in the milk | Sulistyowati et al. (2013) |
DIM = days in milk.
Several studies investigated the effects of including yeast to the diets of lactating Holstein cows and the results obtained showed an improved milk yield, a higher concentration of milk fat (Alshaikh et al., 2002; Rezaeian, 2003; Ramsing et al., 2009; Ayad et al., 2013; Jiang et al., 2017a; Dias et al., 2018; Nasiri et al., 2019; Elaref et al., 2020) and increased milk protein (Alshaikh et al., 2002; Jiang et al., 2017a). The increase in milk fat yield could be attributed to the yeast's ability to stimulate acetate production in the rumen (Hučko et al., 2009; Al Ibrahim et al., 2010; Malekkhahi et al., 2016) which is a precursor of milk fat. The increased proliferation of cellulolytic bacteria following yeast supplementation (Wiedmeier et al., 1987; Harrison et al., 1988; Williams 1989; Mao et al., 2013) could also explain the higher milk yield and milk fat yield. This suggests that yeast supplementation could be useful in preventing milk fat depression in lactating cows. In another study, Elaref et al. (2020) added yeast to the diets of ewes and reported an increased milk yield, improved milk fat, increased milk protein, and solids rather than fats. Contrary to the above studies, some authors reported no effect of yeast treatment on milk yield and milk fat in lactating Holstein cows (Ambriz-Vilchis et al., 2017; Al- Mallah, 2018; Ferreira, 2019). Similarly, the milk yield, milk fat, and milk protein of buffalo cows were not affected by yeast supplementation (Ramírez et al., 2007). Typical of animal experiments, contrasting findings are bound to arise probably due to variations in the stage of lactation of the cows and/or the dose, viability, or strains of yeast used.
There are very few studies that investigated the milk fatty acid profile of ruminants. In one of the studies on goats, Sulistyowati et al. (2013) reported an increased concentration of long-chain fatty acids and medium-chain fatty acids in the milk after yeast supplementation and the proportion of short-chain fatty acids and unsaturated fatty acids were observed to decrease. A reduced ratio of n6:n3 fatty acids was also recorded which indicates an improved milk quality. In another study, the inclusion of yeast into the diets of cows increased the composition of linolenic acid (18:3) and the concentration of some essential amino acids in milk (Yalçın et al., 2011). The improvement in the ratio of n6:n3 and linolenic acid composition in the first and second studies respectively could be attributed to the efficacy of yeast in modulating the biohydrogenation of unsaturated fatty acids in the rumen, which lead to its increased deposition in the milk. In contrast to the above studies, Bayat et al. (2015) supplemented live yeast at a dose of 0.5 g/d to dairy cows and reported no effects on the fatty acid profile of milk. The authors also observed no effects on milk yield, milk fat, protein, and lactose. Similarly, Longuski et al. (2009) did not observe any effect on milk fatty acid and its CLA composition after yeast supplementation in lactating cows.
3.2.3. Role of yeast in modulating rumen biohydrogenation to improve meat and milk quality
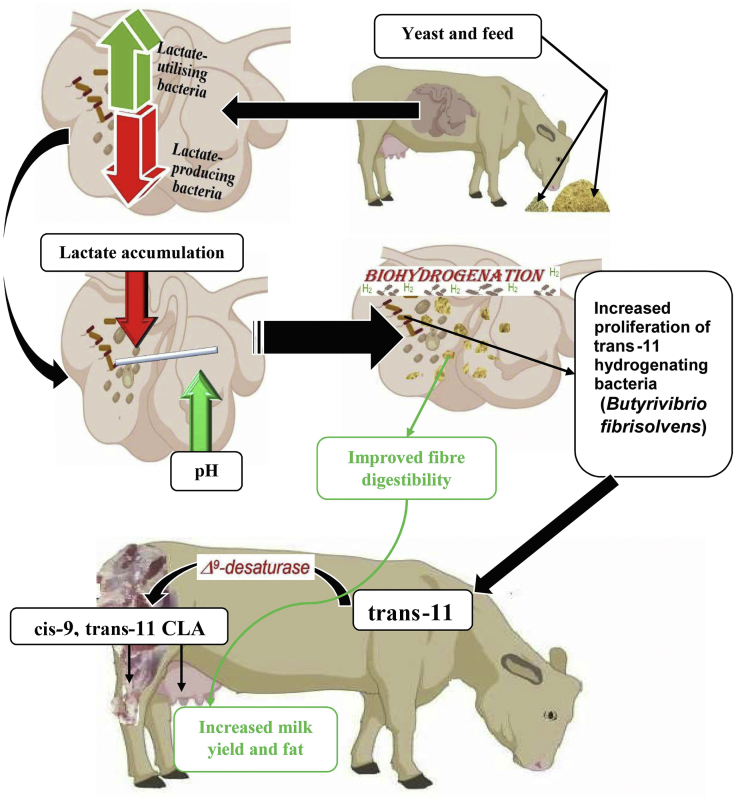
Ruminal biohydrogenation involves the transformation of linoleic acid into cis-9, trans-11 CLA (rumenic acid) followed by its hydrogenation to produce trans-11 (vaccenic acid) and subsequent conversion into stearic acid (Harfoot and Hazlewood 1997; Kim et al., 2000). Several species of bacteria are involved in the biohydrogenation of linoleic and linolenic acids in the rumen. These bacteria can be divided into groups A and B depending on the products synthesized from their fermentation activities. The bacteria in group A are responsible for converting linoleic and linolenic fatty acids into trans-11 (vaccenic acid), whereas group B specialises in the production of stearic acid (18:0). Examples of rumen bacteria belonging to group A include some strains of Butyrivibrio fibrisolvens, and those in group B consist of 2 strains of Fusocillus (Harfoot and Hazlewood, 1997). Yeast has the ability to influence the biohydrogenation pathway in the rumen by encouraging the growth of these bacteria involved in the process. It can also maintain the rumen pH at an appropriate condition to promote or inhibit some steps in the biohydrogenation of unsaturated fatty acids (Julien et al., 2010). Although a decrease in the biohydrogenation of dietary unsaturated fatty acids is beneficial, because higher concentrations of PUFA can be absorbed and deposited into animal products, a change in the biohydrogenation pathway to produce higher amounts of trans-11 and cis-9, trans-11 is more advantageous (Fig. 1). Julien et al. (2010) reported a decrease in the concentration of stearic acid together with an increased concentration of trans-11 C18:1 following yeast supplementation in Holstein cows. The authors ascribed this to the ability of yeast to stimulate the growth of B. fibrisolvens which are the main bacteria responsible for the biohydrogenation process in the rumen (Kepler et al., 1966; Harfoot and Hazlewood, 1988; Martin and Jenkins, 2002). The efficacy of yeast in increasing the ruminal population of B. fibrisolvens as speculated earlier was confirmed in a study on Holstein calves conducted by Xiao et al. (2016). An increase in the ruminal production of trans-11 C18:1 implies that a higher amount of cis-9, trans-11 CLA will be deposited into milk and meat due to the conversion of trans-11 to cis-9, trans-11 CLA in the tissue by the enzyme Δ9-desaturase (Bauman et al., 2000, Bauman et al., 2003).
Fig. 1.
Major effects of yeast supplementation on rumen fermentation and quality of products in ruminants. CLA = conjugated linoleic acid.
Rumen pH is another important factor affecting ruminal biohydrogenation (Sun et al., 2017). In a study conducted by Troegeler et al. (2006), the inclusion of yeast increased the rumen pH above 6.0 which lead to a reduction in the concentration of stearic acid in the rumen fluid. The authors also reported an increased proportion of linoleic acid and oleic acid which they attributed to a reduction in the biohydrogenation of unsaturated fatty acids in the rumen. To further support this, Lee (2013) studied the effect of pH on rumen biohydrogenation and reported a decreased biohydrogenation of linoleic, linolenic, and oleic acid at a culture pH of 5.5, and a higher biohydrogenation of the PUFA was seen at a pH of 6.5. This could be due to the inhibitory effects of low pH on the growth and activities of some biohydrogenating bacteria (Anaerovibrio lipolytica and B. fibrisolvens) at a rumen pH below 5.6 (Fuentes et al., 2009). In a related study, Choi et al. (2005) investigated the effects of pH on biohydrogenation and the results obtained revealed that the concentration of cis-9, trans-11 CLA was found to increase when the culture pH rises above 5.6. However, the authors recorded a higher quantity of trans-10, cis-12 CLA at pH 5.6 to 6.3 before cis-9, trans-11 CLA predominated in the culture. Fuentes et al. (2009) confirmed the synthesis of trans-10 C18:1 and trans-10 cis-12 CLA at a rumen pH of 5.6. Similarly, Sun et al. (2017) reported an increase in the production of trans-10, cis-12 CLA under a low pH condition. This is possible because different enzymes and pathways are involved in the formation of both trans-10, cis-12 CLA and cis-9, trans-11 CLA (Wallace et al., 2007). This suggests that the bacteria responsible for the synthesis of the biohydrogenation intermediate trans-10, cis-12 CLA thrives well in low pH conditions which could explain why low rumen pH causes milk fat depression. Therefore, a pH range between 5.6 and 6.3 is suggested for increased synthesis of trans-11 (vaccenic acid) and cis-9, trans-11 in the rumen. This means that the composition of CLA in the meat and milk of ruminant animals can be increased through dietary modification (Bauman et al., 2000), which in turn alters the rumen pH to effect a change in the population of microbes involved in ruminal biohydrogenation (Harfoot and Hazlewood, 1997; Bauman et al., 2000).
The ability of yeast to stimulate the growth of ciliate protozoa (Tripathi and Karim, 2011) could also contribute to improving meat and milk quality in ruminants. Ciliate protozoa are known to integrate cis-9, trans-11 CLA, and trans-11, 18:1 into their cells. The fatty acids within the cells of these protozoa eventually become available for absorption in the small intestine after the microbes get degraded during acidic digestion in the abomasum (Francisco et al., 2019). Ciliate protozoa are also important in the maintenance of rumen pH by competing with amylolytic bacteria in the assimilation of starch particles within the rumen when high grain diets are fed (Mendoza et al., 1993). However, pH is very critical to the survival of ciliate protozoa and they are usually killed when subjected to rumen pH lower than 5.5 for more than 15 h in a day (Franzolin and Dehority, 1996). This further corroborates the earlier findings which suggest that a rumen pH range between 5.6 and 6.3 is ideal for an increased flow of CLA and other PUFAs out of the rumen to be absorbed in the small intestine.
4. Areas of future research
There is a wide research gap in the area of meat and milk quality improvement and yeast has the potential to alter the composition of rumen fatty acid profile and possibly the fatty acid profile of animal products like milk and meat. Very few studies investigate the effects of yeast on the fatty acid profile and its conjugated isomers in animal products. Different strains of yeast need to be tested at different doses and viability levels on the fatty acid profile of animal products as well as its vaccenic acid and rumenic acid composition. This will enable researchers to comprehend the mechanism of the action of yeast and ascertain the ideal strain and dose to obtain a positive effect. Transcriptomics should be employed to identify the mRNA transcript expression levels of genes like stearoyl-CoA desaturase, fatty acid synthase, and elongase of very long chain fatty acids 6 due to their association with intramuscular fat and fatty acid composition in the muscle.
5. Conclusion
Yeast supplementation has been reported to increase feed intake and growth rate in young ruminants as well as improve milk production and milk fat in dairy cows. This could be due to the ability of yeast to stimulate fibre digestion and stabilise the rumen pH. The supplementation of yeast at the right dose and viability level in a concentrate diet could counteract the acidotic effects of high concentrate diets in the rumen by competing with lactate-producing (S. bovis and Lactobacillus) bacteria for available sugar and providing nutrients to encourage the growth of lactate-utilising bacteria (M. elsdenii). M. elsdenii is known to convert lactate into butyrate and propionate leading to a decrease in the accumulation of lactate. This causes an increase in rumen pH to support the proliferation of some beneficial bacteria like B. fibrisolvens. This group of bacteria (B. fibrisolvens) is responsible for the biohydrogenation of linoleic acid to produce trans-11 (a precursor of cis-9, trans-11) and cis-9, trans-11. Moreover, ciliate protozoa also decrease the production of stearic acid in the rumen by incorporating trans-11, cis-9, and trans-11 into their cells to protect them from being reduced to stearic acid. Interestingly, both thrive well at rumen pH above 5.6. The addition of yeast into the diet of ruminants has been reported to alter the rumen biohydrogenation pathway to synthesise more of the beneficial biohydrogenation intermediates (trans-11 and cis-9, trans-11). This implies that more dietary sources of linoleic acid, linolenic acid, and oleic acid along with beneficial biohydrogenation intermediates (cis-9, trans-11-CLA, and trans-11) would escape complete biohydrogenation in the rumen to be absorbed into milk and meat. However, several factors such as the dosage of yeast, the viability of yeast, age of the animal, specie, and breed of animal, physiological status of animal, duration of yeast treatment, diet composition, and environment may affect the response of an animal to yeast supplementation.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This work was supported by the Jiangsu Agricultural Science and Technology Innovation Fund (CX (19) 1006). The authors are also grateful to the Chinese Scholarship Council for the Ph.D. scholarship offered to Abdulmumini B. Amin.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adams M.C., Luo J., Rayward D., King S., Gibson R., Moghaddam G.H. Selection of a novel direct-fed microbial to enhance weight gain in intensively reared calves. Anim Feed Sci Technol. 2008;145:41–52. [Google Scholar]
- Al Ibrahim R.M., Kelly A.K., O'Grady L., Gath V.P., McCarney L., Mulligan F.J. The effect of body condition score at calving and supplementation with Saccharomyces cerevisiae on milk production, metabolic status, and rumen fermentation of dairy cows in early lactation. J Dairy Sci. 2010;93:5328. doi: 10.3168/jds.2010-3201. [DOI] [PubMed] [Google Scholar]
- Al- Mallah O. Effect of yeast supplement to the rations differed in degradable protein in milk production and components in cow. Mesopotamia J Agric. 2018;46(2):158–165. [Google Scholar]
- Alshaikh M.A., Alsiadi M.Y., Zahran S.M., Mogawer H.H., Aalshowime T.A. Effect of feeding yeast culture from different sources on the performance of lactating Holstein cows in Saudi Arabia. Asian-Australas J Anim Sci. 2002;15(3):352–356. [Google Scholar]
- AlZahal O., Rustomo B., Odongo N.E., Duffield T.F., McBride B.W. Technical note: a system for continuous recording of ruminal pH in cattle. J Anim Sci. 2007;85:213–217. doi: 10.2527/jas.2006-095. [DOI] [PubMed] [Google Scholar]
- Ambriz-Vilchis V., Jessop N.S., Fawcett R.H., Webster M., Shaw D.J., Walker N. Effect of yeast supplementation on performance, rumination time, and rumen pH of dairy cows in commercial farm environments. J Dairy Sci. 2017;100(7):5449–5461. doi: 10.3168/jds.2016-12346. [DOI] [PubMed] [Google Scholar]
- Arowolo M.A., He J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: a review. Anim Nutr. 2018;4(3):241–249. doi: 10.1016/j.aninu.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad M.A., Benallou B., Saim M.S., Smadi M.A., Meziane T. Impact of feeding yeast culture on milk yield, milk components, and blood components in Algerian dairy herds. J Veterinar Sci Technol. 2013;4:135. [Google Scholar]
- Bach A., Iglesias C., Devant M. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim Feed Sci Technol. 2007;136:156–163. [Google Scholar]
- Bach A., López-García A., González-Recio O., Elcoso G., Fàbregas F., Chaucheyras-Durand F. Changes in the rumen and colon microbiota and effects of live yeast dietary supplementation during the transition from the dry period to lactation of dairy cows. J Dairy Sci. 2019;102(7):6180–6198. doi: 10.3168/jds.2018-16105. [DOI] [PubMed] [Google Scholar]
- Bauman D.E., Baumgard L.H., Corl B.A., Griinari J.M. Biosynthesis of conjugated linoleic acid in ruminants 1. J Anim Sci. 2000;77(suppl_E):1–15. [Google Scholar]
- Bauman D.E., Corl B.A., Peterson D. The biology of conjugated linoleic acids in ruminants. In: Sebedio J.-L., Chrisite W.W., Adlof R., editors. Advances in conjugated linoleic acid research. AOCS Press; Champaign, IL: 2003. pp. 146–173. [Google Scholar]
- Bayat A.R., Kairenius P., Stefański T., Leskinen H., Comtet-Marre S., Forano E. Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. J Dairy Sci. 2015;98(5):3166–3181. doi: 10.3168/jds.2014-7976. [DOI] [PubMed] [Google Scholar]
- Beauchemin K.A., Rode L.M., Yang W.Z. Relationship between low ruminal pH and fibre digestion or efficiency of microbial protein synthesis in cattle. Can J Anim Sci. 2000;80:761. [Abstract] [Google Scholar]
- Beauchemin K.A., Yang W.Z., Morgavi D.P., Ghorbani G.R., Kautz W. Effects of bacterial direct-fed microbials and yeast on site and extent of digestion, blood chemistry, and subclinical ruminal acidosis in feedlot cattle. J Anim Sci. 2003;81:1628–1640. doi: 10.2527/2003.8161628x. [DOI] [PubMed] [Google Scholar]
- Bhatt R.S., Sahoo A., Karim S.A., Gadekar Y.P. Effects of Saccharomyces cerevisiae and rumen bypass-fat supplementation on growth, nutrient utilisation, rumen fermentation, and carcass traits of lambs. Anim Prod Sci. 2018;58(3):530–538. [Google Scholar]
- Bonilla C.J.A., LLamas L.G., Gutierrez A., Campos R. UANL, Monterrey; N.L., M´exico.: 1992. Efecto del uso de un cultivo de levaduras y del nivel de proteina en el suplemento, sobre el aprovechamiento de dietas a base de rastrojo dema'ız. Memorias del 5◦ Congreso Nacional de Producci'onOvina. Asociaci'on Mexicana de T´ecnicos Especialistas en Ovinocultura, A.C., Facultad de Medicina Veterinaria; pp. 33–47. [Google Scholar]
- Buntyn J.O., Schmidt T.B., Nisbet D.J., Callaway T.R. The role of direct-fed microbials in conventional livestock production. Ann Rev Anim Biosci. 2016;4:335–355. doi: 10.1146/annurev-animal-022114-111123. [DOI] [PubMed] [Google Scholar]
- Cabrera E.J.I., Mendoza M.G.D., Aranda I.E., Garcia-Bojalil C., B´arcena G.R., Ramos J.J.A. Saccharomyces cerevisiae and nitrogenous supplementation in growing steers grazing tropical pastures. Anim Feed Sci Technol. 2000;83:49–55. [Google Scholar]
- Chademana I., Offer N.W. The effect of dietary inclusion of yeast culture on digestion in the sheep. Anim Prod. 1990;50:483. [Google Scholar]
- Chaucheyras F., Fonty G., Bertin G., Gouet P. Effects of live Saccharomyces cerevisiae cells on zoospore germination, growth, and cellulolytic activity of the rumen anaerobic fungus, Neocallimastix frontalis MCH3. Curr Microbiol. 1995;31:201–205. doi: 10.1007/BF00298373. [DOI] [PubMed] [Google Scholar]
- Chaucheyras F., Fonty G., Bertin G., Salmon J.M., Gouet P. Effects of a strain of Saccharomyces cerevisiae (levucell SC), a microbial additive for ruminants, on lactate metabolism in vitro. Can J Microbiol. 1996;42:927–933. doi: 10.1139/m96-119. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Fonty G. Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077. Reprod Nutr Dev. 2001;41:57–68. doi: 10.1051/rnd:2001112. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Fonty G. Effect and mode of action of live yeast in the rumen. Biologia. 2006;61:741–750. [Google Scholar]
- Chaucheyras-Durand F., Walker N.D., Bach A. Effects of active dry yeasts on the rumen microbial ecosystem: past, present and future. Anim Feed Sci Technol. 2008;145(1):5–26. [Google Scholar]
- Chen L., Shen Y., Wang C., Ding L., Zhao F., Wang M. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front Microbiol. 2019;10:162. doi: 10.3389/fmicb.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikwanha O.C., Vahmani P., Muchenje V., Dugan M.E.R., Mapiye C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res Int. 2018;104:25–38. doi: 10.1016/j.foodres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Chiquette J., Allison M.J., Rasmussen M. Use of Prevotella bryantii25A and a commercial DFM during subacute acidosis challenge in mid-lactation dairy cows. J Dairy Sci. 2012;95:5985–5995. doi: 10.3168/jds.2012-5511. [DOI] [PubMed] [Google Scholar]
- Choi N.-J., Imm J.Y., Oh S., Kim B.-C., Hwang H.-J., Kim Y.J. Effect of pH and oxygen on conjugated linoleic acid (CLA) production by mixed rumen bacteria from cows fed high concentrate and high forage diets. Anim Feed Sci Technol. 2005;123–124:643–653. [Google Scholar]
- Cole N.A., Purdy C.W., Hutcheson D.P. Influence of yeast culture on feeder calves and lambs. J Anim Sci. 1992;70:1682–1690. doi: 10.2527/1992.7061682x. [DOI] [PubMed] [Google Scholar]
- Commun L., Mialon M.M., Martin C., Baumont R., Veissier I. Risk of subacute ruminal acidosis in sheep with separate access to forage and concentrate. J Anim Sci. 2009;87:3372–3379. doi: 10.2527/jas.2009-1968. [DOI] [PubMed] [Google Scholar]
- Cotta M., Forster R. The family Lachnospiraceae, including the genera Butyrivibrio, lachnospira and roseburia. The Prokaryotes. 2006;4:1002–1021. [Google Scholar]
- Desnoyers M., Giger-Reverdin S., Bertin G., Duvaux-Ponter C., Sauvant D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J Dairy Sci. 2009;92:1620–1632. doi: 10.3168/jds.2008-1414. [DOI] [PubMed] [Google Scholar]
- Dias A.L.G., Freitas J.A., Micai B., Azevedo R.A., Greco L.F., Santos J.E.P. Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J Dairy Sci. 2018;101(1):201–221. doi: 10.3168/jds.2017-13241. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Ellis J.L., Kebreab E., Strathe A.B., López S., France J. Ruminal pH regulation and nutritional consequences of low pH. Anim Feed Sci Technol. 2012;172(1):22–33. [Google Scholar]
- Domínguez-Vara I.A., González-Muñoz S.S., Pinos-Rodríguez J.M. Effects of feeding selenium-yeast and chromium-yeast to finishing lambs on growth, carcass characteristics, and blood hormones and metabolites. Anim Feed Sci Technol. 2009;152(1):42–49. [Google Scholar]
- Edwards E., Mustvangwa T., Topps J.H., Paterson G.F.M. The effects of supplemental yeast culture on patterns of rumen fermentation and growth performance of intensively fed bulls. Anim Prod. 1990;50:579. [Abstract] [Google Scholar]
- Elaref M.Y., Hamdon H.A.M., Nayel U.A., Salem A.Z.M., Anele U.Y. Influence of dietary supplementation of yeast on milk composition and lactation curve behavior of Sohagi ewes, and the growth performance of their newborn lambs. Small Rumin Res. 2020:106176. [Google Scholar]
- Erasmus L.J., Botha P.M., Kistner A. Effect of yeast culture supplement on production, rumen fermentation, and duodenal nitrogen flow in dairy cows. J Dairy Sci. 1992;75(11):3056–3065. doi: 10.3168/jds.S0022-0302(92)78069-2. [DOI] [PubMed] [Google Scholar]
- Faccenda A., Zambom M.A., de Avila A.S., Schneider C.R., Werle C.H., Anschau F.A. Performance and milk composition of Holstein cows fed with dried malt bagasse and selenium-enriched Saccharomyces cerevisiae. Livest Sci. 2020;238:104081. [Google Scholar]
- Ferreira G. Short communication: production performance and nutrient digestibility of lactating dairy cows fed diets with and without addition of a live-yeast supplement. J Dairy Sci. 2019;102(12):11057–11060. doi: 10.3168/jds.2019-17265. [DOI] [PubMed] [Google Scholar]
- Fomenky B.E., Chiquette J., Bissonnette N., Talbot G., Chouinard P.Y., Ibeagha-Awemu E.M. Impact of Saccharomyces cerevisiae boulardii CNCMI-1079 and Lactobacillus acidophilus BT1386 on total lactobacilli population in the gastrointestinal tract and colon histomorphology of Holstein dairy calves. Anim Feed Sci Technol. 2017;234:151–161. [Google Scholar]
- Fonty G., Chaucheyras-Durand F. Effects and modes of action of live yeasts in the rumen Biologia. Bratisl.) 2006;61:741–750. [Google Scholar]
- Food and Agriculture Organization of the United Nations, FAO . FAO; London UK: 2015. Word agriculture towards 2015/2030: an FAO perspective. [Google Scholar]
- Francisco A.E., Santos-Silva J.M., Portugal AP V., Alves S.P., Bessa RJ B. Relationship between rumen ciliate protozoa and biohydrogenation fatty acid profile in rumen and meat of lambs. PloS One. 2019;14(9) doi: 10.1371/journal.pone.0221996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzolin R., Dehority B.A. Effects of ruminal PH and feed intake on defaunation in sheep fed high concentrate diets. Rev Bras Zootec. 1996;25(6):1207–1215. [Google Scholar]
- Fuentes M.C., Calsamiglia S., Cardozo P.W., Vlaeminck B. Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in a dual-flow continuous culture. J Dairy Sci. 2009;92(9):4456–4466. doi: 10.3168/jds.2008-1722. [DOI] [PubMed] [Google Scholar]
- Galvao K.N., Santos J.E., Coscioni A., Villasenor M., Sischo W.M., Berge A.C. Effect of feeding live yeast products to calves with failure of passive transfer on performance and patterns of antibiotic resistance in fecal Escherichia coli. Reprod Nutr Dev. 2005;45:427–440. doi: 10.1051/rnd:2005040. [DOI] [PubMed] [Google Scholar]
- Geng C.Y., Ren L.P., Zhou Z.M., Chang Y., Meng Q.X. Comparison of active dry yeast (Saccharomyces cerevisiae) and yeast culture for growth performance, carcass traits, meat quality and blood indexes in finishing bulls. J Anim Sci. 2016;87:982–988. doi: 10.1111/asj.12522. [DOI] [PubMed] [Google Scholar]
- Guedes C.M., Gonçalves D., Rodrigues M.A.M., Dias-da-Silva A. Effects of a Saccharomyces cerevisiae yeast on ruminal fermentation and fibre degradation of maize silage in cows. Anim Feed Sci Technol. 2008;145:27–40. [Google Scholar]
- Harfoot C.G., Hazlewood G.P. Lipid metabolism in the rumen. In: Hobson P.N., editor. The rumen microbial ecosystem. 2nd ed. Elsevier; London, UK: 1997. pp. 382–426. [Google Scholar]
- Harfoot C.G., Hazlewood G.P. Lipid metabolism in the rumen. In: Hobson P.N., editor. The rumen microbial ecosystem. Elsevier Applied Science Publishers; London, UK: 1988. pp. 285–322. [Google Scholar]
- Harrison G.A., Hemken R.W., Dawson K.A., Harmon R.J., Barker K.B. Influence of addition of yeast culture supplement to diets of lactating cows on ruminal fermentation and microbial populations. J Dairy Sci. 1988;71:2967–2975. doi: 10.3168/jds.S0022-0302(88)79894-X. [DOI] [PubMed] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman D.D., Sorensen S.J., Momont P.A., Albin R., Cole N.A. Effect of yeast culture on steer performance, apparent diet digestibility, and carcass measurements when used in a barley and potato finishing diet. Prof Anim Sci. 1998;14(3):173–177. [Google Scholar]
- Hučko B., Bampidis V.A., Kodeš A., Christodoulou V., Mudřik Z., Poláková K., Plachý V. Rumen fermentation characteristics in pre-weaning calves receiving yeast culture supplements. Czech J Anim Sci. 2009;54(10):435–442. [Google Scholar]
- Jiang Y., Ogunade I.M., Arriola K.G., Qi M., Vyas D., Staples C.R. Effects of the dose and viability of Saccharomyces cerevisiae. 2. Ruminal fermentation, performance of lactating dairy cows, and correlations between ruminal bacteria abundance and performance measures. J Dairy Sci. 2017;100(10):8102–8118. doi: 10.3168/jds.2016-12371. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Ogunade I.M., Qi S., Hackmann T.J., Staples C.R., Adesogan A.T. Effects of the dose and viability of Saccharomyces cerevisiae. 1. Diversity of ruminal microbes as analyzed by Illumina MiSeq sequencing and quantitative PCR. J Dairy Sci. 2017;100:325–342. doi: 10.3168/jds.2016-11263. [DOI] [PubMed] [Google Scholar]
- Julien C., Marden J., Enjalbert F., Bayourthe C., Troegeler A. Live yeast as a possible modulator of polyunsaturated fatty acid biohydrogenation in the rumen. Rev Med Vet (Toulouse) 2010;161(8–9):391–400. [Google Scholar]
- Kawas J.R., García-Castillo R., Garza-Cazares F., Fimbres-Durazo H., Olivares-Sáenz E., Hernández-Vidal G. Effects of sodium bicarbonate and yeast on productive performance and carcass characteristics of light-weight lambs fed finishing diets. Small Rumin Res. 2007;67(2):157–163. [Google Scholar]
- Kepler C.R., Hirons K.P., Mcneill J.J., Tove S.B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J Biol Chem. 1966;241:1350–1354. [PubMed] [Google Scholar]
- Kim Y.J., Liu R.H., Bond D.R., Russell J.B. Effect of linoleic acid concentration on conjugated linoleic acid production by Butyrivibrio fibrisolvens A38. Appl Environ Microbiol. 2000;66:5226–5230. doi: 10.1128/aem.66.12.5226-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel C.R., Rust S.R., Zang G., Gilliland S.E. Bacterial direct-fed microbials in ruminant diets: performance response and mode of action. J Anim Sci. 2003;81(2):120–132. [Google Scholar]
- Křížová L., Richter M., Třináctý J., Říha J., Kumprechtová D. The effect of feeding live yeast cultures on ruminal pH and redox potential in dry cows as continuously measured by a new wireless device. Czech J Anim Sci. 2011;56(1):37–45. [Google Scholar]
- Kung L., Jr., Hession A.O. Preventing in vitro lactate accumulation in ruminal fermentation by inoculation with Megasphaera elsdenii. J Anim Sci. 1995;73:250–256. doi: 10.2527/1995.731250x. [DOI] [PubMed] [Google Scholar]
- Lara E.C., Bragiato U.C., Rabelo C.H.S., Messana J.D., Reis R.A. Inoculation of corn silage with Lactobacillus plantarum and Bacillus subtilis associated with amylolytic enzyme supply at feeding. 1. Feed intake, apparent digestibility, and microbial protein synthesis in wethers. Anim Feed Sci Technol. 2018;243:22–34. [Google Scholar]
- Laarman A.H., Ruiz-Sanchez A.L., Sugino T., Guan L.L., Oba M. Effects of feeding a calf starter on molecular adaptations in the ruminal epithelium and liver of Holstein dairy calves. J Dairy Sci. 2012;95:2585–2594. doi: 10.3168/jds.2011-4788. [DOI] [PubMed] [Google Scholar]
- Lee Y. Effect of pH on conjugated linoleic acid (CLA) formation of linolenic acid biohydrogenation by ruminal microorganisms. J Microbiol. 2013;51(4):471–476. doi: 10.1007/s12275-013-1070-z. [DOI] [PubMed] [Google Scholar]
- Lesmeister K.E., Heinrichs A.J., Gabler M.T. Effects of supplemental yeast (Saccharomyces cerevisiae) culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J Dairy Sci. 2004;87:1832–1839. doi: 10.3168/jds.S0022-0302(04)73340-8. [DOI] [PubMed] [Google Scholar]
- Liu Y.Z., Lang M., Zhen Y.G., Chen X., Sun Z., Zhao W. Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on growth performance, carcass traits and the fatty acid profile of the longissimus dorsi muscle in lambs. J Anim Physiol Anim Nutr. 2019;103(5):1274–1282. doi: 10.1111/jpn.13128. [DOI] [PubMed] [Google Scholar]
- Loesche W.J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969;18:723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuski R.A., Ying Y., Allen M.S. Yeast culture supplementation prevented milk fat depression by a short-term dietary challenge with fermentable starch. J Dairy Sci. 2009;92(1):160–167. doi: 10.3168/jds.2008-0990. [DOI] [PubMed] [Google Scholar]
- Lynch H.A., Martin S.A. Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal micro- organism fermentation. J Dairy Sci. 2002;85:2603–2608. doi: 10.3168/jds.S0022-0302(02)74345-2. [DOI] [PubMed] [Google Scholar]
- Maamouri O., Selmi H., M’hamdi N. Effects of yeast (Saccharomyces cerevisiae) feed supplement on milk production and its composition in Tunisian Holstein friesian cows. Sci Agric Bohem. 2014;45(3):170–174. [Google Scholar]
- Maggioni D., Marques J. de A., Perotto D., Rotta P.P., Ducatti T., Matsushita M. Bermuda grass hay or sorghum silage with or without yeast addition on performance and carcass characteristics of crossbred young bulls finished in feedlot. Asian-Australas J Anim Sci. 2009;22(2):206–215. [Google Scholar]
- Magrin L., Gottardo F., Fiore E., Gianesella M., Martin B., Chevaux E. Use of a live yeast strain of Saccharomyces cerevisiae in a high-concentrate diet fed to finishing Charolais bulls: effects on growth, slaughter performance, behaviour, and rumen environment. Anim Feed Sci Technol. 2018;241:84–93. [Google Scholar]
- Martin S.A., Jenkins T.C. Factors affecting conjugated linoleic acid and trans-C18:1 fatty acid production by mixed ruminal bacteria. J Anim Sci. 2002;80:3347–3352. doi: 10.2527/2002.80123347x. [DOI] [PubMed] [Google Scholar]
- Malekkhahi M., Tahmasbi A.M., Naserian A.A., Danesh-Mesgaran M., Kleen J.L., AlZahal O. Effects of supplementation of active dried yeast and malate during sub-acute ruminal acidosis on rumen fermentation, microbial population, selected blood metabolites, and milk production in dairy cows. Anim Feed Sci Technol. 2016;213:29–43. [Google Scholar]
- Malik R., Bandla S. Effect of source and dose of probiotics and exogenous fibrolytic enzymes (EFE) on intake feed efficiency, and growth of male buffalo (Bubalus bubalis) calves. Trop Anim Health Prod. 2010;42:1263–1269. doi: 10.1007/s11250-010-9559-5. [DOI] [PubMed] [Google Scholar]
- Mao H.L., Mao H., Wang J.K., Liu J.X., Yoon I. Effects of Saccharomyces cerevisiae fermentation product on in vitro fermentation and microbial communities of low-quality forages and mixed diets. J Anim Sci. 2013;91:3291–3298. doi: 10.2527/jas.2012-5851. [DOI] [PubMed] [Google Scholar]
- McAllister T.A., Beauchemin K.A., Alazzeh A.Y., Baah J., Teather R.M., Stanford K. Review: the use of direct fed microbials to mitigate pathogens and methane production in cattle. Can J Anim Sci. 2011;91:193–211. [Google Scholar]
- Mendoza G.D., Britton R.A., Stock R.A. Influence of ruminal protozoa on site and extent of starch digestion and rumen fermentation. J Anim Sci. 1993;71:1572–1578. doi: 10.2527/1993.7161572x. [DOI] [PubMed] [Google Scholar]
- Milewski S., Zaleska B., Bednarek D., Tański Z., Sobiech P., Ząbek K. Effect of yeast supplements on selected health-promoting properties of lamb meat. Bull Vet Inst Pulawy. 2012;56(3):315–319. [Google Scholar]
- Milewski S., Zaleska B. The effect of dietary supplementation with Saccharomyces cerevisiae dried yeast on lambs meat quality. J Anim Feed Sci. 2011;20(4):537–545. [Google Scholar]
- Miller-Webster T., Hoover W.H., Holt M. Influence of yeast culture on ruminal microbial metabolism in continuous culture. J Dairy Sci. 2002;86:2009–2014. doi: 10.3168/jds.S0022-0302(02)74277-X. [DOI] [PubMed] [Google Scholar]
- Nami Y., Vaseghi Bakhshayesh R., Manafi M., Hejazi M.A. Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT. 2019;111:876–882. [Google Scholar]
- Nasiri A.H., Towhidi A., Shakeri M., Zhandi M., Dehghan-Banadaky M., Pooyan H.R. Effects of Saccharomyces cerevisiae supplementation on milk production, insulin sensitivity and immune response in transition dairy cows during hot season. Anim Feed Sci Technol. 2019;251:112–123. [Google Scholar]
- Newbold C.J. Probiotics for ruminants. Anim Res. 1996;45(Suppl. 1):329–335. [Google Scholar]
- Nisbet D.J., Martin S.A. Effect of dicarboxylic acids and Aspergillus oryzae fermentation extract on lactate uptake by the ruminal bacterium Selenomonas ruminantium. Appl Environ Microbiol. 1990;56:3515–3518. doi: 10.1128/aem.56.11.3515-3518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noziére P., Steinberg W., Silberberg M., Morgavi D.P. Amylase addition increases starch ruminal digestion in first-lactation cows fed high and low starch diets. J Dairy Sci. 2014;97:2319–2328. doi: 10.3168/jds.2013-7095. [DOI] [PubMed] [Google Scholar]
- Ogunade I.M., Lay J., Andries K., McManus C.J., Bebe F. Effects of live yeast on differential genetic and functional attributes of rumen microbiota in beef cattle. J Anim Sci Biotechnol. 2019;10(1):68. doi: 10.1186/s40104-019-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsi F., Fortina R., Tassone S., Bodas R., López S. Effects of inactivated and live cells of Saccharomyces cerevisiae on in vitro ruminal fermentation of diets with different forage:concentrate ratio. J Agric Sci. 2012;150(2):271–283. [Google Scholar]
- Peng Q., Cheng L., Kang K., Tian G., Al-Mamun M., Xue B. Effects of yeast and yeast cell wall polysaccharides supplementation on beef cattle growth performance, rumen microbial populations and lipopolysaccharides production. J Integr Agric. 2020;19(3):810–819. [Google Scholar]
- Pinloche E., McEwan N., Marden J.P., Bayourthe Auclair C.E., Newbold C.J. The effects of a probiotic yeast on the bacterial diversity and population structure in the rumen of cattle. PloS One. 2013;8(7) doi: 10.1371/journal.pone.0067824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva G., Belladonna S., Fusconi Sicbladi F. Effects of yeast culture on dairy cow performance, ruminal fermentation, blood components, and milk manufacturing properties. J Dairy Sci. 1993;76:2717–2722. doi: 10.3168/jds.S0022-0302(93)77608-0. [DOI] [PubMed] [Google Scholar]
- Putnam D.E., Schwab C.G., Socha M.T., Whitehouse N.L., Kierstead N.A., Garthwaite B.D. Effect of yeast culture in the diets of early lactation dairy cows on ruminal fermentation and passage of nitrogen fractions and amino acids to the small intestine. J Dairy Sci. 1997;80:374–384. doi: 10.3168/jds.S0022-0302(97)75947-2. [DOI] [PubMed] [Google Scholar]
- Raghebian M., Dabiri N., Yazdi A.B., Bahrani M.J., Shomeyzi J., Raghebian A., Hatami P. Probiotic effect on meat quality and carcass parameters of Iranian zandi lambs. J Livestock Sci. 2007;8:163–168. [Google Scholar]
- Ramírez J.F., Medina S., Garcê N., Cifuentes T. Effects of the supplementation with yeast (Saccharomyces cerevisiae) on milk yield, and milk components of water buffalo cows from northeast of Colombia. Ital J Anim Sci. 2007;6(sup2):502–503. [Google Scholar]
- Ramsing E.M., Davidson J.A., French P.D., Yoon I., Keller M., Peters-Fleckenstein H. Effects of yeast culture on peripartum intake and milk production of primiparous and multiparous Holstein Cows 1. Prof Anim Sci. 2009;25(4):487–495. [Google Scholar]
- Rezaeian M. The effects of Saccharomyces cerevisiae on feed intake, milk yield and composition in lactating Holstein cows. Proc Br Soc Anim Sci. 2003;2003:129. 2017/11/20. [Google Scholar]
- Robinson J.A., Smolenski W.J., Greening R.C., Ogilvie R.L., Bell R.L., Barsuhn K., Peters J.P. Prevention of acute acidosis and enhancement of feed intake in the bovine by Megasphaera elsdenii 407A. J Anim Sci. 1992;70(Suppl. 1):310. [Abstract] [Google Scholar]
- Rodríguez-Gaxiola M.A., Domínguez-Vara I.A., Barajas-Cruz R., Contreras-Andrade I., Morales-Almaráz E., Bórquez-Gastelum J.L. Effect of enriched-chromium yeast on growth performance, carcass characteristics and fatty acid profile in finishing Rambouillet lambs. Small Rumin Res. 2020;188:106118. [Google Scholar]
- Rose A.H. Alltech Technical Publications; Nicholasville, Kentucky: 1987. Live yeast, A microorganism for all species: a theoretical look at its mode of action; pp. 113–118. [Google Scholar]
- Rufino L.D.A., Pereira O.G., Ribeiro K.G., Valadares Filho S.C., Cavali J., Paulino P.V.R. Effect of substitution of soybean meal for inactive dry yeast on diet digestibility, lamb's growth and meat quality. Small Rumin Res. 2013;111(1):56–62. [Google Scholar]
- Sales J. Effects of Saccharomyces cerevisiae supplementation on ruminal parameters, nutrient digestibility and growth in sheep: a meta-analysis. Small Rumin Res. 2011;100(1):19–29. [Google Scholar]
- Silva RG da, In do Prado, Matsushita M., Souza NE de. Dietary effects on muscle fatty acid composition of finished heifers. Pesquisa Agropecuária Brasileira . scielo. 2002;37:95–101. [Google Scholar]
- Slattery J., MacFabe D.F., Frye R.E. The significance of the enteric microbiome on the development of childhood disease: a review of prebiotic and probiotic therapies in disorders of childhood. Clin Med Insights Pediatr. 2016;10:91–107. doi: 10.4137/CMPed.S38338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulistyowati E., Sudarman A., Wiryawan K.G., Toharmat T. Quality of milk fatty acid during late lactation in dairy goat fed on PUFA-diet supplemented with yeast and curcuma Xanthorrhiza roxb. J Indonesian Trop Anim Agric. 2013;38(4):247–256. [Google Scholar]
- Sun L., Toyonaga M., Ohashi A., Matsuura N., Tourlousse D.M., Meng X.Y. Isolation and characterization of Flexilinea flocculi gen. nov., sp. nov., a filamentous, anaerobic bacterium belonging to the class Anaerolineae in the phylum Chloroflexi. Int J Syst Evol Microbiol. 2016;66:988–996. doi: 10.1099/ijsem.0.000822. [DOI] [PubMed] [Google Scholar]
- Sun Y., Allen M., Lock A. 375 Young Scholar Presentation: effects of dietary factors and rumen pH on rumen biohydrogenation pathways and risk of milk fat depression. J Anim Sci. 2017;95(suppl_2):181–182. [Google Scholar]
- Thrune M., Bach A., Ruiz-Moreno M., Stern M.D., Linn J.G. Effects of Saccharomyces cerevisiae on ruminal pH and microbial fermentation in dairy cows: yeast supplementation on rumen fermentation. Livest Sci. 2009;124(1):261–265. [Google Scholar]
- Titi H.H., Dmour R.O., Abdullah A.Y. Growth performance and carcass characteristics of Awassi lambs and Shami goat kids fed yeast culture in their finishing diet. Anim Feed Sci Technol. 2008;142(1):33–43. [Google Scholar]
- Tripathi M.K., Karim S.A. Effect of yeast cultures supplementation on live weight change, rumen fermentation, ciliate protozoa population, microbial hydrolytic enzymes status and slaughtering performance of growing lamb. Livest Sci. 2011;135(1):17–25. [Google Scholar]
- Troegeler A., Marden J., Bayourthe C., Moncoulon R., Enjalbert F. INRA-RRI symposium, 21-23 jun 2006, Aberdeen, Scotland. 2006. Effects of live yeast on the fatty acid biohydrogenation by ruminal bacteria. (Unpublished) [Google Scholar]
- Vita N., Valette O., Brasseur G., Lignon S., Denis Y., Ansaldi M. The primary pathway for lactate oxidation in Desulfovibrio vulgaris. Front Microbiol. 2015;6:606. doi: 10.3389/fmicb.2015.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R.J., McKain N., Shingfield K.J., Devillard E. Isomers of conjugated linoleic acids are synthesised via different mechanisms in ruminal digesta and bacteria. J Lipid Res. 2007;48:2247–2254. doi: 10.1194/jlr.M700271-JLR200. [DOI] [PubMed] [Google Scholar]
- Webb E.C., O'Neill H.A. The animal fat paradox and meat quality. Meat Sci. 2008;80(1):28–36. doi: 10.1016/j.meatsci.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Wiedmeier R.D., Arambel M.J., Walters J.L. Effect of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J Dairy Sci. 1987;70:2063–2068. doi: 10.3168/jds.S0022-0302(87)80254-0. [DOI] [PubMed] [Google Scholar]
- Williams P.E.V. Alltech Tech. Publishers; Nicholasville, KY: 1989. The mode of action of yeast culture in ruminal diets. A review of the effect on rumen fermentation patterns. Biotechnology in the feed industry. [Google Scholar]
- Wood J.D., Richardson R.I., Nute G.R., Gianesella M., Martin B., Chevaux E. Effects of fatty acids on meat quality: a review. Meat Sci. 2004;66(1):21–32. doi: 10.1016/S0309-1740(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Xiao J.X., Alugongo G.M., Chung R., Dong S.Z., Li S.L., Yoon I. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: ruminal fermentation, gastrointestinal morphology, and microbial community. J Dairy Sci. 2016;99(7):5401–5412. doi: 10.3168/jds.2015-10563. [DOI] [PubMed] [Google Scholar]
- Yalçın S., Yalçın S., Can P., Gürdal A.O., Bağcı C., Eltan Ö. The nutritive value of live yeast culture (Saccharomyces cerevisiae) and its effect on milk yield, milk composition and some blood parameters of dairy cows. Asian-Australas J Anim Sci. 2011;24(10):1377–1385. [Google Scholar]
- Yang W.Z., Beauchemin K.A., Vedres D.D., Ghorbani G.R., Colombatto D., Morgavi D.P. Effects of direct-fed microbial supplementation on ruminal acidosis, digestibility, and bacterial protein synthesis in continuous culture. Anim Feed Sci Technol. 2004;114(1):179–193. [Google Scholar]
- Zhou B., Wang H., Luo G., Niu R., Wang J. Effect of dietary yeast chromium and l-carnitine on lipid metabolism of sheep. Biol Trace Elem Res. 2013;155(2):221–227. doi: 10.1007/s12011-013-9790-9. [DOI] [PubMed] [Google Scholar]
- Zhu W., Wei Z., Xu N. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J Anim Sci Biotechnol. 2017;8(1):36. doi: 10.1186/s40104-017-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]