Abstract
This review aims to highlight the effects of ochratoxin A (OTA) in the feed of meat-producing animals. The accumulation of OTA in feed and its distribution in various farm animals were compared and evaluated. Primarily, the oral administration of OTA-contaminated feed and the predisposition in an animal's vital organ were critically examined in this work. The collated reports show that OTA directly associated with endemic nephropathy and its high concentration leads to degeneration of liver cells, and necrosis of intestinal and lymphoid tissues. At present, limited reports are available in the recent literature on the problems and consequences of OTA in feed. Therefore, this review focused on the OTA carryover from feed to farm animals and the interaction of its secondary metabolites on their biochemical parameters. Hence, this report provides greater insights into animal health related to OTA residues in meat and meat products. This article also explores mitigation strategies that can be used to prevent the carryover effects of OTA in livestock feeds and the effects in the food chain.
Keywords: Ochratoxin A, Feed, Livestock, Meat, Mitigation strategy
1. Introduction
Due to climate change and unpredicted rainfall, the frequency and level of fungal contamination in feedstuffs may expand. In general, wet seasons may postpone the grain development, which results in more formation of mycotoxin, particularly in maize. Similarly, Fusarium toxins mainly occur during the period of harvesting and post-harvesting stages (Bernhoft et al., 2012). In animals, mycotoxicosis is associated with feed related, non-infectious, non-transferable, non-irresistible, and non-detectable growth specific mechanisms (Pappas et al., 2016). Disorders that are initiated by mycotoxin cascade various infections in farm animals, characterizing the complexity of the infection mechanism. The occurrence of mycotoxins in feed relies upon a few factors such as climatic conditions, multisets of microbes in specific crops, and storing practices. The feed blend, degree of feed components, feed handling procedures, and storage practices (Egbontan et al., 2017) are significant factors associated with the production of multiple mycotoxins. It was reported that mycotoxin contamination in feed reduced the productivity and immune suppression in livestock (Grenier and Oswald, 2011). Amongst the common mycotoxins such as aflatoxin (AF), deoxynivalenol (DON), ochratoxin A (OTA), zearalenone (ZEN) and fumonisins (FUM), OTA represents a crucial issue in the feed of livestock animals that causes adverse effects to a human following consumption (Denli and Perez, 2010). Nevertheless, there are no recent, detailed reports on OTA contamination in feed and its impact on meat-producing animals. Therefore, this review article discusses all the significant interactions of OTA from feed to farm animals. Accumulation of OTA in various organs and its concentration in specific meat products were compared and evaluated. The main aim is to mitigate OTA contamination in feed, and uncover intervention strategies to prevent carryover of OTA from animals to humans.
2. Production of OTA in feed
OTA is produced primarily by fungal species Aspergillus ochraceus, Penicillium viridicatum and Penicillium cyclopium (FAO, 2004), this raising significant public concern across the globe. A. ochraceus is a filamentous fungus with biseriate conidiophores characteristics. It was found to occupy a variety of environmental functions including soil and agricultural commodities such as cereal grains, peanuts, cottonseed and fruits (D'Mello, 2001). OTA delivered by A. ochraceus is consistent under acidic conditions that endure at ordinary cooking temperatures, which could be diffused in the animals' food chain. Further, OTA was considered as one of the active carcinogenic agents (Nazieh and Khalaileh, 2018). Nevertheless, OTA impairs animal productivity which might remains in the meat and meat products. The fungal species readily colonizes into feeds and forages during post-harvest transportation and in storage time. Likewise, rodents, birds and insects may facilitate contamination by causing visible lesions on plants, that provide entry into the plant for fungal spores (Abdel-Wahhab and Kholif, 2008). All these activities are facilitated by physicochemical parameters including water activity (aw), temperature, presence of oxygen, nature of the substrate and pH conditions. These are the key determinants to proliferate and produce OTA (D'Mello, 2001). However, OTA-contaminated feed has a greater economic impact on monogastric animals (poultry and pig) than ruminant animals (cattle, sheep, and goats). It has been reported that ruminant animals are more resistant to OTA toxicity than monogastric animals (Denli and Perez, 2010). OTA has shown nephrotoxic, hepatotoxic, and teratogenic actions in animal species as well as in humans. A recent survey on 74,821 feed samples collected from 100 countries detected that OTA occurred in 15% of the samples (Gruber-Dorninger et al., 2019). These researchers identified that climate change is the crucial factor for all mycotoxin formation in South-Asia, Sub-Saharan Africa, and Southeast Asia. Other factors like inconsistent rainfall and temperature are also associated with high OTA formation in feed (Gruber-Dorninger et al., 2019).
3. Contamination of OTA in the feed of meat-producing animals
3.1. Contamination of OTA in poultry feed and distribution in poultry
Poultry feed ingredients are comprised of the following composition: 1) a blend of cereals with maize as the primary energy source, 2) animal-derived proteins including fish, meat, and bone meal, and 3) plant-derived proteins from soybean and peanuts. Maize is the prevalent grain utilized in poultry, and can be contaminated by mycotoxins from Aspergillus spp., Fusarium spp. and Penicillium spp. during handling and storage. Feed ingredients such as maize, peanut cake, wheat, and other cereals tested in Nigeria were reported to contain OTA at 10 μg/kg. In contrast, the concentration of other mycotoxins was found to be ≤ 647 μg/kg with a mean of 114 μg/kg among 20% of samples (Akinmusire et al., 2019). Thus, mycotoxin contamination in feed varies depending on the regions. However, maize grains collected from farmers and traditional markets in the Kupang region of Indonesia were found to contain OTA at 20.38 and 20.39 μg/kg DM, respectively (Nalle et al., 2019). The upper limit OTA set by the European Commission (EC, 2006) for poultry feed was 0.1 mg/kg. The upper limit for OTA in poultry feed is 0.1 mg/kg, which was set by the European Commission (EC, 2006), and the transformation from feed to various parts of the poultry body varies. For instance, OTA a decrease in thymus to serum albumins and globulin (alpha-, beta-and gamma-globulin) was found. Although these parameters were identified in the bird's hematology, OTA was not detected in liver and renal cells (Pozzo et al., 2013).
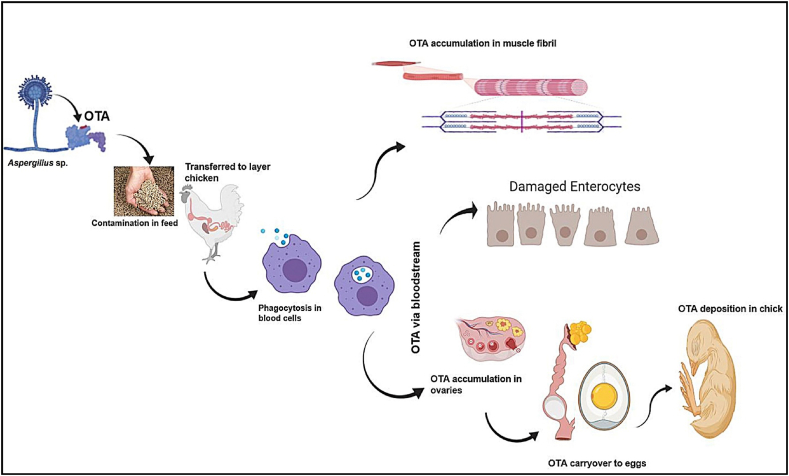
Further, the occurrence of OTA in chicken meat and eggs showed contamination of 41% and 35%, respectively. The liver of layer chickens contained OTA at 2.41 μg/kg, but no trace of OTA was detected in the wings, legs, and chest of domestic chickens (Nazieh and Khalaileh, 2018). Nevertheless, the poultry feed contained Aspergillus flavus and Penicillium citrinum spp., which was observed at approximately 46% of OTA, and found to be high concentration compared to other studies (Rosa et al., 2009). Further, laboratory-based experiments showed significant changes in chicks fed with OTA. For example, contaminated chick feed (200 μg/kg of OTA) that was given for 35 d manifested in degeneration of epithelial cells on proximal convoluted tubules with cytoplasmic swelling in the kidney region. Similarly, the liver region exhibited adverse necrosis and hepatic haemorrhages (Mujahid et al., 2019). A greater accumulation of OTA was detected in the kidney (1.14 μg/kg) and heart (0.073 μg/kg) of broiler chicks (Arbreaker breed) (Mujahid et al., 2019). Another experiment showed similar outcomes on fertile eggs that were injected with OTA in ovo (12.5 ng/egg). After 19 d of incubation, the blood and organ samples exhibited a high degree of thyroid hormone, and liver tissue showed megalocytosis and degeneration of liver cells in OTA injected chicks. These results confirmed that OTA exposure during the embryonic stage would lead to extensive oxidative damage and organ damage (Elsayed et al., 2019). The schematic representation of OTA carryover from feed to various parts of chicken is given in Fig. 1.
Fig. 1.
Schematic representation of ochratoxin A (OTA) carryover from feed to various parts of the chicken.
3.2. Contamination of OTA in pig feed and distribution
The most frequently observed mycotoxin metabolites in pig feed were DON, ZEA, and OTA. The toxicokinetics study showed that OTA was detected to a greater degree in urine samples of swine, but that it varies in every species (Schelstraete et al., 2020). In Costa Rica, 19 out of 57 feed samples were detected with multi-mycotoxins, and this feed sample exposed more than 50 μg/kg of OTA in pigs and sows (Persi et al., 2014; Leiva et al., 2019). In northwest Italy, 0.22 to 38.4 μg/kg OTA was observed from 15 different swine farms with moisture of ≥120 μg/kg in every feed samples. However, this amount was lower than the standard set by the European Commission for swine feed of 50 μg/kg of OTA (Pozzo et al., 2010). According to Malagutti et al. (2005), swine fed containing OTA (25 μg/kg) resulted in a high concentration of OTA in the final meat product. Safety limit standards set by the Italian Ministry of Health for OTA were 1 μg/kg in pork meat.
One study reported that the presence of OTA was ≥3.65 μg/kg in liver samples (67%) of swine from various meat production units in France (Milićević et al., 2009). Similarly, a study undertaken over 3 consecutive years in Poland reported a level of OTA in the range of 0.2 to 5.0 μg/kg in 430 meat samples, mainly from pig kidneys, poultry livers and fish muscle. Among them, 94 samples were contaminated with a maximum concentration of 10 μg/kg of OTA (Pietruszka et al., 2017). Vojvodina, Serbia had an average of 1.36 to 3.97 μg/kg of OTA in 14.74% of the pig kidney samples (Horvatovic et al., 2019). These levels of OTA in pig meat were higher than the maximum limit set by various European Union (EU) countries. For instance, Denmark and Estonia have set the limit of ≤10.0 μg/kg in pig kidney and liver (Hort et al., 2018), while Slovakia has set a limit of 5 μg/kg for meat, and pork meat from Italy should be ≤ 1 μg/kg (Duarte et al., 2010). Further, when the dietary concentration of OTA increased, it leads to endemic swine nephropathy and degeneration of liver cells and necrosis in intestinal and lymphoid tissues (Santos Pereira et al., 2019). A similar outcome was reported by Persi et al. (2014). When pigs were orally dosed with 300 μg/kg of OTA for 30 d, elevated OTA accumulated in kidney, lungs and adipose tissues. A minimum concentration of OTA was detected in their final products such as black pudding frankfurters (14.02 μg/kg), liver sausage (13.77 μg/kg) and pâté (9.33 μg/kg).
3.3. Contamination of OTA in dairy cattle feed
Cattle feed is the most vulnerable to substrates for mould growth, whereas commercial feeds are usually contaminated with OTA (25%) followed by green gram feed. A. flavus growth was commonly observed in legume blends and other cereal blended feeds which became a growth substrate for Penicillium sp. and Fusarium as well (Bhagya et al., 2019). Data published on contamination of mycotoxin in natural feed showed 24.8 μg/kg of OTA in various regions of Saudi Arabia (Al-Jaal et al., 2019). Nevertheless, 37.5% silage samples had 0.1 to 169 ng/g OTA in Pakistan, which is higher than the standard set by the European Commission proposed for OTA (Sultana et al., 2013). In contrast with formula feed, approximately 77.8% silage samples were recorded with the presence of OTA in dairy feed (Zhang et al., 2019).
3.4. Distribution of OTA in milk from farm animals
Mycotoxin has been suggested to have an estrogenic effect because of its binding action on estrogen receptors. This could cause disruptive action on reproductive glands or mammary glands and could also be transferred in the milk of mammals. According to Zhang et al. (2019), the OTA and alpha ochratoxin (OTα) were distinguishable in urine samples of dairy cows ranging between 1.8 ng/mL and 324.6 ng/mL, respectively. Further, OTA was detected in milk, plasma, and various other tissues of dairy cows. Similarly, OTA levels in 120 raw milk samples tested in Egypt-based dairy animals (cow, buffalo, sheep, and goat) did not meet the standard limit. Comparable results were found in fresh milk samples in Germany (Turkoglu and Keyvan, 2019). Moreover, the extensive levels of OTA and OTα that were recorded in the milk were due to the high exposure of OTA (13.3 mg/kg body weight) in dairy feed. Kamal et al. (2019) stated that OTA gets degraded into less toxic OTα under rumen microbiota, and is finally excreted in urine. However, processing temperatures also decrease OTA in milk. For example, Turkey-based research on raw milk, pasteurized and ultra-high temperature (UHT) milk samples contained OTA of 137 ± 57 ng/L, 135 ± 8 ng/L, and 85 ± 4 ng/L, respectively (Gross et al., 2019). However, donkey milk samples showed the absence of OTA, even though the feed samples were contaminated with ≤4 μg/kg of OTA (Boudra et al., 2013). A minimal transfer of OTA from feed to sheep milk was also noticed. A. ochraceus contaminated feed (237.3 μg/kg) provided to dairy sheep had 3.5 μg/L of OTA in milk (Ogunade et al., 2018).
In contrast, cow milk samples in Norway based milk samples showed a greater accumulation of OTA. 11 ng/L OTA in 6 out of 40 samples of conventional milk resulted in 15 ng/L in fresh milk being observed (Ferrufino-Guardia et al., 2000). Further, oral administration of naturally contaminated feed (15.6 μg/kg of OTA) for rabbits exhibited a greater OTA accumulation in blood plasma and milk. This result confirmed that contaminated feed fed during lactation could easily carryover into the milk (Hashimoto et al., 2016). Similarly, OTA at various dosages given to lactating cows did not show variation in the milk yield, but 0.2 μg/kg of OTA detected in the plasma of dairy cows (Akkaya and Bal, 2013).
3.5. Contamination of OTA in beef cattle feed
Beef cattle feed samples at Marmara and Mediterranean, Turkey, contained multiple mycotoxins with the highest concentration of total mycotoxin (5.23 μg/kg) and OTA of 1.68 μg/kg (Rezaei et al., 2015). All these feed samples had a moisture content of 2.6% to 3.0% (Rezaei et al., 2015). Still, these values were considered to be low across various regions of Turkey compared to 250 μg/kg of OTA limit set by European Food Safety Authority and the Turkish Ministry of Agriculture (Ekici et al., 2016). A similar study conducted in at Markazi Province, Iran gave greater insights on fungal contamination, with dairy cattle feed that contained 16,436.75 ± 6.32 CFU/g of the fungal colony and beef cattle feed possessing 291,839.6 ± 5.91 CFU/g (Rezaei et al., 2015). However, Ekici et al. (2016), reported that the presence of OTA in ruminant feed mix varied depending upon the season. For example, in Turkey, the highest concentration of OTA (57.69 μg/kg) and other mycotoxins (total AF, 13.57 μg/kg; AFB1, 8.54 μg/kg) was recorded during winter, followed by autumn, summer, and spring seasons (Ekici et al., 2016). Both toxigenic fungal species and mycotoxin contaminations corresponded in the crude material with the presence of Aspergillus sp. (OTA) (Juan et al., 2019).
3.6. Distribution of OTA in beef meat products
OTA was found at a high concentration ranging between 77% and 68% in the blood- and liver-sausages from the commercial market in Germany (Scheuer and Gareis, 2002). A comparison was made with Italian samples, which observed with 45% of OTA residues in sausages (Iacumin et al., 2009). The majority of beef luncheon (40 samples) from Qena city of Egypt, had 4.1 to 7.1 ppm of OTA with the presence of Aspergillus tubingensis and Aspergillus niger between 4.6 and 8.2 ppm (Hussein and Gherbawy, 2019). Bahobail (2016) confirmed that A. niger isolated from various origins could produce more OTA compared to other fungal species. Nevertheless, OTA contamination in any meat products could develop either from OTA-delivering fungi on the external surface of meat items (Iacumin et al., 2009) or fungal species from non-meat substances in the form of grains and spices (Sultana et al., 2013). As per the Food and Agriculture Organization (FAO, 2004), it is suggested that the tolerable limit for OTA be extended to ≤5 μg/kg.
3.7. Distribution of OTA in rabbit meat
Feed samples were tested for contamination over a period of 8 months in Córdoba province, of Argentina. Whereby, 12% of rabbit feeds were found to contain OTA ranging between 15 and 25 ng/g. These feeds mainly contaminated with toxigenic fungi species including Aspergillus candidus, A. flavus, Aspergillus terreus, Aspergillus parasiticus, Penicillium implicatum, Penicillium minioluteum, Penicillium crustosum and Penicillium citrionigrum (Scheuer and Gareis, 2002). Although contaminated feed had adverse effects, a combination of bioactive compounds suppressed ill-effects in rabbits. For instance, a contaminated diet (19 μg/kg of OTA) + 0.4 mg/kg Bacillus subtilis and Bacillus licheniformis blended with 0.1% of manna oligosaccharides (MOS) in the rabbit feed showed positive changes and normal histology of liver and kidney cells. This contrasts with feed containing B. subtilis and B. licheniformis, which showed degeneration in the kidney with slight modification in mononuclear inflammatory cell (Magnoli et al., 2005). Therefore, inclusion of Bacillus in the diet showed that positive effects on hinders the development of OTA. Similarly, MOS inhibits the secretion pathway of phenylalanine-t-RNA which diminishes the production of OTA (Salama et al., 2019). Moreover, Mourão et al. (2006) reported that MOS at 1 g/kg in the rabbit diet improved cecal volatile fatty acid concentration for healthy intestinal morphology.
3.8. Contamination of OTA in fish feed
Fish feeds were detected with various toxigenic fungi including Aspergillus sp. (86.6%), Penicillium sp. (23.3%), Fusariam sp. (6.6%), Mucor sp. (10%) and Rhizopus sp. (3.3%) (Parussolo et al., 2019). Among them, Aspergillus sp. and Penicillium sp. were the most predominant fungi found in fish feed at farms. However, Penicillium and Fusarium were commonly observed in Brazil fish farms instead of Aspergillus sp. (Barbosa et al., 2013). Almost 10 - 25% of fish feed displayed the presence of A. niger which was found to produce less OTA (Cardoso, 2011). According to Kholife et al. (2019), 66.67% of feed samples had a high admissible limit of OTA (5 μg/kg) compared to AFB1. However, none of these feed samples detected with A. ochraceus on culture. Comparable values were observed in rainbow trout feed samples of Argentina, which exhibited high OTA of 5.26 μg/kg. While fish feed samples from Egypt, Brazil, Portugal, Kenya and Mexico reported an absence of OTA, other mycotoxins were found in samples from these countries (Marijani et al., 2019). Supamattaya et al. (2005) reported that 1,000 μg/kg of OTA administered orally to black tiger shrimp had traces of OTA with no toxicological lesions being found in shrimps.
3.9. Distribution of OTA in meat products
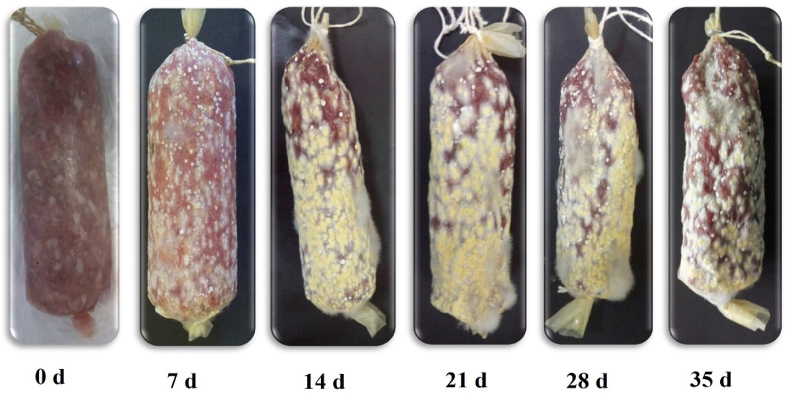
Modern meat products consist of meat and non-meat ingredients in their product formulation, with OTA liable to accumulate from both sources. Sausage, semi-dry sausage, and dry-meat products from Croatian meat products showed a presence of OTA in 99% of samples (14 out of 15 samples) (Markov et al., 2013). Markov et al. observed that game sausage from rabbit meat showed 2.21 to 2.37 μg/kg of OTA, but minimum OTA was recorded in Roe deer meat (0.05 to 1.37 μg/kg). Semi-dry sausages showed a higher concentration of OTA in Slavonian sausage (2.03 to 2.31 μg/kg) than Kranjska sausage (0.05 to 3.28 μg/kg) (Markov et al., 2013). Almost 64% of commercial sausage samples were contaminated with OTA (7.83 μg/kg). However, conversion of OTA from feed (15.6 μg/kg) to meat were more likely to be recorded in the liver (12.7% in rabbit liver) than in the mammary glands (8.8%) and muscle (3.1%) (Ferrufino-Guardia et al., 2000). OTA (5 to 100 μg/kg) dosed orally in cows had no residual accumulation of OTA in the liver, the kidney, muscles and jejunoileal of the cow (Hashimoto et al., 2016). According to Karmi (2019), 72% to 96% of OTA derived from the meat of Basterma, Burger, luncheon, minced meat, and kofta. While, the maximum concentration of OTA was 2.5 μg/kg in Basterma meat, and the minimum concentration was 1.03 μg/kg in minced meat. They identified a method to reduce OTA residue in burger samples using various probiotics strains. For instance, Saccharomyces serevisiae (3%) reduces 71.1% of OTA (0.43 μg/kg) and Lactobacillus acidophilus (3%) reduces 61% of OTA (0.57 μg/kg). In Egypt, the beef luncheon and burger patties had 100% of OTA from 50 meat products (Scudamore, 2005). Fig. 2 shows the growth rate and occurrence of OTA by Aspergillus westerdijkiae on salami sausage wrapped up with collagen kept in the chamber at 20 °C for 35 d.
Fig. 2.
Growth of ochratoxin A (OTA) by Aspergillus westerdijkiae on the surface of Italian Salami sausage (Parussolo et al., 2019).
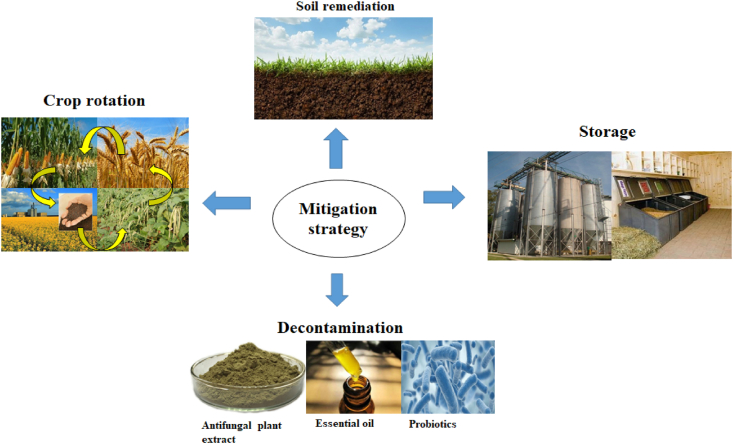
4. Mitigation strategy for prevention of OTA
4.1. Cropping system
OTA crossovers mainly result from poor agriculture practices and meat products formulation, which allow the growth of toxigenic fungi in crops. Penicillium verrucosum growth was predominant in the cool-temperate areas, whereas A. ochraceus found during hot atmospheres (Bernhoft et al., 2012). Good agronomic practices, cropping techniques, foliar fungicide applications and tillage practices can control the formation of Fusarium Head Blight. Though these are universal accessible, they did not show promising results in field trials (Iqbal et al., 2016). Rotation of cropping might lessen Fusarium attacks and mycotoxin production (Fig. 3).
Fig. 3.
Schematic representation of mitigation strategy for prevention of ochratoxin A (OTA) in feed.
4.2. Feed decontamination method
Bhatti et al. (2019) reported that the inclusion of 0.5 to 2.0 g/kg of Trichosporon mycotoxinivorans (TR) in contaminated feed (OTA, 0.15 to 1.0 mg/kg feed) effectively suppressed the immunotoxicity of OTA but feed contained TR alone had no response on the growth of broiler chicks. Ducks exposed to 16% of mouldy corn (OTA at 2 mg/kg) with curcumin at 400 mg/kg given for 21 d showed promising changes in body weight, with average daily feed intake and average daily gain equivalent to normal ducks (Ruan et al., 2019). On the other side, antioxidant biomarkers (glutathione peroxidase, and superoxide dismutase) were increased, while interleukin-10 and tumour necrosis factor-α were decreased. Therefore, curcumin is a potent antioxidant and demonstrates an anti-inflammatory function to suppress the adverse effects of OTA in duck feed.
The addition of clay in the feed mixture displayed an effective action against OTA. Khatoon et al. (2018) identified a positive reaction on broiler chicks when they were fed contaminated feed containing bentonite clay (5 to 20 g/kg) in OTA (0.15 to 1.0 mg/kg). This exhibited no harmful effects such as immunosuppression, lymph proliferative responses to mitogen, or phagocytic action. Rajendran et al. (2020), reported that H-β zeolite (1 kg/t of feed) as a toxin binder against OTA in poultry displayed better action. Some antifungal strains from Solanum indicum L. (green berries), suppressed major OTA producers like A. niger, Aspergillus carbonarius and A. ochraceus, with 8% of green berry extract (antifungal fraction) inhibiting all Aspergillus strains growth in the feed. Similarly, a natural fungicide action was observed from flower buds and essential oil of S. aromaticum (5 g of extract/kg), which inhibited the proliferation of A. flavus and A. parasiticus on rice seeds (Gonçalves et al., 2019).
4.3. Efficacy of OTA binders in livestock
Feed additives supplementation on OTA-contaminated feed reduces OTA adsorption in the GIT, which decreases the ill effect of OTA in animal health. Aoudia et al. (2009) reported a reduced OTA concentration in plasma, kidney and liver by including 1% of micronized wheat fibres in OTA (118 μg/kg) contaminated feed. Stoev et al. (2002) added artichoke, and Curcuma longa powder in broiler feed, which had an effective reduction of OTA in feed and muscle meat. Similarly, Santin et al. (2002) identified that 0.25% of aluminosilicate in OTA (2 mg/kg) contaminated feed did not alter the macroscopic and microscopic structure of broiler muscle tissue. According to Raju and Devegowda (2000), the contaminated diet (OTA, 2 mg/kg) supplied with esterified-glucomannan (1 g/kg) showed increased body weight gain. It decreased liver weight, adrenals and GGT activity in broilers. Besides, bentonite added in OTA (0.1 mg/kg) contaminated feed, had maintained regular chicken performance under mycotoxicosis (Pappas et al., 2016). Also, bentonite clay (20 g/kg) supplementation in OTA polluted feed, positively influenced the immune system in the broiler (Khatoon et al., 2018). Further, OTA (0.3 and 1 mg/kg of feed) contaminated diet mixed with toxin binder did not observe with OTA in eggs (Krogh et al., 1976). However, later research (Juszkiewicz et al., 1982) reported the presence of OTA in the eggs of layer hens fed with OTA (10 mg/kg of body weight). Similarly, Saccharomyces cerevisiae (0.4 g/kg feed) reduced the effects of OTA in sheep (Blank and Wolffram, 2009). However, in recent times more innovative approaches have been explored for the mitigation of OTA effects in feed. To conclude, our reports focused on reducing the economic loss suffered by agriculturalists and rectifying the hazards to farm animals.
5. Climate change as an impact on feed and livestock
Drought conditions during flowering and development stages of maize (July–August), could proliferate mycotoxins growth (Hove et al., 2016). Many reports revealed that long-term drought and elevation of temperatures during the developing season confined the growth of A. Flavus and inhibited the regular fertilization in maize plants (Alberts et al., 2019). Increased heat stress might decrease milk production in cows and buffalos and reverse action found in the winter season. As per the available data on mycotoxin, it was noticed that hazard levels were raised in numerous places around the world. There has been an almost 62% to 80% increase in the Middle East and Asian regions respectively. Further, 66% of contaminated feed samples possess multiple-mycotoxin or specific mycotoxin strains. This deposition varies due to climate, seasonal and weather change (Abbas, 2019).
6. Conclusion and future perspective
Biocontrol, physical and chemical methods of decontamination in feed against mycotoxins are practiced globally. Still, OTA could be an alarming health concern in farm animals. The discussed methodologies are effectively reducing OTA in feed and feed supplements of livestock. However, the contaminated feeds carryover from feed to meat-producing animals was identified from the available database. Some techniques such as feed additives, natural fungicides, clay materials, and phytochemicals showed potent action against OTA as well as lessening the adverse effects in livestock. Therefore, the addition of antifungal natural extracts, essential oils and probiotics could prove efficient in preventing the formation of toxigenic fungi species in feed. However, a holistic approach should be implemented in agricultural practices that would prevent OTA formation at the farm to break the chain of causation.
Author contributions
Conceptualization: B. Balasubramanian and I. H. Kim; Data curation: A. R. Ganesan, B. Balasubramanian and I. Andretta; Roles/Writing original draft: A. R. Ganesan and B. Balasubramanian; Writing review & editing: R. Jha, A. G. Bakare, S. Park, I. Andretta, I. H. Kim.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by National Research Foundation (NRF) of South Korea (Grant No: 2018R1C1B5086232) funded by Korean Government (MEST). We thank Dr. Prashneel R. Gounder, School of Communication, Language and Literature, Fiji National University for his valuable assistance in English editing and proof reading.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Balamuralikrishnan Balasubramanian, Email: geneticsmurali@gmail.com, bala.m.k@sejong.ac.kr.
In Ho Kim, Email: inhokim@dankook.ac.kr.
References
- Abbas M. Mycotoxins-impact and management strategies. Intech Open; 2019. Co-occurrence of mycotoxins and its Detoxification strategies. [DOI] [Google Scholar]
- Abdel-Wahhab M.A., Kholif A.M. Mycotoxins in animal feeds and prevention strategies: a review. Asian J Anim Sci. 2008;2(1):7–25. [Google Scholar]
- Akinmusire O.O., El-Yuguda A.D., Musa J.A., Oyedele O.A., Sulyok M., Somorin Y.M., Krska R. Mycotoxins in poultry feed and feed ingredients in Nigeria. Mycotoxin Res. 2019;35(2):149–155. doi: 10.1007/s12550-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya M.R., Bal M.A. Regional distribution of aflatoxin and ochratoxin A contaminated beef and dairy cattle feeds in Turkey. Anim Health Prod Hygiene. 2013;2:162–166. [Google Scholar]
- Alberts J., Rheeder J., Gelderblom W., Shephard G., Burger H.M. Rural subsistence maize farming in South Africa: risk assessment and intervention models for reduction of exposure to fumonisin mycotoxins. Toxins. 2019;11(6):334. doi: 10.3390/toxins11060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jaal B., Salama S., Al-Qasmi N., Jaganjac M. Mycotoxin contamination of food and feed in the Gulf Cooperation Council countries and its detection. Toxicon. 2019;171(5):43–50. doi: 10.1016/j.toxicon.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Aoudia N., Callu P., Grosjean F., Larondelle Y. Effectiveness of mycotoxin sequestration activity of micronized wheat fibres on distribution of ochratoxin A in plasma, liver and kidney of piglets fed a naturally contaminated diet. Food Chem Toxicol. 2009;47:1485–1489. doi: 10.1016/j.fct.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Bahobail A. Studies on mycobiota associated with wheat bran and soybean seeds in Jeddah city, Saudia Arabia. Aust J Basic appl Sci. 2016;10(16):176–184. [Google Scholar]
- Barbosa T.S., Pereyra C.M., Soleiro C.A., Dias E.O., Oliveira A.A., Keller K.M., Rosa C.A. Mycobiota and mycotoxins present in finished fish feeds from farms in the Rio de Janeiro State, Brazil. Int Aquat Res. 2013;5(1):3. [Google Scholar]
- Bernhoft A., Torp M., Clasen P.E., Løes A.K., Kristoffersen A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit Contam A. 2012;29(7):1129–1140. doi: 10.1080/19440049.2012.672476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagya A., Begum S.R., Kiran S., Surekha M. Incidence and Mycotoxigenic fungi associated with cattle feeds in North Telangana region, India. Int J Curr Microbiol Appl Sci. 2019;8(4):2247–2253. [Google Scholar]
- Bhatti S.A., Khan M.Z., Saleemi M.K., Hassan Z.U. Impact of dietary Trichosporon mycotoxinivorans on ochratoxin A induced immunotoxicity; in vivo study. Food Chem Toxicol. 2019;132:110696. doi: 10.1016/j.fct.2019.110696. [DOI] [PubMed] [Google Scholar]
- Blank R., Wolffram S. Effects of live yeast cell supplementation to high concentrate diets on the toxicokinetics of ochratoxin A in sheep. Food Addit Contam A. 2009;26:119–126. doi: 10.1080/02652030802320600. [DOI] [PubMed] [Google Scholar]
- Boudra H., Saivin S., Buffiere C., Morgavi D.P. Toxicokinetics of ochratoxin A in dairy ewes and carryover to milk following a single or long-term ingestion of contaminated feed. J Dairy Sci. 2013;96(10):6690–6696. doi: 10.3168/jds.2013-6707. [DOI] [PubMed] [Google Scholar]
- Cardoso F. Universidade Federal do Piauí; Teresina, Brasil: 2011. Monitoramento de fungos toxigenicos e aflatoxina em raçaes utilizadas na pisicultura em Teresina, Piauí, Brasil. Doctoral dissertation, Master's Thesis. [Google Scholar]
- D'Mello J.P.F. Mycotoxins in plant products. In: D'Mello J.P.F., editor. Food safety. CABI Publishing Wallingford; UK: 2001. [Google Scholar]
- Denli M., Perez J.F. Ochratoxins in feed, a risk for animal and human health: control strategies. Toxins. 2010;2(5):1065–1077. doi: 10.3390/toxins2051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte S.C., Lino C.M., Pena A. Mycotoxin food and feed regulation and the specific case of ochratoxin A: a review of the worldwide status. Food Addit Contam. 2010;27(10):1440–1450. doi: 10.1080/19440049.2010.497166. [DOI] [PubMed] [Google Scholar]
- Egbontan A.O., Afolabi C.G., Kehinde I.A., Enikuomehin O.A., Ezekiel C.N., Sulyok M., Warth B., Krska R. A mini-survey of moulds and mycotoxins in locally grown and imported wheat grains in Nigeria. Mycotoxin Res. 2017;33(1):59–64. doi: 10.1007/s12550-016-0264-8. [DOI] [PubMed] [Google Scholar]
- Ekici H., Yildirim E., Yarsan E. The effect of seasonal variations on the occurrence of certain mycotoxins inconcentrate feeds for cattle collected from some provinces in Turkey. Turk J Vet Anim Sci. 2016;40(3):298–303. [Google Scholar]
- Elsayed M.A.E., Mohamed N.E., Hatab M.H., Elaroussi M.A. Oxidative stress of in-ovo ochratoxin A administered during chick embryonic development. Brazilian J Poult Sci. 2019;21(1) [Google Scholar]
- European Commission Regulation (EC) No. 1881/2006 http://europe.eu.int/eurlex/lex/LexUriserv/site/en/oj/2006/1/9120040528en00010052.pdf
- FAO Assessing quality and safety of animal feeds. 2004. http://www.fao.org/3/y5159e/y5159e00.htm#Contents
- Ferrufino-Guardia E.V., Tangni E.K., Larondelle Y., Ponchaut S. Transfer of ochratoxin A during lactation: exposure of suckling via the milk of rabbit does fed a naturally-contaminated feed. Food Addit Contam. 2000;17(2):167–175. doi: 10.1080/026520300283522. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) 2004. Worldwide regulations for mycotoxins in food and feed in 2003. Summary of study, carried out for the Food and Agriculture Organization (FAO), by FAO as Food and Nutrition Paper No. 81. [Google Scholar]
- Gonçalves A., Gkrillas A., Dorne J.L., Dall'Asta C., Palumbo R., Lima N., Giorni P. Pre-and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Compr Rev Food Sci Food Saf. 2019;18(2):441–454. doi: 10.1111/1541-4337.12420. [DOI] [PubMed] [Google Scholar]
- Grenier B., Oswald I.P. Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4(3):285–313. [Google Scholar]
- Gross M., Ploetz C.P., Gottschalk C. Immunochemical detection of mycotoxins in donkey milk. Mycotoxin Res. 2019;35(1):83–87. doi: 10.1007/s12550-018-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: a Ten-Year survey. Toxins. 2019;11(7):375. doi: 10.3390/toxins11070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Katsunuma Y., Nunokawa M., Minato H., Yonemochi C. Influence of repeated ochratoxin A ingestion on milk production and its carryover into the milk, blood and tissues of lactating cows. Anim Sci J. 2016;87(4):541–546. doi: 10.1111/asj.12466. [DOI] [PubMed] [Google Scholar]
- Hort V., Nicolas M., Minvielle B., Maleix C., Desbourdes C., Hommet F., Guérin T. Ochratoxin A determination in swine muscle and liver from French conventional or organic farming production systems. J Chromatogr B. 2018;1092:131–137. doi: 10.1016/j.jchromb.2018.05.040. [DOI] [PubMed] [Google Scholar]
- Horvatovic M.P., Radovic I., Glamocic D., Jajic I., Krstovic S., Mirkov M., Vasiljevic V. The occurrence of ochratoxin A in kidneys of healthy pigs from Vojvodina province, Serbia. IOP Publ IOP Conf Series: Earth Env Sci. 2019;333(1) [Google Scholar]
- Hove M., De Boevre M., Lachat C., Jacxsens L., Nyanga L.K., De Saeger S. Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe. Food Contr. 2016;69:36–44. [Google Scholar]
- Hussein M.A., Gherbawy Y. Genotypic identification of ochratoxigenic Aspergilli that contaminated beef luncheon and their protease activity. Rendiconti Lincei Scienze Fisiche e Naturali. 2019;30(4):767–773. [Google Scholar]
- Iacumin L., Chiesa L., Boscolo D., Manzano M., Cantoni C., Orlic S., Comi G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009;26(1):65–70. doi: 10.1016/j.fm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Iqbal S.Z., Asi M.R., Hanif U., Zuber M., Jinap S. The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 2016;210:135–140. doi: 10.1016/j.foodchem.2016.04.104. [DOI] [PubMed] [Google Scholar]
- Juan C., Oueslati S., Mañes J., Berrada H. Multimycotoxin determination in Tunisian farm animal feed. J Food Sci. 2019;84(12):3885–3893. doi: 10.1111/1750-3841.14948. [DOI] [PubMed] [Google Scholar]
- Juszkiewicz T., Piskorska-Pliszczynska J., Wisniewska H. Mycotoxins and Phycotoxins, 5th International IUPAC symposium on Mycotoxins and Phytotoxins. Technical University; Vienna, Austria: 1982. Ochratoxin A in laying hens: tissue deposition and passage into eggs; pp. 122–125. [Google Scholar]
- Kamal R.M., Mansour M.A., Elalfy M.M., Abdelfatah E.N., Galala W.R. Quantitative detection of aflatoxin M1, ochratoxin and zearalenone in fresh raw milk of cow, buffalo, sheep and goat by UPLC XEVO-TQ in Dakahlia Governorate. Egypt J Vet Sci. 2019;3:114. [Google Scholar]
- Karmi M. Detection of Aflatoxins and Ochratoxin A residues in meat products with mmelioration by probiotics. Zagazig Vet J. 2019;47(2):213–221. [Google Scholar]
- Khatoon A., Khan M.Z., Abidin Z.U., Bhatti S.A. Effects of feeding bentonite clay upon ochratoxin A–induced immunosuppression in broiler chicks. Food Addit Contam A. 2018;35(3):538–545. doi: 10.1080/19440049.2017.1411612. [DOI] [PubMed] [Google Scholar]
- Kholife M., Moawad A., Diab A., El-keredy A. Mycological examination of fish feed stuff with special reference to mycotoxin production. Slovenian Vet Res. 2019;56(22-Suppl) [Google Scholar]
- Krogh P., Elling F., Hald B., Jylling B., Petersen V.E., Skadhauge E., Svendsen C.K. Experimental avian nephropathy. Acta Pathol Microbiol Scand A. 1976;84:215–221. [PubMed] [Google Scholar]
- Leiva A., Méndez G., Rodríguez C., Molina A., Granados-Chinchilla F. Chemical assessment of mycotoxin contaminants and veterinary residues in Costa Rican animal feed. Int J Food Contam. 2019;6(1):5. [Google Scholar]
- Magnoli C., Hallak C., Astoreca A., Ponsone L., Chiacchiera S.M., Palacio G., Dalcero A. Surveillance of toxigenic fungi and ochratoxin A in feedstuffs from Córdoba province, Argentina. Vet Res Commun. 2005;29(5):431–445. doi: 10.1007/s11259-005-5507-7. [DOI] [PubMed] [Google Scholar]
- Malagutti L., Zannotti M., Scampini A., Sciaraffia F. Effects of Ochratoxin A on heavy pig production. Anim Res. 2005;54(3):179–184. [Google Scholar]
- Marijani E., Kigadye E., Okoth S. Occurrence of fungi and mycotoxins in fish feeds and their impact on fish health. Internet J Microbiol. 2019;2019:1–17. doi: 10.1155/2019/6743065. Article ID 6743065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov K., Pleadin J., Bevardi M., Vahčić N., Sokolić-Mihalak D., Frece J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Contr. 2013;34(2):312–317. [Google Scholar]
- Milićević D.R., Jurić V.B., Vuković D.Z., Mandić M.M., Baltić T.M. Residue of ochratoxin a in swine tissues: risk assessment. Arch Oncol. 2009;17(3–4):56–60. [Google Scholar]
- Mourão J.L., Pinheiro V., Alves A., Guedes C.M., Pinto L., Saavedra M.J., Kocher A. Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation of fattening rabbits. Anim Feed Sci Technol. 2006;126(1–2):107–120. [Google Scholar]
- Mujahid H., Hashmi A.S., Khan M.Z., Tayyab M., Shehzad W. Protective effect of yeast sludge and whey powder against Ochratoxicosis in Broiler Chicks. Pakistan Vet J. 2019 doi: 10.29261/pakvetj/2019.077. [DOI] [Google Scholar]
- Nalle C.L., Angi A.H., Supit M.A.J., Ambarwati S. Aflatoxin and Ochratoxin A contamination in corn grains and sago (putak meal) from different sources for poultry in West Timor, Indonesia. Int J Poultry Sci. 2019;18(8):353–360. [Google Scholar]
- Nazieh I., Khalaileh A.L. Prevalence of ochratoxin A in poultry feed and meat from Jordan. Pakistan J Biol Sci. 2018;21:239–244. doi: 10.3923/pjbs.2018.239.244. [DOI] [PubMed] [Google Scholar]
- Ogunade I.M., Martinez-Tuppia C., Queiroz O.C.M., Jiang Y., Drouin P., Wu F., Adesogan A.T. Silage review: mycotoxins in silage: occurrence, effects, prevention, and mitigation. J Dairy Sci. 2018;101(5):4034–4059. doi: 10.3168/jds.2017-13788. [DOI] [PubMed] [Google Scholar]
- Pappas A., Tsiplakou E., Tsitsigiannis D., Georgiadou M., Iliadi M., Sotirakoglou K., Zervas G. The role of bentonite binders in single or concomitant mycotoxin contamination of chicken diets. Br Poultry Sci. 2016;57:551–558. doi: 10.1080/00071668.2016.1187712. [DOI] [PubMed] [Google Scholar]
- Parussolo G., Oliveira M.S., Garcia M.V., Bernardi A.O., Lemos J.G., Stefanello A., Copetti M.V. Ochratoxin A production by Aspergillus westerdijkiae in Italian-type salami. Food Microbiol. 2019;83:134–140. doi: 10.1016/j.fm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Persi N., Pleadin J., Kovačević D., Scortichini G., Milone S. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Sci. 2014;96(1):203–210. doi: 10.1016/j.meatsci.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Pietruszka K., Piątkowska M., Jedziniak P. Occurrence of ochratoxin a in animal tissues and feeds in Poland in 2014–2016. J Vet Res. 2017:483–487. doi: 10.1515/jvetres-2017-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo L., Cavallarin L., Nucera D., Antoniazzi S., Schiavone A. A survey of ochratoxin A contamination in feeds and sera from organic and standard swine farms in northwest Italy. J Sci Food Agric. 2010;90(9):1467–1472. doi: 10.1002/jsfa.3965. [DOI] [PubMed] [Google Scholar]
- Pozzo L., Salamano G., Melli E., Gennero M.S., Doglione L., Cavallarin L., Schiavone A. Feeding a diet contaminated with ochratoxin A for chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 1. Effects on growth and slaughter performance, haematological and serum traits. J Anim Physiol an N. 2013;97:13–22. doi: 10.1111/jpn.12050. [DOI] [PubMed] [Google Scholar]
- Rajendran R.M., Umesh B., Chirakkal H. Assessment of H-βzeolite as an ochratoxin binder for poultry. Poultry Sci. 2020;99:76–88. doi: 10.3382/ps/pez535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M.V.L.N., Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin) Br Poultry Sci. 2000;41:640–650. doi: 10.1080/713654986. [DOI] [PubMed] [Google Scholar]
- Rezaei M., Pourfard I.M., Yahyaei M., Gholamrezaei M., Ghasemikhah R., Bonchenari M.K. Evaluation of some dairy and beef cattle feed samples for fungal contamination in Markazi Province of Iran. Int J Curr Microbiol Appl Sci. 2015;4(6):1139–1146. [Google Scholar]
- Rosa C.A.R., Keller K.M., Keller L.A.M., Gonza´lez Pereyra M.L., Pereyra C.M., Dalcero A.M. Mycological survey and ochratoxin A natural contamination of swine feedstuffs in Rio de Janeiro State, Brazil. Toxicon. 2009;53:283–288. doi: 10.1016/j.toxicon.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Ruan D., Wang W.C., Lin C.X., Fouad A.M., Chen W., Xia W.G., Zheng C.T. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. 2019;13(1):42–52. doi: 10.1017/S1751731118000678. [DOI] [PubMed] [Google Scholar]
- Salama M., Morsy W., Mohamed R., El-Midany S. Effect of some feed-additives on the growth performance, physiological response and histopathological changes of rabbits subjected to ochratoxin-a feed contamination. Slovenian Vet Res. 2019;56(22-Suppl) [Google Scholar]
- Santin E., Paulillo A.C., Maiorka P.C., Alessi A.C., Krabbe E.L., Maiorka A. The effects of ochratoxin/aluminosilicate interaction on the tissues and humoral immune response of broilers. Avian Pathol. 2002;31:73–79. doi: 10.1080/03079450120106642. [DOI] [PubMed] [Google Scholar]
- Santos Pereira C., Cunha S., Fernandes J.O. Prevalent mycotoxins in animal feed: occurrence and analytical methods. Toxins. 2019;11(5):290. doi: 10.3390/toxins11050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer R., Gareis M. Occurrence of ochratoxin A and B in spices. Mycotoxin Res. 2002;18:62. doi: 10.1007/BF02946698. [DOI] [PubMed] [Google Scholar]
- Schelstraete W., Devreese M., Croubels S. Comparative toxicokinetics of Fusarium mycotoxins in pigs and humans. Food Chem Toxicol. 2020;137:111140. doi: 10.1016/j.fct.2020.111140. [DOI] [PubMed] [Google Scholar]
- Scudamore K.A. Prevention of ochratoxin A in commodities and likely effects of processing fractionation and animal feeds. Food Addit Contam. 2005;22:17–25. doi: 10.1080/02652030500309392. [DOI] [PubMed] [Google Scholar]
- Stoev S.D., Djuvinov D., Mirtcheva T., Pavlov D., Mantle P. Studies on some feed additives giving partial protection against ochratoxin A toxicity in chicks. Toxicol Lett. 2002;135:33–50. doi: 10.1016/s0378-4274(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Sultana N., Rashid A., Tahira I., Hanif H.U., Hanif N.Q. Distribution of various mycotoxins in compound feed, total mix ration and silage. Pakistan Vet J. 2013;33(2) [Google Scholar]
- Supamattaya K., Sukrakanchana N., Boonyaratpalin M., Schatzmayr D., Chittiwan V. Effects of ochratoxin A and deoxynivalenol on growth performance and immuno-physiological parameters in black tiger shrimp (Penaeus monodon) Songklanakarin J Sci Technol. 2005;27(Suppl 1):S91–S99. [Google Scholar]
- Turkoglu C., Keyvan E. Determination of aflatoxin M1 and ochratoxin A in raw, pasteurized and UHT milk in Turkey. Acta Sci Vet. 2019;47 [Google Scholar]
- Zhang Z., Fan Z., Nie D., Zhao Z., Han Z. Analysis of the carry-over of ochratoxin A from feed to milk, blood, urine, and different tissues of dairy cows based on the establishment of a reliable LC-MS/MS method. Molecules. 2019;24(15):2823. doi: 10.3390/molecules24152823. [DOI] [PMC free article] [PubMed] [Google Scholar]