Abstract
This study aimed to assess the impact of seasonal thermal stress on oxidative stress, immune response, and stress hormones of lactating dairy cows in subtropical regions with different levels of temperature-humidity index (THI). A total of 32 healthy lactating Holstein dairy cows experienced 4 seasons (8 cows/season). The physiological parameters were categorized into low THI (LTHI, THI = 42.97 ± 0.95) in winter, moderate THI (MTHI, THI = 61.84 ± 0.42) in spring and autumn, and high THI period (HTHI, THI = 86.09 ± 0.23) in summer. The blood samples were collected twice in each season to measure oxidative stress, inflammatory and hormonal parameters. Our results showed THI had a positive correlation with the rectal temperature (R2 = 0.821, P < 0.001) and respiratory rate (R2 = 0.816, P < 0.001). Dry matter intake, milk yield and fat percentage also significantly differed among groups (P < 0.05). Compared with the MTHI group, the LTHI group exhibited a significant increase in malondialdehyde (MDA) level (P < 0.001), and the HTHI group displayed a significant increase in levels of cortisol, interleukin (IL)-10, IL-1β and tumor necrosis factor-α (P < 0.001). Opposite changes in serum endotoxin and immunoglobulin G levels were observed with the increasing THI (P < 0.001). LTHI notably increased the triiodothyronine level, although the thyroxine level was reduced by LTHI and HTHI compared with the MTHI group. In conclusion, LTHI and HTHI conditions may induce different degrees of oxidative stress, inflammation response, and stress hormone imbalances on lactating dairy cows, therefore environmental management is necessary for the health of dairy cows in extreme weather conditions.
Keywords: Thermal stress, Oxidative stress, Immune response, Stress hormones, Lactating dairy cows
1. Introduction
Although advances in nutrition management and environmental control technology have been reported, thermal stress remains a concern for the health and welfare of dairy cows in tropical and subtropical regions (Qu et al., 2015). Thermal stress occurs when animals are exposed to temperatures that exceed their thermal comfort threshold, and heat production is imbalanced with heat dissipation (Bagath et al., 2019). The temperature-humidity index (THI), combining the effect of environmental temperature and humidity, has been commonly used to indicate the degree of thermal stress of dairy cows (Mader et al., 2006). Concerns have been raised regarding the oxidative stress induced by thermal stress in lactating cows (Kurokawa et al., 2016). Oxidative stress is characterized as an imbalance of antioxidants and oxidative molecules, such as reactive oxygen species and lipid peroxides (Sordillo and Aitken, 2009). Production of metabolic heat load and oxidative molecules during lactation would increase during thermal stress conditions (Tao et al., 2018), leading to metabolic disorder and infectious disease (Sordillo and Aitken, 2009).
Thermal stress initiates alterations in biological functions including feed intake, rectal temperature, respiratory rate, hormone secretion, and oxidative stress of dairy cows (da Costa et al., 2015; Kurokawa et al., 2016). However, knowledge on the effect of seasonal heat and cold stress on the endocrine status and immunity of lactating dairy cows needs further research. Turk et al. (2015) reported that cows exposed to hot summers showed negative energy balance and lower antioxidant status compared with cold winter cows. Ihsanullah et al. (2017) observed an increase in the concentrations of serum cortisol and protein in freshly parturiated primiparous dairy cows under heat stress. Further, Webster et al. (2008) reported immune response activation in terms of inflammatory cytokines and heat shock protein in dry-period Holstein cows exposed to extreme cold-wet environments, indicating changes of oxidative and endocrine status under different thermal environment.
Enzymatic antioxidants could eliminate the unpaired valence electrons of oxidative molecules by their extra electrons and play a crucial role in controlling oxidative balance (Suresh et al., 2009). Previous studies have revealed the reduced levels of total anti-oxidizing capability (T-AOC), superoxide dismutase (SOD) in heat-stressed cows, and increased lipid peroxidation leading to oxidative stress (Megahed et al., 2008; Safa et al., 2019). It is often stated that oxidative damage has been involved in immune function impairment (Sordillo and Aitken, 2009), which is indicated by immunoglobulin G (IgG), endotoxin and proinflammatory cytokines, such as interleukin (IL)-10, IL-1β, and tumor necrosis factor (TNF)-α (Esposito et al., 2014). A better understanding of the physiological variation on lactating dairy cows during extreme environmental conditions can help to improve their welfare and prevent thermal stress-related economic loss. Therefore, the objective of this study is to investigate the effects of seasonal thermal stress on the oxidative status, immune function, and stress hormones of lactating dairy cows.
2. Materials and methods
The protocol (HZAUCA-2017-010) of this experiment was approved by the Institution Animal Care and Use Committee at Huazhong Agricultural University (Wuhan, China), and the animal experiment was conducted in accordance with the National Institute of Health Guidelines for the Care and Use of Experiment Animals (Beijing, China).
2.1. Animals
A total number of 32 healthy lactating Holstein dairy cows (body weight: 627 ± 58 kg; parity: 2 to 3; 120 to 180 d in lactation; milk yield: 21.14 ± 3.57 kg/d) were selected (8 cows per season) and housed in a free-stall barn. The cows were fed with a total mixed ration at 06:00 and 18:00 before milking. During the experimental period, the animals had free access to feed and water. Feed formula and nutrients are reported in Table 1.
Table 1.
Ingredients and nutrient composition of the diets1 (% of dry matter).
| Ingredients | Content | Nutrient composition | Content |
|---|---|---|---|
| Corn silage | 30.45 | NEL3, MJ/kg | 6.80 |
| Straw | 16.24 | Crude protein | 15.26 |
| Apple meal | 1.53 | Neutral detergent fiber | 42.25 |
| Syrup | 1.32 | Acid detergent fiber | 28.95 |
| Corn | 18.52 | Calcium | 0.92 |
| Soybean meal | 12.56 | Phosphorous | 0.44 |
| Peanut meal | 4.66 | ||
| Distillers dried grains with solubles | 4.46 | ||
| Corn husk | 7.57 | ||
| Calcium carbonate | 0.50 | ||
| Calcium hydrogen phosphate | 0.68 | ||
| Sodium bicarbonate | 0.58 | ||
| Sodium chloride | 0.35 | ||
| Premix2 | 0.58 | ||
| Total | 100 |
Fed with total mixed ration.
Formulated to provide (per kilogram of dry matter) Fe 400 mg, Cu 660 mg, Mn 600 mg, Zn 3,000 mg, Co 30 mg, Se 100 mg, I 6.26 mg, vitamin A 1,000,000 IU, vitamin D 700,000 IU, vitamin E 1500 IU.
Calculated according to NRC (1989).
2.2. Experimental design
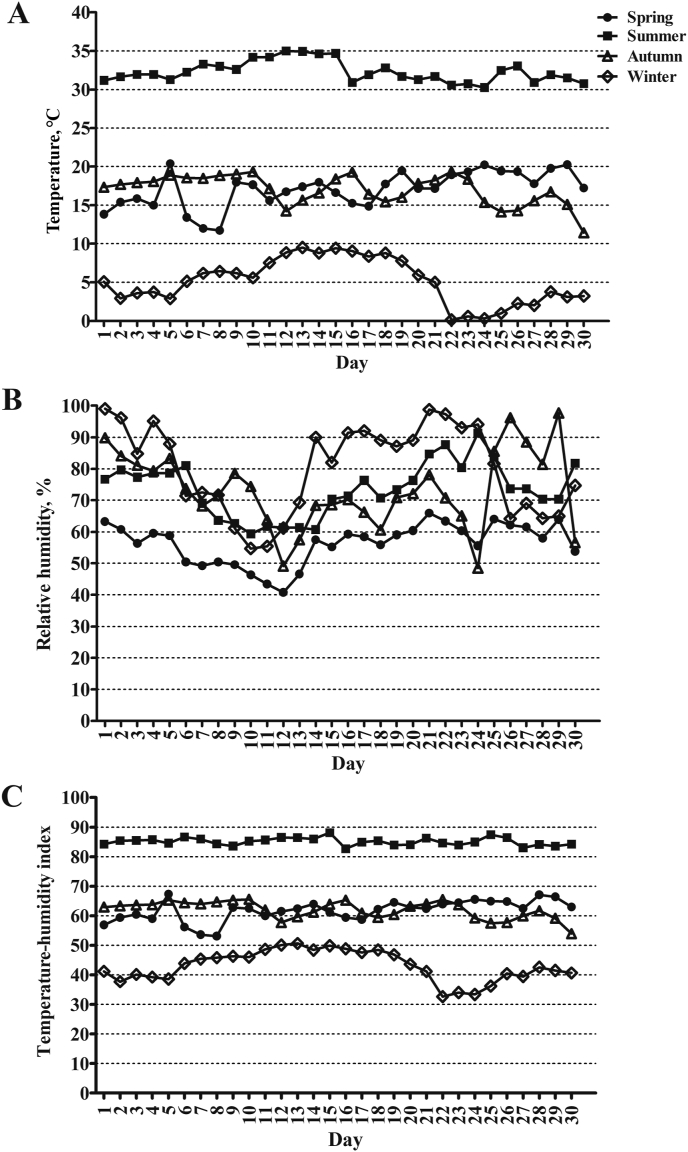
This study was conducted at the Academy of Agricultural Sciences commercial dairy farm (latitude and longitude were 30°28′N, 114°16′E) located in Wuhan, China. The area was characterized by a subtropical monsoon climate. Four experiments were respectively carried out in different seasons with 30 d and 8 cows in each experiment as follows: the first experiment was conducted in spring (4th April to 3rd May), the second experiment in summer (12th July to 10th August), the third experiment in autumn (19th October to 17th November), and the 4th experiment in winter (28th December to 26th January). The daily temperature, relative humidity, and the calculated THI in different seasons are shown in Fig. 1. To detect the effect of THI on thermal tolerance, antioxidant status, immune response and serum hormones of Holstein dairy cows, the physiological parameters were categorized into low THI (LTHI) in winter with mean daily THI of 42.97 ± 0.95, moderate THI (MTHI) in spring and autumn with mean daily THI of 61.84 ± 0.42, and high THI period (HTHI) in summer with mean daily THI of 86.09 ± 0.23.
Fig. 1.
Environmental conditions in the experimental period of different seasons: (A) daily temperature, (B) daily relative humidity, (C) daily temperature-humidity index (THI).
2.3. Environmental measures and thermal tolerance parameters
Ambient temperature and relative humidity were recorded 4 times daily (06:00, 08:00, 12:00, and 18:00) at the barn area using an electronic thermometer and hygrometer (Zhengzhou Boyang Instrument Factory, China). THI was calculated using the following equation (Mader et al., 2006):
| THI = [0.8 × Ambient temperature (°C)] + [(Relative humidity (%)/100) × (Ambient temperature - 14.4)] + 46.4. |
Thermal tolerance data were measured 3 times daily (06:00, 12:00, and 18:00) for 6 d. Rectal temperature was measured with a clinical thermometer inserted 3 cm into the rectum and held in place for 5 min, then the temperature was recorded. Respiratory rate was determined using a stopwatch to count the flank movements of the individual cows for one minute and was expressed as breaths per minute (bpm).
2.4. Feed intake, chemical analysis of diet and milk performance
During each season's experimental period, the total mixed ration amounts offered and refused were measured daily during d 22 to 27 to determine the dry matter intake (DMI). Representative samples of total mixed ration (150 g) and refusals were taken daily during d 22 to 27 of each period. Samples of feeds and refusals were oven-dried at 65 °C, and ground with a Wiley mill grinder through a 1-mm screen. Standard methods (AOAC, 2012) were applied to analyze dry matter (DM; method 934.01), crude protein (CP) using an automatic Kjeldahl system (Kjeltec 8400, Foss, Sweden; method 981.10), calcium (method 968.08) and phosphorus (method 965.1). Determinations of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were carried out according to Van Soest et al. (1991). Predicted net energy of lactation (NEL) was calculated according to NRC (1989).
Cows were milked twice daily (6:00 and 18:00) and milk yield was recorded daily for individual cows. Milk samples were taken daily during d 22 to 27 of each period. Daily milk samples were mixed according to the ration of the morning and afternoon milk yield for each cow and stored at −20 °C for analysis of milk protein, fat, and lactose using Milko-Scan FT 6000 (Foss Electric, Denmark). The 4% fat-corrected milk (FCM) was calculated using the following equation (NRC, 2001):
| 4% FCM (kg/d) = [0.4 × Milk yield (kg/d)] + [15 × Milk fat yield (kg/d)]. |
Daily milk protein, fat, and lactose yields were calculated by multiplying the daily milk yield by the percentage of a specific component.
2.5. Blood sampling and analysis
Blood samples were collected through the jugular vein before morning feeding on d 15 and 30 of each experimental season, and samples were placed into separation gel coagulation promoting tubes, followed by centrifugation within 2 h at 3,000 ×g for 15 min. The obtained serum was immediately stored at −20 °C for further analysis.
The levels of glutathione peroxidase (GSH-Px, A005-1-2), SOD (A001-1-2), T-AOC (A015-1-2), catalase (CAT, A007-1-1), alkaline phosphatase (ALP, A059-1-1), and malondialdehyde (MDA, A003-1-2) in the serum were assessed using commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions, respectively. The optical densities were measured at 412, 550, 520, 405, 520, and 532 nm using a microplate reader (model 320, Labsystems Multiskan MS, Finland) for GSH-Px, SOD, T-AOC, CAT, ALP and MDA levels, respectively.
The levels of heat shock protein (HSP) 70 (H264-2), IL-10 (H009), IL-1β (H002), TNF-α (H052), IgG (H106), cortisol (H094), epinephrine (H208), triiodothyronine (H222) and thyroxine (H223) in the serum were determined by commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions, respectively. The ELISA results were obtained by using a microplate reader (DNM-9602, Pulang, New technology, Beijing, China) at a wavelength of 450 nm.
2.6. Data analysis
Statistical analysis was performed by using SPSS software (SPSS v. 21, SPSS Inc.; Chicago, IL, USA). Significance analysis of the environmental parameters, thermal tolerance, milk yield, DMI, the biomarkers of oxidative stress, immune response, and serum hormones in different seasons was conducted by using one-way ANOVA in SPSS. The relationships between THI value and respiratory rate, as well as rectal temperature, were assessed by using the Pearson correlation coefficient. The significant differences were presented at the levels of P < 0.05.
3. Results
3.1. Environmental conditions and physiological parameters
Environmental conditions in southern China typically ranged from hot-humid to cold-wet weather when this study was carried out. The values of THI varied from 50 to 68 in spring and autumn periods, which were characterized as MTHI. However, the entire summer experimental period, that was characterized as HTHI, presented high daily temperature and THI, which exceeded the critical values of 25 °C and 72, respectively. The cold winter with low temperature and high relative humidity was thus characterized as LTHI (Fig. 1).
Average daily temperature (32.31 ± 0.25 °C vs. 17.02 ± 0.28 °C) and THI (86.09 ± 0.23 vs. 61.84 ± 0.42) were significantly higher in the HTHI group than in the MTHI group. In contrast, LTHI exhibited a significantly decreased average daily temperature (5.11 ± 0.54 °C) and THI (42.97 ± 0.95) compared to MTHI (Table 2).
Table 2.
Descriptive statistics of environmental conditions, rectal temperature and respiratory rate in Holstein lactating cows.
| Item | THI periods |
P-value | ||
|---|---|---|---|---|
| LTHI | MTHI | HTHI | ||
| Mean daily temperature, °C | 5.11 ± 0.54c | 17.02 ± 0.28b | 32.31 ± 0.25a | <0.001 |
| Mean daily RH, % | 80.11 ± 2.56a | 76.72 ± 1.17ab | 73.58 ± 1.56b | 0.057 |
| Mean daily THI | 42.97 ± 0.95c | 61.84 ± 0.42b | 86.09 ± 0.23a | <0.001 |
| Rectal temperature, °C | 38.19 ± 0.05c | 38.66 ± 0.03b | 39.91 ± 0.05a | <0.001 |
| Respiratory rate, bpm | 21.47 ± 0.61c | 38.23 ± 0.78b | 103.71 ± 1.04a | <0.001 |
THI = temperature-humidity index; LTHI = low THI; MTHI = moderate THI; HTHI = high THI; RH = relative humidity; bpm = breaths per minute.
a-c Means within a row with different superscripts differ (P < 0.05).
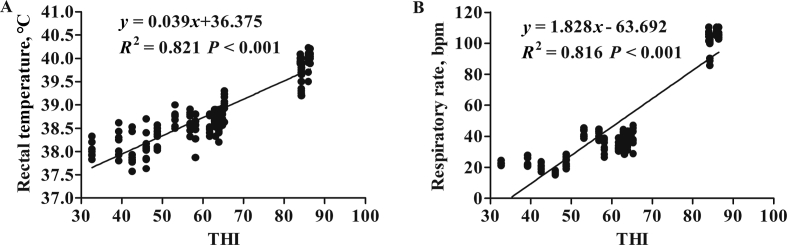
Rectal temperature and respiratory rate also significantly varied according to the THI levels. Dairy cows exhibited higher rectal temperature under HTHI (39.91 ± 0.05) than under MTHI (38.66 ± 0.03) and LTHI (38.19 ± 0.05), respectively (P < 0.05). A similar result was observed on the alterations of respiratory rate with increasing THI (Table 2). Regression analysis revealed the strong positive correlation between THI and rectal temperature and respiratory rate (Fig. 2).
Fig. 2.
Relationship between physiological parameters and temperature-humidity index (THI): (A) rectal temperature and (B) respiratory rate. Points indicate individual observations; solid lines represent simple linear regression equations. R2 represents the correlation coefficient.
3.2. DMI and milk performance
The DMI was significantly affected by THI levels (P < 0.001) since it approximately reduced by 19.54% and increased by 11.05%, respectively for HTHI and LTHI when compared with MTHI. There was also a significant difference in daily milk yield (P < 0.001) with the lowest milk yield in HTHI (15.86 ± 0.12), and a reduction of 33.67% from MTHI to HTHI. Moreover, HTHI cows produced 4% less FCM per day than LTHI and MTHI cows (P < 0.05). Although no differences were detected between groups for milk protein and lactose percentages, the milk fat percentage was significantly decreased in the HTHI group (P < 0.05) since it approximately decreased by 7.42% when compared with MTHI. Milk protein, fat, and lactose yields were significantly affected by THI (P < 0.001) with the highest value in HTHI and the lowest value in HTHI (Table 3).
Table 3.
Effect of temperature-humidity index (THI) on dry matter intake (DMI) and milk performance of Holstein lactating cows.
| Item | THI periods1 |
P-value | ||
|---|---|---|---|---|
| LTHI (42.97) | MTHI (61.84) | HTHI (86.09) | ||
| DMI, kg/d | 30.46 ± 0.33a | 27.43 ± 0.22b | 22.07 ± 0.30c | <0.001 |
| Milk yield, kg/d | 21.06 ± 0.91b | 23.91 ± 0.31a | 15.86 ± 0.12c | <0.001 |
| 4% FCM2, kg/d | 22.41 ± 0.49a | 23.69 ± 0.64a | 15.23 ± 0.31b | <0.001 |
| Protein, % | 3.07 ± 0.07 | 3.01 ± 0.07 | 3.24 ± 0.08 | 0.183 |
| Fat, % | 4.42 ± 0.15a | 4.04 ± 0.15ab | 3.74 ± 0.05b | 0.061 |
| Lactose, % | 5.08 ± 0.05 | 5.13 ± 0.03 | 5.09 ± 0.05 | 0.711 |
| Protein yield, kg/d | 0.65 ± 0.02a | 0.71 ± 0.02a | 0.51 ± 0.01b | <0.001 |
| Fat yield, kg/d | 0.93 ± 0.03a | 0.95 ± 0.04a | 0.59 ± 0.01b | <0.001 |
| Lactose yield, kg/d | 1.07 ± 0.01b | 1.23 ± 0.02a | 0.81 ± 0.01c | <0.001 |
LTHI = low THI; MTHI = moderate THI; HTHI = high THI; FCM = fat-corrected milk.
a-c Means within a row with different superscripts differ (P < 0.05).
The data in parentheses were the average THI value.
4%FCM, fat-corrected milk, calculated according to NRC (2001).
3.3. Oxidative stress biomarkers
As shown in Table 4, significant changes were observed in the levels of antioxidant enzymes, MDA, and HSP70 in serum. HTHI cows exhibited a significantly lower level of SOD than LTHI and MTHI cows (P < 0.05). T-AOC was significantly lower in LTHI cows than that in MTHI cows (P < 0.05). Meanwhile, higher levels of the CAT and MDA were found in LTHI compared to MTHI and HTHI (P < 0.05). Compared with MTHI and LTHI period, the HTHI period exhibited a significantly decreased activity of ALP (P < 0.05). The level of serum HSP70 was significantly increased with an increase in THI (P < 0.05).
Table 4.
Effect of temperature-humidity index (THI) on antioxidant status of Holstein lactating cows.
| Item | THI periods1 |
P-value | ||
|---|---|---|---|---|
| LTHI (42.97) | MTHI (61.84) | HTHI (86.09) | ||
| T-AOC, U/mL | 0.45 ± 0.08b | 1.17 ± 0.11a | 0.94 ± 0.06a | <0.001 |
| GSH-Px, U/mL | 12.31 ± 3.00b | 41.88 ± 11.70ab | 53.43 ± 9.32a | 0.061 |
| SOD, U/mL | 214.04 ± 5.01a | 230.55 ± 19.75a | 108.91 ± 4.98b | 0.001 |
| CAT, U/mL | 24.28 ± 2.24a | 4.47 ± 1.16b | 4.31 ± 0.06b | <0.001 |
| MDA, nmol/mL | 2.95 ± 0.11a | 1.92 ± 0.17b | 1.84 ± 0.71b | <0.001 |
| ALP, U/L | 67.63 ± 2.08a | 62.37 ± 3.11a | 33.04 ± 1.53b | <0.001 |
| HSP70, ng/mL | 24.55 ± 0.90c | 31.29 ± 0.75b | 41.81 ± 0.76a | <0.001 |
LTHI = low THI; MTHI = moderate THI; HTHI = high THI; T-AOC = total anti-oxidizing capability; GSH-Px = glutathione peroxidase; SOD = superoxide dismutase; CAT = catalase; MDA = malondialdehyde; ALP = phosphatase; HSP70 = heat shock protein 70.
a-c Means within a row with different superscripts differ (P < 0.05).
The data in parentheses were the average THI value.
3.4. Immune responses
Biomarkers of immunity function such as cytokines, endotoxin, and IgG were significantly influenced by THI (Table 5). The concentration of IL-10 was significantly increased with the increase in THI (P < 0.05). The concentrations of 1L-1β and TNF-α in serum were significantly higher in HTHI cows than in MTHI cows (P < 0.05). However, the IgG and endotoxin levels were respectively increased and decreased in HTHI compared with MTHI, and opposite results were observed in the LTHI group (P < 0.05).
Table 5.
Effect of temperature-humidity index (THI) on immune responses of Holstein lactating cows (pg/mL).
| Item | THI periods1 |
P-value | ||
|---|---|---|---|---|
| LTHI (42.97) | MTHI (61.84) | HTHI (86.09) | ||
| IL-10 | 137.29 ± 8.39c | 167.15 ± 3.68b | 190.86 ± 3.27a | <0.001 |
| IL-1β | 156.39 ± 6.87b | 154.81 ± 3.21b | 235.98 ± 5.13a | <0.001 |
| TNF-α | 77.38 ± 2.73b | 76.68 ± 1.95b | 104.92 ± 1.48a | <0.001 |
| IgG | 18.27 ± 0.33b | 20.08 ± 0.26a | 13.10 ± 0.41c | <0.001 |
| Endotoxin | 99.91 ± 2.64b | 79.01 ± 2.86c | 113.86 ± 1.69a | <0.001 |
LTHI = low THI; MTHI = moderate THI; HTHI = high THI; IL = interleukin; TNF-α = tumor necrosis factor-α; IgG = immunoglobulin G.
a-c Means within a row with different superscripts differ (P < 0.05).
The data in parentheses were the average THI value.
3.5. Serum hormones
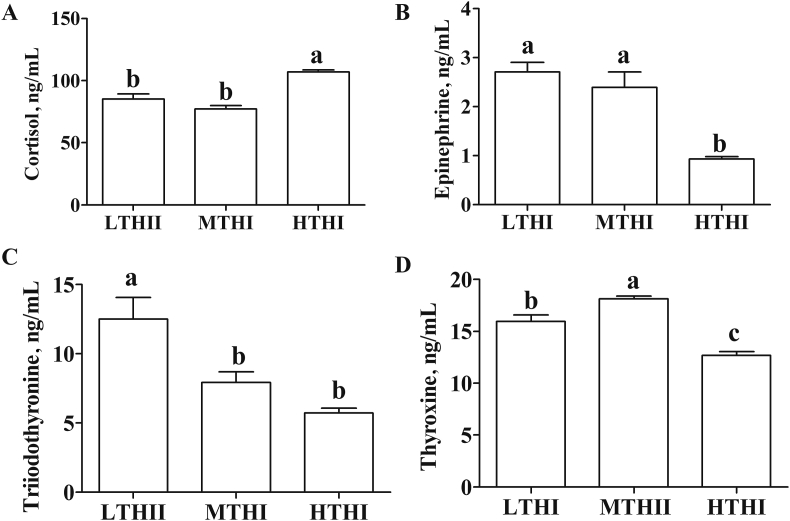
As shown in Fig. 3, The level of serum cortisol was significantly higher in HTHI than in MTHI and LTHI (P < 0.05, Fig. 3A). However, HTHI cows exhibited a significantly lower level in epinephrine and thyroxine than MTHI cows (P < 0.05, Fig. 3B and D). LTHI significantly increased the triiodothyronine level while significantly decreasing the thyroxine level, as compared with MTHI (P < 0.05, Fig. 3C and D).
Fig. 3.
Effect of temperature-humidity index (THI) on stress hormones of Holstein lactating cows: (A) cortisol, (B) epinephrine, (C) triiodothyronine, (D) thyroxine. LTHI = low THI; MTHI = moderate THI; HTHI = high THI. Values with different letters (a, b, c) differ (P < 0.05).
4. Discussion
THI has been well known as a good indicator to evaluate the degree of thermal stress condition on dairy cows (Mader et al., 2006). The rectal temperature and respiratory rate have been considered as visible physiological indicators for determining the thermal regulatory responses of dairy cows to heat stress (da Costa et al., 2015; Alfonzo et al., 2016). This could be further supported by the strong positive correlation between THI value and rectal temperature and respiratory rate in this study. The rectal temperature of dairy cows was reported to be about 39.4 °C when dairy cows were exposed to 75 to 90 THI condition, and the respiratory rate was also positively related to THI values (da Costa et al. 2015), which was consistent with the data in the HTHI period in this study. In contrast, the decreased respiratory rate of cows under LTHI conditions might contribute to the reduced heat dissipation for maintaining body temperature and energy balance (Alfonzo et al., 2016). Moreover, the change in DMI was also a protective mechanism to maintain heat balance by regulating metabolic rate and heat production (Qu et al., 2015). Hill and Wall (2017) reported an 11.5% increase in DMI in dairy cows under cold condition (THI = 38.9) compared with hot exposure (THI = 73.9). By contrast, DMI was reduced 0.51 kg for every 1 unit increase in THI between 73 and 83 units (West et al., 2003), which supported our results. Notably, inconsistencies in milk yield and DMI responses to LTHI condition are likely due to disrupted energy metabolism under continuous cold-wet condition (Webster et al., 2008). Moreover, it was reported that heat stress inhibited the secretary function of the mammary gland (Qu et al., 2015). In the current study, milk protein and lactose percentage were not affected by different levels of THI and this was in accordance with the study performed by Nasr (2016). However, the milk fat percentage tended to decrease from LTHI to MTH. In agreement, a previous study (Lambertz et al., 2014) under a temperate climate reported a reduction in milk fat percentage when THI increased from 35 to 85. These results suggest that cold exposure could activate thermoregulatory responses just like heat stress.
Although dairy cows were traditionally characterized to be sensitive to heat stress and to be cold-tolerant (Liang et al., 2013), in this study, the antioxidant status and immune system were found to be affected by both LTHI and HTHI. T-AOC that represents total antioxidants present in blood and body fluid reflect the compensatory capacity against external stimuli (Suresh et al., 2009). SOD, as an essential antioxidant enzyme, could eliminate superoxide radicals in the body (Lei et al., 2015). Reduced T-AOC and SOD levels were observed in HTHI and LTHI cows in this study. Similar results were found in previous studies involving dairy cows challenged with oxidative stress (Megahed et al., 2008; Zhao et al., 2018). The main source of MDA was the peroxides of polyunsaturated fatty acids in biological systems, and the increased lipid mobilization to gain energy was an adaptation to cold stress (Turk et al., 2015). A sharp increase in MDA level under the LTHI condition is indicative of negative energy balance under cold stress (Giri et al., 2019), as well as the reduction in antioxidant capacity and the increase in free radicals (Kurokawa et al., 2016). The reduced ALP activity of dairy cows under the HTHI condition could be associated with energy metabolism and endocrine acclimation responses under a hot environment, both resulting from decreased gut and liver activity (Abeni et al., 2007). These data indicate that the HTHI and LTHI condition impaired antioxidant capacity and thus induced oxidative stress. The previous study of dairy cows reported the expression of HSP70 was increased under heat stress (THI = 86.83) and was reduced in cold stress (THI = 60.52) (Kumar et al., 2018). Similar results in HSP70 were also observed in dairy cows exposed to HTHI (86.09) and LTHI (42.97) in this study. The alteration in HSP70 expression could regulate ROS generation and mitochondrial protein oxidation (Zhang et al., 2014), and such a change was associated with the activity of immune cells in response to stressful environments (Do Amaral et al., 2011).
Oxidative damage was reported to be one of the causes of immune functional impairment under heat stress conditions (Sordillo and Aitken, 2009). In addition to oxidative damage, we also observed the alteration on levels of systematic inflammation markers in lactating dairy cows under HTHI and LTHI conditions. It has been reported that heat stress inhibits the immune function of dairy cows (Caroprese et al., 2009; Safa et al., 2019). TNF-α and IL-1β are known as pro-inflammatory cytokines, that directly promote an inflammatory response, while IL-10 mediated an anti-inflammatory response in dairy cows (Esposito et al., 2014). In agreement with previous studies (Caroprese et al., 2009; Min et al., 2016), HTHI conditions resulted in an increase in the levels of TNF-α, IL-1β, and IL-10, which might be explained by the protective mechanism activated by heat stress to control the risk of immune function impairment. Additionally, a reduction in IL-10 concentration, together with the increased levels of TNF-α and IL-1β were reported in cold-stressed broilers (Qing et al., 2014). These results suggest an important role of cytokines in immune systems under heat and cold stress conditions.
Endotoxins, as immunogenic molecules released from Gram-negative bacteria, were reported to promote the secretion of inflammatory cytokines and stress hormones (e.g. cortisol) and could induce localized or systematic inflammation (Kvidera et al., 2017). Serum endotoxin has usually been used for the evaluation of gut barrier function and responses under stress conditions (Kvidera et al., 2017). In the present study, the concentration of serum endotoxin was significantly increased in cows under HTHI and LTHI conditions, which was in accordance with previous reports that thermal stress directly impaired gut integrity and activated an immune response in rats and cows (Kaushik and Kaur, 2005; Koch et al., 2019). LTHI and HTHI conditions in this study inhibited the competence of humoral immune responses by decreasing secretion of serum IgG, since high IgG concentration could improve the immunity in lactating dairy cows (Xu et al., 2017). Moreover, the transfer of IgG from the bloodstream to the mammary gland was related to milk synthesis. If the degree of endogenous inflammation exceeded the adjustment capacity of immune systems, lactation performance would be deteriorated (McCarthy et al., 2016).
A cascade of stress hormones is an important pathway of endocrine adaptation to stress conditions. Cortisol secretion was activated as an anti-inflammatory response by targeting the cytokine, inflammatory proteins, and chemokines (Bagath et al., 2019). It has been reported that reduced feed intake under heat stress could promote the mobilization of triglycerides of adipose tissue, which was a favorable lipid pattern to offset insufficient energy intake (Qu et al., 2015). The decreased epinephrine had been found in organisms with metabolic syndrome (Lee et al., 2001). In this study, the increased cortisol level and reduced epinephrine level HTHI cows might be explained by an energy imbalance, a consequence of the reduction in DMI, and by organism immune dysfunction under heat stress (Turk et al., 2015). In addition, the thyroid hormone of mammals plays an important thermoregulatory role in maintaining metabolic rates under thermal stress conditions (Weitzel et al., 2017; Hu et al., 2019). Thyroxine is the predominated hormone secreted by the thyroid gland and must be deiodinated to triiodothyronine to be biologically active (Senese et al., 2018). Reduced feed intake under heat stress has been reported to cause depression of the thyroid gland activity and lead to decreased thyroid hormone levels (Aleena et al., 2016). In the current study, the levels of thyroxine and triiodothyronine generally decreased in lactating cows during the HTHI period, which might be a result of thyroid gland hypo-function, and also an attempt to reduce metabolic rate under heat stress. Interestingly, but contrary to the previous finding which reported increased thyroxine and triiodothyronine levels in dairy cows after severe cold exposure (Hu et al., 2019), a decreased thyroxine level accompanied with an increased triiodothyronine level were observed in LTHI cows in this study. This physiological response may result from the accelerated conversion of thyroxine to triiodothyronine under cold stress (Hangalapura et al., 2004), thus promoting oxygen consumption and heat production to maintain basal metabolism (Aleena et al., 2016).
5. Conclusions
In summary, our results clearly show that oxidative stress, inflammation response and endocrine imbalance of lactating dairy cows occur in hot summer and cold-wet winter in comparison with comfortable seasons, i.e. spring and autumn. It is recommended that environmental managements be undertaken in dairy housing systems to aid adaptation to extreme weather.
Author contributions
Han Li: conceptualization, methodology, formal analysis, writing - original draft; Yifeng Zhang: validation, formal analysis; Rong Li: investigation; Yan Wu: investigation; Dingran Zhang: visualization; Hongrun Xu: data duration; Yangdong Zhang: resources; Zhili Qi: conceptualization, project administration, funding acquisition.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This work was supported by Chinese Key Research and Development Program (2016YFD0500507 and 2018YFD0501605), Fundamental Research Funds for the Central Universities (2662018PY079), and Open Fund of the State Key Laboratory of Animal Nutrition Open Project (2004DA125184F1721).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abeni F., Calamari L., Stefanini L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int J Biometeorol. 2007;52:87–96. doi: 10.1007/s00484-007-0098-3. [DOI] [PubMed] [Google Scholar]
- Aleena J., Pragna P., Archana P.R., Sejian V., Bagath M., Krishnan G. Significance of metabolic response in livestock for adapting to heat stress challenges. Asian J Anim Sci. 2016;10:224–234. [Google Scholar]
- Alfonzo E.P.M., da Silva M.V.G.B., dos Santos Daltro D., Stumpf M.T., Dalcin V.C., Kolling G. Relationship between physical attributes and heat stress in dairy cattle from different genetic groups. Int J Biometeorol. 2016;60:245–253. doi: 10.1007/s00484-015-1021-y. [DOI] [PubMed] [Google Scholar]
- AOAC . 19th ed. 2012. Official methods of analysis of AOAC International. Gaithersburg, MD, USA. [Google Scholar]
- Bagath M., Krishnan G., Devaraj C., Rashamol V., Pragna P., Lees A. The impact of heat stress on the immune system in dairy cattle: a review. Res Vet Sci. 2019;126:94–102. doi: 10.1016/j.rvsc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Caroprese M., Marzano A., Entrican G., Wattegedera S., Albenzio M., Sevi A. Immune response of cows fed polyunsaturated fatty acids under high ambient temperatures. J Dairy Sci. 2009;92:2796–2803. doi: 10.3168/jds.2008-1809. [DOI] [PubMed] [Google Scholar]
- da Costa A.N.L., Feitosa J.V., Montezuma P.A., de Souza P.T., de Araújo A.A. Rectal temperatures, respiratory rates, production, and reproduction performances of crossbred Girolando cows under heat stress in northeastern Brazil. Int J Biometeorol. 2015;59:1647–1653. doi: 10.1007/s00484-015-0971-4. [DOI] [PubMed] [Google Scholar]
- Do Amaral B., Connor E., Tao S., Hayen M., Bubolz J., Dahl G. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J Dairy Sci. 2011;94:86–96. doi: 10.3168/jds.2009-3004. [DOI] [PubMed] [Google Scholar]
- Esposito G., Irons P.C., Webb E.C., Chapwanya A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim Reprod Sci. 2014;144:60–71. doi: 10.1016/j.anireprosci.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Giri A., Kumar B.V., Kalia S., Raj T., Chaurasia O. Evaluation of antioxidant status in serum and milk of Jersey cross-bred in different seasons reared under high-altitude stress condition. Biol Rhythm Res. 2019;50:726–738. [Google Scholar]
- Hangalapura B.N., Nieuwland M.G.B., Buyse J., Kemp B., Parmentier H.K. Effect of duration of cold stress on plasma adrenal and thyroid hormone levels and immune responses in chicken lines divergently selected for antibody responses. Poult Sci. 2004;83:1644–1649. doi: 10.1093/ps/83.10.1644. [DOI] [PubMed] [Google Scholar]
- Hill D.L., Wall E. Weather influences feed intake and feed efficiency in a temperate climate. J Dairy Sci. 2017;100:2240–2257. doi: 10.3168/jds.2016-11047. [DOI] [PubMed] [Google Scholar]
- Hu L., Ma Y., Liu L., Kang L., Brito L.F., Wang D.S. Detection of functional polymorphisms in the hsp 70 gene and association with cold stress response in Inner-Mongolia Sanhe cattle. Cell Stress Chaperones. 2019;24:409–418. doi: 10.1007/s12192-019-00973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihsanullah Qureshi M.S., Suhail S.M., Akhtar S., Khan R.U. Postpartum endocrine activities, metabolic attributes and milk yield are influenced by thermal stress in crossbred dairy cows. Int J Biometeorol. 2017;61:1–9. doi: 10.1007/s00484-017-1335-z. [DOI] [PubMed] [Google Scholar]
- Kaushik S., Kaur J. Effect of chronic cold stress on intestinal epithelial cell proliferation and inflammation in rats. Stress. 2005;8:191–197. doi: 10.1080/10253890500245953. [DOI] [PubMed] [Google Scholar]
- Koch F., Thom U., Albrecht E., Weikard R., Nolte W., Kuhla B. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc Natl Acad Sci Unit States Am. 2019;116:10333–10338. doi: 10.1073/pnas.1820130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J., Madan A.K., Kumar M., Sirohi R., Yadav B., Reddy A.V. Impact of season on antioxidants, nutritional metabolic status, cortisol and heat shock proteins in Hariana and Sahiwal cattle. Biol Rhythm Res. 2018;49:29–38. [Google Scholar]
- Kurokawa Y., Yamashita R., Okita M., Yoshitoshi R., Sugino T., Obitsu T. A comparison of plasma glucose and oxidative status in lactating dairy cows in Summer and Autumn. Anim Sci J. 2016;87:1212–1217. doi: 10.1111/asj.12548. [DOI] [PubMed] [Google Scholar]
- Kvidera S.K., Horst E.A., Fernandez M.V.S., Abuajamieh M., Ganesan S., Gorden P.J. Characterizing effects of feed restriction and glucagon-like peptide 2 administration on biomarkers of inflammation and intestinal morphology. J Dairy Sci. 2017;100:9402–9417. doi: 10.3168/jds.2017-13229. [DOI] [PubMed] [Google Scholar]
- Lambertz C., Sanker C., Gauly M. Climatic effects on milk production traits and somatic cell score in lactating Holstein-Friesian cows in different housing systems. J Dairy Sci. 2014;97:319–329. doi: 10.3168/jds.2013-7217. [DOI] [PubMed] [Google Scholar]
- Lee Z.S., Critchley J.A., Tomlinson B., Young R.P., Thomas G.N., Cockram C.S. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism. 2001;50:135–143. doi: 10.1053/meta.2001.19502. [DOI] [PubMed] [Google Scholar]
- Lei X.G., Zhu J.H., Cheng W.H., Bao Y., Ho Y.S., Reddi A.R. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 2015;96:307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Wood C., McQuerry K., Ray D., Clark J., Bewley J. Influence of breed, milk production, season, and ambient temperature on dairy cow reticulorumen temperature. J Dairy Sci. 2013;96:5072–5081. doi: 10.3168/jds.2012-6537. [DOI] [PubMed] [Google Scholar]
- Mader T.L., Davis M.S., Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci. 2006;84:712–719. doi: 10.2527/2006.843712x. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Yasui T., Felippe M., Overton T. Associations between the degree of early lactation inflammation and performance, metabolism, and immune function in dairy cows. J Dairy Sci. 2016;99:680–700. doi: 10.3168/jds.2015-9694. [DOI] [PubMed] [Google Scholar]
- Megahed G., Anwar M., Wasfy S., Hammadeh M. Influence of heat stress on the cortisol and oxidant-antioxidants balance during oestrous phase in buffalo-cows (Bubalus bubalis): thermo-protective role of antioxidant treatment. Reprod Domest Anim. 2008;43:672–677. doi: 10.1111/j.1439-0531.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Min L., Zheng N., Zhao S., Cheng J., Yang Y., Zhang Y., Wang J. Long-term heat stress induces the inflammatory response in dairy cows revealed by plasma proteome analysis. Biochem Bioph Res Co. 2016;471:296–302. doi: 10.1016/j.bbrc.2016.01.185. [DOI] [PubMed] [Google Scholar]
- Nasr M.A.F. The impact of crossbreeding Egyptian and Italian buffalo on milk yield and composition under subtropical environmental conditions. J Dairy Res. 2016;83:196–201. doi: 10.1017/S0022029916000194. [DOI] [PubMed] [Google Scholar]
- NRC . 6th ed. 1989. Nutrient requirements of dairy cattle. Washington, DC. [Google Scholar]
- NRC . 7th ed. 2001. Nutrient requirements of dairy cattle. Washington, DC. [Google Scholar]
- Qing Z.F., Wei Z.Z., Ping Q.J., Dong Y.H., Ming L., Shu L. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19:635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M., Wei S., Chen Z., Wang G., Zheng Y., Yan P. Differences of hormones involved in adipose metabolism and lactation between high and low producing Holstein cows during heat stress. Anim Nutr. 2015;1:339–343. doi: 10.1016/j.aninu.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa S., Kargar S., Moghaddam G.A., Ciliberti M.G., Caroprese M. Heat stress abatement during the postpartum period: effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J Anim Sci. 2019;97:122–132. doi: 10.1093/jas/sky408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senese R., de Lange P., Petito G., Moreno M., Goglia F., Lanni A. 3, 5-Diiodothyronine: a novel thyroid hormone metabolite and potent modulator of energy metabolism. Front Endocrinol. 2018;9:427. doi: 10.3389/fendo.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo L.M., Aitken S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol. 2009;128:104–109. doi: 10.1016/j.vetimm.2008.10.305. [DOI] [PubMed] [Google Scholar]
- Suresh D.R., Annam V., Pratibha K., Prasad B.M. Total antioxidant capacity - a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci. 2009;16:61. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Orellana R.M., Weng X., Marins T.N., Dahl G.E., Bernard J.K. Symposium review: the influences of heat stress on bovine mammary gland function. J Dairy Sci. 2018;101:5642–5654. doi: 10.3168/jds.2017-13727. [DOI] [PubMed] [Google Scholar]
- Turk R., Podpečan O., Mrkun J., Flegar-Meštrić Z., Perkov S., Zrimšek P. The effect of seasonal thermal stress on lipid mobilisation, antioxidant status and reproductive performance in dairy cows. Reprod Domest Anim. 2015;50:595–603. doi: 10.1111/rda.12534. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Webster J.R., Stewart M., Rogers A.R., Verkerk G.A. Assessment of welfare from physiological and behavioural responses of New Zealand dairy cows exposed to cold and wet conditions. Anim Welf. 2008;17:19–26. [Google Scholar]
- Weitzel J.M., Viergutz T., Albrecht D., Bruckmaier R., Schmicke M., Tuchscherer A. Hepatic thyroid signaling of heat-stressed late pregnant and early lactating cows. J Endocrinol. 2017;234:129–141. doi: 10.1530/JOE-17-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.W., Mullinix B.G., Bernard J.K. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J Dairy Sci. 2003;86:232–242. doi: 10.3168/jds.S0022-0302(03)73602-9. [DOI] [PubMed] [Google Scholar]
- Xu H., Huang W., Hou Q., Kwok L.Y., Sun Z., Ma H. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci Bull. 2017;62:767–774. doi: 10.1016/j.scib.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Zhang F.J., Weng X.G., Wang J.F., Zhou D., Zhu Y.H. Effects of temperature-humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J Anim Sci. 2014;92:3026–3034. doi: 10.2527/jas.2013-6932. [DOI] [PubMed] [Google Scholar]
- Zhao F., Wu T., Zhang H., Loor J.J., Wang M., Peng A., Wang H. Jugular infusion of arginine has a positive effect on antioxidant mechanisms in lactating dairy cows challenged intravenously with lipopolysaccharide. J Anim Sci. 2018;96:3850–3855. doi: 10.1093/jas/sky250. [DOI] [PMC free article] [PubMed] [Google Scholar]