Abstract
Two studies were conducted to investigate the effect of Bacillus amyloliquefaciens CECT 5940 (BA) as a probiotic on growth performance, amino acid digestibility and bacteria population in broiler chickens under a subclinical necrotic enteritis (NE) challenge and/or fed diets with different levels of crude protein (CP). Both studies consisted of a 2 × 2 factorial arrangement of treatments with 480 Ross 308 mix-sexed broiler chickens. In study 1, treatments included 1) NE challenge (+/−), and 2) BA (1.0 × 106 CFU/g of feed) supplementation (+/−). In study 2, all birds were under NE challenge, and treatments were 1) CP level (Standard/Reduced [2% less than standard]) and 2) BA (1.0 × 106 CFU/g of feed) supplementation (+/−). After inducing NE infection, blood samples were taken on d 16 for uric acid evaluation, and cecal samples were collected for bacterial enumeration. In both studies, ileal digesta was collected on d 35 for nutrient digestibility evaluation. In study 1, the NE challenge reduced body weight gain (BWG), supressed feed conversion ratio (FCR) and serum uric acid levels (P < 0.001). Supplementation of BA increased BWG (P < 0.001) and reduced FCR (P = 0.043) across dietary treatments, regardless of challenge. Bacillus (P = 0.030) and Ruminococcus (P = 0.029) genomic DNA copy numbers and concentration of butyrate (P = 0.017) were higher in birds fed the diets supplemented with BA. In study 2, reduced protein (RCP) diets decreased BWG (P = 0.010) and uric acid levels in serum (P < 0.001). Supplementation of BA improved BWG (P = 0.001) and FCR (P = 0.005) and increased Ruminococcus numbers (P = 0.018) and butyrate concentration (P = 0.033) in the ceca, regardless of dietary CP level. Further, addition of BA reduced Clostridium perfringens numbers only in birds fed with RCP diets (P = 0.039). At d 35, BA supplemented diets showed higher apparent ileal digestibility of cystine (P = 0.013), valine (P = 0.020), and lysine (P = 0.014). In conclusion, this study suggests positive effects of BA supplementation in broiler diets via modulating gut microflora and improving nutrient uptake.
Keywords: Bacillus amyloliquefaciens, Necrotic enteritis, Growth performance, Reduced protein diet, Bacteria population, Gut function
1. Introduction
The ban of in-feed antimicrobial growth promoters has caused enteric challenges specifically necrotic enteritis (NE) to become a significant economical concern for poultry farmers, especially in the subclinical form, due to a lack of obvious symptoms and thus delayed treatment (Shojadoost et al., 2012). Necrotic enteritis disease is estimated to cause US$6 billion annual loss for the global poultry industry (Wade and Keyburn, 2015). This disease is initiated by the proliferation of the necrotic enteritis B-like toxin-producing Clostridium perfringens strains in the gut (Keyburn et al., 2008). Excessive and extensive use of antimicrobials, coupled with an increased interest in food safety and development of antimicrobial-resistant human pathogenic bacteria, resulted in a great deal of development in finding alternatives to antibiotics.
Probiotics have shown to be a candidate, presenting beneficial effects on animal health and welfare (Vuong et al., 2016). In particular, Bacillus-based probiotics have shown immense reliability due to their spore-forming capacity, which can withstand environmental stress such as pelletising of feed and a range of gastrointestinal conditions including low pH (Shivaramaiah et al., 2011). These bacteria are widely used as probiotics in both human and veterinary nutrition. It has been proposed that the Bacillus bacterium can exert positive effects by competitive exclusion of intestinal pathogens and secretion of antimicrobial compounds that can inhibit the growth of pathogenic bacteria (Cartman et al., 2008). This group of bacteria can also produce enzymes such as, amylase, trypsin and lipase that could potentially aid nutrient digestion benefiting the host. One of the Bacillus species that has shown positive effects on broiler production is Bacillus amyloliquefaciens (Lei et al., 2015). Although this soil bacterium is very similar to Bacillus subtilis, differences between their colonization abilities and enzymes products have been previously observed (Reva et al., 2004; Welker and Campbell, 1967). It is believed that B. amyloliquefaciens CECT 5940 (BA) possesses a wide range of antimicrobial activities that can ultimately improve health in broilers (Kadaikunnan et al., 2015; Lei et al., 2015).
Apart from NE disease, another concern for the poultry industry is the high cost of protein sources and environmental concerns related to high nitrogen excretion. Excessive dietary protein is known to be one of the predisposing factors that succumb broiler chickens to NE (Cooper and Songer, 2010). In recent years, efforts have been made to decrease the crude protein (CP) level in diets which will lead to reduced nitrogen concentration in broiler excreta and litter (Torres-Rodriguez et al., 2005). Considering that dietary protein has a direct effect on water consumption, providing reduced crude protein (RCP) diets can reduce water consumption and litter moisture (Hernandez et al., 2012), thus decreasing the incidence of footpad dermatitis (FD). In addition, supplementation of diets with probiotics has shown to improve performance in birds fed RCP diets (Katoch et al., 2017; Suartika et al., 2014). We hypothesized that reducing dietary protein under challenge conditions might negatively impact the growth performance of broiler chickens due to reduced ability of birds to utilize the available nutrients and also due to partitioning of higher nutrients towards maintenance rather than for growth. On the other hand, probiotics have shown to increase the availability of nutrients, and significantly improve performance in birds fed with RCP diets (Suartika et al., 2014). They have also shown to increase digestive enzyme activity, protein efficiency and nutrient retention in broilers (Angel et al., 2005; Murugesan et al., 2014). In this context, this study aimed to examine if B. amyloliquefaciens CECT 5490 could improve the gut environment and reduce the negative effects of NE in chickens, and also have a positive impact on performance, particularly in birds fed RCP diets.
2. Materials and methods
2.1. Ethics statement
This experiment was approved by the Animal Ethics Committee of the University of New England (Approval No. AEC17-127). All broiler management procedures including health care, husbandry and use of laboratory animals fulfilled the requirements of the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC, 2013).
2.2. Experimental design and bird management
Two studies were conducted to evaluate the impact of BA supplementation both under NE challenge and in the presence of diets with different levels of CP. In both studies, birds (mixed-sex, day-old Ross-308 broiler chickens) were obtained from Baiada hatchery in Tamworth, NSW, Australia. On arrival, all birds were weighed, feather sexed, and the same ratio of males to females was allocated to each pen. Both studies were designed as a 2 × 2 factorial arrangement consisting of 480 birds assigned to 32 floor pens with 4 treatments, 8 replicates, and 15 birds per pen. In study 1, the factors were NE challenge (yes or no) and BA probiotic supplementation (yes or no). In study 2, all birds were challenged with NE, and the treatments were dietary CP level (standard or reduced) and BA probiotic supplementation (yes or no). A flow chart of the experimental design is shown in Fig. 1.
Fig. 1.
Flow diagram of experimental design. (A) Study 1. (B) Study 2. NE = necrotic enteritis; CP = crude protein level; BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
All pens were within the same environmentally controlled facility, equipped with tube feeders and cup drinkers. Chickens had ad libitum access to feed and water. The lighting, relative humidity, and temperature were maintained following Ross 308 guidelines (Aviagen, 2014).
2.3. Diets and bird performance
The ingredients and nutrient composition of the diets for both experiments are shown in Table 1. The diets were formulated based on wheat and soybean meal. The standard protein (SCP) diets were formulated to meet the CP levels in Ross 308 guidelines (Aviagen, 2014) (starter 23%, grower 21.5% and finisher 19.5%) and the RCP diet was formulated to have 2% less CP compared to SCP diets (starter 21%, grower 19.5% and finisher 17.5%). The reduction in RCP diet was obtained with additional l-valine, l-isoleucine and l-arginine, which were also supplied to the diets to ensure the same amino acid (AA) levels in both treatments. Phytase was included in all treatments at 0.01% (Quantum Blue, AB vista Feed Ingredients, Marlborough, UK). Titanium dioxide was added as an indigestible marker at 0.5% in the grower and finisher diets.
Table 1.
Ingredients and nutritional content of the experimental diets (%, DM basis).
| Item | Starter (d 0 to 10) |
Grower (d 11 to 24) |
Finisher (d 25 to 35) |
|||
|---|---|---|---|---|---|---|
| SCP | RCP | SCP | RCP | SCP | RCP | |
| Ingredients | ||||||
| Wheat | 39.9 | 49.6 | 42.6 | 52.3 | 49.2 | 59.1 |
| Soybean meal | 33.2 | 24.1 | 29.2 | 20.1 | 22.9 | 13.5 |
| Sorghum | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Canola oil | 2.83 | 1.38 | 3.93 | 2.47 | 3.89 | 2.38 |
| Dicalcium phosphate | 1.27 | 1.33 | 1.09 | 1.15 | 0.98 | 1.05 |
| Limestone | 1.24 | 1.26 | 1.16 | 1.18 | 1.12 | 1.14 |
| Salt | 0.55 | 0.55 | 0.55 | 0.56 | 0.48 | 0.48 |
| dl-methionine | 0.34 | 0.38 | 0.31 | 0.35 | 0.28 | 0.32 |
| l-lysine-HCl 78% | 0.27 | 0.52 | 0.25 | 0.50 | 0.27 | 0.53 |
| l-threonine | 0.16 | 0.27 | 0.14 | 0.25 | 0.12 | 0.23 |
| Vitamin premix1 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Trace mineral premix2 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| l-valine | – | 0.12 | – | 0.12 | – | 0.13 |
| l-isoleucine | – | 0.11 | – | 0.11 | – | 0.12 |
| l-arginine | – | 0.18 | – | 0.19 | – | 0.22 |
| Choline chloride 60% | 0.03 | 0.07 | 0.03 | 0.07 | 0.03 | 0.07 |
| Phytase | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Titanium dioxide | – | – | 0.50 | 0.50 | 0.50 | 0.50 |
| Nutrients content | ||||||
| AMEn, kcal/kg | 3,000 | 3,000 | 3,090 | 3,090 | 3,150 | 3,150 |
| CP | 23.0 | 21.0 | 21.5 | 19.5 | 19.5 (19.8)3 | 17.5 (17.6)3 |
| Choline, mg/kg | 1,700 | 1,700 | 1,600 | 1,600 | 1,500 | 1,500 |
| Linoleic acid | 1.68 | 1.25 | 1.99 | 1.56 | 1.98 | 1.53 |
| Ca | 0.96 | 0.96 | 0.87 | 0.87 | 0.81 | 0.81 |
| Av. P | 0.48 | 0.48 | 0.44 | 0.44 | 0.41 | 0.41 |
| Dig. Lys | 1.20 | 1.20 | 1.10 | 1.10 | 0.98 (1.08)3 | 0.98 (0.96)3 |
| Dig. Met | 0.62 | 0.62 | 0.57 | 0.57 | 0.51 (0.53)3 | 0.51 (0.50)3 |
| Dig. Arg | 1.33 | 1.33 | 1.22 | 1.22 | 1.07 (1.13)3 | 1.07 (1.04)3 |
| Dig. Ile | 0.83 | 0.83 | 0.77 | 0.77 | 0.68 (0.79)3 | 0.68 (0.73)3 |
| Dig. Val | 0.90 | 0.90 | 0.84 | 0.84 | 0.75 (0.88)3 | 0.75 (0.82)3 |
CP = crude protein; SCP = standard protein; RCP = reduced protein.
Vitamin premix supplied the following per kilogram of diet: retinol, 12,000 IU; cholecalciferol, 5,000 IU; tocopheryl acetate, 75 mg, menadione, 3 mg; thiamine, 3 mg; riboflavin, 8 mg; niacin, 55 mg; pantothenate, 13 mg; pyridoxine, 5 mg; folate, 2 mg; cyanocobalamin, 16 mg; biotin, 200 mg; cereal-based carrier, 149 mg; mineral oil, 2.5 mg.
Trace mineral premix supplied the following per kilogram of diet: Cu (sulfate), 16 mg; Fe (sulfate), 40 mg; I (iodide), 1.25 mg; Se (selenate), 0.3 mg; Mn (sulfate and oxide), 120 mg; Zn (sulfate and oxide), 100 mg; cereal-based carrier, 128 mg; mineral oil, 3.75 mg.
Analyzed content in parenthesis.
The probiotic, B. amyloliquefaciens CECT 5940, provided by Evonik Nutrition & Care GmbH had an approximate spore count of 2 × 109 CFU/g. Five hundred grams of probiotic was added on top of 1 t of feed, and diets were pelleted at 65 to 70 °C. Final feeds were checked for the spore count and they met the expected spore count level 1 × 106 CFU/g of feed.
Birds were fed in starter (d 0 to 10), grower (d 11 to 24), and finisher (d 25 to 35) phases. Birds and feed were weighed on arrival and on d 10, 24, and 35, and mortality was recorded daily. Body weight gain (BWG), feed intake (FI), and mortality adjusted feed conversion ratio (FCR) were calculated for all phases.
2.4. Necrotic enteritis challenge
On d 9, birds in the challenged groups were inoculated per os with 1 mL of Eimeria strains, a suspension of 5,000 sporulated oocysts of field strains both of Eimeria acervulina and Eimeria maxima, and 2,500 sporulated oocysts of Eimeria brunetti (Eimeria Pty Ltd, Ringwood, Vic, Australia). Non-challenged groups were gavaged with sterile PBS. On d 14 and 15, birds in the challenged group were inoculated per os with 1 mL of C. perfringens EHE-NE18 (108 CFU/mL), whereas the non-challenged birds were inoculated with sterile thioglycolate broth.
2.5. Sample collection, lesion scoring and uric acid evaluation
Four birds (2 males and 2 females) per pen were randomly selected and weighed on d 16. These birds were then electrically stunned, and blood samples were immediately collected from the jugular vein of both male birds. Blood samples were collected into vacutainers that contained spray-coated silica and a polymer gel for serum separation, and the samples were stored at 4 °C before being centrifuged at 1,500 × g at 4 °C for 10 min to separate the serum. Serum samples were separated and stored at −20 °C until analysis. Serum samples were analyzed for uric acid concentration using an integrated chemistry analyzer (Siemens Dimension Xpand Plus, Newark, NJ, US).
The whole jejunum (from the pancreatic loop to Meckel's diverticulum) was excised from all the sampled birds and examined for lesion scores according to Broussard et al. (1986). Two experienced personnel with no knowledge of the treatment allocation of the birds performed the lesion scoring. Ileal and cecal pH values were measured by inserting an EcoScan 5/6 pH meter (Envirosensors spear tip pH probe, Australia) probe directly into the distal ileum and caeca. Cecal and ileal contents were then separately collected into sterile 50-mL tubes. Approximately 1 mL of the pooled cecal contents were placed in sterile 2-mL Eppendorf tubes, snap-frozen in liquid nitrogen, and stored at −20 °C for DNA extraction. The remaining samples were stored at −20 °C for short-chain fatty acid (SCFA) evaluation.
2.6. Digesta collection, carcass parameters and footpad lesion measurements
Four birds (2 males and 2 females) per pen were randomly selected, weighed, electrically stunned and euthanized by cervical dislocation on d 35. Abdominal fat (considered as the fat from the proventriculus surrounding the cloaca and adjacent to the abdominal muscle) was taken out and weighed. Abdominal fat was then expressed as a percentage of live BW. The digesta contents from the distal ileum from male birds were collected and pooled by gently squeezing the digesta into 50-mL plastic containers and stored at −20 °C for nutrient digestibility analysis. All sampled birds were then processed for breast (skinless and boneless), thigh (with skin and bone) and drumstick weights as a proportion of determinations of live weight. All individual birds from each pen were scored for FD on d 35. The score of FD was determined according to the method of Allain et al. (2009). A 10-point (ranging from 0 to 9) scale was used based on the extent and appearance of lesions: 0 indicated no lesions and 9 the most macroscopic deep lesions.
2.7. Microbial analysis of cecal contents
The method described by Kheravii et al. (2017) was used for DNA extraction. In brief, approximately 60 mg of frozen cecal samples were placed into a 2-mL Eppendorf tube that contained 300 mg of glass beads (0.1 mm). With the use of QIAxtractor DNA Reagents, and QIAxtractor DNA plasticware (Qiagen, Inc., Doncaster, VIC, Australia), samples were lysed with 300 μL of Qiagen Lysis Buffer with cells disrupted by shaking the tubes in a bead beater mill (Retsch GmbH & Co, Haan, Germany) for 5 min at 30 Hz frequency. Then the samples were placed in a heating block for 2 h at 55 °C followed by 5 min centrifugation at 20,000 × g. The X-tractor-Gene automated DNA extraction system (Corbett Life Science, Sydney, Australia) was used for DNA extraction. Reagents (DXB, DXW, DXF, and DXE) were placed in their specific locations in the robotics machine together with 200 μL of the lysate transferred automatically into loading block. Then 400 μL of the binding buffer (DXB) was added to the 200-μL lysate and incubated for 6 min, and then 500 μL of the lysed samples were transferred into capture columns and vacuumed at 30 kPa for 3 min. Following this, 600 μL of DXW was transferred to the capture columns and vacuumed for 30 kPa for 2 min, 600 μL of DXF was transferred to the columns and vacuumed at 35 kPa for 1 min, and DNA was dried by vacuuming again at 25 kPa for 5 min. Finally, an elution block was used to elute the extracts by the addition of 60 μL of DXE and the samples were vacuumed at 30 kPa for 2 min. The resulting DNA samples were measured on a Nanodrop 8000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) for assessment of quantity and purity. DNA with ratios of A260 to A280 and A260 to A230 being greater than 1.8 were considered of high purity and were stored at −20 °C. The extracted cecal DNA were diluted 20 times in Milli-Q water, and the real-time quantitative polymerase chain reaction (qPCR) was performed using a SensiMix-SYBRR (Bioline, Sydney, Australia) reagent. The SYBRGreen (SensiMix SYBR No-Rox, Bioline, Sydney, Australia) reagent was used in qPCR, and a volume of 10 μL or the reaction contained 5 μL of 2 × SensiMix, 300 mmol/L of each primer and 2 μL of DNA template. The genome copies of Bacillus, Bacteroides. Bifidobacteria, Lactobacillus, Ruminococcus spp. and for C. perfringens, SensiFAST Probe SYBR No-ROX (Bioline, Sydney, Australia) were used (Table 2). The PCR was performed in duplicate, and if the difference of the threshold cycle (Ct) values between the duplicates were greater than 0.5, the assay of the sample was repeated. Rotorgene 6500 real-time (Corbett Research, Sydney, Australia) PCR was used to perform PCR, and a threshold cycle average from the duplicate samples was used for data analysis. The concentrations of the plasmid DNA were measured using NanoDrop ND-8000 (Thermo Fisher Scientific, Waltham, MA) prior to the serial dilutions. The number of target DNA copies was calculated from the mass of DNA, taking into account the size of the amplicon inserted in the plasmid. Bacteria quantity were expressed as log10 (genomic DNA copy number)/g digesta.
Table 2.
Primers used for the qPCR analysis of different bacteria groups.
| Target group | Primer/probe sequence (5ʹ–3ʹ) | Amplicon length, bp | Annealing temperature, °C | Reference | |
|---|---|---|---|---|---|
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTT GA R-TCA TCC CCA CCT TCC CC GGT |
92 | 63 | Zhang et al. (2015) | |
| Bacteroides spp. | F-GAG AGG AAG GTC CCC CAC R-CGC TAC TTG GCT GGT TCA G |
108 | 63 | Layton et al. (2006) | |
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC R-CTT CTC CGG CAT GGT GTT G |
106 | 63 | Requena et al. (2002) | |
| Clostridium perfringens | F-GCA TAA CGT TGA AAG ATG G R-CCT TGG TAG GCC GTT ACC C TaqMan probe: 5ʹ-FAM-TCA TCA TTC AAC CAA AGG AGC AAT CC-TAMRA-3ʹ |
120 | 60 | Kheravii et al. (2018) | |
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG R-AGC AGT AGG GAA TCT TCC A |
186 | 63 | Wise and Siragusa (2007) | |
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT R-CCA GGT GGA TWA CTT ATT GTG TTAA |
157 | 63 | Ramirez-Farias et al. (2008) | |
qPCR = real time quantitative PCR; F = forward primer; R = reverse primer.
2.8. Short chain fatty acids analysis of cecal contents
The SCFA analysis followed the method described by Jensen et al. (1995). In brief, approximately 1 g of ceca was weighed, and 1 mL of internal standard (0.01 mol/L methyl butyric acid) was added, the solution was vortexed and centrifuged for 20 min at 15,000 × g at 5 °C. Then, 1 mL of the supernatant was transferred to 8-mL vials, and 0.5 mL of concentrated HCl (36%) and 2.5 mL of ether was added. The mixture was vortexed, centrifuged at 1,000 × g for 15 min at 5 °C. The 400 μL of the resulting supernatant was then mixed with 40 μL of N-tert-butyldimethylsilyl-N-methyl trifluoroacetamide. Samples were then vortexed and heated on a heating block at 80 °C for 20 min. Before analyzing samples on a Varian CP3400 CX gas Chromatograph (Varian Analytical Instruments, Palo Alto, CA, USA), the vials were kept at room temperature for 48 h. SCFA concentrations were expressed as micromole per gram digesta.
2.9. Amino acid digestibility
Ileal digesta samples collected on d 35 were freeze-dried at −50 °C for 5 d and finely ground with an electrical grinder to pass through a 0.5-mm sieve to ensure a homogenous mixture. The diets and digesta samples were analyzed for dry matter (DM) by placing duplicate samples in a drying oven at 105 °C for 36 h to constant weight. The diet and freeze-dried digesta samples were analyzed for AA content by an amino acid analyzer, according to AOAC (2005) by method 930.15 at Evonik's AMINOLab in Singapore. Titanium dioxide (indicator) concentrations were determined in duplicate for diets and digesta samples by the colorimetric method described by Short et al. (1996). The coefficient of apparent ileal digestibility of the AA was calculated with the following formula:
Coefficients of ileal apparent digestibility (CIAD) = [100 − (100 × Id × AAdc/Idc × AAd)]/100,
where Id is content of the indicator in DM of diet, AAdc is amino acid content in DM of digesta, Idc is indicator content in DM of digesta, AAd is AA content of DM of diet.
2.10. Litter moisture
On d 35, approximately 1 kg of pooled litter from 6 locations of the pen (around the feeder, drinkers, middle, and corners) was collected and immediately stored in 2 zip tight bags to avoid any evaporation and/or moisture absorption of the samples. Samples were stored at −20 °C for later analysis. For evaluating the litter moisture, plastic bags were kept in room temperature for 5 h to thaw, and approximately 0.5-kg subsamples were obtained and weighed accurately before drying in the oven at 105 °C for 24 h. The moisture content was calculated according to the method presented by Barker et al. (2013).
2.11. Statistical analysis
The data were analyzed by SPSS statistics package version 22 (IBM Corporation, Armonk, NY, United States). Intestinal lesions, FD scoring and mortality data were analyzed using the non-parametric Kruskal–Wallis test as the data was not normally distributed. Mean values of the treatments were compared within the confidence interval adjusted by Tukey test. All significant differences were determined at P < 0.05.
3. Results
3.1. Broiler performance (studies 1 and 2)
The effects of BA and NE challenge on broiler performance in the periods from 0 to 10 d, 0 to 24 d, and 0 to 35 d are presented in Table 3. In study 1, the NE challenge significantly affected bird performance by reducing BWG (P < 0.001) and FI (P < 0.001) and increasing FCR (P < 0.001) during d 0 to 24 and overall period of the trial. However, the supplementation of BA significantly increased BWG (P = 0.001, P < 0.001) and FI (P = 0.007, P < 0.001) and resulted in lower FCR (P = 0.017, P = 0.043) in the 0 to 24 and 0 to 35 d periods, respectively. Nevertheless, no interaction between BA and NE challenge was observed in these periods. The effects of BA supplementation and dietary CP level on performance in the periods from 0 to 10 d, 0 to 24 d and 0 to 35 d are shown in Table 4. Results in study 2 shows a significant reduction of BWG with the RCP diet (P = 0.01) in the overall period of the experiment (d 0 to 35), whereas the BA supplementation significantly improved BWG (P = 0.001) regardless of the CP level. Birds fed RCP diets showed a significantly higher FCR from d 0 to 24 (P < 0.001) and d 0 to 35 (P = 0.006). However, BA supplementation significantly reduced FCR regardless of dietary CP level over 0 to 24 d (P = 0.022) and 0 to 35 d (P = 0.005). No interaction between BA and CP levels was observed (P > 0.05). Overall mortality was not significantly affected by NE and BA supplementation and different CP levels (data not shown).
Table 3.
Performance of broiler chickens in response to probiotic and necrotic enteritis challenge.1
| Treatments |
BWG, g |
FI, g |
FCR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Challenge | BA, g/t | 0 to 10 d | 0 to 24 d | 0 to 35 d | 0 to 10 d | 0 to 24 d | 0 to 35 d | 0 to 10 d | 0 to 24 d | 0 to 35 d | |
| No | 0 | 129 | 787 | 1,737 | 140 | 1021 | 2453 | 1.084 | 1.298 | 1.412 | |
| No | 500 | 133 | 871 | 1,903 | 141 | 1107 | 2644 | 1.066 | 1.271 | 1.389 | |
| Yes | 0 | 130 | 676 | 1,543 | 140 | 925 | 2343 | 1.077 | 1.356 | 1.520 | |
| Yes | 500 | 132 | 718 | 1,658 | 140 | 962 | 2435 | 1.056 | 1.342 | 1.490 | |
| SEM | 0.93 | 13.8 | 22.6 | 1.59 | 14.3 | 19.7 | 0.008 | 0.010 | 0.011 | ||
| P-value | |||||||||||
| Source | |||||||||||
| Challenge | 0.903 | <0.001 | <0.001 | 0.803 | <0.001 | <0.001 | 0.624 | <0.001 | <0.001 | ||
| BA | 0.166 | 0.001 | <0.001 | 0.948 | 0.007 | <0.001 | 0.242 | 0.017 | 0.043 | ||
| Challenge × BA | 0.741 | 0.056 | 0.300 | 0.796 | 0.114 | 0.326 | 0.958 | 0.143 | 0.800 | ||
| Main effect | |||||||||||
| Challenge | |||||||||||
| No | 131 | 829a | 1,820a | 141 | 1,064a | 2,548a | 1.075 | 1.284b | 1.401b | ||
| Yes | 131 | 698b | 1,600b | 140 | 945b | 2,386b | 1.067 | 1.349a | 1.505a | ||
| BA | |||||||||||
| 0 | 130 | 735b | 1,640b | 141 | 976b | 2,398b | 1.081 | 1.325a | 1.466a | ||
| 500 g/t | 133 | 795a | 1,780a | 140 | 1,035a | 2,546a | 1.061 | 1.306b | 1.440b | ||
BWG = body weight gain per bird; FI = feed intake per bird; FCR = feed conversion ratio; BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (total number of birds per replicate).
Table 4.
Performance of broiler chickens in response to probiotic and crude protein levels under necrotic enteritis challenge.1
| Treatments |
BWG, g |
FI, g |
FCR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CP level | BA, g/t | 0 to 10 d | 0 to 24 d | 0 to 35 d | 0 to 10 d | 0 to 24 d | 0 to 35 d | 0 to 10 d | 0 to 24 d | 0 to 35 d |
| Standard2 | 0 | 130 | 657 | 1,543 | 140 | 897 | 2,343 | 1.112 | 1.365 | 1.520 |
| Standard2 | 500 | 132 | 718 | 1,658 | 140 | 962 | 2,469 | 1.089 | 1.342 | 1.490 |
| Reduced3 | 0 | 128 | 674 | 1,480 | 148 | 979 | 2,344 | 1.130 | 1.455 | 1.586 |
| Reduced3 | 500 | 129 | 660 | 1,574 | 145 | 920 | 2,389 | 1.115 | 1.397 | 1.520 |
| SEM | 0.97 | 11.6 | 17.1 | 1.59 | 15.7 | 19.0 | 0.009 | 0.011 | 0.010 | |
| P-value | ||||||||||
| Source | ||||||||||
| CP | 0.173 | 0.380 | 0.010 | 0.050 | 0.520 | 0.269 | 0.073 | <0.001 | 0.006 | |
| BA | 0.557 | 0.309 | 0.001 | 0.610 | 0.915 | 0.022 | 0.129 | 0.022 | 0.005 | |
| CP × BA | 0.653 | 0.117 | 0.698 | 0.761 | 0.051 | 0.256 | 0.497 | 0.304 | 0.254 | |
| Main effect | ||||||||||
| CP | ||||||||||
| Standard | 131 | 687 | 1,600a | 140 | 930 | 2,406 | 1.100 | 1.353b | 1.505b | |
| Reduced | 129 | 667 | 1,527b | 146 | 950 | 2,367 | 1.122 | 1.426a | 1.552a | |
| BA | ||||||||||
| 0 | 129 | 665 | 1,512b | 144 | 938 | 2,344b | 1.120 | 1.410a | 1.553a | |
| 500 g/t | 131 | 689 | 1,616a | 142 | 941 | 2,429a | 1.102 | 1.369b | 1.505b | |
BWG = body weight gain/bird; FI = feed intake/bird; FCR = feed conversion ratio; BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (total number of birds per replicate).
Standard crude protein level according to recommendation.
Reduced crude protein diets with 2% lower protein than standard diets.
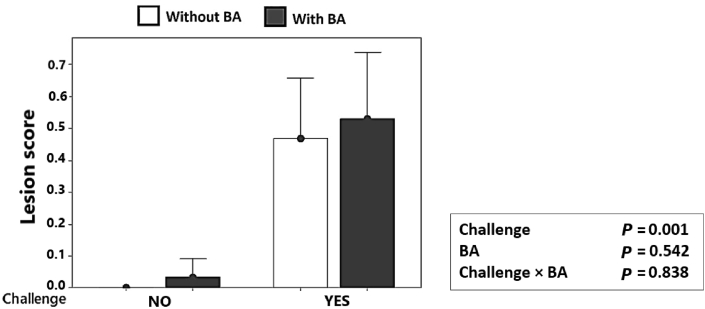
3.2. Intestinal lesion score (studies 1 and 2)
As illustrated in Fig. 2, on d 16, in study 1, the NE challenge resulted in a significantly higher prevalence of lesions in the jejunum (P = 0.001) compared to the non-challenged birds, and probiotic supplementation had no significant effect on lesion score (P > 0.05). In study 2, there were no significant interactions between dietary level and supplementing BA on intestinal lesion scores (data not shown).
Fig. 2.
Effect of Bacillus amyloliquefaciens CECT 5940 (BA; 1.0 × 106 CFU/g of feed) and necrotic enteritis challenge on jejunum lesion scores at d 16. Values are means ± SEM. NO, non-challenged; YES, necrotic enteritis challenge. Birds did not have lesion scores higher than 2.
3.3. Meat and fat pad percentage (studies 1 and 2)
Study 1 results are illustrated in Table 5, which shows that on d 16, the NE challenge reduced ileal pH (P < 0.001). On d 35, the NE challenge reduced breast yield (P < 0.001) relative to the body weight. Footpad dermatitis lesions were significantly higher in NE challenged birds (P = 0.024). Furthermore, litter moisture and overall mortality were not significantly affected by either the NE challenge or BA supplementation (P > 0.05). In study 2, RCP fed birds had a significantly higher fat pad percentage relative to their body weights (P < 0.001) compared to birds fed the SCP diets. On the other hand, birds fed RCP diets had significantly lower (P = 0.013) litter moisture compared to birds fed SCP diets. Meat yield and overall mortality were not significantly affected by CP level or BA supplementation (Table 6).
Table 5.
Day 16 ileum and cecum pH, and d 35 meat and fat yield, litter moisture, footpad dermatitis (FD) and overall mortality in responses to the necrotic enteritis challenge and probiotic supplementation.
| Treatments |
pH1 |
Meat and fat yield,1 % |
Litter moisture,2 % | FD3 | Overall mortality, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| Challenge | BA, g/t | Ileum | Cecum | Breast | Thigh | Fat pad | |||
| No | 0 | 6.18 | 6.55 | 19.4 | 10.1 | 0.96 | 24.7 | 0.010 | 2.50 |
| No | 500 | 6.20 | 6.69 | 20.5 | 9.87 | 0.90 | 29.7 | 0.087 | 2.50 |
| Yes | 0 | 5.65 | 6.38 | 17.9 | 9.75 | 0.96 | 27.6 | 0.163 | 2.50 |
| Yes | 500 | 5.79 | 6.45 | 18.4 | 9.58 | 0.97 | 27.8 | 0.193 | 3.33 |
| SEM | 0.06 | 0.07 | 0.25 | 0.09 | 0.01 | 1.47 | 0.135 | 1.62 | |
| P-value | |||||||||
| Source | |||||||||
| Challenge | <0.001 | 0.130 | <0.001 | 0.112 | 0.058 | 0.874 | 0.024 | 0.802 | |
| BA | 0.434 | 0.451 | 0.046 | 0.350 | 0.255 | 0.393 | 0.906 | 0.802 | |
| Challenge × BA | 0.550 | 0.786 | 0.386 | 0.727 | 0.121 | 0.443 | 0.443 | 0.802 | |
| Main effect | |||||||||
| Challenge | |||||||||
| No | 6.19a | 6.62 | 19.9a | 9.9 | 0.92 | 27.2 | 0.098b | 2.50 | |
| Yes | 5.72b | 6.41 | 18.1b | 9.7 | 0.96 | 27.7 | 0.178a | 2.91 | |
| BA | |||||||||
| 0 | 5.92 | 6.47 | 18.6b | 9.90 | 0.96 | 26.1 | 0.136 | 2.50 | |
| 500 g/t | 5.99 | 6.57 | 19.3a | 9.72 | 0.94 | 28.8 | 0.140 | 2.91 | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (2 birds per replicate).
Each value represents the mean of 8 replicates.
Each value represents the mean of 8 replicates (total number of birds per replicate).
Table 6.
Day 16 ileum and cecum pH, d 35 meat yield, fat pad, litter moisture and footpad dermatitis (FD) and overall mortality in responses to crude protein levels and probiotic supplementation.
| Treatments |
pH1 |
Meat yield,1 % |
Litter moisture,2 % | FD3 | Overall mortality, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| CP | BA, g/t | Ileum | Cecum | Breast | Thigh | Fat pad | |||
| Standard4 | 0 | 5.65 | 6.38 | 18.1 | 9.75 | 0.96 | 27.6 | 0.163 | 2.50 |
| Standard4 | 500 | 5.79 | 6.45 | 18.3 | 9.66 | 0.97 | 27.8 | 0.193 | 3.33 |
| Reduced5 | 0 | 5.70 | 6.38 | 17.2 | 9.47 | 1.08 | 16.9 | 0.087 | 3.33 |
| Reduced5 | 500 | 5.66 | 6.38 | 18.1 | 9.74 | 1.06 | 23.5 | 0.082 | 0.83 |
| SEM | 0.05 | 0.06 | 0.18 | 0.07 | 0.01 | 1.56 | 0.132 | 1.40 | |
| P-value | |||||||||
| Source | |||||||||
| CP | 0.714 | 0.787 | 0.149 | 0.453 | <0.001 | 0.013 | 0.001 | 0.573 | |
| BA | 0.643 | 0.798 | 0.186 | 0.551 | 0.483 | 0.243 | 0.615 | 0.573 | |
| CP × BA | 0.385 | 0.820 | 0.278 | 0.213 | 1.000 | 0.282 | 0.504 | 0.263 | |
| Main effect | |||||||||
| CP | |||||||||
| Standard | 5.72 | 6.41 | 18.2 | 9.70 | 0.963b | 27.7a | 0.178a | 2.92 | |
| Reduced | 5.77 | 6.38 | 17.7 | 9.60 | 1.068a | 20.2b | 0.084b | 2.08 | |
| BA | |||||||||
| 0 | 5.67 | 6.38 | 17.7 | 9.61 | 1.01 | 22.3 | 0.125 | 2.08 | |
| 500 g/t | 5.72 | 6.41 | 18.2 | 9.69 | 1.00 | 25.6 | 0.138 | 2.92 | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (2 birds per replicate).
Each value represents the mean of 8 replicates.
Each value represents the mean of 8 replicates (total number of birds per replicate).
Standard crude protein level according to recommendation.
Reduced crude protein diets with 2% lower protein than standard diets.
3.4. Cecal bacterial quantification (studies 1 and 2)
As shown in Table 7, in study 1, the NE challenge significantly increased C. perfringens (P < 0.001), and Bacteroides numbers (P = 0.001), whereas decreased Bifidobacterium (P = 0.036), Bacillus (P = 0.002) and Ruminococcus (P < 0.001) populations in the caeca at d 16. However, the supplementation of BA significantly increased Bacillus and Ruminococcus populations in the caeca (P = 0.030 and P = 0.029, respectively). In study 2, BA supplementation significantly increased the number of Ruminococcus (P = 0.018) and Bifidobacterium (P = 0.027) in the cecum, regardless of the protein level. A significant interaction was observed between CP level and BA (P = 0.039), where the RCP diet with supplementation of BA resulted in the reduction of C. perfringens content in the cecum. Neither BA nor CP level had an impact on the other groups of bacteria evaluated (P > 0.05) (Table 8).
Table 7.
Bacterial population in cecal content of birds in response to necrotic enteritis challenge and probiotic supplementation at d 16.1
| Treatment |
Bacterial quantity (log10 genomic DNA copy number/g digesta) |
||||||
|---|---|---|---|---|---|---|---|
| Challenge | BA, g/t | Bacillus | Bacteroides | Bifidobacterium | Lactobacillus | C. perfringens | Ruminococcus |
| No | 0 | 7.96 | 9.83 | 6.92 | 9.30 | 6.22 | 10.1 |
| No | 500 | 8.30 | 10.0 | 6.98 | 9.32 | 5.84 | 10.2 |
| Yes | 0 | 7.06 | 10.3 | 5.50 | 9.40 | 9.70 | 9.62 |
| Yes | 500 | 7.74 | 10.5 | 6.42 | 9.51 | 10.2 | 9.91 |
| SEM | 0.13 | 0.08 | 0.24 | 0.04 | 0.51 | 0.06 | |
| P-value | |||||||
| Source | |||||||
| Challenge | 0.002 | 0.001 | 0.036 | 0.069 | <0.001 | <0.001 | |
| BA | 0.030 | 0.089 | 0.281 | 0.401 | 0.530 | 0.029 | |
| Challenge × BA | 0.256 | 0.878 | 0.352 | 0.647 | 0.370 | 0.193 | |
| Main effect | |||||||
| Challenge | |||||||
| No | 8.13a | 9.93b | 6.95a | 9.31 | 5.99b | 10.2a | |
| Yes | 7.43b | 10.4a | 5.96b | 9.46 | 9.92a | 9.77b | |
| BA | |||||||
| 0 | 7.55b | 10.1 | 6.21 | 9.35 | 8.83 | 9.88b | |
| 500 g/t | 8.02a | 10.3 | 6.70 | 9.41 | 8.56 | 10.0a | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates.
Table 8.
Bacterial populations in cecal content of necrotic enteritis challenged birds in response to dietary crude protein level and probiotic supplementation at d 16.1
| Treatment |
Bacterial quantity (log10 genomic DNA copy number/g digesta) |
||||||
|---|---|---|---|---|---|---|---|
| CP | BA, g/t | Bacillus | Bacteroides | Bifidobacterium | Lactobacillus | C. perfringens | Ruminococcus |
| Standard2 | 0 | 7.06 | 10.4 | 5.50 | 9.40 | 10.4a | 9.62 |
| Standard2 | 500 | 7.74 | 10.5 | 6.92 | 9.51 | 10.3a | 9.88 |
| Reduced3 | 0 | 7.28 | 10.6 | 5.12 | 9.42 | 10.3a | 9.80 |
| Reduced3 | 500 | 7.45 | 10.6 | 5.84 | 9.42 | 9.50b | 9.95 |
| SEM | 0.13 | 0.03 | 0.23 | 0.04 | 0.04 | 0.08 | |
| P-value | |||||||
| Source | |||||||
| CP | 0.814 | 0.201 | 0.159 | 0.657 | 0.083 | 0.136 | |
| BA | 0.127 | 0.761 | 0.027 | 0.528 | 0.047 | 0.018 | |
| Crude protein × BA | 0.353 | 0.298 | 0.264 | 0.566 | 0.039 | 0.476 | |
| Main effect | |||||||
| CP | |||||||
| Standard | 7.43 | 10.50 | 6.09 | 9.46 | 10.32 | 9.76 | |
| Reduced | 7.37 | 10.61 | 5.48 | 9.42 | 9.95 | 9.88 | |
| BA | |||||||
| 0 | 7.18 | 10.5 | 5.33b | 9.41 | 10.3 | 9.71b | |
| 500 g/t | 7.60 | 10.6 | 6.33a | 9.47 | 9.92 | 9.91a | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates.
Standard crude protein level according to recommendation.
Reduced crude protein diets with 2% lower protein than standard diets.
3.5. Short chain fatty acid concentration (studies 1 and 2)
The SCFA concentrations were measured in the cecal content collected on d 16 in both studies (Table 9, Table 10). In study 1, NE challenge significantly reduced acetate (P = 0.001) and butyrate (P < 0.001) concentrations and increased lactate (P = 0.001) level in the cecal contents. The BA supplementation increased butyrate (P = 0.017) concentration in the cecum. Formate, propionate, isobutyrate, isovalerate, valerate, and succinate concentrations were not significantly affected by either the NE challenge or BA supplementation (P > 0.05). No interaction between NE challenge and BA supplementation was observed for SFCA (P > 0.05).
Table 9.
Concentration of short-chain fatty acids in cecal content in responses to the necrotic enteritis challenge and probiotic supplementation in chickens at d 16.1
| Treatment |
Short chain fatty acid concentration, μmol/g |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Challenge | BA, g/t | Formate | Acetate | Propionate | Isobutyrate | Butyrate | Isovaleric | Valerate | Lactate | Succinate |
| No | 0 | 1.16 | 86.0 | 3.84 | 0.71 | 30.9 | 0.42 | 0.92 | 0.79 | 13.3 |
| No | 500 | 1.88 | 90.0 | 3.79 | 0.67 | 34.7 | 0.34 | 0.84 | 0.78 | 16.2 |
| Yes | 0 | 1.21 | 64.5 | 5.45 | 0.85 | 12.7 | 0.56 | 1.20 | 27.9 | 15.3 |
| Yes | 500 | 0.93 | 68.3 | 4.66 | 0.79 | 19.2 | 0.45 | 1.00 | 18.3 | 8.84 |
| SEM | 0.17 | 3.26 | 0.31 | 0.05 | 1.95 | 0.03 | 0.06 | 3.59 | 1.24 | |
| P-value | ||||||||||
| Source | ||||||||||
| Challenge | 0.286 | 0.001 | 0.055 | 0.188 | <0.001 | 0.063 | 0.101 | 0.001 | 0.292 | |
| BA | 0.716 | 0.623 | 0.500 | 0.706 | 0.017 | 0.181 | 0.319 | 0.433 | 0.331 | |
| Challenge × BA | 0.150 | 0.651 | 0.476 | 0.723 | 0.425 | 0.473 | 0.452 | 0.288 | 0.058 | |
| Main effect | ||||||||||
| Challenge | ||||||||||
| No | 1.45 | 87.9a | 3.82 | 0.69 | 32.7a | 0.38 | 0.89 | 0.80b | 14.8 | |
| Yes | 1.08 | 66.5b | 5.04 | 0.81 | 15.7b | 0.50 | 1.09 | 23.1a | 12.2 | |
| BA | ||||||||||
| 0 | 1.18 | 74.7 | 4.73 | 0.78 | 21.2b | 0.49 | 1.06 | 15.61 | 14.48 | |
| 500 g/t | 1.31 | 77.6 | 4.31 | 0.74 | 26.4a | 0.41 | 0.94 | 11.29 | 12.13 | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (2 birds per replicate).
Table 10.
The concentration of short-chain fatty acids in the cecal content of necrotic enteritis challenged birds in responses to the dietary crude protein level and probiotic supplementation in chickens at d 16.1
| Treatment |
Short chain fatty acid concentration, μmol/g |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude protein | BA, g/t | Formate | Acetate | Propionate | Isobutyrate | Butyrate | Isovaleric | Valerate | Lactate | Succinate |
| Standard2 | 0 | 1.02 | 64.5 | 5.45 | 1.04 | 15.3 | 0.56 | 1.49 | 17.5 | 15.3 |
| Standard2 | 500 | 0.83 | 68.3 | 4.66 | 0.79 | 15.0 | 0.45 | 1.21 | 16.7 | 13.6 |
| Reduced3 | 0 | 0.79 | 67.8 | 6.93 | 1.44 | 15.6 | 0.75 | 1.82 | 30.3 | 10.9 |
| Reduced3 | 500 | 1.42 | 84.4 | 7.04 | 0.90 | 24.6 | 0.40 | 1.61 | 10.2 | 18.7 |
| SEM | 0.13 | 2.37 | 0.40 | 0.08 | 1.33 | 0.04 | 0.10 | 0.62 | 1.37 | |
| P-value | ||||||||||
| Source | ||||||||||
| CP | 0.402 | 0.030 | 0.017 | 0.080 | 0.039 | 0.467 | 0.054 | 0.610 | 0.834 | |
| BA | 0.293 | 0.020 | 0.706 | 0.004 | 0.033 | 0.003 | 0.195 | 0.034 | 0.186 | |
| CP × BA | 0.139 | 0.126 | 0.570 | 0.308 | 0.079 | 0.123 | 0.915 | 0.057 | 0.087 | |
| Main effect | ||||||||||
| CP | ||||||||||
| Standard2 | 0.92 | 66.5b | 5.04b | 0.91 | 15.2b | 0.50 | 1.34 | 17.2 | 14.4 | |
| Reduced3 | 1.15 | 76.1a | 6.99a | 1.16 | 20.4a | 0.56 | 1.71 | 19.2 | 15.0 | |
| BA | ||||||||||
| 0 | 0.89 | 66.3b | 6.28 | 1.27a | 15.4b | 0.66a | 1.67 | 23.9a | 12.9 | |
| 500 g/t | 1.18 | 76.7a | 5.98 | 0.85b | 20.8a | 0.42b | 1.43 | 12.8b | 16.5 | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed).
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates (2 birds per replicate).
Standard crude protein level according to recommendation.
Reduced crude protein diets with 2% lower protein than standard diets.
In study 2, RCP fed birds had higher acetate (P = 0.030), propionate (P = 0.017), and butyrate (P = 0.039) concentrations in the cecal contents compared to birds fed the SCP diets (Table 10). Supplementation of BA significantly decreased isobutyrate (P = 0.004), isovalerate (P = 0.003) and lactate (P = 0.034) concentrations whereas increased acetate (P = 0.020) and butyrate (P = 0.033) concentrations in the cecum. No interaction between CP level × BA was observed for SCFA (P > 0.05).
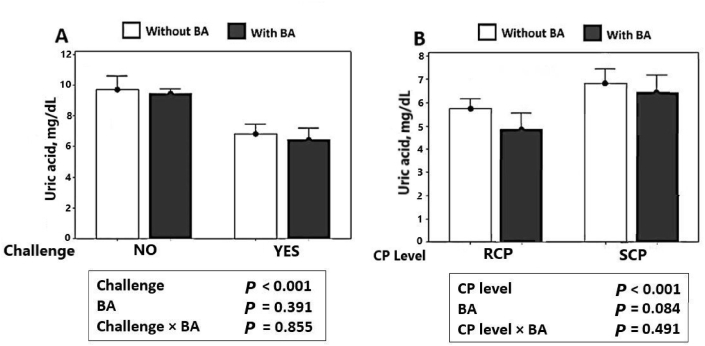
3.6. Serum uric acid (studies 1 and 2)
Serum uric acid levels on d 16 of both studies are shown in Fig. 3, illustrating a significant decrease in uric acid levels induced by the NE challenge (P < 0.001) (Fig. 3A) in study 1. As shown in Fig. 3B, in study 2, birds fed with RCP diets had significantly lower uric acid concentrations compared to SCP diets (P < 0.001).
Fig. 3.
Effect of Bacillus amyloliquefaciens CECT 5490 (BA; 1.0 × 106 CFU/g of feed) on serum uric acid levels in broilers at d 16. (A) Effect of BA and necrotic enteritis (NE) challenge on serum uric acid levels. (B) Effect of CP level and BA supplementation on serum uric acid levels. CP = crude protein; NO, non-challenged; YES, NE challenged; RCP = reduced protein (2% lower than standard diets); SCP = standard protein.
3.7. Apparent ileal amino acid digestibility (studies 1 and 2)
The apparent ileal amino acid digestibility in response to the NE challenge and BA supplementation in broiler chickens at d 35 is shown in Table 11. No significant effect of NE challenge was observed in the digestibility coefficients (P > 0.05) and no interactions were observed between NE challenge and BA supplementation (P > 0.05). However, BA supplementation improved the digestibility of cystine (P = 0.006), valine (P = 0.047), glycine and serine (P = 0.010).
Table 11.
Apparent ileal amino acid digestibility in responses to the necrotic enteritis challenge and probiotic supplementation in broiler chickens at d 35.1
| Treatment |
Apparent ileal amino acid digestibility, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| Challenge | BA, g/t | Lys | Met | Cys | Arg | Val | Gly | Ser |
| No | 0 | 86.2 | 87.6 | 73.1 | 87.2 | 79.6 | 76.8 | 78.9 |
| No | 500 | 87.1 | 88.4 | 74.8 | 87.1 | 80.9 | 78.7 | 80.6 |
| Yes | 0 | 85.4 | 88.2 | 73.5 | 87.3 | 79.0 | 76.3 | 78.2 |
| Yes | 500 | 86.6 | 89.1 | 76.3 | 87.4 | 81.0 | 78.6 | 80.7 |
| SEM | 0.27 | 0.26 | 0.43 | 0.29 | 0.40 | 0.40 | 0.40 | |
| P-value | ||||||||
| Source | ||||||||
| Challenge | 0.257 | 0.224 | 0.211 | 0.718 | 0.764 | 0.656 | 0.726 | |
| BA | 0.067 | 0.104 | 0.006 | 0.993 | 0.047 | 0.010 | 0.010 | |
| Challenge × BA | 0.765 | 0.700 | 0.445 | 0.945 | 0.645 | 0.755 | 0.631 | |
| Main effect | ||||||||
| Challenge | ||||||||
| No | 86.6 | 88.0 | 73.9 | 87.1 | 80.3 | 77.8 | 79.7 | |
| Yes | 86.0 | 88.6 | 74.9 | 87.4 | 80.0 | 77.4 | 79.5 | |
| BA | ||||||||
| 0 | 85.9 | 87.9 | 73.3b | 87.2 | 79.4b | 76.6b | 78.6b | |
| 500 g/t | 86.9 | 88.7 | 75.5a | 87.3 | 81.0a | 78.6a | 80.6a | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed); Lys = lysine; Met = methionine; Cys = cysteine; Arg = arginine; Val = valine; Gly = glycine; Ser = serine.
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates.
The apparent ileal amino acid digestibility in responses to the CP level and BA supplementation (study 2) in challenged broiler chickens at d 35 are shown in Table 12. Supplementation of BA improved the digestibility of lysine (P = 0.014), cysteine (P = 0.013), and valine (P = 0.020). The RCP diet had significantly higher valine digestibility compared to SCP (P = 0.004). No interaction was observed between CP level and BA supplementation (P > 0.05).
Table 12.
Apparent ileal amino acid digestibility in responses to the crude protein level and probiotic supplementation in broiler chickens at d 35.1
| Treatment |
Apparent ileal amino acid digestibility, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| CP level | BA, g/t | Lys | Met | Cys | Arg | Val | Gly | Ser |
| Standard2 | 0 | 86.1 | 88.2 | 74.3 | 87.3 | 79.0 | 74.6 | 79.1 |
| Standard2 | 500 | 86.6 | 89.1 | 76.3 | 87.4 | 81.0 | 78.6 | 80.7 |
| Reduced3 | 0 | 83.9 | 88.0 | 73.2 | 88.0 | 81.6 | 74.6 | 76.6 |
| Reduced3 | 500 | 87.4 | 88.1 | 76.9 | 89.0 | 83.7 | 77.2 | 79.1 |
| SEM | 0.44 | 0.29 | 0.59 | 0.30 | 0.47 | 0.54 | 0.58 | |
| P-value | ||||||||
| Source | ||||||||
| CP | 0.404 | 0.402 | 0.896 | 0.058 | 0.004 | 0.079 | 0.079 | |
| BA | 0.014 | 0.451 | 0.013 | 0.337 | 0.020 | 0.075 | 0.075 | |
| CP × BA | 0.063 | 0.485 | 0.414 | 0.485 | 0.874 | 0.284 | 0.507 | |
| Main effect | ||||||||
| CP | ||||||||
| Standard2 | 86.3 | 88.6 | 75.2 | 87.4 | 80.1b | 77.8 | 79.8 | |
| Reduced3 | 85.7 | 88.1 | 75.1 | 88.5 | 82.5a | 76.0 | 77.9 | |
| BA | ||||||||
| 0 | 85.0b | 88.1 | 73.7b | 87.7 | 80.4b | 76.0 | 77.8 | |
| 500 g/t | 87.1a | 88.6 | 76.6a | 88.3 | 82.3a | 77.8 | 79.9 | |
BA = Bacillus amyloliquefaciens CECT 5940 (1.0 × 106 CFU/g of feed); Lys = lysine; Met = methionine; Cys = cysteine; Arg = arginine; Val = valine; Gly = glycine; Ser = serine.
a, bMeans in a column not sharing the same superscripts are significantly different according to Tukey test (P < 0.05).
Each value represents the mean of 8 replicates.
Standard crude protein level according to recommendation.
Reduced crude protein diets with 2% lower protein than standard diets.
4. Discussion
In the current study, the supplementation of BA as an in-feed probiotic improved growth performance and increased beneficial bacterial populations in the NE challenged chickens. It also improved FCR and enhanced ileal digestibility of several important amino acids in broilers fed diets containing different levels of protein under a subclinical necrotic enteritis challenge. The results of this study suggest that the inclusion of BA in broiler feed could act as an additive to alleviate the adverse effects of subclinical NE challenge and improve feed efficiency in birds fed with standard or RCP diets, thus leading to improved performance and production.
A successful subclinical NE challenge was induced after the inoculation of birds with Eimeria and C. perfringens shown by impaired performance and higher intestinal lesion scores, but no significant NE-induced mortality was observed (Mot et al., 2013). Birds fed BA supplemented diets had higher BWG and lower FCR compared to those fed the control diet. This improved performance observed as a result of the addition of BA has also been reported in other studies indicating the effectiveness of some BA strains as feed additives (Ahmed et al., 2014; Lei et al., 2015). Such improved growth performance by Bacillus spp. in broilers has primarily been related to their ability to regulate the intestinal microbiota. They can exert antimicrobial activity by producing antimicrobial peptides and lytic enzymes (Boottanun et al., 2017), resulting in a reduction of harmful bacterial colonization and further facilitating the intestinal micro-ecosystem (Guo et al., 2006). In agreement with our previous study (Gharib-Naseri et al., 2019), the current study showed that NE challenge led to a reduced population of beneficial bacteria such as Ruminococcus spp. and Bacillus spp. However, the supplementation of BA significantly increased the numbers of these two groups of bacteria (Ruminococcus spp. and Bacillus spp.) in the cecal contents.
Bacillus amyloliquefaciens can produce peptide antibiotics such as lantibiotic, subtilisin, and mersacidin that can suppress the growth of potentially pathogenic bacteria (Tang et al., 2018; Ulyanova et al., 2011), and this may lead to a more suitable intestinal environment for beneficial bacteria such a Ruminococcus spp. to develop. Changes in the microbiota have direct effect on the SCFA concentrations in the gut (den Besten et al., 2013). Cao et al. (2018) reported that supplementation of BA changed cecal metabolomics such as glyceride and amino acid contents and increased Ruminococcus spp. numbers in the ceca of broilers. The Ruminococcus spp. are known as butyrate-producing bacteria which utilize complex plant-derived carbohydrates, especially fibrous content, to produce butyrate (Apajalahti and Vienola, 2016). In the current results, the increased concentration of butyrate in both studies is in line with the increased numbers of Ruminococcus numbers. On the other hand, Ruminococcus spp. is also able to produce digestive enzymes such as cellulase, xylanase, and cellobiose (Flint et al., 1991; Pettipher and Latham, 1979; Saburi et al., 2010), therefore, can contribute to improved digestibility of nutrients and thus enhanced performance. Although the supplementation of BA affected the SCFA concentrations in the birds, however these changes did not have an impact on the intestinal pH. Metabolomic approaches have shown that addition of BA in broiler diets increases the concentrations of threonine, monopalmitin, and allose in broiler cecal digesta, which are all related to amino acid and glyceride metabolism (Cao et al., 2018).
Amino acid digestibility measurements showed that BA supplementation increased digestibility of amino acids, such as lysine, cysteine, valine, and serine. This increase is possibly due to the production and secretion of enzymes such as α-amylase, cellulase, lipase, and protease by BA strains (Deb et al., 2013; Kanmani et al., 2015; Lee et al., 2008). These enzymes can increase the digestibility of proteins, carbohydrates, and lipids in broilers (Salim et al., 2013). Farhat-Khemakhem et al. (2018) reported that BA could ferment and hydrolyse complex carbohydrates and oligosaccharides, some being anti-nutrients such as non-starch polysaccharides, which leads to improved digestibility of feed components. Furthermore, the positive effects of BA supplementation on nutrient digestibility have also been previously observed in pigs and mice (Blavi et al., 2019; Geeraerts et al., 2015). Positive alteration in gut microbiota, followed by improved digestibility of nutrients may be the underlying reason for the improved meat yield in this study.
In the current study, the effect of BA on C. perfringens reduction was observed in RCP diets. Previous reports have shown a robust activity of BA against C. perfringens in vitro (Geeraerts et al., 2016). It is also well known that C. perfringens proliferation is influenced by the presence of amino acids and protein in the diet (Titball et al., 1999) and there is a possible imbalance of amino acids other than the 6 essential ones supplemented in the RCP diets (Barekatain et al., 2018). Therefore, lowered amino acids may have contributed to the reduced C. perfringens together with BA. Wilkie et al. (2005) has shown that dietary amino acids such as glycine can be an important factor in C. perfringens growth in chicken intestine. The RCP diets may have unbalanced substrate for C. perfringens growth and reduced their ability to survive against BA.
Furthermore, quorum sensing (QS) communication plays a key role in triggering the infectious activity of pathogenic bacteria, such as sporulation, biofilm formation, and virulence (Cooksley et al., 2010). The BA strain can produce an enzyme, N-acyl-homoserine-lactone lactonase (AHL) (Yin et al., 2010), which hydrolyses the QS signaling molecules of Gram-negative bacteria and disturb their pathogenic activities (Alina et al., 2015; de Oliveira et al., 2018). The mechanism by which bacterial communication (QS) can be interrupted is referred to as quorum quenching (QQ). The QQ activity of the Clostridium family is known to be related to the Agr-like QS system, which plays a key role in the pathogenesis on NE (Yu et al., 2017). The information regarding QQ activity of Bacillus strains is scarce and further investigation can be suggested on possible QQ activity of this family of bacteria on Agr-like QS system activity.
The current study revealed that the NE challenge reduced the serum uric acid level. A similar reduction has been observed with Eimeria infections in broilers (Allen and Fetterer, 2002). The formation of each molecule of uric acid requires one molecule of glycine to build the purine ring (Patience, 1990), and RCP diets have shown to reduce the net portal fluxes of glycine in broiler chickens (Wu et al., 2018). The decrease in serum uric acid in the RCP fed birds is likely due to the reduced availability of glycine for uric acid synthesis in the liver (Namroud et al., 2008, Waldroup et al., 2005). Further, the lower uric acid levels in the NE challenged birds could be due to the lower feed intake on these group of birds (Donsbough et al., 2010). Considering that uric acid is produced at the last stage of protein metabolism, the lower feed consumption in the challenged birds means less substrate available for the production of uric acid. On the other hand, damaged intestinal villi caused by Eimeria and C. perfringens (Liu et al., 2012) and the negative effects of these infections on intestinal nutrient uptake can all lead to malabsorption of nutrients (Guo et al., 2014), which could be another reason for the lower serum uric acid level observed in the NE challenged birds compared to the non-challenged group.
Reducing dietary protein is an important way of reducing the amount of the undigested protein reaching the hindgut that increases the substrate available for infectious bacteria to proliferate. However, RCP diets are known to deteriorate broiler performance through sub-optimum supply of essential and nonessential amino acids (Aftab et al., 2006; Hilliar et al., 2020). Furthermore, the combination of an RCP diet and a subclinical NE infection can cause a further challenge for birds similar to those in the field situation. Therefore, adding gut health feed additives might be beneficial to mitigate the negative impact of these challenges.
Our results showed lower serum uric acid levels in birds fed RCP diets, as observed in other studies (Hernández et al., 2013; Swennen et al., 2011). El-Katcha et al. (2014) and Swennen et al. (2005) reported a negative correlation between plasma uric acid level and protein retention, which indicates a reduced serum uric acid level when proteins are utilized by birds to synthesis muscle. Protein retention was not measured in the current study; however, the lower uric acid level in the RCP-fed birds could mean higher retention of protein in these birds. Furthermore, the observed increase in fat pad percentage in birds fed with RCP diets is in agreement with previous studies (Hilliar et al., 2020). Barekatain et al. (2018) suggest that lowering CP can increase the starch content of the diet, thus, affect intestinal glucose transport and subsequent fat deposition in birds. As presented in previous studies, RCP diets reduce water intake (Alleman and Leclercq, 1997), which can lead to decreased litter moisture mostly by lowering the risk of water spillage by less frequent visits to the water lines. Birds fed with RCP diets had less litter moisture in the present results, which is in line with previous reports (Kamran et al., 2010). Wet litter and sticky droppings are key factors for the onset of ulceration of the feet leading to FD in broiler because they can soften the footpad whereas providing a suitable environment for microorganisms to grow (Martland, 1985; Shepherd and Fairchild, 2010). The FD lesions were mitigated by the use of RCP diets in the present study.
Based on the current findings, it may be concluded that supplementation of broiler diets with B. amyloliquefaciens CECT 5940 at a level of 500 g/t of diet showed benefits in promoting growth performance, improving gut microbiota profile and increasing amino acid digestibility in birds. Challenged birds fed with RCP diets supplemented with this probiotic showed improved performance traits, which could have been related to the reduced numbers of C. perfringens numbers and improved amino acid digestibility. Further studies are warranted to provide more evidence to elucidate the underlying mechanisms of how this Bacillus strain can improve the intestinal bacterial profile and amino acid digestibility, thus improving health and performance in broiler chickens.
Author contributions
Kosar Gharib-Naseri: animal trial, laboratory experiments, statistical analysis, study designed and writing. Juliano Cesar de Paula Dorigam: study design, feed formulation, data evaluation, manuscript review. Kiran Doranalli: study design, feed formulation, manuscript review. Natalie Morgan: methodology, data collection, manuscript review. Robert A. Swick: study design, data evaluation, data analysis, manuscript review. Mingan Choct: study design, manuscript review. Shu-Biao Wu: coordinator, study design, data collection, statistical analyses, critical manuscript review.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The authors thank Evonik Nutrition & Care GmbH, Germany for funding this project, Ms. Petrina Young of Eimeria Pty Ltd for providing Eimeria species, Prof. Robert Moore for providing Clostridium perfringens EHE-18, and Mrs. Shuyu Song for her help and guidance with the lab work.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aftab U., Ashraf M., Jiang Z. Low protein diets for broilers. Worlds Poult Sci J. 2006;62:688–701. [Google Scholar]
- Ahmed S.T., Islam M., Mun H.S., Sim H.J., Kim Y.J., Yang C.J. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult Sci. 2014;93:1963–1971. doi: 10.3382/ps.2013-03718. [DOI] [PubMed] [Google Scholar]
- Alina S.O., Constantinscu F., Petruţa C.C. Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Rom Biotechnol Lett. 2015;20:10737–10750. [Google Scholar]
- Allain V., Mirabito L., Arnould C., Colas M., Le Bouquin S., Lupo C. Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. Br Poult Sci. 2009;50:407–417. doi: 10.1080/00071660903110901. [DOI] [PubMed] [Google Scholar]
- Alleman F., Leclercq B. Effect of dietary protein and environmental temperature on growth performance and water consumption of male broiler chickens. Br Poult Sci. 1997;38:607–610. doi: 10.1080/00071669708418044. [DOI] [PubMed] [Google Scholar]
- Allen P., Fetterer R. Interaction of dietary vitamin E with Eimeria maxima infections in chickens. Poult Sci. 2002;81:41–48. doi: 10.1093/ps/81.1.41. [DOI] [PubMed] [Google Scholar]
- Angel R., Dalloul R., Doerr J. Performance of broiler chickens fed diets supplemented with a direct-fed microbial. Poult Sci. 2005;84:1222–1231. doi: 10.1093/ps/84.8.1222. [DOI] [PubMed] [Google Scholar]
- AOAC . 18th edition. Association of Official Analytical Chemists; Arlington, VA, USA: 2005. Official Methods of Analysis. [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim Feed Sci Technol. 2016;221:323–330. [Google Scholar]
- Aviagen . 2014. Ross Broiler Management Manual; pp. 10–35. [Google Scholar]
- Barekatain R., Nattrass G., Tilbrook A., Chousalkar K., Gilani S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult Sci. 2018;0:1–14. doi: 10.3382/ps/pey563. [DOI] [PubMed] [Google Scholar]
- Barker K., Coufal C., Purswell J., Davis J., Parker H., Kidd M. In-house windrowing of a commercial broiler farm during early spring and its effect on litter composition. J Appl Poult Res. 2013;22:551–558. [Google Scholar]
- Blavi L., Jørgensen J.N., Stein H.H. Effects of Bacillus amyloliquefaciens and Bacillus subtilis on ileal digestibility of AA and total tract digestibility of CP and gross energy in diets fed to growing pigs. J Anim Sci. 2019;97:727–734. doi: 10.1093/jas/sky432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boottanun P., Potisap C., Hurdle J.G., Sermswan R.W. Secondary metabolites from Bacillus amyloliquefaciens isolated from soil can kill Burkholderia pseudomallei. AMB Express. 2017;7:1–11. doi: 10.1186/s13568-016-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard C.T., Hofacre C.L., Page R.K., Fletcher O.J. Necrotic enteritis in cage-reared commercial layer pullets. Avian Dis. 1986;30:617–619. [PubMed] [Google Scholar]
- Cao G., Zhan X., Zhang L., Zeng X., Chen A., Yang C. Modulation of broilers' caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J Anim Physiol Anim Nutr. 2018;102:909–917. doi: 10.1111/jpn.12856. [DOI] [PubMed] [Google Scholar]
- Cartman S.T., La Ragione R.M., Woodward M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl Environ Microbiol. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksley C.M., Davis I.J., Winzer K., Chan W.C., Peck M.W. Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic Clostridium botulinum. Appl Environ Microbiol. 2010;76:4448–4460. doi: 10.1128/AEM.03038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Namroud N., Shivazad M., Zaghari M. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poult Sci. 2008;87:2250–2258. doi: 10.3382/ps.2007-00499. [DOI] [PubMed] [Google Scholar]
- NHMRC . 8th edition. 2013. Australian code for the care and use of animals for scientific purposes. [Google Scholar]
- de Oliveira M.J.K., Sakomura N.K., de Paula Dorigam J.C., Doranalli K., Soares L., Viana GdS. Poult Sci A 2nd Latin American Scientific Conference. 2018. Bacillus amyloliquefaciens CECT 5940 alone or in combination with antibiotic growth promoters improves performance in broilers under enteric pathogen challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb P., Talukdar S.A., Mohsina K., Sarker P.K., Sayem S.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus. 2013;2:154. doi: 10.1186/2193-1801-2-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsbough A., Powell S., Waguespack A., Bidner T., Southern L. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult Sci. 2010;89:287–294. doi: 10.3382/ps.2009-00401. [DOI] [PubMed] [Google Scholar]
- El-Katcha M.I., Soltan M.A., El-Kaney H.F., Karwarie E. Growth performance, blood parameters, immune response and carcass traits of broiler chicks fed on graded levels of wheat instead of corn without or with enzyme supplementation. Alex J Vet Sci. 2014;40:95–111. [Google Scholar]
- Farhat-Khemakhem A., Blibech M., Boukhris I., Makni M., Chouayekh H. Assessment of the potential of the multi-enzyme producer Bacillus amyloliquefaciens US573 as alternative feed additive. J Sci Food Agric. 2018;98:1208–1215. doi: 10.1002/jsfa.8574. [DOI] [PubMed] [Google Scholar]
- Flint H.J., McPherson C.A., Martin J. Expression of two xylanase genes from the rumen cellulolytic bacterium Ruminococcus flavefaciens 17 cloned in pUC13. Microbiology. 1991;137:123–129. doi: 10.1099/00221287-137-1-123. [DOI] [PubMed] [Google Scholar]
- Geeraerts S., Delezie E., Ducatelle R., Haesebrouck F., Devreese B., Van Immerseel F. Vegetative Bacillus amyloliquefaciens cells do not confer protection against necrotic enteritis in broilers despite high antibacterial activity of its supernatant against Clostridium perfringens in vitro. Br Poult Sci. 2016;57:324–329. doi: 10.1080/00071668.2016.1169246. [DOI] [PubMed] [Google Scholar]
- Geeraerts S., Ducatelle R., Haesebrouck F., Van Immerseel F. Bacillus amyloliquefaciens as prophylactic treatment for Clostridium difficile-associated disease in a mouse model. J Gastroenterol Hepatol. 2015;30:1275–1280. doi: 10.1111/jgh.12957. [DOI] [PubMed] [Google Scholar]
- Gharib-Naseri K., Kheravii S.K., Keerqin C., Morgan N., Swick R., Wu S. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult Sci. 2019;0:1–11. doi: 10.3382/ps/pez480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Liu D., Zhao X., Li C., Guo Y. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poult Sci. 2014;93:94–103. doi: 10.3382/ps.2013-03188. [DOI] [PubMed] [Google Scholar]
- Guo X., Li D., Lu W., Piao X., Chen X. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek. 2006;90:139–146. doi: 10.1007/s10482-006-9067-9. [DOI] [PubMed] [Google Scholar]
- Hernandez F., Lopez M., Martinez S., Megias M.D., Catala P., Madrid J. Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1- to 48-day-old broilers. Poult Sci. 2012;91:683–692. doi: 10.3382/ps.2011-01735. [DOI] [PubMed] [Google Scholar]
- Hernández F., Megias M., Orengo J., Martinez S., Lopez M., Madrid J. Effect of dietary protein level on retention of nutrients, growth performance, litter composition and NH3 emission using a multi-phase feeding programme in broilers. Span J Agric Res. 2013;11:736–746. [Google Scholar]
- Hilliar M., Hargreave G., Girish C., Barekatain R., Wu S.-B., Swick R. Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets. Poult Sci. 2020;99:1551–1563. doi: 10.1016/j.psj.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M., Cox R., Jensen B.B. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. J Anim Sci. 1995;61:293–304. [Google Scholar]
- Kadaikunnan S., Rejiniemon T.S., Khaled J.M., Alharbi N.S., Mothana R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob. 2015;14:1–11. doi: 10.1186/s12941-015-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran Z., Sarwar M., Nisa M., Nadeem M.A., Mahmood S. Effect of low levels of dietary crude protein with constant metabolizable energy on nitrogen excretion, litter composition and blood parameters of broilers. Int J Agric Biol. 2010;12:401–405. [Google Scholar]
- Kanmani P., Kumaresan K., Aravind J. Gene cloning, expression, and characterization of the Bacillus amyloliquefaciens PS35 lipase. Braz J Microbiol. 2015;46:1235–1243. doi: 10.1590/S1517-838246420141068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoch S., Dev S., Rajput R. Effect of probiotic supplementation in broiler birds offered feed formulated with lower protein densities. Int J Livest Res. 2017;5:90–101. [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:1–11. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Effect of oat hulls as a free choice feeding on broiler performance, short chain fatty acids and microflora under a mild necrotic enteritis challenge. Anim Nut. 2018;4:65–72. doi: 10.1016/j.aninu.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.B. Potential of pelleted wheat straw as an alternative bedding material for broilers. Poult Sci. 2017;96:1641–1647. doi: 10.3382/ps/pew473. [DOI] [PubMed] [Google Scholar]
- Layton A., McKay L., Williams D., Garrett V., Gentry R., Sayler G. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Kim B.K., Lee B.H., Jo K.I., Lee N.K., Chung C.H. Purification and characterization of cellulase produced by Bacillus amyoliquefaciensdl-3 utilizing rice hull. Bioresour Technol. 2008;99:378–386. doi: 10.1016/j.biortech.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Lei X., Piao X., Ru Y., Zhang H., Peron A., Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australas J Anim Sci. 2015;28:239–246. doi: 10.5713/ajas.14.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo S., Guo Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012;41:291–298. doi: 10.1080/03079457.2012.684089. [DOI] [PubMed] [Google Scholar]
- Martland M. Ulcerative dermatitis in broiler chickens: the effects of wet litter. Avian Pathol. 1985;14:353–364. doi: 10.1080/03079458508436237. [DOI] [PubMed] [Google Scholar]
- Mot D., Timbermont L., Delezie E., Haesebrouck F., Ducatelle R., Van Immerseel F. Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathol. 2013;42:179–184. doi: 10.1080/03079457.2013.778955. [DOI] [PubMed] [Google Scholar]
- Murugesan G.R., Gabler N.K., Persia M.E. Effects of direct-fed microbial supplementation on broiler performance, intestinal nutrient transport and integrity under experimental conditions with increased microbial challenge. Br Poult Sci. 2014;55:89–97. doi: 10.1080/00071668.2013.865834. [DOI] [PubMed] [Google Scholar]
- Patience J. A review of the role of acid-base balance in amino acid nutrition. J Anim Sci. 1990;68:398–408. doi: 10.2527/1990.682398x. [DOI] [PubMed] [Google Scholar]
- Pettipher G.L., Latham M.J. Characteristics of enzymes produced by Ruminococcus flavefaciens which degrade plant cell walls. Microbiology. 1979;110:21–27. [Google Scholar]
- Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2008;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- Requena T., Burton J., Matsuki T., Munro K., Simon M.A., Tanaka R. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl Environ Microbiol. 2002;68:2420–2427. doi: 10.1128/AEM.68.5.2420-2427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva O.N., Dixelius C., Meijer J., Priest F.G. Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiol Ecol. 2004;48:249–259. doi: 10.1016/j.femsec.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Saburi W., Yamamoto T., Taguchi H., Hamada S., Matsui H. Practical preparation of epilactose produced with cellobiose 2-epimerase from Ruminococcus albus NE1. Biosci Biotechnol Biochem. 2010;74:1003531–10035312. doi: 10.1271/bbb.100353. [DOI] [PubMed] [Google Scholar]
- Salim H., Kang H., Akter N., Kim D., Kim J., Kim M. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci. 2013;92:2084–2090. doi: 10.3382/ps.2012-02947. [DOI] [PubMed] [Google Scholar]
- Shepherd E., Fairchild B. Footpad dermatitis in poultry. Poult Sci. 2010;89:2043–2051. doi: 10.3382/ps.2010-00770. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Pumford N.R., Morgan M.J., Wolfenden R.E., Wolfenden A.D., Torres-Rodriguez A. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult Sci. 2011;90:1574–1580. doi: 10.3382/ps.2010-00745. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res. 2012;43:1–12. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short F., Gorton P., Wiseman J., Boorman K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 1996;59:215–221. [Google Scholar]
- Suartika I.G., Sumadi I., Bidura I. Effect of probiotic supplementation on low protein diet on broiler performance. E-J Anim Sci Uday Uni. 2014;3:1–10. [Google Scholar]
- Swennen Q., Geraert P.A., Mercier Y., Everaert N., Stinckens A., Willemsen H. Effects of dietary protein content and 2-hydroxy-4-methylthiobutanoic acid or dl-methionine supplementation on performance and oxidative status of broiler chickens. Br J Nutr. 2011;106:1845–1854. doi: 10.1017/S0007114511002558. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Janssens G., Millet S., Vansant G., Decuypere E., Buyse J. Effects of substitution between fat and protein on feed intake and its regulatory mechanisms in broiler chickens: endocrine functioning and intermediary metabolism. Poult Sci. 2005;84:1051–1057. doi: 10.1093/ps/84.7.1051. [DOI] [PubMed] [Google Scholar]
- Tang R.Y., Wu Z.L., Wang G.Z., Liu W.C. The effect of Bacillus amyloliquefaciens on productive performance of laying hens. Ital J Anim Sci. 2018;17:436–441. [Google Scholar]
- Titball R.W., Naylor C.E., Basak A.K. The Clostridium perfringens α-toxin. Anaerobe. 1999;5:51–64. doi: 10.1006/anae.1999.0191. [DOI] [PubMed] [Google Scholar]
- Torres-Rodriguez A., Sartor C., Higgins S., Wolfenden A., Bielke L., Pixley C. Effect of Aspergillus meal prebiotic (fermacto) on performance of broiler chickens in the starter phase and fed low protein diets. J Appl Poult Res. 2005;14:665–669. [Google Scholar]
- Ulyanova V., Vershinina V., Ilinskaya O. Barnase and binase: twins with distinct fates. FEBS J. 2011;278:3633–3643. doi: 10.1111/j.1742-4658.2011.08294.x. [DOI] [PubMed] [Google Scholar]
- Vuong C.N., Chou W.-K., Hargis B.M., Berghman L.R., Bielke L.R. Role of probiotics on immune function and their relationship to antibiotic growth promoters in poultry, a brief review. Int J Probiotics Prebiotics. 2016;11:1–6. [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Waldroup P., Jiang Q., Fritts C. Effects of supplementing broiler diets low in crude protein with essential and nonessential amino acids. Int J Poult Sci. 2005;4:425–431. [Google Scholar]
- Welker N., Campbell L.L. Comparison of the α-amylase of Bacillus subtilis and Bacillus amyloliquefaciens. J Bacteriol. 1967;94:1131–1135. doi: 10.1128/jb.94.4.1131-1135.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie D.C., Van Kessel A.G., White L.J., Laarveld B., Drew M.D. Dietary amino acids affect intestinal Clostridium perfringens populations in broiler chickens. Can J Anim Sci. 2005;85:185–193. [Google Scholar]
- Wise M., Siragusa G. Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol. 2007;102:1138–1149. doi: 10.1111/j.1365-2672.2006.03153.x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. Rev Bras Cienc Avic. 2018;9:1–14. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.T., Xu L., Fan S.S., Xu L.N., Li D.C., Liu Z.Y. Isolation and characterization of an AHL lactonase gene from Bacillus amyloliquefaciens. World J Microbiol Biotechnol. 2010;26:1361–1367. [Google Scholar]
- Yu Q., Lepp D., Gohari I.M., Wu T., Zhou T., Yin X. The Agr-like quorum sensing system is required for necrotic enteritis pathogenesis in poultry caused by Clostridium perfringens. Infect Immun. 2017:1–16. doi: 10.1128/IAI.00975-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen D., Yu B., He J., Yu J., Mao X. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J Anim Sci. 2015;93:2967–2976. doi: 10.2527/jas.2014-8820. [DOI] [PubMed] [Google Scholar]