Abstract
The objective of this study was to develop equations for estimating ileal digestible crude protein (CP) and metabolizable energy (ME) contents of meat meal (MM) and meat and bone meal (MBM) as feed ingredients for pigs based on in vitro assays. Test ingredients were 4 sources of MM and 3 sources of MBM. Ash and CP contents of the ingredients ranged from 3.8% to 33.1% and 46.8% to 82.9% (as-is basis), respectively. In vitro ileal disappearance (IVID) of CP was determined and ileal digestible CP content was calculated by multiplying CP content by IVID of CP. In vitro total tract disappearance (IVTTD) of dry matter (DM) was determined and ME was calculated using gross energy, CP contents, and IVTTD of DM. The IVID of CP and IVTTD of DM ranged from 77.2% to 88.7% and from 82.7% to 92.4%, respectively. Calculated ileal digestible CP and ME contents ranged from 37.8% to 73.5% DM and 2,405 to 3,905 kcal/kg DM, respectively. Ash contents were negatively correlated (P < 0.001) with CP (r = −0.99), in vitro ileal digestible CP (r = −0.97), gross energy (r = −1.00), in vitro digestible energy (r = −0.97), and adjusted ME (r = −0.97). The most fitting equations for ileal digestible CP and adjusted ME were: ileal digestible CP (% DM) = 11.91 − 0.90 × Ash (% DM) + 0.74 × IVID of CP (%) (R2 = 0.99) and adjusted ME (kcal/kg DM) = 130.85 − 50.90 × ash (% DM) + 47.06 × IVTTD of DM (%) (R2 = 0.99). To validate the accuracy of the prediction equations for ME, mean bias and linear bias were determined using a regression analysis. Calculated ME values of MM and MBM were in a good agreement with data obtained from animal experiments based on a statistically insignificant bias in the models. In conclusion, ME concentrations of MM and MBM as swine feed ingredients can be calculated using ash concentration and in vitro disappearance of dry matter.
Keywords: Animal offal, Energy, In vitro disappearance method, Protein
1. Introduction
Animal offal is defined as rendered products from mammal tissues with or without bone, exclusive of blood, hair, hoof, horn, hide trimmings, manure, stomach, and rumen contents (AAFCO, 2016). As rendered products including meat meal (MM) and meat and bone meal (MBM) contain high energy and crude protein (CP) concentrations, these ingredients can be used as energy and protein sources in swine diets (Hendriks et al., 2002). In addition, the indispensable amino acid proportions in MM and MBM are very similar to the requirements of pigs (NRC, 2012).
An accurate nutritional evaluation for swine diets is important to maximize growth performance and to minimize nitrogen excretion and feed costs (Kil et al., 2013; Lee et al., 2017; Son et al., 2019). Nutritional values of MM and MBM reported in the literature are highly variable likely due to different processing methods and sources of raw material (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009; Wang and Parsons, 1998). For this reason, prediction equations for estimating metabolizable energy (ME) of MBM in pigs have been developed using chemical compositions of MBM (Castilho et al., 2015; Olukosi and Adeola, 2009).
In vitro assays are highly correlated with data obtained from animal experiments and are time-saving and inexpensive for estimating ileal digestible amino acids (AA; Boisen and Fernández, 1995) and energy digestibility values (Boisen and Fernández, 1997; Park et al., 2012) of feed ingredients. To our knowledge, very limited information is available on the relationship between nutritional values and in vitro disappearance of nutrients in MM and MBM with a wide range of nutrient compositions. Therefore, the objective of the present study was to develop novel prediction equations for estimating ileal digestible CP and ME concentrations in MM and MBM fed to pigs based on in vitro assays.
2. Materials and methods
2.1. Test ingredients and in vitro disappearance assays
The ingredients tested in the present work were 4 sources of MM and 3 sources of MBM originated from pig offal. These samples were finely ground using a coffee grinder and used for in vitro assays and chemical analyses. To simulate digestion and absorption processes in the stomach and the small intestine, in vitro ileal disappearance (IVID) of dry matter (DM) in the test ingredients was determined using a 2-step procedure (Boisen and Fernández, 1995). Briefly, 1 g of a ground ingredient sample was transferred into 100-mL conical flasks. A 25 mL of sodium phosphate buffer solution (0.1 mol/L, pH 6.0) and 10 mL of HCl (0.2 mol/L, pH 0.7) were added. To simulate digestion conditions in the stomach, the pH was adjusted 2.0, and 1 mL of freshly prepared pepsin solution (10 mg/mL; ≥250 units/mg solid, P7000, pepsin from porcine gastric mucosa, Sigma–Aldrich, St. Louis, MO, USA) was added to the samples. Test flasks were incubated in a shaking incubator at 39 °C for 6 h as the final procedure of the first step. After the incubation, 10 mL of sodium phosphate buffer solution (0.2 mol/L, pH 6.8) and 5 mL of NaOH (0.6 mol/L, pH 13.8) were added. Then, the pH was adjusted to 6.8. Thereafter, 1 mL of freshly prepared pancreatin solution (50 mg/mL; 4 × USP, P1750, pancreatin from porcine pancreas, Sigma–Aldrich, St. Louis, MO, USA) was added. After incubation in a shaking incubator at 39 °C for 18 h, 5 mL of 20% sulfosalicylic acid solution was added and samples were left for 30 min at room temperature to precipitate the indigestible protein. The samples were then filtered through pre-dried and pre-weighed glass filter crucibles (Filter Crucibles CFE Por. 2, Robu, Hattert, Germany) containing 400 mg of celite. Test flasks were rinsed twice with 1% sulfosalicylic acid solution, and 20 mL of 95% ethanol and 99.5% acetone were added twice to the glass filter crucibles. Glass filter crucibles with undigested residues were dried at 80 °C for 24 h. After conducting the 2-step procedure, undigested residues on filter crucibles were collected for analyzing CP concentration to determine IVID of CP (Akonjuen et al., 2019).
To simulate total tract digestion and absorption, in vitro total tract disappearance (IVTTD) of DM in the ingredients was determined using 3-step procedure (Boisen and Fernández, 1997). The first and second steps were similar to the IVID procedure. In the third step of the IVTTD procedure, 10 mL of 0.2 mol/L EDTA solution was added to the samples. The pH was then adjusted to 4.8. Samples were supplemented with 0.5 mL of multi-enzyme (V2010, Viscozyme, Sigma–Aldrich, St. Louis, MO, USA) as a substitute for microbial enzymes, and incubated in a shaking incubator for 18 h at 39 °C. After incubation, the samples were then filtered and the undigested residues were collected and dried as previously described in the IVID procedure except for the drying condition (at 130 °C for 6 h).
2.2. Chemical analysis
The test ingredients were analyzed for gross energy (GE) using a bomb calorimetry (Parr 1261, Parr Instrument Co., Moline, IL, USA). Dry matter in the test ingredients was analyzed (Ahn et al., 2014). Crude protein (method 990.03), ether extract (EE; method 920.39), and ash (method 942.05) in the test ingredients were also analyzed as described in AOAC International (2016). The test ingredients were also analyzed for calcium (Ca; method 978.02) and phosphorus (P; method 946.06) concentrations using a Perkin Elmer Avio 200 ICP-OES (Perkin Elmer, Shelton, CT, USA) as described in AOAC International (2016). Amino acids in the test ingredients were liberated from the protein by hydrolysis with 6 mol/L HCl at 110 °C for 24 h under nitrogen atmosphere. Performic acid oxidation occurred before acid hydrolysis for methionine and cysteine. Amino acids in hydrolysates were determined by HPLC after post-column derivatization (method 982.30; AOAC International, 2016).
2.3. Calculation and statistical analysis
The IVID or IVTTD of DM and IVID of CP were calculated with the following equations (Akonjuen et al., 2019):
| IVID or IVTTD of DM (%) = [(DMTI − DMUR)/DMTI] × 100 , |
where DMTI and DMUR are the weight (g) of DM concentrations in the test ingredient and undigested residues, respectively.
| IVID of CP (%) = [(DMTI × CPTI) − (DMUR × CPUR)]/(DMTI × CPTI) × 100, |
where CPTI and CPUR are CP concentrations (%) expressed as DM basis in the test ingredient and undigested residues, respectively.
Based on the determined IVTTD of DM and GE values, in vitro digestible energy (IVDE) in the feed ingredient was calculated with the following equation:
| IVDE (kcal/kg DM) = IVTTD of DM (%) × GE (kcal/kg DM). |
Metabolizable energy was calculated using an equation as follows (Sung and Kim, 2021):
| ME (kcal/kg DM) = 0.97 × IVDE (kcal/kg DM) – 3.86 × CP (% DM). |
A correction factor based on the average value of ME:GE (0.652) of MBM (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009) was used to make an adjusted ME value with a following equation:
| Adjusted ME (kcal/kg DM) = ME (kcal/kg DM) – 659. |
Data were analyzed using Mixed procedure of SAS (SAS Inst. Inc., Cary, NC, USA). The model included a test ingredient as a fixed variable. Least square means for IVID of DM, IVID CP, and IVTTD of DM for each test ingredient were calculated. Correlation coefficients among the chemical compositions (GE, CP, and ash), IVID of DM and CP, IVTTD of DM, in vitro ileal digestible crude protein (IVID CP), IVDE, and adjusted ME in the test ingredient were determined by CORR procedure of SAS. Prediction equations for ileal digestible CP and ME of the test ingredient were generated by PROC REG of SAS using ileal digestible CP or ME as a dependent variable and in vitro disappearance of CP or DM and ash in the test ingredients as independent variables. The statistical significance and tendency were determined as P < 0.05 and P < 0.10, respectively. Redundant variables were excluded based on root mean square error, R2, and P-values. The accuracy of prediction equations for ME concentrations of the present work was assessed by regressing the published ME values (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009) of MM and MBM minus the calculated ME values on each calculated value centered to the mean (Sung and Kim, 2019). To validate the accuracy of prediction equations for ME, only data corresponding to the range of nutrient compositions used for developing equations of the present work were employed.
3. Results
Gross energy, CP, and ash concentrations in 4 sources of MM and 3 sources of MBM ranged from 3,701 to 5,912 kcal/kg, 46.8% to 82.9%, and 3.8% to 33.1% (as-is basis), respectively (Table 1). The AA contents in MM were greater than those in MBM. However, the concentrations of Ca and P of MM were less than those of MBM. In vitro ileal disappearance of CP and IVTTD of DM in MM and MBM ranged from 77.2% to 88.7% and from 82.7% to 92.4%, respectively (Table 2). Ileal digestible CP and adjusted ME concentrations of MM and MBM ranged from 37.8% to 73.5% DM and 2,405 to 3,905 kcal/kg DM, respectively (Table 3).
Table 1.
Analyzed energy concentration and chemical composition of 4 sources of meat meal and 3 sources of meat and bone meal (as-is basis, %).
| Item | Meat meal |
Meat and bone meal1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | 1 | 2 | 3 | Mean | |
| Dry matter | 98.54 | 98.41 | 97.93 | 97.84 | 98.18 | 97.44 | 97.02 | 95.56 | 96.67 |
| GE, kcal/kg | 5,857 | 5,912 | 4,843 | 4,797 | 5,352 | 4,573 | 4,180 | 3,701 | 4,151 |
| CP | 82.58 | 82.92 | 65.72 | 62.91 | 73.53 | 61.65 | 55.68 | 46.82 | 54.71 |
| Ether extract | 16.16 | 15.20 | 12.56 | 12.38 | 14.07 | 12.39 | 10.95 | 13.81 | 12.38 |
| Ash | 3.80 | 4.19 | 17.41 | 20.25 | 11.41 | 22.03 | 29.58 | 33.07 | 28.23 |
| Indispensable amino acid | |||||||||
| Arginine | 5.45 | 5.28 | 4.35 | 4.12 | 4.80 | 3.98 | 3.67 | 3.17 | 3.60 |
| Histidine | 1.65 | 1.69 | 1.20 | 1.23 | 1.44 | 1.18 | 0.98 | 0.70 | 0.95 |
| Isoleucine | 2.06 | 2.09 | 1.69 | 1.67 | 1.87 | 1.57 | 1.31 | 0.87 | 1.25 |
| Leucine | 4.52 | 4.58 | 3.84 | 3.72 | 4.16 | 3.54 | 2.96 | 2.10 | 2.87 |
| Lysine | 4.11 | 4.08 | 3.26 | 3.12 | 3.64 | 2.99 | 2.67 | 1.84 | 2.50 |
| Methionine | 1.27 | 1.23 | 1.01 | 0.95 | 1.11 | 0.94 | 0.81 | 0.57 | 0.77 |
| Phenylalanine | 2.48 | 2.49 | 2.13 | 2.05 | 2.29 | 1.96 | 1.68 | 1.26 | 1.63 |
| Threonine | 2.50 | 2.54 | 2.15 | 2.05 | 2.31 | 1.97 | 1.69 | 1.24 | 1.63 |
| Valine | 2.91 | 2.92 | 2.50 | 2.42 | 2.69 | 2.29 | 1.99 | 1.47 | 1.92 |
| Dispensable amino acid | |||||||||
| Alanine | 5.81 | 5.62 | 4.65 | 4.57 | 5.16 | 4.35 | 4.08 | 3.46 | 3.96 |
| Aspartic acid | 5.70 | 5.70 | 4.77 | 4.55 | 5.18 | 4.39 | 3.87 | 3.00 | 3.75 |
| Glutamic acid | 9.78 | 9.77 | 7.97 | 7.81 | 8.83 | 7.55 | 6.63 | 5.26 | 6.48 |
| Glycine | 10.83 | 10.12 | 8.39 | 7.98 | 9.33 | 7.68 | 7.67 | 7.29 | 7.55 |
| Proline | 7.05 | 6.64 | 5.40 | 5.08 | 6.04 | 4.90 | 4.84 | 4.48 | 4.74 |
| Serine | 2.92 | 2.91 | 2.52 | 2.31 | 2.66 | 2.24 | 2.03 | 1.64 | 1.97 |
| Tyrosine | 1.69 | 1.71 | 1.47 | 1.40 | 1.57 | 1.36 | 1.06 | 0.74 | 1.05 |
| Calcium | 0.32 | 0.47 | 5.70 | 6.67 | 3.29 | 8.28 | 11.03 | 14.37 | 11.23 |
| Phosphorus | 0.62 | 0.73 | 3.13 | 3.59 | 2.02 | 4.36 | 5.41 | 6.97 | 5.58 |
GE = gross energy; CP = crude protein.
Meat and bone meal was defined as rendered product from mammal tissues containing minimum of 4.0% phosphorus (AAFCO, 2016).
Table 2.
In vitro ileal disappearance (IVID) of crude protein (CP) and in vitro total tract disappearance (IVTTD) of DM in meat meal and meat and bone meal (%).1
| Item | Meat meal |
Meat and bone meal |
SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | |||
| IVID of DM | 87.0ab | 85.1b | 88.8a | 81.8c | 82.6c | 86.7ab | 78.0d | 0.47 | <0.001 |
| IVID of CP | 87.7ab | 84.7b | 88.7a | 80.4c | 80.1c | 88.0ab | 77.2c | 0.74 | <0.001 |
| IVTTD of DM | 84.8cd | 82.7d | 89.5b | 85.2c | 85.0c | 92.4a | 86.6c | 0.46 | <0.001 |
SEM = standard error of the mean.
a-d Within a row means without a common superscript differ (P < 0.05).
Each least square mean represents 3 observations.
Table 3.
Calculated energy (kcal/kg DM) and in vitro ileal digestible crude protein (IVID CP, % DM) concentrations of 4 sources of meat meal and 3 sources of meat and bone meal.
| Item | Meat meal |
Meat and bone meal |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | |
| IVID CP1 | 73.5 | 71.3 | 59.5 | 51.7 | 50.7 | 50.5 | 37.8 |
| IVDE2 | 5,040 | 4,966 | 4,426 | 4,179 | 3,988 | 3,979 | 3,355 |
| ME3 | 4,565 | 4,492 | 4,034 | 3,806 | 3,624 | 3,638 | 3,065 |
| Adjusted ME4 | 3,905 | 3,832 | 3,375 | 3,146 | 2,964 | 2,979 | 2,405 |
IVDE = in vitro digestible energy; ME = metabolizable energy.
IVID CP = concentration of CP × in vitro ileal disappearance of CP.
IVDE = concentration of gross energy × in vitro total tract disappearance of DM.
ME = 0.97 × IVDE (kcal/kg DM) – 3.86 × CP concentration (% DM) (Sung and Kim, 2021).
Adjusted ME energy was calculated using gross energy-to-ME ratio (0.652) based on in vivo data (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009).
Ash was negatively correlated (P < 0.001) with CP (r = −0.99), IVID CP (r = −0.97), GE (r = −1.00), IVDE (r = −0.97), and adjusted ME (r = −0.97; Table 4). The most fitting equations for ileal digestible CP and ME of MM and MBM were as follows:
| Ileal digestible CP (% DM) = 11.91 − 0.90 × ash (% DM) + 0.74 × IVID of CP (%) (R2 = 0.99; Eq. 1 in Table 5) |
| Adjusted ME (kcal/kg DM) = 130.85 – 50.90 × ash (% DM) + 47.06 × IVTTD of DM (%) (R2 = 0.99; Eq. 3 in Table 5). |
Table 4.
Correlation coefficients between chemical components and in vitro ileal disappearance (IVID) of crude protein (CP), in vitro ileal digestible CP (IVID CP), in vitro total tract disappearance of dry matter (DM), in vitro digestible energy (IVDE), and adjusted metabolizable energy (ME) of meat meal and meat and bone meal (n = 7).
| Item | Correlation coefficient (r) |
|||||||
|---|---|---|---|---|---|---|---|---|
| EE | CP | IVID of CP | IVID CP | GE | IVTTD of DM | IVDE | Adjusted ME | |
| Ash | –0.68 | –0.99∗∗∗ | 0.47 | –0.97∗∗∗ | –1.00∗∗∗ | 0.60 | –0.97∗∗∗ | –0.97∗∗∗ |
| EE | 0.65 | 0.00 | 0.59 | 0.65 | –0.71 | 0.55 | 0.54 | |
| CP | 0.51 | 0.98∗∗∗ | 1.00∗∗∗ | –0.56 | 0.98∗∗∗ | 0.98∗∗∗ | ||
| IVID of CP | 0.66 | 0.48 | 0.42 | 0.65 | 0.66 | |||
| IVID CP | 0.97∗∗∗ | –0.40 | 1.00∗∗∗ | 0.99∗∗∗ | ||||
| GE | –0.59 | 0.98∗∗∗ | 0.97∗∗∗ | |||||
| IVTTD of DM | –0.41 | –0.39 | ||||||
| IVDE | 1.00∗∗∗ | |||||||
EE = ether extract; GE = gross energy.
∗∗∗P < 0.001.
Table 5.
Regression equations for ileal digestible crude protein (CP, % DM) and metabolizable energy (ME, kcal/kg DM) concentration of meat meal and meat and bone meal.
| Item | Regression coefficient parameter |
Statistical parameter |
|||||
|---|---|---|---|---|---|---|---|
| Intercept | Ash | In vitro disappearance1 | RMSE | R2 | P-value | ||
| Ileal digestible CP | Eq. 1 | 11.91 | –0.90 | 0.74 | 1.25 | 0.99 | <0.001 |
| SE | 11.06 | 0.05 | 0.13 | – | – | – | |
| P-value | 0.342 | <0.001 | 0.004 | – | – | – | |
| Eq. 2 | 76.29 | –1.04 | – | 3.45 | 0.94 | <0.001 | |
| SE | 2.63 | 0.12 | – | – | – | – | |
| P-value | <0.001 | <0.001 | – | – | – | – | |
| Adjusted ME | Eq. 3 | 130.85 | –50.90 | 47.06 | 57.36 | 0.99 | <0.001 |
| SE | 744.58 | 8.91 | 2.48 | – | – | – | |
| P-value | 0.869 | 0.006 | <0.001 | – | – | – | |
| Eq. 4 | 4,055.20 | –43.04 | – | 144.84 | 0.94 | <0.001 | |
| SE | 110.42 | 5.00 | – | – | – | – | |
| P-value | <0.001 | <0.001 | – | – | – | – | |
RMSE = root mean square error; SE = standard error.
In vitro disappearance represents in vitro ileal disappearance of CP in ileal digestible CP and in vitro total tract disappearance of dry matter in adjusted ME.
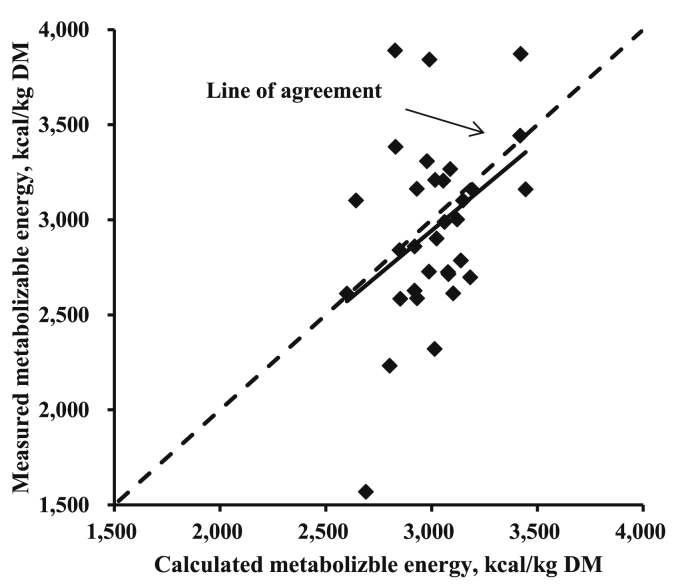
Based on the published ME values (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009) of MM and MBM, the values were plotted against calculated ME values using an equation developed in the present work employing ash as an independent variable (Fig. 1). When the prediction equation was tested using the determined ME data, the intercept (–57.66 ± 79.74; P = 0.475) and the slope (–0.07 ± 0.40; P = 0.859) were not different from 0.
Fig. 1.
Comparison of determined and calculated metabolizable energy (ME, kcal/kg DM) of meat meal and meat and bone meal using the determined ME values (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009; n = 32). The prediction equation ME (Eq. (4)) was: ME (kcal/kg DM) = 4,055.20 – 43.04 × ash (% DM). Using the regression analysis (determined – calculated ME vs. calculated ME – average of calculated ME), the intercept (– 57.66 ± 79.74; P = 0.475) and slope (– 0.07 ± 0.40; P = 0.859) were not different from 0.
4. Discussion
Considering high CP and AA contents in MM and MBM, these ingredients can be used as protein and energy sources in swine diets. Although standardized ileal digestible CP (Kong et al., 2014; Navarro et al., 2018; Stein et al., 2001) and energy concentrations (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009) in MM and MBM have been reported, the nutritional values were largely variable. In addition, very little information on estimating digestible CP and ME concentrations with a wide range of nutrient compositions of MM and MBM is available. Therefore, the objective of the present study was to develop equations for estimating ileal digestible CP and ME contents of MM and MBM by pigs based on in vitro assays.
The samples of animal offal in the present work were divided into 2 groups according to the definition of MM and MBM in AAFCO (2016). The rendered products containing less than 4.0% of P were considered as MM. The animal offal that contained over 4.0% of P and had Ca:P less than 2.2 were considered as MBM.
The chemical compositions of CP, ash, Ca, P, and AA concentrations of 3 sources of MBM used in the present work were within the range of the previous data (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009; Sulabo and Stein, 2013). However, the CP and AA concentrations of MM were relatively greater compared with the values in a previous study (Cromwell et al., 1991). The deviations of nutrient compositions among MM sources are likely due to the inclusion rate of soft tissues and bone fractions of animals. These results were supported by the relatively greater coefficient of variation values in nutrients of MM with different ratios of soft tissues and bones (Fontaine et al., 2001).
A negative correlation between ash and GE was observed (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009; Wang and Parsons, 1998), which is in agreement with the present work. This observation is reasonable because ash content is not an energy source due to the lack of organic compounds. Therefore, the inclusion rate of bone fractions relative to soft tissues in animal offal results in greater ash concentrations but less CP and GE concentrations (Garcia and Phillips, 2009). In previous studies, the negative correlation between ash and GE (r = –0.93) was stronger than that between ash and CP (r = –0.59) (Adedokun and Adeola, 2005). In the present work, however, the correlation coefficient between ash and GE (r = –1.00) and ash and CP (r = –0.99) were very similar. The reason for this discrepancy appears to be due to the range in nutrient compositions of MM and MBM employed in the experiments. While CP ranges from 49.7% to 61.9% DM in the work by Adedokun and Adeola (2005), CP ranged from 49.0% to 84.3% DM in the present work. A wide range of nutrient compositions results in stronger correlation coefficients (Choi et al., 2020). In the present work, the samples of MM and MBM were selected to maximize nutrient variability.
In the present work, strong correlations between ash and other nutrients were observed. However, in vitro disappearance of CP and DM was not correlated with nutrients. As MM and MBM contains a very limited quantity of fibers, the insignificant correlation between in vitro CP and DM disappearance and nutrients is reasonable. Similarly, the variability of ileal digestibility of CP (Wang and Parsons, 1998) and total tract digestibility of energy (Adedokun and Adeola, 2005) in MBM could not be explained by nutrient compositions. The potential reasons for the digestibility variation in MM and MBM include the protein quality produced by processing methods (Hendriks et al., 2004) and the raw materials of soft tissues (poultry, swine, and beef; Márquez et al., 2005). Protein in bone fractions contains relatively greater collagen concentrations than soft tissues, which results in low digestibility of AA (Garcia and Phillips, 2009). Further research is warranted to determine the relationship between nutrient composition and digestibility coefficients. In addition, high-temperature processing is conducted to prevent pathogenic bacteria and viruses from contaminating MM and MBM (Meeker and Meisinger, 2015), which potentially decreases the ileal AA digestibility by lowering protein quality (Johnson et al., 1998).
Digestible CP and AA (Hendriks et al., 2002; Wang and Parsons, 1998) and energy concentrations (Castilho et al., 2015; Wang and Parsons, 1998) of MM and MBM were negatively correlated with ash, which agrees with the present work. This observation is likely due to the fact that ash is negatively correlated with GE and CP.
The IVID of DM and IVTTD of DM simulate the 2-step digestion and 3-step digestion of pigs, respectively (Boisen and Fernández, 1995, 1997). In vitro ileal disappearance of CP was greater than the apparent ileal digestibility of CP from in vivo (Akonjuen et al., 2019; Cho and Kim, 2011). The reason for the inconsistency between the in vitro and in vivo data may be the basal endogenous losses of CP and energy (Boisen and Fernández, 1995, 1997). Apparent ileal digestibility of CP and total tract digestibility of energy do not consider the basal endogenous losses of CP and energy, but the in vitro assay may represent the standardized ileal or total tract digestibility that was not affected by the basal endogenous losses of CP and energy, respectively. Standardized ileal digestibility values of CP in MM and MBM were reported in previous studies (Kong et al., 2014; Navarro et al., 2018; Stein et al., 2001). However, the basal endogenous loss of energy was not reported. Therefore, to estimate in vivo energy digestibility data from the in vitro disappearance values, we used a correction factor (659 kcal/kg DM) to estimate the ME value based on the data in the literature (Adedokun and Adeola, 2005; Olukosi and Adeola, 2009).
In the present work, the ash concentration was used as a main independent variable to develop the prediction equations for ileal digestible CP and adjusted ME concentrations in MM and MBM (Table 5). The addition of CP and GE in the models did not improve the accuracy of the predictions as ash was very strongly correlated with CP and GE. In addition, ash is practically very convenient as an independent variable because ash content is easy to determine.
The ME values of MM and MBM calculated by using equation (4) in Table 5 were relatively in agreement with the determined ME values (Fig. 1). As the number of determined ileal digestible CP of MM and MBM was very limited compared with that of ME values in the literature, the equation for ileal digestible CP was not validated.
5. Conclusions
Ash contents in MM and MBM from pig offal have a very strong negative correlation with protein and energy contents. Metabolizable energy concentrations of MM and MBM as swine feed ingredients can be estimated using ash concentration and in vitro disappearance of DM.
Author contributions
Hyunjun Choi: conceptualization, methodology, formal analysis, writing – original draft; Chang Sik Won: investigation, formal analysis; Beob Gyun Kim: conceptualization, supervision, writing – review & editing.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AAFCO. Official . Association of American Feed Control Officials; West Lafayette, IN, USA: 2016. Association of American Feed Control Officials. [Google Scholar]
- Adedokun S.A., Adeola O. Metabolizable energy value of meat and bone meal for pigs. J Anim Sci. 2005;83:2519–2526. doi: 10.2527/2005.83112519x. [DOI] [PubMed] [Google Scholar]
- Ahn J.Y., Kil D.Y., Kong C., Kim B.G. Comparison of oven-drying methods for determination of moisture content in feed ingredients. Asian-Australas J Anim Sci. 2014;27:1615–1622. doi: 10.5713/ajas.2014.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akonjuen M.B., Hong B., Choi H., Kim B.G. Digestibility of energy and crude protein in Korean rice wine residues fed to pigs. Am J Anim Vet Sci. 2019;14:183–189. doi: 10.3844/ajavsp.2019.239.243. [DOI] [Google Scholar]
- AOAC International . 20th ed. Association of Official Analytical Chemist International; Gaithersburg, MD, USA: 2016. Official methods of analysis. [Google Scholar]
- Boisen S., Fernández J.A. Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mixtures for pigs by in vitro analyses. Anim Feed Sci Technol. 1995;51:29–43. doi: 10.1016/0377-8401(94)00686-4. [DOI] [Google Scholar]
- Boisen S., Fernández J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim Feed Sci Technol. 1997;68:277–286. doi: 10.1016/S0377-8401(97)00058-8. [DOI] [Google Scholar]
- Castilho R.A., Pozza P.C., de Oliveira N.T.E., Sangali C.P., Langer C.N., Nunes R.V. Equations to predict the metabolizable energy of meat and bone meal for growing pigs. Cienc Agrotecnol. 2015;39:565–573. doi: 10.1590/S1413-70542015000600003. [DOI] [Google Scholar]
- Cho J.H., Kim I.H. Evaluation of the apparent ileal digestibility (AID) of protein and amino acids in nursery diets by in vitro and in vivo methods. Asian-Australas J Anim Sci. 2011;24:1007–1010. doi: 10.5713/ajas.2011.10435. [DOI] [Google Scholar]
- Choi H., Sung J.Y., Kim B.G. Neutral detergent fiber as an independent variable increases the accuracy of prediction equation for digestible energy in feeds for growing pigs. Asian-Australas J Anim Sci. 2020;33:615–622. doi: 10.5713/ajas.19.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell G.L., Stahly T.S., Monegue H.J. Amino acid supplementation of meat meal in lysine-fortified, corn-based diets for growing-finishing pigs. J Anim Sci. 1991;69:4898–4906. doi: 10.2527/1991.69124898x. [DOI] [PubMed] [Google Scholar]
- Fontaine J., Hörr J., Schirmer B. Near-infrared reflectance spectroscopy enables the fast and accurate prediction of the essential amino acid contents in soy, rapeseed meal, sunflower meal, peas, fishmeal, meat meal products, and poultry meal. J Agric Food Chem. 2001;49:57–66. doi: 10.1021/jf000946s. [DOI] [PubMed] [Google Scholar]
- Garcia R.A., Phillips J.G. Physical distribution and characteristics of meat and bone meal protein. J Sci Food Agric. 2009;89:329–336. doi: 10.1002/jsfa.3453. [DOI] [Google Scholar]
- Hendriks W., Butts C., Thomas D., James K., Morel P., Verstegen M. Nutritional quality and variation of meat and bone meal. Asian-Australas J Anim Sci. 2002;15:1507–1516. doi: 10.5713/ajas.2002.1507. [DOI] [Google Scholar]
- Hendriks W.H., Cottam Y.H., Morel P.C.H., Thomas D.V. Source of the variation in meat and bone meal nutritional quality. Asian-Australas J Anim Sci. 2004;17:94–101. doi: 10.5713/ajas.2004.94. [DOI] [Google Scholar]
- Johnson M.L., Parsons C.M., Fahey G.C., Jr., Merchen N.R., Aldrich C.G. Effects of species raw material source, ash content, and processing temperature on amino acid digestibility of animal by-product meals by cecectomized roosters and ileally cannulated dogs. J Anim Sci. 1998;76:1112–1122. doi: 10.2527/1998.7641112x. [DOI] [PubMed] [Google Scholar]
- Kil D.Y., Kim B.G., Stein H.H. Feed energy evaluation for growing pigs. Asian-Australas J Anim Sci. 2013;26:1205–1217. doi: 10.5713/ajas.2013.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C., Kang H.G., Kim B.G., Kim K.H. Ileal digestibility of amino acids in meat meal and soybean meal fed to growing pigs. Asian-Australas J Anim Sci. 2014;27:990–995. doi: 10.5713/ajas.2014.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.A., Jo H., Kong C., Kim B.G. Use of digestible rather than total amino acid in diet formulation increases nitrogen retention and reduces nitrogen excretion from pigs. Livest Sci. 2017;197:8–11. doi: 10.1016/j.livsci.2016.12.013. [DOI] [Google Scholar]
- Márquez E., Bracho M., Archile A., Rangel L., Benítez B. Proteins, isoleucine, lysine and methionine content of bovine, porcine and poultry blood and their fractions. Food Chem. 2005;93:503–505. doi: 10.1016/j.foodchem.2004.10.030. [DOI] [Google Scholar]
- Meeker D.L., Meisinger J.L. Companion animals symposium: rendered ingredients significantly influence sustainability, quality, and safety of pet food. J Anim Sci. 2015;93:835–847. doi: 10.2527/jas.2014-8524. [DOI] [PubMed] [Google Scholar]
- Navarro D.M.D.L., Mathai J.K., Jaworski N.W., Stein H.H. Amino acid digestibility in six sources of meat and bone meal, blood meal, and soybean meal fed to growing pigs. Can J Anim Sci. 2018;98:860–867. doi: 10.1139/cjas-2017-0217. [DOI] [Google Scholar]
- NRC . 11th ed. National Research Council, National Academy Press; Washington, DC, USA: 2012. Nutrient requirements of swine. [Google Scholar]
- Olukosi O.A., Adeola O. Estimation of the metabolizable energy content of meat and bone meal for swine. J Anim Sci. 2009;87:2590–2599. doi: 10.2527/jas.2009-1775. [DOI] [PubMed] [Google Scholar]
- Park C.S., Son A.R., Kim B.G. Prediction of gross energy and digestible energy in copra meal, palm kernel meal, and cassava root fed to pigs. J Anim Sci. 2012;90:221–223. doi: 10.2527/jas.53954. [DOI] [PubMed] [Google Scholar]
- Son A.R., Park C.S., Park K.R., Kim B.G. Amino acid digestibility in plant protein sources fed to growing pigs. Asian-Australas J Anim Sci. 2019;32:1745–1752. doi: 10.5713/ajas.19.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H.H., Kim S.W., Nielsen T.T., Easter R.A. Standardized ileal protein and amino acid digestibility by growing pigs and sows. J Anim Sci. 2001;79:2113–2122. doi: 10.2527/2001.7982113x. [DOI] [PubMed] [Google Scholar]
- Sulabo R.C., Stein H.H. Digestibility of phosphorus and calcium in meat and bone meal fed to growing pigs. J Anim Sci. 2013;91:1285–1294. doi: 10.2527/jas.2011-4632. [DOI] [PubMed] [Google Scholar]
- Sung J.Y., Kim B.G. Prediction equations for digestible and metabolizable energy concentrations in ingredients and diets for pigs based on chemical composition. Anim Biosci. 2021;34:306–311. doi: 10.5713/ajas.20.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J.Y., Kim B.G. Prediction models for apparent and standardized total tract digestible phosphorus in swine diets. Anim Feed Sci Technol. 2019;255:114224. doi: 10.1016/j.anifeedsci.2019.114224. [DOI] [Google Scholar]
- Wang X., Parsons C.M. Effect of raw material source, processing systems, and processing temperatures on amino acid digestibility of meat and bone meals. Poult Sci. 1998;77:834–841. doi: 10.1093/ps/77.6.834. [DOI] [PubMed] [Google Scholar]