Abstract
This study investigated the effects of isomaltooligosaccharide (IMO) and Bacillus in perinatal diets on the duration of farrowing and post-weaning estrus, serum reproductive hormone concentrations, and gut microbiota and its metabolites of sows. Multiparous sows (n = 130) were fed diets without IMO (control, CON group), or diets containing only IMO (IMO group), IMO and Bacillus subtilis (IMOS group), IMO and Bacillus licheniformis (IMOL group), and IMO and B. subtilis and B. licheniformis (IMOSL group), respectively. The results indicate that the duration of farrowing and post-weaning estrus was shorter in sows in the IMOS, IMOL, and IMOSL groups, and the weaning-estrous interval was lower in sows in the IMOL greoup. In addition, the lowest fecal score was observed in the IMOL group during d 106 to 112 of gestation. Sows in most of the treatment groups had a higher concentration of serum prolactin and prostaglandin at farrowing, but a lower serum concentration of estradiol, oxytocin, and progesterone on d 18 of lactation than sows in the CON group. The treatment groups had a higher abundance of Candidatus Methanoplasma and Bacillus and a lower abundance of Escherichia-Shigella in their feces at farrowing. Furthermore, the treatment groups had higher concentrations of total short-chain fatty acids (SCFA) in feces at farrowing and a higher concentration of branched fatty acids in feces on d 18 of lactation. Furthermore, the abundance of Bacillus in feces was positively correlated with serum prostaglandin concentrations and fecal total SCFA of sows at farrowing, but was negatively correlated with the duration of farrowing. Overall, dietary IMO and Bacillus supplementation affected the concentration of serum reproductive hormones and the duration of farrowing and post-weaning estrus, and the gut microbiota is a key factor.
Keywords: Sow, Isomaltooligosaccharide, Bacillus, Perinatal period, Duration of farrowing, Gut microbiota
1. Introduction
Currently, large-scale pig farms are experiencing many problems, such as the prolonged labor of sows, postweaning anestrus, and estrus return, which can lead directly to a reduction in the producer's economic benefits. Although the prolonged labor of sows is caused mainly by their own physiological conditions, it is also affected by the feed, environment (Oliviero et al., 2010), and other factors (Yun et al., 2013, 2015). At farrowing, the sow undergoes substantial hormonal and metabolic changes during a very short period of time (Bo and Uvnäs-Moberg, 2007). For instance, the concentrations of serum luteinizing hormone (LH), estradiol, and progesterone are very low in sows with anestrus after weaning (Cox et al., 1988). Therefore, the reproductive hormones secreted by sows are extremely important to the labor process during farrowing. Researchers have found that reproductive hormones are closely related to the gut microbiota (Cremon et al., 2009; Frankenfeld et al., 2014), as these microbes and their metabolites can regulate the secretion of hormones (Homma et al., 2005; Viorica et al., 2010). Meanwhile, hormones can also regulate the gut microbes and their metabolites in a feedback effect (Chen and Madak-Erdogan, 2016; Li et al., 2009). Additionally, constipation is a common disorder associated with pregnancy and the postpartum period (Bradley et al., 2007; Oliviero et al., 2009). During late gestation, the increasing volume of the fetus oppresses the intestinal tract, and autocrine hormones inhibit intestinal peristalsis (e.g., progesterone can inhibit the contraction of smooth muscle) (Gill et al., 1985). Prolonged labor and increased stillbirth rates are more common in constipated sows (Gill et al., 1985; Oliviero et al., 2010). Therefore, constipation is another important factor leading to prolonged labor during late gestation. Furthermore, these sudden changes in reproductive hormone levels and constipation are important factors causing gut microbiota disorder in sows during late gestation (Frankenfeld et al., 2014; Cao et al., 2017), which affect the reproductive performance of the animals.
Postweaning anestrus is not only influenced by feeding management, but is also caused by the insufficient secretion of hormones by the sows (Dyck et al., 1979). Previous treatments have mostly involved injections of exogenous estrogens to induce estrus in sows, which may bring them into a harmful situation (Cox et al., 1988). Some researchers have found that the addition of certain prebiotics during lactation could regulate the body condition of sows (e.g., their intestinal health), improve the estrus rate after ablactation, and reduce the estrus return rate (Duan et al., 2016). Thus, it is essential to provide dietary substances that are able to improve gut microbiota disorders in the perinatal sow.
As a functional oligosaccharide, isomaltooligosaccharide (IMO) can improve the gut microbiota and promote the proliferation of beneficial bacteria, especially Bifidobacteria (Patel and Goyal, 2011). IMO can also improve the body's antioxidant capacity and immunity (Hiroyuki et al., 2005; Kai et al., 2015), and relieve both constipation and diarrhea (Chen et al., 2001; Wang et al., 2016). As a new green and efficient feed additive, IMO is superior to antibiotics and other functional oligosaccharides in stability, palatability, and cost performance. However, many studies (Li et al., 2009; Tan et al., 2015) have reported that single prebiotics play a weak role in regulating the intestinal health of the body. If combined with certain probiotics, the prebiotic effect will be greatly improved (Yu et al., 2016). Therefore, we selected a gram-positive facultative anaerobe (Bacillus) as a probiotic to inhibit the proliferation of harmful bacteria in the intestinal tract by consuming oxygen and secreting bacteriostatic substances (Ying et al., 2018). Bacillus and IMO have been shown to complement each other in improving gut microbiota disorders (Li et al., 2009).
In the present study, we determined the roles of IMO and Bacillus in the perinatal diet of sows by studying whether their addition to the animal diet could affect the duration of farrowing, postweaning estrus, and estrus return. Moreover, we studied the changes in the reproductive hormone levels and gut microbiota as well as their metabolites in sows at farrowing and on d 18 of lactation. The hypothesis of this study is that the addition of IMO and Bacillus to the diets of perinatal sows will affect the animal's secretion of reproductive hormones and relieve any constipation experienced in late gestation by regulating the gut microbiota and their metabolites, thereby shortening the duration of farrowing and the estrus interval, while also reducing the rate of estrus return in the sows.
2. Materials and methods
The protocol of this study was approved by the Institution of Animal Care and Use Committee of the College of Animal Science and Technology, at Hunan Agricultural University (Changsha, China), and was conducted in accordance with the National Institutes of Health (Changsha, China) guidelines for the care and use of experimental animals. The IMO was provided by the Baolingbao Biology Company (Shandong, China). Bacillus subtilis (2.0 × 1011 cfu/g) and Bacillus licheniformis (2.0 × 1011 cfu/g) were provided by the Shandong Kangdien Biotechnology Company (Shandong, China).
2.1. Animals, diets, and sample collection
One hundred and thirty late pregnancy sows (d 85 of gestation, Large White × Landrace) with an initial body weight of 236.20 ± 23.66 kg and parities of 3.20 ± 1.17 were randomly allocated to 1 of 5 dietary treatments, with 26 replicates based on body weight, parity, and back fat. The treatments included the following: 1) a diet without IMO (control, CON group); 2) diet containing 5.0 g/kg IMO (IMO group); 3) diet containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis (IMOS group); 4) diet containing 5.0 g/kg IMO plus 0.2 g/kg B. licheniformis (IMOL group); and 5) diet containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis (IMOSL group). The composition of the basal diet (Appendix Table 1) was formulated in compliance with the National Research Council (1998) nutrient requirements. Sows were housed in 2.0 m × 0.6 m concrete-floored farrowing pens during gestational period from d 85 to 110. From d 110 of gestation to ablactation, the sows were housed indoors in 2.13 m × 0.66 m concrete-floored delivery room pens. The sows and piglets were then individually housed in farrowing pens with crates, slatted floors, and heating pads for the piglets. The sows and piglets had free access to water. The experiment was carried out in the Hunan Zhenghong original pig farm (Zhenghong, Inc., Hunan, China), and the feeding management and immunization procedures were carried out in accordance with the company's standard breeding management.
Seven sows per group were randomly selected for sample collection. Fresh feces were collected directly by massaging the rectum of sows on the farrowing day and d 18 of lactation. Subsequently, 40 fecal samples were transported to the laboratory on dry ice and then stored at −80 °C until analysis. A 10-mL blood sample was collected from the sows’ ear veins on the farrowing day (within 2 h of delivery) and on d 18 of lactation after an overnight fasting period of 16 to 18 h. Serum samples were obtained by centrifuging blood samples at 3,000 × g for 15 min at 4 °C after standing for 1 h at 4 °C. Subsequently, the samples were immediately stored at −80 °C for the analysis of reproductive hormone concentrations.
2.2. Duration of farrowing, post-weaning estrus, and fecal score record
The duration of farrowing was recorded for each sow. Starting at weaning, the weaning-estrous interval of sows within 1 wk after weaning was also recorded, and the estrus rate (0 to 7 d) was calculated. Furthermore, the number of returns to estrus from weaning to the next cycle were recorded, and the rate of return to estrus was calculated. In addition, during gestation period of d 85 to 112, the fecal score of each sow was recorded daily. The fecal score index adopts the 5-point system, that is, the feces score is 1 to 5 points according to the appearance, as shown in Table 1.
Table 1.
Standard of fecal score.
| Score | Evaluation | Based on the score (fecal appearance) |
|---|---|---|
| 1 | Diarrhea | Liquid, unformed, separation of fecal water |
| 2 | Mild diarrhea | Forming, no separation of fecal water |
| 3 | Normal | Soft, moderate particle size, grass green |
| 4 | Slight constipation | Micro hard, smaller particles, yellowish outside and green inside |
| 5 | Severe constipation | Hard, small particles, yellow |
2.3. Analysis of serum reproductive hormone concentrations
Several biomarkers related to the reproductive performance of sows were measured in the animal molecular nutrition laboratory. These biomarkers included serum estradiol, oxytocin, prolactin, progesterone, and prostaglandin concentrations, as markers for reproductive hormones. Serum estradiol, oxytocin, prolactin, progesterone, and prostaglandin were measured using radioimmunoassay (RIA). The kits were provided by the Nanjing Jiancheng Bioengineering Institute (Wuhan Biological Engineering Co., Ltd, Wuhan, China). Samples were measured according to the manufacturer's instructions.
2.4. DNA extraction, 16S rDNA amplification, and 16S rRNA sequencing
DNA was extracted from fecal samples of sows (at farrowing and on d 18 of lactation) using a Stool DNA Isolation Kit (Tiangen Biotech Co., Ltd., Beijing, China). The V4 hypervariable region of the bacterial 16S rRNA gene was amplified by a PCR, where the forward primer was 550F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and the reverse primer was 806R: 5′-GGACTACHVGGGTWTCTAAT-3’. For each fecal sample, a 10-digit barcode sequence was added to the 5′ end of the forward and reverse primers (provided by Allwegene Company, Beijing, China). The volume of the PCR reaction was 25 μL, and included 12.5 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs Inc., Beverly, MA, USA), 2 μL of forward and reverse primers, 30 ng of template DNA, and 7.5 μL of double distilled H2O (ddH2O). Cycling parameters were 98 °C for 1 min, followed by 30 cycles at 98 °C for 10 s, 57 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 10 min. The PCR products were mixed in equidensity ratios and purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific Inc., Schwerte, Germany), then quantified using a real-time PCR, and sequenced at the Allwegene Company in Beijing. The sequences were clustered into operational taxonomic units (OTU) at a similarity level of 97% to generate rarefaction curves and to calculate the richness and diversity indices. OTU representing <0.005% of the population were removed, and taxonomy was assigned using the Ribosomal Database Project (RDP) classifier. The β-diversity was assessed by MANOVA and principal coordinate analysis. Significant differences in α-diversity and OTU counts between the different groups were determined by a one-way analysis of variance (ANOVA) followed by Duncan's multiple comparison test using SPSS software (SPSS statistics 20) (Zhu et al., 2013).
2.5. Analysis of fecal short-chain fatty acids (SCFA)
The concentration of SCFA in feces were analyzed using a gas chromatographic method, as described by Bosch et al. (2008). Approximately 1.5 g of feces were homogenized in 1.5 mL of deionized water. The samples were centrifuged at 15,000 × g at 4 °C for 10 min. Supernatants (1 mL each) were then acidified with 25% metaphosphoric acid at a 1:5 ratio (vol:vol) for 30 min on ice. The sample was injected into a GC 2010 series gas chromatograph (Shimadzu, Japan) equipped with a CP-Wax 52 CB column 30.0 m × 0.53 mm i.d (Chrompack, Netherlands). The injector and detector temperatures were 75 and 280 °C, respectively. Total SCFA were determined as the sum of analyzed acetic acid, propionic acid, butyric acid, and 3-branched fatty acids, namely, isobutyric acid, isovaleric acid and valeric acid. All procedures were performed in triplicate.
2.6. Statistical analysis
Individual sows served as the experimental unit. All statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, United States). Alpha and β-diversity were analyzed with QIIME (v. 1.7.0) and displayed with R software (v. 3.5.1). The differences between groups were compared using one-way ANOVA and Duncan's multiple range test. P-values < 0.05 were used to indicate statistical significance.
3. Results
3.1. Duration of farrowing, post-weaning estrus, and fecal score of sows
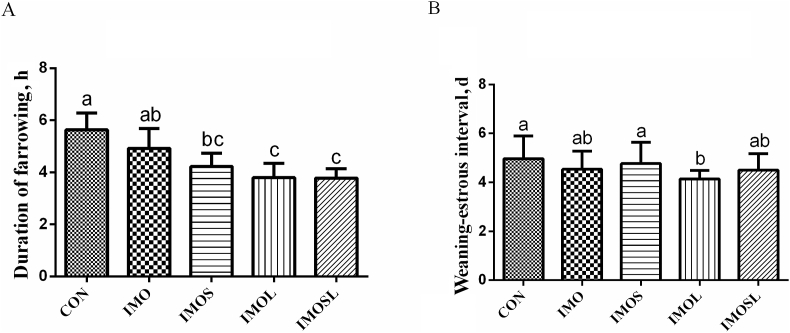
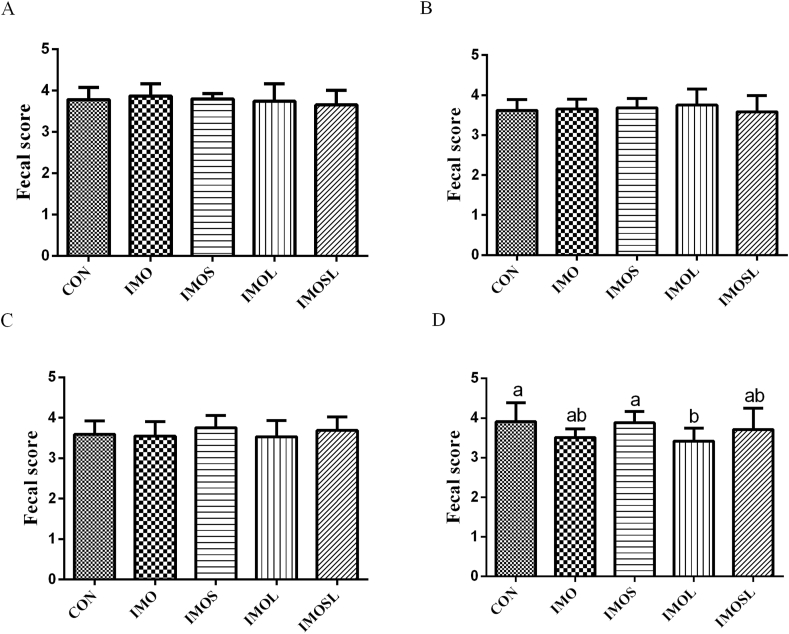
As shown in Fig. 1, sows that were fed the diets containing IMOS, IMOL, and IMOSL (P < 0.05) had a shorter duration of farrowing (Fig. 1A). In addition, the weaning-estrous interval was shorter in sows in the IMOL group (P < 0.05) compared with sows in the CON group (Fig. 1B). The estrus rate was higher in sows in the IMOSL group, and the estrus returning rate was lower in sows in the IMOS, IMOL, and IMOSL groups (P < 0.05, Table 2). Fig. 2 shows the effects of IMO and Bacillus on the sows’ fecal scores during the gestational period of d 85 to 112. There were no differences between any of the groups (P < 0.05) during d 85 to 105 of gestation (Fig. 2A–C). However, during d 106 to 112 of gestation, sows that were fed the diets containing IMOL had a lower fecal score (P < 0.05) compared with the sows that were fed the diets without IMO (Fig. 2D).
Fig. 1.
Duration of farrowing and post-weaning estrus, a crucial factor of the reproductive performance of sows can have economic benefits in pig farms. Isomaltooligosaccharide (IMO) and Bacillus regulated the duration of farrowing (A) and weaning-estrous interval (B) of sows during the perinatal period. Data are presented as means ± SD (n = 20). Values with different lowercase letters (a, b, c) indicate a significant effect of treatment (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
Table 2.
Effects of isomaltooligosaccharide (IMO) and Bacillus on the estrus rate and estrus returning rate of sows during perinatal period (%).
| Item | Treatments1 |
||||
|---|---|---|---|---|---|
| CON | IMO | IMOS | IMOL | IMOSL | |
| The estrus rate (0 to 7d) | 87.88 | 82.35 | 88.00 | 84.62 | 100.00 |
| Estrus returning rate | 9.09 | 8.82 | 0.00 | 3.85 | 0.00 |
CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
Fig. 2.
Pregnant sows, especially in late pregnancy, are prone to constipation due to physiological and feed factors. Effects of isomaltooligosaccharide (IMO) and Bacillus on the fecal score during d 85 to 91 (A), d 92 to 98 (B), d 99 to 105 (C), and d 106 to 112 (D) of gestation of sows. Data are presented as means ± SD (n = 20). Values with different lowercase letters (a, b) are significantly different (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
3.2. Serum reproductive hormone concentrations of sows at farrowing and on d 18 of lactation
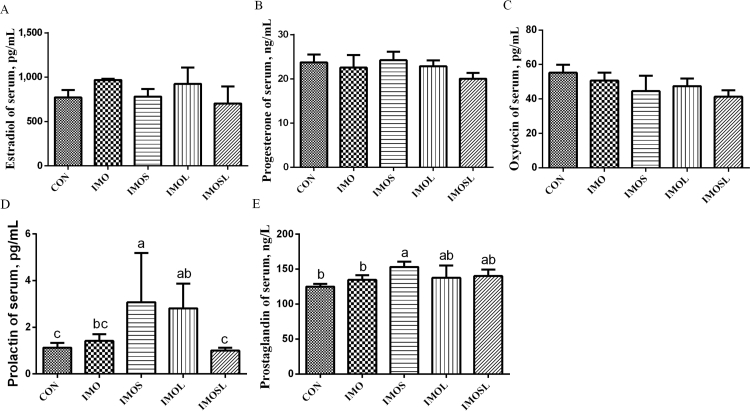
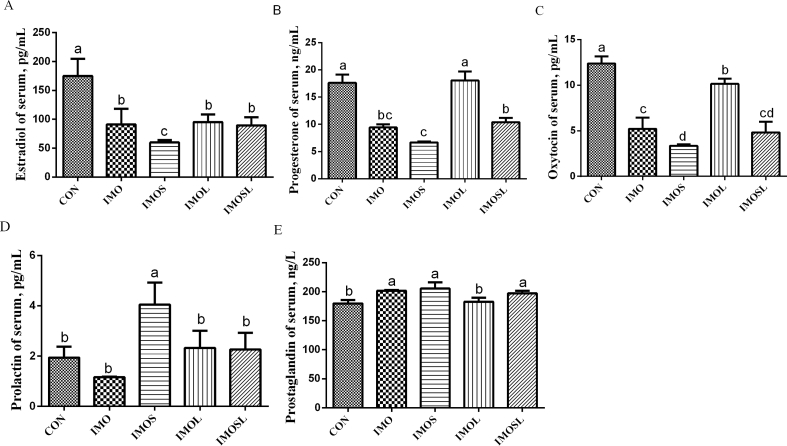
During farrowing, the serum concentration of reproductive hormones in sows' changes, and this can affect the duration of farrowing. In our study, feeding sows’ diets containing IMO or IMO combined with Bacillus did not affect their serum estradiol, oxytocin, or progesterone concentrations (P > 0.05), compared with sows that were fed the CON diet (Fig. 3A–C). Furthermore, sows in the IMOS and IMOL groups had higher concentrations of serum prolactin (P < 0.05) than sows in the CON group (Fig. 3D). We also found a higher concentration of serum prostaglandin (P < 0.05) in sows in the IMOS group (Fig. 3E). During lactation, sows not only need to meet their own maintenance requirements, but they also need to meet lactation requirements. In the present study, we found a lower concentration of serum estradiol and oxytocin in all treatment groups (P < 0.05) compared with the CON group (Fig. 4A, C). Furthermore, sows fed diets containing IMOS had a higher concentration of serum prolactin (P < 0.05) compared with sows fed the CON diet (Fig. 4D). Sows in the IMO, IMOS, and IMOSL groups had lower concentrations of serum progesterone (P < 0.05) compared with sows in the CON group (Fig. 4B), and the serum prostaglandin concentration was higher (P < 0.05) in sows in the IMO, IMOS, and IMOSL groups (Fig. 4E).
Fig. 3.
Isomaltooligosaccharide (IMO) and Bacillus regulate serum reproductive hormone concentration in sows at farrowing. (A) Estradiol, (B) progesterone, (C) oxytocin, (D)prolactin, and (E) prostaglandin. Data are presented as means ± SD (n = 7). Values with different lowercase letters (a, b, c) indicate a significant effect of treatment (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
Fig. 4.
Effect of isomaltooligosaccharide (IMO) and Bacillus on resum hormone concentration in sows at d 18 of lactation. (A) Estradiol, (B) progeterone, (C) oxytocin, (D) prolactin, and (E) prostaglandin. Data are presented as means ± SD (n = 7). Values with different lowercase letters (a, b, c, d) indicate a significant effect of treatment (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
3.3. Gut microbiota diversity and composition
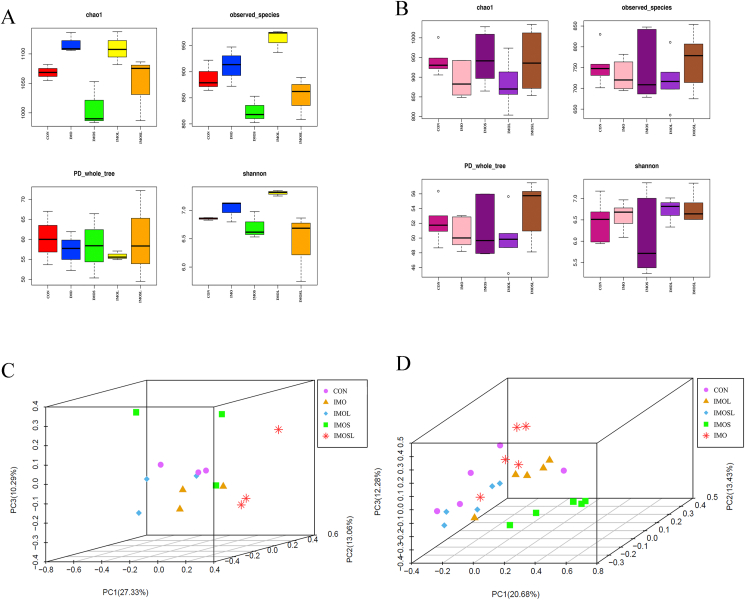
IMO and Bacillus changed the gut microbiota diversity of sows at farrowing and on d 18 of lactation, but the trend was different for these 2 time points. In the present study, the bias-corrected Chao richness estimator (Chao 1) was lower for sows in the IMOS and IMOSL groups (P < 0.05) compared with sows in the IMO group at farrowing (Fig. 5A). Sows fed diets containing IMO and IMOL had higher numbers of observed species and Shannon diversity indices (P < 0.05) compared with sows in the other groups (Fig. 5A). However, there was no difference between any of the groups with regards to the Chao 1 value, number of observed species, PD_whole_tree, and Shannon diversity indices (P > 0.05) on d 18 of lactation (Fig. 5B). Using principal component analysis (PCA) based on OTU, we found that the gut microbiota of sows in the treatment groups were clearly segregated from those in the CON group, especially regarding sows in the IMO plus Bacillus groups, both at farrowing and on d 18 of lactation (Fig. 5C and D).
Fig. 5.
The gut microbiota diversity of sows was altered by addition of isomaltooligosaccharide (IMO) alone and in combination with Bacillus to diets at farrowing and on d 18 of lactation. (A) At farrowing, comparisons of the number of gut microbiota α-diversity containing bias-corrected Chao richness estimator (Chao 1), observed species, PD_whole_tree, and Shannon diversity indices among sows subjected to different dietary treatments. (B) On d 18 of lactation, comparisons of the number of gut microbiota α-diversity containing bias-corrected Chao richness estimator (Chao 1), observed species, PD_whole_tree, and Shannon diversity indices among sows subjected to different dietary treatments. (C) At farrowing, principal component analysis (PCA) based on operational taxonomic units (OTU) among samples of different groups. Each point represents 1 sample. (D) On d 18 of lactation, principal component analysis (PCA) based on OTU among samples of different groups. Each point represents one sample. Data are presented as means ± SEM (n = 3 or n = 5). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
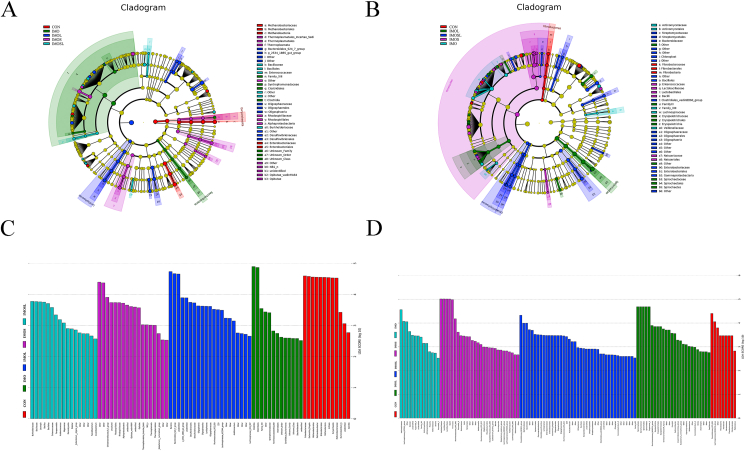
All 40 fecal samples were subjected to 16S rRNA gene sequencing. Illumina Miseq sequencing of the V4 regions of bacterial 16S rRNA genes generated 1,140,552 and 1,052,045 high-quality sequences at farrowing and on d 18 of lactation, respectively. 1,179,085 raw_tags and 1,096,256 raw_tags were filtered to obtain 1,045,821 and 892,661 final_tags at farrowing and on d 18 of lactation, respectively (Appendix Table 2). On the basis of 97% sequence similarity, we obtained 1,423 and 1,331 OTU at farrowing and on d 18 of lactation, respectively. Subsequently, variations in the microbial composition of all groups were explored. Linear discriminant analysis effect size (LEfSe) analysis of the bacterial community was used to filter the significantly different OTU between groups, and the results indicate that there were dramatic differences in microbial composition between the treatment groups and the CON group (Fig. 6). The abundance of Firmicutes was higher, but the abundances of Proteobacteria, Euryarchaeota, and Actinobacteria were remarkably lower in the IMO group sows at farrowing, compared with sows in the CON group (Appendix Table 3). The abundance of Bacteroidetes was greater, and that of Proteobacteria was lower in treatment groups at farrowing, and sows in the IMOL and IMOSL groups had a higher abundance of Bacteroidetes and a lower abundance of Proteobacteria (P < 0.05) compared with sows in the IMO and IMOS groups (Appendix Table 3). However, sows fed diets without IMO or containing IMOS had higher abundances of Spirochaetae and Euryarchaeota (Appendix Table 3). Furthermore, at farrowing, the abundances of Tenericutes and Verrucomicrobia were higher in sows in the IMOS and IMOL group, but the abundance of Actinobacteria was lower in sows in the IMOS group compared with sows in the other groups (P < 0.05) (Appendix Table 3). At the genus level, the abundance of Methanobrevibacter was lower in sows in the IMO, IMOL, and IMOSL groups at farrowing (P < 0.05). In addition, the abundances of Candidatus Methanoplasma and Bacillus were higher, but the abundance of Escherichia-Shigella was lower in sows in all treatment groups (P < 0.05) compared with sows in the CON group at farrowing (Appendix Table 3). On d 18 of lactation, the abundances of Firmicutes and Actinobacteria were higher, and the abundance of Bacteroidetes was higher in sows in the IMO, IMOS, and IMOL groups (P < 0.05) (Appendix Table 4). At the genus level, abundances of Bacteroides, Prevotellaceae NK3B31 group, Prevotellaceae UCG-001, Prevotellaceae UCG-003, Rikenellaceae RC9 gut group and dgA-11 gut group were higher in sows in the IMOSL group on d 18 of lactation (P < 0.05) compared with sows in the CON group (Appendix Table 4). The abundance of Prevotellaceae UCG-001 was higher in sows in the IMO group. Furthermore, the abundances of Lactobacillus, Prevotella 9, and Rikenellaceae RC9 gut group were higher in sows in the IMOS group. The abundances of Parabacteroides, Prevotellaceae NK3B31 group, and dgA-11 gut group were also higher, but the abundance of Bacteroides was lower in sows in the IMOL group on d 18 of lactation (P < 0.05), compared with the CON group (Appendix Table 4).
Fig. 6.
Linear discriminant analysis effect size (LEfSe) analysis of the gut microbiota composition of sows at farrowing and d 18 of lactation. (A) At farrowing, cladogram using LEfSe method indicating the phylogenetic distribution of gut microbiota in sows among all dietary treatment. Each successive circle represents a phylogenetic level. (B) At d 18 of lactation, cladogram using LEfSe method indicating the phylogenetic distribution of gut microbiota in sows among all dietary treatment. Each successive circle represents a phylogenetic level. (C) At farrowing, histogram of the Linear Discriminant Analysis (LDA) score reveals the most differentially abundant taxa between different dietary treatment. (D) At d 18 of lactation, histogram of the LDA scores reveals the most differentially abundant taxa among different dietary treatment. CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
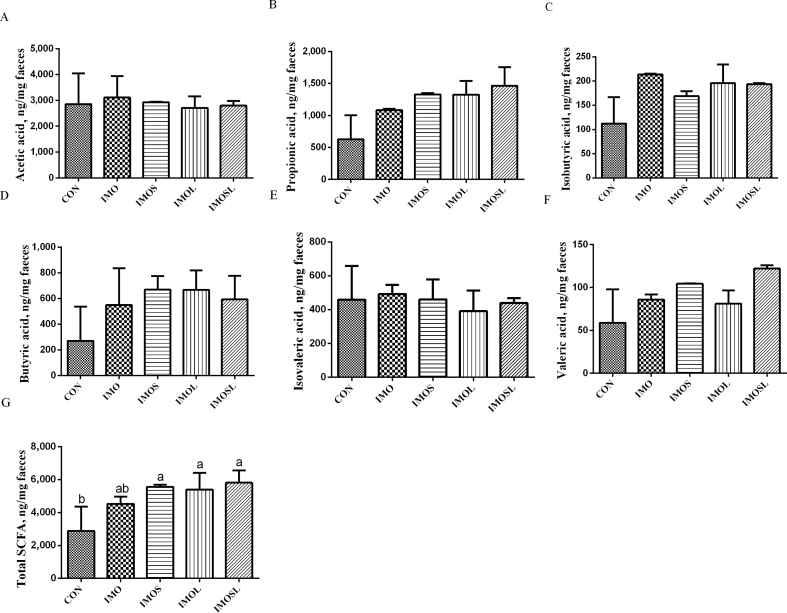
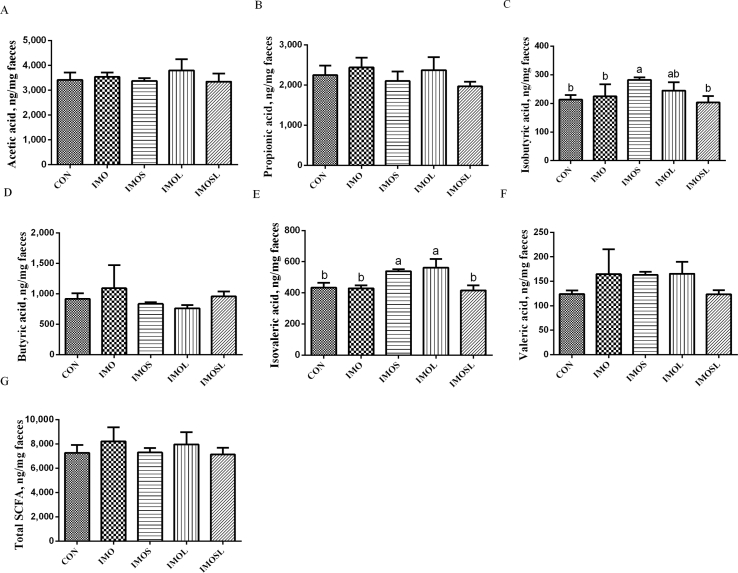
3.4. The concentration of fecal SCFA
Significant changes in gut microbial composition may cause changes in microbial metabolites. Sows fed the diets containing IMOS, IMOL, and IMOSL had higher concentrations (P < 0.05) of fecal SCFA at farrowing (Fig. 7G). Besides, the concentrations of fecal propionic acid, isobutyric acid, and butyric acid were higher in sows in the IMO, IMOS, IMOL, and IMOSL groups, but there were no significant differences (P > 0.05) between these treatments (Fig. 7B–D) at farrowing. In addition, at d 18 of lactation, there were no significant difference in fecal SCFA concentrations between the CON group and treatment groups, except for branched SCFA (P > 0.05), including isobutyric acid and isovaleric acid (Fig. 8). The concentration of fecal isobutyric acid was higher (P < 0.05) in sows in the IMOS group, and the concentration of fecal isobutyric acid was higher (P < 0.05) in sows in the IMOS group and the IMOL group at d 18 of lactation (Fig. 8C, E).
Fig. 7.
Fecal concentrations of gut microbial metabolites in sows at farrowing. (A) Acetic acid, (B) propionic acid, (C) isobutyric acid, (D) butyric acid, (E) isovaleric acid, (F) valeric acid, (G) total short-chain fatty acids (SCFA). Data are presented as means ± SD (n = 7). Values with different lowercase letters (a, b) indicate a significant effect of treatment (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS, IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
Fig. 8.
Fecal concentrations of gut microbial metabolites in sows d18 of lactation. (A) acetic acid, (B) propionic acid, (C) isobutyric acid, (D) butyric acid, (E) isovaleric acid, (F) valeric acid, (G) total short-chain fatty acids (SCFA). Data are presented as means ± SD (n = 7). Values with different lowercase letters (a, b) indicate a significant effect of treatment (P < 0.05). CON group, sows fed diets without IMO; IMO group, sows fed diets containing 5.0 g/kg IMO; IMOS group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus subtilis; IMOL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg Bacillus licheniformis; IMOSL group, sows fed diets containing 5.0 g/kg IMO plus 0.2 g/kg B. subtilis and 0.2 g/kg B. licheniformis.
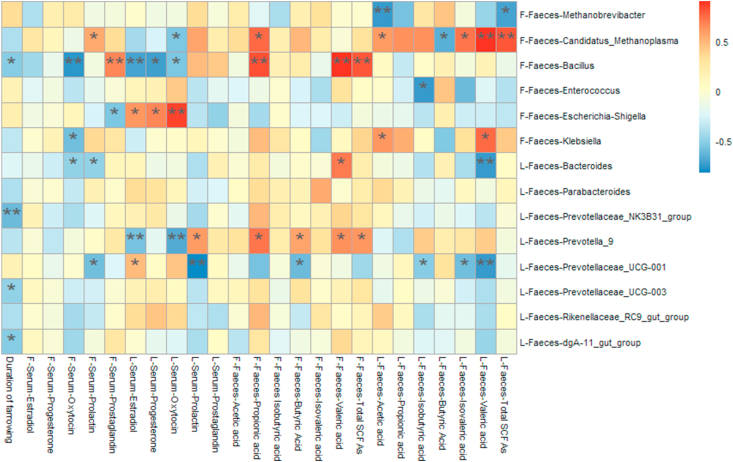
3.5. Correlations between gut microbiota and duration of farrowing and parameters of reproductive hormone in sows
A Spearman correlation analysis was performed to evaluate the potential link between alterations in gut microbiota composition and the duration of farrowing, as well as serum reproductive hormone concentrations in sows (Fig. 9). The genus Bacillus was positively correlated with serum prostaglandin concentrations, fecal propionic acid concentrations, and the concentration of total fecal SCFA of sows at farrowing (P < 0.05), but negatively correlated with the duration of farrowing (P < 0.05). Escherichia-Shigella was negatively correlated with serum prostaglandin concentrations of sows at farrowing (P < 0.05). In addition, Prevotella 9 was positively correlated with serum prolactin concentrations, but negatively correlated with serum estradiol and oxytocin concentrations on d 18 of lactation (P < 0.05). Furthermore, Prevotellaceae UCG 001 was positively correlated with serum prolactin concentrations, but negatively correlated with fecal isobutyric acid, isovaleric acid, and valeric acid concentrations on d 18 of lactation (P < 0.05).
Fig. 9.
Heatmap of the spearman correlations (r) between the gut microbiota significantly modified by different diets treatment and period of sows in. Data are presented as means ± SEM (n = 3, 5 or 7). ∗P < 0.05; ∗∗P < 0.01 (following the Spearman correlation analysis). F, at farrowing; L, at d 18 of lactation.
4. Discussion
A short duration of farrowing is important for piglet survival, as a delay can increase stillborn numbers (Herpin et al., 1996). Sows are generally unresponsive to their piglets during farrowing. The changes seen in the posture and responsiveness to piglets were delayed in sows with a longer parturition, suggesting some involvement of cumulative piglet births on passivity, which has serious implications for both the economic results of the herd and the health of the animals (Jarvis et al., 1999). Many factors may affect the duration of farrowing (Oliviero et al., 2010; Yun et al., 2013), including the breed and age of the sow, length of gestation, number of piglets born, housing, body condition of the sow, and state of constipation (Yun et al., 2015). At farrowing, the sow undergoes substantial hormonal and metabolic changes during a very short period of time (Bo and Uvnäs-Moberg, 2007). Moreover, reproductive hormones not only affect the duration of farrowing directly (Bo and Uvnäs-Moberg, 2007), but also affect it indirectly by regulating the state of constipation during late gestation (Gill et al., 1985). As previously mentioned, constipation is another important factor leading to prolonged labor and can disturb the gut microbiota (de Meij et al., 2016). During the perinatal period, metabolic disorders and an exacerbated inflammatory status were found to be partly due to disorders in the gut microbiota of the sows (Chuanshang et al., 2018), which had a harmful effect on the performance of the animals. In the present study, sows fed diets containing IMO had a shorter duration of farrowing compared with those fed diets without IMO. This was partly because oligosaccharides can relieve constipation by increasing both the water content of feces and the small intestinal transit rate (Wang et al., 2017), and partly because they can restore the gut microbiota (Shi et al., 2017). However, the IMO group showed a weaker effect in shortening the farrowing duration compared with the IMOS, IMOL, and IMOSL groups. This suggests that the oligosaccharide alone has a weaker effect in shortening the farrowing duration of sows compared with IMO combined with Bacillus (Yu et al., 2016; Shi et al., 2017).
In our study, the supplementation of IMO in combination with B. licheniformis shortened the weaning-to-estrus interval, and the supplementation of IMO plus B. licheniformis and B. subtilis increased the estrus rate (0 to 7 d) during the perinatal period. Likewise, Duan et al. (2016) observed a decrease in the weaning-to-estrus interval of sows by adding mannan oligosaccharide to their diets during late gestation. The present study found that sows fed a diet containing IMO and Bacillus had altered concentrations of serum reproductive hormones both at farrowing and at d 18 of lactation. Further, the changes in reproductive hormone levels were different between the 2 periods. There were no differences in the concentrations of serum estradiol, oxytocin, and progesterone among all groups at farrowing, but the concentration of serum prolactin was increased in the IMOS and IMOL groups, and prostaglandin was only increased in the IMOS group at farrowing. Plasma oxytocin concentrations are low or undetectable in late pregnancy. Increases in the oxytocin and estradiol levels are seen at the time of fetus' delivery and at the expulsion of the placenta, while the concentration of progesterone decreases at the same time (Forsling et al., 1979). In addition, there was no evidence that the increases in serum prolactin and prostaglandin could shorten the duration of farrowing. Thus, the reason why the diets containing IMO and Bacillus shortened the duration of farrowing during the perinatal period could be attributed to the relief of constipation through the regulation of the gut microbiota in the sows (Chen et al., 2001; Oliviero et al., 2010). During lactation, the serum prolactin concentration increases due to the stimulation caused by the piglets sucking on the sow's nipples, which leads to lactational anestrus in the sow (Dusza and Krzymowska, 1981). At d 18 of lactation, we showed that adding IMOS to the sow's diet during the perinatal period can cause the sow to maintain a higher lactation state due to the increase of prolactin. Further, the concentrations of serum LH, estradiol, and progesterone are very low in sows with anestrus after weaning (Cox et al., 1988). There is also no difference in serum progesterone concentration at d 18 of lactation between the IMOL and the CON groups, but the serum progesterone concentration in the IMO, IMOS and IMOSL groups are significantly decreased compared with the CON and IMOL group. This could possibly be one reason behind the shorter weaning-estrus interval in the IMOL group (Cox et al., 1988). Therefore, according to these results, we can infer that IMO and Bacillus have different effects on hormone secretion in different periods. In addition, the concentration of serum reproductive hormones at d 18 of lactation did not reflect those after ablactation (Dusza and Krzymowska, 1981).
Most studies have shown that low values of gut microbial diversity and richness are associated with adverse conditions, such as the increase of gut permeability, insulin resistance, and a more pronounced inflammatory phenotype (Emmanuelle et al., 2013; Mokkala et al., 2016). During gestation, especially in late gestation, the gut microbial diversity and richness decrease (Chuanshang et al., 2018). Both prebiotics and probiotics can improve the diversity and richness of the gut microbiota (Shi et al., 2017; Wang et al., 2017). In addition, studies have found that probiotics can regulate the secretion of reproductive hormones (Mnt et al., 2017; Jamilian et al., 2018) by altering the composition of the gut microbiota (Adlercreutz et al., 1984; Baker et al., 2017). These studies prompted us to investigate the effects of IMO and Bacillus on the gut microbiota of sows during the perinatal period. The results suggest the emergence of a dramatic change in the gut microbiota over the different treatments and periods. At farrowing, the highest gut microbial richness and α-diversity values were found in the IMOL group and the lowest in the IMOS group. Interestingly, there were no statistically significant differences in the gut microbial richness and α-diversity values of sows among all groups at d 18 of lactation. The reason for these results may be that the degree of metabolic disturbance in the delivering sow is greater than that in the later period of lactation (Chuanshang et al., 2018). As metabolic disorders of the body can cause a decrease in digestion, more nutrients (that are more conducive to the growth of harmful bacteria) enter into the hindgut and are fermented by microbiota (Bikker et al., 2007; Gaskins, 2001; Williams et al., 2005). This fermentation will further affect the composition of gut microbiota (Chuanshang et al., 2018). Aside to the gut microbial diversity, studies have found substantial shifts in that the phylogenetic composition of the gut microbiota over the different treatments and periods. Firmicutes and Bacteroidetes were the most dominant phyla regardless of breeding stages (Chen et al., 2017; Kong et al., 2017). In our study, the relative abundance of phylum Firmicutes was only higher in the IMO group, compared with the CON group at farrowing. However, sows fed the diets containing IMO and IMOS had a higher relative abundance of phylum Firmicutes than those fed the diets without IMO at d 18 of lactation, whereas Bacteroidetes showed the opposite result. The increase in Firmicutes was considered a means of enhancing the body's capacity for energy acquisition from the diet, which would be a potential advantage to support fetus growth and to prepare the body for the energy needs of lactation (Turnbaugh et al., 2006). Remarkably, a decrease in the Proteobacteria was observed in all treatment groups at farrowing. Additionally, the genera Escherichia and Shigella (phylum Proteobacteria) were reduced in all treatment groups at d 18 of lactation. The Proteobacteria are a minor constituent within a balanced gut-associated microbial community (Eckburg et al., 2005). However, the dysbiotic expansion of the facultatively anaerobic Proteobacteria has been observed under conditions of gut inflammation, including irritable bowel syndrome and inflammatory bowel disease (Morgan et al., 2012). Recent studies have proposed that an expansion of the Proteobacteria could be a potential diagnostic microbial signature of dysbiosis in the gut microbiota and of epithelial dysfunction (Na-Ri et al., 2015). The relative abundance of genus Bacillus improved on d 18 of lactation instead of at farrowing, through the addition of IMO and Bacillus to the sow's perinatal diet. Bacillus can inhibit the proliferation of harmful bacteria by consuming oxygen and secreting bacteriostatic substances into the intestinal tract (Ying et al., 2018). Moreover, genus Bacillus was positively correlated with the fecal propionic acid and fecal total SCFA levels in sows at farrowing, but negatively correlated with the duration of farrowing, which is an important reason behind why the duration of farrowing was shortened by the IMO and Bacillus supplementation to the perinatal diet (Wang et al., 2017).
As the dietary supplementation with IMO and Bacillus had profoundly altered the composition of the sow's gut microbiota at farrowing and lactation, the total SCFA concentration had also changed in all treatment groups at farrowing, resulting in an increase to the branched fatty acid concentration in the IMOS and IMOL groups at d 18 of lactation. Metabolic products from gastrointestinal microbial fermentation, such as SCFA, can stimulate the enteric nervous system and affect gut transit times (Giovanni et al., 2005). Likewise, Wang et al. (2017) demonstrated that oligosaccharides administered as a dietary supplementation increased the water content of feces, reduced the intestinal transit time, modulated the composition of the gut microbiota, and increased the concentration of SCFA in the feces of mice with constipation. Moreover, Shi et al. (2017) have shown that the effects of Lactobacillus cocktails against the cefixime-induced changes in the gut microbiota could be primarily attributed to the beneficial SCFA produced in vivo, which may also be related to the good cell adhesion properties observed in vitro.
5. Conclusion
In conclusion, the present study shows that IMO and Bacillus administered to sows as a dietary supplement during the perinatal period shortened the duration of farrowing and the weaning-estrous interval, altered the gut microbiota composition and increased the concentration of fecal SCFA. Furthermore, the combination of IMO and Bacillus have beneficial effects on gut microbiota and the duration of farrowing and post-weaning estrus. However, the time-dependent effect and the causality of IMO and its combination with Bacillus on the relationship between gut microbiota and serum reproductive hormone concentrations, as well as the underlying mechanism, remain to be further confirmed.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was jointly supported by funding from Double first-class construction project of Hunan Agricultural University (SYL201802009, SYL201802015), and the National Key Research and Development Program of China (2017YFD0500506; 2016YFD0501209). We would like to thank the workers in Allwegene Tech. (Beijing, China) for their experiment on 16s rDNA high-throughput sequencing and data preparation and analysis. The authors are also grateful to the staff at Department of Animal Nutrition and Feed Science of Hunan Agricultural University for their assistance in conducting the experiment. The authors also gratefully acknowledge Hunan Zhenghong Pig Breeding Farm (Zhenghong, Inc., HuNan, China) for their input to this study.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.06.010.
Contributor Information
Xi He, Email: hexi111@126.com.
Zhiyong Fan, Email: fzyong04@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adlercreutz H., Pulkkinen M.O., Hämäläinen E.K., Korpela J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984;20:217–229. doi: 10.1016/0022-4731(84)90208-5. [DOI] [PubMed] [Google Scholar]
- Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Bikker P., Dirkzwager A., Fledderus J., Trevis P., le Huërou-Luron I., Lalle`s J.P. Dietary protein and fermentable carbohydrates contents influence growth performance and intestinal characteristics in newly weaned pigs. Livest Sci. 2007;108:194–197. doi: 10.1016/j.livsci.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Bo A., Uvnäs-Moberg K. Maternal behavior in pigs. Horm Behav. 2007;52:78–85. doi: 10.1016/j.yhbeh.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Bosch G., Pellikaan W.F., Rutten P.G.P., van der Poel A.F.B., Verstegen M.W.A., Hendriks W.H. Comparative in vitro fermentation activity in the canine distal gastrointestinal tract and fermentation kinetics of fiber sources. J Anim Sci. 2008;86:2979–2989. doi: 10.2527/jas.2007-0819. [DOI] [PubMed] [Google Scholar]
- Bradley C.S., Kennedy C.M., Turcea A.M., Rao S.S.C., Nygaard I.E. Constipation in pregnancy: prevalence, symptoms, and risk factors. Obstet Gynecol. 2007;110:1351–1357. doi: 10.1097/01.AOG.0000295723.94624.b1. [DOI] [PubMed] [Google Scholar]
- Cao H., Liu X., An Y., Zhou G., Liu Y., Xu M. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7:10322. doi: 10.1038/s41598-017-10835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lu Y., Lin J., Ko L. Effects of isomalto-oligosaccharides on bowel functions and indicators of nutritional status in constipated elderly men. J Am Coll Nutr. 2001;20:44–49. doi: 10.1080/07315724.2001.10719013. [DOI] [PubMed] [Google Scholar]
- Chen K.L., Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metabol. 2016;27:752–755. doi: 10.1016/j.tem.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Chen L., Xu Y., Chen X., Fang C., Zhao L., Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol. 2017;8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuanshang C., Hongkui W., Huichao Y., Chuanhui X., Siwen J., Jian P. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front Microbiol. 2018;9:1989. doi: 10.3389/fmicb.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.M., Ramirez J.L., Matamoros I.A., Bennett W.A. Estrogen induces estrus unaccompanied by a preovulatory surge in luteinizing hormone in suckled sows. Biol Reprod. 1988;38:592–596. doi: 10.1095/biolreprod38.3.592. [DOI] [PubMed] [Google Scholar]
- Cremon C., Gargano L.L., Morselli-Labate A.M., Santini D., Cogliandro R., De-Giorgio R. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- de Meij T.G., de Groot E.F., Eck A., Budding A.E., Kneepkens C.M., Benninga M.A. Characterization of microbiota in children with chronic functional constipation. PloS One. 2016;11 doi: 10.1371/journal.pone.0164731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.D., Chen D.W., Zheng P., Tian G., Wang J.P., Mao X.B. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim Feed Sci Technol. 2016;218:17–25. doi: 10.1016/j.anifeedsci.2016.05.002. [DOI] [Google Scholar]
- Dusza L., Krzymowska H. Plasma prolactin levels in sows during pregnancy, parturition and early lactation. J Reprod Fertil. 1981;61:131–134. doi: 10.1530/jrf.0.0610131. [DOI] [PubMed] [Google Scholar]
- Dyck G.W., Palmer W.M., Simaraks S. Postweaning plasma concentrations of luteinizing hormone and estrogens in sows: effect of treatment with pregnant mare’s serum gonadotropin or estradiol17β plus progesterone. Can J Anim Sci. 1979;59:159–166. doi: 10.4141/cjas79-019. [DOI] [Google Scholar]
- Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuelle L.C., Trine N., Junjie Q., Edi P., Falk H., Gwen F. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Forsling M.L., Taverne M.A., Parvizi N., Elsaesser, Smidt F.D., Ellendorff F. Plasma oxytocin and steroid concentrations during late pregnancy, parturition and lactation in the miniature pig. J Endocrinol. 1979;82:61–69. doi: 10.1677/joe.0.0820061. [DOI] [PubMed] [Google Scholar]
- Frankenfeld C.L., Atkinson C., Wähälä K., Lampe J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or o-desmethylangolensin from the isoflavone daidzein. Eur J Clin Nutr. 2014;68:526–530. doi: 10.1038/ejcn.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins H.R. Intestinal bacteria and their influence on swine growth. In: Lewis A.J., Southern L.L., editors. Swine nutrition. CRC Press LLC; Boca Raton, USA: 2001. pp. 585–608. [Google Scholar]
- Gill R.C., Bowes K.L., Kingma Y.J. Effect of progesterone on canine colonic smooth muscle. Gastroenterology. 1985;88:1941–1947. doi: 10.1016/0016-5085(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Giovanni B., Vincenzo S., Giovanni B., Cesare C., Giovanni D.N., Roberto D.G. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Herpin P., Le D.J., Hulin J.C., Fillaut M., De M.F., Bertin R. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J Anim Sci. 1996;74:2067–2075. doi: 10.2527/1996.7492067x. [DOI] [PubMed] [Google Scholar]
- Hiroyuki M., Toshiki Y., Noriaki A., Tetsuji T., Yasunobu Y. Isomalto-oligosaccharides polarize Th1-like responses in intestinal and systemic immunity in mice. J Nutr. 2005;135:2857–2861. doi: 10.1089/jmf.2005.8.560. [DOI] [PubMed] [Google Scholar]
- Homma H., Hoy E., Xu D.Z., Lu Q., Feinman R., Deitch E.A. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- Jamilian M., Mansury S., Bahmani F., Heidar Z., Amirani E., Asemi Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:80. doi: 10.1186/s13048-018-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S., Mclean K.A., Calvert S.K., Deans L.A., Chirnside J., Lawrence A.B. The responsiveness of sows to their piglets in relation to the length of parturition and the involvement of endogenous opioids. Appl Anim Behav Sci. 1999;63:195–207. doi: 10.1016/s0168-1591(99)00013-1. [DOI] [Google Scholar]
- Kai M., Li L., Mo T., Pan H., Hong L. Preparation, characterization, and antioxidant activity of an Isomaltooligosaccharide–Iron complex (IIC) J Carbohydr Chem. 2015;34:430–443. doi: 10.1080/07328303.2015.1085551. [DOI] [Google Scholar]
- Kong X.F., Ji Y.J., Li H.W., Zhu Q., Blachier F., Geng M.M. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: effects of food composition at different times of pregnancy. Sci Rep. 2017;6:37224. doi: 10.1038/srep37224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Tan B., Mai K. Dietary probiotic Bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei) Aquaculture. 2009;291:35–40. doi: 10.1080/07328303.2015.1085551. [DOI] [Google Scholar]
- Mnt L., Thybo C.B., Lykkeboe S., Rasmussen L.M., Frette X., Christensen L.P. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017;106:909–920. doi: 10.3945/ajcn.117.153353. [DOI] [PubMed] [Google Scholar]
- Mokkala K., Röytiö H., Munukka E., Pietilä S., Ekblad U., Rönnemaa T. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J Nutr. 2016;146:1694–1700. doi: 10.3945/jn.116.235358. [DOI] [PubMed] [Google Scholar]
- Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na-Ri S., Tae Woong W., Jin-Woo B. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Swine. 10th ed. revised ed. The National Academies Press; Washington, DC: 1998. [Google Scholar]
- Oliviero C., Heinonen M., Valros A., Peltoniemi O. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Oliviero C., Kokkonen T., Heinonen M., Sankari S., Peltoniemi O. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res Vet Sci. 2009;86:314–319. doi: 10.1016/j.rvsc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Patel S., Goyal A. Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol. 2011;27:1119–1128. doi: 10.1007/s11274-010-0558-5. [DOI] [Google Scholar]
- Shi Y., Zhai Q., Li D., Mao B., Liu X., Zhao J. Restoration of cefixime-induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol Res. 2017;200:14–24. doi: 10.1016/j.micres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Vincent M., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Tan C.Q., Wei H.K., Sun H.Q., Long G., Ao J.T., Jiang S.W. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim Feed Sci Technol. 2015;210:254–262. doi: 10.1016/j.anifeedsci.2015.10.013. [DOI] [Google Scholar]
- Viorica B., Mathilde L., Claire B.B., Lionel B., Jean F., Eric H. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2010;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hu L., Yan S., Jiang T., Fang S., Wang G. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 2017;8:1966–1978. doi: 10.1039/c7fo00031f. [DOI] [PubMed] [Google Scholar]
- Wang X.X., Song P.X., Wu H., Xue J.X., Zhong X., Zhang L.Y. Effects of graded levels of isomaltooligosaccharides on the performance, immune function and intestinal status of weaned pigs. Asian-Australas J Anim Sci. 2016;29:250–256. doi: 10.5713/ajas.15.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.A., Bosch M.W., Awati A., Konstantinov S.R., Smidt H., Akkermans A.D.L. In vitro assessment of gastrointestinal tract (GIT) fermentation in pigs: fermentable substrates and microbial activity. Anim Res. 2005;54:191–201. 101051animres2005011. [Google Scholar]
- Ying T., Lei H., Chen X., Xie M., Kong W., Wu Z. Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under aeromonas hydrophila challenge. Probiotics Antimicrob Proteins. 2018;13:1–14. doi: 10.1007/s12602-018-9409-8. [DOI] [PubMed] [Google Scholar]
- Yu T., Zheng Y.P., Tan J.C., Xiong W.J., Wang Y., Lin L. Effects of prebiotics and synbiotics on functional constipation. Am J Med Sci. 2016;353:282–292. doi: 10.1016/j.amjms.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Yun J., Swan K.M., Oliviero C., Peltoniemi O., Valros A. Effects of prepartum housing environment on abnormal behaviour, the farrowing process, and interactions with circulating oxytocin in sows. Appl Anim Behav Sci. 2015;162:20–25. doi: 10.1016/j.applanim.2014.11.006. [DOI] [Google Scholar]
- Yun J., Swan K.M., Vienola K., Farmer C., Oliviero C., Peltoniemi O. Nest-building in sows: effects of farrowing housing on hormonal modulation of maternal characteristics. Appl Anim Behav Sci. 2013;148:77–84. doi: 10.1016/j.applanim.2013.07.010. [DOI] [Google Scholar]
- Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.