Abstract
Background
Colonoscopy requires the intubation of the cecum for screening of colorectal diseases. The conventional position used for colonoscopy is the left lateral position (LLP). However, alternative positions have also been utilized to enhance the success of intubation. Thus, the aim of this study was to perform a meta-analysis of the different positions to determine the effectiveness of the individual positions for successful colonoscopy.
Methods
Medline, Embase and Cochrane trials electronic databases were searched for studies on colonoscopy positions. The primary outcome was defined as the cecal intubation rate. Pooled risk ratios (RR) and 95% confidence intervals (CI) for the rates of cecal intubation were estimated. Secondary outcomes such as the cecal intubation time and adenoma detection rate were further analyzed qualitatively.
Results
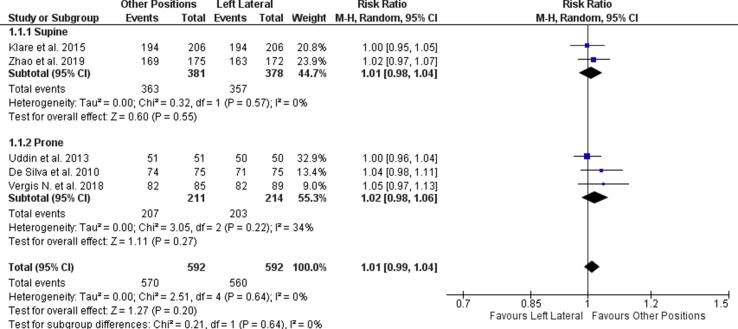
After reviewing 644 identified records, 7 randomized control trials (RCT) studies were included. No significant difference was observed in either comparisons, between the LLP vs. supine position (SP) (RR = 1.01, 95% CI, 0.98 to 1.04, P = 0.55) or the LLP vs. prone position (PP) (RR = 1.02, 95% CI, 0.98 to 1.06, P = 0.27).
Conclusions
Amidst available literature, the use of other positions can be considered when performing colonoscopy. These further highlights that the existential practice is based predominantly on familiarity instead of evidence-based-research.
Keywords: Colonoscopy, Endoscopy, Meta analysis, Patient positioning
Introduction
Worldwide, colorectal cancer has nearly the highest diagnosed malignancies whilst being third in morbidity and second in mortality.1 Colonoscopy is the investigation of choice for diagnosis of colorectal cancer2, 3, 4, 5, 6 and is regarded as the gold standard for all lower gastrointestinal (GI) investigations.5 As such, there is much emphasis placed on improving colonoscopy techniques, so that its efficiency, accuracy and comfort can be improved.6
Traditionally, colonoscopy is conducted in the left lateral position (LLP).7 This position offers protection to sedated patients with an unprotected airway by facilitating easier access to the colon for the operator.8 However, several studies report that performing colonoscopy in the LLP may not be the most optimal.9,10 Anatomically, the left colon is considered as the most difficult section of the colon to navigate during insertion, and even more so for the sigmoid colon. Furthermore, starting in the LLP allows for air to rise out from the left colon, collapsing it, hindering the procedure.
Surrogate measurements of colonoscopy effectiveness primarily include cecal intubation and adenoma detection rates.5,11 Other surrogate measures include colonoscopy completion and complication rates, sedation and patient comfort levels.5 Cecal intubation is vital for the visualization of colonic mucosa and is defined as the insertion of the tip of the colonoscope into the cecal pole, proximal to the ileocecal valve.12 Based on the guidelines by the American Society for Gastrointestinal Endoscopy (ASGE), a cecal intubation rate of at least 90% should be achieved by effective colonoscopists.13
Given the common utility of colonoscopy and its impactful applications, methods to improve the procedure are highly sought after. Certain positions have been reported to straighten the sigmoid colon and enhance the view of the endoscopist as faecal residue and fluid are no longer obstructing.9,14 To facilitate the discussion of a more effective starting position for colonoscopy, this study focuses on comparing colonoscopies in the standard LLP with non-LLPs.
Methods
This study utilizes the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.15
Search strategy
The search was conducted on three electronic databases including Medline, Embase and Cochrane Trials from inception to July 23, 2020 for full text and conference abstracts.16,17 With assistance from a medical librarian, search terms were composed with index and keyword searches on colonoscopy and positions (supplementary material 1). Preceding which, potential abstracts were imported into EndNote X9 (Clarivate Analytics, USA) and duplicates were removed.
Criteria for the selection of studies
Randomized control trials (RCT) were included if they met the following inclusion criteria: Patients who have undergone colonoscopy for screening or diagnostic purposes; Patients were with the starting LLP were compared against other scope positions. Studies reporting the following outcomes including cecal or ileal intubation rate and time, adenoma detection rate, pain score, post-operative questionnaire and analysis. RCT were excluded on the basis of the following criteria: Studies which involved subjects undergoing sigmoidoscopies; Subjects who had undergone emergency colonoscopy; Subjects whom had undergone colonoscopy post-surgery. The first 2 authors independently assessed all publications generated for relevance and conformity to these criteria, and discrepancies were removed with the participation of a third author.
Assessment of bias and data extraction
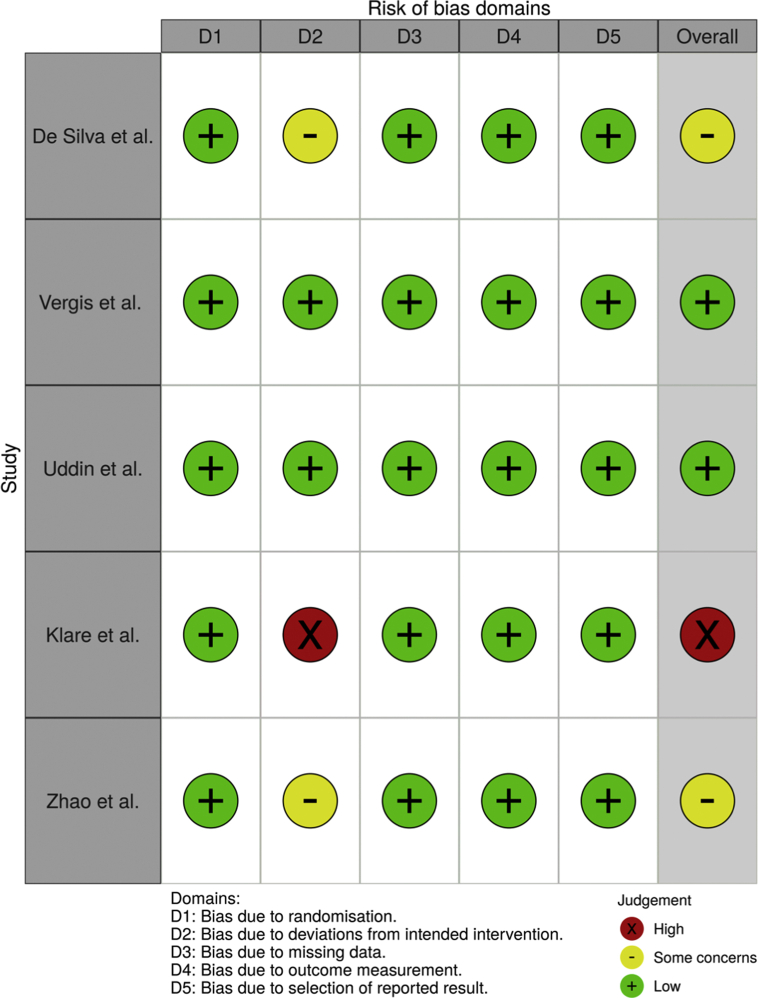
The blinded pair independently reviewed included studies. The Cochrane Risk of Bias 2.0 (Cochrane, UK) was used in the assessment of the bias in individual studies.18 The 2 authors independently appraised the quality of included articles, and any inconsistencies were resolved between with the participation of a third author. The robvis19 tool was used in the depicture of the outcome of assessment from Cochrane Risk of Bias 2.0, with fixed set of 5 domains of bias.
Data extraction was then performed using a pre-designed extraction sheet. The data extracted from each study included the general information (author, year of publication, title, source, country and journal), study characteristics (study design, positions compared, sample sized, baseline characteristics, amount and type of analgesia utilized) and outcomes (successful cecal or ileal intubation rates, adenoma detection rate, the cecal or ileal intubation time and the adverse events). For continuous and dichotomous variables, the mean, standard division and count were extracted. For continuous variables reporting the median and range, calculations proposed by Hozo et al20 were undertaken to derive mean and standard deviation. While for continuous variables reporting the median and interquartile range, calculations proposed by Wan et al21 were undertaken to obtain the mean and standard deviation.
Statistical analysis
When possible, meta-analysis was undertaken with Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). Effect sizes for dichotomous variables were pooled using Mantel Hansel's risk ratio (RR), and a value of P < 0.05 was considered statistically significant. Test for heterogenicity was assessed with Cochran Q-test and I2 with a significance value of at 10% (P < 0.1) or I2 >40 respectively was considered statistically significant for heterogenicity.22,23 Regardless random effects was applied when significant heterogenicity is found. When meta-analysis was deemed inappropriate or insufficient data amount for analysis, a descriptive approach would be undertaken in the presentation of the results. Publication bias for funnel plots was not done since there was less than 10 studies.
Results
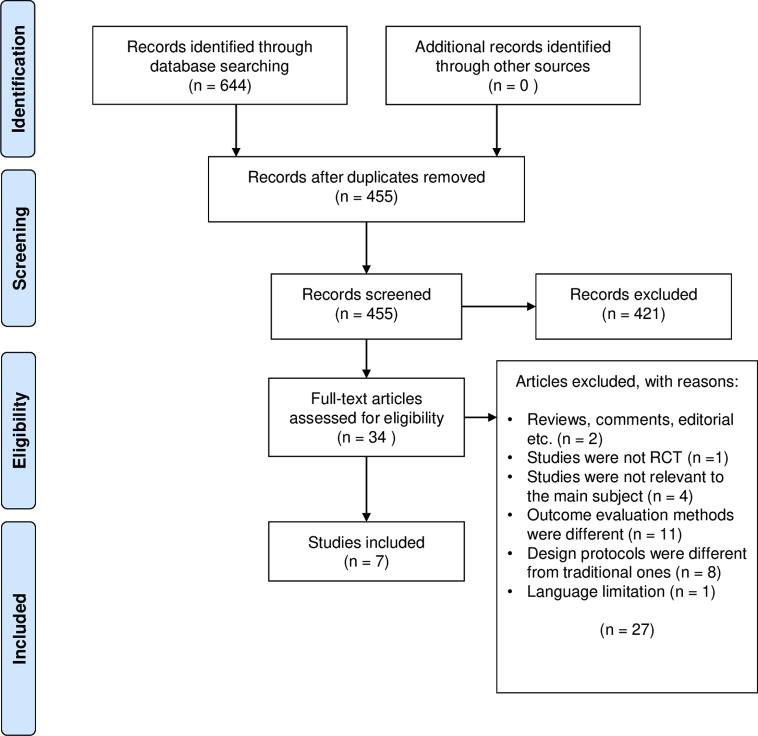
A total of 644 articles were retrieved, 189 duplicates were removed, and 27 studies were excluded after assessing for eligibility and the final 5 randomized controlled trials and 2 conference abstracts were included. A graphical summary of the selection strategy is illustrated as a flowchart in Fig. 1. All 7 of the selected articles were randomized control trials which compared the LLP to supine position (SP) (n = 2), prone position (PP) (n = 3) and right lateral position (RLP) (n = 2). There was a total of 1551 patients; 781 patients in the LLP, 381 in the SP, 211 in the PP and 178 in the RLP. The characteristics of the included studies were reported in Table 1. The risk of bias was assessed for the full text articles and are presented in Fig. 2.
Fig. 1.
PRISMA flow chart.
Table 1.
Characteristics of included studies.
| References | Nature of Article | Country | Sample Size (n) |
Intervention |
Control |
Experience of Endoscopist | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | Age (years) (Mean ± SD) | Type of Analgesia (Dose) | Position | Age (years) (Mean ± SD) | Type of Analgesia (Dose) | |||||
| Klare et al,24 2015 |
FT | Germany | 412 | SP | 56.8 ± 15.4 | Propofol (180 mg) | LLP | 54.9 ± 17.1 | Propofol (200 mg) | NA |
| Zhao et al,25 2019 |
FT | China | 347 | SP | 51.5 ± 14.3 | NA | LLP | 52.8 ± 14.6 | NA | 1 (<1000 Endoscopes) 1 (1000–3000 Endoscopes) 1 (>3000 Endoscopes) |
| Uddin et al,27 2014 |
FT | India | 101 | PP | 62.5 | Midazolam (3.0 mg) Fentanyl (71.5 μg) Meperidine (58.3 mg) None |
LLP | 60.7 | Midazolam (3.1 mg) Fentanyl (62.9 μg) Meperidine (50 mg) None |
3 Experienced |
| Vergis et al,28 2018 |
FT | United Kingdom | 174 | PP | 59 | Midazolam (2 mg) Fentanyl (50 μg) |
LLP | 55 | Midazolam (2 mg) Fentanyl (50 μg) |
1 (>200 Endoscopes) |
| De Silva et al,26 2010 |
FT | Sri Lanka | 150 | PP | 50 ± 17.91 | Midazolam (2.5 mg) Peptidine (25 mg) |
LLP | 55 ± 18.24 | Midazolam (2.5 mg) Peptidine (25 mg) |
9 (<200 Endoscopes) |
| Mocanu et al,30 2017 |
AB | Spain | 188 | RL | 61 | Midazolam (1.04 mg) Peptidine (25 mg) |
LLP | 64 | Midazolam (1.09 mg) Peptidine (25 mg) |
5 (200–500 Endoscopes) |
| Gonzalez et al,29 2017 |
AB | Spain | 179 | RL | NA | NA | LLP | NA | NA | 126 (500–5000 Endoscopes) |
RCT: Randomized control trials; LLP: Left Lateral Position; PP: Prone Position; SP: Supine Position; PP: Prone Position; NA: Datasets were not provided by publication; FT: Full Text Articles; AB: Abstract.
Fig. 2.
Risk of bias assessment of included studies.
Effects of the interventions
LLP vs. SP
Two studies compared the LLP and the SP. No significant difference was observed in the intubation rates across the 2 groups (RR = 1.01, 95% CI, 0.98 to 1.04, P = 0.55, I2 = 0; Fig. 3). Secondary outcomes were reported in Klare et al24 and Zhao et al25 studies. Adenoma detection rates were observed to be higher in the SP as compared to the LLP by Klare et al24 (23.3% SP vs. 18.4% LLP, P = 0.225) and Zhao et al25 (23.1% SP vs. 21.0% LLP, P = 0.64). However, the adenoma detection rate was not significantly higher in the SP than LLP for both studies. The mean intubation time was also observed to be shorter in SP than in the LLP for both Zhao et al25 (315.67 ± 80.74 s LLP vs. 279.17 ± 70.00 s SP) and Klare et al24(1534.48 ± 1408.94 s LLP vs. 1067.09 ± 780.62 s SP).
Fig. 3.
Forest plot of cecal and ileal intubation rate between the left lateral, supine and prone position.
LLP vs. PP
Three studies compared the LLP and the PP. No significant difference was observed in the intubation rates across the 2 groups (RR = 1.02, 95% CI, 0.98 to 1.06, P = 0.27, I2 = 34%, Fig. 3). When considering the secondary outcomes, the intubation time was found to be inconsistent across the three studies. De silva et al26 and Uddin et al27 reported the mean intubation time to be longer in the LLP than in the PP position (68.33 ± 60.74 s vs. 10.67 ± 7.41 s; 550 s vs. 420 s), while Vergis et al28 indicated a shorter intubation time for the LLP than the PP (546.76 ± 126.77 s vs. 723.28 ± 153.00 s). The adenoma detection rate was not reported for all 3 studies.
LLP vs. RLP
Two studies compared the starting LLP to the RLP. Gonzalez et al29 reported a longer median cecal intubation time in the RLP than the LLP (727.54 s vs. 628.69 s). Similarly, Monacu et al30 revealed a longer median cecal intubation time in the RLP for patients with a body mass index >25.0 (654 s RLP vs. 570 s LLP) and those who had previously undergone surgery (702 s RLP vs. 650 s LLP).
Additional outcomes
Four studies reported patient's comfort in relation to the different colonoscopy positions. In the SP, Klare et al24 and Zhao et al25 presented contradicting results. Klare et al24 indicated that there was no significant difference between patient satisfaction levels in the LLP and SP (Visual analogue scale/score (VAS) score: 10 LLP vs. 10 SP, P = 0.430), whilst Zhao et al25 indicated that pain scores were significantly lower in the SP (VAS score: 3.9 ± 1.7 LLP vs. 3.3 ± 1.6 SP, P = 0.002). In the PP, Uddin et al27 and Vergis et al28 reported no significant difference in patient comfort levels (VAS score: 3.7 LLP vs. 3.8 PP, P = 0.79; 4.0 LLP vs. 4.0 PP, P = 0.6).
Two studies compared colonoscopy difficulty, as perceived by endoscopists. Comparing with the LLP, Zhao et al25 indicated that it was easier to conduct colonoscopy in the SP (VAS score: 3.1 ± 1.2 SP vs. 3.7 ± 1.5 LLP, P < 0.001) whilst Vergis et al28 indicated that colonoscopy in the PP is more challenging (VAS score: 5.0 PP vs 4.0 LLP, P = 0.002).
Side effects from colonoscopy were reported by Klare et al,24 who suggested significantly more frequent hypoxemic events in patients with the SP starting than LLP starting (12.14% vs. 6.80%, P = 0.003).
Discussion
Being a cornerstone of lower GI investigations and essential in colorectal cancer diagnosis, colonoscopy is still being fraught with rates of incompletion.31 This meta-analysis is primarily aimed at compiling the available evidence for consideration of differing starting colonoscopy positions from the conventional LLP. Although our results revealed that colonoscopies starting in the SP were no different than those in the LLP in terms of cecal intubation, the cecal intubation times were shorter in the SP, perhaps indicating the relative ease of performing colonoscopy in the SP compared to that of the LLP. Adenoma rates were also noted to be higher in the SP, although the differences were not statistically significant.
Performing colonoscopy in the SP should be considered as it is not predisposed to the disadvantages of LLP, which include the obstruction of the colonoscope due to air rising out from the left colon.25 Hence, endoscopists may have an enhanced view25 and better manoeuvrability32 during colonoscopy. Furthermore, the SP facilitates the application of abdominal pressure, which aids in the colonoscopy process.33 These factors could underpin the rationale for rates of improved cecal intubation rates and better adenoma detection.34
Cecal intubation and adenoma detection are surrogates that can be imprecise due to confounding. Hence, an interesting article by Ahammed et al35 randomised patients to SP and LLP after cecal intubation while studying ileal intubation rates and time. A statistically significant improvement of outcomes was achieved in the SP. Unfortunately, due to its unique nature in the available literature, it cannot be meta-analysed with the other RCTs and the superiority of SP cannot be concluded base on this study alone.
Although not significant, the analysis between PP and LLP colonoscopies revealed that there was a higher rate of intubation in the PP compared to LLP. A lack of significance could be the lack of sufficient sample size rather than the lack of measured effect. Moreover, the reported intubation times were contradictory across the studies. This inconsistency could have arisen from the fact that De silva et al26 examined ileal intubation time while Vergis et al28 examined cecal intubation. The inclusion of trainees in Vergis et al28 study might have also resulted in a differing outcome when compared to De silva et al26 and Uddin et al27
Endoscopist's preferences and experience play an important part in deciding the effectiveness of colonoscopy. Several studies noted the effects of the endoscopist's perception of the difficulty of colonoscopy when the position was PP,28 RLP36 and SP25 was utilised. While one study showed that colonoscopies conducted in the PP position may be more challenging due to increased difficulty in negotiating the left colon,28 others indicated that colonoscopies in the SP and RLP were easier and the extent of difficulty discrepancies were based on endoscopist experience,25,36 with those who are more experienced feeling less of a difference.36 Given this fact, trainees should be exposed to various colonoscopy starting positions and made aware of their choices on a case-by-case basis. For example, in obese patients, starting colonoscopy in the PP has been reported to result in shorter cecal intubation times and decreased need for patient repositioning.10,27
Colonoscopies in various positions have similar patient safety considerations. Excluding Klare et al24 who suggested that significantly more frequent hypoxemic events occurred in the SP compared to the LLP, no known studies have reported adverse effects of starting colonoscopy in non-LLPs. Furthermore, studies included in our study showed that colonoscopies in the non-LLPs were comparable to colonoscopies in the LLP in terms of several surrogate measures such as cecal intubation rates, adenoma detection rates, intubation times and colonoscopy procedural difficulty. Patient comfort is another consideration in determining the viability of colonoscopies in non-LLPs. Studies comparing SP24,25 and PP colonoscopies27,28 with LLP colonoscopies reported that colonoscopies in non-LLPs were well accepted and in fact, Zhao et al25 expressed that less pain was felt by patients.
Despite the proposed advantages of alternative starting positions, there are limitations of the current evidence till date. Firstly, starting position is amongst many other maneuvers that endoscopists commonly adopt like dynamic position change during endoscopy, abdominal pressure33 and administration of antispasmodic agents.37,38 These factors, being potential confounders, are difficult to control in any RCT studying starting positions, as preferences amongst different endoscopists are variable and biased by each individual's training. Furthermore, none of the papers we included had measured perforation rate as an outcome. Thus, we are unable to analyze how the perforation rates differ between the various starting positions of colonoscopy.
Furthermore, this meta-analysis only included 5 RCTs and no cohort studies due to the limited amount of papers available. The inadequate amount of studies could be attributed to the lack of statistically significant data as colonoscopy were mostly performed by experience professionals, who may have similar performance when conducting colonoscopies regardless of starting position.
Conclusions
This meta-analysis has summarised the effectiveness of colonoscopies in various starting positions. In terms of completion rates, both the SP and PP are comparable to conventional LLP whilst for adenoma detection, SP has a slightly higher rate. However, it remains debateable still on which starting position is the most effective especially when other manoeuvres are largely not controlled for within the available RCTs.
Conflicts of interest
None.
Acknowledgement
We would like to express our gratitude to the academic librarian Ms. Annelissa Chin of the National University of Singapore, Yong Loo Lin School of Medicine for assisting us in creating the search strategy.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdtm.2020.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Borsch G., Schmidt G. Endoscopy of the terminal ileum. Diagnostic yield in 400 consecutive examinations. Dis Colon Rectum. 1985;28:499–501. doi: 10.1007/bf02554095. [DOI] [PubMed] [Google Scholar]
- 3.Williams C., Teague R. Colonoscopy. Gut. 1973;14:990–1003. doi: 10.1136/gut.14.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips K.A., Liang S.Y., Ladabaum U. Trends in colonoscopy for colorectal cancer screening. Med Care. 2007;45:160–167. doi: 10.1097/01.mlr.0000246612.35245.21. [DOI] [PubMed] [Google Scholar]
- 5.Rees C., Neilson L. Demonstrating that colonoscopy is high quality. Endosc Int Open. 2015;3:E634–E635. doi: 10.1055/s-0034-1392878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton P.B.W.C. Practical Gastrointestinal Endoscopy: The Fundamentals. 5th ed. Blackwell Publishing Ltd; 2008. Colonoscopy and flexible sigmoidoscopy. [Google Scholar]
- 7.Cappell M.S., Friedel D. The role of sigmoidoscopy and colonoscopy in the diagnosis and management of lower gastrointestinal disorders: technique, indications, and contraindications. Med Clin. 2002;86:1217–1252. doi: 10.1016/s0025-7125(02)00076-7. [DOI] [PubMed] [Google Scholar]
- 8.Willcock H., Gold D.M. Supine colonoscopy: an advantage over left lateral in synchronous proctological surgery. J Laparoendosc Adv Surg Tech. 2016;26:475–477. doi: 10.1089/lap.2015.0609. [DOI] [PubMed] [Google Scholar]
- 9.Vergis N., McGrath A.K., Stoddart C.H., Hoare J.M. Right or left in COLonoscopy (ROLCOL)? A randomized controlled trial of right- vs. Left-sided starting position in colonoscopy. Journal article; randomized controlled trial. Am J Gastroenterol. 2015;110:1576-1581. doi: 10.1038/ajg.2015.298. [DOI] [PubMed] [Google Scholar]
- 10.Desormeaux M.S.M., Friedland S. Colonoscopy in obese patients: a growing problem. J Gastrointestinal Endoscopy. 2008;67 doi: 10.1016/j.gie.2008.03.072. [DOI] [Google Scholar]
- 11.Rees C.J., Bevan R., Zimmermann-Fraedrich K. Expert opinions and scientific evidence for colonoscopy key performance indicators. Gut. 2016;65:2045–2060. doi: 10.1136/gutjnl-2016-312043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullens H.J., Siersema P.D. Quality indicators for colonoscopy: current insights and caveats. World J Gastrointest Endosc. 2014;6:571–583. doi: 10.4253/wjge.v6.i12.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex D.K., Schoenfeld P.S., Cohen J. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S.B., Yang X., Fang J. Effect of left lateral tilt-down position on cecal intubation time: a 2-center, pragmatic, randomized controlled trial. Gastrointest Endosc. 2018;87:852–861. doi: 10.1016/j.gie.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer R.W., Saldanha I.J. How should systematic reviewers handle conference abstracts? A view from the trenches. Letter. Syst Rev. 2019;8:264. doi: 10.1186/s13643-019-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopewell S., McDonald S., Clarke M.J., Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.MR000010.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mcguinness L.A., Higgins J.P.T. Risk, f-bias VISualization (robvis): An R package and Shiny web app for visualizing risk, f-bias assessments. Research Synthesis Methods. 2020 doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 20.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14 doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Klare P., Huth R., Haller B. Patient position and hypoxemia during propofol sedation for colonoscopy: a randomized trial. Endoscopy. 2015;47:1159–1166. doi: 10.1055/s-0034-1392329. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S., Yang X., Meng Q. Impact of the supine position vs. left horizontal position on colonoscopy insertion: a 2-center, randomized controlled trial. Gastrointest Endosc. 2019;89:1193–1201. doi: 10.1016/j.gie.2019.01.009. e1. [DOI] [PubMed] [Google Scholar]
- 26.De Silva A.P., Kumarasena R.S., Perera Keragala S.D. The prone 12 o'clock position reduces ileal intubation time during colonoscopy compared to the left lateral 6 o'clock (standard) position. Comparative Study. BMC Gastroenterol. 2011;11:89. doi: 10.1186/1471-230X-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uddin F.S., Iqbal R., Harford W.V. Prone positioning of obese patients for colonoscopy results in shortened cecal intubation times: a randomized trial. Dig Dis Sci. 2013;58:782–787. doi: 10.1007/s10620-012-2468-x. [DOI] [PubMed] [Google Scholar]
- 28.Vergis N., Scarborough A.J., Morris J.A., Hoare J.M. Prone or left for colonoscopy? A randomized controlled trial of prone vs. Left-sided starting position for colonoscopy. J Clin Gastroenterol. 2018;52:e82–e86. doi: 10.1097/MCG.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 29.Fabiola M., Gonzalez N.B., Casillas Gretel B. Sa1072 comparison between conventional left lateral position and right lateral position as the starting position in colonoscopy. Gastrointest Endosc. 2017;85:AB179–AB180. doi: 10.1016/j.gie.2017.03.391. [DOI] [Google Scholar]
- 30.Mocanu I., Laranjo A., Pires S. Colonoscopy on the left, right? United Eur Gastroent. 2017;5:A461. doi: 10.1177/2050640617725676. [DOI] [Google Scholar]
- 31.Kahi C.J., Boland C.R., Dominitz J.A. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Am J Gastroenterol. 2016;111:337–346. doi: 10.1038/ajg.2016.22. quiz 347. [DOI] [PubMed] [Google Scholar]
- 32.Early D., Larue S., Bhat T. The impact of trendelenberg positioning on ease of colonoscope insertion. Am J Gastroenterol. 2016;111:S145–S146. doi: 10.1038/ajg.2016.354. [DOI] [PubMed] [Google Scholar]
- 33.Waye J.D., Yessayan S.A., Lewis B.S., Fabry T.L. The technique of abdominal pressure in total colonoscopy. Gastrointest Endosc. 1991;37:147–151. doi: 10.1016/S0016-5107(91)70673-1. [DOI] [PubMed] [Google Scholar]
- 34.von Renteln D., Robertson D.J., Bensen S., Pohl H. Prolonged cecal insertion time is associated with decreased adenoma detection. Gastrointest Endosc. Mar 2017;85:574–580. doi: 10.1016/j.gie.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Ahammed S.M., Das K., Sarkar R., Dasgupta J., Bandopadhyay S., Dhali G.K. Patient-posture and Ileal-intubation during colonoscopy (PIC): a randomized controlled open-label trial. Endosc Int Open. 2014;2:E105–E110. doi: 10.1055/s-0034-1365541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath A., Stoddart C., Vergis N., Hoare J.M. Results of the rolcol study: a randomised controlled trial of right vs. left starting position in colonoscopy. Gut. 2015;64:A27–A28. doi: 10.1136/gutjnl-2015-309861.53. [DOI] [PubMed] [Google Scholar]
- 37.Marshall J.B., Patel M., Mahajan R.J., Early D.S., King P.D., Banerjee B. Benefit of intravenous antispasmodic (hyoscyamine sulfate) as premedication for colonoscopy. Gastrointest Endosc. 1999;49:720–726. doi: 10.1016/S0016-5107(99)70289-0. [DOI] [PubMed] [Google Scholar]
- 38.Tamai N., Matsuda K., Sumiyama K., Yoshida Y., Tajiri H. Glucagon facilitates colonoscopy and reduces patient discomfort: a randomized double-blind controlled trial with salivary amylase stress analysis. Eur J Gastroenterol Hepatol. 2013;25:575–579. doi: 10.1097/MEG.0b013e32835e33db. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.