Abstract
In children with desmoid-type fibromatosis (DTF) in whom disease progression occurs after an initial watch-and-wait strategy, prolonged low-dose chemotherapy using vinblastine and methotrexate (VBL-MTX) is currently the standard of care. These conventional drugs have been prospectively evaluated but their efficacy and safety profiles are limited, and alternative therapeutic options are therefore essential. Based on the results of clinical trials, the use of tyrosine kinase inhibitors (TKIs) in the treatment of DTF is currently considered only in adult patients. TKIs such as imatinib show superior therapeutic efficacy to VBL-MTX and tolerable short-term side effects for the treatment of adult DFT, supporting the concept of the use of TKIs for the treatment of paediatric DFT. Moreover, new-generation TKIs, such as pazopanib and sorafenib, have shown improved therapeutic efficacy compared to imatinib in adult non-comparative studies. A tolerable safety profile of TKI therapy in children with disease entities other than DTF, such as leukaemia, has been reported. However, the efficacy and, in particular, the long-term safety of TKIs, including childhood-specific aspects such as growth and fertility, for the treatment of children with DTF should be investigated prospectively, as DFT therapy requires long-term drug exposure.
Subject terms: Paediatric cancer, Paediatric research
Background
Desmoid-type fibromatosis (DTF) is a rare, deep-seated soft-tissue tumour, classified as a ‘locally aggressive fibroblastic/myofibroblastic tumour with intermediate malignancy’—a definition meaning that it has a high propensity for local dissemination and recurrence after resection, but does not metastasise to other organs like a high-grade malignant sarcoma would do. DTF can arise anywhere in the body, and can occur in children and in adults, with a peak incidence in young adults and an overall annual rate of around 2–4 cases per 1,000,000.1–3 Notably, one-third of cases occur in the first decade of life, and DTF accounts for up to 60% of fibrous tumours in childhood, with a peak incidence around 4.5 years and a slight male predominance.
DTF is a complex disease: in the sporadic form, most tumours have a somatic pathogenic variant of the CTNNB1 gene, which encodes ß-catenin, whereas patients with the less frequent form of DTF (<5%)4,5 harbour a germline pathogenic variant of the adenomatous polyposis coli (APC) gene,5–7 which is often associated with familial adenomatous polyposis (FAP). It has been proposed that endocrine factors as well as repetitive trauma might play a role in the pathogenesis of DTF. Interestingly, only in the past 10 years have data supported biological differences according to age,8 with potential molecular differences reported between paediatric and adult DFT.9,10
Although knowledge of the biological and molecular characteristics of DTF is evolving, a standardised treatment approach to this disease still presents a challenge. Surgery—for many years (but no longer) considered the mainstay of treatment—is not curative in many cases, can cause significant deformity, and might promote tumour growth and recurrence.11–15 Over last decade, it has become apparent that DTF can sometimes show spontaneous regression, and stabilisation in a subset of patients (around 27% in children), supporting an initial wait and see approach in cases of non-evolving disease at diagnosis.4,5,16–18 Why some tumours regress, some stabilise, and others progress is largely unknown,19,20 but could be related to the type of somatic CTNNB1 mutation. For those tumours that do progress, various types of systemic therapy, including chemotherapy, hormone therapies and nonsteroidal anti-inflammatory drugs,11,21–24 have been described over the past decade. Prolonged low-dose chemotherapy using vinblastine and methotrexate (VBL-MTX) is currently the standard of care in children with an expected response rate of 31–35% but up to 80% of non-progressive disease (Table 1). Conservative local therapies such as cryoablation have been suggested as treatment approaches for symptomatic residual disease, although cryoablation might have a role in primary treatment or in case of recurrence as well.
Table 1.
Main recent data on treatment with chemotherapy in paediatric patients with DTF.
| Author | Series | Number of cases | Age of patients | Main findings |
|---|---|---|---|---|
| Skapek et al.22 | Phase 2 study from the North American POG 1997–2001 | 28 | <18 years |
Treatment with VBL-MTX; response rate: 31% G3/4 toxicity: 67% 3-year PFS 32.5% (95% CI ± 10) |
| Meazza et al.11 | Retrospective study from the Italian STSC AIEOP 1970–2005 | 94 | <21 years | Various systemic therapy adopted; response rate to systemic therapy: 49% (including also minor responses), plus stabilization in 38%; response rate in frontline 47%, in second-line 50%; response rate to VBL-MTX: 58%; 5-year EFS: 58%, 5-year OS: 99% |
| Oudot et al.12 | Retrospective study from 2 French cancer centres 1976–2005 | 59 | <16 years |
Various systemic therapies Response rate to systemic therapy 33% 10-year PFS 31% (95% CI 20–45), 10-year OS 88% (95% CI 74–95) |
| Skapek et al.29 |
Prospective Phase 2 study from COG 2004–2009 |
59 | <19 years |
Treatment with tamoxifen and sulindac Response rate: 8% 2-year PFS: 36% |
| Orbach et al.4 | Prospective study from the EpSSG 2005–2016 | 163 | <24 years |
From 2005 to 2013; first-line therapy: VBL-MTX. Response to medical therapy 35% with 80% of ‘no progression'. Wait & see and chemotherapy-first strategies do not jeopardize outcome and permit to avoid surgery in 70% of cases. 5-year EFS 31.8% (95% CI 23.6–40.3) |
| Sparber-Sauer et al.28 | Retrospective study from the CWS 1981–2016 | 90 | <18 years |
From 1981 to 2013; response rate to systemic therapy (VAC and VBL-MTX) 39% at 3 months, 53% at 6 months. Response rate to VBL-MTX 47% at 3 months, 58% at 6 months. 5-year EFS 44% (95% CI ± 10), 5-year OS: 100%. |
| Ferrari et al.30 | Retrospective case series from four referral European centres 2008–2016 | 16 | <21 years | Pre-treated/refractory disease, treated with oral hydroxyurea; response rate: 19% major partial response, 37% including also minor responses, 69% considering symptom response or radiological signs of tissue response; No G3–G4 haematological toxicity |
COG Children’s Oncology Group, CWS Cooperative Weichteilsarkom Studiengruppe, EFS event-free survival, EpSSG European pediatric Soft tissue sarcoma Study Group, OS overall survival, PFS progression-free survival, POG Pediatric Oncology Group, STSC AIEOP Soft Tissue Sarcoma Committee of the Italian Association of Pediatric Hematologic Oncology, VAC vincristin/dactinomycin/cyclophosphamide, VBL-MTX vinblastine/methotrexate, CI confidential intervals, G grade.
Owing to its local aggressiveness, frequent locoregional relapses and the need for prolonged and sometimes aggressive treatments, DTF is quite often associated with significant sequelae in children,25 and could therefore be considered a chronic disease for many patients. A report published in 2020 indicated that children previously treated for DTF treated according to the European paediatric Soft Tissue Sarcoma Group (EpSSG) strategy (initial observation, VBL-MTX in case of tumour progression or in life-threatening sites) have lower quality-of-life (QoL) scores compared with those in a healthy population.25 With this in mind, new agents are needed to provide improved tumour control and to limit long-term sequelae for this patient population. Moreover, the results from prospective, randomised clinical trials evaluating the use of tyrosine kinase inhibitors (TKIs) in adults with DTF have prompted us to revise our treatment paradigm for DTF.18,24
In this article, we will outline current approaches to the treatment of paediatric patients with DTF and review the efficacy and toxicity of TKIs in adult patients with DTF before providing an expert opinion on future treatment options and opportunities for paediatric patients with DTF, with a particular focus on these new drugs.
Current management strategies for paediatric DTF
As outlined above in the Background, surgery was once considered the mainstay of treatment for DTF, but this is no longer the case, and systemic therapy is now used to treat the majority of cases of progressive or symptomatic DTF.
Systemic therapy
It is important to mention that treating physicians should approach DTF in a different way to malignant soft tissue sarcomas. The goals of systemic therapy in DTF are not only to promote tumour shrinkage (to permit a subsequent resection, for example), but also to induce growth arrest and tumour stabilisation. Furthermore, the endpoints for the evaluation of treatment results in DTF should be broadened from only tumour response to incorporate symptom control and other important QoL measures as well. In this context, patient perspective, toxicity, and long-term effects of the systemic treatment with VBL-MTX have been well studied in adult and paediatric settings (Tables 1 and 2).11,12,26,27
Table 2.
Main data on treatment with new agent treatment in adults’ patients with DTF.
| Author | Study | Number of cases | Response rate | Toxicity | Outcome |
|---|---|---|---|---|---|
| Chug et al.38 | Phase 2, single arm, trial on imatinib | 51 cases | 6% | G3/4 toxicity: 10% | 12 month PFS: 66% |
| Penel et al.45 | Phase 2, single arm, trial on imatinib | 35 cases | 11% | G3/4 toxicity: 45% | 12 month PFS: 67% |
| Kasper et al.46 | Phase 2, single arm, trial on imatinib | 38 cases | 19% | G3/4 toxicity: 13% | 12 month PAR: 59% |
| Gounder et al.18 | Phase 3, double blind, randomized to sorafenib or placebo | 87 cases | 33% (95% CI: 20-48; sorafenib), 20% (95% CI: 8–38; placebo) | G3 toxicity: 29% (sorafenib), 14% (placebo); G4 toxicity: 4% (sorafenib) | 12 month PFS: 89% (95% CI: 80–99; sorafenib), 46% (95% CI: 32–67; placebo) |
| Agresta et al.8 | Retrospective, comparison between pazopanib and VBL-MTX | 37 cases | 29% (pazopanib), 13% (VBL-MTX) | G3/4 toxicity: 8% | No data on PFS |
| Toulmonde et al.24 | Randomized Phase 2, on pazopanib or VBL-MTX | 72 cases | 37% | G3/4 toxicity: 8% | 12 month PFS: 86% (95% CI: 71–93; pazopanib) and 79% (95% CI: 53–92; VBL-MTX) |
EFS event-free survival, PAR progression arrest rate, PFS progression-free survival, VBL-MTX vinblastine and methotrexate, CI confidential interval, G grade.
Current regimens and their drawback
Most paediatric oncology experts currently consider conventional chemotherapy the first treatment approach in patients with evolving DTF (including patients with worsening symptoms, rapidly growing tumours, or tumours located in a life-threatening site) after an observation period. VBL-MTX constitutes a minimal-morbidity chemotherapy, and is given with a prolonged exposure of at least 6 months (or sometimes up to 12–18 months)4,28 This regimen, with response rates varying between 25 and 49%, is desirable in the paediatric setting due to its known absence of long-term side effects, especially in young children (Table 1). Reported toxicities include mainly tolerable haematological side effects, and no significant long-term effects are known.4,11,28 Other systemic therapies that have been evaluated either prospectively or limited in interpretation based on the retrospective nature of the analysis have shown limited benefit29,30 (Table 1). As has also been seen in adults, the overall response rate (ORR) to chemotherapy is reported to be ~30–50% (or less) in paediatric patients.23,26,31 However, this finding is influenced by many variables, including the definition of tumour response assessment and the study design of the different analyses. The long-term QoL among paediatric patients with a watch and wait strategy did not seem to differ from the QoL in those who received treatment (chemotherapy (predominantly VBL-MTX), surgery).25 Even if the efficacy of radiotherapy in paediatric DTF seems real, the use of this treatment is not recommended as a first-line treatment because of the long-term risks of this technic in front of this benign tumour (growth defect, mutagenic risk).14
Another point to consider when discussing the efficacy of systemic therapy is the challenge of assessing the anti-tumour activity of any medication in DTF. First, as is the case for sarcomas, the response to a given drug is generally evaluated after 2–4 months, but this might underestimate the response rate in DTF, which might sometimes require a longer period of therapy. Second, we know that the classic RECIST dimensional criteria can underestimate the tumour response because the treatment might induce other types of effect, such as fibrotic transformation or reduction of the amount of vital cells, which can potentially correspond to radiological changes in morphology and vascularity rather than changes in size. Furthermore, clinical benefit—for example, pain control or gain-of-function—might be considered as a favourable response even in the absence of frank tumour regression. On the other hand, physicians should be aware that the treatment response might be overestimated, as a reduction in tumour volume or a lack of growth might not necessarily be the result of the drug’s activity, as DTFs can spontaneously regress (or remain dimensionally stable). This concept is best exemplified by the results of a randomised, double-blind, placebo-controlled trial of sorafenib in adult DTF, which demonstrated a durable partial response in 21% of patients receiving placebo treatment.18 Note that the actual rate of spontaneous regression in children with DTF is not clear.
Tyrosine kinase inhibitors in DTF
Overactivation of the Wnt–β-catenin signalling pathway
Research in the field of sarcoma genomics has identified multiple potential drivers of oncogenesis in specific subtypes of soft tissue sarcoma. Alterations in the Wnt–β-catenin signalling pathway probably constitute the major oncogenic driver mechanisms in DTF.32 Both CTNNB1 and APC encode components of the Wnt signalling pathway (β-catenin and adenomatous polyposis coli protein), the dysregulation of which gives rise to the uncontrolled proliferation of fibroblasts. Patients with DTF-associated Gardner-type FAP have inactivating germline mutations of the APC gene on chromosome 5q21, and sporadic desmoids can harbour mutations of APC or CTNNB1.33,34 Using current sensitive detection methods, the incidence of desmoid tumours without CTNNB1 or APC alterations is reported to be less than 5%.32 In more than 85% of patients, the pathogenic somatic CTNNB1 mutations have been reported to affect the N-terminal phosphorylation sites of the β-catenin protein, interfering with its proteolytic degradation. Similarly, the loss of function of APC in the germline pathogenic variant leads to the reduced degradation and consequent intracellular stabilisation of β-catenin.35 Both scenarios result in the accumulation of β-catenin in the nucleus and subsequent transcriptional activation of a number of genes that encode proteins involved in tumorigenic signalling pathways, such as those mediated by the kinases c-KIT, vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR).36 Indeed, the overexpression of VEGF has been identified as a common feature in desmoid tumours, especially in recurrent, aggressive cases.37 Studies are ongoing to confirm whether these mutations are mutually exclusive. Therefore, somatic molecular analysis of CTNNB1 helps to confirm DTF diagnosis, differentiates DTF from other types of fibromatous lesions, encourages offering genetic counselling for patients with DTF without CTNNB1 pathogenic variant, gives a molecular biomarkers risk factor of recurrence and helps to identify possible therapeutic targets. Nevertheless, agents targeting the Wnt pathway are not yet readily available and have not been evaluated in children.
Clinical trials of TKIs in DFT
Why TKIs are efficient in DFT is not totally understood. The presence of KIT or PDGFR in desmoid tumours led to clinical trials of pazopanib and sorafenib, oral TKIs that target VEGF receptors 1, 2 and 3, PDGFRα and β, and c-KIT tyrosine kinases, in adult patients with DFT. However, tumour or serum levels of KIT, PDGFR, PDGF-AA or PDGF-BB, or the CTNNB1 or APC mutation status, have not consistently correlated with responses to TKI therapy.38–40 Alternatively, TKIs might provide an attractive therapeutic approach due to their ability to simultaneously inhibit multiple signalling pathway components, some of which (e.g. AKT, B-RAF and RET) have been implicated in DTF tumorigenesis,9 as well as their ease of administration (orally). Nevertheless, no clear data or consensus exists to decide when to discontinue treatment with a TKI and the rate of tumour recurrence after treatment. The main published data on TKIs in the treatment of adult patients with DTF is outlined below and summarised in Table 2.
Imatinib
Imatinib, a TKI with selectivity for c-KIT, PDGFRα, PDGFRβ and macrophage colony-stimulation factor (M-CSF), has dramatically modified the treatment and outcome of patients with certain solid tumours.41–44 Imatinib was the first TKI evaluated for the treatment of DTF. Three prospective, single arm, non-randomised studies in adults demonstrated potential efficacy of imatinib, with high rates of disease stabilisation (60–80%) despite rather low response rates (6–19%), and the expected well-known toxicity profile of imatinib.38,45,46 However, the lack of randomisation in a disease that has the possibility of spontaneous disease regression and the inclusion of patients in the absence of progressive disease at study entry make it difficult to determine the definitive role of imatinib in this condition.47 Although the efficacy of imatinib in paediatric patients with DTF is unknown, significant pharmacokinetic data and well-established toxicity profiles have been obtained for imatinib in the paediatric setting.48–52 Data on the long-term effects of imatinib in young patients treated over several years for other diseases, such as chronic leukaemia, have been reported and describe issues with potential growth failure, bone-metabolism alterations, endocrinopathies and second malignancies.48,50,53–60
Sunitinib
Sunitinib inhibits cellular signalling by targeting multiple receptor tyrosine kinases such as PDGFR, VEGFR, c-KIT, RET, CD114 and CD135. In paediatric patients with solid tumours, the exposure–response relationships of safety endpoints of sunitinib were mainly driven by sunitinib plasma exposures and were not affected by age, sex, respective baseline safety endpoint values, baseline Eastern Cooperative Oncology Group performance status, or body size.61 However, only one single arm Phase 2 trial has been conducted; it showed an ORR of 26% and 2-year progression-free survival (PFS) of 75% in adults with DTF.62,63
Pazopanib
VEGF overexpression is a common feature of aggressive desmoid tumours.37 Pazopanib is an oral, small molecule TKI that targets VEGFRs 1, 2 and 3, PDGFRα and PDGFRβ, and c-KIT, and is approved for the treatment of soft-tissue sarcomas in adults.8,24,64–68 Pazopanib has been evaluated retrospectively in a limited case series and prospectively in a Phase 2 randomised, adult study for patients with DTF.24,65 In the prospective clinical trial, patients were randomised 2:1 to receive pazopanib or VBL-MTX. The 6-month non-progression rate in the analysis on adults was 84% for pazopanib (versus 45% for VBL-MTX), with response rates similar to those of sorafenib.24 Data on global health status showed a clinically meaningful decrease in pain intensity in adults with DTF receiving this drug, in contrast with patients receiving VBL-MTX.24 Pazopanib has undergone Phase 1 testing in children and found to be generally well-tolerated, with mild haematological and non-haematological toxicities including dose-limiting pancreatic enzyme elevation, hypertransaminitis, proteinuria, canities and hypertension; the recommended Phase 2 dose is 450 mg/m² once daily in tablet form (comparable with the adult dose of 800 mg daily). The Phase 1 powder for oral suspension (PfOS) maximum tolerated dose of 160 mg/m² might result in suboptimal exposure. A Phase 2 study using PfOS at 225 mg/m² has been completed, and the results are pending.69 Data regarding the use of pazopanib in children with DFT are limited.8 In a retrospective analysis in adolescent and young adult patients with DTF (median age 16 years), six patients received pazopanib. Except for one case of oedema, all toxicities responded to dose reduction without sacrificing the objective treatment response.8 The toxicities of pazopanib included hypertension and diarrhoea, but were manageable; however, no data on long-term toxicity were provided. Although seemingly efficacious and generally well-tolerated, long-term effects of pazopanib, particularly with the anticipated chronic use in a paediatric population, are unknown.70
Sorafenib
Sorafenib is an oral, multi-targeted TKI, similar to pazopanib. A retrospective study in adult patients with DTF reported an ORR of 25% and promising disease stabilisation, with an improvement in symptoms in 70% of patients.71 A Phase 3, placebo-controlled, randomised trial of sorafenib in adult patients with progressive DTF showed that the risk of progression could be reduced by a factor of seven in favour of sorafenib.18 Notably, the ORR was 33% for sorafenib, comparable with that of both pazopanib and VBL-MTX. Common toxicities for sorafenib include fatigue, rash, hypertension, thyroid dysfunction and gastrointestinal symptoms.71,72
Sorafenib has undergone Phase 1 and 2 testing in children, showing toxicities similar to those seen in adults, including diarrhoea, rash, fatigue, and liver transaminitis. The recommended dose in children is 200 mg/m2 orally twice daily (comparable with the adult dose of 400 mg/m2 twice daily).73,74 To date, no data exist on the efficacy of this drug in paediatric patients with DFT. The pharmacokinetics and side effects of sorafenib have been better investigated than those of pazopanib73–79 (Table 3). Treatment-related adverse events were reported in 17% of children treated with sorafenib, and no grade 4 or 5 events were reported in the published paediatric Population Pharmacokinetics study.78 Mild skin toxicities are frequently seen in paediatric patients with other tumours (e.g. plexiform neurofibroma) treated with sorafenib.73–78,80,81 Data on long-term effects and fertility are limited.
Table 3.
Main recent data on characteristics, pharmacokinetic data, side effects and efficacy of sorafenib and pazopanib in paediatric patients.
| Author | Series, TKI | MTD | PK/PD | Side effects | Efficacy |
|---|---|---|---|---|---|
| Widemann et al.73 |
Phase 1, sorafenib. 60 patients with solid tumours and refractory leukemia Median age 14 years (range, 4–21) |
200 mg/m2 BID for solid tumours and 150 mg/m2 BID for leukemias. | Substantial interpatient variability for day 1 and steady-state PK parameters. Sorafenib exposure was within the ranges reported in adults. Apparent sorafenib clearance (CL/f) increased significantly with patient age. No correlations of pharmacodynamic parameters to drug exposure or response were observed. | Most common diarrhoea, rash, fatigue, increased ALT/AST | SD for ≥4 cycles in 14 patients with solid tumours and achievement of M1 bone marrow in 2 patients with AML and FLT3ITD |
| Glade Bender et al.69 |
Phase 1, pazopanib. 51 patients with soft tissue sarcoma and other refractory solid tumours Median age 13 years (range, 4–24) |
450 mg/m2 for tablet once daily and 160 mg/m2 for suspension once daily. | There was marked interpatient variability in Cmax and AUC0-24 h. Steady-state trough concentrations Css were reached by day 15 and did not seem to be dose dependent. Mean AUC0-24 h and Css were significantly higher for patients with DLT compared with those without DLT (P = 0.039 and P = 0.04, resp.). | DLT are elevation of lipase, amylase, and ALT, proteinuria and hypertension | PR in 2 evaluable patients; SD for ≥6 cycles in 8 patients |
| Kim et al.75 |
Phase 1, sorafenib. 9 patients with type I neurofibromatosis and plexiform neurofibroma Median age 8 years (range, 6–12) |
Could not be determined. | There was little interpatient variability. | At the starting low dose of 115 mg/m2/dose (n = 5), two patients experienced DLT grade 3 pain. At the de-escalated 80 mg/m2/dose (n = 4), approximately 40% of the pediatric solid tumour MTD, two had DLT grade 3 rash and grade 4 mood alteration, exceeding the MTD. Toxicities appeared to correspond with decreases in quality of life. | No tumour shrinkage observed. |
| Navid et al.76 |
Phase 1, bevacizumab, sorafenib, and low-dose cyclophosphamide. 19 patients with various refractory/recurrent solid tumours. Median age 9 years (range, 1.2–24.5) |
90 mg/m2 BID. | Substantial interpatient exposure variability was observed (6- to 8-fold). Median apparent sorafenib clearance (Cl/f) at 90 mg/m2 was similar to that at 110 mg/m2 (44 vs 39 mL/min/m2). The same hold true for the median sorafenib steady-state concentrations at both dose levels. There was no correlation between sorafenib steady-state concentrations and the development of DLT. Sorafenib steady-state concentrations (day 21) were significantly inversely correlated with inhibition of serum VEGFR2 (P = 0.019) and circulating endothelial cells (P = 0.01). | DLTs during course 1 are grade 3 rash (n = 2), increased lipase (n = 1), anorexia (n = 1), and thrombus (n = 1). With an additional 71 courses of therapy, the most common toxicities ≥grade 3 included neutropenia (n = 9), lymphopenia (n = 9), and rashes (n = 4). | PR in 5/17 evaluable patients, SD in 5/17 |
| Karajannis et al.77 |
Phase 2, sorafenib. 11 patients with recurrent or progressive low-grade astrocytoma. Median age 8.8 years (ranges, 3.0–15.1) |
200 mg/m2 BID | Cmax was 5 µg/mL similar to PK data from a pediatric phase 1 trial using 200 mg/m2 BID (see ref. 68). | Grade 4 ALT elevation rash (n = 1), other grade 3 only each in one patient (HFSR, diarrhoea, AST elevation, headache, mucositis). | Median time to progression was 2.8 months (95% CI, 2.1 - 31.0). Enrollment was terminated early due to this rapid and unexpectedly high progression rate. |
| Okada et al.81 |
Compassionate use, sorafenib. 4 patients with relapsed and refractory neuroblastoma Age 4 to 5 years |
250 mg/m2 BID | - | No adverse events were observed. | Transient anti-tumour activity with PD in all 4 patients |
| Kim et al.74 |
Phase 2, sorafenib. 20 patients with refractory solid tumours (10 rhabdomyosarcoma, 10 Wilms tumour) Median age 11 years [range, 5-21] |
200 mg/m2 BID | Mean (± SD) steady state concentration during cycle 1 day 15 in 10 patients was 6.5 ± 3.9 µg/mL. No relationship between steady-state trough concentrations and change in VEGF and VEGFR2 plasma levels | Seven patients demonstrated DLT during the first cycle of therapy: palmar-plantar erythrodysesthesia, pain, maculo-papular rash, anorexia, fatigue, dyspnea, elevation of ALT, hypoalbuminemia. Five patients with DLT 5 received dose reduction which was tolerable. | No objective responses (RECIST) were observed in 10 evaluable patients. |
| Inaba et al.78 |
Phase 1/2, sorafenib with various other drugs (e.g. bevacizumab, cyclophosphamide, clofarabine, cytarabine). 74 patients, 35 patients with refractory/relapsed leukemia (RELHEM protocol) and 39 patients with refractory/relapsed solid tumours (ANGIO1 protocol) Age ≤31 years (RELHEM) and ≤21 years (ANGIO1) |
200 mg/m2 BID (RELHEM) and 90 mg/m2 BID (ANGIO1) | Older age, Bev/Cyclo regimen, and higher creatinine level were significantly associated with decreased apparent clearance (CL/f, P < 0.0001). Concurrent Clo/AraC administration was associated with sorafenib N-oxide CL/f (P = 7e-4). Sorafenib population apparent clearance was 50% higher and the sorafenib glucuronide population apparent clearance was 22% lower in individuals who received OATP1B1 inhibitors. | A shorter time to development of grade 2/3 HFSR was associated with concurrent Clo/AraC administration (P = 0.0015) but not with sequential Clo/AraC administration, compared with Bev/Cyclo, and with higher stead-state concentrations of sorafenib (P = 0.0004). PK simulations showed that once daily and every-other day sorafenib schedules minimize exposure to steady-state concentrations associated with HFSR. | - |
| Federico et al.80 |
Phase 1, sorafenib with bevacizumab and low-dose cyclophosphamide. 24 patients with solid tumours; Median age 14.5 years (range, 1–22) |
90 mg/m2 BID. | Only PK data for bevacizumab are presented. | Two patients experienced a DLT (grade 3 QTc prolongation, HFSR) during course 1. Most common grade 3/4 non-hematological toxicities were hypertension (n = 4), HFSR (n = 3) and elevated lipase (n = 3), and grade 3/4 hematological neutropenia (n = 7) and lymphopenia (n = 17). Seven patients required 50% dose reduction of sorafenib due to HFSR | 21 patients were evaluable for response. PR in 3 patients, SD in 15 patients, PD in 3 patients. |
| Weiss et al.70 |
Phase 2, pazopanib with ifosfamide-doxorubicin. 81 children and adults patients with advanced soft tissue sarcoma. Median age: 19 years (pazopanib group, n = 42) and 25 years (control group, n = 39); 40% <18 years |
350 mg/m² once daily (patients <18 years) or 600 mg once daily (patients ≥18 years) | Doxorubicin PK data was collected during the dose-finding phase of the study in 7 patients receiving chemotherapy and pazopanib. Doxorubicin clearance (L/h/m2) was similar in study patients and historic controls (24.2 and 24.1, respectively) supporting the safety of administration of pazopanib with doxorubicin-containing chemotherapy. | The most common grade 3/4 toxicities were leukopenia (43%), neutropenia (41%), and febrile neutropenia (41%) in the pazopanib group. 22 (59%) of 37 patients in the pazopanib group had a pazopanib-related serious adverse event. | On the basis of an interim analysis the number of patients with a ≥90% pathological response was 58% in the pazopanib group and 22% in the control group (P = 0.081, 83.8% CI, 16.5–55.8%). Adding pazopanib to neoadjuvant chemo-radiotherapy improved the rate of pathological near complete response. |
AML acute myeloid leukemia, AUC area under the curve, BID bis in die, CI confidential intervals, Cmax maximum concentration, Css steady-state concentration, CL/f apparent total clearance of the drug from plasma after oral administration, COG Children’s Oncology Group, CR complete response, CWS Cooperative Weichteilsarkom Studiengruppe, DLT dose-limiting toxicity, EFS event-free survival, EpSSG European pediatric Soft tissue sarcoma Study Group, FLT3ITO FLT3 internal tandem duplication, HFSR hand-foot skin reaction, MTD maximum tolerated dose, OS overall survival, PD pharmacodynamics, PK pharmacokinetics, PR partial response, PD progressive disease, SD stable disease.
Expert opinion
Unlike for the treatment of malignant soft tissue tumours, the main aim of systemic therapy for DTF is to stabilise tumour growth and minimise tumour-related symptoms. Although low-dose, prolonged chemotherapy using VBL-MTX does achieve this goal in a subset of paediatric patients (at 6 months, the tumour response rate was 69%), approximately a third of patients derive no benefit from this therapy.28 Furthermore, the delivery of chemotherapy, especially in young patients, can be challenging (involving, for example, central venous catheter insertion, risk of systemic infections, need for regular care, weekly clinic visits for drug injections, acute toxic side effects). Owing to the anticipated risk of relapses/progression and need for subsequent lines of therapy, DTF should be viewed as a chronic disease and, thus, QoL with improved functionality should be prioritised as another main endpoint of.25
In an attempt to standardise the approach for both adult and paediatric patients with DTF, a global consensus-based guideline approach to its management was published in 2020.47 The initial watch and wait strategy that is used in adults has now been adopted as the treatment of choice in children.82 However, spontaneous regression is less commonly seen in children than in adults in practice. Furthermore, physicians treating young children have the legitimate concern of the risk of longer-term impairment of structures and organs that are adjacent to the progressively growing tumour, which is not the case for adult patients. As DFT is not a malignant disease, non-cytotoxic approaches with moderate long-term effects should be preferentially investigated in children.
The case for TKIs
Compared with conventional chemotherapy, TKIs appear to show comparable or improved efficacy in DTF, with a more favourable adverse event profile and health-related QoL measures.25 Moreover, the oral application of TKI would facilitate administration in younger patients. When comparing between the different TKIs, imatinib mainly stabilised tumour growth in adults but induced fewer tumour regressions compared with VBL-MTX or other TKIs such as pazopanib and sorafenib. Thus, we would not consider imatinib to be the treatment of first choice for paediatric DTF.47 Furthermore, the clinical data in adults with DTF receiving pazopanib and sorafenib are much more convincing. Both show ORRs similar to those obtained using conventional treatment with VBL-MTX, but differences in PFS are more evident for the TKIs. Compared with the data for pazopanib, data on the pharmacokinetics and safety of sorafenib in children are well investigated, although potential mechanisms of primary resistance and long-term effects of sorafenib are not yet known. The treatment duration of VBL-MTX is typically 6–12 months;28,79 the appropriate duration of treatment with TKIs is currently unknown but, taking into consideration the ‘chronic’ nature of the disease, 6 months would probably not be long enough, especially if this drug is delivered after several relapses, which may reflect a more ‘active' tumour. Well-defined parameters for stopping treatment for TKIs have not yet been established and, owing to the lack of comparative studies, no formalised sequence of therapies exists. MRI shows early change in heterogeneity in responding tumours due to a decrease in cellular area and an increase in fibronecrotic content before dimensional response. The presence of an active residual tumour on MRI or quantified by new technics as radiomics may therefore help to considered to discuss the length of therapy.24,83
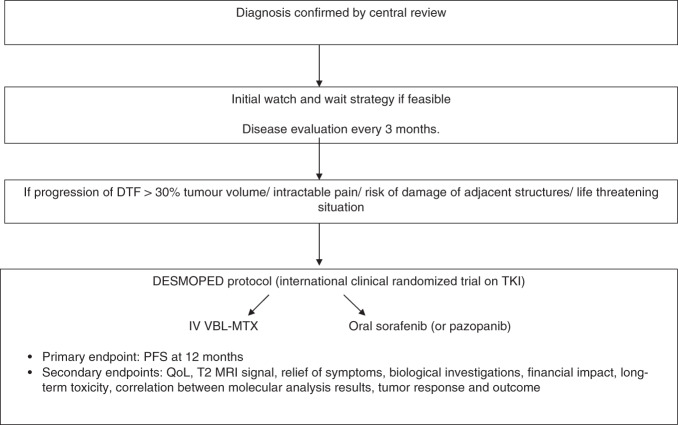
We propose an international prospective multicentre trial to evaluate TKI treatment in children, adolescents and young adults (<21 years) with DFT (Desmoped study; Fig. 1). After an initial watch-and-wait strategy, participants will be randomised (2:1) to TKI (pazopanib or sorafenib) or VBL-MTX (current paediatric standard of care). The indications for systemic treatment (i.e. inclusion in the study) will include frank progressive disease during observation (>30% of tumour volume progression), intractable pain, possible damage of adjacent organs or structures (e.g. inner ear) and/or life-threatening situations (mediastinal tumour, compartment syndrome, etc.). Aesthetic considerations, when tumour occurs in head and neck primaries for instance should be also taken into account.82 Surgery should be avoided in order to avoid stimulating the growth of any residual lesions. Local therapies (e.g. thermal ablation, cryoablation) might be feasible for small, symptomatic lesions, or at the end of systemic therapy. Standard radiotherapy, although effective, has the potential to cause long-term effects, including growth retardation or secondary malignancies, particularly in growing children, and should be avoided. PFS will be the primary endpoint, but other important measures of outcome should include QoL, MRI T2 signal (decrease in volume, T2 hyperintensity decrease), and acute and especially long-term toxicity, including potential effects on fertility.83,84
Fig. 1. Proposal for a prospective international strategy in paediatric DFT.
DTF desmoid-type fibromatosis, iv intravenous, MRI magnetic resonance imaging, QoL quality of life, PFS progression-free survival, MRI magnetic resonance imaging, VBL-MTX vinblastine, methotrexate.
Furthermore, an initial molecular analysis of biopsy specimens should be carried out in order to establish whether the presence of specific mutations could influence the response to future TKI treatment. Panel sequencing, whole-exon sequencing or methylation arrays of the primary tumour might help to limit the use of drugs to those most likely to provide benefit while avoiding toxicity in those agents that might not. Re-biopsy and analysis of circulating tumour DNA during treatment should also be considered to further help in the understanding of which patients respond and why.85 These biological measures might have a significant influence on the ultimate selection of therapy for children with DFT beyond traditional definitions of treatment success, emphasising the importance of their incorporation into study design.
Conclusions
Owing to the unpredictable clinical nature of the disease and emphasis on QoL, the selection of therapy for patients with DTF should be individualised. TKIs—specifically, pazopanib and sorafenib—offer a novel, well-tolerated and effective treatment in adult patients with DTF. However, a lack of data regarding long-term toxicity and standardised stopping parameters has so far made its widespread incorporation into frontline therapy in paediatric patients less clear. Prospective, clinical trials in paediatric DTF patients are urgently needed to appropriately test these newer agents and better understand the age-dependent biological differences compared with adults. We propose a way to consider a prospective protocol on TKIs in children with FDT (Fig. 1). Due to the rarity of the disease, international collaboration for these ongoing efforts is critical.
Acknowledgements
Authors want to thank the ‘SOS Desmoid association' for the review of the manuscript.
Author contributions
M.S.S., D.O. and A.F.: conceptualisation, formal analysis, methodology, writing and editing; F.N., S.H., S.S., N.C., M.C., A.W. and M.S.: formal analysis, methodology, writing and editing.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare no conflict of interest in relation with this manuscript. M.S. is supported by the Robert Bosch Stiftung, Stuttgart, Germany.
Funding information
No specific funding.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Monika Sparber-Sauer, Daniel Orbach
References
- 1.Schmidt BF, Koscielniak E, Pilz T, Treuner J. Radiation therapy in juvenile aggressive fibromatosis. Klin. Padiatr. 1999;211:296–299. doi: 10.1055/s-2008-1043803. [DOI] [PubMed] [Google Scholar]
- 2.Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am. J. Surg. Feb. 1986;151:230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 3.Anthony T, Rodriguez-Bigas MA, Weber TK, Petrelli NJ. Desmoid tumors. J. Am. Coll. Surg. 1996;182:369–377. [PubMed] [Google Scholar]
- 4.Orbach D, Brennan B, Bisogno G, Van Noesel M, Minard-Colin V, Daragjati J, et al. The EpSSG NRSTS 2005 treatment protocol for desmoid-type fibromatosis in children: an international prospective case series. Lancet Child Adolesc. Health. 2017;1:284–292. doi: 10.1016/S2352-4642(17)30045-7. [DOI] [PubMed] [Google Scholar]
- 5.Fiore M, Rimareix F, Mariani L, Domont J, Collini P, Le Pechoux C, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann. Surg. Oncol. 2009;16:2587–2593. doi: 10.1245/s10434-009-0586-2. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 7.Penel N, Coindre JM, Bonvalot S, Italiano A, Neuville A, Le Cesne A, et al. Management of desmoid tumours: a nationwide survey of labelled reference centre networks in France. Eur. J. Cancer. 2016;58:90–96. doi: 10.1016/j.ejca.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Agresta L, Kim H, Turpin BK, Nagarajan R, Plemmons A, Szabo S, et al. Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr. Blood Cancer. 2018;65:e26968. doi: 10.1002/pbc.26968. [DOI] [PubMed] [Google Scholar]
- 9.Trautmann, M., Rehkamper, J., Gevensleben, H., Becker, J., Wardelmann, E., Hartmann, W. et al. Novel pathogenic alterations in pediatric and adult desmoid-type fibromatosis—a systematic analysis of 204 cases. Sci. Rep.25, 10:3368 (2020). [DOI] [PMC free article] [PubMed]
- 10.Meazza C, Belfiore A, Busico A, Settanni G, Paielli N, Cesana L, et al. AKT1 and BRAF mutations in pediatric aggressive fibromatosis. Cancer Med. 2016;5:1204–1213. doi: 10.1002/cam4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meazza C, Bisogno G, Gronchi A, Fiore M, Cecchetto G, Alaggio R, et al. Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer. 2010;116:233–240. doi: 10.1002/cncr.24679. [DOI] [PubMed] [Google Scholar]
- 12.Oudot C, Orbach D, Minard-Colin V, Michon J, Mary P, Glorion C, et al. Desmoid fibromatosis in pediatric patients: management based on a retrospective analysis of 59 patients and a review of the literature. Sarcoma. 2012;2012:475202. doi: 10.1155/2012/475202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woltsche N, Gilg MM, Fraissler L, Liegl-Atzwanger B, Beham A, Lackner H, et al. Is wide resection obsolete for desmoid tumors in children and adolescents? Evaluation of histological margins, immunohistochemical markers, and review of literature. Pediatr. Hematol. Oncol. 2015;32:60–69. doi: 10.3109/08880018.2014.956905. [DOI] [PubMed] [Google Scholar]
- 14.Rutenberg MS, Indelicato DJ, Knapik JA, Lagmay JP, Morris C, Zlotecki RA, et al. External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr. Blood Cancer. 2011;57:435–442. doi: 10.1002/pbc.22916. [DOI] [PubMed] [Google Scholar]
- 15.Buitendijk S, van de Ven CP, Dumans TG, den Hollander JC, Nowak PJ, Tissing WJ, et al. Pediatric aggressive fibromatosis: a retrospective analysis of 13 patients and review of literature. Cancer. 2005;104:1090–1099. doi: 10.1002/cncr.21275. [DOI] [PubMed] [Google Scholar]
- 16.Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG) Ann. Oncol. 2017;28:2399–2408. doi: 10.1093/annonc/mdx323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penel N, Le Cesne A, Bonvalot S, Giraud A, Bompas E, Rios M, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur. J. Cancer. 2017;83:125–131. doi: 10.1016/j.ejca.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, et al. Sorafenib for advanced and refractory desmoid tumors. N. Engl. J. Med. 2018;379:2417–2428. doi: 10.1056/NEJMoa1805052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salas S, Brulard C, Terrier P, Ranchere-Vince D, Neuville A, Guillou L, et al. Gene expression profiling of desmoid tumors by cDNA microarrays and correlation with progression-free survival. Clin. Cancer Res. 2015;21:4194–4200. doi: 10.1158/1078-0432.CCR-14-2910. [DOI] [PubMed] [Google Scholar]
- 20.Colombo C, Miceli R, Lazar AJ, Perrone F, Pollock RE, Le Cesne A, et al. CTNNB1 45F mutation is a molecular prognosticator of increased postoperative primary desmoid tumor recurrence: an independent, multicenter validation study. Cancer. 2013;119:3696–3702. doi: 10.1002/cncr.28271. [DOI] [PubMed] [Google Scholar]
- 21.Meazza C, Alaggio R, Ferrari A. Aggressive fibromatosis in children: a changing approach. Minerva Pediatr. 2011;63:305–318. [PubMed] [Google Scholar]
- 22.Skapek SX, Ferguson WS, Granowetter L, Devidas M, Perez-Atayde AR, Dehner LP, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J. Clin. Oncol. 2007;25:501–506. doi: 10.1200/JCO.2006.08.2966. [DOI] [PubMed] [Google Scholar]
- 23.Azzarelli A, Gronchi A, Bertulli R, Tesoro JD, Baratti D, Pennacchioli E, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92:1259–1264. doi: 10.1002/1097-0142(20010901)92:5<1259::AID-CNCR1446>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Toulmonde M, Pulido M, Ray-Coquard I, Andre T, Isambert N, Chevreau C, et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): a non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. 2019;20:1263–1272. doi: 10.1016/S1470-2045(19)30276-1. [DOI] [PubMed] [Google Scholar]
- 25.Duhil de Benaze G, Vigan M, Corradini N, Minard-Colin V, Marie-Cardine A, Verite C, et al. Functional analysis of young patients with desmoid-type fibromatosis: initial surveillance does not jeopardize long term quality of life. Eur. J. Surg. Oncol. 2020;46:1294–1300. doi: 10.1016/j.ejso.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Skapek SX, Ferguson WS, Granowetter L, Devidas M, Perez-Atayde AR, Dehner LP, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J. Clin. Oncol. 2007;25:501–506. doi: 10.1200/JCO.2006.08.2966. [DOI] [PubMed] [Google Scholar]
- 27.Group DTW. The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur. J. Cancer. 2020;127:96–107. doi: 10.1016/j.ejca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Sparber-Sauer M, Seitz G, von Kalle T, Vokuhl C, Leuschner I, Scheer M, et al. Systemic therapy of aggressive fibromatosis in children and adolescents: report of the Cooperative Weichteilsarkom Studiengruppe (CWS) Pediatr. Blood Cancer. 2018;65:e26943. doi: 10.1002/pbc.26943. [DOI] [PubMed] [Google Scholar]
- 29.Skapek SX, Anderson JR, Hill DA, Henry D, Spunt SL, Meyer W, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr. Blood Cancer. 2013;60:1108–1112. doi: 10.1002/pbc.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari A, Orbach D, Affinita MC, Chiaravalli S, Corradini N, Meazza C, et al. Evidence of hydroxyurea activity in children with pretreated desmoid-type fibromatosis: a new option in the armamentarium of systemic therapies. Pediatr. Blood Cancer. 2019;66:e27472. doi: 10.1002/pbc.27472. [DOI] [PubMed] [Google Scholar]
- 31.Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I. Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur. J. Cancer. 2009;45:2930–2934. doi: 10.1016/j.ejca.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Crago AM, Chmielecki J, Rosenberg M, O’Connor R, Byrne C, Wilder FG, et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer. 2015;54:606–615. doi: 10.1002/gcc.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambo I, Vesely K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol. 2014;50:64–70. [PubMed] [Google Scholar]
- 34.Coindre JM. New WHO classification of tumours of soft tissue and bone. Ann. Pathol. 2012;32:S115–S116. doi: 10.1016/j.annpat.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor) Am. J. Pathol. 1998;153:709–714. doi: 10.1016/S0002-9440(10)65614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enzo MV, Rastrelli M, Rossi CR, Hladnik U, Segat D. The Wnt/beta-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol. Cell Ther. 2015;3:1. doi: 10.1186/s40591-015-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matono H, Tamiya S, Yokoyama R, Saito T, Iwamoto Y, Tsuneyoshi M, et al. Abnormalities of the Wnt/beta-catenin signalling pathway induce tumour progression in sporadic desmoid tumours: correlation between beta-catenin widespread nuclear expression and VEGF overexpression. Histopathology. 2011;59:368–375. doi: 10.1111/j.1365-2559.2011.03945.x. [DOI] [PubMed] [Google Scholar]
- 38.Chugh R, Wathen JK, Patel SR, Maki RG, Meyers PA, Schuetze SM, et al. Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter sarcoma alliance for research through Collaboration (SARC) trial. Clin. Cancer Res. 2010;16:4884–4891. doi: 10.1158/1078-0432.CCR-10-1177. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich MC, Joensuu H, Demetri GD, Corless CL, Apperley J, Fletcher JA, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin. Cancer Res. 2008;14:2717–2725. doi: 10.1158/1078-0432.CCR-07-4575. [DOI] [PubMed] [Google Scholar]
- 40.Heinrich MC, McArthur GA, Demetri GD, Joensuu H, Bono P, Herrmann R, et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor) J. Clin. Oncol. 2006;24:1195–1203. doi: 10.1200/JCO.2005.04.0717. [DOI] [PubMed] [Google Scholar]
- 41.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 42.Maki RG, Awan RA, Dixon RH, Jhanwar S, Antonescu CR. Differential sensitivity to imatinib of 2 patients with metastatic sarcoma arising from dermatofibrosarcoma protuberans. Int. J. Cancer. 2002;100:623–626. doi: 10.1002/ijc.10535. [DOI] [PubMed] [Google Scholar]
- 43.Fields RC, Hameed M, Qin LX, Moraco N, Jia X, Maki RG, et al. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann. Surg. Oncol. 2011;18:328–336. doi: 10.1245/s10434-010-1316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT) Ann. Oncol. 2008;19:821–822. doi: 10.1093/annonc/mdn033. [DOI] [PubMed] [Google Scholar]
- 45.Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann. Oncol. 2011;22:452–457. doi: 10.1093/annonc/mdq341. [DOI] [PubMed] [Google Scholar]
- 46.Kasper B, Gruenwald V, Reichardt P, Bauer S, Rauch G, Limprecht R, et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG) Eur. J. Cancer. 2017;76:60–67. doi: 10.1016/j.ejca.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Desmoid Tumor Working Group The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur. J. Cancer. 2020;127:96–107. doi: 10.1016/j.ejca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Kroschwald LM, Tauer JT, Kroschwald SI, Suttorp M, Wiedenfeld A, Beissert S, et al. Imatinib mesylate and nilotinib decrease synthesis of bone matrix in vitro. Oncol. Lett. 2019;18:2102–2108. doi: 10.3892/ol.2019.10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suttorp M, Metzler M, Millot F, Shimada H, Bansal D, Gunes AM, et al. Generic formulations of imatinib for treatment of Philadelphia chromosome-positive leukemia in pediatric patients. Pediatr. Blood Cancer. 2018;65:e27431. doi: 10.1002/pbc.27431. [DOI] [PubMed] [Google Scholar]
- 50.Suttorp M, Schulze P, Glauche I, Gohring G, von Neuhoff N, Metzler M, et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: results from a phase III trial. Leukemia. 2018;32:1657–1669. doi: 10.1038/s41375-018-0179-9. [DOI] [PubMed] [Google Scholar]
- 51.Suttorp M, Bornhauser M, Metzler M, Millot F, Schleyer E. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Expert Rev. Clin. Pharmacol. 2018;11:219–231. doi: 10.1080/17512433.2018.1398644. [DOI] [PubMed] [Google Scholar]
- 52.Proschmann R, Baldow C, Rothe T, Suttorp M, Thiede C, Tauer JT, et al. Response dynamics of pediatric patients with chronic myeloid leukemia on imatinib therapy. Haematologica. 2017;102:e39–e42. doi: 10.3324/haematol.2016.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroschwald L, Suttorp M, Tauer JT, Zimmermann N, Gunther C, Bauer A. Offtarget effect of imatinib and nilotinib on human vitamin D3 metabolism. Mol. Med. Rep. 2018;17:1382–1388. doi: 10.3892/mmr.2017.7952. [DOI] [PubMed] [Google Scholar]
- 54.Yin XF, Wang JH, Li X, Yu MX, Ma ZX, Jin J. Incidence of second malignancies of chronic myeloid leukemia during treatment with tyrosine kinase inhibitors. Clin. Lymphoma Myeloma Leuk. 2016;16:577–581. doi: 10.1016/j.clml.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Choeyprasert W, Yansomdet T, Natesirinilkul R, Wejaphikul K, Charoenkwan P. Adverse effects of imatinib in children with chronic myelogenous leukemia. Pediatr. Int. 2017;59:286–292. doi: 10.1111/ped.13136. [DOI] [PubMed] [Google Scholar]
- 56.Samis J, Lee P, Zimmerman D, Arceci RJ, Suttorp M, Hijiya N. Recognizing endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic myelogenous leukemia. Pediatr. Blood Cancer. 2016;63:1332–1338. doi: 10.1002/pbc.26028. [DOI] [PubMed] [Google Scholar]
- 57.Tauer JT, Hofbauer LC, Jung R, Gerdes S, Glauche I, Erben RG, et al. Impact of long-term exposure to the tyrosine kinase inhibitor imatinib on the skeleton of growing rats. PLoS ONE. 2015;10:e0131192. doi: 10.1371/journal.pone.0131192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulmer A, Tabea Tauer J, Glauche I, Jung R, Suttorp M. TK inhibitor treatment disrupts growth hormone axis: clinical observations in children with CML and experimental data from a juvenile animal model. Klin. Padiatr. 2013;225:120–126. doi: 10.1055/s-0033-1343483. [DOI] [PubMed] [Google Scholar]
- 59.Jaeger BA, Tauer JT, Ulmer A, Kuhlisch E, Roth HJ, Suttorp M. Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med. Sci. Monit. 2012;18:CR721–CR728. doi: 10.12659/MSM.883599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosokawa T, Hara T, Arakawa Y, Oguma E, Yamada Y. Periosteal reaction possibly induced by pazopanib: a case report and literature review. J. Pediatr. Hematol. Oncol. 2020;42:e822–e825. doi: 10.1097/MPH.0000000000001595. [DOI] [PubMed] [Google Scholar]
- 61.Wang E, DuBois SG, Wetmore C, Khosravan R. Population pharmacokinetics-pharmacodynamics of sunitinib in pediatric patients with solid tumors. Cancer Chemother. Pharmacol. 2020;86:181–192. doi: 10.1007/s00280-020-04106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo JC, Hong YS, Kim KP, Lee JL, Lee J, Park YS, et al. A prospective multicenter phase II study of sunitinib in patients with advanced aggressive fibromatosis. Investig. New Drugs. 2014;32:369–376. doi: 10.1007/s10637-013-0059-0. [DOI] [PubMed] [Google Scholar]
- 63.Wang E, DuBois SG, Wetmore C, Khosravan R. Population pharmacokinetics-pharmacodynamics of sunitinib in pediatric patients with solid tumors. Cancer Chemother. Pharmacol. 2020;86:181–192. doi: 10.1007/s00280-020-04106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 65.Szucs Z, Messiou C, Wong HH, Hatcher H, Miah A, Zaidi S, et al. Pazopanib, a promising option for the treatment of aggressive fibromatosis. Anticancer Drugs. 2017;28:421–426. doi: 10.1097/CAD.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bulut G, Ozluk A, Erdogan AP, Uslu R, Elmas N, Karaca B. Pazopanib: a novel treatment option for aggressive fibromatosis. Clin. Sarcoma Res. 2016;6:22. doi: 10.1186/s13569-016-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin-Liberal J, Benson C, McCarty H, Thway K, Messiou C, Judson I. Pazopanib is an active treatment in desmoid tumour/aggressive fibromatosis. Clin. Sarcoma Res. 2013;3:13. doi: 10.1186/2045-3329-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishida Y, Sakai T, Koike H, Ito K. Pazopanib for progressive desmoid tumours: children, persistant effects, and cost. Lancet Oncol. 2019;20:e555. doi: 10.1016/S1470-2045(19)30543-1. [DOI] [PubMed] [Google Scholar]
- 69.Glade Bender JL, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children’s oncology group phase I consortium report. J. Clin. Oncol. 2013;31:3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss AR, Chen YL, Scharschmidt TJ, Chi YY, Tian J, Black JO, et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:1110–1122. doi: 10.1016/S1470-2045(20)30325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gounder MM, Lefkowitz RA, Keohan ML, D’Adamo DR, Hameed M, Antonescu CR, et al. Activity of sorafenib against desmoid tumor/deep fibromatosis. Clin. Cancer Res. 2011;17:4082–4090. doi: 10.1158/1078-0432.CCR-10-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walko CM, Aubert RE, La-Beck NM, Clore G, Herrera V, Kourlas H, et al. Pharmacoepidemiology of clinically relevant hypothyroidism and hypertension from sunitinib and sorafenib. Oncologist. 2017;22:208–212. doi: 10.1634/theoncologist.2016-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin. Cancer Res. 2012;18:6011–6022. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim A, Widemann BC, Krailo M, Jayaprakash N, Fox E, Weigel B, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr. Blood Cancer. 2015;62:1562–1566. doi: 10.1002/pbc.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim A, Dombi E, Tepas K, Fox E, Martin S, Wolters P, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr. Blood Cancer. 2013;60:396–401. doi: 10.1002/pbc.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Navid F, Baker SD, McCarville MB, Stewart CF, Billups CA, Wu J, et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19:236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16:1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inaba H, Panetta JC, Pounds SB, Wang L, Li L, Navid F, et al. Sorafenib population pharmacokinetics and skin toxicities in children and adolescents with refractory/relapsed leukemia or solid tumor malignancies. Clin. Cancer Res. 2019;25:7320–7330. doi: 10.1158/1078-0432.CCR-19-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skapek SX, Ferguson WS, Granowetter L, Devidas M, Perez-Atayde AR, Dehner LP, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J. Clin. Oncol. 2007;25:501–506. doi: 10.1200/JCO.2006.08.2966. [DOI] [PubMed] [Google Scholar]
- 80.Federico SM, Caldwell KJ, McCarville MB, Daryani VM, Stewart CF, Mao S, et al. Phase I expansion cohort to evaluate the combination of bevacizumab, sorafenib and low-dose cyclophosphamide in children and young adults with refractory or recurrent solid tumours. Eur. J. Cancer. 2020;132:35–42. doi: 10.1016/j.ejca.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada K, Nakano Y, Yamasaki K, Nitani C, Fujisaki H, Hara J. Sorafenib treatment in children with relapsed and refractory neuroblastoma: an experience of four cases. Cancer Med. 2016;5:1947–1949. doi: 10.1002/cam4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul A, Blouin MJ, Minard-Colin V, Galmiche L, Coulomb A, Corradini N, et al. Desmoid-type fibromatosis of the head and neck in children: a changing situation. Int. J. Pediatr. Otorhinolaryngol. 2019;123:33–37. doi: 10.1016/j.ijporl.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 83.Crombe A, Kind M, Ray-Coquard I, Isambert N, Chevreau C, Andre T, et al. Progressive desmoid tumor: radiomics compared with conventional response criteria for predicting progression during systemic therapy—a multicenter study by the French Sarcoma Group. Am. J. Roentgenol. 2020;215:1539–1548. doi: 10.2214/AJR.19.22635. [DOI] [PubMed] [Google Scholar]
- 84.Sheth PJ, Del Moral S, Wilky BA, Trent JC, Cohen J, Rosenberg AE, et al. Desmoid fibromatosis: MRI features of response to systemic therapy. Skeletal Radiol. 2016;45:1365–1373. doi: 10.1007/s00256-016-2439-y. [DOI] [PubMed] [Google Scholar]
- 85.Macagno N, Fina F, Penel N, Bouvier C, Nanni I, Duffaud F, et al. Proof of concept: prognostic value of the plasmatic concentration of circulating cell free DNA in desmoid tumors using ddPCR. Oncotarget. 2018;9:18296–18308. doi: 10.18632/oncotarget.24817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.