Abstract
The demand for genetic testing of hereditary breast cancer genes such as BRCA1 and BRCA2 has continued to increase with the lowering costs of testing, raised awareness in the general public, and implications for breast cancer treatment when a patient is identified as having a germline pathogenic variant. Historically within Australia, patients affected by high genetic risk breast cancers have been referred to a familial cancer centre (FCC) for assessment and testing, resulting in wait times for an appointment for pre- and post-test genetic counselling and an increased demand on the public-funded FCC. To improve patient access and pace of genetic testing, as well as refocus FCC resources, a mainstream clinical genetic testing program was rolled out in September 2017 through the Parkville FCC (PFCC) in Australia at 10 hospital sites. This program enables specialist doctors of eligible patients affected by breast cancer to arrange genetic testing directly at an oncology/surgical appointment and follow up the results as part of the patients’ routine clinical care. In this model, the specialist doctor is responsible for any treatment implications of the genetic test result, and the PFCC is responsible for result interpretation, future cancer risk, family cascade testing and segregation testing where warranted. To date the program has had successful uptake, a notable pathogenic variant detection rate, reduced the burden on the PFCC enabling a reallocation of resources and has streamlined the process of genetic testing for eligible patients. Investigation into the patient and clinician experiences of the mainstream program is required.
Subject terms: Genetic testing, Genetic counselling, Cancer genetics, Breast cancer, Genetic counselling
Introduction
In recent years, familial cancer centres (FCCs) have experienced increased demand on their services, initially in response to the raised awareness of the BRCA1 and BRCA2 genes in the wake of increased media attention surrounding Angelina Jolie’s disclosure of having a BRCA1 pathogenic variant in 2013 [1]. The growth in demand has continued with falling costs of genetic testing, improved funding for genetic testing in the healthcare system [2] and the development of novel treatment implications for cancers that occur in individuals with a germline pathogenic variant (PV) – meaning variants classified as either class 4, likely pathogenic, or class 5, pathogenic, under the ACMG/AMP guidelines [3–5]. Currently these treatment implications include decisions around use of platinum-based chemotherapy for triple negative breast cancer [6, 7], the role of contralateral risk reducing mastectomy at the time of initial surgery [8], PARP inhibitors to treat metastatic disease in BRCA-associated breast cancer [9] and avoidance of radiotherapy in individuals with a germline TP53 PV [10].
In Australia, genetic testing for hereditary breast cancer genes has been accessed through dedicated FCCs, and publicly funded for patients assessed as having a significantly increased probability of finding a PV. This model of care involves the patient being referred by their treating specialist or general practitioner, then attending the FCC initially for an assessment where it is determined whether the patient is eligible for funded testing with pre-test genetic counselling at the FCC if they do proceed with testing. The patient is seen again by the FCC to receive the results and for post-test genetic counselling. With increasing demand, the traditional FCC genetic testing pathway has become less effective, with longer wait times for testing, and for patients with a current breast cancer diagnosis, the need to attend appointments for genetic testing on top of their routine care.
Mainstream genetic testing is a process where genetic testing is offered to a patient by their breast cancer specialist, providing a more streamlined and efficient experience for the patient, particularly where the primary impact of the genetic test result are treatment implications. A mainstream genetic testing program for breast cancer patients operates in Norway and the UK [11, 12], and ovarian mainstream programs have been introduced in Australia and the UK with great success [13, 14].
We now report on the outcomes of a mainstream genetic testing program for breast cancer patients at surgical and oncology clinics, supported by the Parkville Familial Cancer Centre in Victoria, Australia.
Methods
Initial set-up of program
A breast cancer mainstreaming program was introduced at the PFCC in September 2017. The program followed the successful introduction of an ovarian mainstream program, and was modelled on the UK and Australian cancer mainstreaming programs [13, 14] with the aim of addressing the increased demands for hereditary breast cancer genetic testing and the need to streamline the process of treatment focused genetic testing (TFGT) in the breast cancer setting. Upskilling of non-genetic health professionals, including breast surgeons, oncologists and radiation oncologists (referred to here as the ‘breast specialist’ or ‘mainstream clinician’) was completed at each mainstreaming hospital site prior to the roll out of the program - this included a mandatory one-to-two hour in-service presentation, led by the PFCC, covering the relevant principles and implications of genetic testing including interpretation of PV and variants of unknown significance (VUS) for breast cancer patients. Additional communication resources were provided for mainstream clinicians including a frequently asked questions document and suggested script to support a discussion with patients. Practical resources including patient consent form, patient information sheet and populated pathology slips were also provided to each mainstreaming site.
Breast cancer patients were eligible for mainstream testing if they had either a diagnosis of invasive breast cancer at or under age 35, triple negative breast cancer (TNBC) at or under age 60, or were a male with invasive breast cancer at any age. These criteria encompassed the criteria described in the Australian national consensus guidelines published by the Cancer Genetics National Reference Committee, eviQ [15] at the time the program began but distilled into clear clinical groups for implementation in the mainstream setting so as to avoid the need for quantified algorithms, such as BOADICEA [16], for the non-genetic health professionals. Testing was conducted by the NATA accredited Molecular Diagnostic Laboratory at the Peter MacCallum Cancer Centre. Standard testing included targeted gene sequencing of coding regions and splice sites for BRCA1, BRCA2, PALB2, ATM and TP53, on DNA extracted from blood. An exception was made for specific cases where a rapid turnaround of two weeks’ time was required for clinical reasons, where gene testing for BRCA1/2 only occurred. The genes being offered on this panel were determined by whether an Australian national consensus risk management guideline had been published by the Cancer Genetics National Reference Committee, eviQ [15]. At the time the mainstream program began, there was no consensus guideline for management of CHEK2 variants in Australia, and as such the gene was not included in the panel during the first two years of the mainstream program. The Molecular Diagnostic Laboratory is responsible for ongoing review of VUS classification over time. When a VUS is reclassified to class 4 or 5, the PFCC is informed, the clinical implications reviewed and the patient recontacted. Patients who received VUS results were also encouraged to recontact the PFCC in five yearly intervals for review (or sooner if they wished), at which time the PFCC reviews the VUS classification with the Molecular Diagnostic Laboratory.
Patients discussed genetic testing with their breast specialist at an oncology appointment (for active treatment or routine follow up) and were given an information sheet summarising the discussion points. A consent form was completed which included providing any relevant cancer family history information before having blood collected. Patients who wished for a more in-depth discussion were put in contact with the PFCC. The consent form with relevant family history information was sent to the PFCC by the breast specialist, to alert the PFCC to the test request. Test results were sent by the laboratory to the breast specialist, as well as the PFCC. Patients received test results from both their breast specialist (to discuss treatment or risk management implications), as well as the PFCC (to note familial implications). When the mainstream protocol was first implemented the PFCC contacted all patients regarding their test results in writing. Patients identified as having a PV or a suspicious VUS would also receive a telephone call (prior to a letter outlining their result being sent) and be offered an appointment with the PFCC. Patients where no PV or suspicious VUS were identified who also reported a significant personal and/or family history of cancer would receive a questionnaire to gather additional information about the personal and/or family cancer history. Once this information was obtained, a risk assessment was performed to determine whether any further genetic testing was indicated and tailored advice was provided for the patient and family members, either through a letter or the offer of an appointment at the PFCC to discuss the risk assessment in person.
Program refinement
After initial rollout, the mainstream protocol was revised to ensure that upon receipt of a result from the laboratory, the PFCC provided an interpretation for all VUS results (i.e. suspicious or non-suspicious) to the breast specialist via email or phone before the return of the result to the patient. The interpretation was made by the Molecular Diagnostic Laboratory (based on ACMG/AMP guidelines [3–5]), with the capacity for additional clinical interpretation by a clinical geneticist within the PFCC taking in to consideration the patient’s personal and reported family history of cancer. This measure was implemented to ensure accurate result interpretation was given to the patient by their breast specialist, and it was consistent with the interpretation provided to the patient from the PFCC. In addition, due to the large number of patients receiving non-suspicious ATM VUS results following full targeted gene sequencing of coding regions and splice sites for ATM, testing for this gene was restricted to the single PV ATM (NM_000051.3):c.7271T>G that has been reported to be enriched in the Australian population, in line with the Australian national risk management guidelines which apply specifically to this single ATM PV.
During the course of the program, 15 (6.5%) mainstream tests were ordered for patients by clinicians who had not completed the mandatory mainstream upskilling. These tests were halted whilst the PFCC contacted the clinicians and completed a one-on-one upskilling session with them, before the tests could proceed.
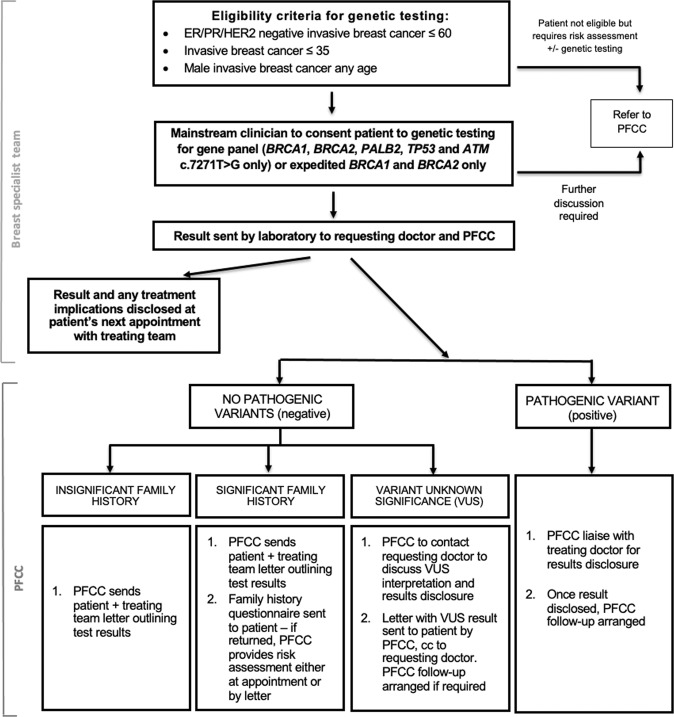
Based on clinician feedback in the program and research around patient preference for results disclosure [17] the protocol was further amended so that the PFCC liaised with breast specialists to determine the optimal setting and timing for disclosure of a PV to align with existing patient appointments. In addition, although all patients with a PV were offered a prompt PFCC appointment, a number of patients declined as they were satisfied with the telephone discussion. These changes were incorporated in the final revised breast cancer mainstream protocol; shown in Fig. 1.
Fig. 1. Revised mainstream genetic testing protocol at the PFCC.
Protocol is modelled on the ovarian mainstream pathways at The Royal Marsden, and PFCC [13, 14].
Data collation
An audit of the PFCC breast mainstream program was conducted between September 2017 and September 2019. With Human Research Ethics Committee (HREC) approval from Peter MacCallum Cancer Centre and The Royal Melbourne Hospital, patient and clinician data for tests ordered through the program, as well as in-service participation during this period was collated, and the results reported below.
Results
Mainstream clinicians
Commencing in September 2017, breast surgical and oncology services from 10 hospitals have participated in the mainstream program, and 92 specialists have completed upskilling. From these clinicians, 57 have ordered a genetic test in the mainstream setting in the first 24 months. A further 15 clinicians who did not complete upskilling have ordered mainstream testing, resulting in a total of 72 clinicians ordering mainstream testing: 42 medical oncologists, 27 surgeons and 3 radiation oncologists. Mainstream testing has been ordered for patients from nine of the 10 hospitals where upskilling was performed.
When asked for feedback, some mainstream clinicians appreciated the efficiency of being able to order genetic testing for their patients directly, rather than referring on to the PFCC. Furthermore, particularly for more complex cases including VUS results, or where assessment for a rare genetic condition was required, mainstream clinicians valued the input and assistance provided by the PFCC.
Patient characteristics
In the first two years of the program, 230 patients have completed mainstream testing across nine hospitals in Victoria, Australia. Of these, 142 (61.7%) qualified due to a diagnosis of TNBC ≤60 years, 85 (37%) had a breast cancer diagnosis ≤35 years (the 17 patients who were diagnosed with TNBC ≤35 years are included in both figures) and 15 (6.5%) were males diagnosed with breast cancer. On review of the clinical information, 5 (2.2%) patients who underwent testing were found not to meet the eligibility criteria.
Mainstream test results
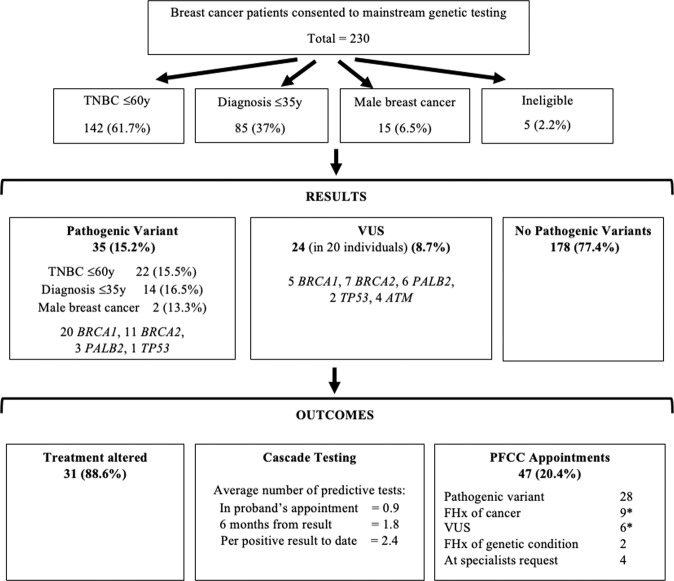
Testing outcomes are outlined in Fig. 2. Expedited testing for BRCA1/2 was requested for only 13 (5.7%) patients, with the standard five gene panel performed in all remaining cases (94.3%). Overall, the detection rate for a PV in one of the five genes was 15.2% (35); 20 BRCA1, 11 BRCA2, 3 PALB2, and 1 TP53 PVs were identified and no ATM PVs. Based on eligibility criteria, the PV detection rate was: 15.5% (n = 22) for TNBC, 16.5% (n = 14) for diagnosis ≤35 years (23.5% (n = 4) of patients with TNBC ≤35 years had a PV identified, and are included in these figures), and 13.3% (n = 2) for male breast cancer. A PV in BRCA1 was identified in a patient who did not meet mainstreaming criteria (the patient had a personal history of three primary breast cancer diagnoses including a TNBC diagnosed >60 years).
Fig. 2. PFCC mainstream program outcomes between September 2017 and September 2019.
*some patient appointments were due to the finding of a VUS and a family history (FHx) of cancer.
Twenty-four VUS were identified in 20 different mainstream patients (VUS detection rate of 8.7%); the majority in BRCA2. These VUS were submitted to the ENIGMA consortium (Evidence-based Network for the Interpretation of Germline Mutant Alleles) for ongoing review of their classification [18, 19]. One of the 24 VUS was downgraded to class 2 (likely benign) during the first two years of the mainstream program period [4, 5]. Three patients had a PV and VUS identified concurrently, and three patients had more than one VUS identified. One TP53 VUS was considered suspicious and two further TP53 VUS required segregation testing to determine if they were de novo or inherited to help clarify their significance. On average, it took 27.6 working days for a test result to become available after blood collection; with a range of 11 working days (BRCA1 and BRCA2 testing only), to 52 working days.
Patient treatment implications
Of the 35 patients found to have a PV, 31 (88.6%) had their cancer treatment altered based on the genetic test result. Treatment implications included women undergoing contralateral prophylactic mastectomy (80.6%) and/or having carboplatin added to chemotherapy (25.8%). Two women were provided with compassionate access to a PARP inhibitor, and in one case a previous plan to provide radiotherapy was revised after a germline TP53 PV was identified. The patient with the suspicious TP53 VUS did not have their treatment altered, as radiotherapy was considered to be strongly clinically indicated due to a high risk of local recurrence.
PFCC involvement in mainstream cases
As the breast specialists and laboratory adapted to the change in the model of care, 52% (n = 120) of the 230 patients who have undergone mainstream testing were managed entirely within the protocol, but the remaining 110 required additional support from the PFCC, although in most cases this was minimal. Common issues leading to additional PFCC involvement included problems with blood collection at local pathology services, mainstream clinicians chasing test results, administrative issues involving interruption of the normal protocol, and mainstream clinicians over-calling VUS results when disclosing results to patients resulting in PFCC contacting patients to clarify interpretation of result. Some patients who were mainstream eligible were referred to PFCC without being offered mainstream genetic testing first but in most cases mainstream referring doctors were simply reminded of the option to offer mainstream testing at the patient’s next treatment appointment.
In total 47 (20.4%) patients in the program required an appointment at the PFCC; 28 due to PV results (six PV patients felt satisfied by telephone discussion only for results, and one PV patient was seen at an interstate FCC), 13 due to the patient either having a significant family history of cancer warranting PFCC review or due to VUS results (or both). Two were due to the presence of another genetic condition in the patient’s family and the remainder involved a genetics health professional being onsite and called upon during their treatment appointment. Forty-nine patients had telephone contact with the PFCC (average length of call was 23 min), with the majority being PV patients, and some due to other family members being referred to PFCC for genetic cancer risk assessment, or another genetic condition being present in the family.
Under the traditional PFCC model of care, on average the 230 mainstream patients would have attended a total of 460 PFCC appointments. Using the mainstream pathway, 413 (90%) PFCC appointments were saved over the two years since its implementation.
Although the PFCC provided additional support in multiple cases, outside of the standard PV patients there were six cases in particular that benefited greatly from having a genetics service involved with the genetic testing; briefly outlined in Table 1.
Table 1.
Mainstream cases that particularly benefited from PFCC involvement.
| Patient age | Mainstream eligibility | Mainstream test ordered by | PFCC involvement in mainstream case |
|---|---|---|---|
| 66 | Male breast cancer | Oncologist | Patient tested negative for the hereditary breast cancer genes, but reported a strong family history of various cancers. Patient completed a family history questionnaire for PFCC to assess whether further genetic testing was indicated and was matched to an existing family in the PFCC database, identifying the patient had a relative with a MEN1 PV. The patient was unaware of the existing familial MEN1 PV, and underwent MEN1 predictive testing at a PFCC appointment; the patient tested negative to the familial MEN1 PV |
| 73 | Male breast cancer | Oncologist | Patient found to have a BRCA2 PV through mainstream testing, was also then identified as having a relative with a different BRCA2 PV. Through consultation with another FCC, the PFCC was able to ensure that the appropriate relatives were able to undergo predictive testing for both BRCA2 PVs |
| 31 | TNBC diagnosis ≤35 years | Oncologist | Patient tested negative for the hereditary breast cancer genes but completed a family history questionnaire and it was determined their relative had likely had RB1 testing in the past due to a history of paediatric retinoblastoma. The patient had an appointment with the PFCC to have a risk assessment performed, and also to discuss future breast cancer risk to aid in treatment decisions |
| 32 | Breast cancer diagnosis ≤35 years | Oncologist | Patient was found to have a BRCA1 PV, and was in a consanguineous relationship; patient’s partner also tested positive for the BRCA1 PV. Both patients had appointments with PFCC, and the PFCC liaised with their reproductive services team where they had had embryos frozen for future use, and discussed the option of preimplantation genetic diagnosis. All relatives of the couple lived overseas where access to genetics services was limited, and so PFCC assisted patients in setting up testing for relatives through an overseas laboratory who offer testing using mail-out saliva kits |
| 44 | Breast cancer diagnosis ≤35 years | Oncologist | Patient had two ATM VUS identified and had a personal history of a young haematological cancer. Patient had a review appointment with PFCC to rule out a potential diagnosis of Ataxia telangiectasia |
| 48 | TNBC diagnosis ≤60 years | Oncologist | Patient tested positive for a BRCA1 PV, but reported she regretted her decision to undergo genetic testing. Patient was reluctant to consider future risk reducing bilateral salpingo-oophorectomy (rrBSO), and was overwhelmed with breast cancer treatment. Although it was challenging to engage the patient who failed to attend multiple appointments and at times did not return phone calls, through careful and supportive interactions with genetic counsellors, the PFCC have been able to keep in contact with the patient whilst she adjusted to the result. The patient has now undergone rrBSO and the PFCC currently await her family members attendance at an upcoming appointment for predictive testing |
When asked for feedback, several patients reflected positively on their experience of mainstream testing, with a common observation being that they appreciated the convenience of having testing through their existing oncology appointments and felt that they may not have had testing if they had to attend the PFCC for separate appointments. One patient expressed regret at having had genetic testing (see Table 1), but ultimately she did engage with the PFCC and underwent risk reducing surgery, as well as communicated with her relatives about the PV identified through her testing.
Cascade testing in relatives
28 PV patients (probands) attended PFCC for an appointment to discuss their genetic test result, and 16 of these patients brought at least one relative (and up to three) who underwent predictive testing at the same time. Within six months of probands receiving their test results, predictive testing had occurred in 74% of the families. With an average follow up of 13 months to date, 2.4 relatives (range 0 to 8) have undergone predictive testing per proband.
Under the traditional PFCC model of care, on average the 25 relatives who attended PFCC with the proband would have attended a total of 50 PFCC appointments. Therefore, a further 25 clinic appointments were saved by the emergence of the hybrid ‘proband results – family predictive testing’ appointment for mainstream probands when compared to the traditional PFCC model of care.
Together over the two years the mainstream program has operated, the PFCC has saved a total of 438 out of 510 (86%) potential appointments through the implementation of the mainstream pathway.
Discussion
Since implementing a breast cancer mainstream program in 2017, there has been excellent uptake by breast cancer specialists with 72 specialists across nine hospitals requesting testing for 230 patients in two years. Testing with the mainstream eligibility criteria generated a decent PV detection rate of 15.2%, with a test turnaround time of five and a half weeks. The majority of PV patients (88.6%) had their cancer treatment changed based on their test result. Cascade testing for family members to date has occurred for the majority of PV patients (77%). Finally, implementing the mainstream pathway has saved the PFCC 438 appointments over a two-year period since the program’s implementation.
The mainstream program’s success after rollout is evident by the breast specialist uptake, and the number of tests requested in the short time it has been running. Similar to a previous study, the PFCC’s mainstream program success appears to rest largely on both the PFCC’s relationship and communication with the cancer speciality teams within the hospitals, as well as multiple ‘mainstreaming champions’ (advocates for mainstreaming, and oncologists & surgeons who also work within the PFCC) at each hospital site [20]. Other groups have previously investigated clinician attitudes towards mainstreaming, and locally the ovarian mainstream program has been received well by the clinicians involved [12–14, 20, 21].
The PV detection rate was well above 10% for each of the chosen eligibility criteria, and the majority of PV carriers had cancer treatment altered due to the test outcome. Similarly to ovarian genetic mainstream programs, this supports the choice to include these high genetic risk patients in the mainstream program, allowing them access to streamlined genetic testing through their breast specialist with support from the PFCC [13].
Uptake of predictive testing in family members of PV carriers was promising, with 74% of families having predictive testing occur within six months of the proband receiving their genetic test result, and 2.4 relatives on average having had testing within 13 months of a proband’s PV result. These figures are in line with previous data collected for PFCC patients who have undergone genetic testing through the standard model of care. These findings also indicate an engagement with adverse health prevention from mainstream patients’ families, meaning there is a health benefit to the families of PV patients who have undergone testing via the mainstream pathway.
Implementing the mainstream protocol has brought about several changes in the PFCC model of care for patients. The first is the reduced number of PFCC appointments required for patients to attend, with the majority of patients not needing to see the PFCC for an appointment at all. This also includes the PV patients, as six were satisfied with a telephone discussion only. The reduced number of PFCC appointments has meant that the genetic testing has been more streamlined and less time consuming for the patients, but has also saved costs for the PFCC, enabling the PFCC to focus its limited resources where they are needed most, caring for patients with high genetic cancer risk and their families.
Another new model of care has emerged for patients with a PV result when they attend the PFCC for an appointment, as over half attended with family members (most commonly first-degree relatives), who underwent predictive testing at the appointment. This phenomenon has shifted the model of care for the PFCC by adjusting to a ‘proband results – family predictive testing’ appointment, where there are different agendas for each patient in the room depending on whether they are discussing results in the context of their cancer diagnosis or wanting to proceed with predictive testing. These family-style appointments have required adaption from the genetic counsellors as often more than one genetic counsellor needs to be present to meet the different patient agendas, or the appointments have to be lengthened and the family members offered time alone to individually discuss any personal concerns around testing. Furthermore, this approach seems to empower individuals and families to engage with genetic testing in a way that feels comfortable to them, with the proband’s agenda seemingly focused on looking after their family members’ health, particularly if they have had their personal needs met at the time of their results disclosure prior to the PFCC appointment. Finally, the family-style appointments have led to a further efficiency of patient care, with additional PFCC appointments saved.
Lastly, the mainstream pathway describes a different model of care compared with the traditional genetic testing process through the PFCC, where the focus is on a support and education model for non-genetic health professionals in facilitating genetic testing, and a collaborative approach between mainstream clinicians and the PFCC in results disclosure. In the setting of a new breast cancer diagnosis, this mainstream model of care enables streamlined genetic testing focused primarily on its implications for the patient’s treatment. This timely approach improves the likelihood of the genetic test results availability to potentially inform the patient’s treatment plan. It also provides mechanisms for involvement from the PFCC when familial implications are identified, or genetic expertise is required. The model utilises and emphasises the different skill sets between the mainstream clinician and genetic expert to ensure the provision of best practice for patients undergoing a mainstream genetic test.
Several challenges have arisen during breast cancer mainstreaming implementation
The majority of these have been resolving administrative issues as the mainstream clinicians, laboratory and PFCC adjust to the mainstream model of care. Whilst the relationship and communication between the PFCC and breast specialists, and the PFCC and genetic testing laboratory are well established, it has been challenging attempting to establish a direct relationship between the genetic testing laboratory and the mainstream clinicians. Historically, all genetic testing has been ordered by the PFCC, and so the laboratory and non-genetics health professionals have not needed to interact in the past. Initially, this meant the mainstream clinicians often use the PFCC as a ‘middle-man’ for test requests and results, rather than communicating with the laboratory directly – this has been unnecessarily time consuming for PFCC staff. To overcome most of these challenges, the PFCC have had to ensure they keep open lines of communication with the laboratory team and the mainstream clinicians and address these issues as they arise. Additionally, as new doctors commenced working at the mainstream hospital sites, we identified mainstream genetic tests ordered by such doctors who had not completed formal upskilling offered as part of the PFCC mainstream program. A consideration moving forward will be to offer annual in-service upskilling sessions to ensure all practicing doctors are upskilled.
At the time of the program’s implementation the eligibility criteria for the mainstream program encompassed more patients than the Australian national guidelines for hereditary breast cancer gene testing. Specifically, the group of women with TNBC diagnosed between age 51 and 60 years would not have automatically qualified for testing under the national guidelines without an assessment of their family history. Fifty-two tests were performed for women in this group with 7 PVs detected (13.5% pick up) indicating that this inclusion was consistent with broader criteria of a 10% probability of detecting a PV that is the major national guideline criteria. Overall the PV detection rate in the mainstream program was encouraging across all criteria, however in the time since implementation public funding for genetic testing of patients with breast cancer has been extended to cover a broader patient group [2] and the Australian guidelines for testing have been updated. As a result, consideration has been given to expanding the eligibility criteria to include all patients with breast cancer and a 10% chance of a PV in the continuing program. In addition, genetic testing eligibility for patients at the PFCC has always remained broader than in the mainstream program, and patients were always able to be referred for specialist assessment to determine genetic testing eligibility by the PFCC outside of the mainstream program. In a further change since the program commenced, the Cancer Genetics National Reference Committee have also released a risk management guideline for CHEK2 truncating PVs [15], and this gene has been added to testing panel for future use. These extensions of the program will continue to improve patient access and streamline the process for eligible patients allowing PFCC resources to be directed to where they add most value and clinical utility.
Whilst there are other areas in genetic counselling such as prenatal genetic screening where a patient undergoes genetic testing with a non-genetics health professional [22], with the change in model of care in the breast cancer genetics mainstreaming program, it will be important to learn more about both patient and mainstream clinician experiences as the program expands, and compare these with the usual standard of care traditionally offered by the PFCC.
Implications for clinical practice
One of the key features of this mainstream program’s success was ensuring ‘mainstreaming champions’ were involved in the oncology and surgical clinics, particularly during its implementation. The importance of being patient-centred and adaptive was paramount for the PFCC, as the mainstreaming program brought about multiple shifts in the usual model of care for both breast cancer patients, and their family members. Finally, an important consideration moving forward is to offer regular upskilling in-service presentation opportunities, and to ensure the lines of communication are open between all mainstreaming parties, including the mainstream clinicians, the laboratory and the PFCC.
Conclusion
Since the introduction of the breast cancer mainstream program in September 2017 at PFCC, it has had a successful uptake by clinicians and patients with 230 mainstream tests being ordered thus far, and a decent overall PV detection rate of 15.2%. The shift in model of care has resulted in a more streamlined process for patients and has reduced the burden on the PFCC with 438 appointments saved over the two years the program has been running. Investigation into the experiences of the mainstream program is required for both patients and clinicians and will help inform its future progress and direction, as well as consideration to expand the eligibility criteria of the program.
Acknowledgements
We thank Professor Bruce Mann and Professor Geoffrey Lindeman for their assistance in implementing the mainstreaming program, as well as all the participating clinicians. Our thanks also to the individuals who underwent testing and who provided important feedback and suggestions to help improve the service.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James PA, Mitchell G, Bogwitz M, Lindeman GJ. The Angelina Jolie effect. The. Med J Aust. 2013;199:646. doi: 10.5694/mja13.11218. [DOI] [PubMed] [Google Scholar]
- 2.Kirk J, Barlow-Stewart KK, Poplawski NK, Gleeson M, Tucker K, Friedlander M. Medicare-funded cancer genetic tests: a note of caution. Med J Aust. 2018;209:193–6. doi: 10.5694/mja17.01124. [DOI] [PubMed] [Google Scholar]
- 3.Jarvik GP, Evans JP. Mastering genomic terminology. Genet Med. 2017;19:491–2. doi: 10.1038/gim.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–91. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poggio F, Bruzzone M, Ceppi M, Ponde NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Annals of oncology: official journal of the European Society for. Med Oncol. 2018;29:1497–508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 7.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28:222–31. doi: 10.1200/JCO.2009.22.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl J Med. 2017;377:523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 10.Heymann S, Delaloge S, Rahal A, Caron O, Frebourg T, Barreau L, et al. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol. 2010;5:104. doi: 10.1186/1748-717X-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grindedal EM, Jørgensen K, Olsson P, Gravdehaug B, Lurås H, Schlichting E, et al. Mainstreamed genetic testing of breast cancer patients in two hospitals in South Eastern Norway. Fam Cancer. 2020;19:133–42. doi: 10.1007/s10689-020-00160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemp Z, Turnbull A, Yost S, Seal S, Mahamdallie S, Poyastro-Pearson E, et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open. 2019;2:e194428. doi: 10.1001/jamanetworkopen.2019.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:29506. doi: 10.1038/srep29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kentwell M, Dow E, Antill Y, Wrede CD, McNally O, Higgs E, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecologic Oncol. 2017;145:130–6. doi: 10.1016/j.ygyno.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 15.eviQ Cancer Genetics National Reference Committee. Risk Management, 2020, https://www.eviq.org.au/cancer-genetics/adult/risk-management2020.
- 16.Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer. 2014;110:535–45. doi: 10.1038/bjc.2013.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tutty E, Petelin L, McKinley J, Young MA, Meiser B, Rasmussen VM, et al. Evaluation of telephone genetic counselling to facilitate germline BRCA1/2 testing in women with high-grade serous ovarian cancer. Eur J Hum Genet: EJHG. 2019;27:1186–96. doi: 10.1038/s41431-019-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen SM, Eccles DM, Romero IL, Al-Mulla F, Balmaña J, Biancolella M, et al. Genetic testing and clinical management practices for variants in non-BRCA1/2 breast (and Breast/Ovarian) cancer susceptibility genes: an international survey by the evidence-based network for the interpretation of germline mutant alleles (ENIGMA) clinical working group. JCO Precis Oncol. 2018;2. [DOI] [PMC free article] [PubMed]
- 19.Spurdle AB, Healey S, Devereau A, Hogervorst FB, Monteiro AN, Nathanson KL, et al. ENIGMA-evidence-based network for the interpretation of germline mutant alleles: an international initiative to evaluate risk and clinical significance associated with sequence variation in BRCA1 and BRCA2 genes. Hum Mutat. 2012;33:2–7. doi: 10.1002/humu.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallowell N, Wright S, Stirling D, Gourley C, Young O, Porteous M. Moving into the mainstream: healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Familial Cancer. 2019;18:293–301. doi: 10.1007/s10689-019-00122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gleeson M, Kentwell M, Meiser B, Do J, Nevin S, Taylor N, et al. The development and evaluation of a nationwide training program for oncology health professionals in the provision of genetic testing for ovarian cancer patients. Gynecol Oncol. 2020;158:431–9. doi: 10.1016/j.ygyno.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Beard CA, Amor DJ, Di Pietro L, Archibald AD. “I’m Healthy, It’s Not Going To Be Me”: exploring experiences of carriers identified through a population reproductive genetic carrier screening panel in Australia. Am J Med Genet Part A. 2016;170:2052–9. doi: 10.1002/ajmg.a.37697. [DOI] [PubMed] [Google Scholar]