Highlights
-
•
Pseudoprogression can be a side effect of SRT of brain metastases.
-
•
CTV-PTV margin reduction to 0 mm did not reduce incidence of pseudoprogression.
-
•
V12 Gy was not associated with incidence of pseudoprogression.
Keywords: Brain metastases, Stereotactic radiotherapy, Margins, Pseudoprogression
Abstract
Purpose
To determine the influence of PTV-margin (0 mm versus 2 mm) on the incidence of pseudoprogression (PP) and local tumour control (LC) in patients treated with stereotactic radiotherapy (SRT) for solitary brain metastases.
Methods
Patients were treated on Novalis LINAC. Three dose schedules were used depending on the PTV-size. The PTV-margin was 2-mm prior to 2015 and 0-mm thereafter. MRI-scans were made every three months including a perfusion MRI-scan when pseudoprogression was suspected. We examined the relation of pseudoprogression and local control with the size of PTV-margin. Besides this, the association of dose-volume data of the whole brain (minus GTV) and pseudoprogression was investigated.
Results
121 patients were analyzed (2-mm margin in 84 patients; 0-mm margin in 37 patients). There was no difference in GTV (7.6 cc versus 9.1 cc p = 0.2). At 24 months there was no difference in incidence of pseudoprogression (49% and versus 33%, p = 0.5) and local control in the 2-mm and 0-mm group (82% and versus 79%, p = 1.0). The size of PTV-margin was not associated with PP. Both margin and volume of brain receiving 12 Gy (V12) were not associated with pseudoprogression in patients treated with single fraction.
Conclusions
PTV-margin reduction did not reduce the incidence of pseudoprogression in LINAC-based-SRT for single brain metastases. We did not find a significant association of GTV-PTV margin or V12Gy with the incidence of pseudoprogression in solitary metastases treated with a single fraction. LC rates were similar, indicating margin reduction seems to be safe.
Introduction
Brain metastases frequently occur in cancer patients (20–40%) and have a substantial impact on quality of life [1], [2]. As systemic treatments become more effective, patients live longer and are at risk to develop brain metastases and an effective local treatment for brain metastases becomes more relevant [3], [4].
Treatment options for patients with 1–3 brain metastases include surgery and stereotactic radiotherapy depending on factors such as performance status, the systemic disease load, the size and the location of the metastases. The local tumour control rate for brain metastases treated with stereotactic radiotherapy (SRT) is up to 93% at 1 year [5], [6], [7] and is comparable to surgery for tumours <4 cm [8]. The non-invasive nature of SRT makes it an excellent treatment option for patients in whom the metastases are not accessible for surgery. Despite this advantage of SRT, it can be a challenge to distinguish between tumour progression and radiation necrosis on MRI-scans during follow-up. The radiological changes seen on follow-up MRI-scans are called pseudoprogression when the changes mimic tumour progression but are actually almost always caused by radiation necrosis. Pseudoprogression can be categorized as symptomatic or asymptomatic. Symptomatic pseudoprogression of the brain has been described in 2–14% of patients after SRT [9], [10]. The incidence of pseudoprogression is likely to increase as more patients have durable responses to new systemic agents. Even when pseudoprogression is asymptomatic, it can cause fear for tumour recurrence and can decrease the quality of life. An attempt to reduce the incidence of both symptomatic and asymptomatic pseudoprogression is therefore important.
Factors that were predictive for pseudoprogression have been identified in retrospective studies. These studies suggest that the normal brain tissue receiving 12 Gy (V12) is predictive for pseudoprogression when SRT is given in a single-fraction [9], [10]. Drawbacks of these studies are that no uniform definition was used to define both pseudoprogression and V12 [11]. In addition to this, previous studies included patients who were treated with whole brain radiotherapy before SRT.
The risk of pseudoprogession may decrease by reducing the planning target volume (PTV) margin, reducing the volume of normal brain tissue irradiated. Three small studies have published conflicting results of the effect of margin reduction on the incidence of pseudoprogression [12], [13], [14]. The primary goal of this study is to determine whether the incidence of pseudoprogression decreases when a smaller PTV-margin is used, and whether local tumour control (LC) and overall survival (OS) are maintained. In addition, associations of margins and dose-volume data of normal brain with pseudoprogression were investigated.
Material and methods
Patients
We retrospectively analyzed patients with solitary brain metastasis treated with stereotactic radiotherapy between 2010–2012 and 2015–2016. We chose these two time periods as different GTV-PTV margins were used: 2-mm GTV-PTV margin prior to 2012 and a 0-mm GTV-PTV as of 2015. The data of patients treated with a 2-mm margin between 2010 and 2012 were already available from a previous analysis. The other group of patients was selected in the period 2015–2016, whom were treated with 0-mm margin. We changed the margin from 2-mm to 0-mm because of relatively high rates of symptomatic pseudoprogression. All patients in this study had a diagnostic Magnetic Resonance Imaging scan (MRI-scan) consisting of five series: a T1-weighted image with and without gadolinium (voxel size 1.1 × 1.1 × 1.3 mm3), a T2-weighted image, a diffusion-weighted series and a MRI-perfusion series. Inclusion criteria were a Karnofsky performance score (KPS) > 60 and a tumour < 4 cm. Exclusion criteria were previous surgical resection or whole brain radiation therapy. 30 patients were excluded due to these reasons. A total of 121 patients met the inclusion criteria.
Treatment
Radiotherapy treatment planning was based on a Computed Tomography scan (CT-scan) and a fused MRI-scan. The CT-scan, with a slice thickness of 2 mm and a pixel size of 0.7 × 0.7 mm, was made while the patient was immobilized using a thermoplastic mask (BrainLAB AG, Feldkirchen, Germany). A customized vacuum mouth piece was used for dentate patients [15] while a standard upper jaw support (Brainlab) was used for patients without teeth. The MRI-scan was used to contour the gross tumour volume (GTV). GTV was defined as the contrast enhanced region on the T1-weighted image with gadolinium. In the majority of cases, treatment started well before two weeks after the MRI-scan was made. If the time between the diagnostic MRI-scan and the start of stereotactic radiotherapy was more than 2 weeks, a new MRI-scan was made for radiotherapy treatment planning. Patients were treated with one of four dose schedules, depending on the PTV size: 1 × 21Gy for a PTV of 1–10 cm3, 1 × 18Gy for a PTV of 10–20 cm3, and for a PTV > 20 cm3 three fractions of 8 Gy were used [7]. The dose was prescribed to the 80% isodose line and the Dmax was mostly > 105%. Treatment plans were made in iPlan (Brainlab). All patients were treated with 6MV photons using a non-coplanar Dynamic Conformal Arc (DCA) technique with three to five arcs. The dose volume histogram (DVH) of the normal brain tissue was exported from the treatment planning system and various dosimetric parameters were derived. Furthermore, we conducted the Paddick’s conformity index (CI) in percentages [16].
Treatment was delivered using the Novalis Classic by Brainlab; a linear accelerator-based radiosurgery system with a micro-multi leaf collimator. The Exactrac system (Brainlab AG) was used for image guidance [15]. Patients were prophylactically treated with dexamethasone (generally 2 times a day 6 mg) one day prior to the first fraction of stereotactic radiotherapy until one day after the last fraction. Treatment planning and delivery technique were essentially unchanged from 2010 to 2016.
Follow-up
Follow-up was generally performed on the day of the second or last treatment fraction, 3–6 weeks after treatment and every three months thereafter. MRI-scans were made every 3 months after treatment or sooner if a patient was symptomatic. For the purpose of this study, the follow-up MRI-scans were interpreted independently by a second experienced neuro-radiologist (EG) to determine a local tumour recurrence or pseudoprogression. In this paper, the term pseudoprogression refers to the radiological manifestation of an inflammatory reaction that, in most cases, is caused by radiation necrosis. Pseudoprogression was defined by a combination of the following MRI criteria [10]: 1) an increase in T1 contrast enhancement in the irradiated area, 2) central hypo-intensity, 3) increased peripheral edema, 4) absence of perfusion, and 5) the absence of highly vascularized nodules within the contrast-enhanced area on the perfusion MRI-scan. In addition to this, subsequent follow-up MRI-scans had to show regression or stability of the contrast enhanced area (in the absence of additional treatment). If subsequent MRI-scans were not available, and the lesion had grown, it was categorized as local recurrence. In case of a progressive lesion that did not fulfil the criteria of pseudoprogression, we scored it as tumour recurrence. Medical hospital records were retrospectively reviewed in patients with signs of pseudoprogression on MRI. Pseudoprogression was symptomatic if the patient had new or progressive neurological symptoms, or if the patient required steroid therapy. We analysed the impact of margin reduction and dose volume parameters on the incidence of pseudoprogression. In this analysis we did not distinguish between asymptomatic and symptomatic pseudoprogression as the incidence of symptomatic pseudoprogression was low.
Data analysis
Descriptive statistics, using the unpaired T-test, Chi-square and Fisher’s exact tests, were used to describe differences between 2-mm and 0-mm groups. For variables that are not normally distributed the Mann-Whitney U test was used. Or if needed a log transformation was computed. In these cases, the data were normally distributed on the log scale.
Local recurrence and pseudoprogression were evaluated at lesion level. Overall survival was evaluated at patient level. Survival time was calculated from the first day of SRT until the date of death or the last date of follow-up. Local tumour control and pseudoprogression were defined as the first day of SRT until the date of local recurrence or pseudoprogression. Local tumour control, overall survival and time to pseudoprogression were estimated using the Kaplan-Meier method. The log-rank test was used to evaluate differences between 2-mm and 0-mm groups. Median follow-up was calculated using the reverse Kaplan-Meier method.
For analysis of factors associated with pseudoprogression a Cox proportional hazards regression model was conducted, with the 0-mm margin group as the reference. Firstly, in the unadjusted model for all treatments we assessed the influence of margin on pseudoprogression. Secondly, the adjusted model tested the influence of gender, KPS, GTV and fractionation schedule [10], [17]. For single fraction treatments the association between pseudoprogression and the V12 as a dichotomous value with the threshold > 10.9 cc was examined as well [10]. We calculated the V12 as total brain minus GTV. Finally, we adjusted for gender, KPS and GTV [10], [17]. In both analysis the GTV was considered as a continuous variable and KPS as a dichotomous variable (≥90 and < 90). P-values ≤ 0.05 (two-sided) were considered to be statistically significant. SPSS Statistics version 22.0 (IBM SPSS Statistics for Windows. Armonk, NY, USA: IBM Corp.) was used for the analyses.
Results
Patient and lesion characteristics
A total of 121 patients (64 females and 57 males) were treated for a solitary brain metastasis between 2010–2012 or 2015–2016. These patients were analyzed for overall survival and 119 patients (62 females and 57 males) were analyzed for pseudoprogression and local control, since 2 patients had no imaging follow-up.
The median follow-up for all patients was 43.2 months (IQR 31.2–79.2 months): 31.2 months (IQR 27.6–34.8 months) in the 0-mm group and 79.2 months in the 2-mm group (IQR 72.0–91.2 months). The clinical characteristics of both patient groups were comparable (Table 1). The percentage of patients with lung cancer was higher in the patients treated with 0-mm margin (73.0% vs 48.8%). In addition, there was a larger V10-V18 in the 2-mm group due to the larger PTV. The prescribed dose was mostly 1 × 21 Gy in the group with 0-mm margin compared to 3 × 8 Gy in the group with 2-mm margin (p ≤ 0.01). As doses were prescribed according to the PTV, this difference can be explained by larger PTVs in the 2-mm margin group. As a consequence of the larger PTV in the 2-mm group the volume of irradiated brain (V10-V18) is increased. There was a significant difference in the Paddick’s CI between two treatment groups (p < 0.001) (Table 1). Fig. 1 shows examples of the dose distributions around two small metastases with 0-mm and 2-mm GTV-PTV margin and two larger metastases with 0-mm and 2-mm GTV-PTV margin. The data in the legend illustrate that the Paddick’s conformity index is higher (better) in larger PTVs. There was no significant difference in maximal dose in the PTV between the 0-mm and 2-mm margin groups for multiple fractions (p = 0.9). However, for the single fractions there was a significant difference in maximal dose (p = 0.03) (Table 1).
Table 1.
Patient-, lesion and treatment characteristics.
| 0-mm margin | 2-mm margin | P Value | ||
|---|---|---|---|---|
| Number of patients | N = 121 | 37 (28.5%) | 84 (71.5%) | |
| Sex Female Male |
23 (62.2%) 14 (37.8%) |
41 (48.8%) 43 (51.2%) |
P = 0.2 |
|
| Median age (years) Mean Range <70 years ≥70 years |
64 64 40–86 |
23 (62.2%) 14 (37.8%) |
57 (67.9%) 27 (32.1%) |
P = 0.5 |
| Primary tumor Lung Breast Melanoma/Renal cell Colorectal Other |
27 (73.0%) 4 (10.8%) 3 (8.1%) 1 (2.7%) 2 (5.4%) |
41 (48.8%) 11 (13.1%) 14 (16.7%) 8 (9.5%) 10 (11.9%) |
P = 0.1 |
|
| Karnofsky status Median Range ≥90 <90 |
80 50–100 |
19 (51.4%) 18 (48.6%) |
33 (39.3%) 51 (60.7%) |
P = 0.2 |
| Localisation Supratentorial Infratentorial |
21 (56.8%) 16 (43.2%) |
63 (75%) 21 (25%) |
P = 0.04 | |
| Volume GTV Mean (in cc) Median (in cc) ≤7 cc >7 cc |
8.6 5.6 |
7.6 4.7 25 (67.6%) 12 (32.4%) |
9.1 5.9 47 (56.0%) 37 (44.0%) |
P = 0.2 P = 0.3 |
| Volume PTV Mean (in cc) Median (in cc) ≤7 cc >7 cc |
12.7 9.2 |
7.6 4.7 25 (67.6%) 12 (32.4%) |
14.9 10.9 25 (29.8%) 59 (70.2%) |
P = 0.001 P < 0.001 |
| Dose 1 × 15 Gy 1 × 18 Gy 1 × 21 Gy 2 × 8 Gy 3 × 8 Gy |
1 23 50 1 46 |
0 (0)% 4 (10.8%) 23 (62.2%) 1 (2.7%) 9 (24.3%) |
1 (1.2%) 19 (22.6%) 27 (32.1%) 0 (0%) 37 (44.0%) |
P = 0.01 |
| Single fraction: V10 Mean in cc (median in cc) V12 Mean in cc (median in cc) V14 Mean in cc (median in cc) V16 Mean in cc (median in cc) V18 Mean in cc (median in cc) |
N = 27 16.4 (11.6) 12.0 (8.3) 8.9 (5.9) 6.5 (4.1) 4.6 (2.3) |
N = 47 17.1 (17.4) 12.9 (13.6) 9.9 (10.7) 7.6 (8.3) 5.5 (6.1) |
P = 0.2 P = 0.2 P = 0.1 P = 0.1 P = 0.04 |
|
| Multiple fraction: V10 Mean in cc (median in cc) V12 Mean in cc (median in cc) V14 Mean in cc (median in cc) V16 Mean in cc (median in cc) V18 Mean in cc (median in cc) |
N = 10 47.1 (39.3) 35.3 (28.8) 27.1 (21.6) 21.1 (16.6) 16.5 (12.7) |
N = 37 60.7 (56.1) 46.5 (42.7) 36.8 (33.9) 29.6 (27.7) 24.0 (22.7) |
P = 0.2 P = 0.1 P = 0.1 P = 0.08 P = 0.06 |
|
| ’Salvage’ WBRT | 5 (13.5%) | 5 (6.0%) | P = 0.3 | |
| Paddick’s CI in % (mean) | 68.2 (13.3) | 78.1 (6.8) | P < 0.001 | |
| Max dose in Gy (mean) Single fraction in Gy (mean) Multiple fractions in Gy (mean) |
27.3 (3.1) 26.1 (1.2) 30.6 (4.3) |
28.2 (4.5) 25.0 (1.9) 32.3 (3.3) |
P = 0.5 P = 0.03 P = 0.9 |
|
Fig. 1.
Dose distributions for patients from 0-mm margin (a and c) and 2-mm margin (b and d) groups, Patients, (a) GTV = PTV = 0.17 cc CI = 44%, (b) GTV = 0.23 cc, PTV = 0.89 cc, CI = 61%, (c) GTV = PTV = 5.32 cc, CI = 76%, (d) GTV = 5.63 cc, PTV = 9.9 cc, CI = 81%.
Pseudoprogression
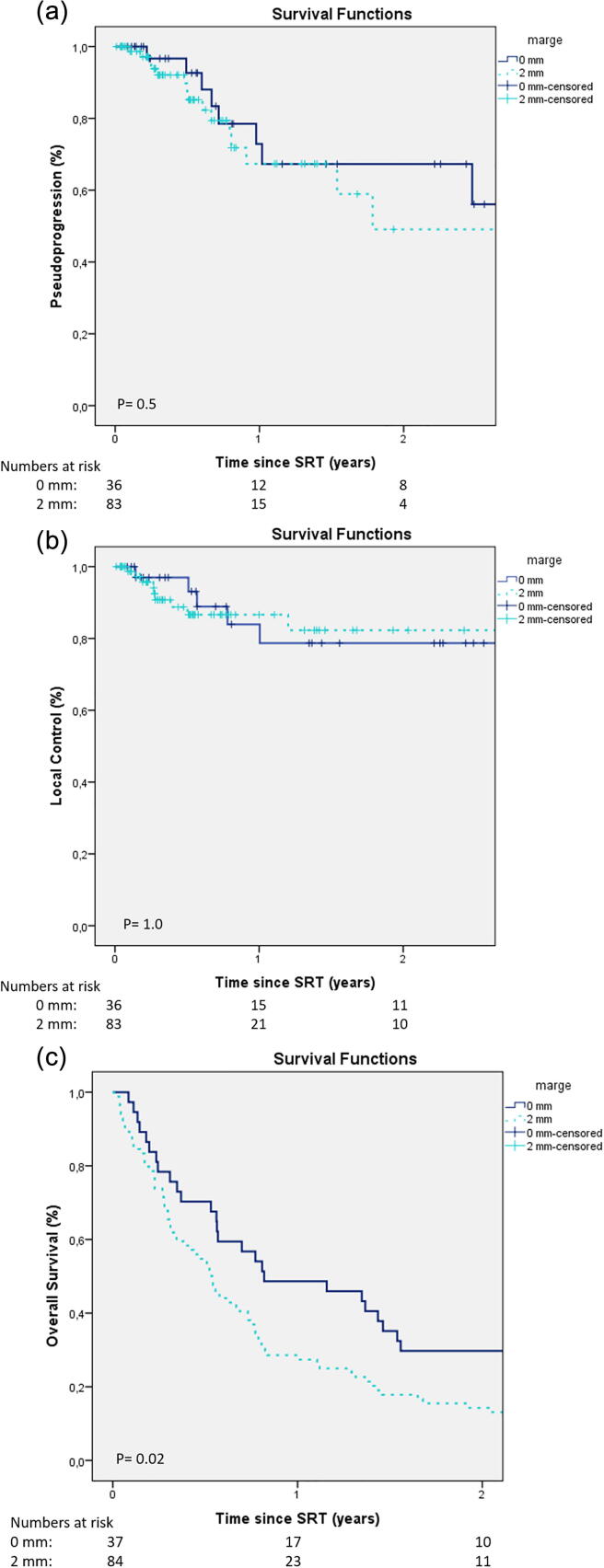
As shown in Fig. 2a. The incidence of pseudoprogression did not decrease significantly when a smaller PTV margin was used. Pseudoprogression in the 0-mm group and the 2-mm group was: 7% versus 15% at 6 months, 33% in both groups at 12 months, and 33% versus 51% at 24 months (p = 0.5). The median time to pseudoprogression was not reached for the 0-mm group, while it was 21.6 months for the 2-mm group. Whole brain radiotherapy as salvage therapy had no influence on the incidence of pseudoprogression (p = 0.9). In the 0-mm group three patients had symptomatic pseudoprogression, all three had neurologic deficits. In the 2-mm group there were also three patients with symptomatic pseudoprogression, one of them had a neurologic deficit and two were only steroid dependent.
Fig. 2.
a: Time to pseudoprogression after stereotactic radiotherapy b: Time to local control after stereotactic radiotherapy. c: Overall survival: Kaplan-Meier analysis. Dark Blue: 0-mm; Light Blue: 2-mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Factors associated with pseudoprogression
Pseudoprogression was not associated with PTV margin after single fraction or fractionated treatments (p = 0.9). Moreover, after single fraction treatments there was no association of pseudoprogression and V12 > 10.9 cm3 (p = 0.5) (Fig. 3).
Fig. 3.
Boxplot correlation between V12 (cc) and yes/no pseudoprogression for single metastasis treated with a single fraction. Boxplot showing median, 1st and 3rd quartile and 95% CI. The asterisks with the numbers refer to single patients, their values are classified as “weak outliers”.
When adjusting for gender, KPS score, and GTV, for both analyses no association was found as well (p ≥ 0.7) (Table 2a, Table 2b).
Table 2a.
Association model for pseudoprogression with all treatments. *Adjusted for gender, Karnofsky and GTV.
| Unadjusted model | HR (95%CI) | p-value |
|---|---|---|
| Margin (0-mm = reference) | 0.9 (0.4–2.3) | 0.9 |
| Adjusted for clinical risk factors* | ||
| Margin (0-mm = reference) | 1.0 (0.4–2.4) | 1.0 |
Table 2b.
Association model for pseudoprogression with only single fraction treatments. *Adjusted for gender, Karnofsky and GTV.
| Unadjusted model | HR (95%CI) | p-value |
|---|---|---|
| Margin (0-mm = reference) | 1.1 (0.4–3.2) | 0.9 |
| V12 > 10.9 (≤10.9 = reference) | 1.4 (0.5–4.6) | 0.5 |
| Adjusted for clinical risk factors* | ||
| Margin (0-mm = reference) | 0.9 (0.3–3.0) | 0.9 |
| V12 > 10.9 (≤10.9 = reference) | 1.3 (0.3–4.9) | 0.7 |
Local control
Local control was maintained when the PTV margin was reduced from 2-mm to 0-mm. Local tumour control did not differ significantly in the 0-mm group and the 2-mm group: 93% versus 87% at 6 months, 79% versus 87% at 12 months and 79% versus 82% at 24 months (p = 1.0) (Fig. 2b).
Survival
Median overall survival was 7 months (IQR 3.6–16.8 months) in the entire cohort; 9.6 months for the group treated in 2015–2016 (0-mm margins) versus 7.2 months for the group treated in 2010–2012 (2-mm margins). Overall survival was significantly better in the group treated in 2015–2016 (0-mm margins) than the group treated in 2010–2012 (2-mm margins): 46% versus 27% at 12 months and 30% versus 13% at 24 months (p = 0.02) (Fig. 2c).
Discussion
This study examined the influence of GTV-PTV margin reduction in patients who had a single brain metastasis and were treated with stereotactic radiotherapy. The reduced PTV-margin did not lead to a significantly lower incidence of pseudoprogression. Local tumour control and overall survival were maintained after reduction of the PTV-margin from 2-mm to 0-mm.
There was no difference in local tumour control and pseudoprogression rates between 0-mm and 2-mm PTV-margin. The results from our study were unexpected, because of the assumption that irradiation of a larger volume of normal brain tissue, i.e. when using larger margins, will lead to a higher risk of pseudoprogression. In the literature, two retrospective series and one small randomized study can be found that studied this question. In a paper by Nataf et al., similar local control rates are reported with 2-mm compared with 0-mm margin, but more symptomatic brain toxicity with 2-mm [12]. Noel et al. however, found a significantly better local control in the 1-mm group in comparison to 0-mm, but no differences in brain toxicity [13]. Interpretation of this study is difficult, because prescribed doses were low (14 Gy) and rates of patients with previous whole brain irradiation were high. Kirkpatrick et al. compared 1- and 3-mm GTV-PTV margin and found no significant difference in terms of local control [14]. In this trial more patients treated with 3-mm margin had brain toxicity, but the difference with the 0-mm group was not significant, possibly because of the small number of patients in the study. These available literature data suggest that, in LINAC-SRT, local control is not worse if GTV-PTV margins are reduced to less than 2-mm, which is consistent with our results. However, the above mentioned studies do not allow conclusions on the influence of margin reduction on pseudoprogression rates.
In our study we found a pseudoprogression rate of 33% at 1 year in both groups (p = 0.5). Only 6 of the 25 patients with pseudoprogression (25%) had neurological symptoms from this pseudoprogression. Other authors report higher rates of neurologic symptoms caused by pseudoprogression [10]. The explanation of this difference is not obvious, (it could be that different methods are used to diagnose pseudoprogression and to differentiate between (symptomatic) pseudoprogression and tumour progression), but symptomatic pseudoprogression remains an important clinical problem, even with our relatively low rates. As the survival of patients with brain metastases is gradually improving, symptomatic pseudoprogression may become even more important. Improved SRT technology, leading to a reduction of the volume of irradiated brain tissue, may be helpful to reduce pseudoprogression rates. Especially because of longer survival rates with i.e., better systemic therapies available, patients are more prone to develop multiple brain metastases. A prospective multicenter study investigated SRT in patients with 1–4 or 5–10 brain metastases [19]. The authors report a similar overall survival and similar treatment-related toxicity rates between the groups with 1–4 and 5–10 metastases. Cumulative volume of metastases, rather than the number, was reported as a significant prognostic factor [18], [19]. A margin reduction could allow us to irradiate more brain metastases without an increase in toxicity. Therefore, it will become more important to investigate the possibilities to reduce the volume of irradiated normal brain tissue. We collected data of multiple brain metastases as well. There will be a second analysis in the future to investigate margin reduction in multiple brain metastases.
In our patients treated with 0-mm margin the pseudoprogression rate did not decrease, while the overall survival was increased in this group (thus the time at risk to develop pseudoprogression was increased). Therefore, we hypothesize that the pseudoprogression rate could have been higher in this group if the margins would not have been reduced. Fortunately, the local progression rate was not higher with 0-mm margin, indicating that margin reduction seems to be safe.
A possible approach to achieve a reduction of the pseudoprogression rate could be to allow a higher dose heterogeneity inside the GTV, thereby increasing the dose drop-off around the PTV. Simultaneously, there may be an improved local control as the GTV mean dose increases [20].
In the unadjusted and adjusted model we did not find an association between pseudoprogression and the V12 > 10.9 cc compared to ≤ 10.9 cc. Blonigen et al. reported a significant risk of pseudoprogression up to 68.8% for V10 > 14.5 cm3 and V12 > 10.8 cm3 [9]. Korytko et al. confirmed the correlation between the V12 and the risk of symptomatic pseudoprogression [21]. Minniti et al. assumed that the V12 may be adopted as the standard method of reporting the dose to the normal brain to estimate the risk of toxicity after SRT [10]. It is not clear why we did not find this association between V12 and PP-rate.
A possible explanation could be the difference in the investigated population compared to other studies. Previous whole brain radiotherapy and patients with multiple metastases were excluded, in contrast to Minniti et al. [10]. Therefore, we will not adopt a policy to prescribe SRT-doses based on the planned V12, as was recently proposed [22].
After investigating the Paddick’s conformity index, we did find a statistically significant difference between the two groups. A smaller (worse) Paddick’s CI for the 0-mm margin group can be explained by the dependence of the CI on PTV [23]. For the 0-mm margin group, the PTV is significantly smaller than for 2-mm group and this resulted in the smaller CI. However, we did not see a higher rate of pseudoprogression in the 2-mm group with the worse CI.
We found a significantly better overall survival in the more recently treated 0-mm group. A plausible explanation is that the improved survival in these patients is due to the introduction of new staging techniques, the trend towards more aggressive local therapy in patients with oligometastases, and the introduction of new systemic treatments such as immunotherapy and targeted therapy. Immunotherapy was not available in the group treated with 2-mm margin in 2010–2012 and not yet wide-spread available in 2015–2016. Only 5 of the 37 lung cancer patients in the 0-mm group (treated in 2015–2016) received immunotherapy. Overall survival rates currently reported in the literature are higher than those reported in our study as a result of the wide spread use of immunotherapy and targeted therapy as a first line treatment in patients with lung cancer and melanoma. Overall survival rates of 69% have been reported in patients with metastasized non-small cell lung cancer treated with chemotherapy and immunotherapy [24]. Not only has survival after one year improved. Long-term survival data are available showing 6-year survival rates of 15% in NSCLC patients, including patients who were progressive on previous therapies [25]. The majority of patients referred for stereotactic radiotherapy in our study had a tumour that qualifies for immunotherapy (lung cancer in 73% and melanoma in 8% in the 0-mm group versus lung cancer in 49% and melanoma in 17% in the 2-mm group). Thus, with current systemic treatment options, we expect survival to improve in patients referred for brain stereotactic radiotherapy. As survival improves, the patient’s time at risk will increase for the occurrence of new metastases, pseudoprogression and local recurrence. We hypothesize that by decreasing the margin, we enable treatment of subsequent metastases, while keeping the risk of toxicity as low as possible. Unfortunately, the impact of the improved overall survival on (pseudo)progression rates and local tumour control are still uncertain and further studies are required to draw conclusions.
Limitations of this study are the relatively small sample size of the 0 mm group and the retrospective character of the study. In this study design small differences in local control and pseudoprogression rates between the groups could have remained undetected. However, we studied a real-life scenario, including all patients treated in a relatively large neuro-oncology unit over multiple years.
We did not include the Radiation Biologically Effective Dose (BED) in our analysis, as there is no consensus on the question how to apply the BED in very high doses per fraction.
In conclusion, SRT is a feasible treatment for patients with brain metastases. In our study PTV-margin reduction from 2- to 0-mm did not reduce the incidence of pseudoprogression in LINAC-SRT for solitary brain metastases. LC rates were not worse, indicating that margin reduction seems to be safe. Pseudoprogression represents the most important late toxicity after SRT, but we have not found a statistically significant association of GTV-PTV margin or V12Gy > 10.9 cc with the incidence of pseudoprogression in solitary brain metastases treated with a single fraction. Further studies are required on the impact of improved overall survival on (pseudo)progression and local tumour control rates after SRT.
Funding
No funding was needed
Ethics approval
The study was approved by the Institutional Research Committee
Availability of data and material
The anonymized research data can be reviewed
Consent for publication
All authors agree to publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Habets EJJ, Dirven L, Wiggenraad RG, Verbeek-De Kanter A, Lycklama À Nijeholt GJ, Zwinkels H, et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: A prospective study. Neuro Oncol 2016; 18. doi: 10.1093/neuonc/nov186. [DOI] [PMC free article] [PubMed]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev 2003; 29: 533–40. doi: 10.1016/S0305-7372(03)00105-1. [DOI] [PubMed]
- 3.Sloot S., Chen Y.A., Zhao X., Weber J.L., Benedict J.J., Mulé J.J. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. 2018;124:297–305. doi: 10.1002/cncr.30946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson B., Yamamoto M., Hanssens P., Beute G., Kawabe T., Koiso T. Does modern management of malignant extracranial disease prolong survival in patients with ≥3 brain metastases? World Neurosurg. 2016;92:279–283. doi: 10.1016/j.wneu.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 5.Banfill K.E., Bownes P.J., St Clair S.E., Loughrey C., Hatfield P. Stereotactic radiosurgery for the treatment of brain metastases: Impact of cerebral disease burden on survival. Br J Neurosurg. 2012;26:674–678. doi: 10.3109/02688697.2012.690913. [DOI] [PubMed] [Google Scholar]
- 6.Kocher M., Wittig A., Piroth M.D., Treuer H., Seegenschmiedt H., Ruge M. Stereotactic radiosurgery for treatment of brain metastases: A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlentherapie Und Onkol. 2014;190:521–532. doi: 10.1007/s00066-014-0648-7. [DOI] [PubMed] [Google Scholar]
- 7.Wiggenraad R., De Kanter A.V., Kal H.B., Taphoorn M., Vissers T., Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother. Oncol. 2011;98:292–297. doi: 10.1016/j.radonc.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Churilla T.M., Chowdhury I.H., Handorf E., Collette L., Collette S., Dong Y. Comparison of local control of brain metastases with stereotactic radiosurgery vs surgical resection. JAMA Oncol. 2019;5:243. doi: 10.1001/jamaoncol.2018.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blonigen B.J., Steinmetz R.D., Levin L., Lamba M.A., Warnick R.E., Breneman J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Minniti G., Clarke E., Lanzetta G., Osti M.F., Trasimeni G., Bozzao A. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6 doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence Y.R., Li X.A., el Naqa I., Hahn C.A., Marks L.B., Merchant T.E. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:20–27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nataf F., Schlienger M., Liu Z., Foulquier J.N., Grès B., Orthuon A. Radiosurgery with or without A 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:766–772. doi: 10.1016/j.ijrobp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Noël G., Simon J.M., Valery C.A., Cornu P., Boisserie G., Hasboun D. Radiosurgery for brain metastasis: Impact of CTV on local control. Radiother Oncol. 2003;68:15–21. doi: 10.1016/S0167-8140(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick J.P., Wang Z., Sampson J.H., McSherry F., Herndon J.E., Allen K.J. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 15.van Santvoort J., Wiggenraad R., Bos P. Positioning accuracy in stereotactic radiotherapy using a mask system with added vacuum mouth piece and stereoscopic X-ray positioning. Int J Radiat Oncol Biol Phys. 2008;72:261–267. doi: 10.1016/j.ijrobp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93:219–222. doi: 10.3171/jns.2000.93.supplement_3.0219. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar R., Wiggenraad R., Verbeek-de Kanter A., Walchenbach R., Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009;23:170–178. doi: 10.1080/02688690902755613. [DOI] [PubMed] [Google Scholar]
- 18.Soffietti R., Abacioglu U., Baumert B., Combs S.E., Kinhult S., Kros J.M. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of neuro-oncology (EANO) Neuro Oncol. 2017;19:162–174. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto M., Serizawa T., Shuto T., Akabane A., Higuchi Y., Kawagishi J. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 20.Lucia F., Key S., Dissaux G., Goasduff G., Lucia A.S., Ollivier L. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother Oncol. 2019;130:132–138. doi: 10.1016/j.radonc.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 21.Korytko T., Radivoyevitch T., Colussi V., Wessels B.W., Pillai K., Maciunas R.J. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006 doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 22.Zindler J.D., Schiffelers J., Lambin P., Hoffmann A.L. Improved effectiveness of stereotactic radiosurgery in large brain metastases by individualized isotoxic dose prescription: an in silico study. Strahlentherapie Und Onkol. 2018;194:560–569. doi: 10.1007/s00066-018-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergalasova I., Liu H., Alonso-Basanta M., Dong L., Li J., Nie K. Multi-institutional dosimetric evaluation of modern day stereotactic radiosurgery (SRS) treatment options for multiple brain metastases. Front Oncol. 2019;9:1–12. doi: 10.3389/fonc.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/nejmoa1801005. [DOI] [PubMed] [Google Scholar]
- 25.Remon J., Passiglia F., Ahn M.-J., Barlesi F., Forde P.M., Garon E.B. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15:914–947. doi: 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized research data can be reviewed