Abstract
Magnesium-based implants are re-emerging as a substantial amendment to standard orthopaedic implants. A brief introduction of magnesium (Mg) as a biodegradable material and basic magnetic resonance imaging (MRI) principles are discussed. This review aims to highlight the current performance of these implants during examinations with MRI. We also aim to summarise comparisons between Mg-based implants with current standards to emphasise the promotion of biodegradable implants in clinical practice. A comprehensive search of current literature on Mg-based implants and the utilisation of MRI in the studies was performed. Additionally, recorded artefact behaviour of Mg-based implants during MRI was investigated. A total of nine studies were included in which MRI was employed to image Mg-based implants. Of those studies, four of the nine discuss artefact production caused by the implants. MRI successfully imaged regions of interest over all and produced fewer artefacts than other materials used in the studies. MRI was employed in contrast angiography, bone growth observation, bone infection healing, and blood perfusion. Imaging capabilities of an implant material are vital to translating products into clinical application. Positive findings presented in this review suggest and support the use of Mg-based implants due to their successful visual compatibility with MRI techniques.
Keywords: Magnetic resonance imaging, Magnesium, Biodegradable implants, Medical imaging, Patient safety
Highlights
-
•
Mg materials have shown to safely degrade in-vivo as a load-bearing implant.
-
•
Magnesium-based implants have successfully been visualised with MRI.
-
•
Magnesium produces lower metallic artefact than titanium and stainless steel.
1. Introduction
Research in magnetic resonance imaging (MRI) has seen a strong increase in growth over the past decade. The recent advancements are warranted by the ability of the modality to provide excellent soft tissue contrast at high resolution. Additionally, MRI extends past other imaging methods by allowing for functional imaging. Metabolic functions such as tissue oxygenation, flow, diffusion, and perfusion can be visualised in MRI [1]. Although other imaging modalities like computed tomography provide detail on bone condition, MRI provides information on surrounding organs, vascular networks, and soft tissue around the implant. Long-term effects are also considered negligible since the modality does not administer any ionising radiation [2]. These assets of MRI make the imaging method favourable in various pre-clinical and clinical settings.
In parallel, significant strides have been accomplished in the area of medical implants. Constant development in the field of material science and engineering have allowed for the possibility to introduce implants into human medical applications. To date, medical implants have been employed in neural, sensory, and spinal environments. Medical implants have functioned as organ stimulation devices as well as tools for cosmetic and dental purposes. Structural implants in the form of stents, braces, rods, heart valves, bones, pins, hip prosthesis, eye, ear, skull implants and knee replacements have also been designed [3]. For a medical device to be successfully implanted, the biocompatibility of the material must be ensured. Previously, metallic implants based on titanium (Ti), stainless steel (SS), and cobalt-chromium alloys have dominated the market as orthopaedic implant material. However, magnesium (Mg) has recently been reintroduced as an appropriate alternative due to its special properties.
The first documented use of Mg in the clinical setting dates to 1878. Edward C. Huse, a physician, successfully implemented Mg wires as blood vessel ligatures [4]. Unfortunately, fast corrosion led to the early abandonment of the biomaterial. Early investigations had shown pure magnesium falling short due to fast corrosion and poor mechanical integrity [5]. Not until later was this main issue addressed by the creation of Mg-based alloys to control the problematic characteristic. To solve this, alloying elements have been introduced to increase corrosion resistance and strengthen material matrix [6]. Such elements include calcium, zinc, manganese, strontium, tin, and silver have been chosen to improve corrosion resistance and material strength [7]. Mg binary alloys have been investigated but resulted in poor yield strength and high corrosion rates [8]. Instead, results from binary alloys have been utilised to develop multi-elemental alloys such as AZ31 [9], AZ91 [10], AM60 [11], LAE442 [12], and WE43 [13]. Notably, Mg-RE-based alloys exhibit good corrosion performance and high strength [8].
Currently, Mg and Mg-based alloys hold many benefits as an implant material option. The ability to safely degrade in-vivo as a load-bearing implant is arguably the most attractive property of the material [14]. As a biomaterial, Mg alloys more similarly align with natural bone than other alternatives. The elastic modulus of Mg alloys (45 GPa) matches relatively closer to that of bone, 3–20 GPa, unlike Ti alloys and SS (110 and 200 GPa, respectively). Additionally, Mg alloys surpass Ti alloys and SS by closely matching human cortical bone density. The similar elastic modulus and densities of Mg alloys and natural bone help prevent negative mechanical defects such as stress-shielding [15].
Not only does Mg appropriately fulfil mechanical stability, but Mg alloys also possess good biocompatibility in terms of Williams definition [16]. Already found in abundance in the human body, Mg is a key element utilised in metabolic processes. Mg is reported to stimulate the growth of bone cells and accelerates the healing of bone tissue [17]. Excess Mg cations, a corrosion product of Mg alloys, do not present risk to the body as it is eliminated in urine [18]. As mentioned previously, the ability of Mg to degrade in a physiological environment is a particular advantage that is unique to the material. Permanent orthopaedic implants which remain in the body have shown to cause inflammatory responses [19,20] as well as further refracture risk [21]. These bodily responses and mechanical failures suggest that temporary solutions be investigated. With the degradation capability of Mg, the need for secondary surgeries to remove the implant is eliminated. Although able to degrade, iron- [22] and polymer-based [23] degradable materials pose as inferior to Mg alloys as they do not stimulate bone growth and poorly match the mechanical properties of bone. Therefore, Mg-based implants present as a biocompatible, biodegradable, lightweight, load-bearing orthopaedic option.
To gain market footing and wider usage as an alternative implant material, medical imaging compatibility must be ensured for successful clinical translation of Mg-based implants. MRI offers the ability to observe soft tissue surrounding Mg-implants as they degrade during patient healing, an essential aspect for physicians. The aim of this review is to highlight and evaluate the current performance and application of Mg-based implants in the MRI environment.
1.1. Basic MRI principles
Magnetic resonance imaging (MRI) makes use of the magnetic properties of certain atomic nuclei. Most prominent is the single proton present in water molecules, therefore present in all biological tissues. An image produced in MRI displays certain radio frequency (RF) signal intensities or phases. These signals originate from human tissue where many of the free hydrogen nuclei align themselves with the direction of the magnetic field after being stimulated by RF signals. Following relaxation, the nuclei lose energy by emitting their own RF signal. Tissue is susceptible to magnetisation due to the presence of protons in the nuclei of hydrogen atoms. Protons contained in human tissue will align stochastically with a strong magnetic field causing Larmor precession [24]. The hydrogen nuclei behave like compass needles that are partially aligned by a strong magnetic field in the scanner. The nuclei will be rotated using radio waves, and they subsequently oscillate in the magnetic field while returning to equilibrium. They emit a radio signal that is detected using antennas (coils) and used for making detailed images after Fourier transformation. The MR signal is sensitive to a broad range of influences, such as chemical surrounding, nuclear mobility, molecular structure, flow, and diffusion. Depending on the tissue or movement of fluids within the area of interest, different levels and processes of relaxation can be captured. MRI is a very flexible technique that provides measures of both structure and function. Through varying different parameters of the imaging protocol, it is possible to manipulate the contrast between the degrees of relaxation.
The imaging process can be viewed as dividing the patient volume into slices, which are then further divided into a matrix of voxels. This localisation is achieved through field gradients. An independent RF signal is then produced from each voxel. Image detail and image noise is determined by voxel size, which should be sized appropriately. Factors such as contrast sensitivity, detail, noise, artefacts, and spatial characteristics of image quality can be adjusted by various protocol factor settings. Therefore, maximum benefit of MRI technology requires well-trained technicians to control the overall process and provide a purposeful image. More importantly and unlike other imaging techniques, MRI does not involve radioactivity or ionising radiation.
1.2. Metals in the MR environment
The effect of magnetic fields involved in the MRI procedure on metallic objects, specifically ferromagnetic materials, is of important safety concern [24]. Ferromagnetic materials may be attracted towards the magnetic bore of the MRI system with great acceleration and force. The magnitude of the force created is proportional to the mass of the object and is also dependent on the proximity of the metallic object to the magnet.
The greatest magnetic field strength is found at the ends of the magnetic bore, allowing for ferromagnetic implants in the patient to be susceptible to the strong field. As a result, the implanted device may become displaced during the entering or exiting of the magnetic bore. Tissue damage may occur if an elongated object is near the vicinity of field. Elongated objects may be torqued along the axis parallel to magnetic field lines. Even weak magnetic field strengths pose risk. Devices such as pacemakers, stimulators, and insulin pumps can be interfered with by a magnetic field.
Additionally, non-magnetic metals may be excellent conductors of electricity. Metallic implants are capable of absorbing radio frequency (RF) energy via induction in the MR environment. The energy deposition into the metallic implants may result in a significant temperature increase of the implant and the surrounding tissue. Since Mg is a metal, Mg-based implants may succumb to this type of heating. Investigations of implant heating have been conducted for materials such as stainless steel [25], deep brain stimulation leads [26], and pacemaker leads [27], in which all resulted in an increase of temperature. However, no reports have been described on heating caused by Mg materials exclusively.
The formation of eddy currents, or localised currents, can also occur in the presence of a magnetic field. Safety guidelines have been created to prevent major patient risk caused by these effects. However, the electrical effects of unlooped conductors, such as rods or wires, are sometimes disregarded. Spark formation across gapped metal objects may occur if through changing magnetic fields a voltage is induced in linear conductors, causing the heating of implanted metallic objects. Resonance phenomena such as standing waves may occur in shorter implants, also resulting in the heating of the conductor ends. For these safety reasons, metallic conductors that are not part of the MR system should not be in near location of the magnet. All patient monitoring equipment must be routed to a safe distance from the magnetic bore. Ultimately, a strong analysis of the effects of metallic implants in a magnetic environment is crucial to ensure the safety of the patient. As far as this review has reached, there have been no documented literature published describing any incident regarding magnesium implants during an MRI procedure.
2. Materials and methods
To begin this review, following the PRISMA guidelines, journal search engines such as Google Scholar and the Mendeley Web Catalogue were utilised in finding articles relating to the following key terms: magnetic resonance imaging, magnesium implants, biodegradable implants, and artefacts. This initial search began on July 1, 2019. Studies were included if magnesium or magnesium-based implants were involved with MRI as a form of imaging modality. Many magnesium-based implant studies were narrowed down to those which utilised MRI in the investigation. Later, studies that discussed artefacts caused by the implants in the MR environment were analysed. No found studies meeting this inclusion criteria were excluded.
3. Results
3.1. Mg-based implant applications and MRI characterisation
Mg-based implants have been successfully implemented in many fields of clinical research. The following section addresses current applications of Mg-based implants and the role of MRI in each respective study. A summary of findings can be seen in Table 1.
Table 1.
Studies administering MRI techniques on Mg implants.
| Year | Author | Study Design | MRI Usage |
|---|---|---|---|
| 2005 | Eggebrecht et al. | Case study | Contrasted MR-angiography successfully applied |
| 2015 | Modrejewski et al. | Case series | Observed bone growth and implant degradation |
| 2016 | Li et al. | Comparison study | Observed healing process of bone infection |
| 2018 | Gigante et al. | Case series | Evaluated screw degradation |
| 2019 | Lai et al. | Prospective study | DCE-MRI observed blood perfusion |
In 2005, Eggebrecht et al. released a case study where a Mg-based stent was implanted into a 54-year-old patient suffering from coronary artery disease. The male patient presented with two-vessel coronary artery disease and stable angina. Treatment prescribed to the patient was the implantation of an absorbable Mg-based stent. The stent was based on a Mg alloy that provided mechanical stability comparable to common SS alternatives. Furthermore, the alloy allowed for controlled complete resorption within approximately two months. To image the stent, x-ray methods could not be utilised as the stent was designed with a percentage of magnesium greater than 90%, resulting in complete radiolucency. However, due to Mg properties, non-invasive follow-up was possible through MRI since the stent produced few metallic artefacts [28]. Contrast-enhanced MR angiography was successfully imaged of the right artery one week after stent implantation. The stented segment is clearly visualised without hindrance of metallic artefacts.
The treatment of chronic osteomyelitis, or bone infection, calls for implantable material with antibacterial properties. A material consisting of Mg and copper (Cu) was proposed by Li et al., in 2016. By testing varying Cu contents (0.05, 0.1, and 0.25 wt %), the group investigated the material's ability to treat methicillin-resistant Staphyloccus aureus-induced (MRSA) osteomyelitis. In-vitro and in-vivo, the biocompatibility and antibacterial capabilities of the material were analysed. MRI was employed in this study to observe the healing process of the bone infection in the left tibias of rabbits at various time periods. Four weeks after MRSA injection, MRI provided visualisation of the cortical bone thickening due to abscess formation, soft tissue swelling, and small amounts of gas production [29]. In combination with other results, the evidence produced from the MR images indicated the potential usage of Mg–Cu alloy material with 0.25 Cu % in orthopaedic infection surgery. It is important to note that x-ray and MRI images were taken throughout this study. Images taken of the Mg implants are indifferent if not clearer when compared to the Ti samples in both imaging modalities.
Mg implants are a load-bearing mechanical alternative to standard Ti and SS-based materials. Today, Mg-based implants can be found in various orthopaedic surgeries where MRI has been applied to assess bone formation, implant degradation, and overall healing post-invasive surgery.
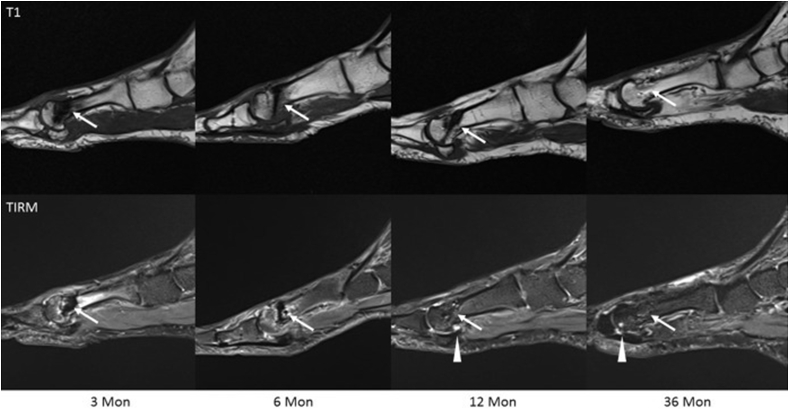
Moreover, implant resorption tracking has been successfully visualised using MRI techniques. Modrejewski et al. analysed the degradation behaviour of Mg alloy screws used in distal metatarsal osteotomies [30]. This was the first clinical study of its kind to focus on implant resorption with an emphasis on MRI analysis as the main imaging method. MRI procedures were administered at 3-, 6-, 12-, and 36-months post-operation with different image sequences seen in Fig. 1. At 3 months, the MRI scans were able to provide identification of partial bone growth between the osteotomy gap. The screw contour was still identifiable due to an obvious signal loss. After 12 months, the residual borders of the osteotomy zone could still be seen. At 36 months, significant signal loss was apparent with the length of the screw no longer identifiable. In this study, Modrejewski et al. accurately employed MRI to track Mg degradation and bone healing.
Fig. 1.
Sample images of Mg screw implants at different time points with two different imaging protocols, T1 and TIRM. Over time, image artefact reduces as implant degrades. Arrow tips point at joint effusion [30].
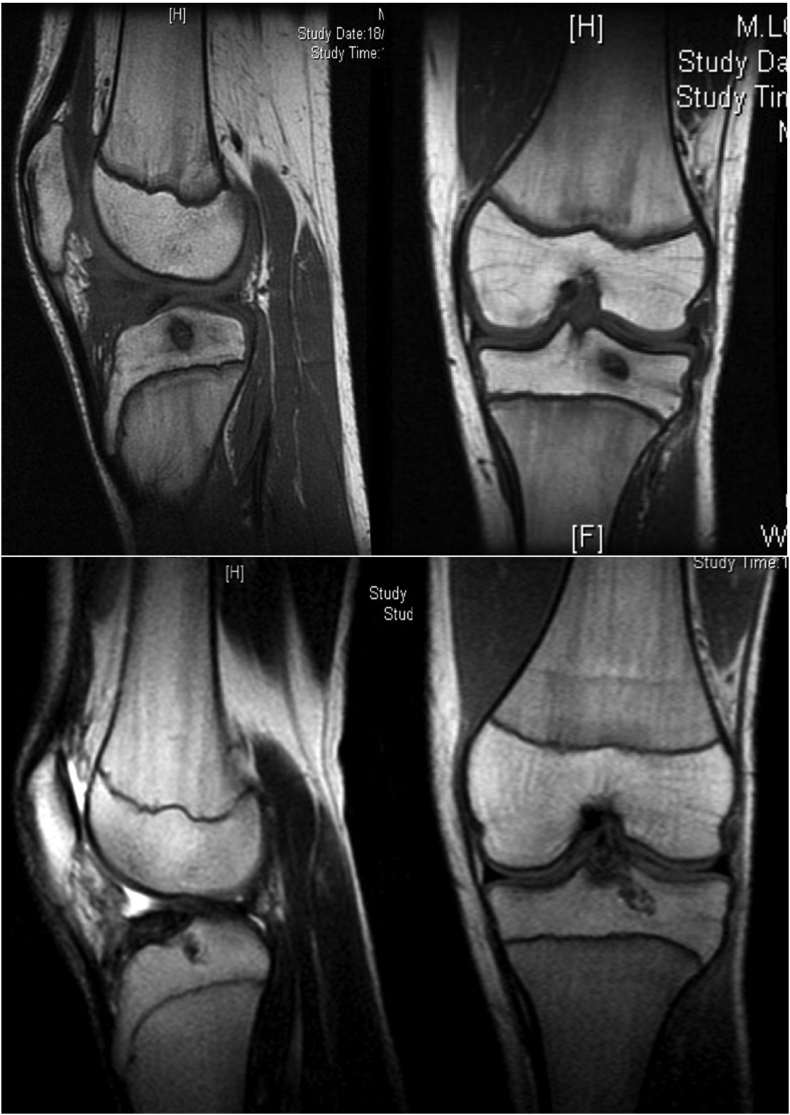
A case series lead by Gigante et al. investigated the treatment of intercondylar eminence fractures with Mg screws. Current literature debates the traditional treatment of tibial spine avulsion fractures with either the implementation of internal fixation devices (screws) or bone tunnel fixation via resorbable sutures. In this study, Gigante et al. explore a third option of applying resorbable Mg screws that combines the best features of the two previously mentioned techniques [31]. Seven patients underwent MRI examination before and after surgery. MRI was chosen as it is the most reliable in imaging meniscal or ligament injuries and was performed after 6- and 12-months post-surgery to evaluate screw degradation. Images seen in Fig. 2 confirm active healing at the fracture site with complete resorption of the fixation device. At 12 months, MRI assessment revealed complete fracture healing, resorption of screws, and newly formed bone.
Fig. 2.
MRI at six months post-operation (top) shows fracture healing and implant resorption. At 12 months (bottom), the fracture is healed, resorption is complete and replaced with new bone-like material [31].
To address bone defect repair, Lai et al. formulated a novel Mg-based porous scaffold as a solution to treat steroid associated osteonecrosis in 2019 [32]. The scaffold was created from Mg powder, polylactide-co-glycolide (PGLA), and β-tricalcium phosphate (β-TCP). The listed elements formed the PGLA/TCP/Mg (PTM) scaffold. The study revolved around the development and testing of the PTM scaffold in-vitro and in-vivo. More specifically, the osteogenic and angiogenic responses of the implant were evaluated in a rabbit model. To assess perfusion function, dynamic contrast-enhanced (DCE-) MRI was employed after 2, 4, and 8 weeks post-surgical implantation. DCE-MRI was utilised to measure the blood perfusion parameter “maximum enhancement” (ME). ME is defined as the maximum percentage of signal intensity increase from a measured baseline signal intensity. DCE-MRI successfully demonstrated a difference in ME between the newly designed scaffold and control groups. When compared to the control, the PTM scaffold promoted greater blood perfusion in early stages. During the study, Lai et al. did not observe any local subcutaneous hydrogen gas cavities. In conjunction with other imaging methods, DCE-MRI results suggested that the PTM scaffold could increase blood perfusion and promote angiogenesis.
3.2. MRI investigations with records of artefact production
Artefacts are observable distortions in MRI images that falsely represent true anatomical measurements. These distortions are produced by perturbations of the static magnetic field and depend on the specific material properties of the implanted device. Moreover, the medical device's magnetic susceptibility characteristic largely affects the distortions generated. Therefore, the evaluation of artefact production is essential when choosing a biomaterial. To date, there has been no investigation on how the production methods of Mg alloys or material preparations affect artifacts in medical imaging explicitly. Table 2 describes the accounts of artefact production in the usage of Mg-based implants.
Table 2.
Studies describing Mg implant artefact production in MRI.
| Year | Author | Implant Type | Artefact Behaviour |
|---|---|---|---|
| 2008 | Ernstberger et al. | Intervertebral spacer | Reduction compared to Ti spacers |
| 2015 | Filli et al. | Pin | Reduction compared to Ti and SS pins |
| 2015/2017 | Belenko et al./Sonnow et al. | Screw | Reduction compared to Ti screws |
| 2017 | Plaass et al. | Screw | Reduction compared to Ti Screws |
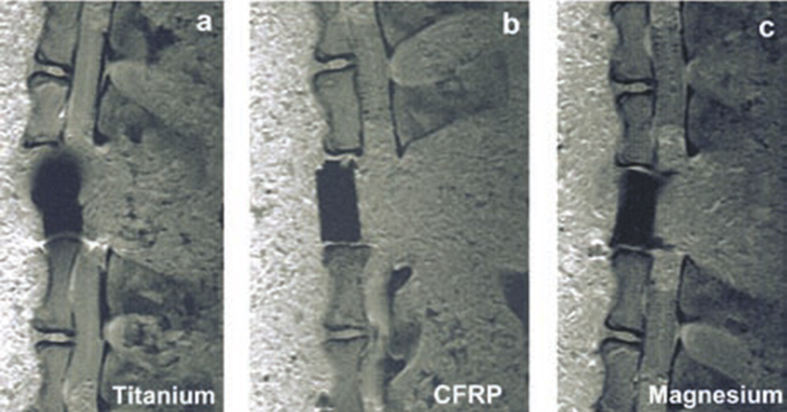
In 2008, Ernstberger et al. investigated the evaluation of Mg compared to Ti and carbon-fibre-reinforced polymers (CFRP) as materials for invertebral test spacers. In particular, the study emphasised artefact production in MRI generated by these materials. Three sizes of each material spacer (nine spacers in total) were implanted into a single Gottingen minipig cadaver spine. The first material was made of TiAl6V4, the second material was made of MgAlMn50, and the third material was made of CFRP which can be seen in Fig. 3. After the MRI was performed, a comparison of artefact production between the sizes and materials was carried out by calculating the total artefact volume (TAV) of each spacer. Using DICOM reader software, the area of the artefacts was measured and multiplied by slice thickness as described by the Debatin multisection slice technique. The MRI evidence revealed the TAV of the Ti-based material to be statistically and significantly greater than the TAV produced by the Mg-based spacers (p < 0.001). The Mg-based spacers were also found to produce almost identical artefact behaviours as the CFRP spacers (p > 0.05). The authors concluded their study suggesting that spinal implants based on Mg material behave similarly to CRFP devices in MRI scans [33]. However, due to the osseoconductive nature of Mg, implants based on Mg alloys provide an extra asset.
Fig. 3.
MRI of various artefact produced by titanium, CFRP, and magnesium [33].
A later study led by Filli et al. analysed the metal-induced artefacts produced by a Mg alloy versus Ti and SS controls. The study focused on the production of these artefacts in computed tomography (CT) and MRI specifically. In the interest of this review, the MRI results of this investigation will be highlighted. Four orthopaedic pins were analysed in this in-vitro study. One pin was made of Ti6Al7Nb, the second was made of SS, and the final two pins were made of MgYNdHRE (two varied diameters). The pins were placed in a phantom filled with CuSO4 solution, presumably to reduce T1 relaxation time. After the MRI scans, the maximum diameter of the artefacts produced by the metallic objects were measured. The experiment revealed that the Mg alloy material produced significantly fewer artefacts than SS (p = 0.019–0.021) [34]. Compared to Ti, the Mg alloy produced less artefacts, although not statistically significant. It is important to note however, the biodegradable Mg alloy induced substantially fewer artefacts in CT when compared to the other controls as well.
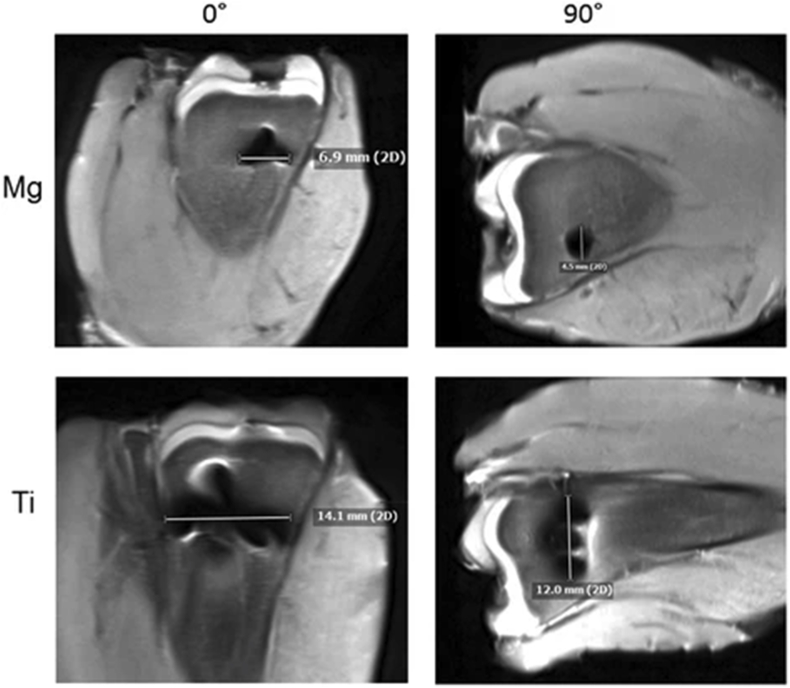
An ex-vivo trial was carried out by Belenko et al., in 2015 to assess the imaging behaviour of Mg implants. Belenko et al. analysed the Mg-based implants in different imaging modalities and compared the performance to traditional Ti-based implants. A CE-approved Mg-based screw (MAGNEZIX©) and an equivalent Ti screw were imaged with the following modalities: digital radiography (DX), multidetector computed tomography (MDCT), high-resolution flat panel CT (FPCT), and MRI. The materials were not only scanned native but were also implanted into fresh chicken tibia to simulate bone and soft tissue. To measure artefacts in MRI, the diameter of signal loss due to metallic distortion was measured. If multiple artefacts were produced, the longest artefact was chosen. In Fig. 4, the Mg-based screw generated less severe and fewer artefacts when compared to the Ti screw (p < 0.005) [35]. Similarly, to the native scans, the ex-vivo assessment revealed the Mg-based screw to be superior to Ti with regards to minor artefact production and signal distortion. Additionally, a metal-artefact reduction sequence technique called WARP was applied to the MRI scans. Unfortunately, the technique was only able to reduce artefacts of the Ti screw with statistical significance (p < 0.001). Regardless of this result, Belenko et al. support the use of Mg-based implants as the material produces less artifacts compared to Ti and out-performed Ti in all of the modalities involved in the study. A few years after this trial was completed, extra supportive findings and results were published into a further descriptive research article by Sonnow et al. where the experiments are discussed in greater detail [36].
Fig. 4.
Artifact production of Magnesium and Titanium in two views of the screw using 3T MRI [36].
A paramount study was published in 2017 by Plaass et al. describing the results of a 3-year randomised clinical trial. The clinical trial focused on the application of Mg screws for the treatment of hallux valgus and the comparison of effectiveness against Ti compression screws. The first mid-term study of its kind, Plaass et al. investigated Mg implants for the fixation of distal metatarsal osteotomies. Twenty-six patients with symptomatic hallux valgus were randomly divided into two cohorts where one group was to be treated with the Mg screw while the other was treated with the Ti screw. Clinical follow up was conducted for three years post-surgery. Various clinical tests evaluating range of motion and patient pain were administered throughout the follow-up period. All MRI scans were evaluated for potential presence of metallic debris resulting from implantation to determine any relations between MRI artefact with potential metallic debris from the surgery procedure. MRI was utilised to assess differences in oedema, soft tissue reaction, bone resorption, and bone healing, which revealed no significant difference between the materials. Despite the presence of debris in three of the Mg patients, metal artefacts were still significantly lower in the Mg-group versus the Ti-group (p < 0.05) [37]. Overall, it was reported that an improvement of the significant measures for all tested clinical scores was observed with no statistically relevant differences between the two patient groups. After the 3-year period, the Mg-based screws were found to be fully degraded but not fully remodelled. The authors note that the volume of remodelling zones decreased over time, suggesting that hydrogen gas was not developed too fast to cause any major tissue displacement. More importantly, the authors suggest that Mg should strongly be considered since the material produced less artefact than Ti.
4. Discussion
Literature discussed in this review describes the interaction of Mg-based implants with magnetic fields produced in MRI procedures. As outlined in the various studies, Mg-based implants present a contemporary option to traditional orthopaedic implants due to their bone-like mechanical properties and degradation capability. As MRI does not involve ionising radiation, the imaging modality favours patient health and limits long-term effects.
An important characteristic of Mg implants is the radiological viewing of gas release due to material degradation. Hydrogen gas is produced during the corrosion process and though non-toxic, gas production may cause local tissue displacement and temporary gas cavities [38]. Of the studies mentioned in this review, only Li et al., Plaass et al., and Lai et al. successfully observe the presence of gas in their MRI results, or lack thereof. Although no reports on gas formation in their study, Sonnow et al. suggest that future investigations be aimed at further differentiation of air, gas formation, and artifacts around implant site [36]. The ability to observe gas formation around Mg implants promote MRI as a compatible imaging modality.
According to the findings stated in this review, Mg-based implants produce minor artefacts during MRI processes when compared to other commercial equivalent implants. As pointed in Table 2, Mg has shown to produce lower artefacts than Ti. Both materials have paramagnetic properties which cause them to create local magnetic field inhomogeneities. However, the degree in which the materials produce metallic artefact are limited by the magnetic susceptibility of each material. The lower magnetic susceptibility property of Mg reduces these distortions when compared to Ti. The significant benefit of lower artefact production is greater image quality of true anatomical location. Higher-detailed imaging allows for surgeons and radiologists to accurately provide correct diagnoses. Furthermore, other biodegradable Mg-based supporting structures and geometries come into the focus of medical applications, such as cardiovascular stents. The performance of Mg-based implants relies heavily on the production process, which has major impact on material properties and therefor magnetic field responses. This is an important factor but is outside the scope of this work.
Within the range of MRI application in the development of Mg-based implants, future investigations should emphasise the later stages of clinical translation further. Specific to MRI, the understanding of Mg-based implant heating is necessary to ensure patient safety. Although there have been accounts of artefact description of Mg-based implants, additional qualitative and quantitative metallic artefact investigations are warranted as suggested by mentioned authors which may provide information on implant degradation state. Finally, MRI has shown to provide significant information on soft tissue and should be utilised in addition with other imaging modalities for an overall multimodal analysis of Mg-based implants.
5. Conclusion
The evidence presented in this review warrants further exploration of Mg-based implants in conjunction with MRI analysis. As shown in the findings, MRI is a viable imaging modality. Further in vivo studies are justified to better quantify artefact production caused by Mg in MRI investigations. Implants based on Mg material display adequate behaviour in the MRI environment and yields promising results for future research and clinical applications.
Authors’ contributions
Espiritu: Study conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of manuscript, Critical revision. Meier: Acquisition of data, Drafting of manuscript, Critical revision. Seitz: Drafting of manuscript. Critical revision
Declaration of competing interest
Syntellix AG is a medical technology manufacturer of metallic and bio-absorbable clinical implants. Authors Espiritu and Seitz are employed as Research Associate and Director of Research and Development, respectively.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 811226.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Gruber B., Froeling M., Leiner T., Klomp D.W. RF coils: a practical guide for nonphysicists. J. Magn. Reson. Imag., Bd. 2018;48(3):590–604. doi: 10.1002/jmri.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidman E., Dean K., Rivera W., Loftus M., Stokes T., Min R. „MRI safety: a report of current practice and advancements in patient preparation and screening, Clin. Imag., Bd. 2015;39(6):935–937. doi: 10.1016/j.clinimag.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Prakasam M., Locs J., Salma-ancane K., Loca D., Largeteau A., Berzina-Cimdina L. Biodegradable materials and metallic implants—a review. J. Funct. Biomater., Bd. 2017;8(4):44. doi: 10.3390/jfb8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huse E.C. Chicago Med J Exam; 1878. A new ligature. [PMC free article] [PubMed] [Google Scholar]
- 5.Seelig M.G. A study of magnesium wire as an absorbable suture and ligature material. Arch Surg., Bd. 1924;8(2):669–680. [Google Scholar]
- 6.Bamberger M., Dehm G. Trends in the development of new Mg Alloys. Annu. Rev. Mater. Res. Bd. 2008;37:505–533. [Google Scholar]
- 7.Radha R., Sreekanth D. Insight of magnesium alloys and composites for orthopedic implant applications - a review. J. Magnes and Alloys, Bd. 2017;5(3):286–312. [Google Scholar]
- 8.Chen Y., Xu Z., Smith C., Sankar J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. Bd. 2014;10(11):4561–4573. doi: 10.1016/j.actbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Zong Y., Yuan G., Zhang X., Mao L., Niu J., Ding W. Comparison of biodegradable behaviors of AZ31 and Mg–Nd–Zn–Zr alloys in Hank's physiological solution. Mater. Sci. Eng.: B, Bd. 2012;177(5):395–401. [Google Scholar]
- 10.Kannan M.B., Raman R.K.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials, Bd. 2008;29(15):2306–2314. doi: 10.1016/j.biomaterials.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Lu S.K., Yeh H.I., Tian T.Y., Lee W.H. 3rd Kuala Lumpur international conference on biomedical engineering, Bd. Vol. 15. 2006. Degradation of magnesium alloys in biological solutions and reduced phenotypic expression of endothelial cell grown on these alloys; pp. 98–101. [Google Scholar]
- 12.Angrisani N., Reifenrath J., Zimmermann F., Eifler R., Meyer-Lindenberg A., Vano-Herrera K., Vogt C. „Biocompatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5 years in a rabbit model. Acta Biomater. Bd. 2016;44(15):355–365. doi: 10.1016/j.actbio.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ye C.H., Zheng Y.F., Wang S.Q., Xi T.F., und, Li Y.D. In vitro corrosion and biocompatibility study of phytic acid modified WE43 magnesium alloy. Appl. Surf. Sci., Bd. 2012;258(8):3420–3427. [Google Scholar]
- 14.Staiger M., Pietak A., Huadmai J., Dias G. „Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials, Bd. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Brar H., Wong J., und, Manuele M. „Investigation of the mechanical and degradation properties of Mg–Sr and Mg–Zn–Sr alloys for use as potential biodegradable implant materials. J. Mech. Behav. Biomed. Mater., Bd. 2012;7:87–95. doi: 10.1016/j.jmbbm.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Williams D.F. Liverpool University Press; 1999. The Williams Dictionary of Biomaterials. [Google Scholar]
- 17.Liu C., Ren Z., Xu Y., Pang S., Yhau X., Zhao Y. „Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Recent Applications of Scanning Microscopy in Surface Engineering. 2018 doi: 10.1155/2018/9216314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornapour M., Celikin M., Cerruti M., Pekguleryuz M. „Magnesium implant alloy with low levels of strontium and calcium: the third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Mater. Sci. Eng.: C, Bd. 2014;35:267–282. doi: 10.1016/j.msec.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Heublein B., Rohde R., Kaese V., Niemeyer M., Hartung W., Haverich A. „Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart, Bd. 2003;89(6):651–656. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böstman O., Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 21.Marcucci G., Brandi M.L. Kyphoplasty and vertebroplasty in the management of osteoporosis with subsequent vertebral compression fractures. Clin. Cases in Miner. Bone Metabol. 2010;7(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- 22.Hermawan H., Dube d., Mantovani D. „Development of degradable Fe-35Mn alloy for biomedical application. Adv. Mater. Res. 2006;15-17:107–112. [Google Scholar]
- 23.Choueka J., Charvet J.L., Koval K.J., Alexander H., James K.S., Hooper K.A., Kohn J. Canine bone response to tyrosine‐derived polycarbonates and poly(L‐lactic acid) J. Biomed. Mater. Res. 1996;31(1):35–41. doi: 10.1002/(SICI)1097-4636(199605)31:1<35::AID-JBM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Sprawls P. Medical Physics Publishing; 2000. Magnetic Resonance Imaging: Principles, Methods, and Techniques. [Google Scholar]
- 25.Nordbeck P., Fidler F., Weiss I., Warmuth M., Friedrich M.T.E.P., Geistert W., Ritter O., Jakob P.M., Ladd M.E., Quick H.H., Bauer W.R. Spatial distribution of RF‐induced E‐fields and implant heating in MRI. Magn. Reson. Med. 2008;60(2):312–319. doi: 10.1002/mrm.21475. [DOI] [PubMed] [Google Scholar]
- 26.Baker K.B., Tkach J.A., Nyenhuis J.A., Phillips M., Shellock F.G., Gonzalez-Martinez J., Rezai A.R. „Evaluation of specific absorption rate as a dosimeter of MRI‐related implant heating. J. Magn. Reson. Imag. 2004;20(2):315–320. doi: 10.1002/jmri.20103. [DOI] [PubMed] [Google Scholar]
- 27.Calcagnini G., Triventi M., Censi F., Mattei E., Bartolini P., Kainz W., Bassen H.I. In vitro investigation of pacemaker lead heating induced by magnetic resonance imaging: role of implant geometry. J. Magn. Reson. Imag. 2008;28(4):879–886. doi: 10.1002/jmri.21536. [DOI] [PubMed] [Google Scholar]
- 28.Eggebrecht H., Rodermann J., Hunold P., Schmermund A., Bose D., Haude M., Erbel R. „Novel magnetic resonance-compatible coronary stent: the absorbable magnesium-alloy stent. Circulation. 2005;112(18) doi: 10.1161/01.CIRCULATIONAHA.104.521641. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Liu L., Wan P., Zhai Z., Mao Z., Ouyang Z., Zu D., Sun Q., Tan L., Ren L., Zhu Z., Hao y., Qu X., Zang K., Dai K. „Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials. 2016;106:250–263. doi: 10.1016/j.biomaterials.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Modrejewski C., Plaass C., Ettinger S., Caldarone F., Windhagen H., Stukenbog-Colsman C., von Falck C., Belenko L. „Degradation behavior of Magnesium-alloy srews after distal metatarsal osteotomies in MRI. Fuß & Sprunggelenk. 2015;13(3):156–161. [Google Scholar]
- 31.Gigante A., Setaro N., Rotini M., Finzi S.S., Marinelli M. „Intercondylar eminence fracture treated by resorbable magnesium screws osteosynthesis: a case series. International Journal of the Care of the Injured. 2018;49(3) doi: 10.1016/j.injury.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 32.Lai Y., Li Y., Cao H., Long J., Wang X., Li L., Li C., Jia Q., Teng B., Tang T., Peng J., Eglin D., Alini M., Grijpma D., Richards G., Qin L. „Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Ernstberger T., Buchhorn G., Heidrich G. „Intervertebral test spacers and postfusion MRI artifacting: a comparative in vitro study of magnesium versus titanium and carbon fiber reinforced polymers as biomaterials. Cent. Eur. J. Med. 2009;4(4):496–500. [Google Scholar]
- 34.Fili L., Luechinger R., Frauenfelder T., Beck S., Guggenberger R., Farshad-Amacker N., Andreisek G. Metal-induced artifacts in computed tomography and magnetic resonance imaging: comparison of a biodegradable magnesium alloy versus titanium and stainless steel controls. Skeletal Radiol. 2015;44:849–856. doi: 10.1007/s00256-014-2057-5. [DOI] [PubMed] [Google Scholar]
- 35.Belenko L., Könneker S., Wacker F., von Falck C. European Society of Radiology; 2015. Biodegradable Magnesium Herbert Screw in Different Modalities - Image Quality and Artifacts. [Google Scholar]
- 36.Sonnow L., Könneker S., Vogt P.M., Wacker F., von Falck C. „Biodegradable magnesium Herbert screw – image quality and artifacts with radiography, CT and MRI. BMC Medical Imaging. 2017;17(16) doi: 10.1186/s12880-017-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaass C., von Falck C., Ettinger S., Sonnow L., Calderone F., Weizbauer A., Reifenrath J., Claassen L., Waizy H., Daniilidis K., Stukenborg-Colsman C., Windhagen H. „Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial. J. Orthop. Sci. 2018;23(2):321–327. doi: 10.1016/j.jos.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Seitz J.M., Eifler R., Bach F.W., Maier H.J. Magnesium degradation products: effects on tissue and human metabolism. J. Biomed. Mater. Res. 2014;102(10):3744–3753. doi: 10.1002/jbm.a.35023. [DOI] [PubMed] [Google Scholar]