Highlights
-
•
Two intensified processes of biodiesel production from palm fatty acid distillate are considered.
-
•
Optimization of a total annual cost is used to find optimal process design parameters.
-
•
Economic evaluation of the intensified biodiesel process is performed.
-
•
Environment evaluation of the intensified biodiesel process is performed.
-
•
The esterification-transesterification intensified process is the best alternative.
Keywords: Biodiesel production, Palm fatty acid distillate, Process intensification, Process design, Economic analysis and environmental analysis
Abstract
Design of the biodiesel production from palm fatty acid distillate (PFAD) using process intensification approach is studied in technical, economic and environmental view points. Firstly, the transport phenomena analysis is performed to select the suitable intensified unit. The reactive distillation is selected and used in esterification – transesterification process and hydrolysis – esterification process. The optimum condition of reactive distillation in esterification – transesterification is achieved when the methanol is fed at the 3rd stage of the 4-stage column and the liquid holdup is maintained at 6 m3. The intensified esterification – transesterification process offers higher biodiesel yield and consumes less energy compared with the intensified hydrolysis – esterification process. The economic analysis shows that the intensified esterification-transesterification process is found to be economically feasible. Finally, environment assessment based on life cycle analysis (LCA) indicates that the environmental impact of both processes are similar.
Nomenclature
- C0
Total investment cost ($)
- Ct
Cash flow in period t ($/year)
- Ea
activated energy (cal mol−1)
- i
interest rate of return (%)
- k0
frequency factor (s−1)
- k
rate constant (s−1)
- n
project life time (year)
- R
Gas constant (J mol−1 K−1)
- t
time period (year)
- T
Temperature (K)
1. Introduction
Presently, the substitution of fossil fuel with alternative energy sources, such as wind, solar, and biomass, has been received increasing attention due to a growing concern on energy shortage and global warming problems. The use of agricultural crops and residues as and energy source is an attractive approach because the biomass derived agricultural product offers the CO2 neutral characteristic.
Biodiesel or fatty acid methyl ester (FAME) is a clean, alternative energy because it releases lower amount of greenhouse and toxic gases when it is combusted [1]. Generally, biodiesel is produced from transesterification process of purified vegetable oils (e.g., palm oil, soybean oil, and rapeseed oil) which contain high amount of triglyceride. Among several transesterification processes (i.e., alkali-catalysed, acid-catalysed, enzymatic-catalysed, and supercritical processes), the alkali-catalysed transesterification process is widely selected for commercial scale because it can be operated in mild conditions [2].
Regarding palm oil refining process, refined palm oil and palm kernel oil are produced as mainly products and palm fatty acid distillate (PFAD) as a byproduct. The main purpose of using vegetable oil is for cooking; therefore, the biodiesel production from vegetable oil reduced the proportion of feed for edible oil production. To relieve this problem, searching for a new, lower-price, alternative feedstock for biodiesel production is an attractive challenge. PFAD, a low value by-product from palm oil refinery, containing some triglyceride seems to be one alternative choice [3]. However, it contains high fatty acid (approximately 85 %), which can react with alkali solution to form an emulsion, resulting in the decrease in biodiesel yield. Therefore, the new process design is needed to achieve the suitable biodiesel production from PFAD. Cho et al. [4] proposed a single-step, non-catalytic process under supercritical conditions. They found that more than 90 % yield of biodiesel could be achieved. A two-step catalytic process under atmospheric pressure including esterification followed by transesterification (esterification-transesterification process) for biodiesel production from PFAD was introduced [5]. Chongkhong et al. [6] found that the optimum condition of the PFAD esterification in a CSTR reactor was achieved at the reaction temperature of 70 °C and the methanol to PFAD molar ratio of 8. Moreover, the production cost of the esterification-transesterification process was found to be competitive with that of the conventional one [7]. The two-step process including hydrolysis and esterification (hydrolysis-esterification process) is another approach for biodiesel production. This process consumes low energy and can handle the raw material that has high moisture and fatty acid content [8]. Machado et al. [9] studied the biodiesel production from soybean oil and ethanol via the hydrolysis-esterification process. They found that the high purity biodiesel of 96.5 % was obtained.
Recently, the biodiesel production process from PFAD is in the research and development phases. As the biodiesel production process consists of several operating units, such as esterification and transesterification reactors, distillation columns, and decanter, a high investment cost is required. Moreover, such integrated process requires high energy input. To deal with these problems, the process intensification approach which combines different phenomena into one unit can be applied. Several intensified processes were proposed to improve the biodiesel production such as reactive distillation, microwave irradiation, ultrasonic cavitation, and hydrodynamic cavitation reactor [[10], [11], [12], [13]]. The use of a reactive distillation could enhance the biodiesel yield from both transesterification and esterification and decrease the investment cost by decreasing the number of operating units [14]. Prasertsit et al. [15] conducted an experiment on biodiesel production via reactive distillation using KOH catalyst. The suitable condition was found when the reboiler temperature and methanol to oil ratio were maintained at 90 °C and 4.5, respectively. Pradana et al. [16], reported that the performance of a reactive distillation was better than a batch reactor; the maximum biodiesel yield of 82.69 % was achieved at 60 °C.
A total annual cost (TAC) including capital and operating costs can be used as a performance indicator. Dai et al., [17] reported that the use of reactive distillation for ethyl acetate production can decrease the TAC by 22.26 %. Simasatitkul et al. [18] found that the TAC and energy consumption of the esterification of oleic acid using reactive distillation could decrease approximately 18.38 % of TAC and 40 %, respectively. Lee et al. [19], reported that the use of reactive distillation for esterification of a mixture of butanol and amyl alcohol decrease of the TAC of the process.
Previously, the studies related to biodiesel production via the intensified process were restricted to palm oil feedstock [20]. However, the use of PFAD has not been widely studied and it is in the research and development phases. Most studies focused only on the effect of operating parameters on technical performance of the biodiesel production from PFAD. To justify which process is suitable, the best performance, the economic and environmental analysis should be considered.
A life cycle assessment (LCA) is a tool for environmental analysis. For example, Cho et al., [21] examined biodiesel production from PFAD in term of green house gas emission. The processing residue led to the most effect on GHG emission comparing with esterification reactor. PFAD could be classified as co – product, by-product and residue of oil palm plant refinery when it produced renewable diesel. PFAD produced to renewable diesel could reduce fossil energy consumption by 77–88% [22]. Furthermore, LCA was also applied with the process intensified unit such as ultrasould- assisted system reactor [23]. Aghbhashlo et al. [23], revealed that the ultrasould- assisted system reactor improved the technical and environmental performance. Under the suitable condition, the electricity was the most affect on human health and climate change. While neutralization section of crude glycerol was the most damange on ecosystem quality.
According to the previous mension, it is evident that process intensification offers more benefits in terms of technical, economical and environmental aspects. Lack of technical economical and environmental information of the intensified biodiesel production from PFAD was concerned in an industrial scale. Therefore, the objective of this study is to complete study in the technical, economical, and environmental view points of the two-step catalytic biodiesel production from PFAD through the intensified processes. Firstly, the insight phenomena of conventional process is analysed to select the suitable intensified process. Then, the performance comparison between the conventional and the selected intensified processes for biodiesel production is examined. Then, the optimum condition of the selected intensified process offering minimum TAC is investigated by varying the operating parameters. The economic analysis using NPV and payback period as the performance indicators is performed to justify which process is the most economically feasible. Finally, the environmental analysis is evaluated based on life cycle assessment (LCA) using LCSoft software. Three aspects in terms of technical, economic, environmental points is identified to determine the sustainable intensified process.
2. Sustainable design of process intensification for biodiesel
Normally, the conventional biodiesel production process consists of transesterification reactor and distillation column. However, the transesterification is limited by chemical equilibrium. To overcome this limitation, a process intensification combining different phenomena into one unit is applied. Different intensified processes have their own inherent characteristic. Therefore, the most suitable process for biodiesel production from PFAD should be considered.
In this section, the selection of suitable intensified process for biodiesel production based on the design methodology reported in the previous work is examined [24,25]. Firstly, unit operations and phenomena of conventional process (esterification – transesterification process) are defined as shown in Supplementary data Table A.1. PFAD is defined as continuous phase while methanol is dispersion phase [26]. It is found that the limitation of transesterification, esterification, and hydrolysis reactors is mass transfer and reaction equilibrium which causes the decrease in biodiesel production rate. To enhance the biodiesel production, the concept of an intensified process which combines the adjacent phenomena in one unit such as reactive distillation, reactive absorption, membrane, and microwave reactors is implemented as shown in Table 1. Then, the binary ratio of the adjacent components for each property is calculated. The intensified process offering the largest binary ratio which indicates the best performance in term of energy consumption. The main component property used to design a reactive distillation involves the vapor pressure, whereas that a reactive extraction and membrane reactor are the solubility. For a microwave reactor, the dipole moment and radius of gyration are considered [27]. Table A.2 in Supplementary data shows the binary ratio calculated from the different properties of each intensified unit for biodiesel production via an esterification-transesterification process. It is found that the reactive distillation consists of mixing (M), two phase mixing (2phM) of liquid-liquid (LL), reaction (Re), heating (H), cooling (C), and phase separation phenomena (e.g., phase change in vapor-liquid (PC-VL), phase separation in vapor-liquid (PS-VL), and phase temperature change in vapor-liquid (PT-VL)). The binary ratio calculated from the vapor pressure of water and methyl esters in the reactive distillation offers the highest value of 5.2 × 105. Therefore, the reactive distillation seems to be the most suitable intensified unit for biodiesel production. It will be intensively studied in the following section.
Table 1.
The intensified processes derived from the combination of the adjacent phenomena.
| Phenomena 1 | Phenomena 2 | Intensified units | Properties |
|---|---|---|---|
| 2phM, | 2phM, | Reactive distillation, | Solubility parameter, |
| Re | PC-VL, | Reactive absorption, | Boiling point, Vapor pressure |
| PT-VL, | Reactive extraction, | ||
| PS-VL, | Membrane reactor, | ||
| H,C | Microwave reactor | ||
| LL | M, | Two liquid phase | Molar volume, |
| PC-VL, | Distillation column, | Solubility parameter, | |
| PT-VL, | Two liquid phase | Dipole moment, | |
| PS-VL, | Absorption, | Solubility parameter, | |
| H,C | Decanter following by distillation | Boiling point, Vapor pressure |
3. Model development
The models of biodiesel production via the esterification-transesterification and via the hydrolysis-esterification reactions through the conventional and intensified processes are developed in Aspen plus V 8.4. The compositions of PFAD used as a model input are shown in reported literature [30] in which the tripalmitin is represented by triglyceride. The nonrandom two liquids (NRTL) activity coefficient model is used to calculate the vapor liquid equilibrium (VLE) of binary components, the missing properties, and the binary interaction parameters of pseudocomponents in PFAD such as tripalmitin, dipalmitin, and monopalmitin. The reactions related in this study are assumed to be irreversible. The transesterification of triglyceride, esterification of free fatty acid (FFA), and hydrolysis of triglycerides are shown in (R1) – (R3), respectively [5,28,29].
| (R1) |
| (R2) |
| (R3) |
The kinetic expressions in which the rate constant based on Arrhenius equation (Eq. (1)) of transesterification of triglyceride, esterification of FFA, and hydrolysis of triglycerides, are shown in Eqs.(2)–(4), respectively. The kinetic constants of these reactions are summarized in Table 2.
| (1) |
| (2) |
| (3) |
| (4) |
Table 2.
The kinetic constants of the reactions related to the biodiesel production.
3.1. Conventional esterification-transesterification process
Fig. 1a shows the flowsheet model of the esterification-transesterification process. Firstly, the PFAD is preheated in E-101 while methanol is mixed with H2SO4 before they are sent to an esterification reactor (R-101) which is simulated using a continuous stirred-tank reactor (CSTR) model. The derived products consisting of methyl esters, water, unreacted FFA, methanol and triglycerides are then sent to a distillation column (D-101), simulated using RADFRAC model, to separate methanol at the top of the column. The separated methanol is recycled while the bottom stream is sent to a decanter (V-101) to separate H2SO4. A mixture of FAME and triglyceride leaving decanter is preheated at the heater (E-102) before it is sent to react with a mixture of methanol and KOH at transesterification reactor (R-102) which simulated using a CSTR model. The product leaving R-102 consisting of FAME, glycerol, unreacted triglycerides and methanol is sent to d-102 to recover some methanol. The bottom stream is sent to separate a mixture of glycerol and water and that of methyl esters and unreacted PFAD at the decanter (V-102). The raw FAME is purified at d-103 and d-104, respectively; to produce 99.5 % purity of methyl esters. The glycerol is separated from water at d-105.
Fig. 1.
(a) Model flowsheet of a conventional esterification-transesterification process. (b) Model flowsheet of an intensified esterification-transesterification process.
3.2. Intensified esterification-transesterification process
An intensified esterification-transesterification process which is modified from the conventional one is shown in Fig. 1b. In this process, the operating units of the esterification and transesterification system (e.g., R-101, d-101, R-102 and d-102 in Fig. 1a) are combined into one reactive distillation (RD-101). Firstly, the PFAD is preheated in heater E-101 before it is sent to react with a mixture of methanol and H2SO4 at RD-101 in which the esterification and transesterification reactions are occurred simultaneously. Water, a lighter component, is separated at the top of column while a mixture of methyl esters, water and H2SO4 is removed at the bottom. Then a mixture of water and H2SO4 is removed at the decanter whereas the crude FAME is sent to purify at distillation column (D-101). Finally, the 99.5 wt% purity of FAME is obtained.
3.3. Conventional hydrolysis-esterification process
A flowsheet model of the conventional hydrolysis-esterification process is shown in Fig. 2a. In this process, a mixture of PFAD and linear alkyl benzene sulfonate and that of water and H2SO4 are simultaneously fed to a hydrolysis reactor (R-101), simulated using a CSTR model, to produce FFA and glycerol. An unreacted water is later separated from the top of a distillation column (D-101) and it is recycled back to R-101, whereas a mixture of glycerol and FFA is separated as a bottom product before it is sent to a decanter. The raw glycerol from decanter is further purified at distillation column (D-105) while the separated FFA is preheated at E-102 and sent to react with a mixture of methanol and H2SO4 at the esterification reactor (R-103). Methanol is recovered at d-102 and the mixture of FAME, H2SO4, water, and unreacted PFAD is separated as a bottom product. Then a mixture of water and H2SO4 are separated from the process at the decanter while the raw FAME is purified at distillation column d-104 and d-105, respectively. Finally, FAME with 99.5 wt% purity is separated as an overhead product of d-105.
Fig. 2.
(a) Model flowsheet of a conventional hydrolysis-esterification process. (b) Model flowsheet of an intensified hydrolysis-esterification process.
3.4. Intensified hydrolysis-esterification process
A flowsheet model of an intensified hydrolysis-esterification process is shown in Fig. 2b. In this process, the hydrolysis reactor and distillation column in hydrolysis section (R-101 and d-101 in Fig. 2a) are combined into to one reactive distillation (RD-101). The esterification reactor and distillation column (R-102 and d-102 in Fig. 2a) in esterification section is also combined to one reactive distillation (RD-102). Firstly, a mixture of PFAD and linear alkyl benzene is fed at the top of RD-102 to react with water and H2SO4 which is fed at the bottom. Hydrolysis of triglyceride takes place in the liquid phase inside RD-101. The unreacted water is removed at the top of the column while the other components e.g. FFA, glycerol, and unreacted PFAD are removed as a bottom product. The glycerol is removed at a decanter (V-101) and later purified at d-101. The other decanter products are preheated at E-102 and fed at the top of RD-102 while a mixture of methanol and H2SO4 is fed at the bottom. The bottom product of RD-102 which mainly contains FAME is sent to purify at d-102. Finally, 99.5 wt% of methyl esters are obtained as an overhead product.
The unit operations of the conventional and intensified processes consist of reactor, mixer, heat exchanger, decanter, reactive distillation, distillation and separator, The model unit blocks in the simulation are given in Table 3.
Table 3.
Description of model unit blocks.
| Process | Block name | Block model | Description |
|---|---|---|---|
| Conventional esterification – transesterification | E-101 | Heater | Heater for heating PFAD |
| E-102 | Heater | Heater for heating product from V-101 | |
| E-103 | Heater | Heater for heating product from d-103 | |
| M-101 | Mixer | Mixer between methanol and H2SO4 | |
| M-102 | Mixer | Mixer between methanol and KOH | |
| R-101 | CSTR | Reactor for esterification reaction of PFAD | |
| R-102 | CSTR | Reactor for transesterification reaction for production of FAME | |
| V-101 | Decanter | Liquid – liquid decanter for separation of FAME and waste water, H2SO4 | |
| V-102 | Decanter | Liquid – liquid decanter for separation of FAME, glycerol and waste KOH | |
| D-101 | Radfrac | Distillation for methanol separation | |
| D-102 | Radfrac | Distillation for methanol separation | |
| D-103 | Radfrac | Distillation for methanol separation | |
| D-104 | Radfrac | Distillation for FAME purification | |
| D-105 | Radfrac | Distillation for glycerol purification | |
| X-101 | SEP | Ideal separator for searation waste KOH | |
| Intensified esterification – transesterification | E-101 | Heater | Heater for heating PFAD |
| M-101 | Mixer | Mixer between methanol and H2SO4 | |
| RD-101 | Radfrac | Reactive distillation for production of FAME through esterification – transesterification reaction | |
| V-101 | Decanter | Liquid – liquid decanter for separation of FAME and waste water, H2SO4 | |
| D-101 | Radfrac | Distillation for FAME purification | |
| Conventional hydrolysis – esterification | M-101 | Mixer | Mixer between PFAD and Alkyl benzene |
| M-102 | Mixer | Mixer between water and KOH | |
| M-103 | Mixer | Mixer between methanol and H2SO4 | |
| E-101 | Heater | Heater for heating product from M-101 | |
| E-102 | Heater | Heater for heating product from V-101 | |
| E-103 | Heater | Heater for heating product from d-103 | |
| R-101 | CSTR | Reactor for hydrolysis reaction of PFAD | |
| R-102 | CSTR | Reactor for sesterification reaction for production of FAME | |
| V-101 | Decanter | Liquid – liquid decanter for separation of FFA, glycerol, waste water and H2SO4 | |
| V-102 | Decanter | Liquid – liquid decanter for separation of FAME and waste H2SO4 | |
| D-101 | Radfrac | Distillation for water separation | |
| D-102 | Radfrac | Distillation for methanol separation | |
| D-103 | Radfrac | Distillation for methanol separation | |
| D-104 | Radfrac | Distillation for FAME purification | |
| D-105 | Radfrac | Distillation for glycerol purification | |
| X-101 | SEP | Ideal separator for searation waste H2SO4 | |
| Intensified hydrolysis - esterification | M-101 | Mixer | Mixer between PFAD and Alkyl benzene |
| M-102 | Mixer | Mixer between water and KOH | |
| E-101 | Heater | Heater for heating product from M-101 | |
| E-102 | Heater | Heater for heating product from V-101 | |
| RD-101 | Radfrac | Reactive distillation for production of FFA through hydrolysis reaction | |
| RD-102 | Radfrac | Reactive distillation for production of FAME through esterification reaction | |
| D-101 | Radfrac | Distillation for water separation | |
| D-102 | Radfrac | Distillation for FAME purification | |
| X-101 | SEP | Ideal separator for searation waste H2SO4 |
4. Results and discussions
4.1. Comparison between conventional and intensified process
The performance of two conventional esterification-transesterification and hydrolysis- esterification processes are compared with their intensified process in term of biodiesel yield and energy consumption. Given the constant PFAD feed rate of 50 kmol/h and 99 wt.% purity of biodiesel, the conventional esterification-transesterification process is operated at 60 °C and atmospheric pressure. The 20 stages of the distillation column for biodiesel purification is required. The conventional hydrolysis-esterification process is operated at 100 °C. For the two intensified process, the 7 stages of the reactive distillation with the reflux ratio of 1 is used as standard condition.
It is found from Table 4 that because the esterification and transesterification reactions are shifted forward to provide higher biodiesel yield, water is separated from the reactive distillation during the biodiesel production process. Regarding the energy consumption, the reboiler of the intensified process consumes 50 % less energy than that of the conventional one because the energy supplied at the reboiler can be utilized in both reaction and separation sections.
Table 4.
Biodiesel yield and energy consumption of conventional process and intensified process.
| Process | Biodiesel yield (%) |
Energy consumption (Btu/hr) |
||
|---|---|---|---|---|
| Conventional process | Intensified process | Conventional process | Intensified process | |
| Esterification-transesterification | 64.8 | 75.89 | 6.25 × 107 | 2.11 × 107 |
| Hydrolysis-esterification | 73 | 76 | 4.07 × 107 | 2.56 × 107 |
4.2. Analysis of the reactive distillation in the intensified biodiesel processes
4.2.1. Esterification-transesterification intensified process
The effect of feed location and number of stages on performance of the reactive distillation which performs the esterification and transesterifications reactions in terms of the yield and concentration of FAME, and energy consumption at the reboiler and condenser, is investigated by varying the methanol feed location of the distillation column which has different number of stages. It is noted that the order of stages is counted from the top of the column in which the condenser is considered as the first stage and the reboiler is the last one.
4.2.1.1. Effect of feed location and number of stages on the production of FAME
It is found in Fig. 3 that based on the PFAD feed location at 2nd stage, the yield of FAME is found to increase when the methanol is fed at the lower stage of the column due to the decrease in vapor boilup. The same trend is found when the number of stages are increased due to an increase in an extent of reaction. However, the yield of FAME approximately 90.5 % is found to be stable for all methanol feed location of 7 – stage RD. Moreover, the concentration of FAME is found to decrease when the feed location of methanol increases whereas the opposite trend is found when the number of stages of the column is increased (Fig. 4). The highest FAME concentration is obtained when methanol is fed at the 2nd stage of 7-stage reactive distillation column. At this condition, the yield and concentration of FAME of 90.5 % and 0.95, respectively are achieved. The result is found to be consistent with the reported literature [20]. It implies that the methanol feed location should be located at the bottom column because the rate of mass transfer and heat transfer is high at this section. As a result, high temperature can be maintained along the column.
Fig. 3.
Effect of number of stages and feed location of methanol on the yield of FAME.
Fig. 4.
Effect of number of stages and feed location of methanol on the concentration of FAME.
4.2.1.2. Effect of feed location and number of stages on the energy consumption
Fig. 5 shows the effect of feed location and number of stages on reboiler duty. The energy required at the reboiler decreases when the feed location of methanol increases due to an increase in methanol concentration at the bottom which can enhance the extent of reaction. However, the opposite trend is observed when the number of stages increases because high amount of energy is required to separate the excess methanol and water byproduct at the top of the column.
Fig. 5.
Effect of number of stages and feed location of methanol on the reboiler duty.
Regarding the energy consumption at condenser, it is found from Fig. 6 that the condenser duty decreases as the number of reactive stage increases. As the concentration of FAME is high at this condition (Fig. 5), the reflux ratio decreases. The inverse effect is found when the feed location of methanol increases due to an increase in FAME yield. It is noted that the number of reactive stages for each methanol feed location has slightly effect on the condenser and reboiler duties.
Fig. 6.
Effect of number of stages and feed location of methanol on the condenser heat duty.
4.2.2. Hydrolysis-esterification process
4.2.2.1. Effect of feed location and number of stages on the production of FFA
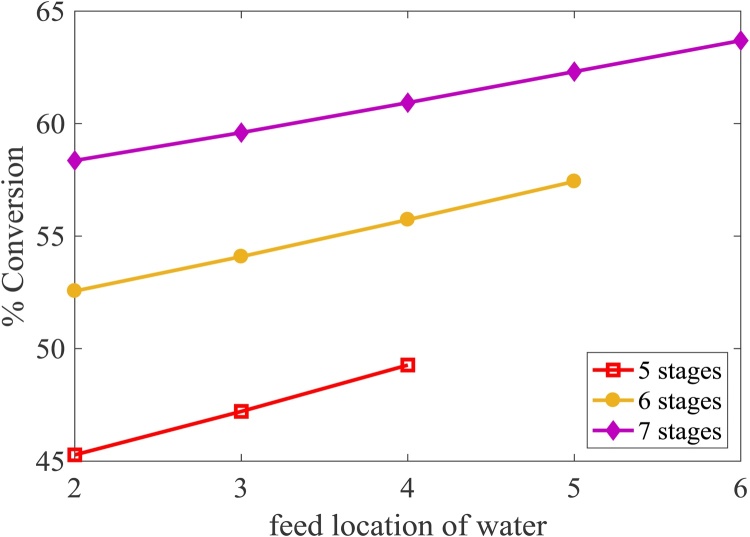
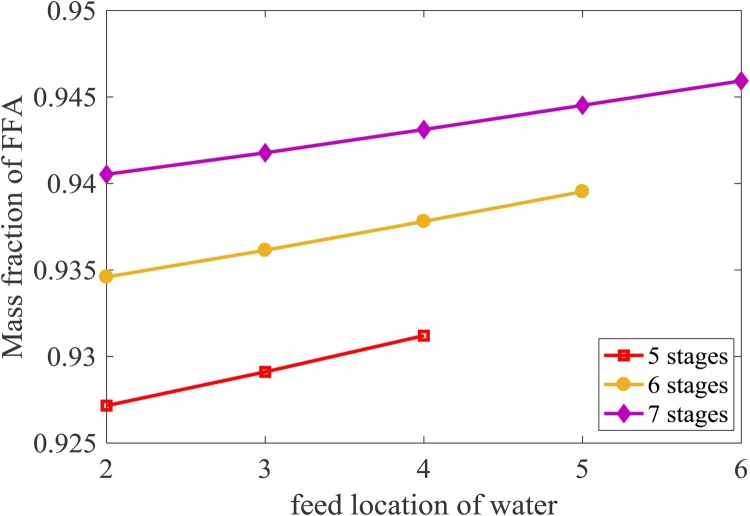
In the hydrolysis-esterification process, the reactive distillation is used in which the hydrolysis reaction occurs to convert triglyceride in PFAD to FFA that is later converted to FAME. Methanol and water are removed at the top of this reactive distillation column while glycerol and FFA are separated as bottom product because the binary ratio based on vapor pressure of water and glycerol (1.4 × 105) is higher than that of glycerol and FFA (242.14). Fig. 7 shows that the conversion of triglyceride increases as the feed location of water increases due to an increase in water concentration at reaction stage which causes an enhancement of hydrolysis reaction. As a result, the FFA concentration is found to increase as shown in Fig. 8. The same trend is found when the number of stages increase due to an increase in resident time and extent of reaction. It can be concluded that the water should be fed at the bottom column.
Fig. 7.
Effect of number of stages and feed location of water on the conversion of triglyceride.
Fig. 8.
Effect of number of stages and feed location of water on the concentration of FFA.
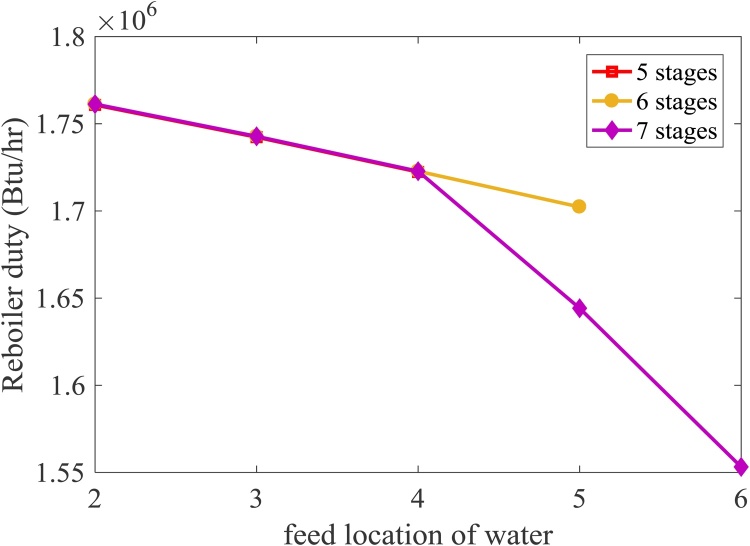
4.2.2.2. Effect of feed location and number of stages on the energy consumption
Fig. 9 shows the effect of change in feed location of water and number of stages on reboiler duty. The reboiler duty decreases as the feed location of water increases due to an increase in water concentration at reactive stages. As a result, the reaction is enhanced and less amount of energy is required at the reboiler. Fig. 9 also indicates that the number of stages does not affect the reboiler duty when the feed location of water is in the range of 2nd -4th stages. Regarding the feed location of water, it is found from Fig. 10 that the condenser duty significantly increases when the feed location of water increases from 2nd to 3rd stages and it seems to be stable when the feed location increases higher than 3rd stage. The effect of number of stages is also investigated. Fig. 10 indicates that the number of stages does not affect the condenser duty. The product leaving this reactive distillation consisting of glycerol and FFA is sent to separate the glycerol at the decanter. The FFA – rich product is further sent to produce the biodiesel via the esterification at the second reactive distillation which operated at the same condition as the one mentioned in the previous section.
Fig. 9.
Effect of number of stages and feed location of water on the reboiler heat duty.
Fig. 10.
Effect of number of stages and feed location of water on the condenser duty.
A comparison of the technical performance of two intensified biodiesel production processes is summarized in Table 5. The result indicates that the esterification-transesterification process offers better performance than the hydrolysis-esterification process in terms of yield and concentration of FAME as well as energy consumption due to the fact that the reaction rates of esterification and transesterification are faster. Although the technical performance of both processes are comparable, the economic evaluation should be additionally performed to justify which process is the most suitable one.
Table 5.
Comparison of yield, and mass fraction of FAME and energy consumption of intensified processes.
| Process | Yield (%) | Mass fraction | Energy (Btu/h) |
| Esterification-transesterification | 90.36 | 0.93 | 4.61 × 106 |
| Hydrolysis - esterification | 87.85 | 0.89 | 7.74 × 106 |
5. Steady state design of reactive distillation columns
TAC is considered for designing a reactive distillation in the two intensified processes, i.e., the esterification-transesterification and hydrolysis-esterification processes based on the biodiesel production rate of 11,000 kg/hr. The design objective is to minimize the TAC, as defined by Eq. (5) which is the summation of annual capital cost (TACcap) and operating cost (TACope) using the payback period of 3 years [31].
| (5) |
where X is the design variables of the reactive distillation (Table 6).
Table 6.
Parameters used in steady state design of reactive distillations and their boundaries.
| Reactive distillation units | Parameters | Range | Units |
|---|---|---|---|
| Esterification - transesterification | Liquid holdup | 2−43 | m3 |
| Number of stages | 5−7 | – | |
| Feed location of methanol | 2−7 | – | |
| Hydrolysis-esterification | Liquid holdup | 1.75 – 14 | m3 |
| Number of stages | 5−8 | – | |
| Feed location of water | 2−8 | – |
The constraints of biodiesel production process are shown in Eqs. (6)–(8):
| (6) |
| P(X) = 0 | (7) |
| Conversion = 95 % | (8) |
Eq. (7) represents the set of equality constraints, e.g., mass and energy balances. The tray sizing function in the Aspen Plus simulator is used to estimate the diameter of reactive distillation. The distance between trays is maintained at 2 ft.
The optimization requires iterative optimization algorithm due to the fact that design variables are defined as discrete variables while reflux ratio and reboiler duty are continuous variables. The optimization procedure is shown in Fig. 11.
Fig. 11.
The optimization procedure of a reactive distillation.
The optimization result indicates that the optimum condition for the reactive distillation depends on reflux ratio. An increase in reflux ratio results to high purity. On the other hand, it also enhance high energy usage. This effect is similar to Gaurav et al., [32] so total annual cost can be obtained under the optimal condition (i.e, number of stages, feed stage, and reflux ratio). The esterification-transesterification process offering the minimum TAC of 4.21 × 105 $/year is achieved when the methanol is fed at the 3rd stage of the 4-stage column and the liquid holdup is maintained at 6 m3. For the first reactive distillation in the hydrolysis-esterification process, the optimum condition offering the lowest TAC of 3.23 × 105 $/year is obtained when the water is fed at the 4th stage of 5 stage column and the liquid holdup is maintained at 2.53 m3. The second reactive distillation provides the lowest TAC of 4.09 × 105 $/year when the number of stages is 5 stages and liquid holdup is 8.5 m3. The optimum parameters for the intensified esterification-transesterification and hydrolysis-esterification processes are summarized in Table 7. It implies that the intensified esterification – transesterification process is preferred due to the lowest TAC. According to Table 7, the suitable number of stages of the reactive distillation column for the first process is 4 stages which is lower than Gaurav et al., [32]; however, the methanol feed location is similar.
Table 7.
Optimal design and operating parameters for the intensified biodiesel processes.
| Main equipment | Symbol | Parameter | Intensified esterification-transesterification process | Intensified hydrolysis-esterification process |
|---|---|---|---|---|
| Reactive distillation unit | RD-101 | Number of stage | 4 | 4 |
| Condenser duty (Btu/hr) x106 | 3.72 | 0.062 | ||
| Reboiler duty (Btu/hr) x106 | 4.67 | 1.92 | ||
| Reactive distillation unit | RD-102 | Number of stage | – | 5 |
| Condenser duty (Btu/hr) x106 | 3.48 | |||
| Reboiler duty (Btu/hr) x106 | 6.68 | |||
| Separation unit | D-101 | Number of stage | 20 | 20 |
| Condenser duty (Btu/hr) x107 | 4.34 | 4.04 | ||
| Reboiler duty (Btu/hr) x107 | 4.86 | 4.66 |
6. Economic evaluation
In order to justify which is the most suitable intensified biodiesel processes from PFAD, the economic evaluation should be additionally performed. The economic feasibility of the optimal esterification-transesterification and hydrolysis-esterification processeses is analysed using the return on investment (ROI), net present value (NPV) and simple payback period (PP) calculated from Eqs. (9)–(11), respectively, as performance indicators.
| (9) |
| (10) |
| (11) |
The economic assumptions are the project lifetime of 10 years and annual interest rate of 7%. The chemical and utility costs related to the designed process are summarized in the supplementary data Table A.3 [[33], [34], [35], [36]]. The total investment cost consists of bare module cost of equipment, total installation cost, and working capital cost (Table 8a ). The total production cost includes raw materials, utilities, maintenance cost, depreciation and general expense as shown in Table 8b . The results indicate that the intensified esterification-transesterification process is more economically attractive than the intensified hydrolysis-esterification one due to the higher value of all the economic indicators as shown in Table 9. In this work, total capital cost ($) per kg biodiesel of 0.07 $/kg biodiesel is lower than biodiesel production from cotton seed oil using reactive distillation (0.327 $/kg biodiesel) [33]. To compare with biodiesel production from feed containing high FFA, this work also provides lower total capital cost than biodiesel production from yellow grease (0.17 $/kg biodiesel) [32].
Table 8a.
Total investment cost of the intensified process for biodiesel production.
| Cost ($ MM/year) | Esterification-transesterification process | Hydrolysis-esterification process |
|---|---|---|
| Reactive distillation | 0.663 | 0.958 |
| Distillation | 6.05 | 5.31 |
| Heater | 0.0924 | 0.194 |
| Bare module cost | 6.94 | 6.60 |
| Purchase equipment delivery | 0.694 | 0.66 |
| Purchase equipment installation | 0.55 | 0.528 |
| Instrumentation and control | 0.181 | 0.172 |
| Piping | 0.55 | 0.528 |
| Electrical system | 0.104 | 0.099 |
| Building | 0.042 | 0.036 |
| Yard improvement | 0.069 | 0.066 |
| Service facilities | 2.08 | 1.98 |
| Land | 0.115 | 0.264 |
| Fixed capital cost | 11.5 | 10.9 |
| Working capital cost | 1.73 | 1.64 |
| Total investment cost | 13.2 | 12.6 |
Table 8b.
Total production cost of process intensification for biodiesel production.
| Cost ($ MM/year) | Esterification-transesterification process | Hydrolysis-esterification process |
|---|---|---|
| Raw materials | 71.9 | 77.8 |
| Labor | 0.779 | 0.779 |
| Supervisory | 0.0779 | 0.0779 |
| Utilities | 0.008 | 0.009 |
| Maintenance | 0.575 | 0.0547 |
| Laboratory | 0.078 | 0.078 |
| Depreciation | 1.15 | 1.09 |
| Local tax | 0.115 | 0.109 |
| Insurance | 0.575 | 0.547 |
| Plant overhead | 0.395 | 0.39 |
| Manufacturing cost | 75.6 | 81.4 |
| General expense | 0.156 | 0.156 |
| Total production cost | 75.8 | 81.6 |
Table 9.
Economic indicators of process intensification for biodiesel production.
| Economic indicators | Esterification-transesterification process | Hydrolysis-esterification process |
|---|---|---|
| Return on investment | 2.25 | 1.93 |
| Net present value ($/year) (x108) | 3.08 | 2.44 |
| Simple payback period (year) | 0.44 | 0.52 |
The uncertain parameters (i.e., price of raw materials and biodiesel) influence the economic performance. Thus, the sensitivity analysis of these parameters is performed, as shown in Fig. 12. It implies that the esterification-transesterification process is more attractive due to the positive NPV and the PFAD price has significant effect on NPV. The total production cost strongly depends on raw material prices. Thus, the fluctuating PFAD price has played an important role on biodiesel selling price. Fig. 13 shows the PFAD price and biodiesel breakeven price since 1996 until 2020. The biodiesel breakeven price varies with PFAD price so the trend of PFAD price and biodiesel breakeven price is similar. The different price between biodiesel breakeven and PFAD prices of the intensified esterification – transesterification process is 0.33 – 0.41 $/kg while that of the intensified hydrolysis - esterification process is 0.36 – 0.50 $/kg. Moreover, when PFAD price is high, the different price between PFAD price and biodiesel breakeven price should be high to make the profit.
Fig. 12.
Effect of parameter uncertainties on NPV.
Fig. 13.
Effect of fluctuation price of PFAD on biodiesel breakeven price.
7. Environmental evaluation
In this section, the environmental impact of the two intensified biodiesel processes is investigated using LCSoft software. The carbon footprint and potential environmental impact (PEI) are used as performance indicators.
7.1. Carbon foot print
A carbon footprint is total amount of greenhouse gases. It represents in a unit of grams carbon dioxide -equivalents per megajoule of energy (g CO2-eq/MJ), or kilograms carbon dioxide -equivalents per kilogram or gallon of biofuel (kg CO2-eq/kg.) The carbon footprint of the intensified esterification – transesterification process and intensified hydrolysis – esterification process are shown in Fig. 14(a) and Fig. 14(b), respectively. The carbon footprint of intensified esterification – transesterification process is 0.488 kg CO2-eq (4.56 × 10−5 kg CO2-eq/kg biodiesel or 9.78 kg CO2-eq/MJ) and intensified hydrolysis –esterification process is 0.474 kg CO2-eq (4.29 × 10−5 kg CO2-eq/kg biodiesel or 3.52 × 10−5 kg CO2-eq/MJ). It implies that the latter process is more environmental friendly than the former. The distillation column for biodiesel purification of both processes presents the highest carbon footprint. It indicates that the use of utility mainly comes from the distillation column.
Fig. 14.
carbon footprint of (a) intensified esterification – transesterification process (b) intensified hydrolysis – esterification process.
7.2. Potential environmental impact (PEI)
The PEI is an indicator representing the average indirect impact of the energy and mass emission on the environment. In this work, 3 types of end –point categories and carbon footprint are evaluated. The first type of end – point categorties consisting of human toxicity non cancer (HTNC), human toxic cancer (HTC), human toxicity by exposure (HTPE), human toxicity by ingestion (HTPI), human toxicity carcinogenics (HTC), and particulate matter causes the human health problem. The second type of end – point categorties includes photochemical ozone formation, Marine eutrophication, photochemistry oxidation potention (PCOP), ozone depletion potential (ODP), global warming potential (GWP), terrestrial eutrophication, marine eutrophication, acidification potential (AP), terrestrial toxicity potential (TTP), aquatic toxicity (ATP), and fresh water ecotoxicity (ET). This type results to biodiversity and ecosystem problem. The last type of end – point involving natural resources that are non-renewable fossil and mineral extraction. To evaluate the environmental impacts, the intensified esterification – transesterification process is dividied into 2 sections (i.e., (1) reaction section which the FAME is produced in RD and (2) separation section which is biodiesel purification), while the intensified hydrolysis –esterification process is divided into four sections (i.e., (1) the first reaction section which is hydrolysis in RD, (2) the first separation section which fatty acid purification, (3) the second reaction section which esterification in RD and (4) the second separation which biodiesel purification). The PEI results of each section are shown in Fig. 15(a) and (b), respectively. Fig. 15(a) shows that the reaction section of esterification – transesterification highly contribute toward the particulate matter, ionizing radiation, terrestrial eutrophication, marine eutrophication, photochemical ozone formation, mineral extraction, ET, HTNC, HTC, PCOP, ODP, ATP and HTPE. These impacts are observed due to waste disposal to soil and water thus this stage damages human health and environmental system quality. While non renewable fuel and GWP are tremendous effect on the separation stage. This is because the energy usage of distillation column release CO2 emission. Fig. 15 (b) shows the environmental impact categorties for the intensified hydrolysis – esterification process. The first reaction section has stronger impact in terms of HTC, HTNC, PCOP and terrestrial eutrophication. Compared with the second one. However, the impact in terms of TTP, HTPI, ATP and nonrenewable fuel of the second reaction section is found to be stronger than the first one. The separation section has the lowest environmental impact. Regarding the GHG emission, the intensified esterification – transesterification process has lower GWP impact (0.46 kg CO2 eq.) than the intensified hydrolysis –esterification process. For the HTNC impact, the intensified esterification – transesterification process has lower HTNC impact (0.53 kg toluene eq.) than the intensified hydrolysis – esterification process because it generates more waste streams from two reactive distillation columns.
Fig. 15.
Relative contribution in the LCA of (a) intensified esterification – transesterification process (b) intensified hydrolysis –esterification process.
Finally, all impact categories are normalized in the same scale (pts) as shown in Fig. 16(a) and (b). The HTNC of the intensified hydrolysis-esterification process is higher than the intensified esterification-transesterification process. The results perform that the strongest PEI is HTNC because the waste cooking oil disposal contains high free fatty acid contents which is polar component. Thus, it implies that the reaction stages provide the highest impact on the human health impact.
Fig. 16.
Normalization environmental impact categories of (a) intensified esterification – transesterification process (b) intensified hydrolysis –esterification process.
8. Conclusions
A systematic sustainable design of process intensification for biodiesel production from PFAD, the byproduct of a palm oil refinery was studied. Transport phenomena analysis indicated that a reactive distillation, the combination of reaction and separation, offers the minimum energy consumption. Then, two intensified processes (i.e. esterification-transesterification and hydrolysis-esterification reactions) was selected to enhance the biodiesel production. The intensified process offered better performance in terms of biodiesel yield and energy consumption compared with the conventional one. The optimal design of the reactive distillation performs by the process optimization of TAC. From the technical aspect, the intensified esterification-transesterification process is preferred than the intensified hydrolysis – esterification process because it decreases number of equipments. Regarding economic aspect, the intensified esterification -transesterification process is also the suitable option with respect to the highest NPV and 0.44 year of simple payback period. Finally, the environmental impact assessment is analysed through LCA. Although both processes result in human health damage and climate change. The intensified esterification – transesterification process is the best option due to the lowest impact categorites. It is noted that the intensified esterification – transesterification process become a sustainable process for biodiesel production because it offers the best performance from technical, economic and environmental points of view.
Declaration of Competing Interest
None.
CRediT authorship contribution statement
Karittha Im-orb: Methodology, Writing - original draft. Amornchai Arpornwichanop: Conceptualization, Visualization, Writing - review & editing. Lida Simasatitkul: Conceptualization, Methodology, Software, Writing - review & editing.
Acknowledgements
The authors would like to acknowledge the support of LCSoft software from PSE for SPEED Company Limited.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2021.e00622.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Abbaszaadeh A., Ghobadian B., Omidkhah M.R., Najafi G. Current biodiesel production technologies: a comparative review. Energy Conver. Manag. J. 2012;63:138–148. [Google Scholar]
- 2.West A., Posarac D., Ellis N. Assessment of four biodiesel production processes using HYSYS. Plant, Bioresource Technol. 2008;99:6587–6601. doi: 10.1016/j.biortech.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Kapor N.Z.A., Maniam G.P., Rahim M.H.A., Yusoff M.M. Palm fatty acid distillate as a potential source for biodiesel production a review. J. Clean. Prod. 2017;143:1–9. [Google Scholar]
- 4.Cho H.J., Kim J.-K., Hong S.W., Yeo Y.-K. A single step non-catalytic esterification of palm fatty acid distillate (PFAD) for biodiesel production. Fuel. 2012;93:373–380. [Google Scholar]
- 5.Aranda D.A., Santos R.T., Tapanes N.C., Ramos A.L., Antunes Q.A. Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catal. Letters. 2008;122:20–25. [Google Scholar]
- 6.Chongkhong S., Tongurai C., Chetpattananondh P., Bunyakan C. Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy. 2007;31:563–568. [Google Scholar]
- 7.Chongkhong S., Tongurai C., Chetpattananondh P. Continuous esterification for biodiesel production from palm fatty acid distillate using economical process. Renew. Energy. 2009;34:1059–1063. [Google Scholar]
- 8.Song C., Liu Q., Ji N., Deng S., Zhao J., Li S., Kitamura Y. Evaluation of hydrolysis – esterification biodiesel production from wet microalgae. Bioresour. Technol. 2016;214:747–754. doi: 10.1016/j.biortech.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Machado G.D., Souza T.L., Aranda D.A.G., Pessoa F.L.P., Castier M., Cabral V.F., Cardozo-Filho V.F. Computer simulation of biodiesel production by hydro-esterification. Chem. Eng. Process. Process. Intensif. 2016;103:37–45. [Google Scholar]
- 10.Ding H., Ye W., Wang Y., Wang X., Li L., Liu D., Gui J., Song C., Ji N. Process intensification of transesterification for biodiesel production from palm oil: microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy. 2018;44:957–967. [Google Scholar]
- 11.Suranani S., Maralla Y., Gaikwad S.M., Sonawane S.H. Process intensification using corning® advanced-flow™ reactor for continuous flow synthesis of biodiesel from fresh oil and used cooking oil. Chem. Eng. Process. Process. Intensif. 2018;126:62–73. [Google Scholar]
- 12.Chuah L.F., Klemes J.J., Yusup S., Bokhari A. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2017;146:181–193. [Google Scholar]
- 13.Oh P.P., Lau H., Chen J., Chong M.F., Choo Y.M. A review on conventional technologies and emerging process intensification (PI) methods for biodiesel production. Renew. Sustain. Energy Rev. 2012;16:5131–5145. [Google Scholar]
- 14.He B.B., Singh A.P., Thompson J.C. A novel continuous-flow reactor using reactive distillation for biodiesel production. Am. Soc. Biol. Eng. 2006;49:107–112. [Google Scholar]
- 15.Prasertsit K., Mueanmas C., Tongurai C. Transesterification of palm oil with methanol in a reactive distillation column. Chem. Eng. Proc. 2013;70:21–26. [Google Scholar]
- 16.Pradana Y.S., Hidayat A., Prasetya A., Budiman A. Biodiesel production in a reactive distillation column catalyzed by heterogeneous potassium catalyst. Energy Procedia. 2017;143:742–747. [Google Scholar]
- 17.Dai S.B., Lee H.Y., Chen C.L. Design and economic evaluation for the production of ethyl lactate via reactive distillation combined with various separation configurations. Ind. Eng. Chem. Res. 2019;58:6121–6132. [Google Scholar]
- 18.Simasatitkul L., Arpornwichanop A., Gani R. Design methodology for bio-based processing: biodiesel and fatty alcohol production. Comput. Chem. Eng. 2013;57:48–62. [Google Scholar]
- 19.Lee H.Y., Yen L.T., Chien I.L., Huang H.P. Reactive distillation for esterification of an alcohol mixture containing n-butanol and n-Amyl alcohol. Ind. Eng. Chem. Res. 2009;48:7186–7204. [Google Scholar]
- 20.Poddar T., Jagannath J., Almansoori A. Biodiesel Production using Reactive Distillation: A Comparative Simulation Study. Energy Procedia. 2015;75:17–22. [Google Scholar]
- 21.Cho H.J., Kim J.-K., Ahmed F., Yeo Y.-K. Life – cycle greenhouse gas emissions and energy balances of a biodiesel from palm fatty acid distillate (PFAD) Appl. Energy. 2013;111:479–488. [Google Scholar]
- 22.Xu H., Lee U., Wang M. Life – cycle energy use and greenhouse gas emissions of palm fatty acid distillate derived renewable diesel. Renew. Sustain. Energy Rev. 2020;134 [Google Scholar]
- 23.Aghbashlo M., Tabatabaei M., Amid S., Hosseinzadeh-Bandbafha H., Khoshnevisan B., Kianian G. Life cycle assessment analysis of an ultrasound-assisted system converting waste cooking oil into biodiesel. Renew. Energy. 2020;151:1352–1364. [Google Scholar]
- 24.Babi D.K., Lutze P., Woodley J.M., Gani R. A process synthesis-intensification framework for the development of sustainable membrane-based operations. Chem. Eng. Proc. 2014;86:173–195. [Google Scholar]
- 25.Babi D.K., Holtbruegge J., Lutze P., Gorak A., Woodley J.M., Gani R. Sustainable process synthesis–intensification. Comput. Chem. Eng. 2015;81:218–244. [Google Scholar]
- 26.Lakshmi C.V., Viswanath K., Venkateshwar S., Satyavathi B. Mixing characteristics of the oil–methanol system in the production of biodiesel using edible and non-edible oils. Fuel Process. Technol. 2011;92:1411–1417. [Google Scholar]
- 27.Lin J., Chen W. Production of biodiesel by transesterification of jatropha oil with microwave heating. J. Taiwan Inst. Chem. Eng. 2017;75:43–50. [Google Scholar]
- 28.Darnoko D., Cheryan M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000;77:1263–1267. [Google Scholar]
- 29.Cho H.J., Kim J.-K., Hong S.W., Yeo Y.-K. Development of a novel process for biodiesel production from palm fatty acid distillate (PFAD) Fuel Process. Technol. 2012;104:271–280. [Google Scholar]
- 30.Anozie A.N., Dzobo J.M. Kinetics of the hydrolysis of palm oil and Palm Kernel oil. Ind. Eng. Chem. Res. 2006;45:1604–1612. [Google Scholar]
- 31.Douglas J. McGraw-Hill; 1988. Conceptual Design of Chemical Process. [Google Scholar]
- 32.Gaurav A., Ng F., Rempel G.L. A new green process for biodiesel production from waste oils via catalytic distillation using a solid catalyst – modeling, economic and environmental analysis. Green Energy Environ. 2016;1:62–74. [Google Scholar]
- 33.Poddar T., Jagannath A., Almansoori A. Use of reactive distillation in biodiesel production: a simulation – based comparison of energy requirements and profitability indicators. Appl. Energy. 2017;(185):985–997. [Google Scholar]
- 34.2015. Commodity and Energy Markets, France. Available from: http://www.commodity3.com/instrument/PFA0MYQ1/pfad-palm-fatty-acid-distillate [November 2015] [Google Scholar]
- 35.2015. Alibaba. Available from https://www.alibaba.com/showroom/linear-alkyl-benzene-price.html [November 2015] [Google Scholar]
- 36.Seider W.D., Seader J.D., Lewin D.R. Wileys; 2004. Product and Process Design Principles: Synthesis, Analysis, and Evaluation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.