Abstract
Background and Aims
Bilirubin encephalopathy/kernicterus is very rare in adults. This study is aimed to investigate the clinical manifestations and genetic features of two patients with UGT1A1-related kernicterus.
Methods
Sanger sequencing analysis was performed to identify UGT1A1 gene mutations in the patients and their families. Bioinformatics analysis was used to predict the potential functional effects of novel missense mutations. Clinical manifestations and biochemical parameters were collected and analyzed.
Results
Two patients with Crigler-Najjar syndrome type II (CNS2) developed kernicterus in adulthood. Sanger sequencing identified a compound heterozygous mutation in the UGT1A1 gene in patient 1, which was inherited from his mother (G71R) and his father (c.-3279T>G; S191F). Patient 2 carried three heterozygous mutations, namely G71R, R209W and M391K; among which, the M391K mutation has not been reported before. Multiple prediction software showed that the M391K mutation was pathogenic. Symptoms were relieved in the two patients after phenobarbital and artificial liver support treatment. Patient 1 also underwent liver transplantation.
Conclusions
Adults with CNS2 are at risk for kernicterus. Phenobarbital treatment is beneficial for maintaining bilirubin levels and preventing kernicterus.
Keywords: Kernicterus, UGT1A1, Crigler-Najjar syndrome type II, Phenobarbital
Introduction
Bilirubin encephalopathy/kernicterus is an uncommon disabling neurologic disease caused by the toxicity of unconjugated bilirubin (UCB) to the basal ganglia and various brain stem nuclei.1 Neonatal jaundice is quite common, affecting 60–80% of newborns, as a whole.2 However, severe hyperbilirubinemia (>20 mg/dL), which may potentially lead to kernicterus and neurodevelopmental complications, is very rare, accounting for less than 2% of newborns.3 The incidence of kernicterus is about 0.2 to 2.7 cases per 100,000 live births.1 Common risk factors may include preterm delivery, hemolytic disease [glucose-6-phosphate dehydrogenase deficiency and ABO hemolysis], perinatal infection and exclusive breastfeeding.1,4
For adults, elevated UCB caused by hemolytic disease and inherited non-hemolytic unconjugated hyperbilirubinemia (bilirubin glucuronidation defects) is relatively common. Bilirubin glucuronidation is regulated by the uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1) enzyme. It is encoded by the UGT1A1 gene, which is located on chromosome 2 (2q37), and covers a promoter, enhancers, and five exons. According to the severity of UGT1A1 enzyme deficiency, inherited unconjugated hyperbilirubinemia can be classified into Crigler-Najjar syndrome type I (CNS1), Crigler-Najjar syndrome type II (CNS2), and Gilbert syndrome (GS).5,6 CNS1 is the most severe form, determined by a complete lack of bilirubin glucuronidation, and patients exhibit a toxic level of hyperbilirubinemia (≥340 µmol/L) shortly after birth.7
CNS1 patients usually suffer from bilirubin encephalopathy, and are prone to death within the first 2 years of their lives.8,9 At present, orthotopic liver transplantation is the only radical treatment.10,11 CNS2 is characterized with not very high bilirubin (from 103 to <340 µmol/L)12 and the bilirubin glucuronidation is less than 10% of normal level but not completely eliminated.13 Although the phenotype of CNS2 is less severe, patients with CNS2 remain vulnerable to brain injury throughout life, especially in the setting of concurrent diseases, after injury, or during surgery.14 Poddar et al.15 reported a case of kernicterus in a CNS2 child due to a dramatic increase in UCB caused by hemolysis. GS is a mild hyperbilirubinemia (from normal level up to 80–100 µmol/L) that occurs in 5–10% of the population,12 with approximately 70% reduction in bilirubin glucuronidation.16 GS is considered as a benign condition without neurological damage and treatment requirement.
At present, there are few reports of kernicterus in adults. Here, we aimed to report two CNS2 adults with UGT1A1 mutations who developed kernicterus.
Methods
Subjects and sample collection
The patients and all family members received careful clinical examinations and laboratory assessments by experienced physicians in Beijing You’an Hospital, Capital Medical University. Fasting blood samples were collected from all participants; clinical manifestations and biochemical parameters were collected and analyzed.
This study was approved by the Ethics Committee of Beijing You’an Hospital, Capital Medical University, and a written informed consent form was obtained from all participants.
DNA extraction and screening for the mutations in UGT1A1
Genomic DNA was extracted from whole blood using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. The promoter, all five exons, exon-intron boundaries, and a region in the distal promoter (the phenobarbital response enhancer module, PBREM) of UGT1A1 were amplified by PCR technology, then purified through agarose gel electrophoresis and sequenced using a 3730XL sequencer (Applied Biosystems Inc., Foster City, CA, USA). Finally, Sanger sequencing data were compared and analyzed by SeqMan software (DNASTAR, Madison, WI, USA).
Bioinformatics analyses
Potential functional effects of novel missense mutations were predicted by PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/index.shtml), SIFT (http://sift.jcvi.org/), PROVEAN (http://provean.jcvi.org/index.php), MutationTaster (http://mutationtaster.org/), FATHMM (http://fathmm.biocompute.org.uk/), InterVar (http://wintervar.wglab.org/), and MutPred2 (http://mutpred.mutdb.org/). The grade of conservation of the mutant nucleotides was determined by PhastCons and PhyloP.
Results
Patient 1
The patient was a 32-year-old man with a 30-year history of jaundice. In May 2020, he lost his appetite after drinking (about 500 mL beer, 20 g ethanol). He took Chinese medicine (unknown pharmaceutical ingredients) for a week, but the symptoms were not alleviated. Then, he developed dizziness, headache, and mild neuropsychological disorder. Liver function results were abnormal, with decreased albumin (23.8 g/L) and increased aspartate aminotransferase (commonly referred to as AST; 148U/L), alanine aminotransferase (commonly referred to as ALT; 54 U/L), total bilirubin (TB, 411.8 µmol/L), indirect bilirubin (266.3 µmol/L) and direct bilirubin (145.5 µmol/L). Blood routine test showed a high proportion of neutrophils (81.5%) and a decrease in hemoglobin (109 g/L). His prothrombin time activity was 42% and the Coombs test was negative. Blood ammonia (16 µg/dL), fasting plasma lipids, autoantibodies profile, anti-neutrophil cytoplasmic antibody, and immunoglobulins (Igs) including IgG, IgA, IgM, and IgE were normal. The viral hepatitis markers were negative. Computed tomography (referred to as CT) and magnetic resonance imaging showed hepatosplenomegaly (Supplementary Fig. 1). Brain CT scans were normal (Supplementary Fig. 1). The patient was diagnosed with acute-on-chronic liver failure (ACLF), CNS2 and kernicterus, and received oral phenobarbital, albumin infusion, and anti-infection therapy.
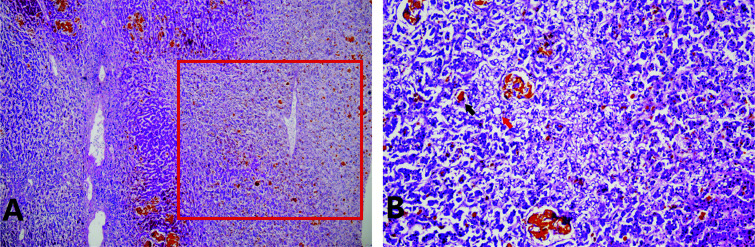
One week after admission, the patient suddenly manifested hematemesis and showed restlessness, then lost consciousness. TB, indirect bilirubin, and blood ammonia levels increased to 532.3 µmol/L, 379.1 µmol/L, and 123 µg/dL, respectively. Gastroscopy revealed cardiac mucosal laceration syndrome (Mallory-Weiss syndrome). The patient was treated with hemostasis, sedation, intramuscular injection of phenobarbital, and artificial liver support treatment of double plasma molecular adsorption system combined with plasma exchange (DPMAS) and plasma exchange. Later, the patient’s consciousness gradually recovered and he underwent liver transplantation. Histopathology of the removed liver showed massive and submassive hepatocyte necrosis, cholestasis, and steatosis (Fig. 1). The patient is now in a relatively stable state. Table 1 and Fig. 2 show his blood test results.
Fig. 1. Liver histopathology of patient 1 revealed massive and submassive necrosis, cholestasis and steatosis.
(A) Hematoxylin-eosin stain, 40 x. (B) Hematoxylin-eosin stain, 200 x. Massive and submassive necrosis, cholestasis and steatosis are indicated by the red box, and black and red arrows, respectively.
Table 1. Biochemical characteristics of patient 1.
| Characteristics | Reference | Days after admission |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 8a | 13b | 15c | 16c | 17c | 18 | 24 | 25c | 26 | ||
| TB (µmol/L) | 5–21 | 411.8 | 513.6 | 532.3 | 616.4 | 570.3 | 353.8 | 453.3 | 417.8 | 524 | 561.5 | 378.5 |
| IB (µmol/L) | <7 | 266.3 | 370.6 | 379.1 | 450.1 | 427.7 | 274.9 | 374.3 | 352.7 | 428.6 | 490.4 | 330.4 |
| DB (µmol/L) | – | 145.5 | 143 | 153.2 | 166.3 | 142.6 | 78.9 | 79 | 65.1 | 95.4 | 71.1 | 48.1 |
| ALB (g/L) | 40–55 | 23.8 | 27.8 | 27.4 | 39.4 | 35.9 | 35.7 | 46.2 | 43.3 | 41.3 | 42.2 | 43.2 |
| BAMR | – | 1.1 | 1.2 | 1.3 | 1.0 | 1.0 | 0.7 | 0.6 | 0.6 | 0.8 | 0.9 | 0.6 |
| ALT (U/L) | 9–50 | 54 | 60 | 62 | 64 | 130 | 81 | 79 | 75 | 108 | 93 | 75 |
| AST (U/L) | 15–40 | 148 | 152 | 147 | 164 | 366 | 207 | 206 | 184 | 207 | 191 | 150 |
| GGT (U/L) | 10–60 | 114 | 99 | |||||||||
| ALP (U/L) | 45–125 | 474 | 412 | |||||||||
| TBA (µmol/L) | <10 | 175.3 | 122.8 | |||||||||
| WBC (×109/L) | 3.5–9.5 | 6.68 | 5.54 | 8.26 | 7.47 | 7.02 | 7.2 | 6.87 | 4.21 | 6.25 | 4.08 | 7.69 |
| N% | 40–75 | 81.5 | 71.1 | 80.1 | 83.2 | 75.8 | 72.8 | 72.7 | 83.4 | 75.9 | 71.6 | 90.1 |
| HGB (g/L) | 130–175 | 109 | 112 | 105 | 80 | 82 | 78 | 85 | 80 | 65 | 62 | 68 |
| PLT (×109/L) | 125–350 | 145 | 138 | 128 | 85 | 87 | 81 | 90 | 89 | 85 | 70 | 75 |
| Amon (µg/dL) | 19–54 | 16 | 25 | 123 | 79 | 20 | 8 | 25 | 48 | 59 | ||
The patient’s neuropsychiatric symptoms deteriorated after hematemesis.
The patient underwent plasma exchange.
The patient underwent DPMAS and plasma exchange.
Abbreviations: %N, proportion of neutrophils; ALB, albumin; ALP, alkaline phosphatase; Amon, blood ammonia; BAMR, Bilirubin-albumin molar ratio; DB, direct bilirubin; GGT, γ-glutamyl transferase; HGB, hemoglobin; IB, indirect bilirubin; PLT, platelets; TBA, total serum bile acid; WBC, white blood cell.
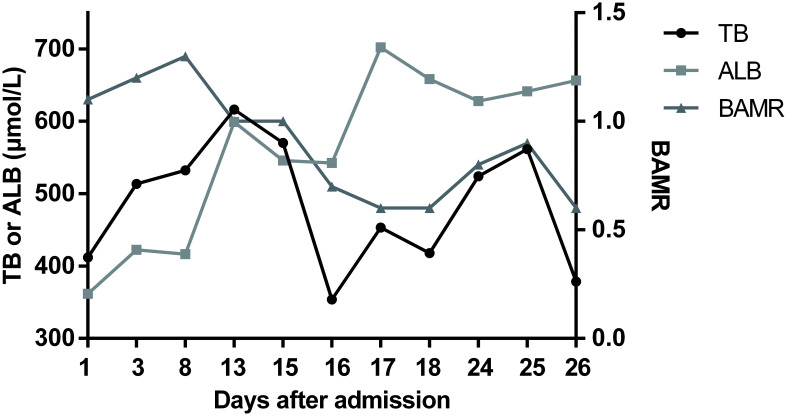
Fig. 2. BAMR in patient 1 after admission.
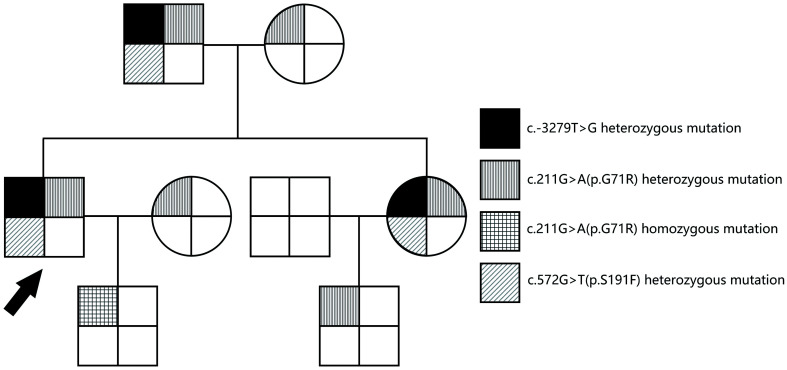
Sanger sequencing of the UGT1A1 gene identified a compound heterozygous mutation in this patient, which was inherited from his mother (c.211G>A, p.G71R) and his father (c.-3279T>G; c.572C>T, p.S191F) (Fig. 3 and Supplementary Fig. 2).
Fig. 3. Family pedigree of patient 1.
The arrow indicates patient 1.
Interestingly, the patient’s elder sister also showed jaundice since childhood. Her TB was about 400 µmol/L. She was diagnosed with CNS2 but had never developed kernicterus. In addition, the patient’s father had mild unconjugated hyperbilirubinemia (TB of 32.1 µmol/L, indirect bilirubin of 23.2 µmol/L) and was diagnosed with GS. They were both also found to carry the same compound heterozygous mutation as the patient (Fig. 3).
Patient 2
The patient was a 35-year-old man. He had been suffering from jaundice since birth, with TB ranging from 70–80 µmol/L. In adulthood, his TB levels were about 300 µmol/L. At the age of 31, he developed right upper abdominal pain and severe jaundice. His TB level had been found to have increased to above 500 µmol/L. The patient was diagnosed with gallbladder stones, cholecystitis and CNS2. After treatment with antibiotics and oral phenobarbital, the patient’s pain was relieved and TB was reduced to 300 µmol/L. Later, the patient experienced repeated abdominal pain, fever, and severe jaundice. In November 2019, he became lethargic and unresponsive after a fever. Then, he developed limb convulsions and urinary incontinence. The patient was admitted on November 15, 2019.
The results of liver function showed decreased albumin (21.5 g/L) and increased AST (222 U/L), ALT (57 U/L), TB (417.4 µmol/L), and direct bilirubin (195 µmol/L). The proportion of neutrophils (88.1%) and blood ammonia (112 µg/dL) were elevated. The Coombs test was negative. The fasting plasma lipids, autoantibodies profile, and anti-neutrophil cytoplasmic antibody were normal. Cerebrospinal fluid results did not suggest central nervous system infection. Abdominal ultrasonography showed gallstones, cholecystitis, and splenomegaly. Brain CT scans were normal.
Sanger sequencing of the UGT1A1 gene identified three heterozygous mutations, namely G71R (c.211G>A), R209W (c. 625C>T), and M391K (c.1172T>A) (Supplementary Fig. 3). The M391K mutation has not been reported before. Seven software programs were used to predict the pathogenicity of the mutation. As shown in Supplementary Table 1, all software programs showed that the M391K mutation was pathogenic or damaging. The PhastCons score of the mutation was 1, and the corresponding PhyloP value was 2.307, suggesting the high conservation of this amino acid.
The patient was treated with antibiotics, sedation, intramuscular injection of phenobarbital, and artificial liver support treatment with DPMAS and plasma exchange. The patient’s neurological status gradually returned to normal. Table 2 and Fig. 4 show the results of his blood investigations.
Table 2. Biochemical characteristics of patient 2.
| Characteristics | Reference | Days after admission |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 5a | 7b | 8b | 9 | 12b | 13 | ||
| TB (µmol/L) | 5–21 | 417.4 | 527.7 | 547.3 | 448.9 | 432.5 | 342.1 | 486.2 | 257.1 |
| IB (µmol/L) | <7 | 222.4 | 320 | 360 | 322.8 | 322.9 | 260.4 | 361.8 | 217.5 |
| DB (µmol/L) | – | 195 | 207.7 | 187.3 | 126.1 | 109.6 | 81.7 | 124.4 | 39.6 |
| ALB (g/L) | 40–55 | 21.5 | 31.7 | 32.8 | 32.1 | 35 | 29.6 | 33.4 | 26.7 |
| BAMR | – | 1.3 | 1.1 | 1.1 | 0.9 | 0.8 | 0.8 | 1.0 | 0.6 |
| ALT (U/L) | 9–50 | 57 | 58 | 59 | 58 | 63 | 71 | 88 | 63 |
| AST (U/L) | 15–40 | 222 | 249 | 233 | 171 | 160 | 171 | 191 | 169 |
| GGT (U/L) | 10–60 | 78 | 76 | 73 | 74 | 112 | |||
| ALP (U/L) | 45–125 | 513 | 460 | 327 | 282 | 334 | |||
| TBA (µmol/L) | <10 | 159.1 | 148.9 | 137.4 | 136.6 | 133.8 | |||
| WBC (×109/L) | 3.5–9.5 | 12.77 | 5.56 | 6.22 | 8.35 | 9.46 | 6.24 | 10.6 | |
| N% | 40–75 | 88.1 | 76.6 | 79.9 | 79.8 | 84.7 | 79 | 86.7 | |
| HGB (g/L) | 130–175 | 116 | 108 | 105 | 102 | 102 | 93 | 93 | |
| PLT (×109/L) | 125–350 | 237 | 161 | 176 | 176 | 188 | 156 | 173 | |
| Amon (µg/dL) | 19–54 | 41 | 107 | 116 | 81 | 74 | 108 | 75 | 89 |
The patient underwent DPMAS and plasma exchange.
The patient underwent plasma exchange.
Abbreviations: %N, proportion of neutrophils; ALB, albumin; ALP, alkaline phosphatase; Amon, blood ammonia; BAMR, Bilirubin-albumin molar ratio; DB, direct bilirubin; GGT, γ-glutamyl transferase; HGB, hemoglobin; IB, indirect bilirubin; PLT, platelets; TBA, total serum bile acid; WBC, white blood cell.
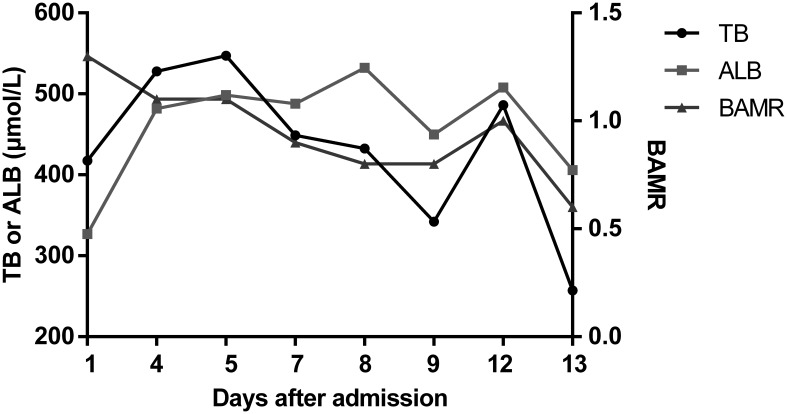
Fig. 4. BAMR in patient 2 after admission.
Discussion
High concentrations of UCB can cause nervous system damage, known as bilirubin encephalopathy or kernicterus. In general, kernicterus is found in infants and young children, especially those who are premature and/or have hemolysis.1,17 On one hand, the blood-brain barrier of newborns and children is immature. On the other hand, the albumin/bilirubin-binding capacity and tissue-binding capacity vary significantly among newborns, and these values are particularly low for premature babies.18 Kernicterus in adults is a rare condition. To date, there are only two published cases.19,20 In one case, the disorder was associated with liver failure.19 The other case was an adult with CNS2, who developed kernicterus after laparoscopic surgery.20 This report describes two new cases of adult kernicterus associated with UGT1A1 mutations (CNS2).
CNS2 is usually caused by missense mutations in the UGT1A1 gene, which reduces enzyme activity but does not eliminate it.21,22 Most patients with CNS2 have homozygous missense mutations or compound heterozygous mutations.23 This explains the milder phenotype and inducibility of the residual enzyme activity by phenobarbital administration. Variants c.211G>A (p.G71R) and c.1456T>G (p.Y486D) are the most frequently reported mutation sites in Asian CNS2 patients.24–26 In our patients, UGT1A1 sequencing analysis was performed. Patient 1 is a compound heterozygote with mutations c.-3279T>G, G71R, and S191F. His father and sister share the same UGT1A1 genotype as him, and were diagnosed with GS and CNS2, respectively. But none of them suffered from kernicterus. The above evidence suggests that the same genotype may result in different phenotypes and clinical manifestations. A possible explanation is that a multifactorial etiology including hormonal, environmental, and genetic factors contributes to the development of inherited diseases. Besides, liver histology suggested massive and submassive hepatocytes necrosis and cholestasis, consistent with ACLF and CNS2, respectively.27,28 Histopathology also showed steatosis, but the related mechanism is not clear. Before the onset of ACLF, Patient 1 drank about 20 g ethanol (500 mL beer) and then took traditional Chinese medicine for 1 week. Considering the relatively small intake of ethanol and the complex composition of Chinese medicine (although the composition is unknown), we speculated that Chinese medicine was the main cause of his acute liver injury, and eventually lead to his ACLF on the basis of Crigler-Najjar syndrome.
Patient 2 is heterozygous for the mutations G71R, R209W and M391K. The mutations G71R, S191F, and R209W are located in exon 1 of the UGT1A1 gene, and have been shown to be associated with CNS2 (moderate hyperbilirubinemia).29 The mutation c.-3279T>G is located in the phenobarbital response enhancer module, which is related to GS (mild hyperbilirubinemia).30 The M391K mutation has not been reported yet. All prediction software indicated that the mutation is pathogenic. PhastCons and PhyloP showed a high degree of amino acid conservation, suggesting that the mutation has a great impact on amino acids and the protein. According to the variant classification criteria of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology,31 the novel M391K mutation is pathogenic to CNS2 in this patient. The effect on the UGT1A1 enzyme’s activity needs to be further verified through cell experimentation. In summary, the mutation site, number, and genotype of UGT1A1 are related to bilirubin level.
The pathogenesis of kernicterus has not been fully elucidated but the main reason is excessive bilirubin production (i.e. hemolysis) and/or insufficient liver glucuronidation, leading to higher levels of free unbound bilirubin. The potential mechanism of UCB neurotoxicity is shown in Fig. 5.18 In our two patients, CNS2 reduced bilirubin glucuronidation. Infection, fever and liver injury cause a decrease in serum albumin levels. Therefore, free unbound bilirubin increased. In infants, a bilirubin-albumin molar ratio (referred to as BAMR) value >0.8 is considered dangerous because bilirubin/albumin binding is unpredictable at these levels.17 However, for adults, the BAMR value predicting kernicterus is still unclear. In our two patients, bilirubin concentrations increased to 616 µmol/L and 547 µmol/L, respectively, with BAMR value >0.8 in both. Then, kernicterus occurred. Therefore, for patients with CNS2, it is important to avoid particularly high bilirubin levels and maintain normal serum albumin levels.
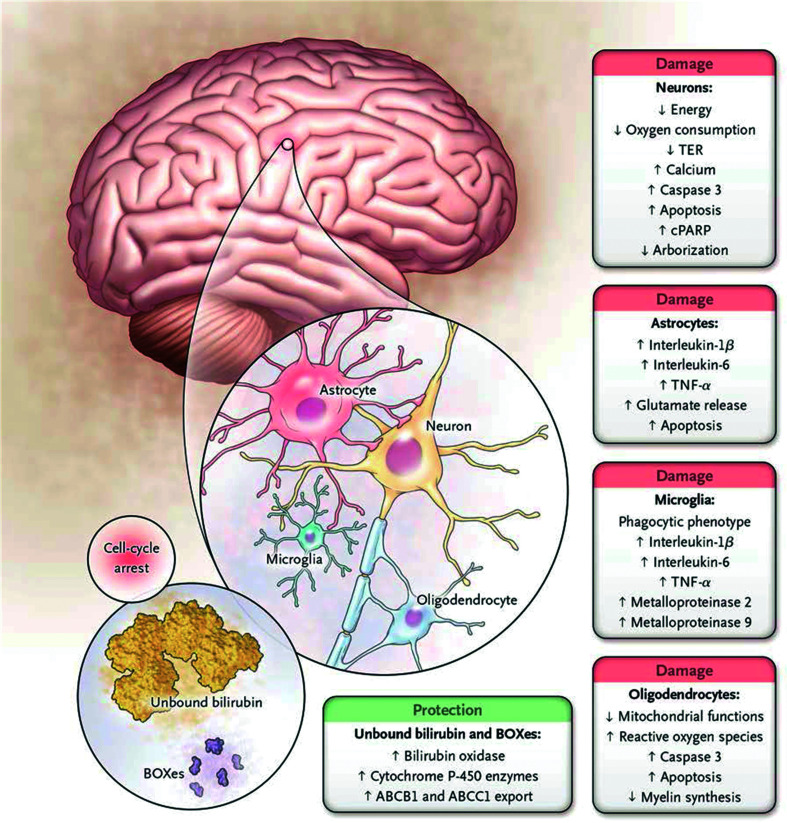
Fig. 5. Cell types and metabolic processes affected by bilirubin in the central nervous system.
The main effects of bilirubin on neurons are decreased oxygen consumption and increased release of calcium and caspase 3, resulting in apoptosis. There is also decreased dendritic and axonal arborization. A similar pattern is observed in oligodendrocytes with increased apoptosis, impairment of the redox state (oxidative stress), and reduced synthesis of myelin. Microglia react to toxic injury associated with bilirubin by increased release of proinflammatory cytokines and metalloproteinase activity as cells manifest a phagocytic phenotype. A similar proinflammatory pattern is observed in astrocytes, with enhanced release of glutamate and apoptosis. At the same time, cells may reduce the intracellular concentration of bilirubin either by extruding the pigment through the ATP-binding cassette transporters or by increasing the formation of the less toxic products through bilirubin oxidation products (BOXes) and/or cytochrome P-450 enzymes (1a1 and 1a2, in particular). These responses are protective, whereas all others result in cell damage; this suggests that once the intracellular concentration of bilirubin exceeds a toxic threshold (still to be defined), the polymorphic metabolic cascade leading to neurotoxicity ensues. (From Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage-mechanism and management approaches. N Engl J Med 2013; 369: 2025). Abbreviations: cPARP, cleaved poly (adenosine diphosphate-ribose) polymerase; TER, transcellular resistance.
Infection, liver damage, and hemolytic disease should also be avoided. It should be noted that CNS2 patients respond to phenobarbital. To prevent severe hyperbilirubinemia and kernicterus, phenobarbital therapy should be adhered to. If the patient develops kernicterus, treatments that eliminate the cause, or those such as albumin supplementation and artificial liver support treatment, are effective. Due to severe necrosis and insufficient regeneration of hepatocytes in patient 1, the bilirubin level still slightly fluctuated in the case of artificial liver support treatment. At the same time, although the patient recovered consciousness after albumin supplementation and artificial liver support treatment, increased bilirubin due to CNS2 may lead to recurrence of kernicterus. At present, liver transplantation is the only treatment option that completely replaces UGT1A1 function and normalizes serum bilirubin levels.
In conclusion, although very rare, adults with CNS2 are at risk of kernicterus. Phenobarbital administration helps maintain bilirubin levels and prevent kernicterus.
Supporting information
Computed tomography (CT) (A) and magnetic resonance imaging (B) of the abdomen showed hepatosplenomegaly. No reduction in signal intensity of the liver parenchyma was found in the out-of-phase image (C). The T2WI image showed a diffuse and mild increase in the signal intensity of the liver parenchyma (D). The brain CT scan was normal (E).
Three heterozygous mutations were found in the UGT1A1 gene of patient 1: c.-3279T>G, c.211G>A (p.G71R), and c.572C>T (p.S191F). II:1, patient 1; I:1, the patient’s father; I:2, the patient’s mother; II:2, the patient’s sister; III:1, the patient’s son; III:2, the patient’s nephew (the son of the patient’s sister).
Three heterozygous mutations were found in the UGT1A1 gene of patient 2: c.211G>A (p.G71R), c. 625C>T (p.R209W), and c.1172T>A (p.M391K).
Abbreviations
- ACLF
acute-on-chronic liver failure
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAMR
bilirubin-albumin molar ratio
- CNS2
Crigler-Najjar syndrome type II
- CT
computed tomography
- DPMAS
double plasma molecular adsorption system combined with plasma exchange
- GS
Gilbert syndrome
- Ig
immunoglobulin
- TB
total bilirubin
- UCB
unconjugated bilirubin
- UGT1A1
uridine diphosphate glucuronosyl transferase 1A1
References
- 1.Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2:610–620. doi: 10.1016/S2352-4642(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 2.Olusanya BO, Ogunlesi TA, Slusher TM. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch Dis Child. 2014;99:1117–1121. doi: 10.1136/archdischild-2013-305506. [DOI] [PubMed] [Google Scholar]
- 3.Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873–878. [PubMed] [Google Scholar]
- 4.Okolie F, South-Paul JE, Watchko JF. Combating the hidden health disparity of kernicterus in black infants: A review. JAMA Pediatr. 2020;174(12):1199–1205. doi: 10.1001/jamapediatrics.2020.1767. [DOI] [PubMed] [Google Scholar]
- 5.Lin JP, Cupples LA, Wilson PW, Heard-Costa N, O’Donnell CJ. Evidence for a gene influencing serum bilirubin on chromosome 2q telomere: a genomewide scan in the Framingham study. Am J Hum Genet. 2003;72:1029–1034. doi: 10.1086/373964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome) Best Pract Res Clin Gastroenterol. 2010;24:555–571. doi: 10.1016/j.bpg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimi A, Rahim F. Crigler-Najjar syndrome: Current perspectives and the application of clinical genetics. Endocr Metab Immune Disord Drug Targets. 2018;18:201–211. doi: 10.2174/1871530318666171213153130. [DOI] [PubMed] [Google Scholar]
- 8.Crigler JF, Jr, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169–180. [PubMed] [Google Scholar]
- 9.Crigler JF, Jr, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus; a new clinical entity. AMA Am J Dis Child. 1952;83:259–260. [PubMed] [Google Scholar]
- 10.van Dijk R, Beuers U, Bosma PJ. Gene replacement therapy for genetic hepatocellular jaundice. Clin Rev Allergy Immunol. 2015;48:243–253. doi: 10.1007/s12016-014-8454-7. [DOI] [PubMed] [Google Scholar]
- 11.Fagiuoli S, Daina E, D’Antiga L, Colledan M, Remuzzi G. Monogenic diseases that can be cured by liver transplantation. J Hepatol. 2013;59:595–612. doi: 10.1016/j.jhep.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Wagner KH, Shiels RG, Lang CA, Seyed Khoei N, Bulmer AC. Diagnostic criteria and contributors to Gilbert’s syndrome. Crit Rev Clin Lab Sci. 2018;55:129–139. doi: 10.1080/10408363.2018.1428526. [DOI] [PubMed] [Google Scholar]
- 13.Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014;146:1625–1638. doi: 10.1053/j.gastro.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr. 2006;165:306–319. doi: 10.1007/s00431-005-0055-2. [DOI] [PubMed] [Google Scholar]
- 15.Poddar B, Bharti B, Goraya J, Parmar VR. Kernicterus in a child with Crigler-Najjar Syndrome Type II. Trop Gastroenterol. 2002;23:33–34. [PubMed] [Google Scholar]
- 16.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 17.Wallenstein MB, Bhutani VK. Jaundice and kernicterus in the moderately preterm infant. Clin Perinatol. 2013;40:679–688. doi: 10.1016/j.clp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage—mechanisms and management approaches. N Engl J Med. 2013;369:2021–2030. doi: 10.1056/NEJMra1308124. [DOI] [PubMed] [Google Scholar]
- 19.Waser M, Kleihues P, Frick P. Kernicterus in an adult. Ann Neurol. 1986;19:595–598. doi: 10.1002/ana.410190614. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Chowdhury NR, Chowdhury JR, Boyer TD. Kernicterus in an adult who is heterozygous for Crigler-Najjar syndrome and homozygous for Gilbert-type genetic defect. Gastroenterology. 1997;112:2099–2103. doi: 10.1053/gast.1997.v112.pm9178703. [DOI] [PubMed] [Google Scholar]
- 21.Clarke DJ, Moghrabi N, Monaghan G, Cassidy A, Boxer M, Hume R, et al. Genetic defects of the UDP-glucuronosyltransferase-1 (UGT1) gene that cause familial non-haemolytic unconjugated hyperbilirubinaemias. Clin Chim Acta. 1997;266:63–74. doi: 10.1016/s0009-8981(97)00167-8. [DOI] [PubMed] [Google Scholar]
- 22.Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297–306. doi: 10.1002/1098-1004(200010)16:4<297::AID-HUMU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Servedio V, d’Apolito M, Maiorano N, Minuti B, Torricelli F, Ronchi F, et al. Spectrum of UGT1A1 mutations in Crigler-Najjar (CN) syndrome patients: identification of twelve novel alleles and genotype-phenotype correlation. Hum Mutat. 2005;25:325. doi: 10.1002/humu.9322. [DOI] [PubMed] [Google Scholar]
- 24.Zheng B, Hu G, Yu J, Liu Z. Crigler-Najjar syndrome type II in a Chinese boy resulting from three mutations in the bilirubin uridine 5′-diphosphate-glucuronosyltransferase (UGT1A1) gene and a family genetic analysis. BMC Pediatr. 2014;14:267. doi: 10.1186/1471-2431-14-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagi T, Nakahara S, Maruo Y. Bilirubin uridine diphosphate-glucuronosyltransferase polymorphism as a risk factor for prolonged hyperbilirubinemia in Japanese preterm infants. J Pediatr. 2017;190:159–162.e1. doi: 10.1016/j.jpeds.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Maruo Y, Nakahara S, Yanagi T, Nomura A, Mimura Y, Matsui K, et al. Genotype of UGT1A1 and phenotype correlation between Crigler-Najjar syndrome type II and Gilbert syndrome. J Gastroenterol Hepatol. 2016;31:403–408. doi: 10.1111/jgh.13071. [DOI] [PubMed] [Google Scholar]
- 27.Fata CR, Gillis LA, Pacheco MC. Liver fibrosis associated with crigler-najjar syndrome in a compound heterozygote: A case report. Pediatr Dev Pathol. 2017;20:522–525. doi: 10.1177/1093526617697059. [DOI] [PubMed] [Google Scholar]
- 28.Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sneitz N, Bakker CT, de Knegt RJ, Halley DJ, Finel M, Bosma PJ. Crigler-Najjar syndrome in The Netherlands: identification of four novel UGT1A1 alleles, genotype-phenotype correlation, and functional analysis of 10 missense mutants. Hum Mutat. 2010;31:52–59. doi: 10.1002/humu.21133. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Luo L, Liu S, Liang C, Bai L, Chen Y, et al. Combined effects of UGT1A1 and SLCO1B1 variants on Chinese adult mild unconjugated hyperbilirubinemia. Front Genet. 2019;10:1073. doi: 10.3389/fgene.2019.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Computed tomography (CT) (A) and magnetic resonance imaging (B) of the abdomen showed hepatosplenomegaly. No reduction in signal intensity of the liver parenchyma was found in the out-of-phase image (C). The T2WI image showed a diffuse and mild increase in the signal intensity of the liver parenchyma (D). The brain CT scan was normal (E).
Three heterozygous mutations were found in the UGT1A1 gene of patient 1: c.-3279T>G, c.211G>A (p.G71R), and c.572C>T (p.S191F). II:1, patient 1; I:1, the patient’s father; I:2, the patient’s mother; II:2, the patient’s sister; III:1, the patient’s son; III:2, the patient’s nephew (the son of the patient’s sister).
Three heterozygous mutations were found in the UGT1A1 gene of patient 2: c.211G>A (p.G71R), c. 625C>T (p.R209W), and c.1172T>A (p.M391K).