Abstract
The gut microbiome plays a key role in the health-disease balance in the human body. Although its composition is unique for each person and tends to remain stable throughout lifetime, it has been shown that certain bacterial patterns may be determining factors in the onset of certain chronic metabolic diseases, such as type 2 diabetes mellitus (T2DM), obesity, metabolic-associated fatty liver disease (MAFLD), and metabolic syndrome. The gut-liver axis embodies the close relationship between the gut and the liver; disturbance of the normal gut microbiota, also known as dysbiosis, may lead to a cascade of mechanisms that modify the epithelial properties and facilitate bacterial translocation. Regulation of gut microbiota is fundamental to maintaining gut integrity, as well as the bile acids composition. In the present review, we summarize the current knowledge regarding the microbiota, bile acids composition and their association with MAFLD, obesity, T2DM and metabolic syndrome.
Keywords: Metabolic-associated fatty liver disease, Metabolic-associated steatohepatitis, Gut microbiota, Gut-liver axis, Dysbiosis, Bile acids
Introduction
The prevalence of metabolic chronic diseases is increasing around the world, mainly due to the increased incidence of obesity and type 2 diabetes mellitus (T2DM). The global prevalence of T2DM is 8.8%, which translates into approximately 422 million afflicted people worldwide; the USA alone has a T2DM prevalence of 25% among its seniors and an alarming amount of obesity, with 604 million adults and 108 million children being obese.1 Metabolic-associated fatty liver disease (MAFLD) affects 25% of the global population,2–9 representing the principal cause of chronic liver disease.10–12
This phenomenon can be associated with the modern lifestyle across industrialized countries that favors a sedentary life and high-calorie diets, thus promoting obesity and other chronic comorbidities. This situation constitutes a serious public health problem.6,13–15
The pathophysiology of these metabolic diseases is very complex and multiple factors seem to play a role in their development and progression; however, the gut microbiota is an emerging topic, as over the past few decades it has been demonstrated to play a critical role in their development. Signals generated by dietary intake and environmental factors disturb the composition of the microbiota, altering intestinal barrier homeostasis as well as the bile acid (BA) composition, and activating inflammatory pathways.16
Furthermore, it has been demonstrated that diet not only determines calorie intake but also impacts composition of the BA pool and gut microbiota, along with the intestinal barrier homeostasis.17
Throughout this paper, we will discuss the role of gut microbiota and BAs composition in metabolic diseases such as T2DM, obesity and MAFLD.
Epidemiology and risk factors of MAFLD
Elements such as ethnicity and gender have been described as determinants of the susceptibility and predictors of the severity and progression of MAFLD. Hispanics are the most susceptible to liver damage, followed by African Americans. Countries with the highest prevalence are USA, Belize, Barbados, and Mexico, with 30% of adults and 10% of adolescents having a MAFLD diagnosis, equaling to more than 80 million MAFLD patients.6 The highest prevalence of MAFLD is between the ages of 50 to 70 years.13 Men are at higher risk than women are; although, after menopause, this protective effect is lost. Life-style related factors, like little physical activity and a high-fat diet, are also important due to the close relationship of MAFLD with other chronic metabolic diseases like T2DM, obesity and metabolic syndrome.6
Genetics and MAFLD
Genetics have always been an important determinant and risk factor for the development of numerous diseases. Several studies have strongly suggested the existence of a hereditary component of MAFLD.18,19
Multiple polymorphisms have been associated with MAFLD, among the most studied are those involving the patatin-like phospholipase domain containing 3 (PNPLA3), TM6SF2, LYPLAL1, GCKR and MBOAT7 genes.20 PNPLA3 has been consistently identified across different genome-wide association studies (commonly known as GWAS). This gene is located on chromosome 22 and encodes up to 81 amino acids related to the synthesis of the enzyme adipose triglyceride lipase, which contributes to the degradation of triacylglycerol in adipose tissue.18 The variant I148M (rs738409) substitutes an isoleucine with a methionine, reducing the capability of the enzyme to catalyze triacylglycerol in lipid droplets as they are gathered in the adipose tissue, leading to the accumulation of lipids within the liver; thus, there is a close relationship with the development of MAFLD.18,21,22 This polymorphism has also been associated with the severity of inflammation and fibrosis progression21,23,24 and, worryingly, some data have also indicated that it might increase the risk of MAFLD-associated hepatocellular carcinoma.18
Other polymorphisms that have been studied in MAFLD patients involve TM6SF2 (rs58542926) and MBOAT7 (rs641738). Their association with the severity of hepatic steatosis has been demonstrated across studies, but a recent study suggested that they are not associated with the severity of hepatic fibrosis.25
On the other hand, the HSD17B13 17-beta hydroxysteroid dehydrogenase 13 polymorphism rs6834314 (located in chromosome 14 and expressed mainly in the liver) has been described to have a protective effect on MAFLD. Although it does not appear to influence hepatocyte lipid accumulation, it might protect against hepatic fibrosis by modulating retinol dehydrogenase activity.26–28 This polymorphism was found to be associated with a protective effect in MAFLD patients, limiting progression from hepatic steatosis to metabolic steatohepatitis and fibrosis progression,27 and thereby reducing MAFLD chronic liver disease progression.28
Genetics and gut microbiota
It is believed that the gut microbiota composition is influenced, at least in part, by genetics, as it has been found that family members share similar microbial signatures.29 Moreover, studies made in twins found that the gut microbiota was more similar among the monozygotic twins rather than the dizygotic twins.30 The idea of a genetic influence on the microbiome and its dysbiosis is not far from the findings obtained from studies of other metabolic disorders; unfortunately, no studies have been made yet to characterize the specific genes and processes underlying this specific interaction.29
Intestinal barrier homeostasis
The intestinal barrier is constituted of several components that assure its homeostasis and prevent the translocation of pathogen and inflammatory factors, protecting the liver in this gut-liver axis. The main components of the intestinal barrier are the mucosal epithelium, tight junction proteins (TJPs) and the immune cellular barrier.
Mucosal epithelial barrier
Mucus represents the first anatomical barrier between the epithelial cells and the intestinal microbiota. The glycoprotein-rich mucus layer also serves as a source of nutrition and growth for some bacterial species. When fiber intake is deprived, the thickness of the mucus layer is decreased,31 which may lead to intestinal inflammation and a reduction in the physical barrier between the microbiota and the epithelial cells.32
The mucus layer also represents a source of immunoglobulins, mainly IgA, and antimicrobial peptides, that hinder the translocation of “good” bacteria. The mucus layer composition and thickness have to be balanced enough to prevent commensal microbial washout due to BAs and peristaltic movements, and to conserve the integrity of the intestinal barrier.33 Therefore, diet is very important to preserve the mucosal barrier integrity, protecting against intestinal pathogen translocation and inflammation.33,34
TJ proteins “leaky gut”
Among the intestinal TJs are those that hold the epithelial cells together, maintaining the integrity of the intestinal barrier. Such TJs are the ZO-1 (zonula occludens-1), occludin, claudin-1 and claudin-4. It has been shown in experimental studies in rodents that a fructose-enriched diet decreases the concentration of the TJs, as well as the concentration of adherent junction proteins, leading to an increase of bacterial endotoxin levels and contributing to the leaky gut condition.35 In other studies, it has also been described that, in MAFLD over-nourished patients (high-fat or fructose-enriched diet), the concentration of ZO-1 and occludin is decreased, favoring bacterial translocation to the circulation, and supporting increase lipopolysaccharide (LPS) levels in the blood (known as metabolic endotoxemia). When the LPS is detected by Toll-like receptors in the liver, the characteristic low-grade inflammation associated with hepatic steatosis and fibrosis is triggered.36
Correlations between increased intestinal permeability and other alterations (like obesity, insulin resistance, and elevated lipid profile) have been demonstrated.37 As described above, MAFLD patients have an increased gut permeability, and even though bacterial translocation is a relatively physiological process and the liver can regulate the bacterial concentrations through their elimination via Kupffer cells, the significant increase of harmful bacterial substances promote the production of chemokines, inflammatory cytokines, vasoactive factors, and reactive oxygen species, which lead to hepatocyte apoptosis and activation as well as proliferation of stellate cells and development of fibrosis through TGFβ.38,39
Immune cells barrier
Several immune cells contribute to reinforcement of the intestinal barrier. These include lymphocytes (T cells [CD4+ Th17, CD4+T regulatory]), natural killer T cells, dendritic cells, and mononuclear phagocytes.16 The intestinal immune barrier itself is composed of intraepithelial lymphocytes that include a diversity of T cells, mainly CD4+ and T regulatory cells.40 Natural killer T cells are also important components for the recognition and discrimination between foreign and self-antigens, when activated by antigen presenting cells triggering immune responses. They can also be activated by pro-inflammatory cytokines.41
Dendritic cells are another important cellular component of the innate immune system that constitute the intestinal barrier. They induce T cell activation, produce interleukin (IL)-23 and induce a T helper immune response.42 Dendritic cells also express TJs and have the capacity to open epithelial tight junctions, playing an important role in preserving epithelial barrier integrity.43
Role of BAs in intestinal microbiota
BA composition and receptors also play a key role in the gut microbiota composition as well as in signaling pathways that regulate metabolic syndrome. They also seem to be part of the progression of hepatic fibrosis in patients with MAFLD.
BA composition
BAs are essential for fat-nutrients absorption and are classified as primary (PBA) and secondary (SBA). They are synthetized in the liver from cholesterol and transformed from PBA to SBA by the gut microbiota. They can be either conjugated or deconjugated. The BA signaling pathways and BA pool are controlled by the gut microbiota through different reactions (dihydroxylation and deconjugation).17 It has been described that SBAs increase the risk of metabolic diseases. Their composition is importantly determined by gut microbiota and dysbiosis disrupts the PBA/SBA ratio.44 Furthermore, BAs may also alter the gut microbiome by inhibiting bacterial development and altering the microbiome composition, by acting as a detergent according to the hydroxyl groups and amino acid portion, exerting this deleterious effect on the Bacteroidetes phyla especially.17,44 BAs represent a crucial partaker in liver-microbiota communication, and their composition and concentration seem to have a positive correlation with metabolism and hepatic fibrosis.45,46
The composition of BAs is important in many metabolic diseases, it has been described that patients with T2DM present a change from PBA to SBA with an increase in deoxycholic acid levels. BAs are metabolic integrators that act through signaling pathways that regulate the expression of certain metabolic genes; hence, a tweak in BA signaling might promote and aggravate metabolic syndrome.17
BA receptors and their effect on metabolic diseases
The BA composition is very important in MAFLD development. They play a key role in the modulation of metabolic pathways and in the balance of gut microbiome by acting as signaling molecules to diverse intestinal and liver receptors, such as farnesoid X receptor (FXR), vitamin D receptor and the TGR5 Takeda G protein-coupled receptor, and modulating immune responses in the gut.47–49 Activation of the hepatic receptors also varies depending on the type of BAs, with FXR being triggered preferably by PBAs and TGR5 by SBAs. These receptors modulate several metabolic characteristics, such as glucose tolerance, insulin sensitivity, fatty acid β-oxidation, energy expenditure, very low-density lipoprotein clearance, as well as hepatic inflammation.17,44
Microbial distribution in healthy subjects
Each individual has a unique gut microbiome, mainly shaped early in life by many factors, like the type of birth, milk feeding, weaning period or antibiotic use. The different bacteria species that inhabit the gut have a specific interaction with the host, regulating nutrient metabolism and immunomodulation, and protecting against pathogens or maintaining the gut mucosal barrier. Those specific variations within individuals are crucial in the predisposition to health or disease.50
Even though the gut microbiota tends to be stable throughout adulthood, it may vary due to exercise, lifestyle, dietary habits, pharmaceutical consumption or even metabolic or mental disorders, like stress or depression. An understanding of the healthy composition of the gut microbiota might be helpful in developing interventions that restore or maintain its equilibrium.50,51
Taxonomically, bacteria are classified into phyla, classes, order, families, genera, and species.
The gut microbiota is mainly composed of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria and Verrucomicrobia, with the first two accounting for up to 95% of the entire pool. The Firmicutes phylum contains over 200 different genera (Lactobacillus, Clostridium, Enterococcus, Ruminococcus, etc.), but Clostridium is the most representative; meanwhile, Bacteroidetes’ predominant genera are Bacteroides and Prevotella.50
Clinical implications
Gastrointestinal microbiome in obesity, T2DM, MAFLD and alcohol consumption
Several studies have analyzed the composition and role of the gut microbiome in different states of disease and have found correlations with metabolic pathologies, such as obesity, T2DM, atherosclerosis, MAFLD, irritable bowel syndrome, even some cutaneous problems, like atopic dermatitis, and psoriasis, among others (Table 1).9,50–64
Table 1. Comparative view of the healthy gut bacterial composition and its alterations during disease.
| Phylum | Species | |
|---|---|---|
| Healthy/Normal | Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, Verrucombia | Lactobacillus, Clostridium, Enterococcus, Ruminococcus, Bacteroides, Prevotella |
| Obesity | Firmicutes (↑) Bacteroidetes (↓) | Akkermansia muciniphila (↓)/Tuminococcaceae, Rikenellaceae, Mollicutes (↑) |
| Diabetes | Firmicutes (↑) Bacteroidetes (↓) | Roseburia, Eubacterium hallii, Faecalibacterium prausnitzii (↓)/Lactobacillus gasseri, Streptococcus mutans, E. coli (↑) |
| MAFLD | Firmicutes (↑), Actinomycetes (↑), Proteobacteria (↑), Bacteroidetes (↓), Fusobacteria (↓), Lentisphaerae (↓), Proteobacteria(↓), Thermus(↓), Verrucomicrobia (↓) | Prevotella, Porphyromonas, Veillonella (↑) |
Obesity
Most of the evidence that links changes in the gut microbiome with obesity was obtained from animal studies. Mouse and human microbiota have close similarities, mostly due to the shared characteristic predominance of Firmicutes and Bacteroidetes.14
Studies of genetically obese mice revealed a lower abundance of Bacteroidetes, whereas the Firmicutes showed higher composition for species such as Tuminococcaceae and Rikenellaceae, with Mollicutes being the most common. Recent studies have demonstrated that Akkermansia muciniphila, a mucin-degrading bacterium that lives in the mucus layer, is decreased in genetically and induced obese mice.53
Available human studies have shown that obese individuals have several specific variations in the gut microbiome; the most evident was a decreased microbial diversity. Healthy individuals with high bacterial richness have been associated with abundant microbial species, such as Faecalibacterium, Bifidobacterium, Lactobacillus and Akkermansia; those with low bacterial richness were dominant in Bacteroides and Ruminococcus.53
Interestingly, the lack of microbial diversity enhances calorie harvesting, which is mainly associated with the increase in Firmicutes species that provide the capacity of metabolizing polysaccharides that would normally be excreted;52 consequently, this leads to more adiposity, systemic inflammation and a tendency towards insulin resistance and dyslipidemia. It is worth mentioning that diet is a substantial point in this microbial richness; indeed, studies have found partial restoration of the gut microbiome in obese individuals with energy-restricted diets.65
Furthermore, studies have shown that the gut microbiome may influence weight gain by affecting host gene expression and modification of metabolic or inflammation pathways; therapeutic methods that regulate this interaction could be useful in the future.66
Still other studies have shown a correlation between the gut microbiome and the metabolic state through fecal microbiota transfer from obese mice to lean ones, which leads to increased fat mass, as well as the inverse situation, by transferring fecal matter from bypass operated mice to non-operated lean mice, with a final observation of reduced fat mass.67
The characteristic low-grade chronic inflammation in obesity can also be partly related to the alteration of the gut microbiome, evidence indicates that gut microbes exacerbate adipose tissue inflammation via increased gut permeability and increased circulating LPS.68
Emerging evidence based on animal studies has shown that short chain fatty acids (SCFAs), like butyrate, propionate and acetate, have an important role in obesity (apart from their normal signaling functions between the gut microbiome and the host metabolism), by increasing de novo lipogenesis in the liver and general lipid accumulation; however, despite human studies finding higher levels of SCFAs in obese individuals, the exact relationship with the pathophysiology remains to be clarified by more specific research.60 Hypotheses suggest that the effects SCFAs have in obesity involve intestinal anaerobic bacteria produced by fermentation of indigestible polysaccharides; these contribute almost 200 kcal to the human body, thus increasing lipogenesis and accumulation in adipose tissue. However, other lines of evidence show that SCFAs might be beneficial for cardio metabolic health.69
T2DM
Evidence shows that obese people with insulin resistance have an altered gut microbiota composition characterized by an increase in the Firmicutes:Bacteroidetes ratio compared to that in healthy people.54 However, related studies of diagnosed T2DM patients showed that this ratio is instead decreased, further accompanied by a reduction of other functional bacteria, like Bifidobacterium.55,56 A lower abundance of butyrate-producing microbes, such as Roseburia intestinalis and F. prausnitzii, and increased Lactobacillus species (L. gasseri, S. mutans) has also been reported in T2DM patients.57
A modification in the Bacteroidetes:Firmicutes ratio has been associated with a higher expression of microbial genes that encode carbohydrate metabolism-related enzymes. This altered fermentation profile may lead to an increased capacity to harvest energy from the diet and subsequent establishment of poor energy homeostasis, which leads to hyperglycemia (as well as hyperlipidemia).54 It is worth mentioning that the excess of adipose tissue is also a cause of insulin resistance, due to the increased production of adipokines, like resistin; on the other hand, the harmful effects of hyperglycemia are widely known, going from nephropathies, neuropathies, retinopathy to cardiovascular disease. Other effects of gut dysbiosis in diabetes are the enrichment in membrane transport of sugars, decreased butyrate synthesis, and an exaggerated oxidative stress response.54,70
MAFLD
Few studies have analyzed the composition of the gut microbiome in MAFLD patients. Unfortunately, the results have not been homogeneous, probably because of differences in sample sizes, variations within countries, and the unavoidable individual properties of each gut microbiota. Nevertheless, the fact that all studies showed the presence of dysbiosis confirms its role in the disease. Overall, at the phylum level, and with aid of animal studies, we can suggest that the general diversity is reduced, with increased Firmicutes, Actinomycetes and reduced Bacteroidetes, Fusobacteria, Leptosphaeria, Proteobacteria, Thermus and Verrucomicrobia.58–60 Studies have obtained contradictory results for Actinobacteria and Bacteroidetes. Additionally, research comparing MAFLD patients and controls has revealed that certain bacteria species, such as Prevotella and Porphyromonas, are increased.61 In patients with metabolic steatohepatitis, Proteobacteria species are consistently enriched; in cirrhosis, oral bacteria, such as Prevotella or Veillonella, can be observed in the distal colon. Studies that analyzed the microbial profile of cirrhotic patients with hepatocellular carcinoma (HCC) found increased fecal counts of Escherichia coli, as well as other Gram-negative bacteria, such as Atopobium, Collinsella, Eggerthella and Coriobacterium; others that studied the microbiota of only HCC-diagnosed patients found that Lactobacillus spp., Bifidobacterium spp., and Enterococcus spp. were reduced, with an additional increased concentration of H2S- and CH3SH-producing bacteria: Fusobacterium, Filifactor, Eubacterium, Parvimonas and Treponema.61,62
Finally, it is important to mention that some bacterial signatures can overlap between MAFLD and other metabolic diseases, due to their close relationship.
Recent studies with mice have shown that the gut microbiome’s state within itself may predispose to the development of MAFLD. In an experimental study, mice were fed for 16 weeks with a fat-rich diet and classified into two groups: “responders” with liver damage, and those that did not. Ultimately, transplant of the fecal microbiota from these groups into new microbe-free mice showed the mice that received “responder” microbiota were lean but had a propensity towards steatosis and insulin resistance.58
Pathologies like dysbiosis and small intestine bacterial overgrowth are closely correlated with MAFLD. The excess of gut bacteria leads to an increased lipid permeability and intake. These bacteria also tend to be translocated to the circulation, where they activate inflammatory pathways via the recognition of LPS. Ultimately, the Toll-like receptor 4 expression increases, production of IL-8 increases and insulin signaling decreases, leading to an increase in the influx of free fatty acids and a vicious cycle of lipotoxicity.54
MAFLD is closely related with obesity and insulin resistance, mainly because of the toxic effects the lipid excess causes in the liver tissue. The pathophysiology of lipotoxicity in the liver revolves around three main points: low-grade inflammation, autoimmunity, and oxidative stress. It occurs when the hepatic capacity to store or export lipids is exceeded by the fatty acid intake (from peripheral tissues or by de novo synthesis). The imbalance of gut microbiota (dysbiosis), as already mentioned, leads to inflammation; this process activates the Kupffer cells and prompts recruitment of other leukocytes to the tissue, initiating a cascade of proinflammatory cytokines and chemotactic factors, which provoke autoimmunity. Stellate cells are also activated by the cytokines and initiate an overproduction of extracellular matrix, which consequently supports progressive fibrosis.71 If these fibrotic and inflammatory processes are not regulated, steatosis can quickly progress into metabolic steatohepatitis.
The crucial point that determines the progression of liver steatosis into metabolic steatohepatitis resides within the production of fibrotic factors such as tumor necrosis factor (TNF)-α, Fas ligand and TGFβ, being mainly regulated by activation of the recurrent nuclear factor kappa-B (NF-κB) in hepatocytes (once again, the consequence of sustained inflammation). It is also important to highlight the importance of Kupffer cell activation and lymphocyte recruitment, because apart from the effects mentioned before, leukocyte presence in the liver tissue is a classic histopathological sign of metabolic steatohepatitis.72
Increasing evidence suggests that the gut-liver axis can take part in the onset of hepatocellular carcinoma, particularly due to the dysbiosis-induced endotoxemia. For example, gut microbial metabolites that act as antigens, like lipoteichoic acids in the case of Gram-positive bacteria or LPS in Gram-negative bacteria, can induce synthesis of the prostaglandin E2 PGE2 by hepatic stellate cells, which reduces the antitumoral activity of CD8+ lymphocytes.61 Another pathway that promotes hepatic carcinogenesis is the Toll-like receptor 4 recognition of pathogen-associated molecular patterns; this generates a cascade that activates NF-κB, which promotes the synthesis and release of inflammatory cytokines (IL-1, IL-6, TNF-α), thereby perpetuating liver inflammation. Nevertheless, the key point is that NF-κB may also induce antiapoptotic genes (TRAF-1 and TRAF-2), having important carcinogenic consequences. Toll-like receptor 4 is also expressed in hepatic stellate cells, where it is involved in the regulation of hepatocytogen epiregulin, an epidermoid growth factor with a potent mitogen effect on liver cells (Fig. 1).63
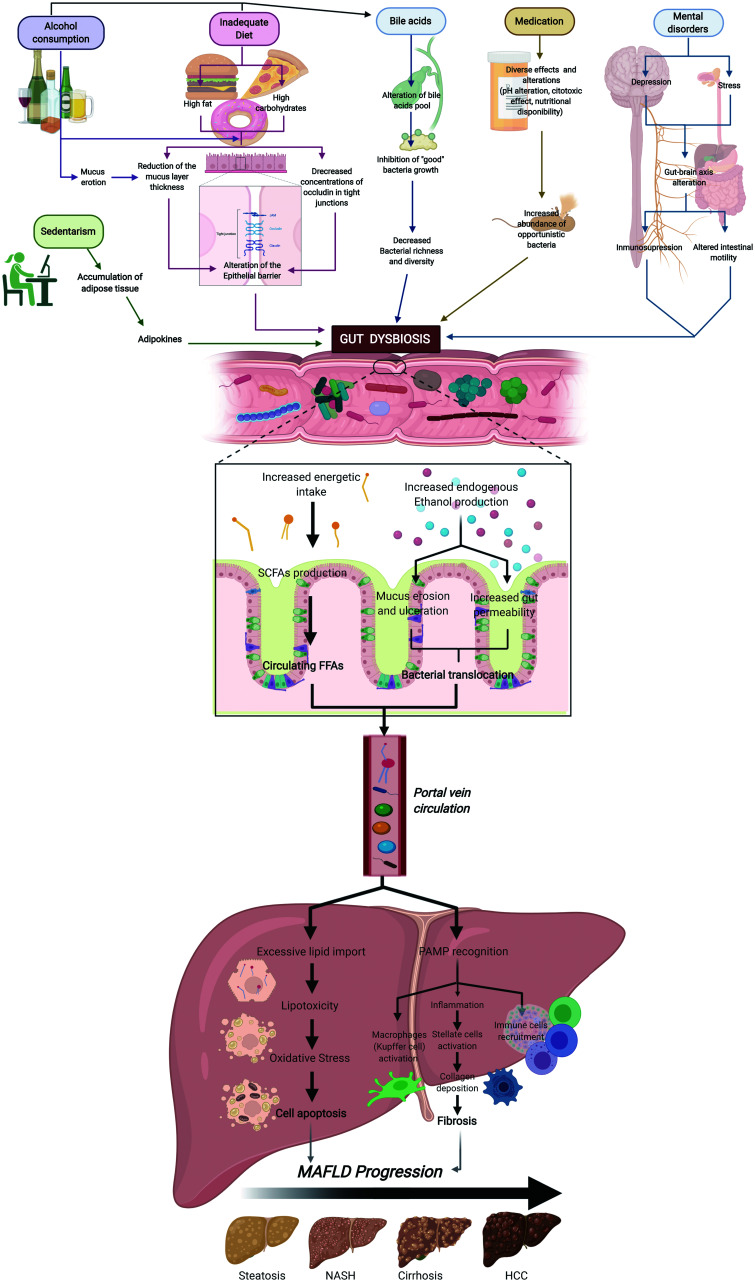
Fig. 1. Factors that promote gut dysbiosis and its effect on MAFLD.
Factors such as alcohol consumption, sedentarism, inadequate diet, medication and mental disorders may lead, through diverse mechanisms, to the onset of gut dysbiosis. Alteration of the normal gut bacteria conformation enhances the energetic intake through SCFA production; this excessive energy is then converted into FFAs through anabolism in the enterocytes. At the same time, ethanol-producing bacteria increase the endogenous levels of this metabolite, which then induces mucus erosion and increases gut permeability, leading to bacterial translocation. The transport of bacteria, related antigens, and FFAs to the liver through the portal vein generates lipotoxicity in the hepatocytes, with inflammation occurring because of PAMP recognition, and ultimately immune cell recruitment. All the mechanisms mentioned favor cell apoptosis and fibrosis, and hence MAFLD severity progression. Abbreviations: FFA, free fatty acids; NASH, nonalcoholic steatohepatitis; PAMPs, pathogen-associated molecular patterns.
Lean non-alcoholic fatty liver disease continues to be an outstanding and interesting topic to discuss. In a study performed by Chen et al.,64 lean healthy subjects were found to have different microbiota and BA composition compared to lean non-alcoholic fatty liver disease patients, which have increased BA levels and bacteria species involved with BA metabolism, such as those in the Clostridium genus, the Ruminococcaceae family, and the Dorea genus, and a reduction of protective bacteria, like Marvinbryantia and Christensellenaceae R7 group. The authors discussed that lean non-alcoholic fatty liver disease patients have a better metabolic profile, with less insulin resistance and dyslipidemia compared to non-lean MAFLD patients, suggesting a different pathophysiology. In another study, Eslam et al.9 also demonstrated that non-obese non-alcoholic fatty liver disease patients have an increase in BA levels and FXR that confers a metabolic adaptation; nevertheless, the progression of non-alcoholic fatty liver disease in lean patients continues to be a matter of debate.9
Alcohol consumption
Mice models have demonstrated that 2 weeks of alcohol consumption is enough to induce an evident increment in gut permeability, endotoxemia and hepatic injuries. Ethanol, similar to what happens with an inadequate hypercaloric diet, has detrimental effects on the gut epithelial integrity by altering the stability of TJs. It also has been observed that it induces mucus erosion and ulcerations, mainly by modifying glycosylation of the mucus. In addition, alcohol may also induce changes in the microbiota composition (dysbiosis), an increased predominance of Proteobacteria, and a decrease in Firmicute species like Faecalibacterium prausnitzii which are important for the reinforcement of intercellular gut connections through the production of butyric acid. Other bacterial products, like SCFAs, can be altered after alcohol consumption, leading to the further destabilization of the intestinal barrier integrity. Furthermore, ethanol also has an influence on BAs, as it reduces the expression of FXR and stimulates CYP7A1 hepatic activity, leading to a higher BA pool; this prevents the growth of beneficial bacterial species and promotes stellate cell activation.73
Treatment of MAFLD
Treatment of MAFLD with probiotics
Current interventions for MAFLD management are focused on drug administration in order to control lipid levels, diabetes and TNF-α production, while others try to encourage dietary and lifestyle modifications, despite poor patient compliance.74 However, in the past few years, as the knowledge about gut-liver relationship has grown, several efforts have been directed towards the development of new strategies using this information. Two approaches to modulate gut dysbiosis have been established: 1) the untargeted methods, that use diet, probiotics, and antibiotics; and 2) microbiota-targeted therapy, which specifically aims at certain bacteria and host metabolites. Throughout this section we will discuss the findings related to beneficial effects of probiotic administration on the onset/treatment of MAFLD.
Probiotics are defined by the World Health Organization as a “live microorganism that—when administered in adequate amounts—confers a health benefit on the host,” not to be confused with prebiotics, which are compounds in food that induce the growth or activity of microorganisms. Probiotics must be able to survive and transit the gut, as well as be able to grow and multiply in order to benefit the host.75 Several probiotics, like Streptococcus, Bifidobacterium and Lactobacillus have been commercialized as fermented dairy products due to beneficial effects on the survivability of the gut epithelium and the promotion of anti-inflammatory cytokines, as well as interaction with the immune system.74 The expected effects of these probiotics are reversion of adverse gut microbiota growth and its consequences related to the constant inflammation through the recognition of LPS, production of ethanol, alteration of the BA metabolism, SCFAs metabolism, cellular stress, and so forth; ultimately, the desired outcome is returning the microbiota to a healthy state.
Multiple animal-based studies have shown significant therapeutic effects in fatty liver mice models. Administration of probiotics could prevent the onset of liver steatosis and improve steatohepatitis and fibrosis. The mechanisms behind these protective effects are the reduction of hepatic lipid accumulation, less endotoxemia, oxidative stress and activation of anti-inflammatory pathways through the modulation of NF-κB and TNF production, as well as the regulation of collagen production.72,75,76 For example, a study conducted by Xin et al.75 showed prevention of the onset of hepatic steatosis and cellular apoptosis in mice fed with a high-fat diet through the administration of the probiotic Lactobacillus johnsonii BS15; the end result was an improvement in hepatic inflammation and oxidative stress. A more recent study by Liang et al.77 gave a compound of probiotics to a group of mice fed with a high-fat diet and also showed an improvement in gut dysbiosis and a reduction of the hepatic lipid deposition. VSL#3 is a multi-strain probiotic that contains eight different species (Lactobacillus plantarum, Lactobacillus delbrueckii, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis and Streptococcus thermophilus), and is the most studied therapy in animals and humans. In mouse-based studies, its effects have included the modulation of NF-κB and TNF, and antifibrotic effects through TGF-β modification.75,77
Despite these promising results, it is important to bear in mind that animal models have their limitations. The mice used have been germ-free and even though their intestinal microbiota resembles that of the human, they are not the same. Nonetheless, the findings point to the potential benefits of pharmacologic intervention with probiotics.76
In terms of clinical studies, few have been conducted to explore the role of probiotics as a treatment therapy for any of the MAFLD stages, mostly due to the novelty of these discoveries.78 Among the currently available studies, the results have been measured by biochemical parameters or through hepatic histology.
Human studies have shown, through double-blind trials, that the administration of some Lactobacillus species, like rhamnosus and acidophilus, in 20 obese children and 30 adults with diagnosed MAFLD, respectively, influence the reduction of hypertransaminasemia.78–83 Other studies, using administration of other species of Lactobacillus (bulgaricus, plantarum), also found improvement in aspartate aminotransferase, as well as the reduction of total cholesterol and low density lipoprotein cholesterol.84,85
Other randomized trials have identified better effects on the disease through the administration of combined probiotics. Administration to adolescents of a capsule containing Lactobacillus acidophilus, Bifidobacterium lactis, Bifidobacterium bifidum and Lactobacillus rhamnosus showed a reduction in alanine aminotransferase, lipid profile and hepatic fat content compared to a placebo group after 12 weeks.79 A meta-analysis conducted by Ma et al.85 highlighted the beneficial impacts of probiotic therapy with Lactobacillus, Bifidobacterium, and Streptococcus, by reducing hepatic fat content, cholesterol, and alanine aminotransferase levels. The widely studied multi-strain probiotic VSL#3 has also been demonstrated to protect the intestine in humans by enhancing the barrier integrity, dampening endotoxemia and reducing oxidative stress, thereby leading to an improvement in chronic liver diseases.66,86 A 24-week trial conducted by Bakhshimoghaddam et al.13,87 studied 102 MAFLD patients divided into the following three groups: one control, and two intervention groups with intake of either 300 g of symbiotic yogurt or conventional yogurt. The authors concluded, after ultrasonography, that the MAFLD scores in those that consumed the symbiotic had decreased aspartate aminotransferase, alanine aminotransferase, and steatosis compared to the other groups. Some other studies have found a decrease in fibrosis levels after treatment with probiotics, apart from the results already mentioned.88,89
Unfortunately, and despite their effectiveness in other stages of MAFLD, studies on the effect of probiotics on cirrhosis have been controversial. Few studies have analyzed the use of probiotics as therapy for HCC; nonetheless, the ones available have presented encouraging data through positive effects. It has been observed that they favor liver function recovery and reduce complications in patients who undergo hepatic resection.90 Drugs like norfloxacin and rifaximin, the latter being capable of inducing overgrowth of beneficial bacteria such as Bifidobacterium, Faecalibacterium, and Lactobacillus, have favored an increase in the survival of patients with cirrhosis and HCC, as well as prevented associated complications, like hepatic encephalopathy, portal hypertension, and spontaneous bacterial peritonitis.63
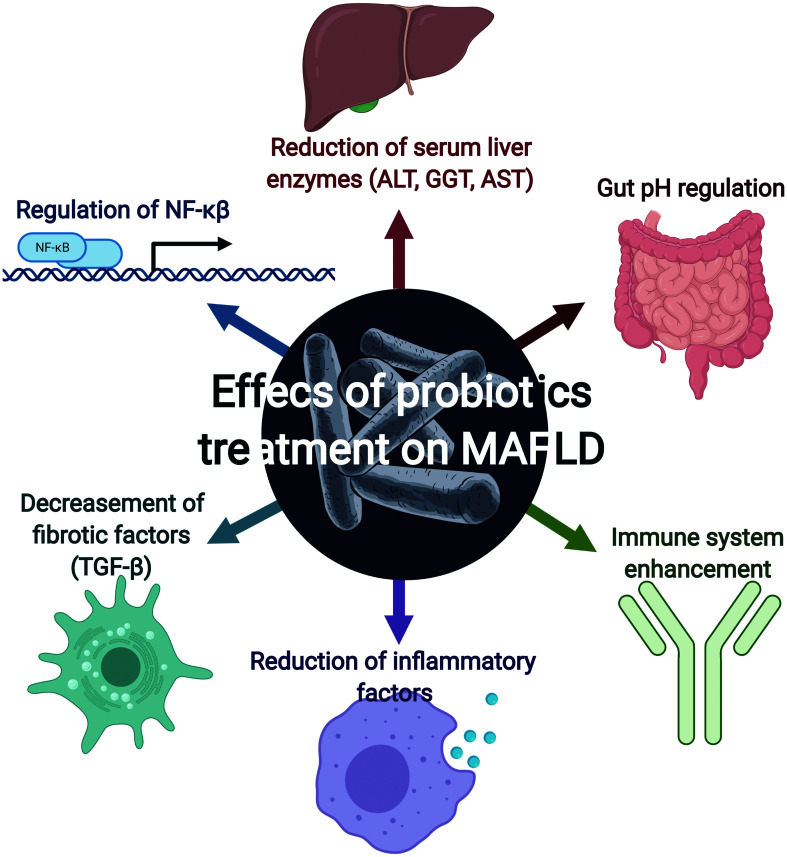
A meta-analysis conducted by Pan et al.60 compared the mechanisms of action of a wide variety of probiotics used in MAFLD treatment and found that the most predominant was the reduction of inflammatory factors (C-reactive protein and TNF-α). Other less determinant findings were the regulation of NF-κB and a reduction of serum liver enzymes (alanine aminotransferase, gamma-glutamyltranspeptidase, and aspartate aminotransferase) and fibrotic factors (TGF-β).
Probiotics may also have antagonistic actions against specific microorganisms, reducing the number and effects, while others ensure the intestine has an adequate pH by releasing products like butyric acid, lactic acid, and propionic acid. They can also enhance immunity by activating macrophages, antibody effectiveness and even competitively against other pathogens for nutrients and growth factors (Fig. 2).91
Fig. 2. Effects of probiotic treatment on MAFLD.
Several trials have demonstrated that probiotic administration has beneficial effects on MAFLD patients; the most relevant are regulation of NF-κB, gut pH regulation, decrease of fibrotic factors (such as TGF-β), reduction of serum liver enzymes, and enhancement of the immune system.
As a side note, we consider it important to mention that probiotics have a wide variety of beneficial effects apart from direct gut microbiota regulation and reduction of carcinogenesis; for example, benefits have been found on mental health, mainly related with the regulation of depression through the increase of serotonin production (Table 2).91
Table 2. Main results and discoveries of MAFLD treatment with probiotics.
| Results and discoveries | |
|---|---|
| Animal trials (mice) | Reduction of hepatic lipid accumulation, less endotoxemia, oxidative stress and activation of anti-inflammatory pathways Modulation of NF-κB, TNF and fibrotic factors |
| Hepatic steatosis (single strain probiotics) | Reduction of hypertransaminasemia Reduction of aspartate aminotransferase, low density lipoprotein, and total cholesterol levels |
| Hepatic steatosis (multi-strain probiotics) | Reduction of alanine aminotransferase and aspartate aminotransferase levels Less hepatic fat content Reduction of cholesterol levels Enhancement of the gut barrier |
| Hepatocellular carcinoma | Increased liver function recovery and reduced complications after hepatic resection |
Treatment of MAFLD with prebiotics
As already defined, prebiotics are substrates that are metabolized by the microbiota and promote the growth of beneficial bacteria. Oligofructose is a mixture of indigestible fermentable dietary fiber, which has been demonstrated to reduce liver oxidative stress and inflammation as well as to improve the intestinal barrier integrity.66 Lactulose, another prebiotic, has shown great ability to promote the growth of Bifidobacteria and Lactobacillus, as well as to exert protective effects against endotoxemia by reducing Gram-negative bacteria, thereby reducing the circulating LPS levels, inflammation and liver damage.
Several other beneficial metabolic effects have been attributed to prebiotics; for example, they can reduce de novo lipogenesis, improve blood glucose control and control weight gain. Although prebiotic administration had been demonstrated as an effective therapy for restoring the normal gut microbiota, it may need to be provided in combination with other interventions in order to fully improve MAFLD.92
Treatment of MAFLD diet and exercise therapies
As discussed earlier, the onset and severity of MAFLD, obesity, insulin resistance and other chronic metabolic diseases are closely correlated with the lifestyle of the afflicted individual. The following paragraphs provide a summary of the related evidence and proposed therapies for the two pivotal elements of a lifestyle-focused treatment: diet and exercise.
Both clinical and basic research have produced robust evidence that physical exercise has a beneficial effect on MAFLD, by reducing hepatic fat content through the activation of various metabolic pathways that improve the systemic sensibility to insulin and degradation of fatty acids and glucose. Ultimately, these processes prevent excessive fatty acid influx to the liver and mitochondrial and hepatocellular damage from cellular stress. In terms of treatment regimen, many have shown effects on liver fat content, but there is no evidence as to prioritizing one over the others; rather, the selection of a training method should be based on the preferences, capability, and likelihood of continuation of each individual patient. Two regimens worth mentioning are aerobic exercise which, even if done at low intensity and volume, has a beneficial effect on the reduction of hepatic fat content, and resistance training, which could be an alternative that provides the same improvements and results for patients who are unable to follow the aerobic regimen. It is worth mentioning that reduction of the hepatic fat content can be achieved even without an overall weight loss.93
On the other hand are the dietary interventions. Even though they are very controversial in terms of the determination of the most optimal regimen, they remain as a key factor in the evolution and improvement of MAFLD. In general, the recent literature reports that diets based on antioxidant intake and reduction of fatty processed foods have a better impact on metabolic health. A famous model that follows these recommendations is the Mediterranean diet, characterized by the consumption of plant-based foods and fish, and reduced meat and dairy products.94 Future dietary approaches might include a fasting regimen (every other day fasting regimen) as experiments in mouse models have demonstrated that it selectively stimulates beige fat development within white adipose tissue, through modification of the gut microbiota composition, which drastically ameliorates obesity, insulin resistance and hepatic steatosis. Although the underlying mechanisms are poorly understood, the participation of microbial fermentation products, such as lactate and acetate, and the upregulation of the monocarboxylate transporter 1 expression in beige cells are some of the main proposed protagonists.95
Vitamin supplementation approaches have also been suggested as treatment for MAFLD, specifically the administration of vitamin D. This is not only because vitamin D, in particular, is a molecule with notorious anti-fibrotic, anti-inflammatory, and insulin-sensitizing properties, but also because epidemiological research has found a relationship between hypovitaminosis D and the progress of liver fibrosis. Even though several pathophysiological pathways link MAFLD with vitamin D, the results from trials are still controversial and require further work; so far, available evidence supports that certain populations of MAFLD patients may benefit from vitamin D supplementation, such as those with shorter disease duration and mild to moderate liver damage.96
Treatment of MAFLD with fecal microbial transplantation
This technique involves transferring functional microbiomes from the feces of healthy individuals to the gastrointestinal tract of patients with MAFLD. Studies in mouse models treated with fecal transplant from lean or obese individuals have shown the consequence of induction of a microbiota signature similar to that of the donor; thus, obese mice that received microbiota of lean donors responded with a significant reduction in the adiposity and an increased insulin sensitivity, and vice versa, with lean mice that received microbiota from obese donors. Other studies applying 6-week to 8-week fecal transplant therapies to high-fat diet-induced non-alcoholic steatohepatitis mouse models, conducted to corroborate the effects, also found that the intervention increases the abundance of beneficial bacteria, alleviates endotoxemia, and reduces the severity of hepatic damage.97 This therapy is not only viable for MAFLD patients, but also for other metabolic diseases, as studies have demonstrated its therapeutic effects on T2DM, ulcerative colitis and metabolic syndrome, associated with healthy microbiota, improved insulin sensibility and normalized blood lipid levels.95 Despite these promising findings, further clinical trials in humans are required to fully confirm the benefits of this procedure, as there are still many unanswered questions, like what is the best way to implant the fecal matter, what is the risk of infection, and what are the long-term therapeutic effects.97
Conclusions
The tendency towards a sedentary lifestyle is turning out to be severely detrimental to the population’s metabolic state; the growing burden of chronic diseases is not the only consequence, as it also affects the quality of life of millions of people and adds economic burdens worldwide. The gut microbiome is an important determinant of health state and tendency towards disease, and even though it is unique in each person, recent studies have found certain patterns that tend to be constant throughout life, as is seen in healthy people with a predominance of the Firmicutes and Bacteroidetes phyla.
Alterations of the normal composition of the microbiome (gut dysbiosis) should be treated as a priority research topic, due to their close relationship with the onset or severity of other pathologies, such as T2DM, obesity and MAFLD, through mechanisms that provoke systemic inflammation, metabolism alteration, infiltration of lipids to non-adipose tissue, and promotion of fibrosis, among others. Apart from drug administration, probiotic supplementation may be a safe and low-cost approach to improve the disease state in patients, especially with multiple-strain probiotics, which have shown ability to reduce inflammatory factors (C-reactive protein and TNF-α), regulation of NF-κB, a decrease of serum liver enzymes (alanine aminotransferase, gamma-glutamyltranspeptidase, and aspartate aminotransferase) and fibrotic factors (TGF-β).
It is important that countries implement measures to control this pandemic of metabolic diseases. In general, people must have a clear understanding of the consequences they have on health and know that specific lifestyle changes can make them exponentially healthier.
Future directions
There is still much research left to be done before we fully understand the interactions between the host and the gut microbiota, the mechanisms of action of some specific bacteria strains and, ultimately, effects on health and disease. For example, the identification of ethanol-producing bacteria responsible for the increase of this endogenous metabolite would be a great step in the right direction. Another area of opportunity is to widen the knowledge about the effects of SCFAs in the pathogenesis of MAFLD. Trials that study the role of gut dysbiosis on diabetes represent a field that remains largely unexplored.
We consider it is also important to carry out epidemiological studies that analyze the prevalence of gut dysbiosis and its correlated chronic metabolic diseases. The information obtained is expected to help in decision-making and the implementation of public health measures.
Abbreviations
- APC
antigen presenting cells
- BA
bile acids
- MAFLD
metabolic associated fatty liver disease
- T2DM
type 2 diabetes mellitus
- GWAS
genome wide association studies
- TJPs
tight junction proteins
- AJPs
adherent junction proteins
- DC
dendritic cells
- PBA
primary bile acids
- SBA
secondary bile acids
- FXR
farnesoid X receptor
- TGR5
takeda G protein coupled receptor
- FMT
fecal microbiota transfer
- SCFAs
short chan fatty acids
- IR
insulin resistance
- HCC
hepatocellular carcinoma
- SIBO
small intestine bacterial overgrowth
- LPS
lipopolysaccharide
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 3.Valencia-Rodríguez A, Vera-Barajas A, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Looking into a new era for the approach of metabolic (dysfunction) associated fatty liver disease. Ann Hepatol. 2020;19:227–229. doi: 10.1016/j.aohep.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40:1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 6.López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, et al. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166–178. doi: 10.1016/S1665-2681(19)30879-8. [DOI] [PubMed] [Google Scholar]
- 7.Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, et al. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73–79. doi: 10.1016/S2468-1253(20)30294-6. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Fan JG, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol Hepatol. 2020;5:713–715. doi: 10.1016/S2468-1253(20)30090-X. [DOI] [PubMed] [Google Scholar]
- 10.Méndez-Sánchez N, Zamarripa-Dorsey F, Panduro A, Purón-González E, Coronado-Alejandro EU, Cortez-Hernández CA, et al. Current trends of liver cirrhosis in Mexico: Similitudes and differences with other world regions. World J Clin Cases. 2018;6:922–930. doi: 10.12998/wjcc.v6.i15.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Méndez-Sánchez N, Valencia-Rodríguez A. Caveats for the implementation of global strategies against non-alcoholic fatty liver disease. J Hepatol. 2020;73:220. doi: 10.1016/j.jhep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond) 2018;18:245–250. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6:65–72. doi: 10.1016/S2468-1253(20)30340-X. [DOI] [PubMed] [Google Scholar]
- 16.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap—bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 18.Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645–655. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 19.Anstee QM, Day CP. The genetics of nonalcoholic fatty liver disease: Spotlight on PNPLA3 and TM6SF2. Semin Liver Dis. 2015;35:270–290. doi: 10.1055/s-0035-1562947. [DOI] [PubMed] [Google Scholar]
- 20.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Qu HQ, Rentfro AR, Grove ML, Mirza S, Lu Y, et al. PNPLA3 polymorphisms and liver aminotransferase levels in a Mexican American population. Clin Invest Med. 2012;35:E237–E245. doi: 10.25011/cim.v35i4.17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LZ, Xin YN, Geng N, Jiang M, Zhang DD, Xuan SY. PNPLA3 I148M variant in nonalcoholic fatty liver disease: demographic and ethnic characteristics and the role of the variant in nonalcoholic fatty liver fibrosis. World J Gastroenterol. 2015;21:794–802. doi: 10.3748/wjg.v21.i3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinchilla-López P, Ramírez-Pérez O, Cruz-Ramón V, Canizales-Quinteros S, Domínguez-López A, Ponciano-Rodríguez G, et al. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann Hepatol. 2018;17:250–255. doi: 10.5604/01.3001.0010.8644. [DOI] [PubMed] [Google Scholar]
- 24.Pontoriero AC, Trinks J, Hulaniuk ML, Caputo M, Fortuny L, Pratx LB, et al. Influence of ethnicity on the distribution of genetic polymorphisms associated with risk of chronic liver disease in South American populations. BMC Genet. 2015;16:93. doi: 10.1186/s12863-015-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basyte-Bacevice V, Skieceviciene J, Valantiene I, Sumskiene J, Petrenkiene V, Kondrackiene J, et al. TM6SF2 and MBOAT7 gene variants in liver fibrosis and cirrhosis. Int J Mol Sci. 2019;20:1277. doi: 10.3390/ijms20061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Belyaeva OV, Brown PM, Fujita K, Valles K, Karki S, et al. 17-beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas H. An HSD17B13 variant reduces cirrhosis risk. Nat Rev Gastroenterol Hepatol. 2018;15:328. doi: 10.1038/s41575-018-0016-7. [DOI] [PubMed] [Google Scholar]
- 29.Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr. 2017;147:1468S–1475S. doi: 10.3945/jn.116.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho YE, Kim DK, Seo W, Gao B, Yoo SH, Song BJ. Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-mediated oxidative and nitrative stress. Hepatology. 2019 doi: 10.1002/hep.30652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, et al. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem. 2015;26:1183–1192. doi: 10.1016/j.jnutbio.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 37.De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, et al. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40:2906–2916. doi: 10.1111/liv.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 39.Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, et al. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814–4834. doi: 10.3748/wjg.v25.i33.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald BD, Jabri B, Bendelac A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2018;18:514–525. doi: 10.1038/s41577-018-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 42.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 43.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 44.Talavera-Urquijo E, Beisani M, Balibrea JM, Alverdy JC. Is bariatric surgery resolving NAFLD via microbiota-mediated bile acid ratio reversal? A comprehensive review. Surg Obes Relat Dis. 2020;16:1361–1369. doi: 10.1016/j.soard.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Chen L, Wang H, Cai W, Xie Q. Modulation of bile acid profile by gut microbiota in chronic hepatitis B. J Cell Mol Med. 2020;24:2573–2581. doi: 10.1111/jcmm.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2017;16:s15–s20. doi: 10.5604/01.3001.0010.5494. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Palmiotti A, Kuipers F. Emerging roles of bile acids in control of intestinal functions. Curr Opin Clin Nutr Metab Care. 2021;24:127–133. doi: 10.1097/MCO.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 48.Cruz-Ramón V, Chinchilla-López P, Ramírez-Pérez O, Méndez-Sánchez N. Bile acids in nonalcoholic fatty liver disease: New concepts and therapeutic advances. Ann Hepatol. 2017;16(Suppl 1):S58–S67. doi: 10.5604/01.3001.0010.5498. [DOI] [PubMed] [Google Scholar]
- 49.Méndez-Sánchez N. Bile acids in health and disease foreword. Ann Hepatol. 2017;16(Suppl 1):S3. doi: 10.5604/01.3001.0010.5492. [DOI] [PubMed] [Google Scholar]
- 50.Lau E, Carvalho D, Freitas P. Gut microbiota: Association with NAFLD and metabolic disturbances. Biomed Res Int. 2015;2015:979515. doi: 10.1155/2015/979515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cresci GA, Bawden E. Gut microbiome: What we do and don’t know. Nutr Clin Pract. 2015;30:734–746. doi: 10.1177/0884533615609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muñoz-Garach A, Diaz-Perdigones C, Tinahones FJ. Gut microbiota and type 2 diabetes mellitus. Endocrinol Nutr. 2016;63:560–568. doi: 10.1016/j.endonu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woldeamlak B, Yirdaw K, Biadgo B. Role of gut microbiota in type 2 diabetes mellitus and its complications: Novel insights and potential intervention strategies. Korean J Gastroenterol. 2019;74:314–320. doi: 10.4166/kjg.2019.74.6.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61:69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 56.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 58.Boursier J, Diehl AM. Nonalcoholic fatty liver disease and the gut microbiome. Clin Liver Dis. 2016;20:263–275. doi: 10.1016/j.cld.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Gómez-Zorita S, Aguirre L, Milton-Laskibar I, Fernández-Quintela A, Trepiana J, Kajarabille N, et al. Relationship between changes in microbiota and liver steatosis induced by high-fat feeding-A review of rodent models. Nutrients. 2019;11:2156. doi: 10.3390/nu11092156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan X, Wen SW, Kaminga AC, Liu A. Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sci Rep. 2020;10:8848. doi: 10.1038/s41598-020-65051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campo L, Eiseler S, Apfel T, Pyrsopoulos N. Fatty liver disease and gut microbiota: A comprehensive update. J Clin Transl Hepatol. 2019;7:56–60. doi: 10.14218/JCTH.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abenavoli L, Luzza F, Mendez-Sanchez N. Probiotics supplementation in the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8:632–634. doi: 10.21037/hbsn.2019.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Méndez-Sánchez N, Valencia-Rodriguez A, Vera-Barajas A, Abenavoli L, Scarpellini E, Ponciano-Rodriguez G, et al. The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer. Hepatoma Res. 2020;6:5. doi: 10.20517/2394-5079.2019.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: A distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71:1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 65.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 66.Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci. 2016;73:737–755. doi: 10.1007/s00018-015-2083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: Moving beyond associations. Cell Host Microbe. 2017;22:589–599. doi: 10.1016/j.chom.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Kim KN, Yao Y, Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients. 2019;11:2512. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luca M, Di Mauro M, Di Mauro M, Luca A. Gut microbiota in alzheimer’s disease, depression, and type 2 diabetes mellitus: The role of oxidative stress. Oxid Med Cell Longev. 2019;2019:4730539. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B, Cordova-Gallardo J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int J Mol Sci. 2018;19:2034. doi: 10.3390/ijms19072034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang X, Zheng J, Zhang S, Wang B, Wu C, Guo X. Advances in the Involvement of Gut Microbiota in Pathophysiology of NAFLD. Front Med (Lausanne) 2020;7:361. doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meroni M, Longo M, Dongiovanni P. Alcohol or gut microbiota: Who is the guilty? Int J Mol Sci. 2019;20:4568. doi: 10.3390/ijms20184568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 75.Vandenplas Y, Huys G, Daube G. Probiotics: an update. J Pediatr (Rio J) 2015;91:6–21. doi: 10.1016/j.jped.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: A new insight into therapeutic strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang Y, Liang S, Zhang Y, Deng Y, He Y, Chen Y, et al. Oral administration of compound probiotics ameliorates HFD-induced gut microbe dysbiosis and chronic metabolic inflammation via the G protein-coupled receptor 43 in non-alcoholic fatty liver disease rats. Probiotics Antimicrob Proteins. 2019;11:175–185. doi: 10.1007/s12602-017-9378-3. [DOI] [PubMed] [Google Scholar]
- 78.Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98:6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 79.Xie C, Halegoua-DeMarzio D. Role of probiotics in non-alcoholic fatty liver disease: Does gut microbiota matter? Nutrients. 2019;11:2837. doi: 10.3390/nu11112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, et al. The therapeutic implications of the gut microbiome and probiotics in patients with NAFLD. Diseases. 2019;7:27. doi: 10.3390/diseases7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdel Monem SM. Probiotic therapy in patients with nonalcoholic steatohepatitis in Zagazig University hospitals. Euroasian J Hepatogastroenterol. 2017;7:101–106. doi: 10.5005/jp-journals-10018-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 84.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–262. doi: 10.1016/S1665-2681(19)31364-X. [DOI] [PubMed] [Google Scholar]
- 85.Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 86.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. J Nutr. 2018;148:1276–1284. doi: 10.1093/jn/nxy088. [DOI] [PubMed] [Google Scholar]
- 88.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 89.Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662–668. doi: 10.1017/S0007114517000204. [DOI] [PubMed] [Google Scholar]
- 90.Rifatbegovic Z, Mesic D, Ljuca F, Zildzic M, Avdagic M, Grbic K, et al. Effect of probiotics on liver function after surgery resection for malignancy in the liver cirrhotic. Med Arh. 2010;64:208–211. [PubMed] [Google Scholar]
- 91.Sharifi-Rad J, Rodrigues CF, Stojanović-Radić Z, Dimitrijević M, Aleksić A, Neffe-Skocińska K, et al. Probiotics: Versatile bioactive components in promoting human health. Medicina (Kaunas) 2020;56:433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen HT, Huang HL, Li YQ, Xu HM, Zhou YJ. Therapeutic advances in non-alcoholic fatty liver disease: A microbiota-centered view. World J Gastroenterol. 2020;26:1901–1911. doi: 10.3748/wjg.v26.i16.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18:89–101. doi: 10.3727/105221617X15124844266408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, et al. Diet and non-alcoholic fatty liver disease: The mediterranean way. Int J Environ Res Public Health. 2019;16:3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26:672–685.e4. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barchetta I, Cimini FA, Cavallo MG. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): An update. Nutrients. 2020;12:3302. doi: 10.3390/nu12113302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]