Abstract
Background and Aims
To compare the efficacy and safety of physical thermal ablation (PTA), including radiofrequency ablation (RFA) and microwave ablation (MWA), combined with sorafenib and physical thermal ablation alone for the control and treatment of hepatocellular carcinoma (HCC) according to the available literature.
Methods
Comprehensive searches were performed on PubMed, Embase, CNKI, the Cochrane Library, China Biomedical Literature Database (known as CBM), Weipu Journal, and Wanfang Database. Meta-analysis was performed using Revman 5.3 software.
Results
A total of 15 studies, consisting of 2,227 HCC patients, were selected and included in this meta-analysis. Compared with the RFA-alone group, the patients in the RFA+sorafenib group had longer 1-, 2-, and 3-year overall survival (all p<0.05), better overall efficacy (p<0.0001), longer radiofrequency interval (p<0.001), and lower 2-year recurrence rate (p=0.02). The 1-year overall survival (p=0.003) and overall efficacy (p=0.002) of the MWA+sorafenib group were also higher than those of the MWA-alone group. The incidences of adverse reactions in the RFA+sorafenib group, such as hand-foot skin reactions (p<0.001), diarrhea and constipation (p=0.0001), hypertension (p=0.009), and alopecia (p<0.001), were significantly higher than those in the RFA-alone group.
Conclusions
RFA or MWA combined with sorafenib has produced a better therapeutic effect on HCC than physical thermal ablation alone; however, adverse reactions have been obvious. It is necessary to evaluate the safety of combination therapy, and pay close attention to the adverse reactions that develop in patients.
Keywords: Physical thermal ablation, Radiofrequency ablation, Microwave ablation, Sorafenib, Hepatocellular carcinoma, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor in the world. About 700,000 people die of HCC worldwide each year, with nearly half of those cases being from China.1,2 Currently, the main treatments include liver transplantation, surgical resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), transarterial chemoembolization (TACE), and sorafenib.3 Following development of medical technology and establishment of different prognosis scoring systems, like the Italian Liver Cancer tumor staging system and the Barcelona clinical liver cancer staging system, there are more therapy options for HCC patients.4
Surgical resection is considered to be the first-line treatment for HCC, but surgery is not always feasible due to factors such as multiple lesions, poor position, and patient status.5 The early symptoms of liver cancer are not obvious, resulting in many patients having advanced liver cancer when they are diagnosed and missing the optimal window for surgery. The scarcity of liver sources and high costs also limit the widespread application of liver transplantation. Therefore, an effective and less invasive alternative therapy, physical thermal ablation (PTA), has been developed. PTA of the liver includes RFA and microwave ablation (MWA). Although the physical mechanisms of the two are different, they both target the tumor through imaging technology and insert the electrode into the tumor precisely. When the temperature of the tumor tissue reaches a certain level, the protein will be denatured to shrink the tumor.
A meta-analysis on the effects of RFA and hepatic resection in the treatment of liver cancer conducted by Xu et al.6 showed that, compared with the hepatic resection group, the RFA group had similar 1-year overall survival (OS), lower 5-year OS, higher incidence of overall recurrence, shorter hospitalization duration and lower complication rate. Which means, compared with surgery, thermal ablation has the advantages of short duration and less complications. However, HCC patients treated with thermal ablation alone have a high recurrence rate and an unsatisfactory long-term prognosis.7
Sorafenib is a multi-targeted kinase inhibitor that inhibits the proliferation and differentiation of tumor cells by inhibiting the activity of B-Raf, Raf-1 and kinases in the Ras/Raf/MEK/ERK signaling pathway;8 it can also reduce angiogenesis by inhibiting hepatocyte cytokine receptor (such as c-Kit), vascular endothelial growth factor receptors (such as the vascular endothelial growth factor receptors VEGFR-2, VEGFR-3), platelet-derived growth factor receptors (such as the platelet-derived growth factor receptor PDGFR-β), etc.9 A meta-analysis of 1,462 patients with unresectable HCC showed that compared with placebo, sorafenib improved disease control rate and reduced the risk of tumor progression and mortality.10 A number of studies also pointed out that sorafenib alone or in combination with other therapies can prolong the survival of HCC patients.11–13 However, sorafenib might delay the tissue repair after thermal ablation and adversely affect normal liver tissue. Therefore, the overall advantage of sorafenib in combination with PTA needs to be balanced, after considering its clinical efficacy effects and adverse effects.
Meta-analysis can provide a higher level of evidence for clinical decision-making by combining disaggregated data.14 This study summarized the literature on the efficacy of PTA combined with sorafenib in the treatment of HCC, to explore the safety and efficacy of this combination therapy objectively.
Methods
Search strategy
A comprehensive literature search was conducted by two searchers on the PubMed, Embase, CNKI, Cochrane Library, China Biomedical Literature (known as CBM), Weipu Journal, and Wanfang databases on October 25–26, 2020 to identify articles published before September 2020. We collected randomized controlled trials (RCTs), controlled clinical trials, and cohort studies comparing RFA or MWA with sorafenib and PTA alone in the treatment of HCC, and reviewed the references to supplement with any missing studies. The search strategy was on the basis of the following terms: (physical thermal ablation) OR ((radiofrequency ablation OR (RFA) OR (RF ablation)) OR ((microwave ablation) OR (MWA) OR (MW ablation)) AND (sorafenib) AND ((Carcinoma, Hepatocellular) OR (HCC) OR (liver cancer) OR (liver tumor)).
Eligibility criteria
Inclusion criteria were: (1) English or Chinese language; (2) RCTs or high-quality cohort studies, quality score Jadad ≥3, Newcastle-Ottawa scale ≥5; (3) observation group treated with RFA/MWA combined with sorafenib, and control group treated with RFA/MWA alone; (4) participant Child-Pugh A/B; and (5) with data for at least one efficacy indicator (recurrence rate, survival rate, complications, radio frequency interval, etc.). Exclusion criteria were: (1) systematic review, meta-analysis, animal experiments, case reports, comments or letters; or (2) lack of required data in the results.
Quality evaluation and data extraction
Two researchers respectively scored the RCTs and non-RCTs according to the Jadad scale and the Newcastle-Ottowa scale, and independently extracted the original data according to the PICO principle (patient, intervention, comparison, and outcome), including basic information, safety indicators and effectiveness indicators.
The basic information included the first author, publication time, nationality of the patients, patient number of each group, sex ratio, age, type of study, and Child-Pugh classification. The safety indicators are the incidence of major adverse reactions, which included hand-foot skin reaction (referred to as HFSR), diarrhea and constipation, hypertension, alopecia, pyrexia, and fatigue. The effectiveness evaluation indicators included OS, recurrence rate, and overall efficacy. According to the World Health Organization solid tumor efficacy criteria, the treatment effect can be divided into four levels, namely complete remission, partial remission, the progression of the disease, and stable disease. The overall efficacy was defined as (complete remission+partial remission)/total number×100%. Different opinions on a controversial issue were solved through consultation with the third investigator.
Statistical methods
Meta-analysis and sensitivity analysis were performed using Revman 5.3 software. The categorical variables were described by odds ratio (OR) and the corresponding 95% confidence interval (CI). The continuous variables were described by mean difference and the corresponding 95% CI. The χ2 test was used to assess heterogeneity. A fixed-effects model was applied when there was no or low heterogeneity (I2<50%, p>0.1) and a random-effects model was applied when there was moderate or high heterogeneity (I2≥50%, p≤0.1). The publication bias was evaluated by funnel plot analysis and Egger’s test, using Stata software. A p-value of <0.05 (two-tailed) was considered statistically significant.
Results
Search results and basic information of the original literature
The process of literature screening is shown in Fig. 1. According to the criteria, this meta-analysis finally included 15 studies (3 RCTs, 5 controlled clinical trials, and 7 retrospective cohort studies).15–29 Among these, 14 studies were high-quality and one was medium quality. A total of 2,227 patients were enrolled, of whom 1,100 were treated with PTA plus sorafenib and 1,127 were treated with PTA alone. The basic information of the studies is summarized in Table 1.
Fig. 1. Inclusion procession.
Table 1. Basic characteristics of the studies.
| Study | Nation | Type | No. of patients | Age in yearsa | Gender, male/female | Child-Pugh A, n | Quality scoreb | |
|---|---|---|---|---|---|---|---|---|
| Bruix 201515 | Spain, China, Japan | RCT | RFA+so | 556 | 58 (24–85) | 451/105 | 541 | 5 |
| RFA | 558 | 60 (19–83) | 461/97 | 538 | ||||
| Yu 201816 | China | RCT | RFA+so | 23 | 58.19±4.34 | 17/6 | 13 | 3 |
| RFA | 23 | 58.25±4.31 | 16/7 | 14 | ||||
| Fu 202017 | China | RCT | RFA+so | 51 | 57.4±3.8 | 34/17 | 32 | 4 |
| RFA | 51 | 57.6±3.9 | 35/16 | 30 | ||||
| Kan 201518 | China | CCT | RFA+so | 30 | 53.7±9.6 | 24/6 | 12 | 6 |
| RFA | 32 | 52.4±8.9 | 25/7 | 18 | ||||
| Zhang 201519 | China | CCT | RFA+so | 52 | 28–65 (51.2±13.4) | 28/24 | 22 | 6 |
| RFA | 68 | 31/37 | 43 | |||||
| Wu 201620 | China | CCT | RFA+so | 45 | 48±11 | 28/17 | – | 6 |
| RFA | 45 | 50±9 | 30/15 | |||||
| Gong 201721 | China | CCT | RFA+so | 40 | 55.7±13.6 | 23/17 | – | 7 |
| RFA | 50 | 53.9±12.4 | 28/22 | |||||
| Sun 201122 | China | cohort | RFA+so | 15 | 59.5 (35–80) | 11/4 | 7 | 6 |
| RFA | 15 | 12/3 | 9 | |||||
| Feng 201423 | China | cohort | RFA+so | 64 | 49.7±11.2 | 59/5 | 64 | 6 |
| RFA | 64 | 50.9±10.9 | 59/5 | 64 | ||||
| Fukuda 201424 | Japan | cohort | RFA+so | 15 | 72.8±7.9 | 6/9 | 15 | 7 |
| RFA | 30 | 72.1±8.0 | 8/22 | 25 | ||||
| Li 201425 | China | cohort | RFA+so | 8 | 53±6.8 | 5/3 | – | 5 |
| RFA | 12 | 48±11.1 | 8/4 | |||||
| Zhu 201826 | China | cohort | RFA+so | 40 | 55.5±10.9 | 3/37 | 33 | 6 |
| RFA | 66 | 54.1±10.1 | 5/61 | 48 | ||||
| Hua 201227 | China | cohort | MWA+so | 42 | 57.2 (38–74) | 28/14 | 32 | 5 |
| MWA | 48 | 54.7 (39–72) | 32/16 | 37 | ||||
| Zheng 201328 | China | CCT | MWA+so | 44 | 56.2 (38–74) | 30/14 | 34 | 5 |
| MWA | 50 | 55.7 (39–72) | 33/17 | 38 | ||||
| Sun 201829 | China | cohort | MWA+so | 45 | 48.5±7.2 | 31/14 | 41 | 6 |
| MWA | 45 | 47.6±7.1 | 30/15 | 40 | ||||
Age recorded with mean±standard deviation or median (interquartile range).
Jadad score and Newcastle-Ottawa scale were used for RCTs and non-RCTs respectively.
Abbreviations: CCT, control clinical trial; MWA, microwave ablation; RCT, randomized clinical trial; RFA, radiofrequency ablation; so, sorafenib.
OS of HCC patients in the PTA+sorafenib group and the PTA-alone group
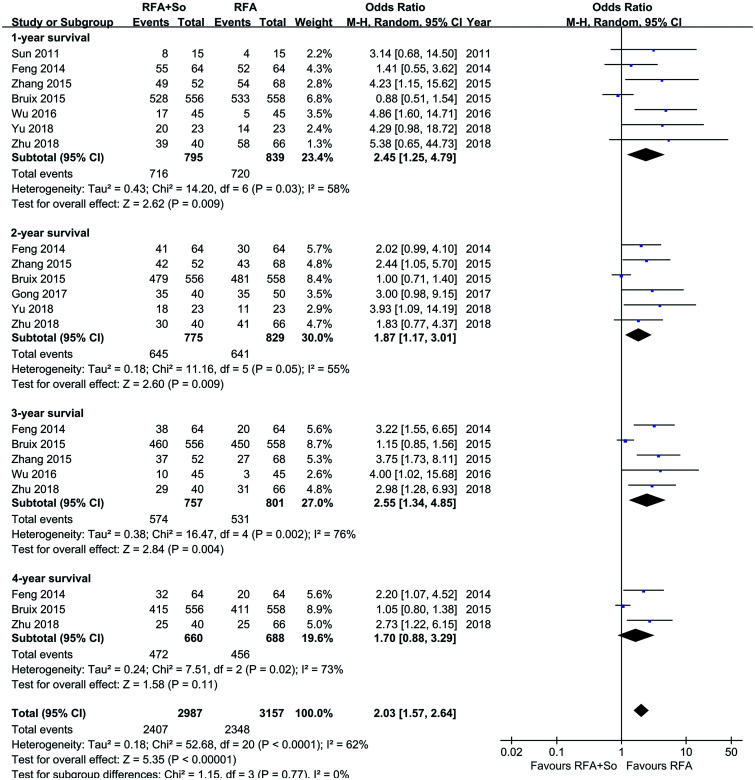
Seven studies, involving 1,634 individuals, reported the OS rate. The random-effects model was used because of the low grade of heterogeneity in the literature reporting OS rates at 1, 2, and 3 years OS rates (I2=58%, 55%, and 76%, respectively). Overall, the 1- , 2- and 3-year OS rates of HCC patients in the RFA+sorafenib group were significantly higher than those of the RFA-alone group (1-year OS: OR=2.45, 95% CI: 1.25–4.79, p=0.009; 2-year OS: OR=1.87, 95% CI: 1.17–3.01, p=0.009; 3-year OS: OR=2.25, 95% CI: 1.34–4.85, p=0.004) (see Fig. 2).
Fig. 2. OS in the RFA+sorafenib group and the RFA-alone group.
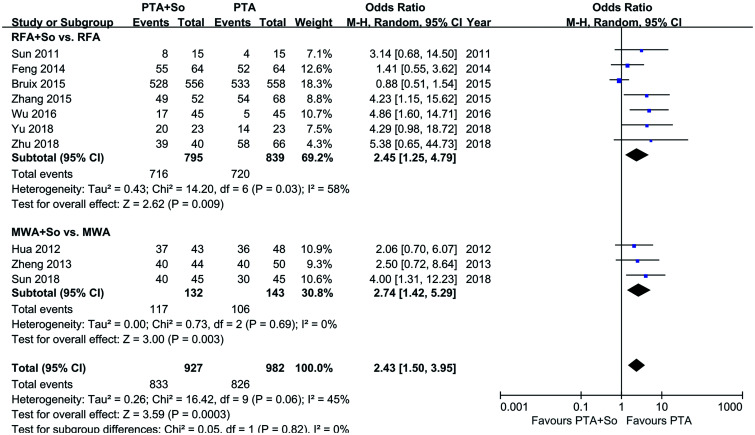
MWA is another major category of physical thermal ablation, and we performed a subgroup analysis to summarize the overall survival rates of the two ablation methods. The result showed that MWA combined with sorafenib also significantly increased HCC patients’ 1-year OS, with an OR of 2.74 (95% CI=1.42–5.29, p=0.009). Coupled with the results of RFA, it can be considered that HCC patients treated with PTA and sorafenib had a higher 1-year OS than those treated with PTA-alone (OR=2.43, 95% CI=1.50–3.95, p=0.003) (see Fig. 3).
Fig. 3. Subgroup analysis of 1-year OS in the RFA and MWA treatment groups.
Recurrence rates of HCC patients in the RFA+sorafenib group and RFA-alone group
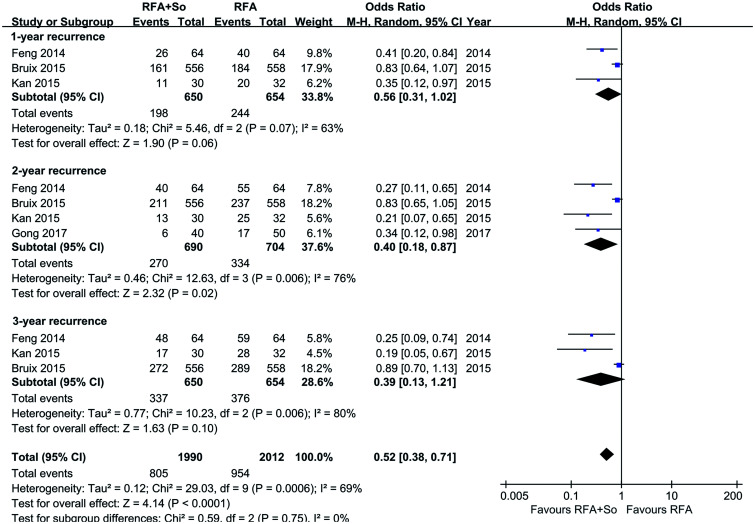
A total of four articles with 1,394 individuals provided information on recurrence rates. After merging them with a random-effects model, the OR of the 2-year recurrence rate was 0.40 (95% CI=0.18–0.87, p=0.02), indicating that the 2-year recurrence rate of HCC patients in the RFA+sorafenib group was lower than that of the RFA-alone group (see Fig. 4).
Fig. 4. Recurrence rates in the RFA+sorafenib group and the RFA-alone group.
Overall efficacy of physical thermal ablation of HCC patients
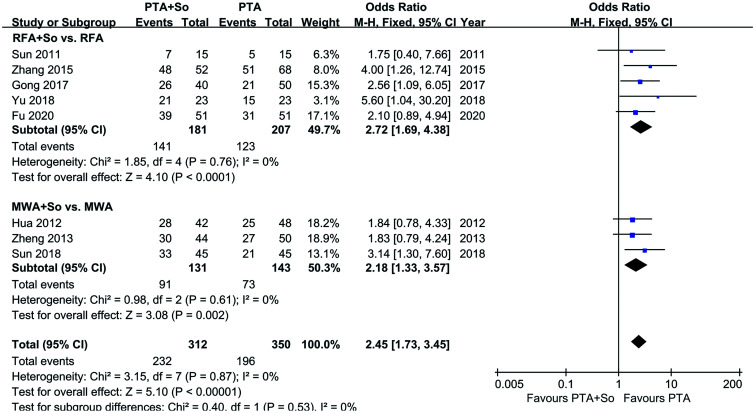
Eight of the studies, involving 562 individuals, mentioned overall efficacy and were divided into two subgroups, according to different thermal ablation methods, four of which used RFA and three of which used MWA. A fixed-effects model was applied, as the studies were homogeneous (I2=0%, p>0.10). Subgroup analysis showed that the overall efficacy of RFA combined with sorafenib for HCC patients was better than that of RFA alone (OR=2.72, 95% CI: 1.69–4.38, p<0.0001). The efficacy of MWA combined with sorafenib was also better than that of MWA alone (OR=2.18, 95% CI: 1.33–3.57, p=0.002). Overall, 312 patients were treated with PTA and sorafenib and 350 patients were treated with PTA alone; the total OR was 2.45 (95% CI=1.73–3.45, p<0.001), indicating that the overall efficacy of PTA combined with sorafenib was significantly better than that of PTA alone (see Fig. 5).
Fig. 5. Subgroup analysis of overall efficacy of RFA and MWA in HCC patients.
The radiofrequency interval of patients also indirectly reflects the effect of treatment. Three studies with 200 individuals documented the patient’s radiofrequency interval, and a fixed-effects model was used since the heterogeneity test yielded results of p=0.21 and I2=36%. The radiofrequency interval of HCC patients treated with RFA and sorafenib was longer than that of RFA alone (95% CI: 1.28–1.94, p <0.001), and the effect of combination therapy can be considered to be superior (see Supplementary Fig. 1.).
Adverse effects in the RFA+sorafenib group and the RFA-alone group
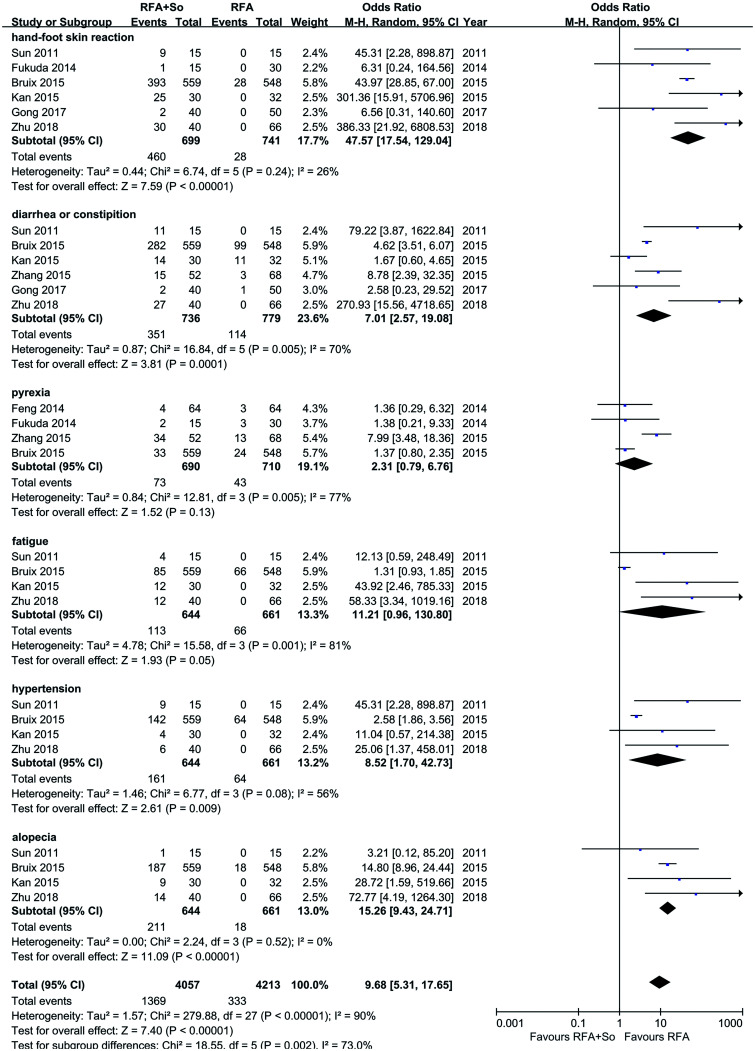
A total of nine studies, involving 1,561 individuals, reported adverse effects after treatment. The incidences of adverse reactions, such as HFSR (OR=47.57, 95% CI: 17.54–129.04, p<0.01), diarrhea and constipation (OR=7.01, 95% CI: 2.57–19.08, p=0.005), hypertension (OR=8.52, 95% CI: 1.70–42.73, p=0.009), and alopecia (OR=15.26, 95%CI: 9.43–24.71, p<0.01), in the combination therapy group were significantly higher than those in the PTA-alone group (see Fig. 6).
Fig. 6. Adverse effects of HCC patients in the RFA+sorafenib group and the RFA-alone group.
Sensitivity analysis and publication bias
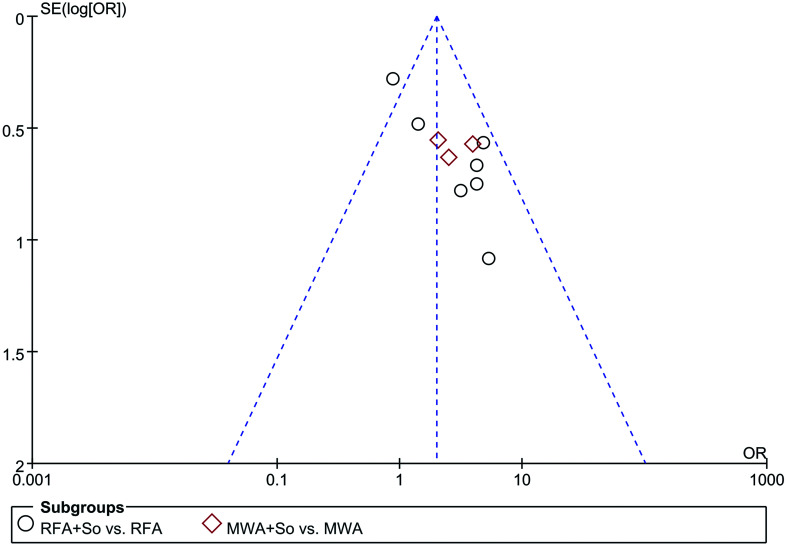
The sensitivity analysis showed that the study conducted by Bruix et al.15 significantly affected the calculated ORs of OS and recurrence rate. After excluding this trial, the I2 value declined to 0%. The funnel plot of the 1-year OS revealed asymmetry; however, after excluding the Bruix 201515 study, the Egger’s test results yielded p=0.107, indicating that there was no substantial publication bias (see Fig. 7). Further reading and evaluation found that this study was a high-quality RCT, recorded a number of indicators, provided results that were credible, and had application value. The reason why the results were different from others might be due to the variety of ethnicity (Spain, China, and Japan) and large sample size (n=1,114). In summary, we retained this high-quality study.
Fig. 7. Funnel plot of 1-year OS with 95% CI to assess publication bias.
Discussion
Ablation combined with chemotherapy has been widely used in cancer treatment, such as for small cell lung cancer, advanced renal cell carcinoma, etc.30,31 In the treatment of HCC, PTA has the advantages of little trauma and quick recovery, and can be applied as treatment of multiple times. However, the size of the lesion and the existence of heat dissipation make it difficult to ablate completely, resulting in a higher risk of local recurrence. When the diameter of the tumor is more than 3.0 cm, it is more likely to recur.32,33 Therefore, reducing the recurrence rate of tumors after thermal ablation has become the focus of treatment improvement.
RFA+sorafenib: Higher survival rate and efficiency, longer radiofrequency interval and lower recurrence rate
Sorafenib, a kinase inhibitor, has been shown to have a synergistic effect in combination with RFA. It has the function of inhibiting angiogenesis in tumors, thereby reducing heat loss and indirectly enhancing ablation. Sorafenib itself also inhibits tumor cell proliferation and differentiation. From the perspective of evidence-based medicine, in order to explore whether the therapeutic effect of RFA combined with sorafenib is better than using RFA alone, a total of 15 studies were included in the meta-analysis, 12 of which were about RFA and included 939 patients treated with RFA plus sorafenib and 1,014 patients treated with RFA alone. We summarized the original literature and found that the RFA+sorafenib group had higher 1-, 2-, and 3-year OS and lower 2-year recurrence rate compared with the RFA-alone group; RFA combined with sorafenib also significantly extended the RF interval, which indirectly reduced the RFA-related adverse effect, and also reduced the pain and financial burden of patients.
However, the survival and recurrence indicators of the RFA+sorafenib group were not always better than the RFA-alone group. The 4-year survival rate and the 1- and 3-year recurrence rates were not significantly different between the two groups. Probably due to (1) a large-sample-size study,15 there was no difference in the 1- and 3 recurrence rates between the two groups, since that study had a large weight in the meta-analysis, and (2) few studies reported the 4-year OS and, the 1- and 3-year recurrence rates and the heterogeneity was significant.
Sorafenib brings significant adverse reactions
Sorafenib is a tyrosine kinase inhibitor that inhibits various receptors, such as RAF-1, VEGFR-2, and FLT-3, and has been used for first-line treatment of liver cancer, with millions of patients benefiting from it.11 Our meta-analysis showed that combined use with sorafenib can significantly improve the effect of RFA, but the incidence of adverse reactions was significantly higher. Studies have suggested that the mechanism of HFSR may be that sorafenib can inhibit VEGF and PDGF, and damage the capillaries. When the hands and feet are subjected to direct pressure, the vessels are again mechanically damaged, thus prompting an inflammatory response and blister formation.34 As we know, severe adverse effects may lead to the suspension of treatment and ultimately affect the patient’s survival. There were also studies suggesting that diarrhea in HCC patients treated with sorafenib predicts better OS.35,36 Reig et al.37 believed that the development of dermatological adverse events within 60 days after the start of sorafenib was associated with better survival. Regardless of whether the adverse reaction can directly affect survival, it may affect the quality of life and cause a dose change or interruption of sorafenib, which may limit the anti-tumor effect. Therefore, standardized treatment and dose adjustment of sorafenib are necessary to improve the survival and life-quality of HCC patients.
RFA and MWA
Both RFA and MWA are PTA techniques. The mechanism of RFA is that the polar molecules in the tumor will run at high speed under the influence of high-voltage, generating heat to kill tumor cells. The MWA electrode emits microwaves, and the polarity of the water molecules in the tumor is changed by the voltage to form an alternating electric field to generate heat. MWA has higher thermal efficiency, faster heating speed, better heat dissipation resistance,38 the ablation range is larger, the operation time is also shorter, and the MWA consumables are relatively inexpensive, which can reduce the economic burden on patients. Compared with RFA, the development of MWA was relatively late, first put into clinical application in China and Japan. Therefore, there were few MWA studies and limited survival index in this meta-analysis.
From the subgroup analysis of the existing literature, the total effective rate and 1-year survival rate of the combination group were higher than in the control group. There have been studies comparing the efficacy and safety of RFA and MWA, but the findings are still inconclusive. After summarizing the high-quality RCTs, this can serve as a topic of our next evaluation.
Limitations and summary
The studies selected for this meta-analysis were not all RCTs. Retrospective cohort studies have selection and recall biases, and the number of original articles was limited. In addition, the entire study cohort for this meta-analysis was incomprehensive in regards to race, and most of the research population was Chinese, with some Japanese and Spanish. The 2015 epidemiological survey report showed that nearly 27% of the world’s cancer deaths are from China, and HCC is the second most common cause of cancer-related mortality in China, after lung cancer.39 Due to hepatitis B virus infection, aflatoxin exposure, alcohol abuse and environmental pollution, China has become the country with the highest incidence of liver cancer (about 55% of the world’s full rate) and with the largest number of deaths.40 China has a long way to go to control the incidence and mortality of liver cancer, which may be one of the important reasons why most of the research population in this meta-analysis was Chinese. Except for overall efficacy and radiofrequency interval, the heterogeneity of other indicators was remarkable. This may be due to differences in sample size, tumor size and number, patient age, and previous treatment history.
Chen et al.41 have also conducted a meta-analysis of the efficacy of RFA combined with sorafenib in patients with HCC. Their results showed no significant difference in OS and recurrence rates, but only included five articles of RFA+sorafenib vs. RFA alone. In addition, their meta-analysis also included literature that did not only use RFA as a control group, which might affect the overall reliability. Our study strictly screened out 15 original studies, and our conclusions are different from theirs.
Nowadays, the ideal therapy for HCC is still being explored. A comprehensive comparative analysis of the scoring system for HCC published in the World Journal of Hepatology told us that an appropriate scoring system should be selected according to the patient’s situation and a personalized strategy for HCC patients should be developed.4 The characteristics and liver function of the patients determine whether the treatment is curative or only palliative, or a combination of the two, as mentioned in this study (RFA+sorafenib). Therefore, the formulation of HCC treatment strategy needs the combination of multiple disciplines, such as hepatobiliary surgery, interventional radiology, and oncology. Personalized settings and adjustments would be needed at any time, according to the patient’s progression, adverse reactions and complications.
According to the current meta-analysis, PTA combined with sorafenib in the treatment of HCC is better than RFA or MWA alone. Patients who undergo the combination therapy should be closely observed for changes in skin, blood pressure, body temperature, gastrointestinal reactions, etc., to reduce the dose or discontinue the drug if necessary, and actively initiate symptomatic treatment. Although the subgroup analysis and random-effects models were applied in this study, the heterogeneity between studies may still affect the reliability of the results. The superiority of PTA plus sorafenib over PTA-alone still needs to be confirmed by more high-quality studies.
Conclusions
RFA or MWA combined with sorafenib has better efficacy than PTA alone; however, the adverse reactions are obvious. It is necessary to evaluate the safety of combination therapy and pay close attention to the adverse reactions of patients.
Supporting information
Abbreviations
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HFSR
hand-foot skin reaction
- MWA
microwave ablation
- OR
odds ratio
- OS
overall survival
- PEI
percutaneous ethanol injection
- PTA
physical thermal ablation
- RCT
randomized controlled trial
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
References
- 1.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Rubin J, Ayoub N, Kaldas F, Saab S. Management of recurrent hepatocellular carcinoma in liver transplant recipients: a systematic review. Exp Clin Transplant. 2012;10:531–543. doi: 10.6002/ect.2012.0085. [DOI] [PubMed] [Google Scholar]
- 4.Campigotto M, Giuffrè M, Colombo A, Visintin A, Aversano A, Budel M, et al. Comparison between hepatocellular carcinoma prognostic scores: A 10-year single-center experience and brief review of the current literature. World J Hepatol. 2020;12:1239–1257. doi: 10.4254/wjh.v12.i12.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PH, Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, et al. When to perform surgical resection or radiofrequency ablation for early hepatocellular carcinoma?: A nomogram-guided treatment strategy. Medicine (Baltimore) 2015;94:e1808. doi: 10.1097/MD.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: Systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287:461–472. doi: 10.1148/radiol.2017162756. [DOI] [PubMed] [Google Scholar]
- 7.Korkusuz Y, Gröner D, Raczynski N, Relin O, Kingeter Y, Grünwald F, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28:929–935. doi: 10.1007/s00330-017-5039-x. [DOI] [PubMed] [Google Scholar]
- 8.Peng ZW, Zhang YJ, Chen MS, Lin XJ, Liang HH, Shi M. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol. 2010;36:1054–1060. doi: 10.1016/j.ejso.2010.08.133. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 10.Shen A, Tang C, Wang Y, Chen Y, Yan X, Zhang C, et al. A systematic review of sorafenib in Child-Pugh A patients with unresectable hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:871–880. doi: 10.1097/MCG.0b013e3182a87cfd. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 12.Wu FX, Chen J, Bai T, Zhu SL, Yang TB, Qi LN, et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17:645. doi: 10.1186/s12885-017-3545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Maio M, Daniele B, Perrone F. Targeted therapies: Role of sorafenib in HCC patients with compromised liver function. Nat Rev Clin Oncol. 2009;6:505–506. doi: 10.1038/nrclinonc.2009.114. [DOI] [PubMed] [Google Scholar]
- 14.de’Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, et al. Role of sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation. Prog Transplant. 2016;26:348–355. doi: 10.1177/1526924816664083. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 16.Yu NS, Yan PJ, Zheng YY, Lu BC. Effect of radiofrequency ablation combined with sorafenib on liver function in patients with advanced hepatic carcinoma. Chinese Journal of General Practice. 2018;16:754–756. doi: 10.16766/j.cnki.issn.1674-4152.000205. [DOI] [Google Scholar]
- 17.Fu Z, Chen H, Wang Y, Xu Z, Zhang X. Analysis on efficacy and prognostic factors of radiofrequency ablation combined with sorafenib in the treatment of advanced hepatocellular carcinoma. Chongqing Yixue. 2020;49:714–717. doi: 10.3969/j.issn.1671-8348.2020.05.006. [DOI] [Google Scholar]
- 18.Kan X, Jing Y, Wan QY, Pan JC, Han M, Yang Y, et al. Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium-sized hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2015;19:247–255. [PubMed] [Google Scholar]
- 19.Zhang H, Guan Q, Ren W, Gu J. Radiofrequency ablation plus sorafenib for hepatocellular carcinoma. J Chin Pract Diagn Ther. 2015;29:409–411. doi: 10.13507/j.issn.1674-3474.2015.04.036. [DOI] [Google Scholar]
- 20.Wu XY, Zhang YZ, Zhang Y, Ma HN, Huang B. Effect of radiofrequency ablation combined with sorafenib in treating primary hepatocellular carcinoma. Chinese medicine. 2016;11:688–690. [Google Scholar]
- 21.Gong Q, Qin Z, Hou F. Improved treatment of early small hepatocellular carcinoma using sorafenib in combination with radiofrequency ablation. Oncol Lett. 2017;14:7045–7048. doi: 10.3892/ol.2017.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun JJ, Zhao HJ, Li W, Miao FH, Wang NY. Clinical study of radiofrequency ablation therapy in combination with sorafenib for advanced hepatocellular carcinoma. J Clini Hepatol. 2011;27:1093–1098. [Google Scholar]
- 23.Feng X, Xu R, Du X, Dou K, Qin X, Xu J, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol. 2014;109:1891–1899. doi: 10.1038/ajg.2014.343. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda H, Numata K, Moriya S, Shimoyama Y, Ishii T, Nozaki A, et al. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation—propensity score matching analysis. Radiology. 2014;272:598–604. doi: 10.1148/radiol.14131640. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Ni HB, Mao CC, Li W, Liu JP, Kang Y, et al. Clinical study of radiofrequency ablation therapy and sorafenib for hepatocellular carcinoma. Journal of Medical Forum. 2014;35:10–11. [Google Scholar]
- 26.Zhu K, Huang J, Lai L, Huang W, Cai M, Zhou J, et al. Medium or large hepatocellular carcinoma: Sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288:300–307. doi: 10.1148/radiol.2018172028. [DOI] [PubMed] [Google Scholar]
- 27.Hua XD, He ZY. Therapeutic effects of sorafenib combined with transcatheter arterial chemoembolization and microwave ablation on postsurgical recurrent hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2012;34:790–792. doi: 10.3760/cma.j.issn.0253-3766.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Zheng S, Li L. Therapeutic effects of sorafenib combined with transcatheter arterial chemoembolization and microwave ablation on postsurgical recurrent hepatocellular carcinoma. Chinese Hepatology. 2013;18:291–293. doi: 10.14000/j.cnki.issn.1008-1704.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Xu E, Qiu C, Dong Y. Effect of microwave ablation combined with sorafenib in the treatment of primary liver cancer. The Practical Journal of Cancer. 2018;33:664–667. doi: 10.3969/j.issn.1001-5930.2018.04.043. [DOI] [Google Scholar]
- 30.Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142. doi: 10.1007/s00270-014-0895-0. [DOI] [PubMed] [Google Scholar]
- 31.Gang G, Hongkai Y, Xu Z. Sorafenib combined with radiofrequency ablation in the treatment of a patient with renal cell carcinoma plus primary hepatocellular carcinoma. J Cancer Res Ther. 2015;11:1026. doi: 10.4103/0973-1482.150405. [DOI] [PubMed] [Google Scholar]
- 32.White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256–263. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 33.Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13:486–491. doi: 10.1007/s11605-008-0727-0. [DOI] [PubMed] [Google Scholar]
- 34.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 35.Mir O, Coriat R, Boudou-Rouquette P, Durand JP, Goldwasser F. Sorafenib-induced diarrhea and hypophosphatemia: mechanisms and therapeutic implications. Ann Oncol. 2012;23:280–281. doi: 10.1093/annonc/mdr525. [DOI] [PubMed] [Google Scholar]
- 36.Koschny R, Gotthardt D, Koehler C, Jaeger D, Stremmel W, Ganten TM. Diarrhea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology. 2013;84:6–13. doi: 10.1159/000342425. [DOI] [PubMed] [Google Scholar]
- 37.Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 40.Tsochatzis EA, Meyer T, Burroughs AK. Hepatocellular carcinoma. N Engl J Med 2012;366:92;author reply 92-93. doi: 10.1056/NEJMc1112501. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Ma X, Liu X, Cui X. Sorafenib combined with radiofrequency ablation as treatment for patients with hepatocellular carcinoma: a systematic review and meta-analysis. J BUON. 2017;22:1525–1532. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.