Neonatal diagnosis of congenital toxoplasmosis is based on a combination of serological and molecular tests. Maternal screening and treatment differ according to national policies and may impact the sensitivity of diagnostic methods in infants at birth.
KEYWORDS: congenital toxoplasmosis, Toxoplasma gondii, serology, IgM, IgA, Western blot, maternal treatment, neonatal diagnosis, qPCR
ABSTRACT
Neonatal diagnosis of congenital toxoplasmosis is based on a combination of serological and molecular tests. Maternal screening and treatment differ according to national policies and may impact the sensitivity of diagnostic methods in infants at birth. In this multicenter study, 115 neonates born to 61 treated (53%) and 54 (47%) untreated women were retrospectively included in three centers (France, Serbia, and the United States) to assess the impact of maternal anti-Toxoplasma treatment on the performance of neonatal workup at birth (neosynthesized anti-Toxoplasma IgM, IgA, and IgG and quantitative PCR [qPCR]) using univariate and multivariate approaches. Independently of the time of maternal seroconversion, the serological techniques were impacted differently by maternal treatment. The detection of IgM by immunosorbent agglutination assay (ISAGA) and Western blotting (WB) dropped from 90.7% and 88.2% in untreated neonates to 53.3% and 51.9% in treated neonates (P < 0.05), whereas IgM enzyme-linked immunosorbent assay (ELISA) and IgA ISAGA were not significantly affected by maternal treatment. A 2-fold reduction in the sensitivity of neosynthesized IgG by WB was also observed in the case of treatment during pregnancy (37.7% versus 82.3%). Interestingly, the effect of treatment was shown to be duration dependent, especially for IgM detection, when the treatment course exceeded 8 weeks, whatever the therapy. The sensitivity of Toxoplasma PCR in blood was also lowered by maternal treatment from 39.1% to 23.2%. These results highlight that anti-Toxoplasma therapy during pregnancy may set back biological evidence of neonatal infection at birth and underline the need for a careful serological follow-up of infants with normal workup.
INTRODUCTION
Toxoplasmosis is a widespread protozoan foodborne infection (1, 2) affecting about one-third of humans, with great differences in prevalence according to geographical area (3). Hence, public health policies vary among countries, as the disease burden is diversely appreciated (4, 5). Indeed, Toxoplasma gondii infection is largely asymptomatic, except in immunocompromised subjects. It can also be responsible for congenital infection when primary infection is acquired during pregnancy. The only way to know if a pregnant woman has acquired toxoplasmosis during pregnancy is to determine her serological status at the beginning of gestation and repeat serological testing regularly until delivery if it shows an absence of protective immunity. Such serological screening has been implemented in some countries, including France, Austria, Belgium, and Italy (6–8), to allow early maternal anti-Toxoplasma therapy and reduce vertical transmission and severe fetal damage (9–15). The usual treatment consists of spiramycin (SPI) administration until delivery (16), but if prenatal diagnosis demonstrates the presence of Toxoplasma DNA in amniotic fluid (AF), it is strongly recommended to switch SPI to the combination of pyrimethamine and sulfadiazine (PYR-SD), as the latter is more potent in reducing fetal sequelae (17). However, in about 10% of cases, neonates are diagnosed after birth, despite a negative prenatal diagnosis, and in about 30% of cases, prenatal diagnosis is not performed because of late infection during gestation (18). Therefore, postnatal diagnosis is essential to diagnose infected neonates and start treatment, as recommended (19–21).
The clinical and biological workup for postnatal diagnosis of congenital toxoplasmosis relies on a combination of methods, including parasite detection in the placenta or AF collected during delivery (18), cord blood, or newborn blood by PCR and serological screening (22, 23). The serological workup is now well established (6, 21, 24) and usually relies on the detection of specific IgA or IgM in neonate serum. As specific IgG antibodies developed by the mother are transferred to the fetal compartment, the only way to detect specific IgG synthesized by the neonate is to characterize anti-Toxoplasma IgG profiles of mother and newborn paired serum samples at birth by Western blotting (WB). This technique has been added to the routine serological workup for many years in most reference laboratories in France (25, 26) and is widely used across Europe. Sensitivity data for these techniques have been published over the years but show various performances according to studies and countries.
A possible impact of maternal treatment has been suggested for parasite detection in the placenta or IgM detection in neonates, with higher rates of positive quantitative PCR (qPCR) or positive IgM results, respectively, when mothers had received SPI than when they had received PYR-SD (18, 27). However, both studies were conducted in France, where women usually receive specific therapy during pregnancy; thus, comparison of the sensitivities of biological tests in neonates born to treated and untreated mothers was not addressed. Recently, Olariu et al. (28) reported the possible impact of maternal treatment on IgA and IgM detection, but the number of treated cases was small, and the trimester of infection, which was shown to influence IgA and IgM antibody detection in the neonate, was not taken into account.
Therefore, the aim of the present study was to assess the impact of maternal anti-Toxoplasma treatment during pregnancy on the sensitivity of various tests in neonates at birth using a multivariate analysis performed on data from three countries with different national policies, i.e., maternal screening and treatment (France), occasional maternal screening (Serbia), and no maternal screening (United States).
MATERIALS AND METHODS
Ethics.
All analyses were performed during routine workup as implemented in the three participating centers. Data were recorded anonymously. The study design was approved by the local ethics committee of the University Hospital of Rennes (approval number 20.08).
Patients.
All congenitally infected infants diagnosed over a 10-year period (2010 to 2019) in the three laboratories (Dr. Jack S. Remington Laboratory for Specialty Diagnostics [JSRLSD], Palo Alto, CA; National Reference Laboratory for toxoplasmosis from Serbia [CNLTS]; and Rennes University Hospital Parasitology Lab [RUH-PL]) were retrospectively included if a blood sample had been analyzed at birth or during the first month of life. Diagnosis of congenital toxoplasmosis relied on a positive prenatal diagnosis (parasite DNA detection by qPCR on amniotic fluid) and/or detection of specific IgM or IgA in peripheral blood at >7 days of life, positive Toxoplasma qPCR in peripheral blood or cerebrospinal fluid (CSF), or detection of neosynthesized IgG or IgM by Western blotting.
Other relevant data were recorded for analysis: age of gestation at the time of maternal infection; type, date of onset, and duration of anti-Toxoplasma-targeted therapy; gender of the newborn; and clinical signs at birth or during the first year of life. When the date of maternal infection could not be known with accuracy, due to the absence of early serological screening, the estimation was indicated as “1st, 2nd, or 3rd trimester” according to clinical or imaging findings or serological results during pregnancy or as “undetermined” when discovered only after birth.
Serological methods.
Anti-Toxoplasma IgM was detected by enzyme-linked immunosorbent assay (ELISA) (Platelia Toxo IgM; Bio-Rad, Marnes-la-Coquette, France) and immunosorbent agglutination assay (ISAGA) (bioMérieux, Marcy-l’Etoile, France) at CNLTS and RUH-PL and by ISAGA only at JSRLSD. Anti-Toxoplasma IgA was detected by an IgA ISAGA (bioMérieux) at CNLTS and RUH-PL and by an in-house ELISA at JSRLSD. Comparison of maternal and neonatal IgG and IgM antibody profiles was performed using the Toxoplasma WB IgG/IgM assay (Ldbio, Lyon, France) at CNLTS and RUH-PL.
ELISA indexes for IgM and IgA were positive when ≥1 and negative when <0.8. For performance calculation, ELISA indexes in the gray zone were merged with positive results. ISAGA IgM and IgA scores were positive when ≥3, according to the manufacturer’s recommendations. When a positive result was obtained for a cord blood sample using a quantitative test, it was taken into account only if it could be confirmed on peripheral blood or if WB could rule out contamination of cord blood by maternal IgM (different profiles).
WB profiles of the mother and her baby were compared to determine if additional bands were observed in the neonatal serum (neosynthesis by the infected neonate). When cord blood samples were tested, if the IgM patterns of the mother and baby were similar, the cord blood was considered contaminated by the maternal serum and was excluded from the study. When maternal and neonatal patterns differed by only one faint band, the WB was considered doubtful. In both instances, if there were no other arguments in favor of the diagnosis of infection, all tests were repeated on a neonatal blood sample collected several days later.
Toxoplasma real-time PCR.
For parasite DNA detection, 200 μl of total blood or CSF was extracted using a QIAamp DNA minikit (Qiagen), and 5 μl of DNA was used for amplification. At CNLTS and RUH-PL, Toxoplasma-specific quantitative real-time PCR targeted the repetitive rep529 sequence and was carried out as previously described (18, 29, 30). At JSRLSD, PCR was done using two target sequences from the B1 gene (22).
Statistical analysis.
All serological and PCR results were included as dichotomous variables (positive/negative), with borderline test results classified as positive, and were analyzed according to maternal treatment.
A univariate analysis was performed on population characteristics according to treatment group. The association of the different test results with qualitative variables (gender, type of sample, trimester of pregnancy, and treatment group) was analyzed using Pearson’s χ2 test or Fisher’s exact test. For continuous variables (age at the time of sampling and duration of treatment) for which the skewness and Kurtosis tests showed a non-Gaussian distribution, we used the nonparametric Mann-Whitney U test, and the distributions were displayed as medians with interquartile ranges (25th and 75th percentiles).
Multivariate analysis was then carried out, and a logistic regression model was set up with each particular test result as an outcome variable and the trimester of infection, gender, newborn age at sampling (in days), center, and maternal treatment (yes/no) as covariates. The duration of treatment (by categories of ≤8/>8 weeks or in number of weeks) and the type of treatment (SPI or PYR-SD with or without SPI) were further analyzed for the treated group. Only variables that were found to be significant by univariate analysis, with a P value of <0.2, were tested forward by stepwise Wald logistic regression. The odds ratios (ORs) and 95% confidence intervals (CIs) were used to describe the associated factors if the P value was <0.05.
All statistical tests and procedures were performed using the IBM SPSS 21 statistical package (IBM, NY, USA).
RESULTS
Patient files.
A total of 115 mother-neonate pairs were included from the 3 centers: 46 from France, 26 from Serbia, and 43 from the United States. Congenital infection was diagnosed antenatally (n = 27) and/or after birth on the basis of neosynthesized antibody detection or positive PCR on newborn samples (see Table S1 in the supplemental material). The first positive postnatal test result was obtained as soon as birth until 1 year of age when treatment was stopped (Table S1). Neonates were aged 0 to 35 days (median, 3 days [interquartile range, 1, 11]) when the first blood sample was obtained and included in the study. The time of sampling was earlier for newborns from treated mothers than for those from untreated ones as a result of the follow-up protocol implemented in cases of maternal Toxoplasma infection in Europe (Table 1). Blood samples included 46 cord blood and 69 peripheral blood samples. Most samples were taken before 5 days of life (n = 70) (Table 1). The frequency of detection of specific IgG, IgM, or IgA neosynthesized antibodies did not depend on the sample type in any of the analyzed diagnostic tests (P > 0.05). Maternal seroconversion occurred during the first trimester (T1), T2, and T3 in 14 (12.2%), 28 (24.3%), and 36 (31.3%) cases, respectively, while the date of maternal infection remained unknown in 37 (32.2%) cases, of which 34 were symptomatic (Table 1).
TABLE 1.
Population characteristics (n = 115) and comparison according to treatment groupa
| Characteristic | Value for group by maternal treatment during pregnancy |
Univariate analysis P valueb | |
|---|---|---|---|
| No (n = 54) | Yes (n = 61) | ||
| Sex ratio (no. of M/F infants) | 0.69 (22/32) | 1.10 (32/29) | 0.209 (NS) |
| No. (%) of infants with type of bloodsample | <0.0001 | ||
| Cord blood (n = 46) | 4 (7.4) | 42 (68.8) | |
| Peripheral blood (n = 69) | 50 (92.6) | 19 (31.1) | |
| Median time of blood sampling(days) (interquartile range) | 10 (4–17) | 1 (0–3) | <0.0001 |
| No. (%) of infants of age (days of life) | |||
| 0–4 (n = 70) | 16 (29.6) | 54 (88.6) | |
| 5–14 (n = 21) | 20 (37.0) | 1 (1.6) | |
| 15–35 (n = 24) | 18 (33.3) | 6 (9.8) | |
| No. (%) of infants with trimester ofmaternal infection | 0.063 (NS) | ||
| T1 (n = 14) | 4 (7.4) | 10 (16.4) | |
| T2 (n = 28) | 2 (3.7) | 26 (42.6) | |
| T3 (n = 36) | 11 (20.4) | 25 (41.0) | |
| ND (n = 37) | 37 (68.5) | 0 | |
| No. (%) of infants with clinical manifestation during the 1st year of life | <0.0001 | ||
| Yes (n = 60) | 41 (75.9) | 19 (31.1) | |
| No (n = 53) | 11 (20.4) | 42 (68.9) | |
| ND (n = 2) | 2 (3.7) | 0 | |
| No. (%) of patients with type oftreatment | NA | NA | |
| SPI alone | 34 (55.7) | ||
| SPI and then PYR-SD | 19 (31.1) | ||
| PYR-SD alone | 8 (13.1) | ||
M, male; F, female; NA, not applicable; ND, not determined; NS, not significant; SPI, spiramycin; PYR-SD, pyrimethamine-sulfadiazine.
Pearson’s χ2 test or Fisher’s exact test was used to compare distributions in treated and untreated groups.
Anti-Toxoplasma-targeted therapy during pregnancy was given to 61 (53.0%) pregnant women. The proportions of treated women did not differ according to the trimester of seroconversion (Table 1). As expected, the median duration of treatment was significantly shorter when infection was diagnosed in T3 (median, 5 weeks [interquartile range, 2, 7]) than in T2 (median, 12 weeks [7.25, 14]) and T1 (median, 17.5 weeks [8, 24]) (P < 0.001). Treatment consisted of SPI alone (n = 34), PYR-SD (n = 8), or SPI followed by PYR-SD (n = 19). Interestingly, the proportions of infections acquired during the first, second, and third trimesters did not differ among treated and untreated mothers (Table 1), ruling out a possible interplay of these two parameters in the interpretation of the results.
The incidence of Toxoplasma-related clinical manifestations within the first year of follow-up reached 53.1% (60/113) (Table 1). The main disorders included cerebral lesions, i.e., intracranial calcifications, ventriculomegaly, and meningitis in 47/60 (78.3%); ocular lesions in 26/60 (43.3%); and growth retardation/prematurity in 7/60 (11.7%) neonates. The rate of newborns with clinical signs was significantly lower when treatment was given during pregnancy (31.1% versus 75.9% in treated and untreated groups, respectively) (Table 1).
Maternal treatment is associated with lower frequencies of detection of anti-Toxoplasma IgM and IgG, but not IgA, in the neonate.
As a first approach to investigate the impact of maternal anti-Toxoplasma treatment on the results of diagnostic methods, we calculated the overall sensitivity of each test for prenatally treated and untreated infants. The overall sensitivity of IgM detection by ELISA and/or ISAGA was lower in infants born to treated mothers (P < 0.0001) (Table 2). However, when analyzing each technique separately, ELISA-based IgM detection was not affected by treatment, whereas the sensitivity of the IgM ISAGA dropped from 90.7% to 53.3% when treatment was administered (P < 0.0001) (Table 2). WB-based IgM detection was also significantly lower in the treated group, as it was positive in only 55.8%, compared to 88.2% without treatment (P < 0.05). This reduction was also observed when gathering doubtful IgM WB profiles (only one additional faint band obtained with the neonate’s profile compared to the mother’s IgM profile) with negative results instead of the positive ones (P = 0.009) (data not shown). A >2-fold reduction of neosynthesized IgG detection by WB was also observed in neonates from treated mothers, at 82.3% versus 37.7% (P < 0.01). No significant differences were observed in the detection of specific IgA (ELISA or ISAGA) according to maternal treatment (Table 2).
TABLE 2.
Sensitivity of serological tests and qPCR in newborns according to treatment group (n = 115)
| Diagnostic method | Overall sensitivity (%) according to maternal treatment (no. of positive infants/total no. of infants) |
Univariate analysis P value (Fisher’s test) | ||
|---|---|---|---|---|
| All (n = 115) | Untreated (n = 54) | Treated (n = 61) | ||
| IgAa (ELISA or ISAGA) | 71.8 (79/110) | 75.5 (40/53) | 68.4 (39/57) | 0.416 |
| IgA ELISA | 72.1 (31/43) | 73.0 (27/37) | 66.7 (4/6) | 1 |
| IgA ISAGA | 71.6 (48/67) | 81.2 (13/16) | 68.6 (35/51) | 0.526 |
| IgMa (ELISA or ISAGA) | 73.9 (85/115) | 90.7 (49/54) | 59.0 (36/61) | 0.0001 |
| IgM ELISA | 59.2 (42/71) | 68.8 (11/16) | 56.4 (31/55) | 0.564 |
| IgM ISAGA | 71.1 (81/114) | 90.7 (49/54) | 53.3 (32/60) | <0.0001 |
| IgM WB | 63.8 (44/69) | 88.2 (15/17) | 55.8 (29/52) | 0.0198 |
| IgG WB | 48.6 (34/70) | 82.3 (14/17) | 37.7 (20/53) | 0.0018 |
| qPCR | 33.3 (28/84) | 43.6 (17/39) | 24.4 (11/45) | 0.104 |
| Blood | 28.8 (19/66) | 39.1 (9/23) | 23.2 (10/43) | 0.254 |
| CSF | 50.0 (9/18) | 50.0 (8/16) | 50.0 (1/2) | 1 |
IgM and IgA ELISA values in the gray zone were grouped with positive results.
To further analyze the differences observed between ISAGA and ELISA for IgM detection, we evaluated the sensitivity on serum samples analyzed concomitantly with both techniques (Serbian and French neonates). The results showed that poorer ISAGA sensitivity was associated with maternal treatment, whereas the ELISA sensitivity was equal in both the treated and untreated groups (Table 3).
TABLE 3.
Comparison of sensitivities of ISAGA and ELISA for IgM detection (n = 70) according to treatment group
| Test | Sensitivity (%) according to maternal treatment (no. of positive infants/total no. of infants)a |
P value (Fisher’s test) | |
|---|---|---|---|
| Treated | Untreated | ||
| IgM ELISAb | 55.6 (30/54) | 68.8 (11/16) | 0.400 |
| IgM ISAGA | 51.9 (28/54) | 81.2 (13/16) | 0.045 |
This analysis included only neonates for whom both IgM ELISA and IgM ISAGA were performed on the same sample.
IgM ELISA values in the gray zone were grouped with positive results.
As the absence of specific IgM detection in the neonate could be due solely to the physiological disappearance of antibodies before birth when infection occurred in early pregnancy, we then performed a multivariate analysis to confirm the results obtained by the univariate analysis. Interestingly, the results showed that treatment significantly altered IgM detection by ISAGA and WB (P < 0.001 and P < 0.01, respectively) as well as neosynthesized IgG detection by WB (Table 4), thus confirming the results of the univariate analysis. Multivariate analysis also showed that the trimester of maternal infection influenced the positivity of these serological tests, with more frequent positivity for infections acquired during the third trimester, while the age of the newborn at the time of sampling had no effect (Table 4 and Table S2). Unexpectedly, gender was significantly associated with IgM detection, with male and female infants being most likely to have positive ISAGA IgM and positive WB IgM, respectively (Table 4 and Table S2).
TABLE 4.
Multivariate analysis of the effects of treatment, trimester of maternal infection, and age at blood sampling on the sensitivity of diagnostic tests
| Diagnostic method (outcome Variable) |
P value (OR [95% CI]) for covariateb |
|||
|---|---|---|---|---|
| Treatment | Trimester of maternal infection | Male gender | Newborn age | |
| IgM ISAGA (n = 144) | 0.000 (0.117 [0.041–0.333]) | 0.001 (3.417 [1.648–7.085]) | 0.021 (2.680 [1.160–6.192]) | 0.104 |
| IgM WB (n = 69) | 0.002 (0.049 [0.007–0.322]) | 0.000 (6.623 [2.461–17.874]) | 0.003 (0.174 [0.055–0.547]) | 0.292 |
| IgG WB (n = 70) | 0.005 (0.115 [0.025–0.518]) | 0.007 (2.931 [1.348–6.372]) | ND | 0.242 |
| qPCRa (n = 80) | 0.480 (0.292 [0.068–0.990]) | ND | ND | 0.007 (1.091 [1.024–1.162]) |
Blood or CSF.
ND, not determined because the P value in univariate analysis was <0.2 (results not shown).
The duration of anti-Toxoplasma therapy during pregnancy is unequally associated with a reduced sensitivity of serological tests.
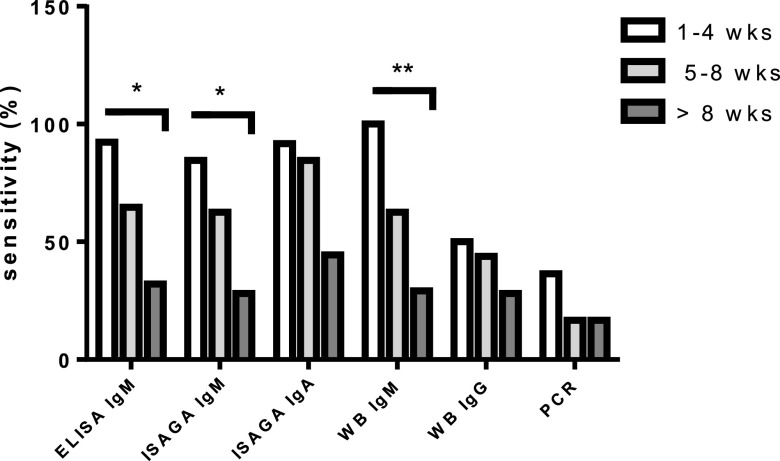
To further analyze if the duration of maternal anti-Toxoplasma therapy influenced the sensitivity of diagnostic methods, we divided the group of 61 infants from treated mothers into two subgroups according to the length of maternal treatment, i.e., ≤8 weeks or >8 weeks of any treatment. Interestingly, the duration of treatment interfered heterogeneously with the detection of IgM, IgA, or IgG. The detection of specific IgA, regardless of the method, was not altered, but when distinguishing the ISAGA and ELISA, it appeared that ISAGA sensitivity was significantly reduced if the treatment course was >8 weeks (P < 0.05) (Table 5). The sensitivity of IgM detection, whatever the technique used (ELISA, ISAGA, or WB), was dramatically decreased when treatment exceeded 8 weeks (P < 0.001, P < 0.01, and P = 0.001, respectively) (Table 5). Overall, the range of IgM detection decreased from 72 and 79% to 29 and 32% when treatment lasted ≤8 weeks and >8 weeks, respectively, depending on the method. In contrast, IgG detection by WB was not significantly modified according to the cutoff of 8 weeks of treatment (Table 5). The duration of treatment seems to have a progressive effect, as illustrated in Fig. 1.
TABLE 5.
Sensitivity of serological tests and qPCR in newborns from treated mothers (n = 61) and comparison according to a treatment duration of ≤8 or >8 weeks
| Diagnostic test | Sensitivity (%) according to duration of treatment (no. of positive infants/total no. of infants) |
Univariate analysis P valuea | Multivariate analysisb |
||
|---|---|---|---|---|---|
| ≤8 wks (n = 33) | >8 wks (n = 28) | P value | OR (95% CI) | ||
| IgA (ELISA or ISAGA) (n = 57) | 78.1 (25/32) | 56.0 (14/25) | 0.091 | 0.079 | NA |
| IgA (ELISA) (n = 6) | 33.3 (1/3) | 100 (3/3) | 1 | ND | NA |
| IgA (ISAGA) (n = 51) | 82.8 (24/29) | 50.0 (11/22) | 0.017 | 0.009 | 0.161 (0.041–0.629) |
| IgM (ELISA or ISAGA) (n = 61) | 75.6 (25/33) | 39.3 (11/28) | 0.005 | 0.04 | 0.208 (0.070–0.619) |
| IgM (ELISA) (n = 55) | 76.7 (23/30) | 32.0 (8/25) | 0.001 | 0.048 | 0.288 (0.083–0.972) |
| IgM (ISAGA) (n = 60) | 71.9 (23/32) | 32.1 (9/28) | 0.004 | 0.035 | 0.117 (0.026–0.517) |
| IgM (WB) (n = 52) | 78.6 (22/28) | 29.2 (7/24) | 0.001 | 0.001 | 0.112 (0.032–0.396) |
| IgG (WB) (n = 53) | 46.4 (13/28) | 28.0 (7/25) | 0.256 | ND | NA |
| qPCR (n = 45) | 30.8 (8/26) | 15.8 (3/19) | 0.309 | ND | NA |
By Fisher’s exact test.
Multivariate analysis was done using the treatment duration as a qualitative variable (>8 or ≤8 weeks); data for other covariates are not shown. NA, not applicable; ND, not determined because the P value in the univariate analysis was <0.2.
FIG 1.
Sensitivity of diagnostic tests according to the duration of treatment. Statistical significance is indicated (*, P < 0.05; **, P < 0.01). Data obtained for IgA ELISA are not shown because only 6 infants had been treated in utero.
Multivariate analysis using the same cutoff for treatment duration (≤8 weeks or >8 weeks) confirmed these findings (Table 5). Additionally, when taking into account the precise duration of treatment (number of weeks), a longer duration of treatment was associated with lower sensitivities of the IgA ISAGA as well as IgM detection by any technique but with no impact on neosynthesized IgG detection by WB (Table 6). Overall, among the 61 treated neonates, no statistically significant difference (P > 0.05) was found between neonates treated with SPI and PYR-SD with or without SPI in any serological test results (Table 6).
TABLE 6.
Sensitivity of diagnostic tests according to treatment duration in weeks
| Diagnostic test | Statistical significance of treatment duration in wks on diagnostic testa |
|||||
|---|---|---|---|---|---|---|
| Any treatment (n = 61) |
SPI (n = 34) |
PYR-SD ± SPI (n = 27) |
||||
| P valueb (Mann-Whitney test) | P value for regression analysisc (OR [95% CI]) | P valueb (Mann-Whitney test) | P value for regression analysis (OR [95% CI]) | P valueb (Mann-Whitney test) | P value for regression analysis (OR [95% CI]) | |
| IgA (ISAGA) | 0.052 | 0.047 (0.929 [0.864–0.999]) | 0.069 | 0.056 | 0.375 | ND |
| IgM (ELISA) | 0.000 | 0.001 (0.518 [0.581–0.962]) | 0.052 | 0.052 | 0.346 | ND |
| IgM (ISAGA) | 0.001 | 0.003 (0.815 [0.440–0.956]) | 0.030 | 0.040 (0.859 [0.743–0.993]) | 0.002 | 0.004 (0.864 [0.778–0.953]) |
| IgM (WB) | 0.000 | 0.001 (0.857 [0.761–0.965]) | 0.000 | 0.027 (0.872 [0.758–0.901]) | 0.000 | 0.023 (0.845 [0.730–0.977]) |
| IgG (WB) | 0.149 | 0.155 | 0.435 | ND | 0.755 | ND |
| qPCR | 0.124 | 0.193 | 0.615 | ND | 0.065 | 0.042 (0.102 [0.011–0.902]) |
Data for other covariates are not shown. ND, not determined because the P value in the univariate analysis was <0.2; SPI, spiramycin; PYR-SD, pyrimethamine-sulfadiazine.
Comparison of the mean durations of treatment in patients with negative and positive test results.
Calculated on sample sizes of 51, 55, 60, 52, 53, and 45, respectively, for the various tests.
Prenatal therapy is associated with decreased sensitivity of the detection of parasite DNA in the neonate at birth.
Eighty-four neonate specimens, consisting of blood (n = 66) or CSF samples (n = 18), were also collected for Toxoplasma detection by qPCR. The sensitivity dropped from 43.6% to 24.4% in neonates from untreated and treated mothers, respectively (Table 2). When the precise duration of treatment was analyzed for each type of treatment, regression analysis showed that there was no effect of SPI alone, whereas there was a significant time-dependent effect of PYR-SD on DNA detection by PCR (Table 6).
DISCUSSION
The laboratory diagnosis of congenital toxoplasmosis at birth requires the use of multiple techniques to prove infection of the neonate through the detection of either antibody neosynthesis by the newborn or circulating parasite DNA. Postnatal screening of neonates is paramount in three situations: (i) when evocative clinical signs are present at birth, and the mother has not benefited from Toxoplasma serological screening during gestation; (ii) when the mother acquired toxoplasmosis during gestation, and the prenatal diagnosis was negative; and (iii) when the mother acquired toxoplasmosis, but prenatal diagnosis was not performed (late infection during pregnancy).
As most French women with primary infection during pregnancy are given specific treatment, we included data on infants from Serbia, where maternal screening has been adopted only recently, and the United States, where women are diagnosed and treated only in cases of ultrasound anomalies. We chose to include only early samples because we wanted to draw conclusions on the effect of maternal treatment on the early diagnosis of congenital toxoplasmosis. To ensure perfect interpretation of IgM or IgA detection in cord blood samples, we considered them as positive only if IgM neosynthesis by the newborn was confirmed by WB, thus ruling out maternal contamination for both IgM and IgA.
Using univariate and multivariate analyses, we found strong evidence that maternal treatment was associated with a dramatic decrease in the sensitivity of IgM and neosynthesized IgG in the neonate. Whereas a direct impact of treatment on parasite replication and dissemination was expected, as it is assumed to reduce parasite loads (27), the demonstration of an impact on antibody synthesis by the newborn is a novelty. Indeed, it could be hypothesized that the absence of IgM or IgA detection in newborns was due to transient synthesis by the fetus, particularly if infection occurred early in gestation. Indeed, we observed that the positivity of tests was associated with the trimester of infection (Table 4), with a higher detection rate for infections acquired during the third trimester (see Table S2 in the supplemental material), which also coincides with shorter courses of treatment. However, there were no differences in the time of infection among treated and untreated mothers (Table 1); thus, the time of infection cannot bias the conclusions on the effect of treatment. Taken together, data from the multivariate analysis clearly demonstrated an association of treatment with a lower sensitivity of IgM and neosynthesized IgG antibody detection that could be explained by delayed synthesis. Additionally, this effect was time dependent, with an apparent cutoff of 8 weeks of any treatment (SPI or PYR-SD) for most serological tests. Besides, the ISAGA was more impacted by maternal treatment than the ELISA. Since the IgM ISAGA uses “global” antigens from entire parasites, this finding suggests that maternal treatment could be more likely to affect the ability of the fetus to react against surface antigens than against cytoplasmic antigens. Why SPI and PYR-SD administered to the mother had roughly the same effect on the fetus or neonate antibody response is difficult to explain, as SPI is known as hardly transferring through the placental barrier. A previous study by Gilbert et al. found no impact of maternal treatment on the sensitivity of IgM detection by multivariate analysis, but the group treated with PYR-SD was compared to a control group, including both SPI-treated and untreated mothers (31).
In contrast to our results, Naessens et al. investigated IgM detection in peripheral blood samples from 86 congenitally infected newborns and found no significant effect of treatment despite an apparent decrease of sensitivity (85% in the untreated group versus 25% in the treated group; P = 0.19); they attributed it to the gestational age of maternal infection rather than prenatal treatment (32). However, the vast majority of mothers (75%) had been treated, and the duration of treatment was not known with accuracy, nor was the time of maternal infection, as monthly serological screening was not the current practice in most centers. In a Brazilian study primarily aiming to measure the duration of IgM synthesis by infected infants, Lago et al. observed that in 23/28 neonates whose mothers had been diagnosed with seroconversion during pregnancy, IgM was detected during the first month of life. However, the difference in sensitivity between the treated (75%) and untreated (88%) groups was not statistically significant (P = 0.4) (33).
While IgA detection appeared not to be affected by treatment using univariate analysis, there was a trend toward a time-dependent effect of treatment on infants from treated mothers, irrespective of the type of treatment. Again, this was significant only with the ISAGA, but it must be acknowledged that there was a small number of samples analyzed by an ELISA. Interestingly, it has been shown in experimental murine infection that specific IgA synthesis was the least affected of all antibody classes by several anti-Toxoplasma chemotherapeutic regimens (34).
In contrast, despite a significant effect of treatment on the detection of neosynthesized IgG by WB, it was not related to the duration of maternal treatment. It was not related to the time of sampling, i.e., the age of the newborn, either (Table 4). In infants from untreated mothers, neosynthesized IgG was found in 4/5 (80%) in the early days after birth (0 to 4 days) and in 7/9 (78%) from days 15 to 35 (data not shown). Few reports are available on the detection of neosynthesized IgG using WB. In a cohort of 55 neonates, a French multicenter study reported a sensitivity of IgG WB similar to ours at birth (37.7% in the treated group), increasing to 40% during the first 10 days of life and to 63.3% at between 0.5 and 1.5 months (35). Similar findings were reported in Brazil, where IgG WB was positive in 40% (6/15) of infected newborns born to treated mothers, up to 3 months of age (36). In another Brazilian series of newborns aged up to 1 month and born to women who had not been given treatment during pregnancy, the sensitivity of IgG WB reached 73.5% (130/177), which is not far from the results obtained in our untreated group (82.3%) (37). However, they found a lower sensitivity of IgM WB (54.8%) than that of IgG WB.
In a previous study, Olariu et al. analyzed IgM and IgA detection in infants from 25 treated and 164 untreated mothers and similarly found that IgM was less frequently detected in infants of treated mothers (44%) than in those of untreated mothers (86.6%) (P < 0.001) (28). The detection of IgA was not modified according to maternal treatment either (P = 0.06). However, they did not take into account the gestational age at the time of maternal infection, which is a major factor impacting the sensitivity of serological tests (27, 32). In addition, samples were obtained within the first 6 months of life, whereas in the present study, we focused on the results obtained early after birth (mean age of 7 days).
It is interesting to note that in cases of maternal anti-Toxoplasma treatment, the detection of IgA was the most sensitive approach to detect neonatal infection, outperforming IgM- and IgG-based screening (68.4% versus 59% and 38%, respectively), while it was less sensitive in untreated cases.
Still, the overall sensitivities of IgA and IgM detection (71.8% and 73.9%, respectively) are quite high compared to those in previous reports, which showed sensitivity ranges from 47.8 to 72.5% and 44 to 67.5% for IgA and IgM, respectively, irrespective of the technique (24, 27, 31, 35).
Whereas the contribution of PCR testing in AF has been extensively evidenced for prenatal diagnosis, the use of PCR on newborn blood or CSF at birth has been sparsely investigated (22). In our cohort, the sensitivity of blood PCR was low (28.6%), but it is interesting to note that it ranged from 23% to 39% in treated and untreated infants, respectively. Unexpectedly, while univariate analysis was significant, multivariate analysis showed that treatment onset did not impact PCR positivity. However, among treated neonates, the longer the treatment, the lower the sensitivity of PCR provided that the treatment was PYR-SD, which is in agreement with the expected in utero antiparasitic effect of this bitherapy. The trimester of pregnancy had no impact on the positivity of PCR, while it might be expected to find more positive PCR results when infection occurred at the end of pregnancy due to a shorter course of treatment. These findings may suffer from a bias of center, as the proportion of positive cases was higher at JSRLSD (38% versus 23% at RUHPL), but 37 cases were excluded from the multivariate analysis because of missing data; thus, the power of the analysis may have been impaired.
The strength of our study is that it was performed on a well-balanced cohort of prenatally treated and untreated infants diagnosed prospectively in three reference centers. A limitation can result from the uncertainty of the time of maternal infection in some U.S. cases, as no serological follow-up was undertaken, which led us to discard 32% of cases for the multivariate analysis.
Taken together, our data provide evidence that anti-Toxoplasma therapy in the mother may contribute to a setback of serological confirmation in the neonate and underline the need for careful serological follow-up of neonates even if the workup at birth is normal. We also recall that prenatal diagnosis can detect at most 90% of infected fetuses; thus, postnatal serological follow-up is essential.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education, Science, and Technological Development of Serbia through project number III 41019 and by a grant to the Institute for Medical Research (contract number 451-03-68/2020-14/200015). We also received a grant from Campus France (PHC Pavle Savic number 40734WC) (Serbian project number 451-03-01963/2017-09/15).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Blaga R, Aubert D, Thébault A, Perret C, Geers R, Thomas M, Alliot A, Djokic V, Ortis N, Halos L, Durand B, Mercier A, Villena I, Boireau P. 2019. Toxoplasma gondii in beef consumed in France: regional variation in seroprevalence and parasite isolation. Parasite 26:77. doi: 10.1051/parasite/2019076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousseau A, Carbona SL, Dumètre A, Robertson LJ, Gargala G, Escotte-Binet S, Favennec L, Villena I, Gérard C, Aubert D. 2018. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite 25:14. doi: 10.1051/parasite/2018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas G, Roussos N, Falagas ME. 2009. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Torgerson PR, Mastroiacovo P. 2013. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91:501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, Laboudi M, El Mansouri B, Ibrahimi A, Rhajaoui M, Quinn F, Murugesan M, Seghrouchni F, Gómez-Marín JE, Peyron F, McLeod R. 2018. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerg Microbes Infect 7:165. doi: 10.1038/s41426-018-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert-Gangneux F, Dardé M-L. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomasoni LR, Meroni V, Bonfanti C, Bollani L, Lanzarini P, Frusca T, Castelli F. 2014. Multidisciplinary approach to congenital Toxoplasma infection: an Italian nationwide survey. New Microbiol 37:347–354. [PubMed] [Google Scholar]
- 8.Prusa A-R, Kasper DC, Sawers L, Walter E, Hayde M, Stillwaggon E. 2017. Congenital toxoplasmosis in Austria: prenatal screening for prevention is cost-saving. PLoS Negl Trop Dis 11:e0005648. doi: 10.1371/journal.pntd.0005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Systematic Review on Congenital Toxoplasmosis Study Group, Thiébaut R, Leproust S, Chêne G, Gilbert R. 2007. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet 369:115–122. doi: 10.1016/S0140-6736(07)60072-5. [DOI] [PubMed] [Google Scholar]
- 10.Hotop A, Hlobil H, Gross U. 2012. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 54:1545–1552. doi: 10.1093/cid/cis234. [DOI] [PubMed] [Google Scholar]
- 11.Cortina-Borja M, Tan HK, Wallon M, Paul M, Prusa A, Buffolano W, Malm G, Salt A, Freeman K, Petersen E, Gilbert RE, European Multicentre Study on Congenital Toxoplasmosis. 2010. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med 7:e1000351. doi: 10.1371/journal.pmed.1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieffer F, Wallon M, Garcia P, Thulliez P, Peyron F, Franck J. 2008. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr Infect Dis J 27:27–32. doi: 10.1097/INF.0b013e318134286d. [DOI] [PubMed] [Google Scholar]
- 13.Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, Kopp CB, Binquet C. 2013. Congenital Toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 56:1223–1231. doi: 10.1093/cid/cit032. [DOI] [PubMed] [Google Scholar]
- 14.Prusa A-R, Kasper DC, Pollak A, Olischar M, Gleiss A, Hayde M. 2015. Amniocentesis for the detection of congenital toxoplasmosis: results from the nationwide Austrian prenatal screening program. Clin Microbiol Infect 21:191.e1–191.e8. doi: 10.1016/j.cmi.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Robert-Gangneux F. 2014. It is not only the cat that did it: how to prevent and treat congenital toxoplasmosis. J Infect 68:S125–S133. doi: 10.1016/j.jinf.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinovic N, Guegan H, Stäjner T, Belaz S, Robert-Gangneux F. 2019. Treatment of toxoplasmosis: current options and future perspectives. Food Waterborne Parasitol 15:e00036. doi: 10.1016/j.fawpar.2019.e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelbrot L, Kieffer F, Sitta R, Laurichesse-Delmas H, Winer N, Mesnard L, Berrebi A, Le Bouar G, Bory J-P, Cordier A-G, Ville Y, Perrotin F, Jouannic J-M, Biquard F, d’Ercole C, Houfflin-Debarge V, Villena I, Thiébaut R, TOXOGEST Study Group. 2018. Prenatal therapy with pyrimethamine + sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: a multicenter, randomized trial. Am J Obstet Gynecol 219:386.e1–386.e9. doi: 10.1016/j.ajog.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Robert-Gangneux F, Dupretz P, Yvenou C, Quinio D, Poulain P, Guiguen C, Gangneux J-P. 2010. Clinical relevance of placenta examination for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J 29:33–38. doi: 10.1097/INF.0b013e3181b20ed1. [DOI] [PubMed] [Google Scholar]
- 19.Moncada PA, Montoya JG. 2012. Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment. Expert Rev Anti Infect Ther 10:815–828. doi: 10.1586/eri.12.58. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado YA, Read J, Committee on Infectious Diseases. 2017. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 139:e20163860. doi: 10.1542/peds.2016-3860. [DOI] [PubMed] [Google Scholar]
- 21.Peyron F, L’ollivier C, Mandelbrot L, Wallon M, Piarroux R, Kieffer F, Hadjadj E, Paris L, Garcia-Meric P. 2019. Maternal and congenital toxoplasmosis: diagnosis and treatment recommendations of a French multidisciplinary working group. Pathogens 8:24. doi: 10.3390/pathogens8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olariu TR, Remington JS, Montoya JG. 2014. Polymerase chain reaction in cerebrospinal fluid for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J 33:566–570. doi: 10.1097/INF.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 23.Pomares C, Montoya JG. 2016. Laboratory diagnosis of congenital toxoplasmosis. J Clin Microbiol 54:2448–2454. doi: 10.1128/JCM.00487-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murat J-B, Hidalgo HF, Brenier-Pinchart M-P, Pelloux H. 2013. Human toxoplasmosis: which biological diagnostic tests are best suited to which clinical situations? Expert Rev Anti Infect Ther 11:943–956. doi: 10.1586/14787210.2013.825441. [DOI] [PubMed] [Google Scholar]
- 25.Tissot Dupont D, Fricker-Hidalgo H, Brenier-Pinchart MP, Bost-Bru C, Ambroise-Thomas P, Pelloux H. 2003. Usefulness of Western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis 22:122–125. doi: 10.1007/s10096-003-0887-5. [DOI] [PubMed] [Google Scholar]
- 26.Robert-Gangneux F, Commerce V, Tourte-Schaefer C, Dupouy-Camet J. 1999. Performance of a Western blot assay to compare mother and newborn anti-Toxoplasma antibodies for the early neonatal diagnosis of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis 18:648–654. doi: 10.1007/s100960050366. [DOI] [PubMed] [Google Scholar]
- 27.Bessières MH, Berrebi A, Rolland M, Bloom MC, Roques C, Cassaing S, Courjault C, Séguéla JP. 2001. Neonatal screening for congenital toxoplasmosis in a cohort of 165 women infected during pregnancy and influence of in utero treatment on the results of neonatal tests. Eur J Obstet Gynecol Reprod Biol 94:37–45. doi: 10.1016/S0301-2115(00)00300-6. [DOI] [PubMed] [Google Scholar]
- 28.Olariu TR, Press C, Talucod J, Olson K, Montoya JG. 2019. Congenital toxoplasmosis in the United States: clinical and serologic findings in infants born to mothers treated during pregnancy. Parasite 26:13. doi: 10.1051/parasite/2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomares C, Estran R, Press CJ, Bera A, Ramirez R, Montoya JG, Robert Gangneux F. 2020. Is real-time PCR targeting Rep 529 suitable for diagnosis of toxoplasmosis in patients infected with non-type II strains in North America? J Clin Microbiol 58:e01223-19. doi: 10.1128/JCM.01223-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stajner T, Bobic B, Klun I, Nikolic A, Srbljanovic J, Uzelac A, Rajnpreht I, Djurkovic-Djakovic O. 2016. Prenatal and early postnatal diagnosis of congenital toxoplasmosis in a setting with no systematic screening in pregnancy. Medicine (Baltimore) 95:e2979. doi: 10.1097/MD.0000000000002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert RE, Thalib L, Tan HK, Paul M, Wallon M, Petersen E, European Multicentre Study on Congenital Toxoplasmosis. 2007. Screening for congenital toxoplasmosis: accuracy of immunoglobulin M and immunoglobulin A tests after birth. J Med Screen 14:8–13. doi: 10.1258/096914107780154440. [DOI] [PubMed] [Google Scholar]
- 32.Naessens A, Jenum PA, Pollak A, Decoster A, Lappalainen M, Villena I, Lebech M, Stray-Pedersen B, Hayde M, Pinon JM, Petersen E, Foulon W. 1999. Diagnosis of congenital toxoplasmosis in the neonatal period: a multicenter evaluation. J Pediatr 135:714–719. doi: 10.1016/s0022-3476(99)70090-9. [DOI] [PubMed] [Google Scholar]
- 33.Lago EG, Oliveira AP, Bender AL. 2014. Presence and duration of anti-Toxoplasma gondii immunoglobulin M in infants with congenital toxoplasmosis. J Pediatr (Rio J) 90:363–369. doi: 10.1016/j.jped.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Alvarado-Esquivel C, Niewiadomski A, Schweickert B, Liesenfeld O. 2011. Antiparasitic treatment suppresses production and avidity of Toxoplasma gondii-specific antibodies in a murine model of acute infection. Eur J Microbiol Immunol (Bp) 1:249–255. doi: 10.1556/EuJMI.1.2011.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinon JM, Dumon H, Chemla C, Franck J, Petersen E, Lebech M, Zufferey J, Bessieres MH, Marty P, Holliman R, Johnson J, Luyasu V, Lecolier B, Guy E, Joynson DH, Decoster A, Enders G, Pelloux H, Candolfi E. 2001. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J Clin Microbiol 39:2267–2271. doi: 10.1128/JCM.39.6.2267-2271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capobiango JD, Monica TC, Ferreira FP, Mitsuka-Breganó R, Navarro IT, Garcia JL, Reiche EMV. 2016. Evaluation of the Western blotting method for the diagnosis of congenital toxoplasmosis. J Pediatr (Rio J) 92:616–623. doi: 10.1016/j.jpedp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Machado AS, Andrade GMQ, Januário JN, Fernandes MD, Carneiro ACAV, Carneiro M, Carellos EVM, Romanelli RMC, Vasconcelos-Santos DV, Vitor RWA. 2010. IgG and IgM Western blot assay for diagnosis of congenital toxoplasmosis. Mem Inst Oswaldo Cruz 105:757–761. doi: 10.1590/s0074-02762010000600005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.