Surrogate neutralization assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that can be done without biosafety level 3 containment and in multiple species are desirable. We evaluate a recently developed surrogate virus neutralization test (sVNT) in comparison to 90% plaque reduction neutralization tests (PRNT90) in human, canine, cat, and hamster sera. With PRNT90 as the reference, sVNT had sensitivity of 98.9% and specificity of 98.8%. Using a panel of immune sera corresponding to other coronaviruses, we confirm the lack of cross-reactivity to other coronaviruses in SARS-CoV-2 sVNT and PRNT90, except for cross-reactivity to SARS-CoV-1 in sVNT.
KEYWORDS: SARS coronavirus 2, COVID-19, serology, neutralization, seroepidemiology, human, animal, SARS-CoV-2, antibody, canine, cat, hamster, surrogate virus neutralization
ABSTRACT
Surrogate neutralization assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that can be done without biosafety level 3 containment and in multiple species are desirable. We evaluate a recently developed surrogate virus neutralization test (sVNT) in comparison to 90% plaque reduction neutralization tests (PRNT90) in human, canine, cat, and hamster sera. With PRNT90 as the reference, sVNT had sensitivity of 98.9% and specificity of 98.8%. Using a panel of immune sera corresponding to other coronaviruses, we confirm the lack of cross-reactivity to other coronaviruses in SARS-CoV-2 sVNT and PRNT90, except for cross-reactivity to SARS-CoV-1 in sVNT.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in 2019 to cause a pandemic. For seroepidemiology studies and in outbreak investigations, it is important to detect antibody responses in humans and animals to ascertain evidence of past infection with SARS-CoV-2. Antibody assays that are transferable across species are desirable because SARS-CoV-2 infects pets and other farmed animals (e.g., mink) (1–3), for monitoring antibody responses in experimental animal models (4), and in studies to identify the natural animal reservoir of SARS-CoV-2. Enzyme-linked immunosorbent assays (ELISAs) of the virus spike receptor binding domain (RBD), which has the fewest cross-reactive epitopes in common with other coronaviruses, or of the whole spike protein or nucleoprotein are widely used for detection of antibody in humans (5–7). Virus neutralization assays are used for confirming positive results but require handling live virus in biosafety level 3 (BSL-3) containment facilities or the use of a pseudotyped virus (6).
ELISAs used for detection of antibody in other animal species require assays to be reoptimized with the relevant species-specific anti-Ig conjugate for detecting immunoglobulins of each species. For some species, relevant anti-Ig reagents may not be available. The currently available generic alternative has been the use of virus neutralization test (VNTs), which usually involve handling live virus in BSL-3 containment. A “surrogate” VNT (sVNT) that can be done in BSL-2 containment has recently been reported (8). It is an assay that relies on competitive inhibition of the interaction of ACE-2 receptor coated on an ELISA plate with horseradish peroxidase (HRP)-labeled virus spike receptor binding domain. We used a panel of sera from patients or animals with real-time PCR (RT-PCR)-confirmed SARS-CoV-2 infection and corresponding controls to evaluate this sVNT in comparison to the “gold standard” 90% plaque reduction neutralization tests (PRNT90).
MATERIALS AND METHODS
Sera.
Sera from patients with RT-PCR-confirmed SARS-CoV-2 infection (n = 205) and negative controls (n = 196) collected from blood donors in 2017 were used (6). Among the 205 SARS-CoV-infected humans, 38 were <20 years of age, 68 were 21 to 40 years of age, 55 were 41 to 60 years of age, and 44 were older than 60 years, with 119 of the overall cohort being male. Disease severity ranged from asymptomatic (n = 18) to mild (n = 166) to severe, with the latter defined as disease severe enough to require >3 liters of supplemental oxygen per min (n = 19); disease severity data were unavailable for two individuals. Dogs (n = 4) and two cats with RT-PCR-confirmed SARS-CoV-2 infection were included in the study (1, 9). Dog sera (n = 40) obtained from a study of canine influenza in 2017 (10) and sera from cats (n = 59) submitted for routine veterinary diagnostic testing in 2020 were used as controls. Hamster sera from experimentally infected hamsters and controls were available from a previous study (4).
Coronavirus immune sera.
Immune sera specific for alphacoronaviruses (porcine respiratory coronavirus, feline infectious peritonitis virus, canine coronavirus, and porcine transmissible gastroenteritis virus), betacoronaviruses (SARS-coronavirus), and gammacoronaviruses (infectious bronchitis virus) were obtained from BEI Resources (animal CoV reagents were supplied to BEI Resources by Linda Saif (http://www.beiresources.org/About/BEIResources.aspx) or generated by Linda Saif (see Table 2). Stanley Perlman provided mouse serum to mouse hepatitis virus (strains A59 and JHM) (Table 1). The homologous antibody titers to the immunizing virus were also provided by Linda Saif or Stanley Perlman (Table 2).
TABLE 2.
SARS-CoV-2 sVNT and PRNT90 cross-reactivity with immune sera corresponding to other coronavirusesa
| Genus | Antiserum | Homologous Ab titer by ELISA or virus neutralization (VN) as indicated | PRNT90 titer |
sVN % inhibition |
sVN result |
|---|---|---|---|---|---|
| Alphacoronavirus | Gnotobiotic pig antiserum to porcine respiratory coronavirus—NR-460 | ELISA 1:1,200b | <1:10 | 8.39 | Negative |
| Guinea pig antiserum to feline infectious peritonitis virus—NR-2518 | ELISA 1:2,000b | <1:10 | 13.17 | Negative | |
| Guinea pig antiserum to canine coronavirus—NR-2727 | ELISA 1:4,094c | <1:10 | 17.20 | Negative | |
| Gnotobiotic pig antiserum to porcine transmissible gastroenteritis virus—NR-458 | ELISA 1:1,400b | <1:10 | 12.76 | Negative | |
| Betacoronavirus | Guinea pig anti-SARS-CoV—NR-10361 | VN 1:2,560c | <1:10 | 81.14 | Positive |
| Rabbit antiserum for SARS-CoV S protein (control)—NRC-769 | VN <1:10 | <1:10 | 13.68 | Negative | |
| Rabbit antiserum for SARS-CoV S protein (low titer)—NRC-770 | VN 1:80 | <1:10 | 78.44 | Positive | |
| Rabbit antiserum for SARS-CoV S protein (medium titer)—NRC-771 | VN 1:160 | <1:10 | 75.30 | Positive | |
| Rabbit antiserum for SARS-CoV S protein (high titer)—NRC-772 | VN 1:640 | 1:10 | 79.59 | Positive | |
| Human SARS convalescent plasma (E222P) | VN 1:160e | <1:10 | 28.39 | Positive | |
| Human SARS convalescent plasma (E229P) | VN 1:320e | <1:10 | 36.39 | Positive | |
| Mouse hepatitis virus (A59 strain) infected mouse | VN 1:1000d | <1:10 | 14.76 | Negative | |
| Hyperimmunized mouse serum to mouse hepatitis virus (JHM strain) | VN 1,1778d | <1:10 | 9.73 | Negative | |
| Gammacoronavirus | Guinea pig antiserum to infectious bronchitis virus—NR-2515 | ELISA 1:50,000b | <1:10 | 15.16 | Negative |
Abbreviations: Ab, antibody; ELISA, enzyme-linked immunosorbent assay; PRNT90, 90% plaque reduction neutralization test; SARS-CoV, severe acute respiratory syndrome coronavirus; sVN, surrogate virus neutralization. sVN inhibition of ≥20% is regarded as positive (pos) and <20% as negative (neg). All homologous antibody titers represent ELISA titers except for antibody titers to SARS-CoV and mouse hepatitis virus, which represent neutralizing antibody titers.
Homologous antibody titer data obtained from BEI Resources.
Homologous antibody titer data obtained from Linda Saif.
Homologous antibody titer data obtained from Stanley Perlman.
Neutralizing antibody titers from the Malik Peiris laboratory.
TABLE 1.
Performance characteristics of sVNT in comparison with PRNT90a
| Study group | No. sVNT pos/PRNT90 pos (PRNT titer and sVN% inhibition) |
No. sVNT neg/ PRNT90 neg |
No. sVNT pos/ PRNT90 neg |
sVNT neg/ PRNT90 pos |
Total |
|---|---|---|---|---|---|
| SARS-CoV-2 infected and control human sera | |||||
| <10 days after illness onset | 6b | 22 | 1 | 1 | 30 |
| ≥10 days after illness onset | 166b | 5 | 3 | 1 | 175 |
| Control humans collected in 2017 | 0 | 196 | 0 | 0 | 196 |
| SARS-CoV-2 infected and control canine sera | |||||
| SARS-CoV-2 infected dogs | 3 (PRNT titers 1:40, 1:80, and 1:160; sVNT inhibition 33, 66, and 78, respectively) | 1 (acute serum, later seroconverted) | 0 | 0 | 4 |
| Control dogs | 0 | 40 | 0 | 0 | 40 |
| SARS-CoV-2 infected and control cats | |||||
| SARS-CoV-2 infected cats | 2 (PRNT titers 1:320 and 1:80; sVNT inhibition 83 and 75, respectively) | 0 | 0 | 0 | 2 |
| Control cats | 0 | 59 | 0 | 0 | 59 |
| SARS-CoV-2 infected and control hamsters | |||||
| SARS-CoV-2 infected hamsters | 3 (PRNT titers 1:320, 1:640, and 1:640; sVNT inhibition 83, 83, and 83, respectively) | 0 | 0 | 0 | 3 |
| Control hamsters | 0 | 2 | 0 | 0 | 2 |
| Total | 180 | 325 | 4 | 2 | 511 |
PRNT90, 90% plaque reduction neutralization test; sVNT, surrogate virus neutralization test.
Correlations between PRNT90 titers and percent surrogate virus neutralization (sVNT%) inhibition levels shown in Fig. 1.
Serological tests.
SARS-CoV-2 surrogate virus neutralization test kits were obtained from GenScript, Inc., NJ, USA, and the tests were carried out according to the manufacturer’s instructions. The test sera (10 μl) and positive and negative controls were diluted 1:10 and mixed with an equal volume of horseradish peroxidase (HRP) conjugated to SARS-CoV-2 spike receptor binding domain (RBD) (6 ng) and incubated for 30 min at 37°C. A 100-μl volume of each mixture was added to each well on the microtiter plate coated with ACE-2 receptor. The plate was sealed and incubated at room temperature for 15 min at 37°C. Plates were then washed with wash solution and tapped dry, and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) solution was added to each well and incubated in the dark at room temperature for 15 min. The reaction was stopped by addition of 50 μl of Stop Solution to each well and the absorbance read at 450 nm in an ELISA microplate reader. The assay validity was based on values representing optical density at 450 nm (OD450) for positive and negative results falling within the range of recommended values. On the basis of the assumption that the positive and negative controls gave the recommended OD450 values, percent inhibition of each serum was calculated as follows: percent inhibition = (1 − sample OD value/negative-control OD value) × 100. Percent inhibition values of ≥20% are regarded as positive results, while percent inhibition values of <20% are regarded as negative results (8).
SARS-CoV-2 90% plaque reduction neutralization titer was determined as previously described using the virus isolate BetaCoV/Hong Kong/VM20001061/2020 (6). In brief, the assay was performed in duplicate using 24-well tissue culture plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in a biosafety level 3 facility. Serial dilutions of each serum sample were incubated with 30 to 40 plaque-forming units of virus for 1 h at 37°C. The virus-serum mixtures were added onto preformed Vero E6 cell (ATCC CRL-1586) monolayers and incubated for 1 h at 37°C in an incubator in a 5% CO2 atmosphere. The cell monolayer was then overlaid with 1% agarose in cell culture medium and incubated for 3 days, at which time the plates were fixed and stained. The antibody titer was defined as the highest serum dilution that resulted in ≥90% reduction in the number of virus plaques (PRNT90).
SARS-CoV-2 spike receptor binding domain ELISA was carried out as previously described (6). ELISA 96-well plates (Nunc MaxiSorp; Thermo Fisher Scientific) were coated overnight with 100 ng per well of the purified recombinant RBD protein in phosphate-buffered saline (PBS) buffer. The plates were then blocked with 100 μl of ChonBlock blocking/sample dilution ELISA buffer (Chondrex Inc., Redmond, WA, USA) and incubated at room temperature for 2 h. Each serum or plasma sample was tested at a dilution of 1:100 in ChonBlock blocking/sample dilution ELISA buffer and added to the ELISA wells of each plate for 2 h of incubation at 37°C. After extensive washing with PBS containing 0.1% Tween 20, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (GE Healthcare) (1:5,000) was added for 1 h at 37°C. The ELISA plates were then washed five times with PBS containing 0.1% Tween 20. Subsequently, 100 μl of HRP substrate (Ncm TMB One; New Cell and Molecular Biotech Co. Ltd., Suzhou, China) was added into each well. After 15 min of incubation, the reaction was stopped by adding 50 μl of 2 M H2SO4 solution and analyzed on a Sunrise (Tecan, Männedorf, Switzerland) absorbance microplate reader at 450-nm wavelength. The validation and optical density cutoff for a positive result were as described in the previous publication (6).
Statistical analysis and modeling.
We used Pearson’s correlation coefficient to compare correlations between assays.
Ethical statement.
Collection of serum from patients with coronavirus disease 2019 (COVID-19) was approved by the institutional review boards (IRBs) of the respective hospitals, viz. Kowloon West Cluster [KW/EX-20-039 (144-27)], Kowloon Central/Kowloon East cluster (KC/KE-20-0154/ER2), and HKU/HA Hong Kong West Cluster (UW 20-273). The collection of healthy blood donor sera performed in 2017 was approved by the IRBs of The Hong Kong University and the Hong Kong Island West Cluster of Hospitals (IRB reference number UW16-254). The canine sera used were residual sera from samples collected as part of routine clinical care, and the Committee on the Use of Live Animals in Teaching and Research (CULATR) has waived animal ethics approval. Cat sera were residual sera collected as part of routine diagnosis. The experimental study on hamsters was approved by the Committee on the Use of Live Animals in Teaching and Research, The University of Hong Kong (CULATR/5323-20).
RESULTS AND DISCUSSION
Sera from SARS-CoV-2-infected or control humans (n = 401), dogs (n = 44), cats (n = 61), or hamsters (n = 5) with known plaque reduction neutralization titer results were tested in the sVNT (Table 1). The overall concordance between the sVNT and PRNT90 was 98.9%, with 505 of 511 giving agreement between the PRNT90 and sVNT results. Of the six discrepant samples, all from SARS-CoV-2-infected humans, two sera were positive in PRNT (1:10 and 1:20) but negative in sVNT and four sera were positive in sVNT (inhibition of 23%, 32%, 54%, and 72%) but negative in PRNT90; however, three of the latter were positive at the lower-stringency PRNT cutoff value of >50% reduction in plaque counts (Table 1). Of 175 sera from SARS-CoV-2-infected humans obtained 10 or more days after onset of illness, 166 (94.9%) were positive in both assays, with 167 (95.4%) being positive by PRNT and 169 (96.6%) positive by sVNT. It is known that many patients do not seroconvert in the first few days of illness; accordingly, 22 of 30 sera collected prior to day 10 after illness onset were seronegative in both assays. Six sera collected less than 10 days after onset of illness were positive by both sVNT and PRNT90, whereas one serum sample each was positive in one but not the other assay. All 196 control human sera collected in 2017 (prior to the emergence of COVID-19) were negative in both assays. Thus, the sVNT was marginally more sensitive than PRNT90 in detecting antibody responses in persons with confirmed SARS-CoV-2 infection.
Three of 4 sera collected from two RT-PCR-positive dogs were positive in both sVNT and PRNT90. The antibody-negative serum was negative in both assays and was an early specimen collected from a dog (5 days after the dog, which had never developed symptoms, was taken to quarantine) which subsequently seroconverted as shown by results from both assays. The sera from both cats with RT-PCR-confirmed SARS-CoV-2 infections were positive in both assays, while sera from 59 control cats were negative in both assays.
Using a PRNT90 antibody titer of ≥1:10 as the gold standard for a positive antibody result, testing 511 sera from multiple species, the sVNT had 98.9% sensitivity and 98.8% specificity.
The sVNT is not meant to be quantitative as a result of testing a single serum dilution. However, there was a semiquantitative relationship between percent inhibition in the sVNT and the PRNT90 titers (Fig. 1A). The Pearson’s correlation coefficient between log-transformed 90% plaque reduction neutralization titer, calculated as , and percent inhibition in the surrogate virus neutralization test () is 0.84 (P value < 0.01).
FIG 1.
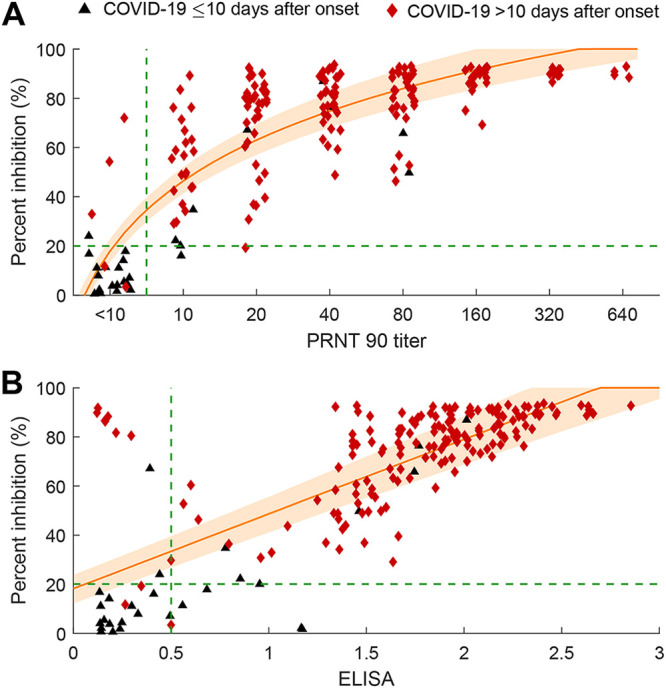
Correlation between percent inhibition in the surrogate virus neutralization test (sVNT) and (A) 90% plaque reduction neutralization test (PRNT90) titer or (B) spike RBD ELISA IgG optical density of 205 sera from humans with results confirmed by RT-PCR. (A) Correlation between PRNT90 and percent inhibition in the sVNT. We fitted a linear log regression model between percent inhibition in the sVNT (sVNT%) and log-transformed PRNT90 titers as follows: . The orange lines indicate estimated percent inhibition, and the orange shading indicates the 95% confidence interval. Blue dots represent the control samples. Black triangles represent samples from confirmed COVID-19 cases obtained less than 10 days since symptom onset, and red diamonds represent samples from confirmed cases obtained 10 days or more after symptom onset. For clarity, the control human sera are not included in the figure as they were negative in all three assays. (B) Correlation between percent inhibition in the sVNT and the spike RBD IgG ELISA optical density. We fitted a linear regression model between percent inhibition in the sVNT and the ELISA OD as follows: .
For reference, we also show the correlation between sVNT and the optical density on the spike RBD IgG antibody ELISA (Fig. 1B). The Pearson’s correlation coefficient between spike RBD ELISA IgG optical density (ELISAOD) and percent inhibition in the surrogate virus neutralization test () is 0.74 (P value < 0.01).
We investigated cross-reactivity of immune sera to a range of alphacoronaviruses, betacoronaviruses, and gammacoronaviruses in the sVNT and PRNT90, including antisera to feline infectious peritonitis virus, canine coronavirus, mouse hepatitis virus, and SARS-CoV (Table 2). Two human SARS-CoV convalescent plasma samples were also included in this assessment. Cross-reactivity in the sVNT was detected with both SARS convalescent human plasma (homologous PRNT90 titers of 1:160 and 1:320) and four of five hyperimmune sera to SARS-CoV. Cross-reactivity in the SARS-CoV-2 PRNT90 was observed only with the high-titer hyperimmune rabbit sera to SARS-CoV (homologous neutralizing antibody titer of 1:640). SARS-CoV-2 and SARS-CoV-1 are closely related sarbecoviruses, and cross-reactivity of antibody binding to the RBD of these two viruses has been previously reported (11). This is unlikely to be a practical problem in testing human sera, as very few humans were previously exposed to SARS-CoV-1, which is known not to have been circulating in the human population after 2004. It is noted that the cross-reactivity of sVNT to SARS-CoV-2 appears greater than that seen with PRNT. Thus, when sVNT is used for testing animal sera, especially bat sera, the possibility of cross-reactivity between closely related viruses within the sarbecovirus group must be kept in mind. In contrast, neither assay had cross-reactivity with immune sera raised to other betacoronaviruses, alphacoronaviruses, or gammacoronaviruses.
In summary, we found excellent concordance between the sVNT and the gold standard PRNT90 for SARS-CoV-2 antibody detection in human, dog, cat, and hamster sera. This assay would be of great utility as a species-independent and specific assay for primary testing for antibodies to sarbecoviruses (SARS-CoV-2 and SARS-CoV-1 and closely related viruses) in humans or animals. For example, in large-scale human seroepidemiology studies where ELISA is used as a screening assay, sVNT may be used to confirm the viral status of sera found to be ELISA positive. Alternatively, when investigating the natural animal reservoirs of sarbecoviruses, it may be used as a primary screening assay of animal sera, irrespective of species. There was a subquantitative relationship between PRNT90 titers and percent inhibition in the sVNT in a single serum dilution. Serial serum dilutions assayed on sVNT may improve quantitation, but this was not attempted in our study. We did not compare its performance with pseudoparticle neutralization assays for antibody, which is an alternative though technically more demanding method for testing for SARS-CoV-2 neutralization. The sVNT has the advantages of technical simplicity, speed (requiring only a few hours for completion), and the fact that it does not require cell culture facilities or BSL-3 containment.
ACKNOWLEDGMENTS
We acknowledge research funding from the Health and Medical Research Fund, Food and Health Bureau, The Government of Hong Kong Special Administration Region (grant COVID190126).
We thank Stanley Perlmann for the mouse hepatitis virus immune sera and relevant serological data. We thank Linfa Wang at Duke-NUS and GenScript for advice on the sVNT.
We declare that we have no conflict of interest.
REFERENCES
- 1.Sit THC, Brackman CJ, Ip SM, Tam KWS, Law PYT, To EMW, Yu VYT, Sims LD, Tsang DNC, Chu DKW, Perera RAPM, Poon LLM, Peiris M. 2020. Infection of dogs with SARS-CoV-2. Nature 586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman A, Smith D, Ghai RR, Wallace RM, Torchetti MK, Loiacono C, Murrell LS, Carpenter A, Moroff S, Rooney JA, Behravesh CB. 2020. First reported cases of SARS-CoV-2 infection in companion animals — New York, March–April 2020. MMWR Morb Mortal Wkly Rep 69:710–713. doi: 10.15585/mmwr.mm6923e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, Tacken MGJ, Rooij MMT, Weesendorp E, Engelsma MY, Bruschke CJM, Smit LAM, Koopmans M, Poel WHM, Stegeman A, Stegeman A. 2020. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 25:2001005. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.23.2001005. Nadia Oreshkova 1, Robert Jan Molenaar 2, Sandra Vreman 1, Frank Harders 1, Bas B Oude Munnink 3, Renate W Hakze-van der Honing 1, Nora Gerhards 1, Paulien Tolsma 4, Ruth Bouwstra 2, Reina S Sikkema 3, Mirriam Gj Tacken 1, Myrna Mt de Rooij 5, Eefke Weesendorp 1, Marc Y Engelsma 1, Christianne Jm Bruschke 6, Lidwien Am Smit 5, Marion Koopmans 3, Wim Hm van der Poel 1, Arjan Stegeman 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, Peiris M, Yen HL. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. 2020. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera RAPM, Mok CK, Tsang OT, Lv H, Ko RLW, Wu NC, Yuan M, Leung WS, Chan JMC, Chik TSH, Choi CYC, Leung K, Chan KO, Chan KCK, Li KC, Wu JT, Wilson IA, Monto AS, Poon LLM, Peiris M. 2020. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill 25:2000421. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, Bruin E, Chandler FD, Yazdanpanah Y, Hingrat QL, Descamps D, Houhou-Fidouh N, Reusken CBEM, Bosch BJ, Drosten C, Koopmans MPG, Haagmans BL. 2020. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis 26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CW, Chia WN, Qin X, Liu P, Chen MIC, Tiu C, Hu Z, Chen VCW, Young BE, Sia WR, Tan YJ, Foo R, Yi Y, Lye DC, Anderson DE, Wang LF. 2020. A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction. Nat Biotechnol 38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 9.Barrs VR, Peiris M, Tam KWS, Law PYT, Brackman CJ, To EMW, Yu VYT, Chu DKW, Perera RAPM, Sit THC. 2020. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerg Infect Dis 26(12). doi: 10.3201/eid2612.202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su W, Kinoshita R, Gray J, Ji Y, Yu D, Peiris JSM, Yen HL. 2019. Seroprevalence of dogs in Hong Kong to human and canine influenza viruses. Vet Rec Open 6:e000327. doi: 10.1136/vetreco-2018-000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv H, Wu NC, Tsang OT, Yuan M, Perera RAPM, Leung WS, So RTY, Chan JMC, Yip GK, Chik TSH, Wang Y, Choi CYC, Lin Y, Ng WW, Zhao J, Poon LLM, Peiris JSM, Wilson IA, Mok CKP. 2020. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep 31:107725. doi: 10.1016/j.celrep.2020.107725. [DOI] [PMC free article] [PubMed] [Google Scholar]


