Abstract
Toxoplasmosis is an infection caused by Toxoplasma gondii, an intracellular protozoan that is often associated with immunocompromised patients and is rare in immunocompetent. A 60-year-old man was admitted with a history of 2 days of headache and right-sided weakness. There was no history of fever, surgeries, or any other comorbid illness. Cerebrospinal fluid showed just mild pleocytosis with 15 cells/mm3, predominantly lymphomononuclear. MRI showed Peripheral enhancing lesion with central diffusion restriction and perivascular enhancing lesion with restricted diffusion with vasogenic edema and leptomeningeal enhancement in the white matter.
Viral serologies, tumor markers, protein electrophoresis were normal. The patient was submitted to brain biopsy, revealing necrotic brain parenchyma with predominantly acute inflammation, with diffuse encephalitis pattern, and cysts with bradyzoites (cystozoites) of Toxoplasma gondii in the brain parenchyma. The central nervous system infection by Toxoplasma gondii can present as meningoencephalitis during primary infection in an immunocompetent, although it is rare. Central nervous system lymphoma is the main differential diagnosis of neurotoxoplasmosis by imaging, especially in our case.
Keywords: Encephalitis, Parasitic infections, Neurotoxoplasmosis, Immunocompetent
Introduction
Toxoplasmosis is an infection caused by Toxoplasma gondii, an intracellular protozoan. Human beings can be infected by ingestion of undercooked, raw meat or water/food containing cysts/oocysts and most individuals are infected inadvertently [9]. Factors such as virulence of the organism, sex, genetic phenomena, and immunity seem to affect the course of the disease and seem to affect the course of infection [13]. Damage to the CNS (central nervous system) by T gondii is characterized by many focuses of enlarging necrosis and microglia nodules [8].
Neurotoxoplasmosis is often associated with immunocompromised patients, however, is often rare in immunocompetent ones. In healthy individuals, the acquired infection is usually asymptomatic causing self-limited lymphadenopathy or mononucleosis-like syndrome [3].
Case report
We present a case of a 60-year-old man who was admitted with 2 days history of headache and right-sided weakness without altered state of consciousness. There was no history of fever, surgeries or any other comorbid illness. Neurologic examination included right hemiparesis. Cerebrospinal fluid showed (CFS) just mild pleocytosis (15 cells/mm3, predominantly lymphomononuclear). Glucose, protein and flow cytometry immunophenotypic analysis were normal. Viral serologies, tumor markers, protein electrophoresis were normal. Immunodeficiency was excluded with T-3 lymphocyte count, serum immunoglobulin levels and normal complement. Computerized tomography of the abdomen and thorax without changes.
MRI showed (Fig. 1, Fig. 2, Fig. 3) perivascular enhancing lesion with restricted diffusion with vasogenic edema and leptomeningeal enhancement in the frontoparietal white matter. Spectroscopy showed an elevated lipid lactate peak. Perfusion demonstrated increased microvascular permeability.
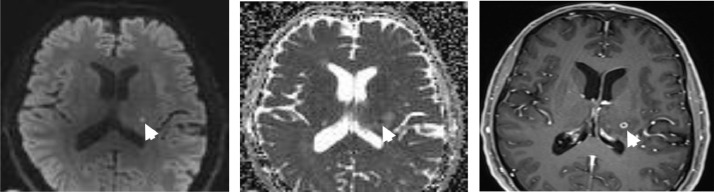
Fig. 1.
Axial DWI (A), ADC (B), and contrast-enhanced T1-weighted image (C). Peripheral enhancing lesion with central diffusion restriction in the left thalamus consistent with microabscess (white arrow).
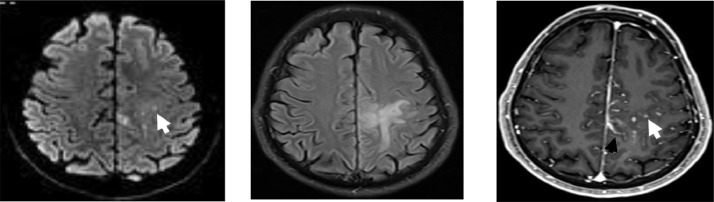
Fig. 2.
Axial DWI (A), FLAIR (C), and contrast-enhanced T1-weighted image (D). Perivascular enhancing lesion with restricted diffusion (white arrow) with vasogenic edema (C) and leptomeningeal enhancement (black arrow) in the frontoparietal white matter.
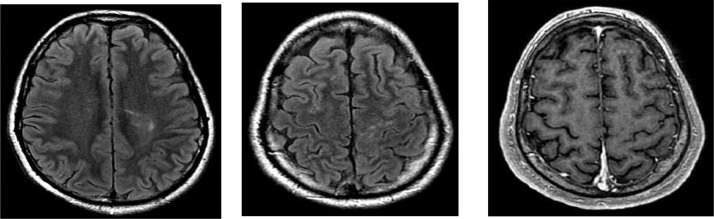
Fig. 3.
Axial FLAIR (A, B) and contrast-enhanced T1-weighted image (C). MRI after treatment showing resolution of perivascular enhancing lesions, vasogenic edema, leptomeningeal enhancement, and microabscess.
Initially, a diagnosis of neoplastic lymphoproliferative disorder was suspected due to the patient's age, history, and imaging features, although it may not rule out inflammatory/infectious diseases. The investigation was complemented with brain biopsy, revealing (Fig. 4) necrotic brain parenchyma with predominately acute inflammation, with diffuse encephalitis pattern and cysts with bradyzoites (cystozoites) of T gondii. Treatment is consisted of sulfadiazine and pyrimethamine for 6 weeks. His neurological features improved completely as well as resonance findings, showing regression of the lesions with no pathological contrast enhancement.
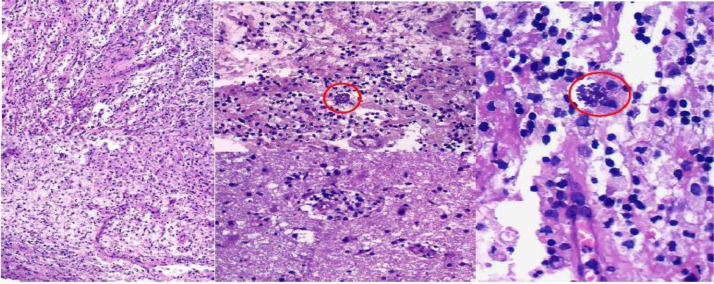
Fig. 4.
Histopathology of brain biopsy (H&E stain). (A) Necrosis, inflammatory infiltrate with macrophages and lymphocytes, original magnification 200×. (B, C) Cysts with bradyzoites (cystozoites) of Toxoplasma gondii (red circle), original magnification 400×, 1000×. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion
Up to one-third of the world's population is infected by T gondii [4]. Infection with T gondii may be subclinical or it may cause clinical signs and symptoms that vary according to the patient's immune status and their clinical situation. Immunocompetent hosts have a primary asymptomatic and self-limited T gondii infection, which usually does not require treatment [2,12].
A definitive diagnosis of neurotoxoplasmosis requires a compatible clinical context and brain imaging and also the detection of the protozoan in a biopsy [1]. The treatment of choice is a combination of sulfadiazine and pyrimethamine for 6 weeks [10]. In an immunocompetent host the probability of CNS infection by T gondii is low and meningoencephalitis as a primary infection is rare in this group of patients [5]. Therefore, the diagnosis is not usually considered initially.
The spectrum of neurological symptoms includes headache, altered mental status, visual disturbances, seizures, cranial nerve abnormalities, and sensory disturbances. The most common neurological signs include motor weakness and speech disturbances [7].
On MRI, neurotoxoplasmosis presents as hypointense lesions on T1-weighted images and may show peripheral hyperintensity. The lesions on T2 and FLAIR images have high or mixed signal intensity. On contrast-enhanced T1-weighted images, the lesions show rim-like enhancement with surrounding hypointense areas. The most common affected areas in CNS include the basal ganglia, corticomedullary junction, white matter and periventricular regions [7,11]. The imaging features reflect the pathogenesis of reactivation and hematogenous spread, with a reduced inflammatory response depending upon the immune status [14].
Central nervous system lymphoma (PCNSL) is the main differential diagnosis of neurotoxoplasmosis by imaging, especially in our case. They have in common findings of unifocal or multifocal involvement that may occur anywhere in the brain, as well as varied patterns of enhancement, edema and mass effect, with hyperintense signal on T2-weighted MRI images and predilection for the basal ganglia [7,11].
PCNSL has predilection for the periventricular and superficial regions, often in contact with ventricular or meningeal surfaces and linear enhancement along perivascular spaces that is highly suggestive of PCNSL. Both perfusion MR imaging and perfusion CT may demonstrate increased microvascular permeability in tumor tissue and MR spectroscopy has demonstrated elevated lipid peaks combined with high Cho/Cr12 [6].
Conclusion
We present a case of an immunocompetent patient with focal signs and neuroimaging demonstrating lesion of undetermined meaning. MRI showed a perivascular enhancing lesion and contact with meningeal surfaces, which suggested the possibility of PCNSL but without enough findings that could rule out infectious or inflammatory disorders, therefore cerebral biopsy was proceeded.
Neurotoxoplasmosis should be considered as an important differential diagnosis in immunocompetent patients with neurological findings that suggest lymphoproliferative disease.
Footnotes
Competing Interests: None.
References
- 1.AIDSinfo. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf [accessed 26.06.19].
- 2.Akturk HK, Sotello D, Ameri A, Abuzaid AS, Rivas AM, Vashisht P. Toxoplasma infection in an immunocompetent host: possible risk of living with multiple cats. Cureus. 2017;9(3):e1103. doi: 10.7759/cureus.1103. Published 2017 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurt C, Tammaro D. Diagnostic evaluation of mononucleosis-like illnesses. Am J Med. 2007;120(10) doi: 10.1016/j.amjmed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7(1) 1–17. 7. [PMC free article] [PubMed] [Google Scholar]
- 5.Dukes CS, Luft BJ, Durak DT. Toxoplasmosis. In: Scheld WM, Whitley RJ, Durak DT, editors. Infections of the central nervous system. Lippincott-Raven; Philadelphia: 1997. pp. 785–804. [Google Scholar]
- 6.Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6):984–992. doi: 10.3174/ajnr.A2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GT, Antelo F, Mlikotic AA. Cerebral toxoplasmosis. RadioGraphics. 2009;29:1200–1205. doi: 10.1148/rg.294085205. [DOI] [PubMed] [Google Scholar]
- 8.Luft BJ, Conley F, Remington JS, Laverdiere M, Wagner KF, Levine JF. Outbreak of central-nervous system toxoplasmosis in western Europe and North America. Lancet. 1983;1(8328):781–784. doi: 10.1016/s0140-6736(83)91847-0. [DOI] [PubMed] [Google Scholar]
- 9.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 10.Nath A, Sinai AP. Cerebral toxoplasmosis. Curr Treat Options Neurol. 2003;5(1):3–12. doi: 10.1007/s11940-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran R, Radhan P, Anand R, Subramanian I, Santosham R, Sai V. CNS toxoplasmosis in an immunocompetent individual. Radiol Case Rep. 2014;1:908. doi: 10.2484/rcr.v9i1.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis [published correction appears in Clin Microbiol Rev. 2012 Jul;25(3):583] Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su C, Howe DK, Dubey JP, Ajioka JW, Sibley LD. Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proc Natl Acad Sci USA. 2002;99(16):10753–10758. doi: 10.1073/pnas.172117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vastava PB, Pradhan S, Jha S, Prasad KN, Kumar S, Gupta RK. MRI features of toxoplasma encephalitis in the immunocompetent host: a report of two cases. Neuroradiology. 2002;44(10):834–838. doi: 10.1007/s00234-002-0852-5. Epub 2002 Aug 24. PMID: 12389133. [DOI] [PubMed] [Google Scholar]