Abstract
Anaphylaxis is a serious allergic life-threatening condition that needs immediate treatment to prevent unfavorable outcomes. The present study revealed that the prevalence of anaphylaxis in children increased with age and the adolescent group has the most frequent anaphylaxis events. Food-induced anaphylaxis was the most common cause of anaphylaxis in pediatric population. This etiology revealed a significant correlation with a known history of food allergy in the study population, P = .029. Anaphylaxis from insect stings associated with cardiovascular symptoms, P < .001 and inclined to be severe. Univariate analysis showed predicted probability of severe anaphylaxis increased with age with an odds ratio of 1.1. This finding strengthens and supports the view that physicians should be aware of severe anaphylaxis reactions in older age group when there is a documented history of insect sting. The overall pediatric anaphylaxis patients in the study population had favorable outcomes. The authors recommended at least 24 hours hospital observation in order to observe biphasic reaction in pediatric anaphylaxis especially in adolescent age group.
Keywords: anaphylaxis, epidemiology, pediatric, treatment
Introduction
Anaphylaxis is a serious allergic life-threatening condition.1 This condition is unpredictable and has various allergic triggers. Several etiological mechanisms of anaphylaxis were described including; immunologic mechanism from Immunoglobulin E (IgE)-dependent (foods, insect stings, drugs, aeroallergens, etc.) and IgE-independent (Non-Steroidal Anti-Inflammatory Drugs), non-immunologic mechanism (direct mast cell stimulation-exercise, ethanol, opioids), and idiopathic anaphylaxis.2 Anaphylaxis has several manifestations such as rashes (typically urticaria),3 angioedema, respiratory symptoms (wheezing, shortness of breath), gastrointestinal symptoms (abdominal pain, diarrhea), and cardiovascular symptoms (hypotension, tachycardia, syncope). Even though there are many faces of its presentation, diagnosis of anaphylaxis can be made by the consensus clinical criterions.2-5 However, in an emergency context with a limitation of patient history taking to find out the causative triggering allergens, some anaphylaxis cases were misdiagnosed.6 This can cause morbidity and mortality consequences since this condition needs an immediate specific treatment.5,7-11 Anaphylaxis in children has various allergic triggers. The European Anaphylaxis Registry confirmed food as the major elicitor of anaphylaxis in children, specifically hen’s egg, cow’s milk and nuts.12 However, the study of anaphylaxis in different geographic areas may have different characteristic and raises the need of awareness to improve prevention and medical care. This became the objective of our study to determine the incidences, causes, characteristics, treatments and outcomes of anaphylaxis in pediatric population in an emergency context of a tertiary care university hospital in Thailand.
Material and Methods
Data Collection
Retrospective data from the official electronic medical record (Health Object® program) used for statistical analysis. All diagnosed anaphylaxis patients at the Emergency Department, tertiary care pediatric referral center, Faculty of Medicine, Khon Kaen University, Thailand, between January 2016 and December 2019 were included in the study. Diagnosis of anaphylaxis based on recorded diagnosis from ICD-10 and confirmed meeting criteria of anaphylaxis evaluated by certified pediatricians.
Statistical Methods
At the end of the study, the collected data were analyzed using STATA software version 10 (StataCorp LP). Descriptive statistical methods-means, standard deviations (SDs), medians, and frequencies-were used to analyze the demographic data. Values of P < .05 are considered to indicate statistical significance. Multinomial logistic regression and post hoc analysis used to test for the association between etiologic factors of anaphylaxis.
Ethical Approval and Informed Consent
The study was approved by the institutional review board of Faculty of Medicine, Khon Kaen University, Thailand (IRB no. #HE631544) before enrolling any participants.
Results
There were 76 anaphylaxis pediatric patients in total at the Emergency Department during the study period. The incidence ranged 1.03 to 3.21/1000-person-year. Table 1 showed incidence of pediatric anaphylaxis during 2016 to 2019 in the study population. The age ranged from 5 months to 18 years old. The median age was 14.54 years, IQR 12.04 to 16.83. The mean age was 13.72, SD 3.88. There were 49 (64.47%) boys and 27 (35.53%) girls. Making male to female ratio was 1.8: 1.
Table 1.
Incidence of Pediatric Anaphylaxis During 2016 to 2019 in the Study Population.
| Year | Pediatric emergency visits (N) | Pediatric anaphylaxis (n) | Incidence/1000 person-year | 95% CI |
|---|---|---|---|---|
| 2016 | 10 689 | 11 | 1.03 | 5.10-1.84 |
| 2017 | 9688 | 16 | 1.65 | 9.40-2.68 |
| 2018 | 9983 | 32 | 3.21 | 2.19-4.52 |
| 2019 | 10 323 | 17 | 1.65 | 9.60-2.64 |
The authors classified patients into 4 different age groups as follows; (i) Infant (<1 year old), (ii) Pre-school age (1-6 years old), (iii) School age (7-12 years old), and (iv) Adolescent (13-18 years old). The most prevalent age group of anaphylaxis patients in the study population was found in adolescent group (52 cases, 68.42%), followed by school age group (19 cases, 25%), pre-school age group (3 cases, 3.95%), and infantile age group (2 cases, 2.63%).
Causes of anaphylaxis identified. Anaphylaxis from food was recorded as the most frequent cause in the present pediatric population (28 cases, 36.8%), followed by idiopathic anaphylaxis, insect stings, drugs, infection-induced, and exercise-induced anaphylaxis. Table 2 describes demographic information of anaphylaxis cases from various different etiologies.
Table 2.
Demographic Information of Anaphylaxis Cases from Different Etiologies.
| Variable | Total (n = 76) | Cause of anaphylaxis | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Idiopathic (n = 24) | Infection (n = 1) | Foods (n = 28) | Insect stings (n = 11) | Exercise (n = 1) | Drugs (n = 11) | |||
| Gender | ||||||||
| Male | 49 (64.47) | 15 (62.5) | 1 (100) | 20 (71.43) | 9 (81.82) | 1 (100) | 3 (27.27) | .080 |
| Female | 27 (35.53) | 9 (37.5) | 0 (0) | 8 (28.57) | 2 (18.18) | 0 (0) | 8 (72.73) | |
| Age (years) | ||||||||
| Mean ± SD | 13.72 (3.88) | 15.46 (2.21) | 8.17 | 12.68 (4.77) | 13.51 (3.46) | 14.08 | 13.24 (3.79) | |
| Age | ||||||||
| ≤1 year | 2 (2.63) | 0 (0) | 0 (0) | 2 (7.14) | 0 (0) | 0 (0) | 0 (0) | .737 |
| 1-6 years | 3 (3.95) | 0 (0) | 0 (0) | 1 (3.57) | 1 (9.09) | 0 (0) | 1 (9.09) | |
| 7-12 years | 19 (25) | 4 (16.67) | 1 (100) | 8 (28.57) | 3 (27.27) | 0 (0) | 3 (27.27) | |
| 13-18 years | 52 (68.42) | 20 (83.33) | 0 (0) | 17 (60.71) | 7 (63.64) | 1 (100) | 7 (63.64) | |
| Underlying disease | ||||||||
| Known food allergy | 10 (13.16) | 0 (0) | 1 (100) | 6 (21.43) | 1 (9.09) | 0 (0) | 2 (18.18) | .029* |
| Atopic dermatitis | 9 (11.84) | 3 (12.5) | 0 (0) | 4 (14.29) | 1 (9.09) | 0 (0) | 1 (9.09) | .988 |
| Allergic rhinitis | 8 (10.53) | 2 (8.33) | 1 (100) | 1 (3.57) | 2 (18.18) | 0 (0) | 2 (18.18) | .042 |
| Asthma | 19 (25) | 5 (20.83) | 0 (0) | 9 (32.14) | 0 (0) | 0 (0) | 5 (45.45) | .169 |
| Fever | ||||||||
| No | 69 (90.79) | 21 (87.5) | 0 (0) | 27 (96.43) | 10 (90.91) | 1 (100) | 10 (90.91) | .045 |
| Yes | 7 (9.21) | 3 (12.5) | 1 (100) | 1 (3.57) | 1 (9.09) | 0 (0) | 1 (9.09) | |
| Length of stay (hours) | ||||||||
| Median (Min-max) | 24 (8-72) | 24 (8-48) | 72 | 24 (24-72) | 24 (24-48) | 24 | 24 (24-48) | .054 |
| Median (IQR) | 24 (24-24) | 24 (24-24) | 72 | 24 (24-48) | 24 (24-24) | 24 | 24 (24-48) | |
| Mean ± SD | 29.89 (14.57) | 24.67 (12.46) | 72 | 34.29 (16.56) | 26.18 (7.24) | 24 | 30.55 (11.21) | |
| Presenting symptoms | ||||||||
| Urticarial rash | 75 (98.68) | 24 (100) | 1 (100) | 28 (100) | 11 (100) | 1 (100) | 10 (90.91) | .307 |
| Angioedema | 24 (31.58) | 4 (16.67) | 0 (0) | 6 (21.43) | 7 (63.64) | 0 (0) | 7 (63.64) | .010* |
| Respiratory | 67 (88.16) | 22 (91.67) | 0 (0) | 23 (82.14) | 10 (90.91) | 1 (100) | 11 (100) | .065 |
| Gastrointestinal | 16 (21.05) | 3 (12.5) | 1 (100) | 10 (35.71) | 1 (9.09) | 0 (0) | 1 (9.09) | .060 |
| Cardiovascular | 7 (9.21) | 2 (8.33) | 0 (0) | 0 (0) | 4 (36.36) | 1 (100) | 0 (0) | <.001* |
Value of P < .05 indicates statistical significance.
There were 46 cases with underlying atopic diseases. These included asthma (19 cases, 25%), atopic dermatitis (9 cases, 11.84%), allergic rhinitis (8 cases, 10.53%), and known food allergy (10 cases, 13.16%). History of known food allergy significantly correlated to the causes of anaphylaxis from food in the study population, P-Value .029, Table 2.
Presenting symptoms of anaphylaxis patients in the study population included urticaria, angioedema, respiratory symptoms, gastrointestinal symptoms, and cardiovascular symptoms. Majority of cases (75 cases, 98.68%) had urticaria. Sixty-seven cases (88.16%) had respiratory symptoms, mostly with the presentation of wheezing, dyspnea, and chest discomfort symptoms. Twenty-four cases (31.58%) had angioedema. Sixteen cases (21.05%) had gastrointestinal symptoms such as abdominal pain, abdominal cramp, and diarrhea. Seven cases (9.21%) had cardiovascular symptoms.
All patients received intramuscular adrenaline as an emergency treatment of anaphylaxis. Seventy-four cases (97.37%) had this treatment at the emergency room. Only 2 cases received the treatment after the hospital admissions. There were 3 cases (3.94%) of biphasic anaphylaxis whom received second doses of intramuscular adrenaline.
Various types of systemic corticosteroids used to treat anaphylaxis patients in the study population. There were intravenous dexamethasone (72.37%), intravenous hydrocortisone (40.79%), and oral prednisolone (56.58%). Antihistamines were put-to-use in the present population. H1-blockers (chlopheniramine, ceterizine) prescribed in most of the patients. H2-blocker (ranitidine) was prescribed in 77 cases (88.16%).
The outcome of anaphylaxis in pediatric population in the present study was good. The mean length of hospital stay was 29.89 hours (SD = 14.57). There was no mortality case recorded in the present study population.
Discussion
The average lifetime prevalence of anaphylaxis is estimated at 0.05% to 2% in the USA and ~3% in Europe.13 However, the pooled community-based prevalence of anaphylaxis in the general Asian population remains unknown. In Thailand, a study in Bangkok during 2008 to 2013 revealed a prevalence of pediatric anaphylaxis at 2.7 to 4.51 cases/1000 pediatric admissions.14 A recent study from the north part of Thailand showed an incidence of pediatric anaphylaxis at 3.0 to 4.9 episodes/100 000 visits during 2007 to 2016.15 The present study had data that are more recently collected during 2016 to 2019. We found a higher incidence of pediatric anaphylaxis in our setting at 1.03-3.21/1000-person-year. This higher incidence in the more recent years had a correlation to the overall world data that have found an increase in incidences of anaphylaxis over the years.
Anaphylaxis is more common in adults compared to young children. Guidelines from the World Allergy Organization (WAO) have reported that teenagers, pregnant women, and elderly are at higher risk for anaphylaxis due to various reasons.15,16 The present study revealed a similar pattern that the rate of anaphylaxis increased with age. The highest prevalence found in pediatric population was in adolescent group (68.42%), however, the present data did not show a statistical difference compared to the other age groups.
The causes of anaphylaxis described and thought to vary among different age groups. Anaphylaxis from food was the most prevalent in pediatric population.10,18-20 The findings correlated to the present study that found food-induced anaphylaxis as the most frequent cause. This etiology has found to increase during the following years. Figure 1 showed yearly percentage of anaphylaxis with the different etiologies.
Figure 1.
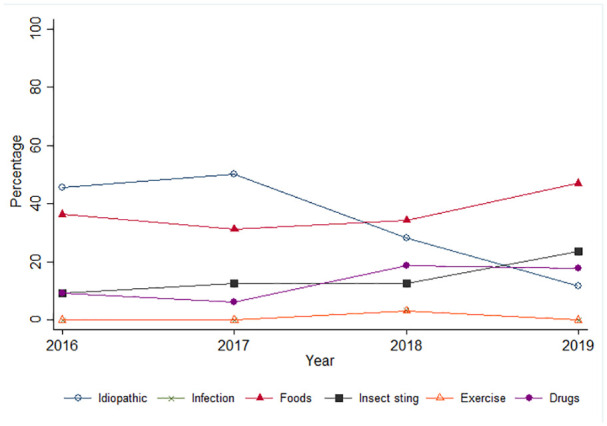
Yearly percentage of anaphylaxis with the different etiologies.
Incidence of food anaphylaxis in people with food allergy was low at all ages.21 This may be because people tend to avoid contact with food that they are allergic to. However, food anaphylaxis in people with food allergy still appeared to be high in young children. This finding is correlated to the present study in pediatric population that revealed a significant difference of the food-induced anaphylaxis when patients had a known history of food allergy compared to the other causes of anaphylaxis, P = .029, Table 2. This finding strengthen supports that food-induced anaphylaxis is still a common cause of anaphylaxis in children and pediatricians should look for this trigger allergen especially in young age groups.
The present study also found that different age groups revealed different anaphylaxis etiologies. Anaphylaxis from insect stings and drugs found to be more frequent in adolescent age group. A different common cause of anaphylaxis in a different age group may help physicians to narrow down for a history taking during the emergency visit.
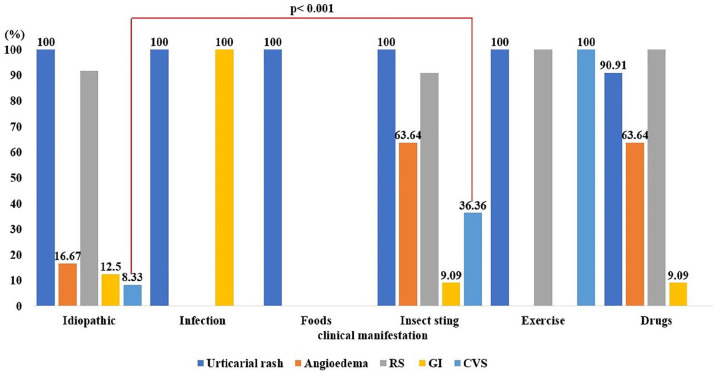
Urticaria was the most common presentation of anaphylaxis in the study population (98.68%), followed by respiratory symptoms (88.16%), angioedema (31.58%), gastrointestinal symptoms (21.05%), and cardiovascular symptoms (9.21%). Having cardiovascular symptoms such as hypotension and tachycardia may indicate severe anaphylaxis.22 The present study revealed that cardiovascular symptoms found were more frequent in anaphylaxis from insect stings compared to other causes, P < .001, Figure 2. Anaphylaxis to insect stings has occurred in 3% of adults and can be fatal even on the first reaction.23 Thus, physicians should be aware of severe anaphylaxis reaction when a history of insect sting is documented. For future prevention of sting anaphylaxis, venom immunotherapy is 75% to 98% effective.23
Figure 2.
Clinical presentation of pediatric anaphylactic patients among different etiologies. Cardiovascular symptoms found were more frequent in anaphylaxis from insect stings compared to other causes, P < .001.
All patients in the study population received intramuscular adrenaline as an emergency treatment of anaphylaxis according to a standard recommendation guideline.5,7,9,16,18 Seventy-four cases (97.37%) had this treatment at the emergency room. Only 2 cases received the treatment after the hospital admissions. The number of proper intramuscular adrenaline for anaphylaxis treatment at the emergency room in the study population was high compared to some other previous reports.11 This is because of the present study retrospectively included patients diagnosed with anaphylaxis in a tertiary care setting. Physicians at the emergency department had proper standard guideline for anaphylaxis treatment. Moreover, in those controversial cases, physicians were able to consult on call pediatricians who were available for 24 hours at all time. However, there might have some anaphylaxis misdiagnosed, or under recorded as a limitation in a retrospective study design.
There were 3 cases (3.94%) of biphasic anaphylaxis whom received second doses of intramuscular adrenaline. All 3 cases found were in adolescent age and all cases were caused from food-induced. The prevalence of biphasic anaphylaxis in the study population was relatively low as compared to adult population. Regarding the development of biphasic reactions, symptoms, the number of systems of symptoms and severity of the initial reactions, and treatment with adrenaline and corticosteroid were not clearly related with biphasic reactions.24 Biphasic anaphylaxis can occur in a variety of times after the first episode. The traditional recommended emergency department observation time is 4 to 6 hours after complete resolution of symptoms for every anaphylaxis patient.25 However, 3 cases in the present study had biphasic reaction at 10, 12, 18 hours after the first episode respectively. There has been great controversy regarding whether the standard of care to observe 4 to 6 hours is evidence-based. From the present findings, however, we recommended at least 24 hours hospital observation in order to observe biphasic reaction in pediatric anaphylaxis especially in adolescent age groups.
Having cardiovascular symptoms and/or biphasic reactions are defined as severe anaphylaxis. Previous studies revealed that some factors such as having underlying asthma was a predictor of severe presentation.22,24,25 Nevertheless, the present study did not find any such association regarding patients’ underlying diseases. However, from univariate analysis, the association of severe presentation found an odds ratio of 1.1 for every year increase in age. This finding showed a trend of severe presentation in older age group. Figure 3 showed predicted probability of severe anaphylaxis based on patient’s age.
Figure 3.
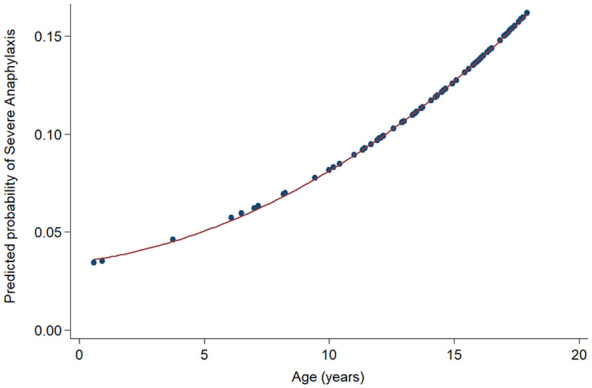
Predicted probability of severe anaphylaxis based on patient’s age, Crude OR (95% CI) 1.10 (0.88-1.38).
The overall pediatric anaphylaxis patients in the study population had favorable outcomes. All patients were hospital admitted. The mean length of hospital stay was 29.89 hours (SD 14.57). No mortality case reported in the present study.
Limitations
The main limitation of this study was a retrospective study design that may have caused missing of some unrecorded data. One third of the study population (31.57%) documented were idiopathic anaphylaxis in which some of them might have unrecorded specific etiologies. Even though some factors did not show a significant statistical information, but the present study has shown a trend that correlated to other previous studies from the other geographic regions of the world. Future prospective study with more sample size may reveal a significant information in Asian children population.
Conclusion
The present study revealed that the prevalence of anaphylaxis increased with age and adolescent group has the most frequent anaphylaxis events. The most common etiology found was in food-induced anaphylaxis. This etiology revealed a significant correlation with a history of known food allergy in the study population, P = .029. This finding strengthen supports that food-induced anaphylaxis is still a common cause of anaphylaxis in children and pediatricians should look for this trigger allergen especially in younger age group. Cardiovascular symptoms that indicated severe anaphylaxis found were more frequent in anaphylactic patients from insect stings, P < .001. Physician should be aware of severe anaphylaxis reactions when there is a documented history of insect sting. The overall pediatric anaphylaxis patients in the study population had favorable outcomes. Three cases of biphasic reactions documented in adolescent age group, all of them had food-induced etiology. The authors recommended at least 24 hours hospital observation in order to observe biphasic reaction in pediatric anaphylaxis especially in adolescent age groups.
Acknowledgments
We would like to acknowledge Mr. Gurdeep Singh, for editing the manuscript via publication clinic KKU, Thailand.
Footnotes
Authors’ Contribution: LT contributed to the conception and design of the study, data analysis, interpretation of findings, drafting the article, revising the article, and final approval of the version submitted. RU, PP, and DM contributed to study conception and data collection. JC contributed to data processing and data analysis, critical revision of the article, and final approval of the version submitted.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Leelawadee Techasatian  https://orcid.org/0000-0003-4668-6792
https://orcid.org/0000-0003-4668-6792
Availability of Data and Material: Data and material are available up on request via corresponding author, L. Techasatian.
References
- 1. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simons FER, Ardusso LRF, Bilò MB, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Techasatian L, Phungoen P, Chaiyarit J, et al. Etiological and predictive factors of pediatric urticaria in an emergency context. BMC Pediatr. 2021;21(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380. [DOI] [PubMed] [Google Scholar]
- 5. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.e1-42. [DOI] [PubMed] [Google Scholar]
- 6. Onsoi W, Chaiyarit J, Techasatian L. Common misdiagnoses and prevalence of dermatological disorders at a pediatric tertiary care center. J Int Med Res. 2020;48: 300060519873490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sicherer SH, Simons FER,; SECTION ON ALLERGY AND IMMUNOLOGY. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139: e20164006. [DOI] [PubMed] [Google Scholar]
- 8. Sidhu N, Jones S, Perry T, et al. Evaluation of anaphylaxis management in a pediatric emergency department. Pediatr Emerg Care. 2016;32:508-513. [DOI] [PubMed] [Google Scholar]
- 9. Russell S, Monroe K, Losek JD. Anaphylaxis management in the pediatric emergency department: opportunities for improvement. Pediatr Emerg Care. 2010;26:71-76. [DOI] [PubMed] [Google Scholar]
- 10. Lee WS, An J, Jung Y-H, et al. Characteristics and treatment of anaphylaxis in children visiting a pediatric emergency department in Korea. BioMed Res Int. 2020; 2020:2014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubus J-C, Lê M-S, Vitte J, et al. Use of epinephrine in emergency department depends on anaphylaxis severity in children. Eur J Pediatr. 2019;178:69-75. [DOI] [PubMed] [Google Scholar]
- 12. Anagnostou K. Anaphylaxis in children: epidemiology, risk factors and management. Curr Pediatr Rev. 2018;14: 180-186. [DOI] [PubMed] [Google Scholar]
- 13. Panesar SS, Javad S, de Silva D, et al. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68:1353-1361. [DOI] [PubMed] [Google Scholar]
- 14. Manuyakorn W, Benjaponpitak S, Kamchaisatian W, Vilaiyuk S, Sasisakulporn C, Jotikasthira W. Pediatric anaphylaxis: triggers, clinical features, and treatment in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2015; 33:281-288. [DOI] [PubMed] [Google Scholar]
- 15. Rangkakulnuwat P, Sutham K, Lao-Araya M. Anaphylaxis: ten-year retrospective study from a tertiary-care hospital in Asia. Asian Pac J Allergy Immunol. 2020; 38:31-39. [DOI] [PubMed] [Google Scholar]
- 16. Simons FER, Ardusso LRF, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193-204. [DOI] [PubMed] [Google Scholar]
- 17. Simons FER, Ebisawa M, Sanchez-Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015; 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nunez J, Santillanes G. Anaphylaxis in pediatric patients: early recognition and treatment are critical for best outcomes. Pediatr Emerg Med Pract. 2019;16:1-24. [PubMed] [Google Scholar]
- 19. De Vera MJ, Tagaro IC. Anaphylaxis diagnosis and management in the Emergency Department of a tertiary hospital in the Philippines. Asia Pac Allergy. 2020;10:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Commins SP. Outpatient emergencies: anaphylaxis. Med Clin North Am. 2017;101:521-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Umasunthar T, Leonardi-Bee J, Turner PJ, et al. Incidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2015;45: 1621-1636. [DOI] [PubMed] [Google Scholar]
- 22. Olabarri M, Vazquez P, Gonzalez-Posada A, et al. Risk factors for severe anaphylaxis in children. J Pediatr. 2020;225:193-197.e5. [DOI] [PubMed] [Google Scholar]
- 23. Golden DBK. Anaphylaxis to insect stings. Immunol Allergy Clin North Am. 2015;35:287-302. [DOI] [PubMed] [Google Scholar]
- 24. Nomura T, Sekii H, Sugita M, Nakahara S. Association between biphasic reactions and the systems of symptoms and treatment in patients with anaphylaxis hospitalized from the emergency department. Acute Med Surg 2020; 7:e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: a review of the literature and implications for emergency management. Am J Emerg Med. 2018;36:1480-1485. [DOI] [PubMed] [Google Scholar]