Abstract
Mesenchymal stem cells (MSCs) possess regenerative and immunomodulatory properties and can control the immune dysregulation that leads to β-cell destruction. Stem-cell transplantation could thus manage insulin-dependent diabetes mellitus (IDDM) in dogs. In this pilot study, we aimed to assess canine adipose tissue-derived MSCs (cAT-MSCs) transplantation as a treatment for canine diabetes mellitus. This study included four dogs with over a year of insulin treatment for IDDM, following diagnosis at the Veterinary Medicine Teaching Hospital of Seoul National University. Allogenic cAT-MSCs were infused intravenously three or five times monthly to dogs with IDDM. Blood and urine samples were obtained monthly. General clinical symptoms, including changes in body weight, vitality, appetite, and water intake were assessed. Three of the four owners observed improvement of vitality after stem cell treatment. Two of the four dogs showed improvement in appetite and body weight, polyuria, and polydipsia. C-peptide has increased by about 5–15% in three of the cases, and fructosamine and HbA1c levels have improved in two of the cases. Hyperlipidemia was resolved in two of the dogs, and there was no concurrent bacterial cystitis in any of the dogs. C-peptide secretion and lipid metabolism are associated with diabetic complications. Improvement in these parameters following the treatment suggests that cAT-MSC transplantation in dogs with IDDM might help to improve their insulin secretory capacity and prevent diabetic complications.
Keywords: C-peptide, diabetes mellitus, dog, hyperlipidemia, mesenchymal stem cell
Canine diabetes mellitus is a common disease, with a prevalence of 0.13% in a cohort study conducted in Sweden [13] and a 0.32% prevalence in one study conducted in the UK [18]. Of 860 dogs diagnosed with diabetes in the Swedish study, the median survival time was 2 years, not including 223 dogs that died at the time of diagnosis. In another study, the 1-year survival rate for canine diabetic cases was 64% [12] and diabetes commonly results in many complications [19, 34]. Thus, canine diabetes mellitus is a common disease that affects the dog’s lifespan and quality of life.
Although 40% of diabetic dogs die due to ketoacidosis or euthanasia on the day of diagnosis with diabetes mellitus [13], subsequent mortality could be due to diverse complications, including retinopathy, nephropathy, neuropathy, gastrointestinal, and infectious complications. These complications are more likely to occur as the disease duration increases [10, 17]. One study reported that diabetic dogs may have a higher prevalence of diabetic microvascular complications when their insulin secretion capacity is reduced; this can be evaluated by measuring fasting serum C-peptide levels [10, 36].
Most cases of diabetes mellitus in dogs are due to insulin-dependent diabetes mellitus (IDDM), which is historically thought to be caused by pancreatitis [2, 21, 37, 41] or autoimmune-mediated beta cell destruction [3, 4, 8, 38]. One study contradicted the commonly held concepts about autoimmune-mediated beta cell destruction [1], but did not evaluate T cell-mediated autoimmune responses. In another study, although there was no direct evidence of B and T lymphocytic infiltration in the pancreatic tissue of diabetic dogs, except for in those with early diabetes, only a few alpha, beta, pancreatic polypeptide, and ghrelin cells remained after the extremely selective destruction of beta cells, suggesting a high likelihood of autoimmune-mediated beta cell destruction as a cause of canine IDDM [38]. Given the immunomodulatory effect of mesenchymal stem cells (MSCs) [10, 24, 26, 30] and their potency for differentiation into insulin secretory cells [26], administration of MSCs to cases with IDDM is likely to lead to improvement in the insulin secretion ability in diabetic cases [7, 20, 22, 43].
Studies on stem cell therapy in diabetes have been conducted in humans and laboratory animals, and it has been reported that treatment with adipose tissue-derived MSCs or bone marrow-derived MSCs is effective and safe [9]. However, there has been no study of the effect of canine adipose tissue-derived MSC (cAT-MSC) therapy on dogs who have been treated for IDDM for more than 1 year. Therefore, this study investigated whether insulin secretion was improved when dogs with IDDM were treated with cAT-MSCs, and whether this treatment prevented or improved diabetic complications.
MATERIALS AND METHODS
Case selection
Four dogs (Table 1) that had already been diagnosed with IDDM at the Medicine Teaching Hospital of Seoul National University Veterinary (SNU-VMTH) were enrolled in the study, with their owners’ consent. The inclusion criterion was that dogs had been diagnosed as IDDM and managed with insulin for more than 1 year. All owners received an explanation of the study in oral and written form, and owners gave written consent for participation before the study commenced. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of SNU (approval number SNU-180321-4).
Table 1. Enrolled diabetic mellitus case details.
| Age | Breed | Sex | BCS | DM duration | |
|---|---|---|---|---|---|
| Case 1 | 11 y | Toy poodle | MC | 5/9 | 1 y |
| Case 2 | 15 y | Toy poodle | FS | 4/9 | 5 y |
| Case 3 | 14 y | Pomeranian | FS | 4/9 | 2 y |
| Case 4 | 11 y | Mongrel | MC | 5/9 | 1 y |
BCS, body condition score; DM, diabetes mellitus; MC, male castrated; FS, female spayed.
Study design
This study was a pilot study to evaluate the safety and efficacy of using cAT-MSCs for dogs with type 1 DM. Dogs who met the inclusion criterion were treated with cAT-MSCs and the therapeutic effect was assessed using the following evaluation criteria. The evaluation tests included changes in overall clinical symptoms, laboratory tests (fasting C-peptide, TG, total cholesterol, fructosamine, HbA1c, treated insulin dose, and urine test) and changes in required insulin dosage. The end of the study was 1 month after the completion of stem cell treatment, and the dogs were evaluated every 1–2 months until that time-point. The DM dogs enrolled in this study had conventional insulin therapy after the end time-point.
Isolation, culture, characterization, and injection of cAT-MSCs
Canine adipose tissue for stem cell isolation was obtained from healthy 4-year-old beagle dogs. The procedure was established with the approval of the IACUC of SNU (approval number SNU-180706-2), and all dogs were negative for major infectious diseases: canine parvo virus, canine coronavirus, and canine distemper virus.
cAT-MSCs were cultured after isolation, according to previously described methods [27, 28]. First, adipose tissue was washed 5 times with a mixture of Dulbecco’s phosphate-buffered saline (DPBS, PAN-Biotech, Aidenbach, Germany) and 1% penicillin–streptomycin (PS; PAN-Biotech). Then, the tissue was cut into small pieces with sterile scissors. Physically excised samples were digested with collagenase type IA (0.1%, Gibco/Life Technologies, Carlsbad, CA, USA) for 60 min at 37°C and were then neutralized in a mixture of 10% fetal bovine serum (FBS, PAN-Biotech) and Dulbecco’s Modified Eagle’s Medium (DMEM, PAN-Biotech). The neutralized adipose tissue mixture was centrifuged at 1,200 × g for 5 min and then the undigested fragments were removed using a 70-µm cell filter (Thermo Fisher Scientific, Rockford, IL, USA). The obtained cells were resuspended in a mixture of 10% FBS, 1% PS, and DMEM and then cultured at 37°C and 5% CO2 at a density of 3,000/cm2. Cell growth plates were washed with DPBS after 5 days to remove unattached cells and then further cultured in fresh medium. The culture medium was newly replaced every 2–3 days until cell confluency reached 70–80%. Then, the plate was subcultured at a density of 10,000/cm2.
The cells were evaluated for stem cell marker expression using flow cytometry prior to the injection experiment. The identified stem cell markers were Clusters of Differentiation (CD) 44-fluorescein isothiocyanate (FITC), CD34-FITC, CD45-phycoerythrin (PE) (BD Biosciences, San Diego, CA, USA), and CD90-allopicosin (eBiosciences, San Diego, CA, USA). Cell fluorescence analysis was performed with a FACS Aria II system (BD Biosciences). These cells were evaluated for their differentiation potential using stempro adipogenesis, osteogenesis, and chondrogenesis differentiation kits (Gibco, Grand Island, NY, USA). The differentiated cells were stained with Oil Red O, Alizarin Red, and Alcian Blue, respectively.
Dogs were treated with chlorpheniramine (0.5 mg/kg, intravenously) 15 min before stem cell treatment to prevent a hypersensitivity reaction. cAT-MSCs (5 × 106 cells/kg) at passage 4, diluted with 10 ml of 0.9% saline, was injected intravenously over a period of 30 min. cAT-MSCs were infused intravenously 3 or 5 times monthly in the dogs.
Safety tests
History was taken and laboratory evaluations performed to monitor adverse events. This included the incidence of hypersensitivity, inflammation, or other conditions around the injection site, neoplasm, and severe hypoglycemia or hyperglycemia. On the day when the stem cells were administered, the hypersensitivity reaction was monitored through evaluating body temperature, heart rate, blood pressure, and the injection site for 30 min after MSC administration. When the dog visited for the next stem cell treatment, we asked the owner about the history of pain at the site after treatment and about side effects suggesting hypoglycemia at home during the previous 1 month. Then, a general physical examination was performed. On the day of treatment, we also measured fasting blood sugar levels to determine whether serious hyperglycemia or hypoglycemia had occurred.
Efficacy tests
Treatment efficacy evaluation included clinical sign monitoring, fasting C-peptide, HbA1c, fructosamine, TG, and total cholesterol levels, treated insulin dose, and urine test evaluations. Monitoring these profiles started after deciding on stem cell therapy.
Clinical sign monitoring: The general clinical symptoms were assessed for changes in body weight, vitality, appetite, and water intake. Vitality was evaluated using a 5-point system: 5. The dog walks lightly, actively responds to external stimuli, including the owner’s call, and there is little change in sleeping time. 4. There is no change in response to walking and external stimuli, but the sleeping time is greatly increased. 3. The dog does not move when walking and the response to external stimuli is poor. 2. Moves only for defecation or urination. 1. The dog cannot move and defecates and urinates in the lying position.
The appetite was also evaluated using a 5-point scale: 5. Eats all of the recommended food in 1 sitting. 4. Eats the recommended amount of food, but does not eat it in 1 sitting. 3. Does not eat the recommended amount of food, but eats more than half. 2. Leaves at least half of the recommended amount of food. 1. No appetite. The amount of water intake was evaluated based on the owner’s observation using a 120-ml cup. We considered that PU/PD occurred if the dog’s water intake was above 100 ml/kg/day [11, 14, 35]. In addition, to evaluate the neurological complications caused by diabetes mellitus, we evaluated tachycardia due to increased sympathetic tone or hypotension due to decreased sympathetic tone [17, 19, 34].
Fasting C-peptide monitoring: To evaluate the insulin secretion ability of the beta cells [36] and the possibility of complications caused by diabetes mellitus, we measured the dog’s fasting C-peptide levels [10, 40]. We collected blood samples when the dogs visited after 12 hr fasting. The serum samples were centrifuged and stored at −80°C until the last MSC treatment. The serum samples were then thawed at room temperature and analyzed using a Dog Insulin C-Peptide ELISA kit (LS Bio, Inc., Seattle, WA, USA).
Blood analysis: Fructosamine, HbA1c, TG, and total cholesterol levels were evaluated by blood analysis. Blood samples were collected after 12 hr fasting. Those results showed the dog’s blood glucose management status during the study and helped to estimate the possibility of diabetes complications [5, 10, 15, 17, 19, 24, 34, 42].
Urine analysis: A urine test was performed to monitor proteinuria [32] and bacterial cystitis [29], which are common complications in diabetic cases. The urine samples were taken by cystocentesis and was used in a urine dip-stick test (Combur-Test strips, Roche, Basel, Switzerland) and urinary sediment test. When proteinuria was found by the urine dip-stick test, a urinary sediment test was performed to evaluate the presence of cystitis, followed by quantitative analysis of urine protein–creatinine ratio.
RESULTS
cAT-MSC characterization
cAT-MSCs used in this study were differentiated into adipocytes, osteocytes, and chondrocytes for characterization. Each cell-type was stained with specific dyes that allowed their identification by microscopic evaluation (Supplementary Fig. 1A–C). cAT-MSC was also characterized by stem cell marker. Fluorescein of CD 44 and CD 90 which are the stem cell positive markers were expressed and CD 34 and CD 45 which are the hematopoietic markers were not detected in cAT-MSC (Supplementary Fig. 1D).
Monitoring of clinical signs
After stem cell treatment, three of 4 owners observed improvement of the respective dogs’ vitality. No tachycardia or hypotension was observed on physical examination during cell injection in any of the cases. Two of 4 dogs showed improvement in appetite, polyuria and polydipsia (PU/PD), and body weight; the weight of these dogs increased by 6.83% and 8.69%, respectively. The other 2 dogs’ body weight decreased by 2.1% and 6% during the treatment period (Table 2).
Table 2. Clinical sign monitoring results during canine adipose tissue-dervied mesenchymal stem cell treatment.
| Treatment | Vitality | Appetite | PU/PD | BW | BP | |
|---|---|---|---|---|---|---|
| Case 1 | Pre-treatment | 5 | 4 | No PU/PD | 4.68 | 140 |
| Post treatment #1 | 5 | 4 | PU/PD | 4.81 | 120 | |
| Post treatment #2 | 5 | 5 | PU/PD | 4.89 | 140 | |
| Post treatment #3 | 5 | 5 | PU/PD | 4.82 | 140 | |
| Post treatment #4 | 5 | 5 | PU/PD | 4.87 | 120 | |
| Post treatment #5 | 5 | 5 | No PU/PD | 5.00 | 130 | |
| Case 2 | Pre-treatment | 4 | 5 | No PU/PD | 2.76 | 110 |
| Post treatment #1 | 4 | 5 | No PU/PD | 2.88 | 130 | |
| Post treatment #2 | 3 | 5 | No PU/PD | 3.00 | 100 | |
| Case 3 | Pre-treatment | 4 | 4 | PU/PD | 2.40 | 140 |
| Post treatment #1 | 5 | 5 | No PU/PD | 2.37 | 130 | |
| Post treatment #2 | 5 | 5 | NoPU/PD | 2.39 | 180 | |
| Post treatment #3 | 5 | 5 | NoPU/PD | 2.35 | 130 | |
| Post treatment #4 | 5 | 5 | NoPU/PD | 2.39 | 90 | |
| Post treatment #5 | 5 | 5 | No PU/PD | 2.35 | 110 | |
| Case 4 | Pre-treatment | 4 | 4 | PU/PD | 5.43 | 160 |
| Post treatment #1 | 5 | 5 | PU/PD | 5.16 | 150 | |
| Post treatment #2 | 5 | 5 | PU/PD | 5.17 | 135 | |
| Post treatment #3 | 5 | 5 | PU/PD | 5.00 | 110 | |
| Post treatment #4 | 5 | 5 | PU/PD | 5.00 | 130 | |
| Post treatment #5 | 5 | 5 | PU/PD | 5.12 | 120 | |
PU/PD, polyuria/polydipsia; BW, body weight; BP, blood pressure.
Fasting C-peptide monitoring
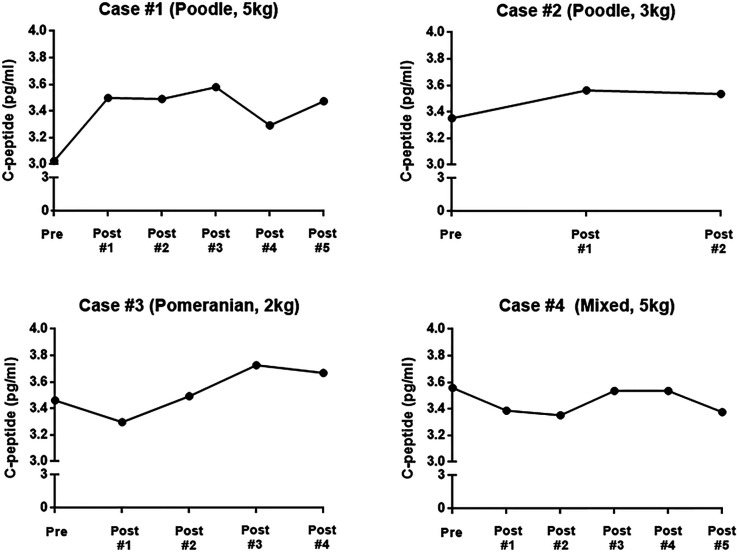
Fasting serum C-peptide levels were measured on the morning of each dog’s visit. C-peptide secretion was increased in 3 of 4 dogs, while 1 dog showed decreased fasting serum C-peptide levels. Dogs with a positive response to treatment showed improvement in C-peptide secretion after 1 or 2 stem cell treatments and improved 5–15% compared with levels before stem cell treatment. During the treatment period, C-peptide of case 1 increased from 3.02 pg/ml to 3.47 pg/ml, that of case 2 increased from 3.35 pg/ml to 3.53 pg/ml, that of case 3 increased from 3.46 pg/ml to 3.67 pg/ml, and that of case 4 decreased from 3.55 pg/ml to 3.37 pg/ml (Fig. 1).
Fig. 1.
Changes of fasting serum C-peptide in 4 dogs during canine adipose tissue-derived mesenchymal stem cell treatment.
Blood analysis and treated insulin dose
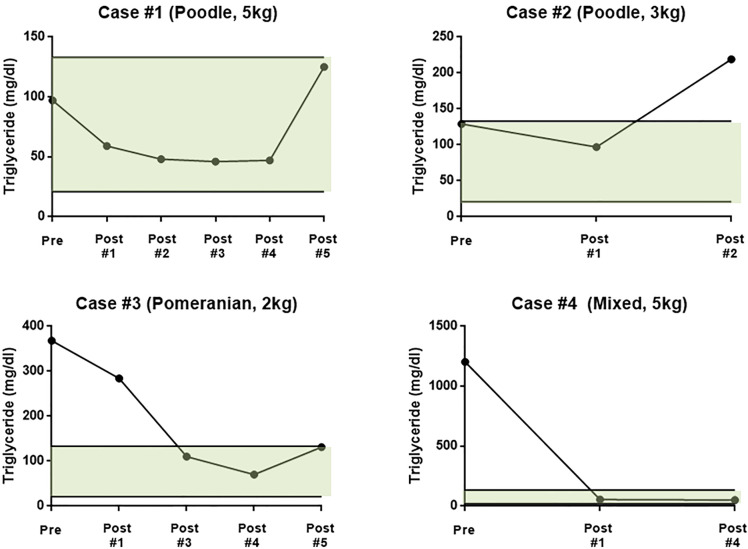
During the study, fructosamine and glycated hemoglobin (HbA1c) levels decreased in 2 of 4 dogs, while the levels in the other dogs were unchanged or increased (Table 3). In case 1, fructosamine decreased from 516 µmol/l to 354 µmol/l after treatment, and HbA1c decreased from 9.8% to 7.4%. The fructosamine concentration of case 3 decreased from 433 µmol/l to 301 µmol/l and the HbA1c levels did not change. Two of the 4 dogs was resolved hyperlipidemia. TG and T.chol was decreased to within the normal range. Otherwise, 1 of the 4 dogs showed increased serum TG levels, above the normal range (Fig. 2). Insulin adjustment was controlled, according to 2018 American Animal Hospital Association Diabetes management guidelines for Dogs and Cats. Insulin doses were reduced in 2 of 4 dogs, unchanged in 1 dog, and increased in 1 dog.
Table 3. Blood test results and treated insulin dose during canine adipose tissue-derived mesenchymal stem cell treatment.
| Treatment | Fructosamine | Hb/A1c | Insulin dose | TG | T. chol | |
|---|---|---|---|---|---|---|
| (µmol/l) | (%) | (U/kg) | (mg/dl) | (mg/dl) | ||
| Case 1 | Pre-treatment | 516 | 9.8 | 0.63 | 97 | 289 |
| Post treatment #1 | 490 | 9.7 | 0.63 | 59 | 259 | |
| Post treatment #2 | 464 | 10.9 | 0.63 | 48 | 243 | |
| Post treatment #3 | 532 | 8.9 | 0.69 | 46 | 287 | |
| Post treatment #4 | 515 | 8.9 | 0.69 | 47 | 319 | |
| Post treatment #5 | 354 | 7.4 | 0.79 | 125 | 320 | |
| Case 2 | Pre-treatment | 212 | 4.0 | 0.73 | 129 | 387 |
| Post treatment #1 | 287 | 6.6 | 0.65 | 97 | 423 | |
| Post treatment #2 | 324 | 6.5 | 0.63 | 219 | 423 | |
| Case 3 | Pre-treatment | 433 | 7.4 | 1.10 | 368 | 410 |
| Post treatment #1 | 382 | 7.4 | 1.10 | 284 | 219 | |
| Post treatment #2 | 368 | 7.8 | 1.10 | - | - | |
| Post treatment #3 | 420 | 7.4 | 1.10 | 110 | 258 | |
| Post treatment #4 | 375 | 8.0 | 1.10 | 70 | 324 | |
| Post treatment #5 | 301 | 7.3 | 1.10 | 131 | 251 | |
| Case 4 | Pre-treatment | 358 | 9.1 | 0.24 | 1,203 | 355 |
| Post treatment #1 | 417 | 9.1 | 0.21 | 54 | 274 | |
| Post treatment #2 | 425 | - | 0.19 | - | - | |
| Post treatment #3 | 338 | 9.9 | 0.20 | - | - | |
| Post treatment #4 | 492 | 11.2 | 0.20 | 51 | 263 | |
| Post treatment #5 | 448 | 8.7 | 0.22 | - | - | |
Hb/A1c, hemoglobin A1c; TG, triglyceride; T. chol, total cholesterol.
Fig. 2.
Changes of serum triglyceride in 4 dogs during canine adipose tissue-derived mesenchymal stem cell treatment. Reference range of serum triglyceride: 21–133 mg/dl.
Urine analysis
Two of 4 dogs showed no evidence of proteinuria during this study, 1 showed improvement in proteinuria, and 1 did not have sufficient continuous evaluation data due to the owner’s refusal for further examination. None of the dogs had bacterial cystitis (Table 4).
Table 4. Urinary test results during canine adipose tissue-derived mesenchymal stem cell treatment.
| Treatment | USG | Dipstick | Sediment | UPC | |
|---|---|---|---|---|---|
| Case 1 | Pre-treatment | 1.037 | Glu 4+ | No inflammatory evidence | - |
| Post treatment #1 | 1.051 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #2 | 1.042 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #3 | 1.039 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #4 | 1.050 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #5 | 1.051 | Glu 4+ | No inflammatory evidence | - | |
| Case 2 | Pre-treatment | - | - | - | - |
| Post treatment #1 | 1.043 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #2 | - | - | - | - | |
| Case 3 | Pre-treatment | 1.018 | Glu 3+, Pro 2+ | No inflammatory evidence | 1.64 |
| Post treatment #1 | 1.025 | Glu 3+, Pro 3+ | No inflammatory evidence | 3.23 | |
| Post treatment #2 | 1.010 | Glu 2+, Pro trace | No inflammatory evidence | 1.89 | |
| Post treatment #3 | 1.025 | Glu 3+, Pro 2+ | No inflammatory evidence | 1.13 | |
| Post treatment #4 | - | - | - | - | |
| Post treatment #5 | 1.014 | Glu 3+, Pro 2+ | No inflammatory evidence | 1.20 | |
| Case 4 | Pre-treatment | 1.018 | Glu 2+, Pro trace | No inflammatory evidence | - |
| Post treatment #1 | 1.021 | Glu 4+, Pro trace | No inflammatory evidence | - | |
| Post treatment #2 | 1.020 | Glu 4+ | No inflammatory evidence | - | |
| Post treatment #3 | 1.023 | Glu 4+, Pro trace | No inflammatory evidence | - | |
| Post treatment #4 | - | - | - | - | |
| Post treatment #5 | - | - | - | - | |
USG, urine specific gravity; UPC, urine protein creatinine ratio.
Adverse effects
To evaluate the safety of this treatment, the dogs’ vitality, inflammation near the injection site, development of a neoplastic mass, and hypoglycemia were examined by means of history taking, physical examination, and blood tests during the course of treatment. cAT-MSCs were administered 18 times in total. No side effects were observed other than in 1 dog who showed pain at the injection site on the day of treatment and a flare at the injection site lasting until the next day, once, after the 5th injection.
DISCUSSION
A previous study showed that dogs with IDDM, treated with insulin for a long period, preserve their insulin secretory capacity [40]. Therefore, stem cell therapy [10, 24,25,26, 30, 31] may involve both transplantation of new beta cells and preservation of the remaining beta cells by stem cells-enhanced regeneration and immunomodulation [23]. Previous reports showed that AT-MSCs, administered by the venous or splenic route, have improved insulin secretory capacity in human diabetic patients and laboratory diabetic model animals [9, 44]. However, to date, no studies have been performed in dogs suffering from IDDM, neither short-term nor long-term research. The present study examined cAT-MSCs transplantation as a treatment for dogs with IDDM, aiming to preserve or improve their pancreatic beta cell function.
We monitored the dogs throughout the treatment to determine the safety of cAT-MSCs transplantation. Only minor side effects were observed in one dog, involving pain at the injection site. To study the therapeutic effect of cAT-MSCs, we performed blood tests, which assessed fasting C-peptide concentration [10, 40], serum TG, total cholesterol [19, 42], fructosamine, and Hb1Ac [5]. We also studied urine sediments to identify potential complications of diabetes mellitus such as bacterial cystitis, proteinuria, and nephrological diseases [34].
As indexes of blood glucose management, fructosamine might be effective when assessing the therapeutic effects of stem cell 2–3 weeks after treatment, while HbA1c can be used for the same purpose 10–14 weeks after treatment. Although fructosamine and HbA1c are good monitoring blood glucose indexes, they are indirect indicators of pancreatic function and are affected by changes in insulin levels [5]. We further tested C-peptide, a substance that is released from the pancreas and is thus a more direct indicator of insulin secretion from pancreatic beta cells [36]. Through C-peptide levels, we could indirectly measure pancreatic insulin releasing function. After stem cell therapy, fasting C-peptide concentration had increased in three of the dogs. This might suggest that the insulin secretory capacity had been improved in these three dogs.
It was reported that in human patients, recently diagnosed with type 1 diabetes mellitus, C-peptide has increased and the requirement for daily insulin dose has decreased after stem cell therapy. Mesenchymal stem cells secrete immune-modulating factors and reduce anti-islet antibodies [33]. Although we selected dogs that were diagnosed as having diabetes for over a year before this study, C-peptide still increased following stem cell therapy. We assume that mesenchymal stem cells reduce the inflammatory process surrounding the pancreatic beta islets through their immunomodulatory effect and by supporting the regeneration of these islets. Moreover, we expect that the therapeutic effect would be even greater if dogs diagnosed with DM would get the therapy shortly after diagnosis.
Human studies found that fasting plasma C-peptide levels decreased with the length of diabetes. They also found that the lower the fasting C-peptide was, the higher was the TG level and the more frequent complications due to diabetes were reported [10]. Therefore, our finding that cAT-MSCs transplantation could improve the lipid metabolism profile and elevate the levels of C-peptide suggests that the treatment could help prevent diabetes-related complications. We thus measured serum TG and total cholesterol to assess fat metabolism. Diabetic dogs are more likely to have hyperlipidemia due to aberrant fat metabolism [19, 42]. When diabetes is properly managed, the hyperlipidemic state improves [15, 24]. Hyperlipidemia could cause pancreatitis, hepatobiliary disease, atherosclerosis, ocular disease, and seizures or other neurologic abnormalities [24, 42]. Therefore, resolving hyperlipemia is an important aspect when managing the complications of diabetes. In the present study, two dogs with improved levels of fructosamine showed resolution of hyperlipidemia and maintained a normal range of serum TG and cholesterol. One of the two dogs, in which there was no improvement in fructosamine levels, showed resolution of severe hyperlipidemia and mild hypercholesterolemia. These results suggest that dogs treated with cAT-MSC might avoid complications because of hyperlipidemia with no medical treatment for hyperlipidemia [24].
Urine was tested to evaluate diabetic nephropathy, which, in diabetic dogs, is associated with glucose utilization at the cellular level and the rate of microvascular complications [29, 32, 34, 39]. The test included a dip-stick test and a urine sedimentation test. Besides, the urine protein–creatinine (UPC) ratio was calculated when the dip-stick test detected proteinuria without cystitis. Two of the dogs showed evidence of proteinuria. This had improved in the dog who underwent continuous UPC assessment. As one dog with proteinuria showed improvement and as no proteinuria occurred in two of the other dogs during six months of follow-up, we suggest that cAT-MSCs treatment could help prevent IDDM-related nephropathy complications. As blood pressure resolved in two of the dogs, it might also affect to ameliorate proteinuria.
One of the most common concurrent diseases, occurring in 15–30% of DM dogs, is bacterial cystitis [16, 19]. During monitoring after the stem cell therapy, we found no sign of bacteria cystitis in the urine sediments test in any of the dogs. The immunomodulation effect of the stem cells could be behind this, but further research is needed to assess the correlation between stem cell therapy and the prevention of infectious complications.
This study has some limitations. We conducted it on only four dogs, and the small sample size limited the possibility to perform a statistical assessment of the treatment effects. In addition, to evaluate insulin secretion, continuous C-peptide measurement is suggested, using a mixed meal tolerance test or a 90-min post-dietary test. This method has been established as a general evaluation method [6], but could not be performed in the current study because the owners refused to allow additional blood sampling. Thus, fasting C-peptide concentration was used, following the suggested association between diabetic complications and C-peptide levels in human [10]. Also, the dogs enrolled in this study were client-owned patients, and they have received conventional veterinary medical treatments including dose adjustment of insulin during stem cell therapy. Therefore, it was impossible to evaluate efficacy of cAT-MSCs on DM with thorough control of other treatments. Further researches are needed to overcome these limitations.
In this study, three of four dogs treated with cAT-MSCs showed improved fasting C-peptide levels, suggesting improved insulin-secretion capacity. cAT-MSC show the potential of resolved hyperlipidemia, hypertension and proteinuria that might be associated with treating nephropathy. Thus, administration of cAT-MSCs could be an additional option for treating diabetes in dogs.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Supplementary Material
Acknowledgments
We are very thankful to the Research Institute for Veterinary Science of Seoul National University and the BK21 PLUS Program for Creative Veterinary Science Research. This study was also supported by the Research Institute of Veterinary Science of Jeju National University.
REFERENCES
- 1.Ahlgren K. M., Fall T., Landegren N., Grimelius L., von Euler H., Sundberg K., Lindblad-Toh K., Lobell A., Hedhammar Å., Andersson G., Hansson-Hamlin H., Lernmark Å., Kämpe O.2014. Lack of evidence for a role of islet autoimmunity in the aetiology of canine diabetes mellitus. PLoS One 9: e105473. doi: 10.1371/journal.pone.0105473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alejandro R., Feldman E. C., Shienvold F. L., Mintz D. H.1988. Advances in canine diabetes mellitus research: etiopathology and results of islet transplantation. J. Am. Vet. Med. Assoc. 193: 1050–1055. [PubMed] [Google Scholar]
- 3.Atkins C. E., Hill J. R., Johnson R. K.1979. Diabetes mellitus in the juvenile dog: a report of four cases. J. Am. Vet. Med. Assoc. 175: 362–368. [PubMed] [Google Scholar]
- 4.Atkins C. E., LeCompte P. M., Chin H. P., Hill J. R., Ownby C. L., Brownfield M. S.1988. Morphologic and immunocytochemical study of young dogs with diabetes mellitus associated with pancreatic islet hypoplasia. Am. J. Vet. Res. 49: 1577–1581. [PubMed] [Google Scholar]
- 5.Bennett N.2002. Monitoring techniques for diabetes mellitus in the dog and the cat. Clin. Tech. Small Anim. Pract. 17: 65–69. doi: 10.1053/svms.2002.33044 [DOI] [PubMed] [Google Scholar]
- 6.Besser R. E., Shields B. M., Casas R., Hattersley A. T., Ludvigsson J.2013. Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care 36: 195–201. doi: 10.2337/dc12-0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson P. O., Schwarcz E., Korsgren O., Le Blanc K.2015. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64: 587–592. doi: 10.2337/db14-0656 [DOI] [PubMed] [Google Scholar]
- 8.Catchpole B., Ristic J. M., Fleeman L. M., Davison L. J.2005. Canine diabetes mellitus: can old dogs teach us new tricks? Diabetologia 48: 1948–1956. doi: 10.1007/s00125-005-1921-1 [DOI] [PubMed] [Google Scholar]
- 9.Dang L. T.T., Phan N. K., Truong K. D.2017. Mesenchymal stem cells for diabetes mellitus treatment: new advances. Biomed. Res. Ther. 4: 1062–1081. doi: 10.15419/bmrat.v4i1.144 [DOI] [Google Scholar]
- 10.De Miguel M. P., Fuentes-Julián S., Blázquez-Martínez A., Pascual C. Y., Aller M. A., Arias J., Arnalich-Montiel F.2012. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr. Mol. Med. 12: 574–591. doi: 10.2174/156652412800619950 [DOI] [PubMed] [Google Scholar]
- 11.DiBartola S. P.1992. Disorders of sodium and water: hypernatremia and hyponatremia. pp. 47–79. In: Fluid Therapy in Small Animal Practice (Di Bartola, S. P. ed.), WB Saunders, Philadelphia. [Google Scholar]
- 12.Doxey D., Milne E. M., Mackenzie C.1985. Canine diabetes mellitus: a retrospective survey. J. Small Anim. Pract. 26: 555–561. doi: 10.1111/j.1748-5827.1985.tb02232.x [DOI] [Google Scholar]
- 13.Fall T., Hamlin H. H., Hedhammar A., Kämpe O., Egenvall A.2007. Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution. J. Vet. Intern. Med. 21: 1209–1216. doi: 10.1111/j.1939-1676.2007.tb01940.x [DOI] [PubMed] [Google Scholar]
- 14.Feldman E. C., Nelson R. W., Reusch C., Scott-Moncrieff J. C.2014. Canine and Feline Endocrinology-e-book, Elsevier Health Sciences, Amsterdam. [Google Scholar]
- 15.Ford R. B.1996. Clinical management of lipemic patients. The Compendium on continuing education for the practicing veterinarian (USA) 18: 1053–1065. [Google Scholar]
- 16.Forrester S. D., Troy G. C., Dalton M. N., Huffman J. W., Holtzman G.1999. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J. Vet. Intern. Med. 13: 557–560. doi: 10.1111/j.1939-1676.1999.tb02209.x [DOI] [PubMed] [Google Scholar]
- 17.Fowler M. J.2008. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 26: 77–82. doi: 10.2337/diaclin.26.2.77 [DOI] [Google Scholar]
- 18.Guptill L., Glickman L., Glickman N.2003. Time trends and risk factors for diabetes mellitus in dogs: analysis of veterinary medical data base records (1970–1999). Vet. J. 165: 240–247. doi: 10.1016/S1090-0233(02)00242-3 [DOI] [PubMed] [Google Scholar]
- 19.Hess R. S., Saunders H. M., Van Winkle T. J., Ward C. R.2000. Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993–1998). J. Am. Vet. Med. Assoc. 217: 1166–1173. doi: 10.2460/javma.2000.217.1166 [DOI] [PubMed] [Google Scholar]
- 20.Ho J. H., Tseng T. C., Ma W. H., Ong W. K., Chen Y. F., Chen M. H., Lin M. W., Hong C. Y., Lee O. K.2012. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and β-cell differentiation in streptozocin-induced diabetic mice. Cell Transplant. 21: 997–1009. doi: 10.3727/096368911X603611 [DOI] [PubMed] [Google Scholar]
- 21.Hoenig M.2002. Comparative aspects of diabetes mellitus in dogs and cats. Mol. Cell. Endocrinol. 197: 221–229. doi: 10.1016/S0303-7207(02)00264-2 [DOI] [PubMed] [Google Scholar]
- 22.Hu J., Wang Y., Wang F., Wang L., Yu X., Sun R., Wang Z., Wang L., Gao H., Fu Z., Zhao W., Yan S.2015. Effect and mechanisms of human Wharton’s jelly-derived mesenchymal stem cells on type 1 diabetes in NOD model. Endocrine 48: 124–134. doi: 10.1007/s12020-014-0219-9 [DOI] [PubMed] [Google Scholar]
- 23.Hussain M. A., Theise N. D.2004. Stem-cell therapy for diabetes mellitus. Lancet 364: 203–205. doi: 10.1016/S0140-6736(04)16635-X [DOI] [PubMed] [Google Scholar]
- 24.Jurewicz M., Yang S., Augello A., Godwin J. G., Moore R. F., Azzi J., Fiorina P., Atkinson M., Sayegh M. H., Abdi R.2010. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 59: 3139–3147. doi: 10.2337/db10-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M. C.2005. Hyperlipidemia disorders in dogs. Compendium 27: 361–370. [Google Scholar]
- 26.Karaoz E., Genç Z. S., Demircan P. Ç., Aksoy A., Duruksu G.2010. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 1: e36–e36. doi: 10.1038/cddis.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karaoz E., Ayhan S., Okçu A., Aksoy A., Bayazıt G., Osman Gürol A., Duruksu G.2011. Bone marrow-derived mesenchymal stem cells co-cultured with pancreatic islets display β cell plasticity. J. Tissue Eng. Regen. Med. 5: 491–500. doi: 10.1002/term.342 [DOI] [PubMed] [Google Scholar]
- 28.Kim H. W., Song W. J., Li Q., Han S. M., Jeon K. O., Park S. C., Ryu M. O., Chae H. K., Kyeong K., Youn H. Y.2016. Canine adipose tissue-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating T cells in rats. J. Vet. Sci. 17: 539–548. doi: 10.4142/jvs.2016.17.4.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King G. L., Shiba T., Oliver J., Inoguchi T., Bursell S. E.1994. Cellular and molecular abnormalities in the vascular endothelium of diabetes mellitus. Annu. Rev. Med. 45: 179–188. doi: 10.1146/annurev.med.45.1.179 [DOI] [PubMed] [Google Scholar]
- 30.Kirsch M.1998. [Incidence of bacterial cystitis in diabetic dogs and cats at the time of diagnosis. Retrospective study for the period 1990–1996]. Tierarztl. Prax. Ausg. K Klientiere. Heimtiere 26: 32–36. [PubMed] [Google Scholar]
- 31.Lange C., Bruns H., Kluth D., Zander A. R., Fiegel H. C.2006. Hepatocytic differentiation of mesenchymal stem cells in cocultures with fetal liver cells. World J. Gastroenterol. 12: 2394–2397. doi: 10.3748/wjg.v12.i15.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzi A., Fracassi F., Dondi F., Gentilini F., Famigli Bergamini P.2008. Ratio of urinary protein to creatinine and albumin to creatinine in dogs with diabetes mellitus and hyperadrenocorticism. Vet. Res. Commun. 32 Suppl 1: S299–S301. doi: 10.1007/s11259-008-9133-z [DOI] [PubMed] [Google Scholar]
- 33.Mesples A., Majeed N., Zhang Y., Hu X.2013. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: preliminary results. Med. Sci. Monit. 19: 852–857. doi: 10.12659/MSM.889525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñana K. R.1995. Long-term complications of diabetes mellitus, Part I: Retinopathy, nephropathy, neuropathy. Vet. Clin. North Am. Small Anim. Pract. 25: 715–730. doi: 10.1016/S0195-5616(95)50064-6 [DOI] [PubMed] [Google Scholar]
- 35.Nichols R.2001. Polyuria and polydipsia. Diagnostic approach and problems associated with patient evaluation. Vet. Clin. North Am. Small Anim. Pract. 31: 833–844. doi: 10.1016/S0195-5616(01)50001-7 [DOI] [PubMed] [Google Scholar]
- 36.Panero F., Novelli G., Zucco C., Fornengo P., Perotto M., Segre O., Grassi G., Cavallo-Perin P., Bruno G.2009. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 32: 301–305. doi: 10.2337/dc08-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polonsky K. S., Pugh W., Jaspan J. B., Cohen D. M., Karrison T., Tager H. S., Rubenstein A. H.1984. C-peptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J. Clin. Invest. 74: 1821–1829. doi: 10.1172/JCI111601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinovitch A.1998. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 14: 129–151. doi: [DOI] [PubMed] [Google Scholar]
- 39.Shields E. J., Lam C. J., Cox A. R., Rankin M. M., Van Winkle T. J., Hess R. S., Kushner J. A.2015. Extreme beta-cell deficiency in pancreata of dogs with canine diabetes. PLoS One 10: e0129809. doi: 10.1371/journal.pone.0129809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffes M. W., Buchwald H., Wigness B. D., Groppoli T. J., Rupp W. M., Rohde T. D., Blackshear P. J., Mauer S. M.1982. Diabetic nephropathy in the uninephrectomized dog: microscopic lesions after one year. Kidney Int. 21: 721–724. doi: 10.1038/ki.1982.88 [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Lovejoy N. F., Faustman D. L.2012. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care 35: 465–470. doi: 10.2337/dc11-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson P. J.2003. Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J. Small Anim. Pract. 44: 306–312. doi: 10.1111/j.1748-5827.2003.tb00159.x [DOI] [PubMed] [Google Scholar]
- 43.Xenoulis P. G., Steiner J. M.2010. Lipid metabolism and hyperlipidemia in dogs. Vet. J. 183: 12–21. doi: 10.1016/j.tvjl.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 44.Xiao N., Zhao X., Luo P., Guo J., Zhao Q., Lu G., Cheng L.2013. Co-transplantation of mesenchymal stromal cells and cord blood cells in treatment of diabetes. Cytotherapy 15: 1374–1384. doi: 10.1016/j.jcyt.2013.06.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.