Abstract
This report describes the cases of two Miniature Dachshunds who were suspected to have immune-mediated hemolytic anemia (IMHA) and were treated with immunosuppressive therapy. However, progression of anemia, increases in C-reactive protein (CRP) and total-bilirubin (T-Bil) levels, splenomegaly, transition to nonregenerative anemia, and thrombocytopenia occurred after the treatment. Splenectomy and bone-marrow aspirations were performed subsequently. Both dogs were diagnosed with hemophagocytic syndrome (HPS) associated with IMHA. Unfortunately, they died 9 and 6 days later. These findings indicate that some cases of refractory IMHA have the pathogenicity of HPS. HPS should be included as a differential diagnosis of refractory IMHA concurrent with thrombocytopenia. Continuously elevated CRP and T-Bil levels may be helpful indicators in the detection of HPS associated with IMHA.
Keywords: autoimmune-associated hemophagocytic syndrome, evans’ syndrome, hemophagocytosis, immune-mediated hemolytic anemia, thrombocytopenia
Canine hemophagocytic syndrome (HPS) is a rare, severe, and life-threatening disease. It is characterized by activation of macrophages and prominent hemophagocytosis throughout the reticuloendothelial system [1, 13, 18,19,20,21]. Canine HPS is divided to two subcategories, namely: idiopathic or secondary. Secondary HPS is characterized by the presence of underlying diseases such as immune-mediated diseases representative of immune-mediated hemolytic anemia (IMHA), infectious diseases, and neoplastic diseases such as lymphoma and myelodysplastic syndrome (MDS). Compared with other types of secondary HPS, the prognosis of HPS associated with IMHA (HPS-IMHA) is very poor [21]. There are very few available studies on canine HPS-IMHA; notably, there is no case report of canine HPS-IMHA [21]. Therefore, the available evidence regarding the detailed clinicopathologic findings and clinical course of the disease is insufficient. Interestingly, recent studies have indicated that the Miniature Dachshund (MD) breed is predisposed to non-neoplastic bone marrow (BM) diseases that induce non-regenerative anemia [2, 17]. Cases of immunosuppressive treatment-resistant MDs with anemia frequently occur in Japan; however, the cause and pathophysiology of such cases have not been clarified yet. Furthermore, there is currently no report of a case of an MD with HPS-IMHA and non-regenerative anemia [17]. In this report, we describe the clinicopathological findings and clinical courses of the cases of two MDs with HPS-IMHA.
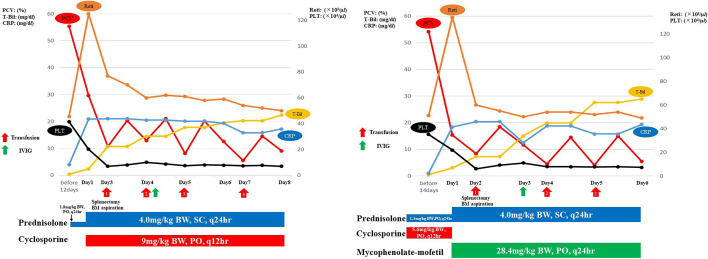
Case 1: An 11-years-old, 5.55-kg, male, neutered Miniature Dachshund was referred to Akiyoshi Animal Clinic with a two-weeks history of anorexia and lethargy that progressed to severe illness. Prior to referral, the dog was administered with prednisolone (1.8 mg/kg body weight [BW], per os [PO], q24 hr) for 12 days for the treatment of presumptive herniated intervertebral disk. Twelve days before presentation, the dog’s packed cell volume (PCV), reticulocytes, platelet count, total bilirubin (T-Bil) concentration, and C-reactive protein (CRP) concentration were normal (Fig. 1).
Fig. 1.
Clinical course of these cases. The graph shows the transition of packed cell volume (PCV), C-reactive protein (CRP), total bilirubin (T-Bil), reticulocytes, and platelet count (PLT), and the dose transition of each drug administered in (A) Case 1 and (B) Case 2. BW, SC, and PO indicate body weight, subcutaneous injection, and per os, respectively.
Physical examination on presentation revealed that the dog had a fever (40.1°C) and pale mucosal membranes; cranial organomegaly was observed on abdominal palpation. A complete blood count (CBC; IDEXX ProCyte Dx Hematology Analyzer; IDEXX Laboratories, Tokyo, Japan) revealed mild regenerative anemia; 7 spherocytes/100× oil immersion field was visualized in the blood smear examination (Fig. 2). The platelet count was within reliable intervals (RIs). A routine plasma biochemistry panel (FUJIFILM DRI-CHEM 7000V; FUJIFILM Co., Tokyo, Japan) revealed severely increased CRP concentration, moderately increased T-Bil concentration, and mildly increased alkaline phosphatase (ALP) activity and triglyceride (TG) concentration (Table 1). Thoracic and abdominal imaging revealed splenomegaly without morphological abnormalities. The results of hemostatic tests performed on citrated (3.8%) plasma at a commercial laboratory (Hoken Kagaku, Yokohama, Japan and FUJIFILM VET Systems, Tokyo, Japan) were within RIs (Table 1), as were the results of the pre- and postprandial total bile acid (TBA) examinations (FUJIFILM DRI-CHEM IMMUNO AU 10V; FUJIFILM Co.) performed. Hemoglobinuria was considered present because microscopic sediment examination, which was performed using a urine dipstick, showed a positive heme reaction in the absence of intact erythrocytes. Direct Coombs tests (FUJIFILM VET Systems) and agglutination tests showed positive results at 4°C and 37°C. The dog was then diagnosed with IMHA [5, 14,15,16]. The results of aerobic and anaerobic blood culture examinations performed on day 1 were negative for bacteria. In addition, real-time PCR was performed at a commercial laboratory (IDEXX Laboratories) on day 1 using peripheral blood in K3EDTA to test for infections, including those caused by Anaplasma phagocytophilum, Babesia canis, Bartonella spp., Ehrlichia canis, Hepatozoon canis, Leishmania spp., Neorickettsia risticii, Rickettsia rickettsii, and canine hemotropic mycoplasmas; all results were negative. On day 1, the prednisolone dose was increased (Prednisolone®, Sanwa Kagaku Kenkyusho Co., Nagoya, Japan; 4 mg/kg BW, SC, q24 hr). Additional therapy included cyclosporine (Atopica®, Kyoritsu Seiyaku Co., Tokyo, Japan; 9 mg/kg BW, PO, q12 hr), dalteparin (Dalteparin Na, Nichi-Iko., Toyama, Japan; 150 U/kg BW, SC, q8 hr), omeprazole (Omepral, Mitsubishi Tanabe Co., Osaka, Japan; 1 mg/kg BW, IV, q12 hr), and antibiotics. On day 3, the anemia, hyperbilirubinemia, increased CRP concentration, and splenomegaly had severely progressed, and the dog suddenly developed thrombocytopenia as well (Fig. 1). Regenerative reaction was observed on presentation but became weaker over time (Fig. 1). The results of hemostatic tests remained within RIs. Spleen aspiration cytology revealed many hemophagocytic macrophages (45 per 10 oil immersion fields) but no neoplastic findings (Fig. 2). Evans’ syndrome (ES) and the other BM disorders were suspected. On day 3, the dog underwent splenectomy and BM aspiration of the right and left humeri and left femur after a whole blood transfusion (20.0 ml/kg BW, total: 110 ml). Splenic histopathologic findings revealed marked extramedullary hematopoiesis, severe diffuse red pulp congestion, absence of both etiologic agents and neoplastic cells, and erythrophagocytic macrophages (Fig. 2). Cytologic examination of three liver lobes, which was performed during the abdominal surgery, revealed only mild vacuolar hepatocyte degeneration but no neoplastic findings. A 500-cell differential count was performed on multiple BM smears and all smears showed almost the same findings: generalized hyperplasia, marked erythroid hyperplasia (myeloid:erythroid [M/E]: 0.41; RIs, 0.9–1.8) [11, 12], moderate to marked megakaryocytic hyperplasia, evidence of hemophagocytosis (3.2% of all nucleated cells [ANC]) of platelet, mature neutrophils, erythroid precursors, and mature erythrocytes (Fig. 2). Macrophages were increased in 10% of ANC [21]. Lymphocytes and plasma cells were present in 7.3% and 3.9% of ANC, respectively, and both values were within RIs [11, 12]. Neoplastic cells and lineage dysplasia were absent, and no infectious agents were observed. ES was then excluded [3]. The dog was diagnosed with HPS-IMHA, indicating progression from IMHA into HPS. The absence of neoplastic findings in BM or spleen and the negative blood culture and real-time PCR results excluded HPS associated with neoplasm and infection [1, 9, 13, 18,19,20,21]. Thereafter, a single infusion of IV immunoglobulin (GAMMAGARD Solvent, Takeda Pharmaceutical Co., Tokyo, Japan; 0.9 g/kg), whole blood transfusions (20 ml/kg BW/day; total: 330 ml), and prednisolone and cyclosporine treatments were administered. The whole blood transfusion was performed a total of 4 times (Fig. 1). However, the dog died on day 9 from refractory hemolytic anemia (Fig. 1).
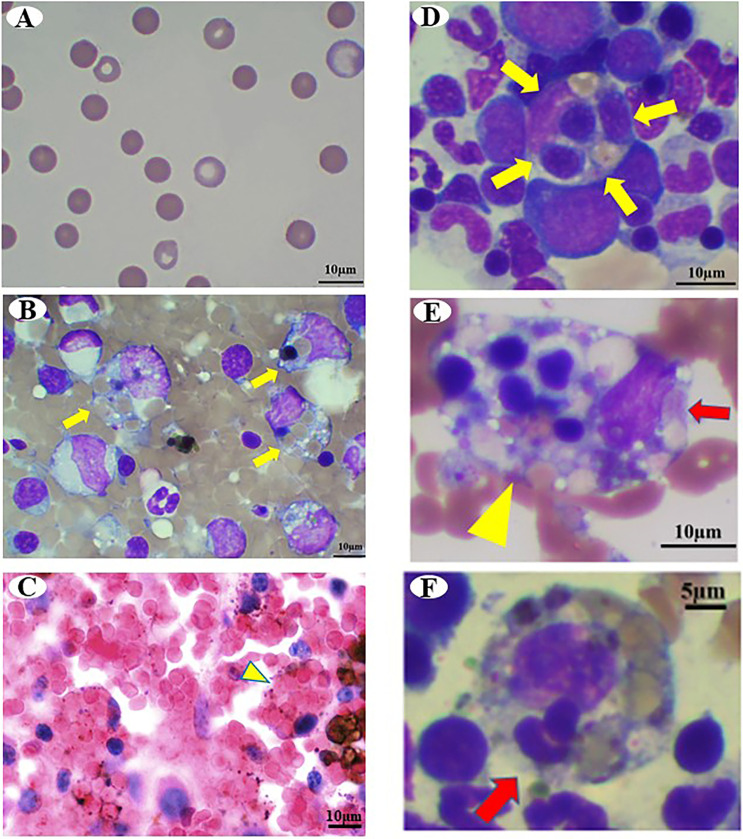
Fig. 2.
Photomicrographs of Case 1. (A) Peripheral blood smear shows increased spherocytes on day 1. Wright Giemsa stain, Bar=10 µm. (B) Cytology of the spleen. Yellow arrows indicate macrophage phagocytosis. Wright Giemsa stain, Bar=10 µm. (C) Histology of the spleen. Yellow arrowheads indicate erythrophagocytic macrophages. Hematoxylin and eosin (H&E) stain, Bar=10 µm. (D–F) Cytology of the bone marrow. (D) Yellow arrows indicate hemophagocytosis of erythroid precursors and mature erythrocytes. Wright Giemsa stain, Bar=10 µm. (E) Yellow arrowhead indicates hemophagocytic macrophage. Red arrows indicate platelets phagocyted by macrophages. Wright Giemsa stain, Bar=10 µm. (F) Red arrow indicates leukocyte phagocyted by macrophages. Wright Giemsa stain, Bar=5 µm.
Table 1. On day 1, the complete blood cell count (CBC), chemistry, and hemostatic test results in case 1 and case 2. Reference intervals are from our laboratory.
| Case 1 | Case 2 | Unit | Reference interval | Case 1 | Case 2 | Unit | Reference interval | ||
|---|---|---|---|---|---|---|---|---|---|
| RBC | 4.69* | 2.14* | ×106/µl | 5.65–8.87 | Total proteins | 7.4 | 7.8 | g/dl | 5.0–7.8 |
| PCV | 29.6* | 15.4* | % | 37.3–61.7 | Albumin | 3.9 | 3.3 | g/dl | 2.6–4.0 |
| Hemoglobin | 9.4* | 5.1* | g/dl | 13.1–20.5 | ALT | 59 | 22 | IU/l | 17–78 |
| MCV | 63.1 | 72 | fl | 61.6–73.5 | AST | 44 | 40 | IU/l | 17–44 |
| MCH | 20 | 23.8 | pg | 21.2–25.9 | ALP | 637* | 486* | IU/l | 47–254 |
| MCHC | 31.8 | 33.1 | g/dl | 32.0–37.9 | GGT | 13 | 14 | IU/l | 5.0–14 |
| Reticulocytes | 125* | 134* | ×103/µl | 10–110 | Total Bilirubin | 2.4* | 3.1* | mg/dl | 0.1–0.8 |
| WBC | 7.76 | 27.69* | ×103/µl | 5.05–16.76 | Ammonia | 27 | 46 | µg/dl | 16–78 |
| Neutrophils | 6.3 | 18.96* | ×103/µl | 2.95–11.64 | Glucose | 117 | 115 | mg/dl | 78–128 |
| Lymphocytes | 1.3 | 2.22 | ×103/µl | 1.05–5.1 | Cholesterol | 288 | 258 | mg/dl | 115–320 |
| Monocytes | 0.15 | 6.46* | ×103/µl | 0.16–1.12 | Triglycerides | 366* | 667* | mg/dl | 30–133 |
| Eosinophils | 0 | 0.02 | ×103/µl | 0.06–1.23 | Urea | 18.2 | 20.5 | mg/dl | 10.0–29.2 |
| Basophils | 0.1 | 0.03 | ×103/µl | 0–0.1 | Creatinine | 1 | 0.5 | mg/dl | 0.4–1.4 |
| Platelets | 202 | 202 | ×103/µl | 148–484 | Phosphorous | 3.5 | 3.7 | mg/dl | 1.9–5.0 |
| PT | 8.2 | 8.1 | sec | 6.0–9.0 | Calcium | 10.2 | 10.1 | mg/dl | 9.3–12.1 |
| APTT | 15 | 15 | sec | 13–19 | C-reactive protein | 20.9* | 18.4* | mg/dl | <0.7 |
| Fibrinogen | 651* | 754* | mg/dl | 160–400 | Sodium | 145 | 148 | mmol/l | 141.0–152.0 |
| AT | 96 | 107 | % | 95< | Potassium | 4 | 3.8 | mmol/l | 3.8–5.0 |
| FDPs | 2.5 | 2.5 | µg/ml | <5 | Chloride | 110 | 112 | mmol/l | 102–117 |
| D-dimer | 0.5 | 0.5 | µg/ml | <2 | Iron | 417* | 458* | µg/dl | 61–240 |
| TAT | <0.2 | <0.2 | ng/ml | <0.2 | TIBC | 685* | 658* | µg/dl | 303–526 |
| UIBC | 268 | 200 | µg/dl | 164–354 | |||||
| Pre TBA | 3.4 | 6.2 | µmol/l | <7.9 | |||||
| Post TBA | 8.8 | 7.8 | µmol/l | <24.5 | |||||
Asterisks indicate that it greatly deviate from the reference interval (RI). RBC, red blood cell; PCV, packed cell volume; MCV, mean cell volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; PT, prothrombin time; APTT, activated partial thromboplastin time; AT, anti-thrombin; FDPs, fibrin degradation products; TAT, thrombin antithrombin complex ; ALT, alanine aminotransferase; AST, asparate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; TIBC, total iron-binding capacity; UIBC, unsaturated iron-binding capacity, TBA, total bile acids.
Case 2: A 12-year-old, 8.92-kg, male, neutered Miniature Dachshund presented to Akiyoshi Animal Clinic with a three-days history of anorexia and lethargy that progressed to severe illness. The dog was diagnosed with immune-mediated thrombocytopenia (IMT) two months ago and was treated with prednisolone and cyclosporine. The IMT improved and was in complete remission. Thus, the doses of the prednisolone [1.1 mg/kg BW, PO, q24 hr] and cyclosporine [5.6 mg/kg BW, PO, q24 hr] were reduced. Prior to admission, the dog was administered these doses for 21 days. Seven days before presentation, the dog’s PCV, reticulocytes, platelet, CRP, and T-Bil were all within normal values (Fig. 1).
On presentation, physical examination revealed fever (40.3°C), pale mucosal membranes, and icterus; cranial organomegaly was observed on abdominal palpation. The IMT and allergic dermatitis resolved completely with the drug regimen. However, results of a CBC revealed severe regenerative anemia but no thrombocytopenia; 8 spherocytes/100× oil immersion field was visualized in the blood smear examination (Fig. 3). A routine plasma biochemistry panel revealed severely increased CRP, moderately increased T-Bil, and mildly increased ALP activity and TG concentration (Table 1). Thoracic and abdominal imaging revealed splenomegaly without morphological abnormalities. The results of the hemostatic tests (Hoken Kagaku and FUJIFILM VET Systems) (Table 1) and pre- and postprandial TBA examinations were all within RIs. Hemoglobinuria was considered present for a reason similar to that of Case 1. Direct Coombs tests and slide agglutination tests showed positive results at 4°C and 37°C. The dog was then diagnosed with IMHA [5, 14,15,16]. On day 1, aerobic and anaerobic blood culture examinations showed negative results for bacteria, just as in Case 1. In addition, real-time PCR was also performed at a commercial laboratory (IDEXX Laboratories) on day 1 using peripheral blood in K3EDTA to test for infections, including those caused by Anaplasma phagocytophilum, Babesia canis, Bartonella spp., Ehrlichia canis, Hepatozoon canis, Leishmania spp., Neorickettsia risticii, Rickettsia rickettsii, and canine hemotropic mycoplasmas; all results were negative. The dog’s serum cyclosporine concentration was within the therapeutic range (541 ng/ml; therapeutic level, 100–600 ng/ml). On day 1, the prednisolone dose was increased to 4 mg/kg BW, SC, q24 hr. Additional therapy included administration of dalteparin (150 U/kg BW, SC, q8 hr), omeprazole (1 mg/kg BW, IV, q12 hr), and antibiotics. Since the dog developed IMHA even though the serum cyclosporine concentration was within the therapeutic range, cyclosporine was substituted with mycophenolate-mofetil (MMF; CELLCEPT, Chugai Pharmaceutical Co, Tokyo, Japan, 28.4 mg/kg BW, PO, q24 hr). On day 2, the anemia, hyperbilirubinemia, increased CRP, and splenomegaly had severely progressed, and the dog suddenly developed thrombocytopenia (Fig. 1). Regardless of the severe anemia, mild regenerative reaction was noted on presentation, but it became weaker over time. (Fig. 1). Results of hemostatic tests remained within RIs. Spleen aspiration cytology revealed many hemophagocytic macrophages (41 per 10 oil immersion fields) but no neoplastic findings (Fig. 3). ES and the other BM disorders were suspected. On day 2, the dog underwent splenic extirpation and BM aspiration of the right and left humeri and left femur after a whole blood transfusion (20.0 ml/kg BW, total: 176 ml). Splenic histopathologic findings revealed marked extramedullary hematopoiesis, severe diffuse red pulp congestion, absence of both etiologic agents and neoplastic cells, and erythrophagocytic macrophages (Fig. 3). Cytologic examination of three liver lobes, which was performed during the abdominal surgery, revealed only mild vacuolar hepatocyte degeneration but no neoplastic findings. A 500-cell differential count was performed on multiple BM smears, and all smears showed almost the same findings. BM aspiration revealed generalized hyperplasia, marked erythroid hyperplasia (M/E: 0.35; RI, 0.9–1.8) [11, 12], moderate to marked megakaryocytic hyperplasia, and evidence of hemophagocytosis (3.5% of ANC) of platelet, mature neutrophils, erythroid precursors, and mature erythrocytes (Fig. 3). Macrophages were increased in 11% of ANC [21]. Lymphocytes and plasma cells were observed in 8.5% and 4.1% of ANC, respectively, and both values were within RIs [11, 12]. Neoplastic cells and lineage dysplasia were absent, and no infectious agents were observed. Thus, ES was excluded [3]. The dog was then diagnosed with HPS-IMHA, indicating progression from IMHA into HPS, just as in Case 1. HPS associated with neoplasm and infection was excluded due to the absence of neoplastic findings in BM or spleen and the negative blood culture and real-time PCR results [1, 9, 13, 18,19,20,21]. Thereafter, a single infusion of IVIG (0.57 g/kg) and whole blood transfusions (20 ml/kg BW /day; total; 352 ml), as well as prednisolone and MMF, were administered. The whole blood transfusion was performed a total of 3 times in total. However, the dog died on day 6 from refractory hemolytic anemia (Fig. 1).
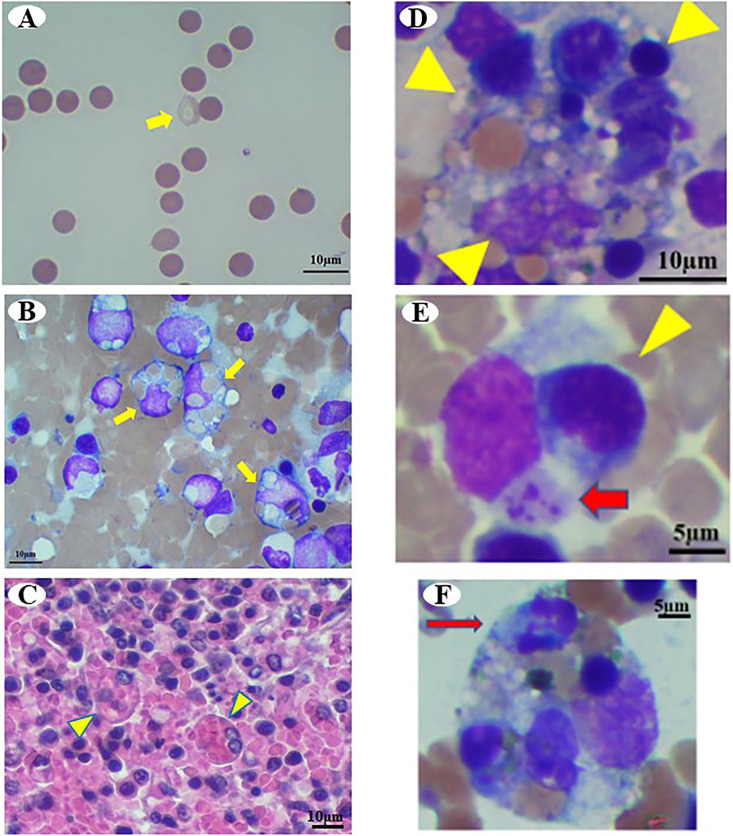
Fig. 3.
Photomicrographs of Case 2. (A) Peripheral blood smear shows increased spherocytes and erythrocyte ghosts (yellow arrow) on day 1. Wright Giemsa stain, Bar=10 µm. (B) Cytology of the spleen. Yellow arrows indicate macrophage phagocytosis. Wright Giemsa stain, Bar=10 µm. (C) Histology of the spleen. Yellow arrowheads indicate erythrophagocytic macrophages. H&E stain, Bar=10 µm. (D–F) Cytology of the bone marrow. (D) Yellow arrowheads indicate hemophagocytosis of erythroid precursors and mature erythrocytes. Wright Giemsa stain, Bar=10 µm. (E) Red arrow and yellow arrowhead indicate platelets and erythroid precursors phagocyted by macrophages. Wright Giemsa stain, Bar=5 µm. (F) Red arrow indicates leukocytes phagocyted by macrophages. Wright Giemsa stain, Bar=5 µm.
Canine HPS is an extremely rare benign proliferative disorder of activated macrophages and is associated with multiple blood cytopenias [1, 21]. Canine HPS is challenging to diagnose either clinically or cytologically. Detailed clinicopathological examinations are necessary for accurate diagnosis of HPS. In 2007, Weiss et al. indicated that the criteria for diagnosis of canine HPS consist of bicytopenia or pancytopenia in the peripheral blood and hemophagocytic macrophages in >2% of ANC in BM aspirates [21]. Therefore, in contrast to IMHA and IMTP or ES, BM aspiration is absolutely required for the diagnosis of HPS. Weiss et al. reported that only five out of 24 dogs with HPS were diagnosed with HPS-IMHA, which progressed into HPS with non-regenerative anemia and thrombocytopenia but without disseminated intravascular coagulation (DIC) from IMHA [21]. Similar to the cases in the present report, some of the five dogs in the Weiss et al. report did not have neutropenia, even though phagocyted neutrophils were observed. In the present report, HPS was diagnosed based on the criteria outlined in a previous report [21], namely: anemia, thrombocytopenia, and 3.2% and 3.5% hemophagocytic macrophages, including phagocytosis of mature erythrocytes, erythroid precursors, neutrophils, and platelets, observed on BM smears. In addition, the underlying disease in both cases was IMHA; thereafter, the IMHA progressed into nonregenerative anemia, indicating thrombocytopenia without DIC. Therefore, we suggest that these clinical courses and BM findings may be important features of HPS-IMHA.
In human medicine, HPS secondary to immune-mediated diseases, such as canine HPS-IMHA, is called autoimmune-associated HPS (AAHS). Kumakura et al. [8] proposed that the diagnosis of AAHS must fulfill the following four criteria; 1) cytopenia (affecting ≥2 of 3 lineages in the peripheral blood and not caused by aplastic or dysplastic BM); 2) histiocytic hemophagocytosis in the BM or other reticuloendothelial systems, including the spleen, liver, or lymph nodes; 3) active phase of underlying autoimmune disease concurrent with hemophagocytosis; and 4) other reactive hemophagocytic syndromes, such as virus- or malignancy-associated syndromes, are able to be excluded [7, 8]. In the present report, each case fulfilled all of the criteria for the diagnosis of human AAHS. We consider that the underlying disease in our cases was IMHA. Additionally, the pathogenesis of our cases was refractory against immunosuppressive therapy in the active phase [4, 5]. The criteria for AAHS in humans include increased hemophagocytic macrophages not only in the BM, but also in the spleen or lymph nodes [7, 8]. Hemophagocytic macrophages in the spleen of dogs with canine HPS have been reported, but the implications of this finding have not been clarified [13]. We consider that cytological examination of the spleen may be a useful diagnostic tool for canine HPS. Further clinical studies and more data are required in the future to clarify a causal association between the absolute value of BM and the spleen in the diagnosis of canine HPS.
Since AAHS is characterized by the presence of blood cell autoantibodies, its pathogenesis is assumed to be caused by the production of autoantibodies or immune complexes, in addition to mediation of cytokines by macrophage activation [7, 8]. In the cases in the present report, antibody-mediated cellular destruction was strongly supported, since both intravascular and extravascular hemolysis were recognized through positive Coombs testing, bilirubinuria, and hemoglobinuria. Furthermore, activation of macrophages was also likely because of the >2% hemophagocytic macrophages including mature erythrocytes, platelets, erythroid precursors, and neutrophils in the BM and increased macrophages (10%, 11%, respectively). Therefore, we suspect that the findings of these two cases are consistent with those of AAHS in humans.
Currently, clinicopathological findings that differentiate canine HPS-IMHA from refractory IMHA are unclear. A definitive diagnostic tool for diagnosing canine HPS-IMHA is BM aspiration [21]. We consider that increased CRP concentrations and hyperbilirubinemia might be characteristic clinicopathological findings for detecting HPS-IMHA. Griebsch et al. reported that CRP and T-Bil concentrations in canine IMHA were significantly decreased after immunosuppressive therapy compared with before therapy, regardless of whether the prognosis was favorable or poor [6]. Continuously increased CRP concentrations, which are refractory to immunosuppressive therapy, and severe hyperbilirubinemia may be indicators of HPS-IMHA, especially if these findings do not improve, regardless of immunosuppressive therapy. However, in the future, accumulation of more cases is required to identify whether non-disease specific markers, such as CRP and T-Bil, are beneficial for detecting HPS-IMHA.
In Japan, a study investigated the breed predisposition of dogs that are diagnosed with non-neoplastic BM disorders, which induce non-regenerative anemia, such as suspected immune-mediated disease [17]. MD was identified as a breed with a predisposition for these disorders, but HPS with non-regenerative anemia was not included in this previous study. MDs in Japan are also commonly predisposed to inflammatory colorectal polyps and sterile panniculitis, which are rare immune-mediated diseases in other breeds [10, 22]. Because the two dogs in the present report were MDs, these findings suggest that Japanese MDs may easily develop rare immune-mediated diseases. Breed-specific genetic aberrations in Japanese MDs may progress to HPS-IMHA owing to immune-mediated antibody cellular destruction. We recommend that veterinarians should be aware of progression of HPS-IMHA in cases of refractory IMHA in Japanese MDs.
In conclusion, HPS may be representative of the severe poor prognosis in some cases of acute refractory canine IMHAs. To the best of our knowledge, the present report is one of the few reports of canine HPS-IMHA. We suggest that BM aspiration be performed immediately in cases of refractory IMHA concurrent with thrombocytopenia to elucidate whether HPS is involved or not. Elevated CRP and T-Bil may be helpful in the detection of HPS. However, as only two cases are included in this report, data accumulated of more canine HPS cases will be needed to further elucidate the pathogenesis of the disease. Forward further analysis must be pushed to find important clinicopathological findings to make diagnosis in canine HPS-IMHA.
CONFLICT OF INTEREST
The authors indicate that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.
Supplementary Material
REFERENCES
- 1.Akiyoshi M., Hisasue M., Neo S., Akiyoshi M., Goto-Koshino Y.2019. A case of hemophagocytic syndrome progressing into large granular lymphoma in a dog. Vet. Clin. Pathol. 48: 71–77. doi: 10.1111/vcp.12704 [DOI] [PubMed] [Google Scholar]
- 2.Assenmacher T. D., Jutkowitz L. A., Koenigshof A. M., de A Lucidi C., Scott M. A.2019. Clinical features of precursor-targeted immune-mediated anemia in dogs: 66 cases (2004–2013). J. Am. Vet. Med. Assoc. 255: 366–376. doi: 10.2460/javma.255.3.366 [DOI] [PubMed] [Google Scholar]
- 3.Bianco D., Hardy R. M.2009. Treatment of Evans’ syndrome with human intravenous immunoglobulin and leflunomide in a diabetic dog. J. Am. Anim. Hosp. Assoc. 45: 147–150. doi: 10.5326/0450147 [DOI] [PubMed] [Google Scholar]
- 4.Carr A. P., Panciera D. L., Kidd L.2002. Prognostic factors for mortality and thromboembolism in canine immune-mediated hemolytic anemia: a retrospective study of 72 dogs. J. Vet. Intern. Med. 16: 504–509. doi: 10.1111/j.1939-1676.2002.tb02378.x [DOI] [PubMed] [Google Scholar]
- 5.Garden O. A., Kidd L., Mexas A. M., Chang Y. M., Jeffery U., Blois S. L., Fogle J. E., MacNeill A. L., Lubas G., Birkenheuer A., Buoncompagni S., Dandrieux J. R. S., Di Loria A., Fellman C. L., Glanemann B., Goggs R., Granick J. L., LeVine D. N., Sharp C. R., Smith-Carr S., Swann J. W., Szladovits B.2019. ACVIM consensus statement on the diagnosis of immune-mediated hemolytic anemia in dogs and cats. J. Vet. Intern. Med. 33: 313–334. doi: 10.1111/jvim.15441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griebsch C., Arndt G., Raila J., Schweigert F. J., Kohn B.2009. C-reactive protein concentration in dogs with primary immune-mediated hemolytic anemia. Vet. Clin. Pathol. 38: 421–425. doi: 10.1111/j.1939-165X.2009.00146.x [DOI] [PubMed] [Google Scholar]
- 7.Kumakura S.2005. Hemophagocytic syndrome. Intern. Med. 44: 278–280. doi: 10.2169/internalmedicine.44.278 [DOI] [PubMed] [Google Scholar]
- 8.Kumakura S., Murakawa Y.2014. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol. 66: 2297–2307. doi: 10.1002/art.38672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAtee B. B., Cummings K. J., Cook A. K., Lidbury J. A., Heseltine J. C., Willard M. D.2017. Opportunistic Invasive cutaneous fungal infections associated with administration of cyclosporine to dogs with immune-mediated disease. J. Vet. Intern. Med. 31: 1724–1729. doi: 10.1111/jvim.14824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmi A., Tsukamoto A., Ohno K., Uchida K., Nishimura R., Fukushima K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2012. A retrospective study of inflammatory colorectal polyps in miniature dachshunds. J. Vet. Med. Sci. 74: 59–64. doi: 10.1292/jvms.11-0352 [DOI] [PubMed] [Google Scholar]
- 11.Rizzi T. E., Meinkoth J. H., Clinkenbeard K. D.2010. . Normal hematology of the dog. pp. 799–810. In: Schalm’s Veterinary Hematology, 6th ed. (Weiss, D. J. and Wardrop, K. J. eds.), Wiley-Blackwell, Ames. [Google Scholar]
- 12.Stockham S. L., Scott M. A.2008. Bone marrow and lymph node. pp. 323–340. In: Fundamentals of Veterinary Clinical Pathology, 2nd ed. (Stockham, S. L. and Scott, M. A. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 13.Suwa A., Shimoda T.2018. Lymphoma-associated hemophagocytic syndrome in six dogs. J. Vet. Med. Sci. 80: 1271–1276. doi: 10.1292/jvms.17-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swann J. W., Skelly B. J.2015. Systematic review of prognostic factors for mortality in dogs with immune-mediated hemolytic anemia. J. Vet. Intern. Med. 29: 7–13. doi: 10.1111/jvim.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swann J. W., Woods K., Wu Y., Glanemann B., Garden O. A.2016. Characterisation of the immunophenotype of dogs with primary immune-mediated haemolytic anaemia. PLoS One 11: e0168296. doi: 10.1371/journal.pone.0168296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swann J. W., Garden O. A., Fellman C. L., Glanemann B., Goggs R., LeVine D. N., Mackin A. J., Whitley N. T.2019. ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 33: 1141–1172. doi: 10.1111/jvim.15463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tani A., Tomiyasu H., Ohmi A., Ohno K., Tsujimoto H.2020. Clinical and clinicopathological features and outcomes of Miniature Dachshunds with bone marrow disorders. J. Vet. Med. Sci. 82: 771–778. doi: 10.1292/jvms.19-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walton R. M., Modiano J. F., Thrall M. A., Wheeler S. L.1996. Bone marrow cytological findings in 4 dogs and a cat with hemophagocytic syndrome. J. Vet. Intern. Med. 10: 7–14. doi: 10.1111/j.1939-1676.1996.tb02017.x [DOI] [PubMed] [Google Scholar]
- 19.Weiss D. J.2001. Cytologic evaluation of benign and malignant hemophagocytic disorders in canine bone marrow. Vet. Clin. Pathol. 30: 28–34. doi: 10.1111/j.1939-165X.2001.tb00253.x [DOI] [PubMed] [Google Scholar]
- 20.Weiss D. J.2002. Flow cytometric evaluation of hemophagocytic disorders in canine. Vet. Clin. Pathol. 31: 36–41. doi: 10.1111/j.1939-165X.2002.tb00276.x [DOI] [PubMed] [Google Scholar]
- 21.Weiss D. J.2007. Hemophagocytic syndrome in dogs: 24 cases (1996–2005). J. Am. Vet. Med. Assoc. 230: 697–701. doi: 10.2460/javma.230.5.697 [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi C., Momoi Y., Kobayashi T., Ide K., Ohno K., Tsujimoto H., Iwasaki T.2007. A retrospective study and gene analysis of canine sterile panniculitis. J. Vet. Med. Sci. 69: 915–924. doi: 10.1292/jvms.69.915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.