Abstract
Recently, developmental exposure to clothianidin (CLO) has been shown to cause reproductive toxicity in male mice, but the effects in female mice remain to be clarified. Pregnant C57BL/6N mice were given a no-observed-adverse-effect-level (NOAEL) dose of CLO until weaning. We then examined ovaries of 3- or 10-week-old female offspring. In the CLO-administered group, morphological changes, a decrease in the immunoreactivity of the antioxidant enzyme glutathione peroxidase 4 (GPx4), and activation of genes in the steroid hormone biosynthesis pathway were observed in 3-week-old mice, and decreases of GPx4 immunoreactivity, 17OH-progesterone and corticosterone levels were observed in 10-week-old mice, along with high rates of infanticide and severe neglect, providing new evidence that developmental exposure to CLO affects juvenile and adult mice differently.
Keywords: clothianidin, developmental stage, DOHaD (developmental origins of health and diseases), female mice, in utero and lactational exposure
Neonicotinoid pesticides (NNs) were developed in the 1980s and are chemically similar to nicotine. NNs exhibit insecticidal effects by binding to the nicotinic acetylcholine receptors (nAChRs) of insects and triggering a response in neuronal cells [3, 24, 25]. NNs bind more readily to insect nAChRs than to mammalian nAChRs [38]. However, recent studies have reported reproductive toxicity [15] and neurobehavioral effects [16, 34, 35, 45] in mammals. In terms of reproductive toxicity, it has been shown that the NN clothianidin (CLO), causes the DNA fragmentation of germ cells and the inhibition of embryonic development in mature quail [18, 19, 37]. Another NN, imidacloprid, has been reported to cause significant changes in ovarian morphology and in follicle stimulating hormone (FSH), luteinizing hormone (LH), and progesterone levels in female rats [21] and to decrease testosterone levels in male rats [12]. We recently reported that CLO passes rapidly through the placental barrier in mice [27]. It has also been shown that in utero and lactational exposure to CLO decrease the numbers of germ cells in male mice [44]. Fetuses are vulnerable to chemicals, and there is concern about the effect of prenatal exposure on the health of future generations. In fact, developmental exposure to NNs induces abnormalities in behavior [4, 31, 36] and delayed sexual maturation [32] in mice. However, there are currently few animal research models for elucidating the role of prenatal and perinatal exposure to NNs in the development of diseases in adult females.
Oxidative stress is an imbalance between the oxidation and antioxidation reactions in the body, resulting in an oxidative state, and oxidative damage causes base damage, as well as strand breaks in DNA, and is thought to be one of the mechanisms by which NNs induce cytotoxicity [8]. In other studies, the oxidative stress of NNs was also found to cause cytoplasmic clumping of follicles [21] and DNA fragmentation of sperm in the reproductive systems of mammals [15] and birds [19, 37].
Hence, in utero and lactational exposure to NNs can have effects on ovarian development through oxidative stress, but it is currently not known how NNs affect the female mammalian genital organs in the next generation. Therefore, we investigated the effects of no-observed-adverse-effect-level (NOAEL) NN exposure during the embryonic and juvenile stages on developmental plasticity and female reproductive potential in the adult stage.
C57BL/6NCrSlc pregnant mice were purchased from Japan SLC (Hamamatsu, Japan) and maintained as described elsewhere [27]. This study was approved by the Institutional Animal Care and Use Committee (Permission #26-05-07) and carried out according to the Kobe University Animal Experimental Regulations. The dams were divided into two groups: a CLO-0 group administered 0 mg CLO/kg body weight/day (18 dams and 36 pups) and a CLO-65 group administered 65 mg CLO/kg body weight/day (17 dams and 33 pups). The administration concentration was set with reference to the NOAEL (ICR female mice: 65.1 mg CLO/kg/day [10, 39]). To eliminate the risk of adverse effects associated with gavage, the dams were given soft gel (MediGel® Sucrarose; ClearH2O, Portland, ME, USA) with and without CLO (extracted from Dantotsu®; Sumitomo Chemical Co., Tokyo, Japan, [15]) as a substitute for filtered water from gestational day 1.5 to postnatal day (PND) 21. Dams ingested the gel actively, and the CLO concentration of 65 mg/kg/day did not affect the amount of gel intake. Each litter was randomly culled to a maximum of six pups on PND 2 to standardize the amount of milk, and litters of three pups or fewer were removed from the experiment for the same reason. One or two pups per litter were used in order to avoid a litter bias. The amount of isolated CLO added to the aqueous solution was calculated as follows: the CLO purity (95%), daily gel intake and average body weight (6 g/day and 22 g for early pregnancy, 6.5 g/day and 30 g for late pregnancy, 16 g/day and 29 g for the first week of lactation, and 20 g/day for the second and third weeks of lactation), total gel weight (60 g: excluding the cup weight). The calculated amount of CLO was dissolved in 600 µl of DMSO (1% volume of gel) and injected into the gel cups, and which were then shaken vigorously to mix the CLO evenly in the gel.
On PND 21 of age of the offspring, blood was collected from the dams and the pups under anesthesia with isoflurane. After the animals were euthanized, the ovaries and blood were collected. The right ovaries were weighed and fixed in 4% paraformaldehyde in phosphate buffer at 4°C for 3 hr or 6 hr, respectively. They were then dehydrated through a graded series of ethanol followed by xylene and embedded in paraffin. The left ovaries were placed in a mixture of Buffer RA1 and TCEP (NucleoSpin plus RNA isolation kit), frozen with liquid nitrogen, and stored in a −80°C freezer. Samples were collected from the 10-week-old mice in the same way, and some of the F1 animals were mated within the group when they reached 10 weeks of age and evaluated for fertility and parental care.
The tissue samples were sectioned at 4-µm thickness by a sliding microtome (SM2000R; Leica Microsystems, Wetzlar, Hesse, Germany), and mounted on a glass slide precoated with 0.2% 3-aminopropyltriethoxysilane (Shin-Etsu Chemical Co., Tokyo, Japan). To detect glutathione peroxidase 4 (GPx4) and manganese superoxide dismutase (MnSOD) immunoreactivity in the ovary, we used the following antibodies: rabbit monoclonal anti-GPx4 (1:50; ab125066; Abcam, Cambridge, UK) and rabbit monoclonal anti-MnSOD (1:300; ab68155; Abcam). The immunoreactivities of the primary antibodies were examined with 3,3-diaminobenzidine tetrahydrochloride solution (EnVision®+ kit/HRP [DAB], Dako, Glostrup, Denmark). Immunostaining of all sections was performed as previously described [28].
For the analysis of steroid hormones (17OH-progestereone and corticosterone), LC-ESI/MS/MS was used as described elsewhere [46]. In brief, steroid hormones and its deuterated or 13C labeled internal standard were detected with multiple reaction monitoring (MRM; 331.2>109.1 and 334.0>100.2 for 17OH-progestereone and 17OH-progesterone-13C3, and 347.2>121.2 and 351.4>121.2 for Corticosterone and Corticosterone D4) in a positive ion mode using a 6495 B Triple Quadrupole LC-MS/MS system from Agilent Technologies (Santa Clara, CA, USA). Chromatography separation was achieved by using Kinetex® Biphenyl column (1.7 µm, 100 × 2.1 mm; Phenomenex, Torrance, CA, USA) and a gradient elution as a flow rate of 0.4 ml/min with the mobile phase A as 0.2 mmol/l NH4F containing distilled water, and B as 0.2 mmol/l NH4F containing methanol. The gradient program was 0–0.5 min 50% B (isocratic), 0.5–10 min 95% B (gradient), and 10–12 min 95% B (isocratic).
Total RNA of the left ovaries of 3- and 10-week-old mice was extracted using a NucleoSpin XS RNA isolation kit (Macherey-Nagel GmbH & Co., Düren, Germany). The effects of CLO on the gene expression profiles in the ovaries of 3-week-old mice were analyzed by the GeneChip system and Clariom S mouse array (Affymetrix, Santa Clara, CA, USA) as described elsewhere [14]. The raw intensity data were normalized using GeneSpring GX 14.9 software (Agilent Technologies) and analyzed using Ingenuity Pathways Analysis (IPA) tools (Ingenuity Systems, Mountain View, CA, USA). The microarray data (.CEL files) were deposited in a public database (Gene Expression Omnibus, accession number: GSE164099).
The changes in gene expression were measured by the quantitative reverse transcription polymerase chain reaction (qRT-PCR) as described previously [14] using specific primers (Supplementary Table 1) on a StepOnePlus (Life Technologies Japan Inc., Tokyo, Japan). The thermal cycling consisted of initial degeneration of 95°C for 30 sec, followed by 40 cycles of denaturing at 95°C for 5 sec and elongation at 60°C for 30 sec. The copy numbers of genes were calculated using standard curves, and normalized with the housekeeping genes β-actin (Actb). All samples were measured in duplicate and the specificity of the PCR products was confirmed by melting curves.
Statistical analysis was performed with Excel statistics 2012 (Version 1.00; SSRI, Tokyo, Japan). Organ weights and hormone data were analyzed by two-way ANOVA followed by Tukey-Kramer post hoc test. Body weights, the number of pups, sex ratio and qRT-PCR data were analyzed by Welch’s t-test, and postnatal days to vaginal opening data were analyzed by Mann-Whitney U-test. Infanticide and neglect data were analyzed by chi-square test. The results were considered significant when the P-value was less than 0.05.
In the 3-week-old mice, the ovary weight (Fig. 1B) and size (Fig. 1C and 1D) of the CLO-65 group were clearly lower than those of the CLO-0 group. There was no significant effect of CLO on the sex ratio of the pups or postnatal days to vaginal opening, but there was a trend toward smaller litter size in the CLO-65 group (P=0.064) ( Supplementary Table 2), and no significant effect of CLO on the body weights of all groups, or ovary weights of 10-week-old mice (Fig. 1A and 1B). The general histological analyses of ovaries by HE staining revealed a clear increase in the intercellular spaces between granulosa cells in the 3-week-old CLO-65 group, but there were no changes in the number of follicles or their composition in either the 3- or 10-week-old CLO-65 group compared to the respective control group (Fig. 1E and 1F, Supplementary Fig. 1A and 1B). Yanai et al. [44] reported that prenatal and postnatal exposure to CLO decreased the testicular weights and number of germ cells in childhood, but in our study there was no significant effect of CLO on the stages of follicle development or the constitution of follicles. Therefore, exposure to CLO may affect cells other than follicles in the fetal ovary.
Fig. 1.
Effect of clothianidin (CLO) on body weight (A) and ovary weight (B) in F1. General histology of 3-week-old ovaries (C–F) and representative examples of hematoxylin and eosin (HE) staining are shown. A: There was no effect of CLO. Data represent the means ± SD of each group and circles show the means of each group (n=20 in each). B: The ovary weights were significantly lower in the CLO-65 group of 3-week-old mice. Data represent means ± SD of each group and circles show the values for individual mice (n=5–16 in each). C, D: Size-reduction ovaries were observed in the CLO-65 group. E, F: Intercellular spaces between granulosa cells (arrows) were observed in the CLO-65 group. Scale bars=500 µm (C, D). Scale bars=100 µm (E, F). *P<0.05 vs. the CLO-0 group.
The results of the immunohistochemical analyses of the ovary visualizing GPx4 and MnSOD are shown in Fig. 2. We assessed the intensity of GPx4 and MnSOD immunoreactivity as an index of oxidative stress in the ovary. Some degree of GPx4 immunoreactivity was observed in the nucleus and cytoplasm of oocytes, granulosa cells, stromal cells, and theca cells in all groups, as well as in the lutein cells of 10-week-old mice (Fig. 2A–D). The intensity of GPx4 immunoreactivity in the granulosa cells, theca cells and lutein cells was decreased by CLO. Some degree of MnSOD immunoreactivity was observed in the cytoplasm of oocytes, granulosa cells, stromal cells, and theca cells in all groups, as well as in the lutein cells of the 10-week-old mice (Fig. 2E–H). There was no effect of CLO on the intensity in any of the groups. Several previous studies have demonstrated that NNs induce oxidative stress [8, 21], which reflects an imbalance between reactive oxygen species (ROS) and antioxidants and can cause cell death. GPx is an antioxidant enzyme that typically uses glutathione (GSH) as a reductant [20]. SOD is an antioxidant enzyme, and MnSOD, a type of SOD, is found in the mitochondria. We previously reported that subchronic administration of CLO caused a decrease in GPx4 and MnSOD in mature quail [37]. In addition, it has been shown that GPx4 uses GSH and that substitution of thiazole ring chlorine by GSH is one of the major metabolic pathways of CLO [20, 39]. Therefore, our present results suggest that GSH may be consumed by CLO administration, and GPx4 activity may also be decreased due to the decrease in GSH. Since GPx4 is involved in the major metabolic pathway of CLO, it is possible that GPx4 is more susceptible to CLO administration than MnSOD.
Fig. 2.
Immunohistochemistry of 3-week-old (A, B, E, F) and 10-week-old (C, D, G, H) ovaries. Representative examples of immunohistochemistry for GPx4 (glutathione peroxidase 4; A–D) and MnSOD (manganese superoxide dismutase; E–H) are shown. A–D: The intensity of GPx4 immunoreactivity was decreased in the CLO-65 group. E–H: There was no effect of CLO on the intensity of MnSOD immunoreactivity. Scale bars=100 µm.
17-Hydroxyprogesterone (17-OH progesterone) and corticosterone were detected in the blood of pups (Table 1). There was a significant main effect of CLO and age with a significant interaction on 17-OH progesterone [F(1, 45)=16.12, P<0.001; F(1, 45)=6.391, P<0.05; F(1, 45)=4.837, P<0.05]. There was also a significant main effect of CLO and age with a significant interaction on corticosterone [F(1, 45)=8.900, P<0.005; F(1, 45)=12.01, P<0.005; F(1, 45)=4.450, P<0.05]. Both hormones were significantly decreased in the 10-week-old CLO-65 group compared to the CLO-0 group. These results showed that in utero and lactational exposure to NNs decreased the levels of hormones related to estrogen and progesterone in adults. A previous study revealed that prenatal and neonatal nicotine exposure changes serum progesterone and estradiol levels in 6-month-old female rats, despite inducing no changes in 4-month-old female rats, suggesting that nicotine disrupts the hypothalamic-pituitary-ovarian function, resulting in ovarian reprogramming and age-related reduced fertility [17]. However, there was no obvious histological effect of CLO on 10-week-old ovaries in our study, which may be because 17OH-progesterone and corticosterone mainly affected the adrenal glands. 17OH-progesterone is produced by the adrenal glands and gonads, and corticosterone by the adrenal glands. NNs have been reported to induce functional impairment in the hypothalamic-pituitary-adrenal axis in rats [1], and to increase adrenaline secretion in PC12D cells [22]. These findings of prenatal effects manifesting in adulthood led to the hypothesis of the developmental origins of health and diseases (DOHaD), and it is assumed that the same thing happened with CLO, which binds to nAChR as well as nicotine.
Table 1. Clothianidin (CLO)-induced alterations at plasma levels of 17OH-progesterone and corticosterone.
| 3-week-old | 10-week-old | |||
|---|---|---|---|---|
| CLO-0 | CLO-65 | CLO-0 | CLO-65 | |
| 17OH-progesterone (ppb) | 7.88 ± 0.75 | 6.23 ± 0.36 | 7.58 ± 1.68 | 1.91 ± 1.06* |
| Corticosterone (ppb) | 445.93 ± 37.34 | 411.31 ± 17.29 | 392.21 ± 44.57 | 190.41 ± 62.78* |
Mean ± SE (n=6–22 in each). *P<0.01 vs. the CLO-0 group.
Infanticide (n=2) and severe neglect (n=1) were observed in the CLO-65 group of the F1 generation, but these effects were not among the pregnancy outcomes in any groups of the F0 generation or in the CLO-0 group of the F1 animals (Fig. 3). Common laboratory mouse strains have been selected for low maternal aggression to increase breeding success, and thus infanticidal mothers are rarely observed under normal husbandry conditions [23, 42]. A previous study reported that in utero and lactational exposures to bisphenol S cause moderate neglect, such as poor cleaning of pups as a pregnancy outcome in the F0 generation, and infanticide and severe neglect as pregnancy outcomes in the F1 generation [7]. Another study reported that environmental tobacco smoke decreases oxytocin in the plasma of rats during the lactation period [26]. Oxytocin binds to RAGE (receptor for advanced glycation end-products) and crosses the blood-brain barrier, and it has been reported that RAGE-knockout mice fail to nurture their pups [43]. Moreover, Sairenji et al. [30] revealed that maternal prolactin could be a key factor for generating nurturing behavior in offspring by activating neural circuits required for the expression of nurturing behaviors. Thus, the effects observed in the F1 animals in our study may have been due to their direct exposure to CLO, poor care from mothers with low oxytocin and prolactin levels, or a combination of these factors.
Fig. 3.
Effects of clothianidin (CLO) on infanticide and severe neglect. In the CLO-administered group of generation F1, 3 out of 9 animals exhibited infanticide and severe neglect behaviors, whereas no animals exhibited these behaviors in the F0 generation (CLO-0: n=13; CLO-65: n=15) or in the CLO-0 group of the F1 generation (n=4).
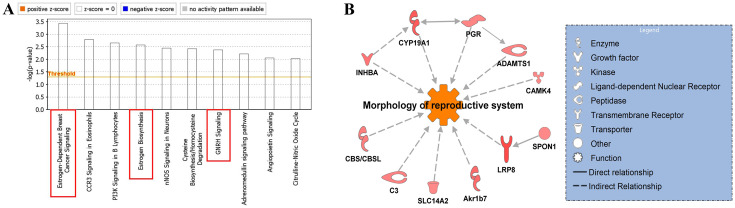
We identified 99 genes (62 up-regulated and 37 down-regulated) that showed 1.5-fold or greater differential expression between the CLO-65 group and CLO-0 group. The top 20 biological functions related to the up-regulated genes are summarized in Table 2. CLO increased the expression of genes associated with reproductive functions with annotations of “Quantity of gonadal cells”, “Quantity of germ cells”, “Quantity of gonads” and “Quantity of ovarian follicles”. The top 10 canonical pathways that were differentially activated or suppressed by the up-regulated genes are shown in Fig. 4A. The affected pathways included those for estrogen-dependent breast cancer signaling, estrogen biosynthesis and GnRH signaling relating to steroid hormone biosynthesis. Networks describing the relationships between a subset of genes and their neighboring genes are presented in Fig. 4B. Canonical pathways associated with the steroid hormone biosynthesis pathway were identified, but it was not clear whether these pathways were activated or suppressed. However, gene sets related to “Quantity of gonadal cells”, “Quantity of germ cells”, “Quantity of gonads” and “Quantity of ovarian follicles” were significantly enriched in the up-regulated genes, suggesting that steroid hormone biosynthesis pathways were activated. Wakabayashi et al. [41] revealed that estrogen administration caused a decrease in ovarian weight and a reduction in ovarian size in rats, but did not change the number of follicles, leading them to conclude that estrogen suppressed the secretion of gonadotropins from the pituitary gland. Hence, the present study suggests that CLO may have activated the estrogen biosynthetic pathway in prepubertal mice, causing a decrease in ovarian weight and size reduction at 3 weeks of age.
Table 2. Top 20 of biological functions of 62 up-regulated genes.
| Functions annotation | P-value | Predicted activation state | Activation z-score | Number of molecules |
|---|---|---|---|---|
| Quantity of gonadal cells | 6.20E-05 | Increased | 2.198 | 5 |
| Fertility | 2.16E-03 | Increased | 2.189 | 5 |
| Litter size | 8.56E-04 | 1.982 | 4 | |
| Quantity of germ cells | 6.52E-04 | 1.951 | 4 | |
| Quantity of gonads | 1.34E-05 | 1.864 | 6 | |
| Development of neurons | 7.02E-05 | 1.761 | 10 | |
| Neuritogenesis | 2.61E-04 | 1.516 | 8 | |
| Long-term potentiation | 4.23E-04 | 1.4 | 5 | |
| Quantity of bone | 4.07E-04 | 1.091 | 5 | |
| Differentiation of bone cells | 4.13E-04 | 1.067 | 6 | |
| Necrosis of epithelial tissue | 5.44E-03 | 1 | 6 | |
| Apoptosis of epithelial cells | 5.58E-03 | 1 | 4 | |
| Quantity of ovarian follicles | 1.61E-06 | 0.927 | 5 | |
| Differentiation of connective tissue cells | 2.67E-04 | 0.713 | 8 | |
| Quantity of osteoclasts | 1.98E-04 | 0.577 | 4 | |
| Sprouting | 5.20E-03 | 0.44 | 5 | |
| Quantity of connective tissue | 1.83E-04 | 0.13 | 8 | |
| Quantity of protein in blood | 2.06E-03 | −0.003 | 6 | |
| Quantity of cytokines | 3.35E-04 | −0.09 | 5 | |
| Concentration of hormone | 5.18E-04 | −0.095 | 6 |
Activation z-score; >2.0 or < −2.0 is significantly predictive.
Fig. 4.
Canonical pathways, and networks of up-regulated genes (62 genes) altered by exposure of clothianidin (CLO) identified by ingenuity pathway analysis (IPA) software. A: Signaling pathways activated or suppressed. Solid squares indicate pathways related to the steroid hormone biosynthesis pathway. B: A gene network map illustrating the interactions of up-regulated genes (red colored) and other molecules (n=2 in each).
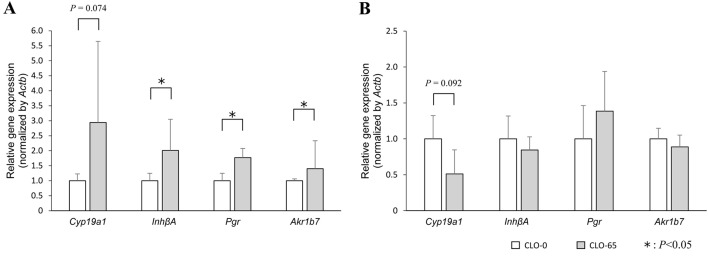
We chose four genes that were found to be significant in the network analyses—i.e., cytochrome P450 family 19 subfamily A member 1 (Cyp19a1), inhibin subunit beta A (InhβA), progesterone receptor (Pgr) and aldo-keto reductase family 1, member B7 (Akr1b7)—for independent verification by qRT-PCR. We found that the expressions of InhβA, Pgr and Akr1b7 were significantly activated, and that the activation-of Cyp19a1 expression approached significance (Cyp19a1: P=0.074; Fig. 5A) in the 3-week-old CLO-65 group, matching the pattern of the microarray results. In 10-week-old mice, there was a downward trend in the gene expression of Cyp19a1 by CLO (P=0.092; Fig. 5B). Exposure of Hs578t cells to NNs resulted in an increase of CYP19 expression and aromatase catalytic activity [6], and such effects have been shown to result in increased biosynthesis of estrogens [5, 9]. NNs cause reproductive toxicity in humans by increasing estrone and estradiol production and inhibiting estriol production [5, 13]. Moreover, we showed that CLO decreased the GPx4 immunoreactivity in granulosa cells, theca cells and lutein cells of 3-week-old mice. Cyp19a1 is expressed in lutein cells [29], InhA in granulosa cells [33], Akr1b7 in theca cells [2], and Pgr in granulosa cells [40] and lutein cells [11]. Thus, in 3-week-old ovaries of the present study, it was suggested that CLO may induce oxidative stress in those cells, and increase the expressions of Cyp19a1, InhA, Pgr, and Akr1b7, or that CLO may act directly to increase Cyp19a1 expression. These effects, in turn, could activate the estrogen biosynthetic pathway. In 10-week-old ovaries, the expression of the Cyp19a1 showed a rather downward trend, contrary to that of 3-week-old ovaries, suggesting that in utero and lactational exposure may have effects in adulthood.
Fig. 5.
Validation of microarray results using qRT-PCR in 3- (A) and 10-week-old mice (B). Gene expression levels of Cytochrome P450 family 19 subfamily A member 1 (Cyp19a1), inhibin subunit beta A (InhβA), progesterone receptor (Pgr) and aldo-keto reductase family 1, member B7 (Akr1b7) were calculated relative to the housekeeping genes, β-actin (Actb), by comparison to the control group. Data represent means ± SD of each group (n=4–5 in each). *P<0.05 vs. the CLO-0 group.
Our present study showed that developmental exposure to NOAEL-dose CLO has different effects on reproduction and developmental plasticity between juvenile and adult animals. This implies that CLO exposure in the fetal and lactation stages is subsequently manifested differently in adult animals, and proper evaluation of these effects will contribute to an improvement of toxicity testing of NNs.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Supplementary Material
Acknowledgments
This work was partly supported by Grants-in-Aid for Scientific Research A (#JP18H04132 to YI) and B (#JP19H04277 to NH), and a Grant-in-Aid for Early-Career Scientists (#JP19K19406 to TH) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a grant from the Japan Environ and Organic-Farming Foundation (#2019004 to NH); and by an “Act Beyond Trust” (GIA) civil grant 2020 (to NH, YI). We also acknowledge financial support from the Nakajima Foundation, the Sumitomo Foundation, the Nihon Seimei Foundation and the Triodos Foundation (to YI).
REFERENCES
- 1.Annabi A., Dhouib I. B., Dkhili H., Bdiri Y., Rejeb I., Gharbi N., El-Fazâa S., Lasram M. M.2015. Mechanisms of imidacloprid-induced alteration of hypothalamic-pituitary-adrenal (HPA) axis after subchronic exposure in male rats. Recent Adv. Biol. Med. 1: 51–59. doi: 10.18639/RABM.2015.01.195931 [DOI] [Google Scholar]
- 2.Baumann C., Davies B., Peters M., Kaufmann-Reiche U., Lessl M., Theuring F.2007. AKR1B7 (mouse vas deferens protein) is dispensable for mouse development and reproductive success. Reproduction 134: 97–109. doi: 10.1530/REP-07-0022 [DOI] [PubMed] [Google Scholar]
- 3.Buckingham S., Lapied B., Corronc H., Sattelle F.1997. Imidacloprid actions on insect neuronal acetylcholine receptors. J. Exp. Biol. 200: 2685–2692. [DOI] [PubMed] [Google Scholar]
- 4.Burke A. P., Niibori Y., Terayama H., Ito M., Pidgeon C., Arsenault J., Camarero P. R., Cummins C. L., Mateo R., Sakabe K., Hampson D. R.2018. Mammalian susceptibility to a neonicotinoid insecticide after fetal and early postnatal exposure. Sci. Rep. 8: 16639. doi: 10.1038/s41598-018-35129-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron-Beaudoin E., Viau R., Hudon-Thibeault A. A., Vaillancourt C., Sanderson J. T.2017. The use of a unique co-culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: The effects of neonicotinoids on aromatase activity and hormone production. Toxicol. Appl. Pharmacol. 332: 15–24. doi: 10.1016/j.taap.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 6.Caron-Beaudoin É., Viau R., Sanderson J. T.2018. Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Perspect. 126: 047014. doi: 10.1289/EHP2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catanese M. C., Vandenberg L. N.2017. Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology 158: 516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duzguner V., Erdogan S.2012. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pestic. Biochem. Physiol. 104: 58–64. doi: 10.1016/j.pestbp.2012.06.011 [DOI] [Google Scholar]
- 9.Eldridge J. C., Tennant M. K., Wetzel L. T., Breckenridge C. B., Stevens J. T.1994. Factors affecting mammary tumor incidence in chlorotriazine-treated female rats: hormonal properties, dosage, and animal strain. Environ. Health Perspect. 102 Suppl 11: 29–36. doi: 10.1289/ehp.94102s1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Agriculture Organization of the United Nations. 2020. FAO Specifications and Evaluations for Agricultural Pesticides Clothianidin. http://www.fao.org/3/ca7726en/ca7726en.pdf [accessed on November 4, 2020].
- 11.Gräs S., Hannibal J., Fahrenkrug J.1999. Pituitary adenylate cyclase-activating polypeptide is an auto/paracrine stimulator of acute progesterone accumulation and subsequent luteinization in cultured periovulatory granulosa/lutein cells. Endocrinology 140: 2199–2205. doi: 10.1210/endo.140.5.6737 [DOI] [PubMed] [Google Scholar]
- 12.Hafez E. M., Issa S. Y., Al-Mazroua M. K., Ibrahim K. T., Rahman S. M. A.2016. The neonicotinoid insecticide imidacloprid: A male reproductive system toxicity inducer-human and experimental study. Toxicol. Open Access 2: 1–8. doi: 10.4172/2476-2067.1000109 [DOI] [Google Scholar]
- 13.Han W., Tian Y., Shen X.2018. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 192: 59–65. doi: 10.1016/j.chemosphere.2017.10.149 [DOI] [PubMed] [Google Scholar]
- 14.Hirano T., Minagawa S., Furusawa Y., Yunoki T., Ikenaka Y., Yokoyama T., Hoshi N., Tabuchi Y.2019. Growth and neurite stimulating effects of the neonicotinoid pesticide clothianidin on human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 383: 114777. doi: 10.1016/j.taap.2019.114777 [DOI] [PubMed] [Google Scholar]
- 15.Hirano T., Yanai S., Omotehara T., Hashimoto R., Umemura Y., Kubota N., Minami K., Nagahara D., Matsuo E., Aihara Y., Shinohara R., Furuyashiki T., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2015. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 77: 1207–1215. doi: 10.1292/jvms.15-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano T., Yanai S., Takada T., Yoneda N., Omotehara T., Kubota N., Minami K., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2018. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 282: 57–63. doi: 10.1016/j.toxlet.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Holloway A. C., Kellenberger L. D., Petrik J. J.2006. Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine 30: 213–216. doi: 10.1385/ENDO:30:2:213 [DOI] [PubMed] [Google Scholar]
- 18.Hoshi N.2021. Adverse effects of pesticides on regional biodiversity and their mechanisms. pp. 235–247. In: Risks and Regulation of New Technologies (Matsuda, T., Wolff, J. and Yanagawa, T. eds.), Springer, Singapore. [Google Scholar]
- 19.Hoshi N., Hirano T., Omotehara T., Tokumoto J., Umemura Y., Mantani Y., Tanida T., Warita K., Tabuchi Y., Yokoyama T., Kitagawa H.2014. Insight into the mechanism of reproductive dysfunction caused by neonicotinoid pesticides. Biol. Pharm. Bull. 37: 1439–1443. doi: 10.1248/bpb.b14-00359 [DOI] [PubMed] [Google Scholar]
- 20.Imai H., Nakagawa Y.2003. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 34: 145–169. doi: 10.1016/S0891-5849(02)01197-8 [DOI] [PubMed] [Google Scholar]
- 21.Kapoor U., Srivastava M. K., Srivastava L. P.2011. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. 49: 3086–3089. doi: 10.1016/j.fct.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 22.Kawahata I., Yamakuni T.2018. Imidacloprid, a neonicotinoid insecticide, facilitates tyrosine hydroxylase transcription and phenylethanolamine N-methyltransferase mRNA expression to enhance catecholamine synthesis and its nicotine-evoked elevation in PC12D cells. Toxicology 394: 84–92. doi: 10.1016/j.tox.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 23.Kohl J., Autry A. E., Dulac C.2017. The neurobiology of parenting: A neural circuit perspective. BioEssays 39: 1–11. doi: 10.1002/bies.201600159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda K., Buckingham S. D., Kleier D., Rauh J. J., Grauso M., Sattelle D. B.2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22: 573–580. doi: 10.1016/S0165-6147(00)01820-4 [DOI] [PubMed] [Google Scholar]
- 25.Nagata K., Song J. H., Shono T., Narahashi T.1998. Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J. Pharmacol. Exp. Ther. 285: 731–738. [PubMed] [Google Scholar]
- 26.Napierala M., Merritt T. A., Mazela J., Jablecka K., Miechowicz I., Marszalek A., Florek E.2017. The effect of tobacco smoke on oxytocin concentrations and selected oxidative stress parameters in plasma during pregnancy and post-partum - an experimental model. Hum. Exp. Toxicol. 36: 135–145. doi: 10.1177/0960327116639363 [DOI] [PubMed] [Google Scholar]
- 27.Ohno S., Ikenaka Y., Onaru K., Kubo S., Sakata N., Hirano T., Mantani Y., Yokoyama T., Takahashi K., Kato K., Arizono K., Ichise T., Nakayama S. M. M., Ishizuka M., Hoshi N.2020. Quantitative elucidation of maternal-to-fetal transfer of neonicotinoid pesticide clothianidin and its metabolites in mice. Toxicol. Lett. 322: 32–38. doi: 10.1016/j.toxlet.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 28.Onaru K., Ohno S., Kubo S., Nakanishi S., Hirano T., Mantani Y., Yokoyama T., Hoshi N.2020. Immunotoxicity evaluation by subchronic oral administration of clothianidin in Sprague-Dawley rats. J. Vet. Med. Sci. 82: 360–372. doi: 10.1292/jvms.19-0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacchi S., D’Ippolito G., Sena P., Marsella T., Tagliasacchi D., Maggi E., Argento C., Tirelli A., Giulini S., La Marca A.2016. The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J. Assist. Reprod. Genet. 33: 95–100. doi: 10.1007/s10815-015-0615-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sairenji T. J., Ikezawa J., Kaneko R., Masuda S., Uchida K., Takanashi Y., Masuda H., Sairenji T., Amano I., Takatsuru Y., Sayama K., Haglund K., Dikic I., Koibuchi N., Shimokawa N.2017. Maternal prolactin during late pregnancy is important in generating nurturing behavior in the offspring. Proc. Natl. Acad. Sci. USA 114: 13042–13047. doi: 10.1073/pnas.1621196114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano K., Isobe T., Yang J., Win-Shwe T. T., Yoshikane M., Nakayama S. F., Kawashima T., Suzuki G., Hashimoto S., Nohara K., Tohyama C., Maekawa F.2016. In utero and lactational exposure to acetamiprid induces abnormalities in socio-sexual and anxiety-related behaviors of male mice. Front. Neurosci. 10: 228. doi: 10.3389/fnins.2016.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheets L. P., Li A. A., Minnema D. J., Collier R. H., Creek M. R., Peffer R. C.2016. A critical review of neonicotinoid insecticides for developmental neurotoxicity. Crit. Rev. Toxicol. 46: 153–190. doi: 10.3109/10408444.2015.1090948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smitz J., Cortvrindt R.1998. Inhibin A and B secretion in mouse preantral follicle culture. Hum. Reprod. 13: 927–935. doi: 10.1093/humrep/13.4.927 [DOI] [PubMed] [Google Scholar]
- 34.Takada T., Yoneda N., Hirano T., Onaru K., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Nimako C., Ishizuka M., Ikenaka Y., Hoshi N.2020. Combined exposure to dinotefuran and chronic mild stress counteracts the change of the emotional and monoaminergic neuronal activity induced by either exposure singly despite corticosterone elevation in mice. J. Vet. Med. Sci. 82: 350–359. doi: 10.1292/jvms.19-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada T., Yoneda N., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Verification of the causal relationship between subchronic exposures to dinotefuran and depression-related phenotype in juvenile mice. J. Vet. Med. Sci. 80: 720–724. doi: 10.1292/jvms.18-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka T.2012. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxicol. Ind. Health 28: 697–707. doi: 10.1177/0748233711422726 [DOI] [PubMed] [Google Scholar]
- 37.Tokumoto J., Danjo M., Kobayashi Y., Kinoshita K., Omotehara T., Tatsumi A., Hashiguchi M., Sekijima T., Kamisoyama H., Yokoyama T., Kitagawa H., Hoshi N.2013. Effects of exposure to clothianidin on the reproductive system of male quails. J. Vet. Med. Sci. 75: 755–760. doi: 10.1292/jvms.12-0544 [DOI] [PubMed] [Google Scholar]
- 38.Tomizawa M., Casida J. E.2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45: 247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930 [DOI] [PubMed] [Google Scholar]
- 39.Uneme H., Konobe M., Akayama A., Yokota T., Mizuta K.2006. Discovery and development of a novel insecticide “clothianidin”. Sumitomo Kagaku 2. pp. 1−14. https://www.sumitomo-chem.co.jp/english/rd/report/files/docs/20060202_h6t.pdf [accessed on November 4, 2020].
- 40.Vanderhyden B. C., Tonary A. M.1995. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol. Reprod. 53: 1243–1250. doi: 10.1095/biolreprod53.6.1243 [DOI] [PubMed] [Google Scholar]
- 41.Wakabayashi H.1962. [Basic studies on the “rebound phenomenon” of ovarian function]. Kita Kanto Igaku 12: 22–40 (in Japanese). [PubMed] [Google Scholar]
- 42.Weber E. M., Algers B., Hultgren J., Olsson I. A.2013. Pup mortality in laboratory mice--infanticide or not? Acta Vet. Scand. 55: 83. doi: 10.1186/1751-0147-55-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto Y., Liang M., Munesue S., Deguchi K., Harashima A., Furuhara K., Yuhi T., Zhong J., Akther S., Goto H., Eguchi Y., Kitao Y., Hori O., Shiraishi Y., Ozaki N., Shimizu Y., Kamide T., Yoshikawa A., Hayashi Y., Nakada M., Lopatina O., Gerasimenko M., Komleva Y., Malinovskaya N., Salmina A. B., Asano M., Nishimori K., Shoelson S. E., Yamamoto H., Higashida H.2019. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2: 76. doi: 10.1038/s42003-019-0325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanai S., Hirano T., Omotehara T., Takada T., Yoneda N., Kubota N., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2017. Prenatal and early postnatal NOAEL-dose clothianidin exposure leads to a reduction of germ cells in juvenile male mice. J. Vet. Med. Sci. 79: 1196–1203. doi: 10.1292/jvms.17-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneda N., Takada T., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Peripubertal exposure to the neonicotinoid pesticide dinotefuran affects dopaminergic neurons and causes hyperactivity in male mice. J. Vet. Med. Sci. 80: 634–637. doi: 10.1292/jvms.18-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L. Quantitative Determination of a Panel of Endogenous Steroids in Human Serum by LC/MS/MS. https://www.agilent.com/cs/library/applications/application-sle-steroids-serum-lcmsms-5994-0949en-agilent.pdf [accessed on November 4, 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.