We analyse data on embryonic mortality under constant-temperature incubation for 15 species of chelonians with temperature-dependent sex determination (TSD). Mortality is lowest near species-specific pivotal temperatures (Tpiv) but increases rapidly above TPiv, consistent with a theory that explains the adaptive significance of TSD. Conservation managers should incubate embryos near TPiv.
Keywords: Ectotherm, hatchling, mortality, pivotal temperature, testudines, thermal stress
Abstract
A common reptile conservation strategy involves artificial incubation of embryos and release of hatchlings or juveniles into wild populations. Temperature-dependent sex determination (TSD) occurs in most chelonians, permitting conservation managers to bias sex ratios towards females by incubating embryos at high temperatures, ultimately allowing the introduction of more egg-bearing individuals into populations. Here, we revisit classic sex allocation theory and hypothesize that TSD evolved in some reptile groups (specifically, chelonians and crocodilians) because male fitness is more sensitive to condition (general health, vigor) than female fitness. It follows that males benefit more than females from incubation environments that confer high-quality phenotypes, and hence high-condition individuals. We predict that female-producing temperatures, which comprise relatively high incubation temperatures in chelonians and crocodilians, are relatively stressful for embryos and subsequent life stages. We synthesize data from 28 studies to investigate how constant temperature incubation affects embryonic mortality in chelonians with TSD. We find several lines of evidence suggesting that warm, female-producing temperatures are more stressful than cool, male-producing temperatures. Further, we find some evidence that pivotal temperatures (TPiv, the temperature that produces a 1:1 sex ratio) may exhibit a correlated evolution with embryonic thermal tolerance. If patterns of temperature-sensitive embryonic mortality are also indicative of chronic thermal stress that occurs post-hatching, then conservation programs may benefit from incubating eggs close to species-specific TPivs, thus avoiding high-temperature incubation. Indeed, our models predict that, on average, a sex ratio of >75% females can generally be achieved by incubating eggs only 1°C above TPiv. Of equal importance, we provide insight into the enigmatic evolution of TSD in chelonians, by providing support to the hypothesis that TSD evolution is related to the quality of the phenotype conferred by incubation temperature, with males produced in high-quality incubation environments.
Introduction
Despite recent and widespread interest in reptile conservation (Roll et al., 2017), reptile populations are declining globally (Todd et al., 2010). Turtles, for example, are among the most imperiled group of vertebrates in the world (Rhodin et al., 2018; Gibbons and Lovich, 2019; Stanford et al., 2020). Natural rates of replacement and population growth are low in most turtle species, and because of high juvenile mortality, slow life histories (e.g. Kondo et al., 2017) and low genetic diversity (Romiguier et al., 2014), populations are unable to adapt to environmental change on the time scale of anthropogenic impacts (Hawkes et al., 2009). Elevated adult mortality of turtles arising from road collisions (Steen and Gibbs, 2004), fishing gear entrapment (Lewison et al., 2004; Bolten et al., 2011), predation (Bolten et al., 2011) and direct consumption as a food source (Conway-Gómez, 2007; Hancock et al., 2017) has therefore resulted in dramatic population declines. At present, 56.3% of data sufficient species (51.9% of all recognized species) are considered critically endangered, endangered or vulnerable by the International Union for Conservation of Nature (Rhodin et al., 2018).
Common turtle conservation initiatives include protecting nesting turtles from natural predation and poachers (Eckert et al., 1999), rehabilitation programs at trauma centers (Feck and Hamann, 2013), education initiatives (Hassan et al., 2017) and captive breeding (Bowkett, 2009). Another common initiative undertaken by zoos, parks, conservation authorities and even private individuals involves incubation of reptile eggs in an ex-situ setting (Eckert et al., 1999), followed by the release of hatchlings or juveniles. Growth of wild populations may then be enhanced by protecting eggs and embryos, which reduces embryonic and hatchling mortality, and/or rearing individuals to larger body sizes before release (Carstairs et al., 2019; Tetzlaff et al., 2019), which may increase survival rates in the wild (Rollinson and Rowe, 2015).
Artificial incubation is an integral part of many conservation initiatives for reptiles. Artificial incubation typically follows the removal of eggs from the wild (e.g. natural nests or gravid females that died on a road) or egg production after captive breeding. Embryos are placed in incubators, hatched under a specified incubation regime and then released into the wild (Páez et al., 2015). Although the physical incubation of reptilian embryos may seem to represent a small or insignificant portion of conservation programs, it is widely recognized that incubation temperature has a profound influence on morphology, performance and fitness components (Bobyn and Brooks, 1994a, 1994b; Booth, 2006; Noble et al., 2018b). Further, some lizards, all crocodilians, the tuatara and most turtles exhibit temperature-dependent sex determination (TSD), where sex is permanently affected by incubation temperature (Valenzuela and Lance, 2004). The evolution and maintenance of TSD may in fact be related to the effect of incubation temperature on fitness components (West, 2009), and as such, exploring artificial incubation regimes for reptiles through the lens of TSD evolution may provide insight into best practices for conservation programs.
In reptiles with TSD, the incubation temperatures experienced by an embryo during the thermosensitive period influences sex, where the thermosensitive period comprises specific anatomical stages that occur roughly during the middle third of embryonic development (Yntema, 1968, 1979; Girondot et al., 2018). Under constant temperature, the temperature–sex reaction norm is known to take three forms (reviewed in Valenzuela and Lance, 2004). The Female-Male-Female (FMF) pattern occurs when males are produced at intermediate temperatures and females are produced at extreme temperatures. FMF is hypothesized to be ancestral, and it is found in some turtles, some lizards and all crocodilians. The Male-Female (MF) pattern occurs when males are produced at cool temperatures and females at hot temperatures. Although turtles are the only taxon to exhibit MF, turtles happen to comprise the majority of extant TSD species, and therefore MF is the most common pattern of TSD. Finally, the Female-Male (FM) pattern is when females are produced at cool temperatures and males at hot temperatures, but this pattern is rare and found only in a few lizards and the tuatara. Evolutionary explanations for TSD in lizards and the tuatara (FM and FMF) may be different than evolutionary explanations for TSD in turtles and crocodilians (MF and FMF) (e.g. Janzen and Phillips, 2006). In particular, squamates are a sister group to the tuatara (Rest et al., 2003), whereas turtles are sister to the archosaurs, which includes crocodilians (Crawford et al., 2012), underlining that fundamentally different patterns of TSD are found in divergent evolutionary lineages.
In general, the evolution of TSD is hypothesized to arise by virtue of a sex-by-temperature interaction for fitness (Charnov and Bull 1977). In short-lived squamates with rapid maturation, the FM pattern of TSD may represent an adaptation to ensure females hatch under the cool conditions that occur relatively early in the season, as to maximize time for growth and achieve high fecundity during their short lives (Pen et al., 2010, see also Conover, 1984). In contrast, turtles and crocodilians mature late and are very long lived, so TSD may be an adaptation to an effect of incubation temperature on adult fitness that occurs over their relatively longer lives (Fig. 1). Specifically, males generally experience competition for mates, and a small proportion of males often capture a large portion of mating opportunities (Bateman, 1948), with males in high ‘condition’ (i.e. vigorous males in good health) securing most mates (Rowe and Houle, 1996). Male fitness is therefore more sensitive to condition than female fitness (Trivers and Willard, 1973), such that TSD evolves in age- and size-structured populations to ensure that males are produced under temperatures that represent a high-quality incubation environment, conferring relatively high post-hatching condition, ultimately enhancing male fitness under strong sexual selection (Trivers and Willard, 1973; Deeming and Ferguson, 1989; Freedberg and Wade, 2004; West, 2009). This hypothesis is an extension of Trivers–Willard (1973).
Figure 1.
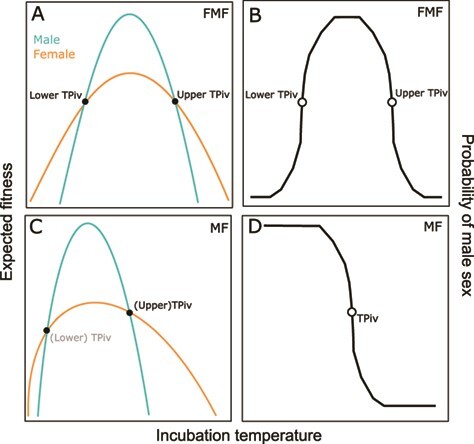
Conceptual model for the evolution of TSD in turtles and crocodilians, with explicit links between reaction norms for sex, TPiv and expected fitness. We hypothesize that males should be produced under the incubation temperatures that result in high condition individuals, as male fitness is more sensitive to condition than female fitness. Sex-specific expected fitness functions are presented for FMF species in panel (a). Here, it suffices to assume that there is an optimal incubation temperature or regime that promotes high fitness, with temperatures becoming increasingly stressful with distance from the optimum temperature. Because male fitness is more sensitive to condition, there must be a sex-by-environment interaction for fitness, which gives rise to (panel b) the FMF reaction norm. Sex-specific expected fitness functions are presented for MF species in panel (c). The concept is the same for MF species, but weak selection to maintain the lower TPiv causes it to drift near or below the thermal boundary of embryonic viability, rendering it invisible in most species. Thus, in panel (c), the lower TPiv (light grey) indicates females may occasionally be produced under extremely low temperatures in MF species, as has been observed in laboratory environments (e.g. Schwarzkopf and Brooks, 1985). The lack of lower TPiv in MF species results in (d) the MF reaction norm for sex.
The FMF pattern can easily be reconciled with this hypothesis (Freedberg and Wade, 2004), where females are produced in extreme environments (i.e. hot and cold incubation environments) and males at intermediate, or ‘optimal’, temperatures (Fig. 1a). The MF pattern can also be reconciled with this hypothesis: because sex is determined by the amount of development that occurs above versus below TPiv (Georges et al., 1994), lower temperatures must have relatively little effect on sexual differentiation, as relatively little development occurs per unit time at low temperatures (Rollinson et al., 2018; Massey et al., 2019). It follows that if an FMF species experiences an evolutionary increase in the lower TPiv (to a greater temperature), then all else being equal, this increase will have a much larger effect on sexual outcomes than would an evolutionary decrease in the lower TPiv. Natural selection to maintain the lower TPiv may therefore be weak in FMF species, especially if the fitness outcome of low temperature incubation is relatively benign. Ultimately, if an evolutionary decrease in the lower TPiv is relatively inconsequential for sex (and perhaps fitness), the lower TPiv may drift to a value near or below the limits of thermal viability of the embryo (Fig. 1). We suggest this process may have given rise to the MF pattern in chelonians.
A central prediction of the focal hypothesis is that, for MF species, fitness should decline with temperature at a relatively faster rate above TPiv versus below TPiv. A similar prediction applies to FMF species, where fitness at female-producing temperatures should be negatively related to the incubation temperature difference from TPiv, whereas fitness should be relatively high across male-producing temperatures. The reasoning for both predictions is that male-producing temperatures should reflect the peak of the performance curve, where a unit change in temperature has little influence on fitness, whereas female-producing temperatures should represent the shoulder of the curve, where fitness changes rapidly with temperature (Fig. 1). Mechanistically, fitness should depreciate rapidly above (upper) TPiv because TPiv evolves to be closer to the upper end of thermal tolerance, rather than the lower end, and reaction rates for most or all processes changes more rapidly near the upper tolerance limit (Sharpe and DeMichele, 1977; Kingsolver, 2009). In contrast, performance metrics should be relatively invariant with respect to temperature in the range of biologically plausible male-producing temperatures. However, male-producing temperatures in MF species that are far cooler than TPiv may indeed result in a decrease in performance, especially at constant temperatures (Bowden et al., 2014), or temperatures that are very cool and otherwise less ecologically relevant.
We have outlined a theoretical ground to suggest that incubation temperature has predictable and long-term effects on phenotypes and fitness components, especially in turtles and crocodilians. Yet, incubation regimes in conservation programs for these taxa are broadly unstandardized. For instance, some head-starting programs allow natural egg incubation (Bona et al., 2012), some allow temperature to fluctuate with above-ground ambient temperature (Tetzlaff et al., 2019) and others incubate different sets of eggs at different constant temperatures (Carstairs et al., 2019). A broad and theoretically informed exploration of how incubation temperature affects fitness in embryos and hatchlings may therefore be useful in developing best practices for artificial incubation in conservation programs.
Our goal is to better understand the evolution of TSD and to inform best practices for artificial incubation programs geared towards the conservation of reptiles, particularly for chelonians. In the present study, we synthesize existing data to explore the fitness consequences of artificial incubation regimes through the lens of TSD theory. We focus on patterns of temperature-sensitive embryonic mortality in chelonians, as chelonians are relatively data rich, and mortality is both a fitness component and is commonly measured. We generalize our mortality findings by centering study-specific incubation temperatures with respect to species-specific pivotal temperatures (TPiv), which is the constant temperature at which the sex ratio is 1:1 (Bull, 1980; Ewert et al., 1994). We focus on a simple explanation for TSD that we believe is specific to chelonians and crocodilians (Fig. 1), which is that males are produced in incubation environments that confer high condition (Charnov and Bull, 1977; Deeming and Ferguson, 1989; Freedberg and Wade, 2004; West, 2009). Several predictions arise from this hypothesis. The first is that embryonic thermal tolerance exhibits a positive correlated evolution with TPiv, where thermal tolerance and TPiv values evolve together. This pattern occurs because embryonic physiology is locally adapted to incubation regimes (Ewert, 1985), and TPiv evolution ensures that extreme temperatures within local regimes produce female hatchlings, consistent with the adaptive explanation for TSD. Therefore, we predict that (i) embryonic mortality will be best explained by the difference between observed incubation temperature and population-specific TPivs, compared to the difference between observed incubation temperature and mean incubation temperature, or simply the observation incubation temperature. We also predict that (ii) mortality will be positively associated with the difference between incubation temperature and TPiv. Next, under the adaptive explanation for TSD explored herein, temperature affects individual quality and males are produced in high-quality thermal environments, but the association between sex and individual quality arises through temperature. We therefore predict that (iii) sex ratio (percent male) will be negatively associated with embryonic mortality, but after accounting for temperature, mortality will not be associated with sex. Finally, if TPiv evolves to delineate high-quality thermal environments (for male production), from low-quality thermal environments (for female production), then (iv) embryonic mortality in MF chelonians should increase with temperature more rapidly above the TPiv, compared to below the TPiv; this prediction also applies to FMF species provided the incubation temperatures examined do not fall below the lower TPiv.
Methods
Data collection
Data were collected from previously published studies collated in the Reptile Development Database (Noble et al., 2018a). Only chelonians were considered in this study, as they are of extreme conservation concern (Rhodin et al., 2018; Gibbons and Lovich, 2019; Stanford et al., 2020), they represent the overwhelming majority of species in the world that exhibit TSD, they feature a pattern of TSD different from squamates and the tuatara (see below), and very limited data were available on crocodilians, especially for TPivs. Thus, studies from the Reptile Development Database were included in the present study if they featured (i) a turtle species with TSD (Sabath et al., 2016), (ii) artificial incubation under constant temperature, (iii) embryonic mortality (or survival) specific to a temperature treatment and (iv) the number of embryos comprising the sample. Data collected from studies fitting inclusion criteria included species, incubation temperature, TPiv, percent mortality per incubation regime, number of eggs incubated and geographic location of egg collection. Exclusion factors included genotypic sex-determined species (GSD), in situ incubation or fluctuating temperature trials. In studies including both TSD and GSD species, and studies including both constant and fluctuating incubation trials, only TSD and constant trial results were extracted. Trials involving temperature shifts were also excluded. Several studies report mortality results in figures and do not specify results textually; in these cases, we extracted data from figures using WebPlotDigitzer (Rohatgi, 2019). We also extracted all available information on substrate moisture; however, moisture was only measured directly in 10 studies. Notably, all studies either reported moisture values or maintained moisture at approximately constant levels across the incubation regime; further, the few studies that manipulated moisture and temperature did so in a factorial manner. It is therefore unlikely that moisture was confounded with temperature in the present study.
Ultimately, 28 studies in the Reptile Development Database fit inclusion criteria for the current study (Supplementary Table 1). In total, 199 data points were collected on a total of 15 different species in 6 different families of turtle, representing 43% of known taxonomic families.
Pivotal temperatures
Some papers that were used in the present study did not explicitly state the population-specific TPiv(s). These values were supplemented with TPivs from other papers studying the exact same population, or the most proximal population of the same species with a TPiv estimate, as TPiv can vary geographically and among species (Bull, 1980; Ewert et al., 1994; Carter et al., 2019). To accomplish this, we performed literature searches to find TPivs of species using the species name ‘pivotal temperature’ and ‘sex ratios’ as search terms. Species-specific TPivs were found, and the closest geographically available population TPiv found was used as the TPiv. In total, 13 studies required supplementation of TPiv from previously published literature (Supplementary Table 1).
There was only one species with FMF TSD, the snapping turtle (Chelydra serpentina), in which both cool and hot temperatures produce females (Fig. 1). For this species, we use only the upper TPiv in our analyses, and essentially treat this FMF species as if it were MF. This is because only three mortality datapoints for this lone FMF species fell below the lower TPiv (and only by ≤2°C); further, high temperature incubation is otherwise the conservation focus of this study. However, we perform all analyses with and without this FMF species to ensure patterns are robust regardless of the type of TSD.
Data analysis
We reasoned that the relationship between temperature and embryonic mortality is likely non-linear (e.g. Amarasekare and Savage, 2012). We therefore allowed flexibility in fixed effects in all models (described below) by using basis splines within the package splines (R Core Team, 2020). We chose basis splines over natural splines as basis splines are not constrained to linearity at the tails, which is important as the tails of our functions are associated with few data points, and basis splines therefore emphasize the uncertainty, rather than minimize it. Except where noted, embryonic mortality was always the dependent variable, and it was weighted by sample size of embryos in each temperature treatment. Given that many species were represented by only one study, we chose to use only StudyID as a random intercept. We explored the possibility of including basis splines as random slopes within StudyID, but these models generally exhibited signatures of overfitting, and results were broadly robust whether or not random slopes were included. Having selected our lone random effect to be applied in all models, our models were subsequently fit using maximum likelihood. Model comparisons outlined below were performed using second-order Akaike information criterion (AICc) and Akaike weights (wi) within the package ‘AICmodavg’ in R (Fabozzi et al., 2014; Mazzerole, 2019); basis spline regression was applied to fixed effects using the bs function in splines and lmer in lme4 (Bates et al., 2015).
We expect that TPiv exhibits a correlated evolution with embryonic thermal tolerance. To address this expectation, we generated three plausible hypotheses for how incubation temperature affects mortality, and we expressed these hypotheses as statistical models (Burnham and Anderson, 2002). The first and simplest hypothesis is that mortality is a function of incubation temperature. In other words, the value of incubation temperature reported in the study from which the data were collected best explains mortality. For clarity we refer to this variable as ‘observed incubation temperature’, or TOBS. Second, we reasoned that TPiv and embryonic thermal tolerance exhibit a correlated evolution, in which case embryonic mortality would be best explained by the difference between TPiv and TOBS. To create this new variable, we subtracted TOBS from species-specific TPivs, and we refer to this new variable as ‘deviation from TPiv’, or ∆TTPiv. Finally, we reasoned that if the mean incubation temperature chosen by researchers is generally similar to the mean temperature of the natural incubation environment, then the mean temperature of a given study provides a good reference point for the average incubation conditions. Mortality may then be related to the extremity of the incubation treatment and would be best explained by the difference between the mean incubation temperature of the study and TOBS. To create this new variable, we subtracted each value of TOBS from the mean temperature of its respective study, and we refer to this new variable as ‘deviation from mean temperature’, or ∆TTμ. We fit the three mixed linear models using maximum likelihood, where the sole fixed-effect predictor of embryonic mortality was TOBS (Model A), ∆TTPiv (Model B), or ∆TTμ (Model C). Models were compared using AICc. We ran these analyses twice, once with all available data, which includes all FMF and MF species (n = 199), and again after excluding FMF species, thereby including only MF species (n = 159).
We also investigated whether there is an association between sex ratio and embryonic mortality, and if so, whether this association is realized over-and-above any temperature effect. This analysis leverages the subset of data for which both temperature and sex ratio are measured at a given incubation temperature. We fit the model that best-explained variation in embryonic mortality (which was Model B, in which temperature is expressed as ∆TTPiv, see Results) and compared this model to two new models: Model D, in which mortality was expressed as a linear effect of sex ratio (proportion of clutch male, or PropMale), and Model E, in which mortality was expressed as a linear effect of PropMale and ∆TTPiv (as in Model B). We ran separate analyses for MF species alone, then for both FMF and MF species together (FM species: 19 studies, 13 species, 130 observations; FMF and MF species: 21 studies, 14 species, 158 observations). Finally, we also fit PropMale as a function of ∆TTPiv, with StudyID as a random effect, simply to visualize how PropMale changes with temperature across broad species.
Theory suggests mortality should increase more rapidly with temperature under female-producing temperatures, compared to male-producing temperatures (Fig. 1). We therefore tested whether the relationship between mortality and temperature is different above the TPiv compared to below the TPiv. We took the absolute value of ∆TTPiv, thereby creating a new variable that we refer to as ‘absolute deviation from TPiv’, or | ∆TTPiv |. We also created a categorical variable Direction of Deviation, which identifies the direction of the temperature difference with respect to TPiv (i.e. above TPiv or below TPiv). We used model selection to compare three models, and continuous fixed effects were fit as basis splines. First, we fit Model F, in which mortality was expressed simply as | ∆TTPiv |. Next, we fit Model G, in which we fit |∆TTPiv | and the additional term Direction of Deviation, which explores whether average mortality is different above versus below the TPiv. Finally, we fit Model H, which included an interaction between Direction of Deviation and | ∆TTPiv |, which explores whether shape of the relationship between embryonic mortality and temperature differs above versus below TPiv. We ran these analyses twice, once with all available data, which includes all FMF and MF species (n = 199), and again after excluding FMF species, thereby including only MF species (n = 159).
Results
We tested whether embryonic mortality is best explained by incubation temperature per se (TOBS, Model A), the difference between incubation temperature and TPiv (∆TTPiv, Model B), or the difference between incubation temperature and the mean incubation temperature of the study (∆TTμ, Model C). We found no support for Model A (k = 6, LogLik = −356.4, ∆AIC = 43.3, wi = 0, n = 199) or Model C (k = 6, LogLik = −353.3, ∆AIC = 37.1, wi = 0, n = 199). Instead, Model B was strongly supported (k = 6, LogLik = −334.7, wi = 1, n = 199). Visualization of Model B indicates that the relationship between ∆TTPiv and embryonic mortality is concave upward, centered below TPiv (Table S1, Fig. 2a). We reran this analysis excluding all FMF species, and Model B remained highly supported (k = 6, LogLik = −258.7, wi = 1, n = 159, Fig. 2b), whereas Model A (k = 6, LogLik = −273.0, ∆AIC = 28.5, wi = 0, n = 159) and Model C (k = 6, LogLik = −278.6, ∆AIC = 39.6, wi = 0, n = 159) remained unsupported.
Figure 2.

The relationship between proportion clutch mortality and incubation temperature deviation from TPiv (∆TTPiv), which is the difference between incubation temperature and species-specific TPivs. In panel (a) all MF and FMF species are included, and in panel (b) FMF species are excluded. Points represent raw mortality data collected from published literature, the size of the point reflects relative sample size of each data point and color reflects study ID. The 95% confidence intervals are shaded in grey
Visualization of sex ratios (PropMale) as a function of ∆TTPiv revealed the expected patterns of variation: when data from FMF and MF species are combined there is a well-defined peak of male producing temperatures below species-specific (upper) TPivs (Fig. 3A), and when MF species are considered alone there was relatively little variation in sex ratios below species-specific TPiv (Fig. 3B). Next, we investigated whether embryonic mortality is best predicted by temperature or sex, and we did so by modelling whether mortality was associated with PropMale, or ∆TTPiv, or both PropMale and ∆TTPiv. Of the three models compared, we found that the model featuring PropMale as the sole predictor of embryonic mortality, Model D, was poorly supported (k = 4, LogLik = 270.3, ∆AIC = 44.3, wi = 0, n = 158). In fact, Model D was poorly supported even though there was a strong association between PropMale and embryonic mortality in this model (bSexRatio ± SE = −1.02 ± 0.210, Fig. 4a). The best-supported model, Model B, featured ∆TTPiv as the sole predictor of embryonic mortality (k = 6, LogLik = −246.0, wi = 0.75, n = 158). Finally, the model featuring both PropMale and ∆TTPiv, Model E, was not supported: it differed from the best model (Model B) by one parameter and likelihoods were nearly identical (Arnold, 2010) (k = 7, LogLik = −246.0, ∆AIC = 2.19, wi = 0.25, n = 158). Importantly, the marginal association between PropMale and embryonic mortality was weak in Model E, i.e. when controlling for ∆TTPiv (bSexRatio = 0.0187 ± 0.322), indicating that temperature, not sex, influences mortality. Next, we excluded all FMF data and reran the above analyses, revealing qualitatively identical results. We found that Model D was unsupported (k = 4, LogLik = −216.9, ∆AIC = 36.2, wi = 0, n = 130) even though there was a non-trivial association between PropMale and embryonic mortality (bSexRatio = −0.638 ± 0.237, Fig. 4B). Model B was again supported (k = 6, LogLik = −196.6, wi = 0.72, n = 130), and the marginal association between PropMale and embryonic mortality was weak when controlling for ∆TTPiv (bSexRatio = −0.200 ± 0.330).
Figure 3.

Visualization of sex ratio (proportion of clutch male) as a function of the difference between observed incubation temperature and species-specific TPivs (∆TTPiv). Fitted line for sex is in blue. In grey, we overlaid the relationship between clutch mortality and ∆TTPiv, estimated from Model B (see Fig. 2). (a) Both MF and FMF species are included in the model, and in (b) FMF species are excluded. Color of points indicates different studies, and size of points indicates relative sample size. The single MF species in panel (b) with a prevalence of females below TPiv is the painted turtle (Schwarzkopf and Brooks, 1985)
Figure 4.

Patterns of clutch mortality as a function of proportion of clutch male for (a) FMF and MF species and (b) MF species only. Color of points indicates different studies. Size of points reflects relative sample size of raw data points
We tested whether patterns of temperature-dependent mortality differed above versus below species-specific TPivs. We compared three models, and we found that the model featuring an interaction between | ∆TTPiv | and Direction of Deviation, Model H, was strongly supported (k = 10, LogLik = −330.7, wi = 1, n = 199). The nature of the interaction in Model H supported the prediction that mortality increases relatively rapidly above the TPiv, compared to below the TPiv. Other models were poorly supported, namely Model F (k = 6, Loglik = −346.3, ∆AIC = 22.5, wi = 0, n = 199) and Model G (k = 7, LogLik = −340.0, ∆AIC = 12.1, wi = 0, n = 199). Next, we excluded FMF species and included only MF species in our analyses. Results were similar, as the only model without the term Direction of Deviation was poorly supported, namely Model F (k = 6, Loglik = −261.3, ∆AIC = 6.92, wi = 0.02, n = 159). Support was otherwise similar between Model G (k = 7, LogLik = −257.2, ∆AIC = 0.93, wi = 0.38, n = 159) and Model H (k = 10, LogLik = −253.4, wi = 0.60, n = 159, Fig. 5B). Visualization again supports the interpretation that mortality increases relatively rapidly above the TPiv, compared to below the TPiv, at least for the first 5–6°C above TPiv (Fig. 5B).
Figure 5.

Clutch mortality as a function of the absolute difference between incubation temperature and species-specific TPivs (|∆TTPiv|) and the direction of the difference (above versus below TPiv) for (a) FMF and MF species and (b) MF species only. Blue indicates raw data points below TPiv, and red indicates raw data points above TPiv. Size of points reflects relative sample size of raw data points
Discussion
The present study presents a simple theory of TSD evolution (Fig. 1) to explore how constant temperature incubation is associated with embryonic mortality in chelonians. We communicate four main findings. First, we show that embryonic mortality is best explained by the temperature difference between species-specific TPiv and incubation temperature (∆TTPiv). Second, and similarly, we find mortality is lowest near TPiv and highest when the difference between TPiv and the incubation temperature is large. We interpret these first two patterns as consistent with the prediction that TPiv exhibits correlated evolution with embryonic thermal tolerance. Third, we found that embryonic mortality was negatively correlated with sex ratio (proportion male) for both MF species alone and for FMF and MF species combined, but that sex ratio was not associated with mortality after accounting for ∆TTPiv. This is consistent with the prediction that temperature, not sex, exhibits an overarching effect on performance, and the prediction that female-producing thermal environments are relatively stressful for embryos. Finally, and relatedly, we demonstrate that embryonic mortality increases relatively rapidly with temperature above the TPiv, compared to below the TPiv, which is also consistent with the prediction that female-producing temperatures are relatively stressful for embryos. Below we explore these results and emphasize their significance in evolution and conservation.
The relatively rapid increase in mortality with temperature above the TPiv has important evolutionary implications. Mechanistically, fitness may depreciate rapidly above the TPiv because TPiv evolves to be closer to the upper end of thermal tolerance, rather than the lower end, as reaction rates for most or all processes change more rapidly near the upper tolerance limit (Sharpe and DeMichele, 1977; Kingsolver, 2009). Indeed, the lowest level of mortality observed in the present study was just below TPiv, suggesting TPiv often falls toward the upper limit of an intermediate thermal range that is optimal for development (Stubbs and Mitchell, 2018). One interpretation of our results, therefore, is that female-producing temperatures coincide, at least roughly, with a departure from optimal incubation temperatures. Indeed, we also found that sex ratio (percent male) per se was negatively correlated with mortality. Although this pattern could arise from differential mortality of male embryos when overall clutch mortality is relatively high, there is no obvious mechanism that would explain this phenomenon. We therefore suggest that the negative correlation between sex ratio (percent male) per se and embryonic mortality arises because female-producing temperatures are relatively stressful. Our findings are therefore important as they are consistent with the simple explanation for TSD explored herein (Fig. 1). Specifically, classic theory suggests females in age-structured populations should produce more sons when sons are expected to become high-condition adults (Trivers and Willard, 1973), where condition refers to general health, energy reserves, vigor and overall quality. Therefore, if incubation temperature has, on average, an effect on the general health and vigor of individuals at adulthood, then a sex by incubation temperature interaction for fitness (Charnov and Bull, 1977) is inexorable when males are produced under favourable incubation temperatures and females under unfavourable incubation temperatures (Freedberg and Wade, 2004). We suggest that this explanation for TSD should be limited to the long-lived chelonians and crocodilians, and not necessarily extended to squamates and the tuatara. This is because chelonians and crocodilians share an evolutionary history, neither group features the FM pattern of TSD, and the longevity of both chelonians and crocodilians results in populations that are highly age- and size-structured, where pronounced variation in male quality exists and can be maintained.
TPivs appear to be heritable and may be under weak selection (Beukeboom and Perrin, 2014), and there is evidence, albeit mixed, that TPivs follow broad environmental gradients in temperature (Ewert et al., 2004, 2005; but see Carter et al., 2019). Microevolution of TPivs is therefore possible, and another prediction from the focal hypothesis (Fig. 1) is that TPiv and embryonic thermal tolerance experience a correlated evolution, as would be necessary to ensure males are produced in relatively high-quality environments. Admittedly, we define ‘tolerance’ fairly broadly, in that tolerance encompasses the range of thermal environments that support normal embryonic development, and we assume that embryonic mortality and tolerance are strongly associated. Nevertheless, we found mortality was best explained by ∆TTPiv (Model B), rather than the difference between observed temperature and the mean temperature used in the study (∆TTμ, Model C), or the observed incubation temperature (Tobs, Model A). One interpretation of this pattern is that TPiv and embryonic thermal tolerance undergo a correlated evolution, although the extent to which this particular finding supports our focal hypothesis is debatable. We suggest that good evidence of a correlated evolution of TPiv and embryonic thermal tolerance would indeed arise if ∆TTPiv (Model B) better explained mortality patterns than deviation from the long-term average environmental temperature, but our variable ∆TTμ (Model C) is the average temperature used in a particular study, which is not necessarily the long-term average temperature of the local environment. In sum, it is unclear if our study provides evidence for correlated evolution between TPiv and embryonic thermal tolerance, consistent with TSD theory (Fig. 1), or whether relatively strong support for our Model B versus Model C arose because researchers choose values of ∆TTμ that do not reflect the typical incubation environments of their focal populations. In any event, it is necessary to study further whether TPivs are strongly linked with thermal tolerance and physiology of the embryo, as this may provide insight into the adaptive significance of TSD.
The overarching message communicated by the present study is that constant temperatures used to produce females are associated with thermal stress, measured here as mortality. Although we did not measure post-hatching phenotypes (but see below), we argue that negative effects of thermal stress are likely to persist beyond the embryo stage. Indeed, persistent phenotypic effects of thermal stress would have ramifications for incubation techniques used in conservation programs, and such effects would be consistent with the simple model for TSD evolution examined herein. Stress is a physiological state in which normal function is disrupted, and stress may have a negative fitness impact (Walker et al., 2005; Klockmann et al., 2017). Our proxy of stress (mortality) represents the most extreme fitness outcome that is possible, as mortality represents an inability to persist, let alone reproduce (Kingsolver, 2009). Thermal stress can be induced at temperatures that do not necessarily induce mortality but may induce phenotypic morbidity (Cavallo et al., 2015; Refsnider et al., 2015; Kingsolver and Woods, 2016). Even within the present study, for example, several studies reported abnormalities in hatchlings reared in high temperature incubation trials (Packard et al., 1987; Zhu et al., 2006), and previous studies suggest extreme temperatures lead to developmental instability (Noble et al., 2018b). We therefore suggest that our findings are probably not restricted to the effect of temperature on embryos: the relationship between temperature and fitness observed herein (i.e. embryonic mortality is lowest just below TPiv, and increases more rapidly above TPiv than below TPiv) may persist into later life by virtue of the thermal stress survivors experienced as embryos.
The form in which thermal stress might persist in post-hatching phenotypes is unclear. However, 13 studies included in the present analysis included a measure of post-hatching growth, performance metrics or mortality, encompassing a total of 23 discrete measurements (Table 1). Fifty-two percent of the measurements indicated a significant temperature effect. Due to the heterogeneity of the papers and fitness metrics, and the multi-level comparisons within some studies, a formal meta-analysis was not possible. However, our inclusion criteria for the present study likely resulted in the exclusion of many studies examining post-hatching growth or performance, such that it may prove fruitful for future research to formally examine post-hatching growth and performance under different incubation regimes, as evidence suggests the impacts of temperature stress may be observed at a variety of life stages (Jonsson et al., 2014; Noble et al., 2018b).
Table 1.
Post-hatching performance and growth temperature effect for papers included in the current study
| Species | Trait | Effect | Is cooler better? | Study |
|---|---|---|---|---|
| Chinemys reevesii | Condition—body condition | No | — | Du et al. (2009) |
| Chinemys reevesii | Condition—body shape | No | — | Du et al. (2009) |
| Maureymys mutica | Condition—size | No | — | Zhao et al. (2015) |
| Maureymys mutica | Condition—size | Yes | No | Zhao et al. (2015) |
| Malaclemys terrapin | Growth | Yes/noa | — | Roosenburg and Kelley (1996) |
| Chrysemys picta | Growth | No | — | Les et al. (2009) |
| Trachemys scripta | Growth | No | — | Les et al. (2009) |
| Emys marmorata | Growth | No | — | Geist et al. (2015) |
| Ocadia sinensis | Growth | No | — | Du et al. (2010) |
| Chinemys reevesii | Growth | Yesb | — | Du et al. (2009) |
| Chinemys reevesii | Growth | Yesb | — | Du et al. (2007) |
| Gopherus agassizii | Growth | Yes | Yes | Spotila et al. (1994) |
| Mauremys mutica | Growth | Yes | Yes | Du et al. (2010) |
| Chelonia mydas | Growth | Yes | Yes | Booth et al. (2004) |
| Gopherus polyphemus | Growth | Yesc | — | Demuth (2001) |
| Chelydra serpentina | Growth—mass | Yes | Yes/nod | Bobyn and Brooks (1994a) |
| Podocnemis lewyana | Growth—straight line carapace | Yes | Yes | Paez et al. (2009) |
| Gopherus polyphemus | Performance—crawl speed | No | — | Demuth (2001) |
| Maureymys mutica | Performance—righting response | No | — | Zhao et al. (2015) |
| Mauerymys mutica | Performance—righting response | Yes | No | Zhao et al. (2015) |
| Caretta caretta | Performance—righting response, crawl test, swim test | Yes | No | Fisher et al. (2014) |
| Chinemys reevesii | Performance—swimming ability | No | — | Du et al. (2009) |
| Chelonia mydas | Performance—swimming trials | Yes | No | Booth et al. (2004) |
aNot able to determine if due to temperature or due to sex
bTemperature effect gone after 3 months
cTemperature effect gone after 271 days
dMay be due to sexual dimorphism, intermediate (male temperature) led to larger size
If artificial incubation is geared toward conservation, then presumably artificial incubation regimes fall within a normal range of incubation temperatures experienced by the focal population. However, conservation managers face at least two reasons that incentivize high-temperature incubation of turtle embryos. The first is that most turtles exhibit TSD, and for turtles, relatively warm temperatures result in an over-production of female hatchlings (Ewert et al., 1994). Given that a major goal of conservation programs is ultimately to increase the number of wild births in the population, and that births are more strongly related to female than male abundance (Girondot et al., 2008), TSD may represent an incentive to incubate at warm temperatures. Second, high temperatures result in faster development rate and hence shorter incubation time, potentially reducing the expense associated with husbandry, further incentivizing high temperature incubation (Du et al., 2006). Despite the incentives associated with high-temperature incubation, our study suggests constant temperature incubation near TPiv results in the highest hatching success and perhaps the least thermal stress. Notably, female production does not require extreme temperatures, as female-biased sex ratios can be produced at temperatures that are only slightly above the TPiv. For example, or analysis suggests that by incubating embryos only 1°C above TPiv (Fig. 3), a sex ratio of over 75% females can be achieved, on average, with a trivial increase in embryonic mortality (and presumably, a trivial increase in post-hatching stress); however, we note that the actual sex ratio achieved will depend on the shape of the temperature-sex reaction norm at the population level (Bentley et al., 2017; Carter et al., 2019). The notion of incubating embryos as near as possible to the TPiv may be especially useful for head starting programs, where incubation protocols can be reviewed to prevent the release of hatchlings or juveniles that underwent significant thermal stress during development.
While some studies suggest head-starting can be beneficial (Cunnington and Brooks, 1996; Crowder and Heppell, 2011; Mullin, 2019), the long-term benefits of head starting are often unclear or negligible (Páez et al., 2015; Spencer et al., 2018), as factors such as predation and adverse environmental conditions result in low natural survival, or even negligible survival, of hatchlings and juveniles in wild environments (Heppell, 1998; Enneson and Litzgus, 2008; Altobelli, 2017). Although the present study does not definitively link incubation environments with long-term survival as we do not examine post-hatching fates, we suggest that incubation of embryos near TPiv may ultimately improve survival prospects of individuals, especially females, released into the wild. As we have pointed out, incubation near TPiv is compatible with sex ratio manipulation for many species and populations with TSD. Indeed, it is desirable when increases in adult recruitment translate into increases in juvenile recruitment, and this requires that careful attention is paid to the sex of released individuals, as population size is not indicative of extinction risk when sex ratios are skewed (Dale, 2001; Clout et al., 2002; Wedekind, 2002; López-Sepulcre et al., 2009).
Data syntheses and analyses are able to identify large-scale patterns and ultimately help strengthen evidence-based decision-making (Stewart, 2010). Indeed, it is useful for conservation managers to periodically review methodologies used for species recovery, and with the substantial increase in reptile conservation organizations since the 1990s, there are likely many different initiatives that leverage artificial incubation (Gibbons and Lovich, 2019). The present study underlines how constant temperature incubation in artificial egg incubation regimes affects embryonic mortality, and possibly post-hatching thermal stress. We emphasize that we studied chelonians in particular, but similar studies of crocodilians, and especially squamates and the tuatara where different patterns of TSD can be found (Valenzuela and Lance 2004), would be useful to uncover how TPiv relates to mortality in those groups. More broadly, we suggest chelonian conservation programs leveraging artificial incubation may benefit from avoiding high-temperature incubation environments, and incubating embryos near species-specific TPivs.
Supplementary Material
Acknowledgements
We thank Anthony Rajkumar and Sami Troendle for constructive comments on the original manuscript and Melanie Massey for help compiling data. We also thank Daniel Noble and two anonymous reviewers for their generous, constructive and extensive feedback that greatly improved the quality of this work.
Funding
Funding was provided by an Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant Number 2016-06469 to N.R.
References
- Altobelli JT (2017) Nest-Site Selection and Neonate Survival of Eastern Box Turtles (Terrapene Carolina Carolina) in Michigan’s Northern Lower Peninsula (Masters Theses). Grand Valley State University. Accessible at https://scholarworks.gvsu.edu/theses/853. [Google Scholar]
- Amarasekare P, Savage V (2012) A framework for elucidating the temperature dependence of fitness. Am Nat 179: 178–191. [DOI] [PubMed] [Google Scholar]
- Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74: 1175–1178. [Google Scholar]
- Bateman A (1948) Intra-sexual selection in Drosophila. Heredity 2: 349–368. doi:10.1038/hyd.1948.21. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67: doi:10.18637/jss.v067.i01. [Google Scholar]
- Bentley BP, Haas BJ, Tedeschi JN, Berry O (2017) Loggerhead sea turtle embryos Caretta caretta regulate expression of stress response and developmental genes when exposed to a biologically realistic heat stress. Mol Ecol 26: 2978–2992. [DOI] [PubMed] [Google Scholar]
- Beukeboom LW, Perrin N (2014) The Evolution of Sex Determination, Ed1st. Oxford University Press, Oxford, United Kingdom, New York, NY, United States of America. [Google Scholar]
- Bobyn ML, Brooks RJ (1994a) Interclutch and interpopulation variation in the effects of incubation conditions on sex, survival and growth of hatchling turtles (Chelydra serpentina). J Zool 233: 233–257. [Google Scholar]
- Bobyn ML, Brooks RJ (1994b) Incubation conditions as potential factors limiting the northern distribution of snapping turtles. Can J Zool 72: 28–37. [Google Scholar]
- Bolten AB, Crowder LB, Dodd MG, MacPherson SL, Musick JA, Schroeder BA, Witherington BE, Long KJ, Snover ML (2011) Quantifying multiple threats to endangered species: an example from loggerhead sea turtles. Front Ecol Environ 9: 295–301. [Google Scholar]
- Bona M, Novotny M, Danko S, Buresova A (2012) Headstarting in a small population of European pond turtles (Emys orbicularis) in Central European conditions: first results. Herpetol Notes 5. [Google Scholar]
- Booth DT (2006) Influence of incubation temperature on hatchling phenotype in reptiles. Physiol Biochem Zool 79: 274–281. [DOI] [PubMed] [Google Scholar]
- Booth DT, Burgess E, McCosker J, Lanyon JM (2004) The influence of incubation temperature on post-hatching fitness characteristics of turtles. Int Congr Ser 1275: 226–223. [Google Scholar]
- Bowden RM, Carter AW, Paitz RT (2014) Constancy in an inconstant world: moving beyond constant temperatures in the study of reptilian incubation. Integr Comp Biol 54: 830–840. [DOI] [PubMed] [Google Scholar]
- Bowkett AE (2009) Recent captive-breeding proposals and the return of the ark concept to global species conservation. Conserv Biol 23: 773–776. [DOI] [PubMed] [Google Scholar]
- Bull JJ (1980) Sex determination in reptiles. Q Rev Biol 55: 3–21. [Google Scholar]
- Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, Ed 2. Springer, New York, NY. [Google Scholar]
- Carstairs S, Paterson JE, Jager KL, Gasbarrini D, Mui AB, Davy CM (2019) Population reinforcement accelerates subadult recruitment rates in an endangered freshwater turtle. Anim Conserv 22: 589–599. [Google Scholar]
- Carter AL, Bodensteiner BL, Iverson JB, Milne-Zelman CL, Mitchell TS, Refsnider JM, Warner DA, Janzen FJ (2019) Breadth of the thermal response captures individual and geographic variation in temperature-dependent sex determination. Funct Ecol 33: 1928–1939. [Google Scholar]
- Cavallo C, Dempster T, Kearney MR, Kelly E, Booth D, Hadden KM, Jessop TS (2015) Predicting climate warming effects on green turtle hatchling viability and dispersal performance. Funct Ecol 29: 768–778. [Google Scholar]
- Charnov E, Bull J (1977) When is sex environmentally determined? Nature 266: 828–830. [DOI] [PubMed] [Google Scholar]
- Clout MN, Elliott GP, Robertson BC (2002) Effects of supplementary feeding on the offspring sex ratio of kakapo: a dilemma for the conservation of a polygynous parrot. Biol Conserv 107: 13–18. [Google Scholar]
- Conover DO (1984) Adaptive significance of temperature-dependent sex determination in a fish. Am Nat 123: 297–313. [Google Scholar]
- Conway-Gómez K (2007) Effects of human settlements on abundance of Podocnemis unifilis and P. expansa turtles in Northeastern Bolivia. Chelonian Conserv Biol 6: 199. [Google Scholar]
- Crawford NG, Faircloth BC, McCormack JE, Brumfield RT, Winker K, Glenn TC (2012) More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett 8: 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder L, Heppell S (2011) The decline and rise of a sea turtle: how Kemp’s ridleys are recovering in the Gulf of Mexico. Solutions 2: 67–73. [Google Scholar]
- Cunnington DC, Brooks RJ (1996) Bet-hedging theory and eigenelasticity: a comparison of the life histories of loggerhead sea turtles (Caretta caretta) and snapping turtles (Chelydra serpentina). Can J Zool 74: 291–296. [Google Scholar]
- Dale S (2001) Female-biased dispersal, low female recruitment, unpaired males, and the extinction of small and isolated bird populations. Oikos 92: 344–356. [Google Scholar]
- Deeming DC, Ferguson MWJ (1989) The mechanism of temperature dependent sex determination in crocodilians: a hypothesis. Am Zool 29: 973–985. [Google Scholar]
- Demuth JP (2001) The effects of constant and fluctuating incubation temperatures on sex determination, growth, and performance in the tortoise Gopherus polyphemus. Can J Zool 79: 1609–1620. [Google Scholar]
- Du W-G, Zheng R-Q, Shu L (2006) The influence of incubation temperature on morphology, locomotor performance, and cold tolerance of hatchling Chinese three-keeled pond turtles, Chinemys reevesii. Chelonian Conserv Biol 5: 294–299. [Google Scholar]
- Du W-G, Shen J-W, Wang L (2009) Embryonic development rate and hatchling phenotypes in the Chinese three-keeled pond turtle (Chinemys reevesii): The influence of fluctuating temperature versus constant temperature. J Therm Biol 34: 250–255. [Google Scholar]
- Du W-G, Wang L, Shen J-W (2010) Optimal temperatures for egg incubation in two Geoemydid turtles: Ocadia sinensis and Mauremys mutica. Aquaculture 305: 138–142. [Google Scholar]
- Eckert KL, IUCN/SSC Marine Turtle Specialist Group, World Wildlife Fund (U.S.), Center for Marine Conservation, United States, National Oceanic and Atmospheric Administration, International Union for Conservation of Nature and Natural Resources, Species Survival Commission (1999) Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group, Washington, DC, (1725 De Sales Street, NW #600, Washington 20036). [Google Scholar]
- Enneson JJ, Litzgus JD (2008) Using long-term data and a stage-classified matrix to assess conservation strategies for an endangered turtle (Clemmys guttata). Biol Conserv 141: 1560–1568. [Google Scholar]
- Ewert MA (1985) Embryology of turtles. In Gans C, Billett F, Maderson PFA, eds, Biology of Reptilia. Vol. 14. Wiley, New York, pp. 75–267. [Google Scholar]
- Ewert MA, Etchberger CR, Nelson CE (2004) Turtle sex-determining modes and TSD patterns, and some TSD pattern correlates. In Valenzuela N, Lance V, eds, Temperature Dependent Sex Determination in Vertebrates. Smithsonian Institute, Washington, DC, pp. 21–32 [Google Scholar]
- Ewert MA, Jackson DR, Nelson CE (1994) Patterns of temperature-dependent sex determination in turtles. J Exp Zool 270: 3–15. [Google Scholar]
- Ewert MA, Lang JW, Nelson CE (2005) Geographic variation in the pattern of temperature-dependent sex determination in the American snapping turtle (Chelydra serpentina). J Zool 265: 81–95. [Google Scholar]
- Fabozzi FJ, Focardi SM, Rachev ST, Arshanapalli BG (2014) Appendix E Model Selection Criterion: AIC and BIC. In The Basics of Financial Econometrics: Tools, Concepts, and Asset Management Applications. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 399–403 [Google Scholar]
- Feck A, Hamann M (2013) Effect of sea turtle rehabilitation centres in Queensland, Australia, on people’s perceptions of conservation. Endang Species Res 20: 153–165. [Google Scholar]
- Fisher LR, Godfrey MH, Owens DW (2014) Incubation Temperature Effects on Hatchling Performance in the Loggerhead Sea Turtle (Caretta caretta). PLoS ONE 9: e114880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg S, Wade MJ (2004) Male combat favours female-biased sex ratios under environmental sex determination. Anim Behav 67: 177–181. [Google Scholar]
- Geist N, Dallara Z, Gordon R (2015) The role of incubation temperature and clutch effects in development and phenotype of head-started western pond turtles (Emys marmorata). 10: 489–503. [Google Scholar]
- Georges A, Limpus C, Stoutjesdijk R (1994) Hatchling sex in the marine turtle Caretta caretta is determined by proportion of development at a temperature, not daily duration of exposure. J Exp Zool 270: 432–444. [Google Scholar]
- Gibbons JW, Lovich JE (2019) Where has turtle ecology been, and where is it going? Herpetologica 75: 4. [Google Scholar]
- Girondot M, Fouillet H, Thinsp CP (1998) Feminizing turtle embryos as a conservation tool. Conserv Biol 12: 353–362. [Google Scholar]
- Girondot M, Monsinjon J, Guillon J-M (2018) Delimitation of the embryonic thermosensitive period for sex determination using an embryo growth model reveals a potential bias for sex ratio prediction in turtles. J Therm Biol 73: 32–40. [DOI] [PubMed] [Google Scholar]
- Hancock JM, Furtado S, Merino S, Godley BJ, Nuno A (2017) Exploring drivers and deterrents of the illegal consumption and trade of marine turtle products in Cape Verde, and implications for conservation planning. Oryx 51: 428–436. [Google Scholar]
- Hassan R, Yahya NK, Ong LM, Kheng LK, Abidin ZZ, Ayob A, Jainal AM (2017) Public awareness program and development of education toolkit for green sea turtle conservation in Sarawak, Malaysia. ISESE 12: 463–474. [Google Scholar]
- Hawkes L, Broderick A, Godfrey M, Godley B (2009) Climate change and marine turtles. Endang Species Res 7: 137–154. [Google Scholar]
- Heppell SS (1998) Application of life-history theory and population model analysis to turtle conservation. Copeia 1998: 367. [Google Scholar]
- Janzen FJ, Phillips PC (2006) Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol 19: 1775–1784. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Jonsson N, Finstad A (2014) Linking embryonic temperature with adult reproductive investment in Atlantic salmon Salmo salar. Mar Ecol Prog Ser 515: 217–226. [Google Scholar]
- Kingsolver JG (2009) The well-temperatured biologist: (American Society of Naturalists Presidential Address). Am Nat 174: 755–768. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Woods HA (2016) Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am Nat 187: 283–294. [DOI] [PubMed] [Google Scholar]
- Klockmann M, Kleinschmidt F, Fischer K (2017) Carried over: heat stress in the egg stage reduces subsequent performance in a butterfly. PLoS One 12: e0180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Morimoto Y, Sato T, Suganuma H (2017) Factors affecting the long-term population dynamics of green turtles (Chelonia mydas) in Ogasawara, Japan: influence of natural and artificial production of hatchlings and harvest pressure. Chelonian Conserv Biol 16: 83–92. [Google Scholar]
- Les HL, Paitz RT, Bowden RM (2009) Living at Extremes: Development at the Edges of Viable Temperature under Constant and Fluctuating Conditions. Physiol Biochem Zool 82: 105–112. [DOI] [PubMed] [Google Scholar]
- Lewison RL, Freeman SA, Crowder LB (2004) Quantifying the effects of fisheries on threatened species: the impact of pelagic longlines on loggerhead and leatherback sea turtles: fisheries effects on sea turtles. Ecol Lett 7: 221–231. [Google Scholar]
- López-Sepulcre A, Norris K, Kokko H (2009) Reproductive conflict delays the recovery of an endangered social species. J Anim Ecol 78: 219–225. [DOI] [PubMed] [Google Scholar]
- Massey MD, Holt SM, Brooks RJ, Rollinson N (2019) Measurement and modelling of primary sex ratios for species with temperature-dependent sex determination. British J Exp Biol 222: jeb190215. [DOI] [PubMed] [Google Scholar]
- Mazzerole MJ (2019) AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC. R package version 2.3-1, https://crain.r-project.org/package=AICcmodavg.
- Mullin DI (2019) Evaluating the Effectiveness of Headstarting for Wood Turtle (Glyptemys insculpta) Population Recovery (Masters of Science). Laurentian University, The Faculty of Graduate Studies. [Google Scholar]
- Noble DWA, Stenhouse V, Riley JL, Warner DA, While GM, Du W-G, Uller T, Schwanz LE (2018a) A comprehensive database of thermal developmental plasticity in reptiles. Sci Data 5: 180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble DWA, Stenhouse V, Schwanz LE (2018b) Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis: incubation temperature and plasticity. Biol Rev 93: 72–97. [DOI] [PubMed] [Google Scholar]
- Packard GC, Packard MJ, Miller K, Boardman TJ (1987) Influence of moisture, temperature, and substrate on snapping turtle eggs and embryos. Ecology 68: 983–993. [Google Scholar]
- Páez VP, Correa JC, Cano AM, Bock BC (2009) A Comparison of Maternal and Temperature Effects on Sex, Size, and Growth of Hatchlings of the Magdalena River Turtle (Podocnemis lewyana) Incubated under Field and Controlled Laboratory Conditions. Copeia 2009: 698–704. [Google Scholar]
- Páez VP, Lipman A, Bock BC, Heppell SS (2015) A plea to redirect and evaluate conservation programs for South America’s podocnemidid river turtles. Chelonian Conserv Biol 14: 205–216. [Google Scholar]
- Pen I, Uller T, Feldmeyer B, Harts A, While GM, Wapstra E (2010) Climate-driven population divergence in sex-determining systems. Nature 468: 436–438. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) Splines. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Refsnider JM, Palacios MG, Reding DM, Bronikowski AM (2015) Effects of a novel climate on stress response and immune function in painted turtles (Chrysemys picta): novel climate effects on turtle physiology. J Exp Zool 323: 160–168. [DOI] [PubMed] [Google Scholar]
- Rest JS, Ast JC, Austin CC, Waddell PJ, Tibbetts EA, Hay JM, Mindell DP (2003) Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome. Mol Phylogenet Evol 29: 289–297. [DOI] [PubMed] [Google Scholar]
- Rhodin AGJ, Stanford CB, Dijk PPV, Eisemberg C, Luiselli L, Mittermeier RA, Hudson R, Horne BD, Goode EV, Kuchling Get al. (2018) Global conservation status of turtles and tortoises (order Testudines). Chelonian Conserv Biol 17: 135. [Google Scholar]
- Rohatgi A (2019) WebPlotDigitizer v4.1. Available: https://automeris.io/WebPlotDigitizer/. Accessed 2019.
- Roll U, Feldman A, Novosolov M, Allison A, Bauer AM, Bernard R, Böhm M, Castro-Herrera F, Chirio L, Collen Bet al. (2017) The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat Ecol Evol 1: 1677–1682. [DOI] [PubMed] [Google Scholar]
- Rollinson N, Holt SM, Massey MD, Holt RC, Nancekivell EG, Brooks RJ (2018) A new method of estimating thermal performance of embryonic development rate yields accurate prediction of embryonic age in wild reptile nests. J Therm Biol 74: 187–194. [DOI] [PubMed] [Google Scholar]
- Rollinson N, Rowe L (2015) Persistent directional selection on body size and a resolution to the paradox of stasis: directional selection on body size. Evolution 69: 2441–2451. [DOI] [PubMed] [Google Scholar]
- Romiguier J, Gayral P, Ballenghien M, Bernard A, Cahais V, Chenuil A, Chiari Y, Dernat R, Duret L, Faivre Net al. (2014) Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature 515: 261–263. [DOI] [PubMed] [Google Scholar]
- Roosenburg WM, Kelley KC (1996) The Effect of Egg Size and Incubation Temperature on Growth in the Turtle, Malaclemys terrapin. J Herpetol 30: 198. [Google Scholar]
- Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B 263: 1415–1421. [Google Scholar]
- Sabath N, Itescu Y, Feldman A, Meiri S, Mayrose I, Valenzuela N (2016) Sex determination, longevity, and the birth and death of reptilian species. Ecol Evol 6: 5207–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf L, Brooks RJ (1985) Sex determination in northern painted turtles: effect of incubation at constant and fluctuating temperatures. Can J Zool 63: 2543–2547. [Google Scholar]
- Sharpe PJH, DeMichele DW (1977) Reaction kinetics of poikilotherm development. J Theor Biol 64: 649–670. [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Van Dyke J, Petrov K, Ferronato B, McDougall MA, Keitel C, Georges A (2018) Profiling a possible rapid extinction event in a long-lived species. Biol Conserv 221: 190–197. doi:10.1016/j.biocon.2018.03.009. [Google Scholar]
- Spotila JR, Zimmerman LC, Binckley CA, Grumbles JS, Rostal DC, List A, Beyer EC, Phillips KM, Kemp SJ (1994) Effects of Incubation Conditions on Sex Determination, Hatching Success, and Growth of Hatchling Desert Tortoises, Gopherus agassizii. Herpetol Monogr 8: 103. [Google Scholar]
- Stanford CB, Iverson JB, Rhodin AGJ, Paul van Dijk P, Mittermeier RA, Kuchling G, Berry KH, Bertolero A, Bjorndal KA, Blanck TEGet al. (2020) Turtles and tortoises are in trouble. Curr Biol 30: R721–R735. [DOI] [PubMed] [Google Scholar]
- Steen DA, Gibbs JP (2004) Effects of roads on the structure of freshwater turtle populations. Conserv Biol 18: 1143–1148. [Google Scholar]
- Stewart G (2010) Meta-analysis in applied ecology. Biol Lett 6: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs JL, Mitchell NJ (2018) The influence of temperature on embryonic respiration, growth, and sex determination in a Western Australian population of green turtles (Chelonia mydas). Physiol Biochem Zool 91: 1102–1114. [DOI] [PubMed] [Google Scholar]
- Tetzlaff SJ, Sperry JH, Kingsbury BA, DeGregorio BA (2019) Captive-rearing duration may be more important than environmental enrichment for enhancing turtle head-starting success. Glob Ecol Conserv 20: e00797. [Google Scholar]
- Todd B, Willson J, Gibbons J (2010) The global status of reptiles and causes of their decline. In Sparling D, Linder G, Bishop C, Krest S, eds, Ecotoxicology of Amphibians and Reptiles, Ed 2nd. CRC Press, Pensacola, FL, pp. 47–67. [Google Scholar]
- Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179: 90–92. [DOI] [PubMed] [Google Scholar]
- Valenzuela N, Lance VA (2004) Temperature-Dependent Sex Determination in Vertebrates. Smithsonian Institution, Washington DC, USA. [Google Scholar]
- Walker BG, Boersma PD, Wingfield JC (2005) Field endocrinology and conservation biology. Integr Comp Biol 45: 12–18. [DOI] [PubMed] [Google Scholar]
- Wedekind C (2002) Manipulating sex ratios for conservation: short-term risks and long-term benefits. Anim Conserv 5: 13–20. [Google Scholar]
- West SA (2009) Sex Allocation. Princeton University Press, Princeton. [Google Scholar]
- Yntema CL (1968) A series of stages in the embryonic development of Chelydra serpentina. J Morphol 125: 219–251. [DOI] [PubMed] [Google Scholar]
- Yntema CL (1979) Temperature levels and periods of sex determination during incubation of eggs of Chelydra serpentina. J Morphol 159: 17–27. [DOI] [PubMed] [Google Scholar]
- Zhao B, Chen Y, Lu H-L, Zeng Z-G, Du W-G (2015) Latitudinal differences in temperature effects on the embryonic development and hatchling phenotypes of the Asian yellow pond turtle, Mauremys mutica: Temperature effects on egg incubation. Biol J Linn Soc 114: 35–43. [Google Scholar]
- Zhu X-P, Wei C-Q, Zhao W-H, Du H-J, Chen Y-L, Gui J-F (2006) Effects of incubation temperatures on embryonic development in the Asian yellow pond turtle. Aquaculture 259: 243–248. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


