Abstract
Objective: Early mobilization and rehabilitation has become common and expectations for physical therapists working in intensive care units have increased in Japan. The objective of this study was to establish consensus-based minimum clinical practice standards for physical therapists working in intensive care units in Japan. It also aimed to make an international comparison of minimum clinical practice standards in this area. Methods: In total, 54 experienced physical therapists gave informed consent and participated in this study. A modified Delphi method with questionnaires was used over three rounds. Participants rated 272 items as “essential/unknown/non-essential”. Consensus was considered to be reached on items that over 70% of physical therapists rated as “essential” to clinical practice in the intensive care unit. Results: Of the 272 items in the first round, 188 were deemed essential. In round 2, 11 of the 62 items that failed to reach consensus in round 1 were additionally deemed essential. No item was added to the “essential” consensus in round 3. In total, 199 items were therefore deemed essential as a minimum standard of clinical practice. Participants agreed that 42 items were not essential and failed to reach agreement on 31 others. Identified 199 items were different from those in the UK and Australia due to national laws, cultural and historical backgrounds. Conclusions: This is the first study to develop a consensus-based minimum clinical practice standard for physical therapists working in intensive care units in Japan.
Keywords: Intensive care units, Physiotherapy, Minimum standards, Education
Early mobilization and rehabilitation in intensive care units (ICU) requires a multi-disciplinary team of doctors, nurses, and physical therapists. Physical therapists must have a minimum level of knowledge, task-related skills, and suitable attitude towards intensive care to work functionally as a member of the ICU multidisciplinary team.
In 2016, Skinner and colleagues explored consensus-based minimum standards of clinical practice for physiotherapists working in critical care settings in Australia and New Zealand1). Using a modified Delphi technique, senior or specialist critical care physiotherapists and appropriate academic staff who met defined eligibility criteria completed three rounds of questionnaires to establish a framework of minimum standards. More recently, Twose and colleagues published minimum standards of clinical practice for physiotherapists working in critical care settings in the United Kingdom2). They followed Skinner and colleagues' research methods and used the same modified Delphi approach. These papers are useful in providing an occupational description for physical therapists working in ICU. However, the activities, scope and role of physical therapists depends on the laws, culture and history of the country. It is therefore necessary to know the minimum standards of clinical practice along with the role of physical therapy when citing and referring to overseas scientific research papers from countries with different laws and cultures relating to physical therapy.
Early mobilization and rehabilitation were not actively carried out in ICU in Japan until quite recently1) despite Level 1 evidence of the benefit of physical therapy intervention in Western countries2). The Japan Society of Intensive Care Medicine (JSICM) organized the Intensive Care Early Rehabilitation Committee (ICERC) in 2014, aiming to establish a suitable system of early rehabilitation in ICU in Japan. The committee planned to develop an evidence-based expert consensus for early rehabilitation in ICU, and this was published in February 20173). In financial year 2018, this led to the introduction of an additional fee for early mobilization and rehabilitation (5,000 yen/patient/day, 14 days upper limit) for ICU. Since then, early mobilization and rehabilitation in Japan has made considerable progress and is now being performed in many institutions. However, it is possible that the knowledge and abilities of physical therapists may have not been fully understood by other medical professions in Japan. Providing documentation of minimum standards of clinical practice for physical therapists will therefore increase understanding of physical therapy and help the critical care team to develop. Establishment of minimum standards of clinical practice for Japanese physical therapists working in ICU is important for core competency development of physical therapists and will show why physical therapists are needed in acute care hospitals. These standards may also be used as a critical care competency list for clinical physical therapists, leading to the provision of entry-level course materials.
The ICERC of the JSICM decided to create minimum standards of clinical practice for physical therapists working in ICU in Japan. The aim of this investigation was to establish a framework for these minimum standards. It also aimed to make an international comparison of minimum standards of clinical practice for physical therapists working in ICU.
Method
Design
This study followed the modified Delphi method used previously1). The Delphi technique is a method of bringing together expert opinion through a series of iterative questionnaires. The process aims to reach a group consensus. Relying on just one expert to determine minimum standards can lead to bias. Delphi techniques are particularly useful when there is no single ‘right' answer, such as decision-making, policy, or long-term prediction.
Participants
Potential participants were recruited from among current members of the JSICM. The Japanese Physical Therapy Association, the professional organization of Japanese physical therapists, has no special interest group for acute care and has no certification system for intensive care practice or competence. Therefore, this survey was conducted among current physical therapist members registered with the JSICM. All were qualified physical therapists with at least five years of experience working in hospitals with ICU.
The inclusion criteria were being (1) a certified respiratory physiotherapist, certified cardiac physiotherapist, or certified physiotherapy specialist in visceral impairment, with certification by the Japanese Physical Therapy Association, or (2) respiratory therapists certified by the joint committee of the three scientific societies (Japanese Association for Thoracic Surgery, The Japanese Respiratory Society and Japanese Society of Anesthesiology) or a Senior instructor certified by the Japanese Association for Cardiac Rehabilitation. The exclusion criteria were having less than five years of work experience in acute care hospitals, less than two years of intensive care experience, or no experience of teaching young physical therapists in ICU and/or not agreeing to participate.
Questionnaire development
The questionnaire used within this study was based on the Australasian questionnaire used by Skinner et al1). The JSICM-ICERC has established an exploratory working group of competency for physical therapists working in ICU. This working group consisted of intensivists, physicians, nurses, physical therapists, occupational therapists, and speech therapists with experience in ICU. First, this working group translated 224 questionnaire items from Skinner's study into Japanese. The translated items were verified by mutual review within the committee to confirm the accuracy of the translation. This working group added 48 more items that were considered potentially necessary for the understanding of healthcare professionals involved in early rehabilitation in Japan. The final version of the questionnaire contained 272 items.
Preparation
The Human Research Ethics Committees of Tokoha University (013013F) approved the study. Written informed consent was obtained in advance by giving potential participants an information sheet with the main purpose, content, and handling of the findings.
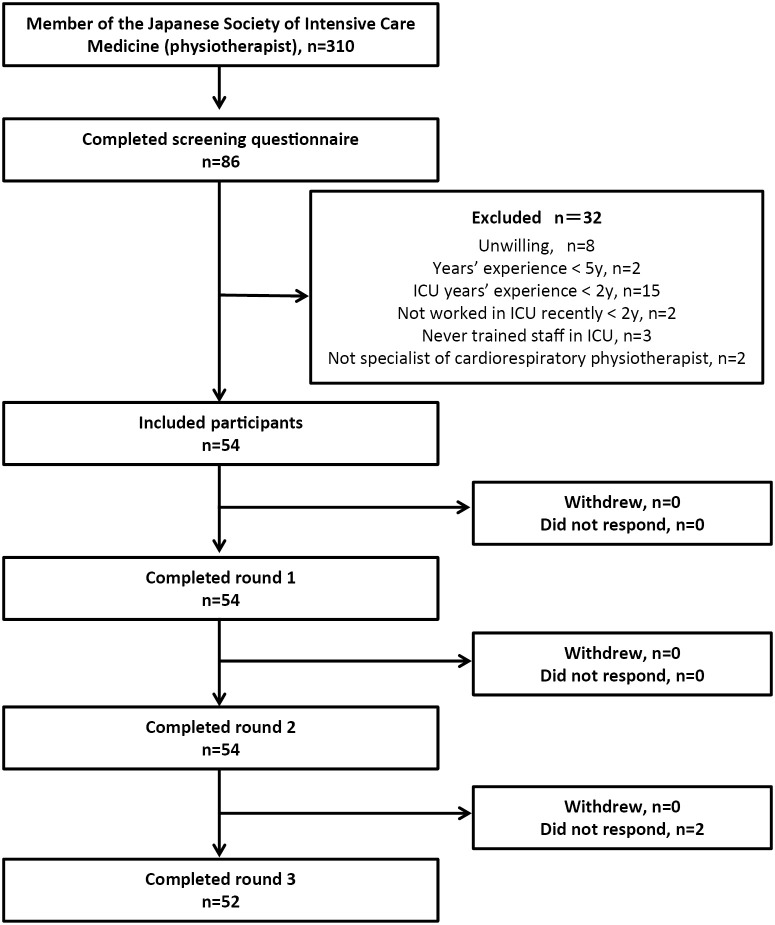
First, an e-mail was sent to all physiotherapist members of the JSICM (n=310) to ask if they wished to participate in this study. Those who were willing to participate were given access to a specific website to answer the questionnaire. All questionnaires including consent forms were distributed electronically via Google Forms (Google, United States). In total, 86 physical therapists (27.7%) expressed their intention to participate and answered questions about the inclusion and exclusion criteria. This process gave 54 physical therapists out of these eligible, who provided informed consent and were registered as the study participants, giving a recruitment success of 17.4% (of 310 invited) (Figure 1).
Figure 1.
Participants selection and completion rate
ICU, intensive care unit.
In line with previous studies, the participants were asked to assess whether each of the 272 items were “Essential” or “Not Essential” as a minimum standard for physical therapists working in ICU. They could also answer that they were “Unsure”. Although we did not specify an upper or lower limit for the number of “Essential” or “Not Essential”, the questionnaire emphasized that participants should:
1) Be accurate and careful when answering;
2) Not give the same answer for all items; and
3) Consider which activities were necessary for physical therapists in the current legal and medical system in Japan.
The first Delphi round began on October 19, 2019 with a response period of three weeks. The response period for the second and third rounds was set at two weeks, with the third round ending on December 21. 2019. Reminders to the responses were sent by email one week and three days before the end of each Delphi round.
In the first round, items that were considered essential by more than 70% of the participants were adopted as minimum standards. Items that were deemed “Not Essential” by more than 70% of participants were excluded from the minimum standards to be consistent with previous studies1,2). Items were also excluded from subsequent rounds if fewer than 30% of participants thought they were essential for clinical practice for physical therapists working in ICU. Items that were considered essential by fewer than 70% of participants were sent to the second round. In the second and third rounds, the same process was used. In all three rounds, items that were considered essential by at least 70% of participants were included in the “Minimum standards of clinical practice for physical therapists working in ICU in Japan” as in previous studies1,2).
The basic attributes and responses of the participants were aggregated in MicrosoftⓇ ExcelⓇ for Office 365 MSO, the average ± standard deviation of the normal distribution was used, and if not suitable, the median (24th, 75th percentile) was shown.
Results
Of the 86 physical therapists who expressed their willingness to participate in the study, 54 physical therapists (62.8%) were eventually participated in the study according to the inclusion and exclusion criteria (Figure 1). Table 1 shows the characteristics of the 54 participants. All participants had at least five years of clinical experience in the ICU as a physical therapist, with 100% being specialist physical therapists (as defined by the Japanese Physical Therapy Association). The average length of clinical experience as a physical therapist for participants was 14 years (11-18 years), and the average length of service in ICUs was 9 years (6-12 years). The participants included three academic faculty members. All these three academic faculty members concurrently work at their related hospitals, so the data for this survey came from total 54 hospitals.
Table 1.
Participants' characteristics
| Invited | Completed round 1 | Completed round 2 | Completed round 3 | ||
|---|---|---|---|---|---|
| (n=54) | (n=54) | (n=54) | (n=52) | ||
| IQR: interquartile range, ICU: intensive care unit *Japanese Physical Therapy Association, ** Japanese Association for Thoracic Surgery, Japanese Respiratory Society and Japanese Society of Anesthesiologists *** Japanese Association of Cardiac Rehabilitation | |||||
| Clinical experience, | Median (IQR) | 14 (11-18) | 14 (11-18) | 14 (11-18) | 13.5 (11-17) |
| Years | Range | 5-31 | 5-31 | 5-31 | 5-31 |
| ICU clinical experience, | Median (IQR) | 9 (6-12) | 9 (6-12) | 9 (6-12) | 9 (6-12) |
| Years | Range | 5-20 | 5-20 | 5-20 | 5-20 |
| ICU experience in senior role, n (%) | 100 (100) | 100 (100) | 100 (100) | 100 (100) | |
| Cardiopulmonary physiotherapy manuscript Publication, n (%) | 0 | 28 (52) | 28 (52) | 28 (52) | 27 (52) |
| 1-4 | 17 (31) | 17 (31) | 17 (31) | 17 (32) | |
| ≧5 | 9 (17) | 9 (17) | 9 (17) | 8 (16) | |
| ICU physiotherapy manuscript Publication, | 0 | 36 (67) | 36 (67) | 36 (67) | 35 (67) |
| n (%) | 1-4 | 14 (26) | 14 (26) | 14 (26) | 13 (25) |
| ≧5 | 4 (7) | 4 (7) | 4 (7) | 4 (8) | |
| Specialist physical therapist (Cardiovascular, respiratory and metabolic disorder) * | 10 (19%) | 10 (19%) | 10 (19%) | ||
| Certified physical therapist (respiratory) * | 19 (35%) | 19 (35%) | 19 (35%) | ||
| Certified physiotherapist (cardiovascular) * | 15 (28%) | 15 (28%) | 15 (28%) | ||
| Certified respiratory therapist** | 46 (85%) | 46 (85%) | 46 (85%) | ||
| Certified cardiac rehabilitation instructor***n (%) | 27 (50%) | 27 (50%) | 27 (50%) | ||
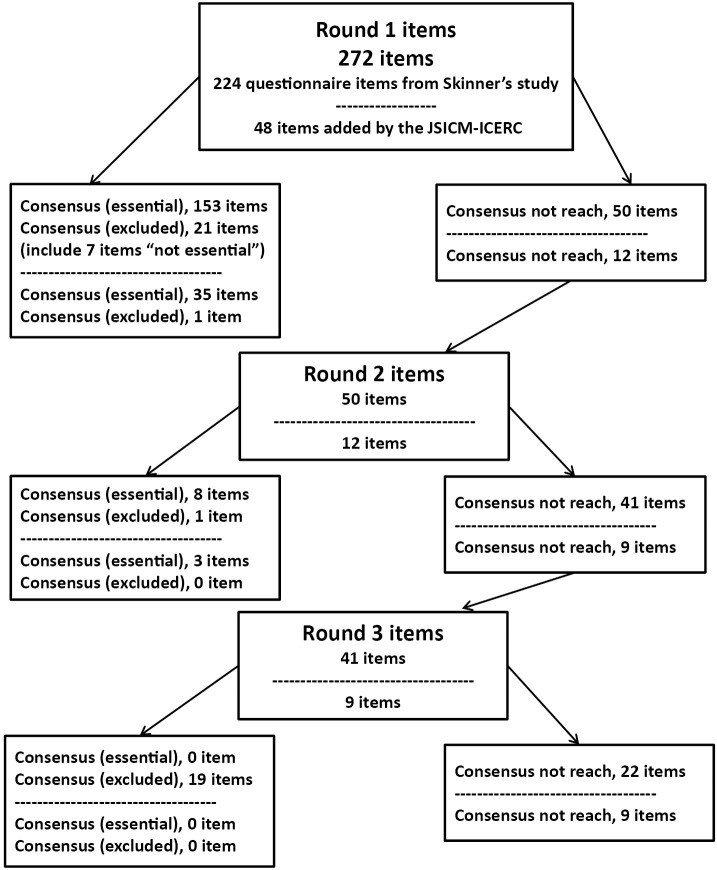
The highest number (%) and lowest number (%) of items selected as “essential” per participant in Round 1 were 260 (95%) and 164 (60%), respectively. Figure 2 shows the three rounds of the Delphi process. Of the 272 items in the first round, 188 were deemed essential. In round 2, 11 of the 62 items that failed to reach consensus in round 1 were also deemed essential. No items were classified as not essential in round 2. In round 3, none of the 50 items that failed to reach consensus in round 2 were deemed essential. There were 31 items on which participants failed to reach agreement.
Figure 2.
Flow of items through the Delphi rounds.
JSICM, the Japanese Society of Intensive Care Medicine; ICERC, the Intensive Care Early Rehabilitation Committee.
In this study, participants took three Delphi rounds to reach a consensus that 161 of the 224 questionnaire items from Skinner's study1) were essential. Table 2 shows the 161 items that were agreed to be essential, and during which round this agreement was reached. Table 2-4 also show comparisons with prior studies in Australia and New Zealand1) and United Kingdom2). Items that were considered essential in this survey but not in the UK and Australia included some cardiovascular items such as knowledge of calcium channel blockers, cardiac output measurements, pulmonary arterial catheter measurements, intra-aortic balloon pump and advanced electrocardiograms. Our survey also confirmed that it is essential for physical therapists to be able to accurately and independently evaluate and interpret the findings from imaging investigations including computed tomography (CT), magnetic resonance imaging (MRI), and echocardiography. Only Japanese physical therapists considered that it was essential for them to be able to interpret and assess nutritional status including food administration, volume and type. Similarly, there was a greater interest in delirium, sedation and analgesia in Japan than in the UK and Australia.
Table 2.
Items agreed to be essential (> 70% of participants said they were “essential”)
| item | Round 1 % |
Round 2 % |
Round 3 % |
AUS-NZ | UK | ||
|---|---|---|---|---|---|---|---|
| AUS-NZ: Australia and New Zealand, UK: the United Kingdom, *: Essential, NRC: did not reach consensus, NE: not essential, N/A: not available ECG: electrocardiogram, PiCCO: pulse contour cardiac output, CO: cardiac output, CI: cardiac index, SVV: stroke volume variation, SVRI: systemic vascular resistance index, PAP: pulmonary artery pressure, ECMO: extracorporeal membrane oxygenation, CPAP: continuous positive airway pressure, PEEP: positive end expiratory pressure, EPAP: expiratory positive airway pressure, PS: pressure support, IPAP: Inspiratory Positive Airway Pressure, SIMV: synchronised intermittent mandatory ventilation, STEMI: ST elevation myocardial infarction, NSTEMI: non-ST elevation myocardial infarction, VAP: ventilator-associated pneumonia, MODS: multiple organ dysfunction syndrome, COPD: chronic obstructive pulmonary disease, PVC: premature ventricular contraction, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, CAM-ICU: confusion assessment method for the intensive care unit, CT: computed tomography, MRI: magnetic resonance imaging, ASIA: American Spinal Cord Injury Association, HCO3: bicarbonate, PaCO2: partial pressure of arterial carbon dioxide, PaO2: partial pressure of arterial oxygen, SPO2: peripheral capillary oxygen saturation, SaO2: arterial oxygen saturation, pH: potential of hydrogen, SvO2: mixed venous oxygen saturation, FiO2: fraction of inspired oxygen ACBT: active cycle of breathing technique, FET: forced expiratory technique, MRC: Medical Research Council, 6MWT: 6-minute walk test, EQ-5D: EuroQol 5 Dimension, AQoL: Assessment of Quality of Life | |||||||
| A physiotherapist is aware or has knowledge of | |||||||
| Key literature that guides evidence-based physiotherapy practice in critical care settings | |||||||
| Key literature that guides evidence-based physiotherapy practice in critical care settings | 100 | ― | ― | * | NRC | ||
| The actions and implications for physiotherapy of the following medications | |||||||
| Analgesia | 93 | ― | ― | * | N/A | ||
| Anti-arrhythmics (e.g. amiodarone, digoxin) | 96 | ― | ― | * | * | ||
| Anti-hypertensives (e.g. beta-blockers, hydralazine) | 98 | ― | ― | * | * | ||
| Bronchodilators | 89 | ― | ― | * | * | ||
| Calcium channel blockers | 87 | ― | ― | NRC | NE | ||
| Sedation and neuromuscular paralyzing agents | 100 | ― | ― | * | * | ||
| Vasopressors/inotropes (e.g. dobutamine, milrinone, adrenaline, dopamine, noradrenaline) | 100 | ― | ― | * | * | ||
| Methods for advanced hemodynamic monitoring, can interpret the measurements and understands the implications for physiotherapy of | |||||||
| Implanted or external pacemakers, and determine the presence of pacing on ECG | 96 | ― | ― | * | NRC | ||
| PiCCO measurements (e.g. CO, CI, SVV, SVRI etc.) | 83 | ― | ― | NE | NE | ||
| Pulmonary arterial catheter measurements (e.g. CO, CI, SVRI, PAP, etc.) | 85 | ― | ― | NE | NE | ||
| A physiotherapist can understand | |||||||
| Equipment (including recognition of equipment), can use/safely apply or handle equipment, understands the implications for physiotherapy of | |||||||
| Arterial lines | 100 | ― | ― | * | * | ||
| Central venous catheters | 98 | ― | ― | * | * | ||
| ECMO | 74 | ― | ― | NE | NE | ||
| Endotracheal tubes and tracheostomy | 100 | ― | ― | * | * | ||
| Indwelling urinary catheters | 91 | ― | ― | * | * | ||
| Intercostal catheters | 100 | ― | ― | * | * | ||
| Intra-aortic balloon pump | 89 | ― | ― | NRC | NE | ||
| Intracranial pressure (ICP) monitors and extra-ventricular drains (EVD) | 91 | ― | ― | * | NE | ||
| Nasogastric tubes | 98 | ― | ― | * | * | ||
| Oxygen therapy devices | 100 | ― | ― | * | * | ||
| Vascath/hemodialysis/continuous veno-venous hemodiafiltration | 94 | ― | ― | * | * | ||
| Wound drains | 100 | ― | ― | * | * | ||
| The key principles of providing the following differing modes of mechanical/assisted ventilation including | |||||||
| Airway Pressure Release Ventilation (APRV) | 76 | ― | ― | NE | NE | ||
| Assist-control | 98 | ― | ― | * | NRC | ||
| BiLevel | 83 | ― | ― | * | * | ||
| CPAP | 98 | ― | ― | * | * | ||
| PEEP/EPAP | 100 | ― | ― | * | * | ||
| Pressure-regulated Volume Control (PRVC) | 81 | ― | ― | * | N/A | ||
| PS/IPAP | 100 | ― | ― | * | * | ||
| SIMV (Volume) / (Pressure) | 93 | ― | ― | * | * | ||
| Weaning protocols | 96 | ― | ― | * | NRC | ||
| Pathophysiology and presenting features, likely medical management and implications for physiotherapy for a range of conditions including | |||||||
| Acute coronary syndrome (e.g. angina, STEMI, NSTEMI) | 100 | ― | ― | * | * | ||
| Acute lung injury/acute respiratory distress syndrome (ARDS) | 98 | ― | ― | * | * | ||
| Burns (cutaneous/inhalational) | 81 | ― | ― | NRC | NE | ||
| Chest trauma | 93 | ― | ― | * | * | ||
| Community acquired/nosocomial/hospital-acquired pneumonia (including VAP) | 100 | ― | ― | * | * | ||
| Guillain-Barre Syndrome | 76 | ― | ― | * | * | ||
| Heart failure | 100 | ― | ― | * | * | ||
| Hepatitis | 72 | ― | ― | NRC | NE | ||
| ICU-acquired weakness (ICU-AW) | 100 | ― | ― | * | * | ||
| Immunocompromise | 65 | 85 | ― | * | * | ||
| Intracerebral hemorrhage/Subarachnoid hemorrhage | 100 | ― | ― | * | * | ||
| Metabolic/electrolyte disturbances | 98 | ― | ― | * | NRC | ||
| Multi-organ failure/MODS | 94 | ― | ― | * | * | ||
| Multi-trauma | 93 | ― | ― | * | NRC | ||
| Obstructive respiratory disease (e.g. asthma, COPD) | 100 | ― | ― | * | * | ||
| Pancreatitis | 76 | ― | ― | * | NRC | ||
| Pleural effusion | 98 | ― | ― | * | * | ||
| Post-abdominal surgery | 98 | ― | ― | * | * | ||
| Post-cardiac surgery | 94 | ― | ― | * | NRC | ||
| Post-surgery other (e.g. orthopaedic, vascular) | 94 | ― | ― | * | N/A | ||
| Post-thoracic surgery | 100 | ― | ― | * | NRC | ||
| Renal failure (acute and chronic) | 100 | ― | ― | * | * | ||
| Respiratory failure (Type I and II) | 100 | ― | ― | * | * | ||
| Restrictive respiratory disease (e.g. pulmonary fibrosis, kyphoscoliosis) | 98 | ― | ― | * | * | ||
| Shock (cardiogenic) | 98 | ― | ― | * | * | ||
| Shock (septic) | 94 | ― | ― | * | * | ||
| Spinal cord injury | 96 | ― | ― | * | * | ||
| Suppurative lung disease (e.g. cystic fibrosis, bronchiectasis) | 74 | ― | ― | * | * | ||
| Systemic inflammatory response syndrome (SIRS) | 89 | ― | ― | * | * | ||
| Thromboembolic disease (e.g. deep vein thrombosis, pulmonary embolus) | 100 | ― | ― | * | * | ||
| Thrombotic cerebrovascular accident | 96 | ― | ― | * | N/A | ||
| Traumatic brain injury | 94 | ― | ― | * | * | ||
| A physiotherapist can accurately/independently (assess and) interpret | |||||||
| Readings from clinical monitoring including | |||||||
| Advanced ECGs (i.e. conduction block, 12-lead ECG) | 80 | ― | ― | NE | NRC | ||
| Basic ECGs (i.e. sinus rhythm/tachycardia/bradycardia, atrial fibrillation, atrial flutter, ventricular tachycardia, ventricular fibrillation, asystole, PVCs) | 98 | ― | ― | * | * | ||
| Blood pressure (systolic, diastolic, and mean arterial blood pressure) | 100 | ― | ― | * | * | ||
| Body temperature | 100 | ― | ― | * | * | ||
| Central venous pressure | 94 | ― | ― | * | NRC | ||
| End tidal carbon dioxide | 83 | ― | ― | * | * | ||
| Fluid intake and output | 96 | ― | ― | * | * | ||
| Heart rate | 100 | ― | ― | * | * | ||
| Nutritional status including feed administration, volume and type | 85 | ― | ― | NE | NE | ||
| SpO2/Pulse oximetry | 100 | ― | ― | * | * | ||
| Findings from laboratory investigations including | |||||||
| Albumin | 94 | ― | ― | NE | NE | ||
| Blood glucose levels | 96 | ― | ― | * | * | ||
| C-reactive protein (CRP) | 100 | ― | ― | NRC | * | ||
| Creatinine kinase (CK) | 98 | ― | ― | NRC | NE | ||
| Hematocrit | 83 | ― | ― | NE | NE | ||
| Hemoglobin | 100 | ― | ― | * | * | ||
| Liver function tests (e.g. ALT, LDH, Bilirubin) | 93 | ― | ― | NE | NE | ||
| Neutrophil counts | 80 | ― | ― | NRC | NE | ||
| Platelets, APTT (activated partial thromboplastin time), INR (international normalized ratio) | 87 | ― | ― | * | * | ||
| Renal function tests (e.g. urea, creatinine) | 93 | ― | ― | * | NRC | ||
| Respiratory function tests (e.g. FEV1, FVC etc.) | 98 | ― | ― | * | NRC | ||
| Troponin | 76 | ― | ― | * | * | ||
| White cell count (WCC) | 98 | ― | ― | * | * | ||
| Findings from imaging investigations (excluding the imaging report) including | |||||||
| Chest radiographs (CXR) | 94 | ― | ― | * | * | ||
| CT - Brain imaging | 74 | ― | ― | NE | NE | ||
| CT - Chest imaging | 81 | ― | ― | NE | NE | ||
| MRI - Brain | 74 | ― | ― | NE | NE | ||
| Skeletal X-rays | 81 | ― | ― | NRC | NE | ||
| Ultrasound - Chest | 74 | ― | ― | NE | NE | ||
| Results from neurological equipment/examinations and functional tests including | |||||||
| Ability to interpret a delirium assessment (e.g. the CAM-ICU) | 96 | ― | ― | NRC | N/A | ||
| Ability to perform a delirium assessment (e.g. the CAM-ICU) | 83 | ― | ― | NE | NE | ||
| An ability to interpret a Glasgow Coma Score (GCS) | 100 | ― | ― | * | * | ||
| An ability to interpret an assessment of cranial nerve function | 93 | ― | ― | NRC | NE | ||
| An ability to interpret an assessment of sedation levels (e.g. Ramsey Sedation Scale, Richmond Agitation-Sedation Scale) | 100 | ― | ― | * | NRC | ||
| An ability to perform a Glasgow Coma Score (GCS) | 93 | ― | ― | NRC | NE | ||
| An ability to perform a neurological examination of motor and sensory functions (e.g. light touch, pain, ASIA score) | 98 | ― | ― | * | NRC | ||
| An ability to perform an assessment of cranial nerve function | 93 | ― | ― | NE | NE | ||
| An ability to perform an assessment of sedation levels | 98 | ― | ― | NE | NE | ||
| Intra-cranial pressure (ICP) monitors (intra-parenchymal, intra-ventricular) and cerebral perfusion pressure (CPP) | 52 | 74 | ― | * | NRC | ||
| Indices from blood gas measurement including | |||||||
| A-a gradient | 81 | ― | ― | NE | NE | ||
| Base excess | 76 | ― | ― | * | * | ||
| HCO3 | 91 | ― | ― | * | * | ||
| Lactate | 87 | ― | ― | NE | NRC | ||
| PaCO2 | 100 | ― | ― | * | * | ||
| PaO2, SpO2, SaO2 | 100 | ― | ― | * | * | ||
| PaO2/FiO2 ratio | 100 | ― | ― | * | NE | ||
| pH | 98 | ― | ― | * | * | ||
| Venous blood gas interpretation (including SvO2) | 74 | ― | ― | NRC | NE | ||
| (assess and interpret) Mechanical ventilation settings/measurements including | |||||||
| Breath types (spontaneous, mandatory, assisted) | 100 | ― | ― | * | * | ||
| Maximum inspiratory pressure (MIP) measurements | 63 | 72 | ― | NE | NE | ||
| Peak inspiratory pressure | 93 | ― | ― | * | * | ||
| Respiratory rate | 100 | ― | ― | * | * | ||
| Static and/or dynamic lung compliance measures | 61 | 72 | ― | NE | NE | ||
| The level of FiO2 | 100 | ― | ― | * | * | ||
| The level of PEEP | 100 | ― | ― | * | * | ||
| The level of PS | 98 | ― | ― | * | * | ||
| Tidal volume | 100 | ― | ― | * | * | ||
| A physiotherapist can | |||||||
| Perform and accurately interpret the results of common respiratory examinations including | |||||||
| Auscultation | 100 | ― | ― | * | * | ||
| Observation of respiratory rate | 100 | ― | ― | * | * | ||
| Palpate the chest wall | 94 | ― | ― | * | * | ||
| Patterns of breathing | 100 | ― | ― | * | * | ||
| Assess | |||||||
| The effectiveness/quality of a patient's cough (on or off mechanical ventilation) | 96 | ― | ― | * | * | ||
| Provide the following techniques, including an understanding of indications, contraindications, evidence for the technique, and progressions | |||||||
| ACBT [breathing control, thoracic expansion and FET] | 83 | * | * | ||||
| Assisted coughing - chest wall | 85 | ― | ― | * | * | ||
| Assisted coughing - subcostal thrusts for spinal cord injuries | 54 | 74 | ― | * | NRC | ||
| Bed exercises (e.g. passive - active - resisted range of motion exercises) | 100 | ― | ― | * | * | ||
| Braces | 74 | ― | ― | NE | NE | ||
| Directed coughing/instructing the patient to cough effectively | 96 | ― | ― | * | * | ||
| Electrical stimulation (e.g. for isolated muscle activation to prevent muscle wasting, such as neuromuscular/functional electrical stimulation) | 78 | ― | ― | NE | NE | ||
| Humidification | 76 | ― | ― | * | * | ||
| Inexsufflator (Cough Assist) | 65 | 70 | ― | NE | * | ||
| Inspiratory muscle training | 78 | ― | ― | NE | NE | ||
| Mobilization of non-ventilated patient (e.g. sitting on edge of bed, stand, hoist or slide transfer to chair, march on spot, walk, use of gait aids) | 100 | ― | ― | * | * | ||
| Mobilization of ventilated patient (e.g. sitting on edge of bed, stand, hoist or slide transfer to chair, march on spot, walk, use of gait aids) | 98 | ― | ― | * | * | ||
| NIV/BiPAP - for use during exercise or mobilization including initiation and titration of | 69 | 70 | ― | NRC | NE | ||
| Patient positioning for prevention of pressure ulcers, management of tone, maintenance of musculoskeletal function | 100 | ― | ― | * | * | ||
| Patient positioning for respiratory care - including use of side lie, sitting upright, postural drainage (modified or head down tilt) | 100 | ― | ― | * | * | ||
| Patient prone positioning in severe respiratory failure/acute lung injury | 87 | ― | ― | NRC | NRC | ||
| Pursed lip breathing | 98 | ― | ― | * | N/A | ||
| Suction via a tracheal tube (Endotracheal tube, tracheostomy, minitracheostomy) | 67 | 72 | ― | * | * | ||
| Supported coughing | 98 | ― | ― | * | * | ||
| Treadmill, cycle ergometry (e.g. Motomed) or stationary bike | 87 | ― | ― | NRC | NE | ||
| A physiotherapist can | |||||||
| Complete musculoskeletal and/or functional assessments including | |||||||
| Ability to assess tone (e.g. utilizing a Modified Ashworth Scale) and reflexes | 100 | ― | ― | * | NRC | ||
| Deep vein thrombosis screening (i.e. color, temperature, touch, swelling, Homan's test) | 93 | ― | ― | * | * | ||
| Dynamometry | 81 | ― | ― | NRC | NE | ||
| Manual muscle testing (e.g. MRC scale) | 98 | ― | ― | * | * | ||
| Objective measures of cardiopulmonary exercise tolerance (e.g. 6-minute walk test; incremental shuttle walk test) | 87 | ― | ― | NRC | NE | ||
| Objective measures of physical function [e.g. the Physical Function ICU Test (PFIT), Timed Up and Go Test (TUG), 6MWT, De-Morton Mobility Index (DEMMI) ] | 93 | ― | ― | * | NE | ||
| Objective measures of quality of life (e.g. Short Form 36, EQ-5D, AQoL) | 72 | ― | ― | NE | NE | ||
| Peripheral edema | 96 | ― | ― | * | * | ||
| Range of motion | 100 | ― | ― | * | * | ||
| Appropriately | |||||||
| Be aware of inotropes and implications for physiotherapy treatment | 100 | ― | ― | * | N/A | ||
| Be aware of sedation and implications for physiotherapy treatment | 98 | ― | ― | * | N/A | ||
| Liaise with medical/nursing staff to increase/decrease inotropes to achieve physiotherapy goals | 85 | ― | ― | * | N/A | ||
| Liaise with medical/nursing staff to increase/decrease sedation to achieve physiotherapy goals | 94 | ― | ― | * | N/A | ||
| A physiotherapist can | |||||||
| Assess and interpret ventilator waveforms | 89 | ― | ― | NE | N/A | ||
| Determine the appropriateness of a patient for extubation | 83 | ― | ― | NRC | NRC | ||
Table 3.
Items that participants agreed to exclude
| Round 1 % |
Round 2 % |
Round 3 % |
AUS-NZ | UK | ||||
|---|---|---|---|---|---|---|---|---|
| AUS-NZ: Australia and New Zealand, UK: the United Kingdom, *: Essential, NRC: did not reach a consensus, NE: not essential, N/A: not available #: Items determined as not essential (consensus >70% “not essential”) NP airway: nasopharyngeal airway, OP airway: oropharyngeal airway, NIV/BiPAP: non-invasive ventilation/ biphasic positive airway pressure, COPD: chronic obstructive pulmonary disease, PEP: positive expiratory pressure. | ||||||||
| The actions and implications for physiotherapy of the following medications | ||||||||
| Prostacyclin | 35 | 22 | ― | NE | NE | |||
| A physiotherapist can understand | ||||||||
| Equipment (including recognition of equipment), can use/safely apply or handle equipment, understands the implications for physiotherapy of | ||||||||
| Sengstaken-Blakemore/Minnesota tubes | 13 | ― | ― | NE | NE | |||
| Pathophysiology and presenting features, likely medical management and implications for physiotherapy for a range of conditions including | ||||||||
| Brain death and organ procurement | 19 | ― | ― | NRC | NRC | |||
| Results from neurological equipment/examinations and functional tests including | ||||||||
| Electroencephalograms (EEG) | 11 | ― | ― | NE | NE | |||
| Extra-ventricular drain (EVD) | 48 | 50 | 29 | * | N/A | |||
| Indices from blood gas measurement including | ||||||||
| P50 | 13 | ― | ― | NE | NE | |||
| A physiotherapist can | ||||||||
| Perform | ||||||||
| a cuff volume and/or pressure test on an endotracheal tube (or tracheostomy) | 35 | 39 | 23 | NRC | NRC | |||
| Swallow assessment | 30 | 43 | 22 | NE | NE | |||
| Provide the following techniques, including an understanding of indications, contraindications, evidence for the technique, and progressions | ||||||||
| Additional rehabilitation techniques (e.g. hydrotherapy, Wii) | 22 | ― | ― | NE | NE | |||
| Assisting bronchoscopy via delivery of secretion mobilization techniques (e.g. vibrations, assisted coughing) during the procedure | 54 | 61 | 28 | NE | NE | |||
| Bronchial lavage (i.e. up to 120 ml in one treatment session administered by bronchoscopy for sputum/organism retrieval for diagnostic purposes) | 13 | ― | ― | # | NE | NE | ||
| Buteyko breathing | 4 | ― | ― | NE | N/A | |||
| Cough stimulation - oropharyngeal catheter stimulation | 19 | ― | ― | * | * | |||
| Cough stimulation - tracheal rub | 15 | ― | ― | * | NE | |||
| Feldenkreis | 2 | ― | ― | NE | NE | |||
| Glottal stacking (frog breathing) | 26 | ― | ― | NE | NE | |||
| Inspiratory hold/sustained maximal inspiration | 50 | 57 | 27 | * | N/A | |||
| Instillation of normal saline into the endotracheal tube (i.e. < 20 ml in one treatment session aimed at increasing sputum yield by diluting and loosening thick secretions) | 9 | ― | ― | # | NRC | * | ||
| Intermittent positive pressure breathing (IPPB, The Bird) | 31 | 33 | 18 | NE | * | |||
| Manual airway clearance techniques - percussion, vibration, chest shaking | 41 | 50 | 27 | * | * | |||
| Manual hyperinflation (MHI) | 39 | 37 | 23 | * | * | |||
| Nasopharyngeal airway suctioning including insertion of NP airway | 39 | 33 | 12 | * | * | |||
| NIV/BiPAP - for Type I or Type II respiratory failure, initiation and titration of e.g. COPD exacerbation with hypercapnia | 39 | 33 | 22 | NE | NRC | |||
| NIV/BiPAP - intermittent, short term applications during physiotherapy to assist secretion mobilization techniques or lung recruitment including initiation and titration of | 65 | 65 | 27 | NRC | NRC | |||
| Oropharyngeal airway suctioning including insertion of OP airway | 37 | 37 | 14 | * | * | |||
| Other breathing techniques (e. g., Buteyko) | 13 | ― | ― | NE | NE | |||
| Performing bronchoscopy independently | 4 | ― | ― | # | NE | NE | ||
| Periodic/intermittent CPAP (non-invasive via mask) including initiation and titration of | 35 | 48 | 22 | NRC | NRC | |||
| Positive pressure devices for airway clearance (e.g. AstraPEP, PariPEP, TheraPEP or oscillating expiratory pressure devices like Acapella, Flutter) | 44 | 56 | 25 | * | NRC | |||
| Splinting and/or casting for the upper and lower limbs | 43 | 44 | 19 | NE | NE | |||
| Ventilator hyperinflation (VHI) via an endotracheal tube or tracheostomy | 20 | ― | ― | NRC | NRC | |||
| Complete musculoskeletal and/or functional assessments including | ||||||||
| Bioimpedance testing of body composition | 43 | 50 | 24 | NE | NE | |||
| Appropriately request/coordinate the following | ||||||||
| Titration of analgesia to achieve physiotherapy goals | 67 | 69 | 27 | * | * | |||
| A physiotherapist can | ||||||||
| Decannulate a tracheostomy | 9 | ― | ― | # | NE | NE | ||
| Determine the appropriateness of tracheostomy decannulation | 50 | 35 | 16 | NRC | NRC | |||
| Extubate a patient | 4 | ― | ― | # | NE | NE | ||
| Intubate a patient | 2 | ― | ― | # | NE | NE | ||
| Lead the co-ordination of cuff deflation trials | 19 | ― | ― | NE | NE | |||
| Lead the co-ordination of speaking valve trials | 28 | ― | ― | NE | NE | |||
| Lead the co-ordination of weaning protocols | 43 | 43 | 20 | NE | NE | |||
| Tracheostomy exchange | 2 | ― | ― | # | NE | NE | ||
Table 4.
Items on which consensus was not reached in any round
| Round 1 % |
Round 2 % |
Round 3 % |
AUS-NZ | UK | |||
|---|---|---|---|---|---|---|---|
| AUS-NZ: Australia and New Zealand, UK: the United Kingdom, *: Essential, NRC: did not reach a consensus, NE: not essential CT: computed tomography, MRI: magnetic resonance imaging, P-V: pressure-volume, FEV1: forced expiratory volume in one second, FVC: Forced vital capacity, PEF: peak expiratory flow | |||||||
| A physiotherapist is aware or has knowledge of | |||||||
| The actions and implications for physiotherapy of the following medications | |||||||
| Mucolytics | 52 | 39 | 50 | * | * | ||
| Nitric oxide | 56 | 50 | 43 | NRC | NE | ||
| The key principles of providing the following differing modes of mechanical/assisted ventilation including | |||||||
| High frequency oscillatory ventilation (HFOV) | 50 | 54 | 38 | NE | NE | ||
| Pathophysiology and presenting features, likely medical management and implications for physiotherapy for a range of conditions including | |||||||
| Fat embolism | 63 | 69 | 44 | * | NRC | ||
| Organ transplantation | 37 | 48 | 37 | NE | NE | ||
| Findings from laboratory investigations including | |||||||
| Procalcitonin | 56 | 61 | 44 | NE | NE | ||
| Sputum cultures | 44 | 52 | 45 | * | NRC | ||
| Findings from imaging investigations (excluding the imaging report) including | |||||||
| CT - Spine imaging | 65 | 63 | 45 | NE | NE | ||
| MRI - Chest | 57 | 57 | 43 | NE | NE | ||
| MRI - Spine | 59 | 63 | 48 | NE | NE | ||
| Indices from blood gas measurement including | |||||||
| Anion gap | 54 | 65 | 46 | NE | NE | ||
| Oxygen content (CaO2) | 61 | 59 | 41 | NE | NE | ||
| (assess and interpret) Mechanical ventilation settings/measurements including | |||||||
| Maximum expiratory pressure (MEP) measurements | 52 | 67 | 37 | NE | NE | ||
| Upper and lower inflection points of P-V curves | 52 | 46 | 30 | NE | NE | ||
| A physiotherapist can | |||||||
| Perform and accurately interpret the results of common respiratory examinations including | |||||||
| Interpret the rapid shallow breathing index (RSBI) | 59 | 61 | 43 | NE | NE | ||
| Measure peak cough flow on or off mechanical ventilation | 50 | 59 | 40 | NE | NE | ||
| Perform | |||||||
| Respiratory function tests (e.g. for measurement of FEV1, FVC, PEF) | 61 | 67 | 46 | * | NE | ||
| Spontaneous breathing trial | 56 | 57 | 32 | NE | NE | ||
| Provide the following techniques, including an understanding of indications, contraindications, evidence for the technique, and progressions | |||||||
| Autogenic drainage | 50 | 59 | 31 | NE | NE | ||
| Collars | 69 | 63 | 35 | NRC | NE | ||
| Oxygen therapy including initiation and titration of oxygen therapy | 63 | 63 | 35 | * | * | ||
| Recruitment maneuvers | 63 | 59 | 31 | NE | NE | ||
Table 3 shows the 41 items that were excluded because less than 30% of participants thought they were essential across the three Delphi rounds. These 41 items included seven items that more than 70% of the participants deemed not essential. Many of these items were also excluded in the UK and Australian surveys, but the following items were considered essential in the UK and Australia: cough stimulation-tracheal rub, manual airway clearance techniques, manual hyperinflation, suctioning, titration of analgesia to achieve physiotherapy goals.
Table 4 shows the 22 items where participants failed to reach a consensus, and the percentage of participants who felt these items were essential. Of the 22 items for which no consensus was reached, 20 items were also determined to be not essential or not reach a consensus in the UK and Australia excluding the knowledge of the actions and implications for physiotherapy of mucolytics and oxygen therapy including initiation and titration.
Finally, Table 5 shows the results for the 48 items added by the JSICM-ICERC. Of these 48 items, 38 items were considered essential, and nine failed to reach consensus.
Table 5.
Items added by the JSICM-ICERC
| Round 1 % |
Round 2 % |
Round 3 % |
||||
|---|---|---|---|---|---|---|
| JSICM-ICERC: The Japan Society of Intensive Care Medicine, the Intensive Care Early Rehabilitation Committee *: Essential, NRC: did not reach a consensus, NE: not essential BIS: bispectral, PICC: peripherally inserted central catheter, NRS: numerical rating scale, VAS: visual analogue scale, BPS: Behavioral Pain Scale, CPOT: Critical-Care Pain. Observation Tool, HADS: Hospital Anxiety and Depression Scale, IES-R: Impact of Event Scale-Revised, I/E ratio: inspiratory/expiratory ratio, ADL: activities of daily living | ||||||
| A physiotherapist is aware or has knowledge of | ||||||
| The actions and implications for physiotherapy of the following medications | ||||||
| Steroids | 94 | ― | ― | * | ||
| Sodium bicarbonate (meiron, etc.) | 63 | 57 | 51 | NRC | ||
| A physiotherapist can understand | ||||||
| Equipment (including recognition of equipment), can use/safely apply or handle equipment, understands the implications for physiotherapy of | ||||||
| Pump catheter for auxiliary circulation (IMPELLA) | 52 | 48 | 41 | NRC | ||
| Epidural catheter | 89 | ― | ― | * | ||
| The key principles of providing the following differing modes of mechanical/assisted ventilation including | ||||||
| NIV (Non-Invasive Ventilation) | 98 | ― | ― | * | ||
| Brain Monitor (BIS Monitor) | 48 | 61 | 51 | NRC | ||
| Indirect calorimeter | 31 | 44 | 37 | NRC | ||
| Peripheral insertion center venous catheter (PICC catheter) | 80 | ― | ― | * | ||
| Pathophysiology and presenting features, likely medical management and implications for physiotherapy for a range of conditions including | ||||||
| Hemorrhagic shock | 93 | ― | ― | * | ||
| Anaphylactic shock | 59 | 78 | ― | * | ||
| Sepsis | 96 | ― | ― | * | ||
| Post-Intensive Care syndrome (PICS) | 96 | ― | ― | * | ||
| Disseminated intravascular coagulation syndrome (DIC) | 87 | ― | ― | * | ||
| Mediastinitis | 91 | ― | ― | * | ||
| Post-resuscitation encephalopathy | 67 | 80 | ― | * | ||
| Postcardiac Syndrome (PCAS) | 78 | ― | ― | * | ||
| Myasthenia gravis (MG) | 76 | ― | ― | * | ||
| Parkinson's disease | 81 | ― | ― | * | ||
| Abandoned syndrome | 96 | ― | ― | * | ||
| A physiotherapist can accurately/independently (assess and) interpret | ||||||
| Readings from clinical monitoring including | ||||||
| Transdermal carbon dioxide fractional pressure (PtcCO2) | 52 | 70 | ― | * | ||
| Findings from laboratory investigations including | ||||||
| Fibrinolytic inspection (FDP, D-dimer) | 94 | ― | ― | * | ||
| Cerebral natriuretic peptide (BNP) | 94 | ― | ― | * | ||
| Electrolytes (sodium, potassium, calcium, magnesium, phosphorus) | 94 | ― | ― | * | ||
| Findings from imaging investigations (excluding the imaging report) including | ||||||
| Abdominal X-rays (free air, ascites, ileus, etc.) | 57 | 65 | 47 | NRC | ||
| Aortic contrast examination | 46 | 56 | 30 | NRC | ||
| Results from neurological equipment/examinations and functional tests including | ||||||
| Ability to assess pain (NRS, VAS, BPS, CPOT, etc.) | 100 | ― | ― | * | ||
| Ability to practice pain assessment (NRS, VAS, BPS, CPOT, etc.) | 98 | ― | ― | * | ||
| Objective evaluation of the spirit (e.g. HADS, IES-R) | 48 | 56 | 45 | NRC | ||
| Objective evaluation of cognitive function | 78 | ― | ― | * | ||
| Indices from blood gas measurement including | ||||||
| Venous blood mixing ratio (shunt rate) | 57 | 50 | 32 | NRC | ||
| Dead cavity ventilation rate | 59 | 63 | 38 | NRC | ||
| (assess and interpret) Mechanical ventilation settings/measurements including | ||||||
| inspiratory time (Ti) | 70 | ― | ― | * | ||
| I/E ratio | 80 | ― | ― | * | ||
| A physiotherapist can | ||||||
| Perform | ||||||
| Kahlek test | 24 | 30 | 14 | NE | ||
| Provide the following techniques, including an understanding of indications, contraindications, evidence for the technique, and progressions | ||||||
| Deep inspiratory and respiratory aids for increased lung capacity under spontaneous breathing | 94 | ― | ― | * | ||
| Preoperative non-smoking guidance | 85 | ― | ― | * | ||
| Complete musculoskeletal and/or functional assessments including | ||||||
| ADL rating | 98 | ― | ― | * | ||
| A physiotherapist can | ||||||
| Safety management (prevention of fall and route removal) | 100 | ― | ― | * | ||
| Infection prevention | 96 | ― | ― | * | ||
| Communication within the team (appropriate reporting and consultation, participation in implementation plan, proposals for cooperation and role sharing) | 98 | ― | ― | * | ||
| Check the fixation of tubes and lines | 96 | ― | ― | * | ||
| Communication with patients with ventilators | 100 | ― | ― | * | ||
| Proper release and mounting of physical restraints | 78 | ― | ― | * | ||
| Communication with patient families | 98 | ― | ― | * | ||
| Patient and Family Engagement/Empowerment | 93 | ― | ― | * | ||
| Conducted multi-job collaboration and multi-job conferences | 100 | ― | ― | * | ||
| ABCDEF Bundle | 98 | ― | ― | * | ||
| Improvement of the environment before and after, during rehabilitation | 100 | ― | ― | * | ||
Discussion
Consensus was reached in three Delphi rounds that 161 (71.9%) of the 224 questionnaire items from Skinner's study1) were essential. Items ranged from pathophysiology and clinical signs and symptoms related to physical therapy, to physical therapy practice. Skinner et al. reported that 132 (58.0%) items of knowledge and skill were deemed essential as a minimum standard of clinical practice in critical care in Australia and New Zealand1). Twose et al. reported that 107 (47.7%) items were considered essential in the UK2). There are several possible reasons why Japanese physical therapists included more items as essential than experienced physical therapists in other Western countries. Physiotherapy in ICU in Japan has developed rapidly since 2017. As multi-disciplinary teams have developed with other critical care health professions, physical therapists may have felt that they needed a wider range of knowledge and skills to practice patient-centered care. Further research is needed to determine what expertise and skills are sought from physical therapists by physicians and nurses in ICU. Internationally, the role of physical therapists working in ICU has not been clearly defined. Their role in early mobilization and rehabilitation overlaps considerably with that of nurses3). It is therefore possible that physical therapists participating in this study thought they should have a broader range of knowledge and skills, comparable to those of nurses. The development of these minimum standards of clinical practice gives clear definition to the role of physical therapists in ICU. This may lead to quality assurance of physiotherapy in critical care and subsequently improve health outcomes for patients4).
Items that were considered essential in our survey but not in the UK and Australia included many cardiovascular items such as knowledge of calcium channel blockers, cardiac output measurements, pulmonary arterial catheter measurements, intra-aortic balloon pump and advanced electrocardiograms. In Japan, the Dohi-Anderson Criteria5,6) and the Guidelines for the Safety Management and Promotion of Rehabilitation Medicine of the Japanese Association of Rehabilitation Medicine7) have been traditionally used as risk management standards in the clinical setting. These include many cardiovascular-related indices, and the importance of cardiovascular indices has long been recognized as a risk management standard for rehabilitation. In addition, the evidence-based expert consensus for early rehabilitation in ICU published by the JSICM includes many cardiovascular items in the inception and cessation criterion for early mobilization and rehabilitation8,9). This may have caused Japanese physical therapists to consider that many cardiovascular items were essential.
Our survey also confirmed that it is essential for physical therapists to be able to accurately and independently evaluate and interpret the findings from imaging investigations including CT, magnetic resonance imaging MRI, and echocardiography. This was different from the surveys in the UK and Australia. One of the reasons for this is that Japanese hospitals have much higher rates of CT and MRI usage than in the UK and Australia. Physical therapists in Japan therefore have easier access to a variety of imaging test results. Japan has 111.49 CTs per million people (2017), which is very high compared to 9.46 in the UK (2014) and 67.2 in Australia (2018)10).
Only Japanese physical therapists considered that it was essential for them to be able to interpret and assess nutritional status including food administration, volume and type. The JSICM has recently published the Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients11,12). The Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN)13), and The European Society for Clinical Nutrition and Metabolism (ESPEN)14) have also published the guideline on clinical nutrition in the ICU. These guidelines strongly recommend nutritional management for severely ill patients according to their condition and stage. In severe sepsis, extensive burns, and severe trauma, the immune response from various hormones (e.g. cortisol, glucagon, and catecholamine) and proinflammatory cytokines that increase catabolism as a biological defense response leads to a rapid development of metabolic reactions and a hyper-catabolic state, leading to severe nutritional disorders15,16). The development of nutritional disorders (hyper-catabolism) worsens the prognosis, including infectious complications, increased mortality, and prolonged hospital stay17). One systematic review found that the rate of malnutrition in acutely ill patients was 38-78%, and malnutrition was an independent predictor of ICU length of stay, ICU readmission, infection incidence, and in-hospital mortality18). The decrease in skeletal muscle associated with poor nutritional status has a significant impact on prognosis19). It is therefore recommended that patients who are at high nutritional risk because of failure of oral intake or other reasons should be screened for nutrition early on during their stay in ICU and started on enteral or parenteral nutrition within 24-48 hours20). In Japan, from April 2020, a new reimbursement will be added for nutritional management, such as enteral nutrition, during the early stages of a stay in ICU, as a way to promote early discharge and return home. The rapid spread of understanding of the importance of nutritional management in intensive care in Japan may therefore have influenced the results of this study. In addition, the dietitians as part of the critical care team has become a regular feature of the critical care team, and there is a clear division of roles within the critical care team for each health professional in Australia and the UK. The presence of dietitians in critical care teams is still not that common although there is a strong interest in nutrition management in critical care in Japan. That might explain some of the difference.
Similarly, there was a greater interest in delirium, sedation and analgesia in Japan than in the UK and Australia. This may be partly because the JSICM recently translated “Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU (2018)”21) in addition to “Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the ICU (2013)”22), so there was heightened interest in this area by Japanese physical therapists during this Delphi process.
A further 48 items that JSICM-ICERC considered necessary for physical therapists in Japan were added to the survey. Japanese physical therapists considered recent concerns such as post-intensive care syndrome (PICS), pain assessment, and cognitive function assessment to be essential. They also believed that they should learn the basics of team medicine, including infection prevention, safety management, and communication within the team, including family members. Consideration of tubes, lines, restraining bands, conferences, bundles, and environmental maintenance were also important common items for the ICU team to promote early withdrawal and rehabilitation, and are characteristic of the minimum standards for physical therapy in Japan.
There were considerably more items considered essential by Japanese physical therapists than their counterparts in the UK and Australia. However, there were also some items that were not considered essential in Japan but were in the UK and Australia. For example, “Instillation of normal saline into the endotracheal tube (i.e. <20 ml in one treatment session aimed at increasing sputum yield by diluting and loosening thick secretions)” was considered essential in the UK. Oxygen therapy including initiation and titration of oxygen therapy, cough stimulation-oropharyngeal catheter stimulation, manual hyperinflation (MHI) and nasopharyngeal airway suctioning including insertion of nasopharyngeal airway were considered essential in both the UK and Australia. In Japan, physical therapists are not permitted to undertake these invasive interventions. This variation therefore reflects differences in national laws. Explaining the knowledge and skills of physical therapists elsewhere will improve understanding of physiotherapy among other healthcare professionals.
The activities, scope and role of physical therapists vary depending on national laws, culture and history. The results of this study provide views of Japanese physical therapists on the minimum standards of clinical practice for physical therapists working in ICU in Japan. However, intensivists, specialist nurses and certified nurses who work in ICU may have different views on what is required of physical therapists. Studies are currently investigating the knowledge and skills required by Japanese physical therapists working in ICU. Using this information, and the international comparisons here, JSICM-ICERC will be able to determine the minimum standards of clinical practice for physical therapists working in ICU in Japan. This will provide a rationale for developing entry-level educational materials for physical therapists and lead to quality assurance for physical therapists.
Conclusions
This study identified 199 items of knowledge and skills that are considered essential as minimum standards of clinical practice for physical therapists working in ICU in Japan. The findings of this study may help to define content on intensive care in undergraduate and post-graduate education programs in physiotherapy in Japan. The minimum standards of clinical practice for physical therapists working in ICU, as considered by experienced physical therapists in Japan, differed from those in the UK and Australia due to national laws, cultural and historical backgrounds. Further studies are necessary to show the utility of this framework in practice.
Funding
The authors received no financial support for the research, authorship, and/or publication with respect to this article.
Acknowledgments
The authors thank Watanabe Daisuke (Juntendo University Shizuoka Hospital) and Mori Yuji (Shizuoka Medical Center) for performing and completing the questionnaire surveys. We also thank Melissa Leffler, MBA, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
References
- 1. Skinner EH, Thomas P, et al.: Minimum standards of clinical practice for physiotherapists working in critical care settings in Australia and New Zealand: A modified Delphi technique. Physiother Theory Pract. 2016; 32: 468-482. [DOI] [PubMed] [Google Scholar]
- 2. Twose P, Jones U, et al.: Minimum standards of clinical practice for physiotherapists working in critical care settings in the United Kingdom: A modified Delphi technique. J Intensive Care Soc. 2019; 20: 118-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaboyer W, Gass E, et al.: Patterns of chest physiotherapy in Australian intensive care units. Journal of Critical Care. 2004; 19: 145-151. [DOI] [PubMed] [Google Scholar]
- 4. Gallesio AO, Ceraso D, et al.: Improving quality in the intensive care unit setting. Critical Care Clinics. 2006; 22: 547-571. [DOI] [PubMed] [Google Scholar]
- 5. Anderson AD: The use of the heart rate as a monitoring device in an ambulation program: a progress report. Arch Phys Med Rehabil. 1964; 45: 140-146. [PubMed] [Google Scholar]
- 6. Dohi Y: Risk and management. Medicina. 1976; 13: 1068-1069. [Google Scholar]
- 7. Japanese Society of Rehabilitation Medicine: Guidelines for the Safety Management and Promotion of Rehabilitation Medicine. 2018. p112. [Google Scholar]
- 8. Takahashi T, Morisawa T, et al.: Current status and future development of acute and cardiac physiotherapies in Japan. Physical Therapy Research. 2020; 23: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ad Hoc Committee for Early Rehabilitation, The Japanese Society of Intensive Care Medicine: Evidence based expert consensus for early rehabilitation in the intensive care unit. J Jpn Soc Intensive Care Med. 2017; 24: 255-303. [Google Scholar]
- 10. Health Care Resources: Medical technology. OECD.Stat. [cited 2020 May 2]; Available from: https://stats.oecd.org/index.aspx?DataSetCode=HEALTH_STAT
- 11. The Committee on Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients, Japanese Society of Intensive Care Medicine: Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients. J Jpn Soc Intensive Care Med. 2016; 23: 185-281. [Google Scholar]
- 12. The Committee on Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients, Japanese Society of Intensive Care Medicine: Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients: Disease-Specific Nutrition Support Therapy. J Jpn Soc Intensive Care Med. 2017; 24: 569-591. [Google Scholar]
- 13. Taylor BE, McClave SA, et al.: Society of Critical Care Medicine; American Society of Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016; 44: 390-438. [DOI] [PubMed] [Google Scholar]
- 14. Singer P, Blaser AR, et al.: ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019; 38: 48-79. [DOI] [PubMed] [Google Scholar]
- 15. Hasselqren PO: Catabolic response to stress and injury: implications for regulation. World J Surg. 2000; 24: 1452-1459. [DOI] [PubMed] [Google Scholar]
- 16. Demling RH and Seigne P: Metabolic management of patients with severe burns. World J Surg. 2000; 24: 673-680. [DOI] [PubMed] [Google Scholar]
- 17. Ali NA, O'Brien JM Jr, et al.: Midwest Critical Care Consortium. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008; 178: 261-268. [DOI] [PubMed] [Google Scholar]
- 18. Lew CCH, Yandell R, et al.: Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. JPEN J Parenter Enteral Nutr. 2017; 41: 744-758. [DOI] [PubMed] [Google Scholar]
- 19. Moisey LL, Mourtzakis M, et al.: Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013; 17: R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McClave SA, Taylor BE, et al.: Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016; 40: 159-211. [DOI] [PubMed] [Google Scholar]
- 21. Devlin JW, Skrobik Y, et al.: Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018; 46: e825-e873. [DOI] [PubMed] [Google Scholar]
- 22. Barr J, Fraser GL, et al.: American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41: 263-306. [DOI] [PubMed] [Google Scholar]