Abstract
Tuberculosis (TB) remains one of the leading causes of death globally. Although abdominal or peritoneal TB is a recognised site for extrapulmonary TB to manifest, the diagnosis is often delayed due to the non-specific nature of the presenting clinical features. We present the diagnostically challenging case of a 32-year-old patient with recurrent episodes of fever and a non-productive cough that was initially treated as community-acquired pneumonia with oral antibiotics. A computed tomography scan of the thorax was unrevealing, aside from a large volume of ascites within the partially imaged upper abdomen. The patient did not report any abdominal symptoms and the abdominal examination was unremarkable. Subsequently, a transvaginal ultrasound, a contrast-enhanced computed tomography scan of the abdomen and pelvis, and magnetic resonance imaging of the abdomen and pelvis confirmed a large volume of ascites in the absence of any definite aetiology. A peritoneal biopsy was required before the diagnosis of peritoneal TB was eventually confirmed. This case highlights the importance of considering peritoneal TB in patients presenting with treatment-resistant chest symptoms and persistent pyrexia of undetermined aetiology, even in the absence of abdominal signs and symptoms.
Keywords: Tuberculosis, Ascites, Peritonitis, Infectious diseases, Tropical Medicine
Introduction
Tuberculosis (TB) is an airborne infection caused by its namesake pathogen, Mycobacterium tuberculosis. Despite developments in targeted antibiotic therapy and vaccines, it remains a significant cause of morbidity and mortality worldwide [1]. Globally, approximately 2 billion people are infected with TB and around 10% of these will develop active TB during their lifetime [2]. Although TB commonly presents with pulmonary signs and symptoms e.g. cough, fever and haemoptysis, the bacterium can spread via haematogenous and lymphatic routes, giving rise to extrapulmonary manifestations. Frequently reported sites of extrapulmonary TB infection include the central nervous system, the lymphatic system, the genitourinary system and the bones and joints [1].
Although peritoneal TB is a recognised site of extrapulmonary infection, the diagnosis is often delayed as it can be mistaken for intra-abdominal malignancies including primary peritoneal carcinoma and ovarian cancer [3]. This is due to the non-specific and insidious presentation of peritoneal TB [4]. Furthermore, tumour markers such as cancer antigen (CA)-125 can be elevated due to peritoneal involvement, complicating matters further [5]. Peritoneal TB has been associated with a mortality rate of up to 60% when undiagnosed however, prompt diagnosis and commencement of therapy can help successfully cure the condition [4].
We present the unusual case of a 32-year-old female who presented with fever and respiratory symptoms in the absence of any abdominal features. She was incidentally found to have a large volume of ascites on cross-sectional imaging of the chest and only after a rigorous work-up was the diagnosis of peritoneal TB confirmed. We highlight and discuss the challenges of diagnosing peritoneal TB, particularly in the absence of abdominal signs and symptoms.
Case report
A 32-year-old female presented to her local emergency department with a 6-week history of non-productive cough and fever. This was her third attendance, having previously been discharged twice on separate occasions with oral antibiotics for presumed community-acquired pneumonia. Despite completing both antibiotic courses, her symptoms had not abated. She denied any chest pain, haemoptysis or night sweats, however reported a recent travel history to India in the last year to visit her mother who had previously contracted and received treatment for TB. This presentation was prior to the COVID-19 pandemic. She reported a past medical history of type II diabetes mellitus (for which she was on metformin monotherapy) and menorrhagia. She had no drug allergies, did not smoke or consume alcohol/narcotics and was otherwise socially independent and in good health.
On assessment, she was tachycardic with a pulse rate of 118 beats per minute and febrile with a temperature of 38°C. She had a respiratory rate of 19 breaths per minute, oxygen saturation of 94% on room air and a pressure of 145/92 mm Hg. The cardiorespiratory and abdominal examinations were unremarkable. Specifically, there was no palpable cervical, axillary or inguinal lymphadenopathy.
Blood tests were suggestive of a non-specific inflammatory response. The results were as follows: haemoglobin level 110 g/L (110-150); white blood cell count 9.12 × 10^9/L (3.5-11.0); platelet count 473 × 10^9/L (140-400); INR 1.2 (0.9-1.12); sodium 132 mmol/L (135-145); potassium 4.3 mmol/L (3.5-5.1); urea 2.5 mmol/L (2.1-7.1); creatinine 75 umol/L (49-92); eGFR 82 mL/min; bilirubin 11 umol/L (0-21); alanine aminotransferase 16 unit/L (10-35); aspartate aminotransferase 34 unit/L (0-35); alkaline phosphatase 76 unit/L (0-129); albumin 32 g/L (35-50); C-reactive protein 194 mg/L (0-5); haemoglobin A1c 58 mmol/mol (20-42); lactate dehydrogenase level 202 unit/L (135-214); procalcitonin level 0.35 ug/L (0-0.5) and amylase 7 unit/L. In addition, serological tests for autoimmune antibodies, hepatitis, cytomegalovirus, Epstein Barr virus and human immunodeficiency virus were all negative.
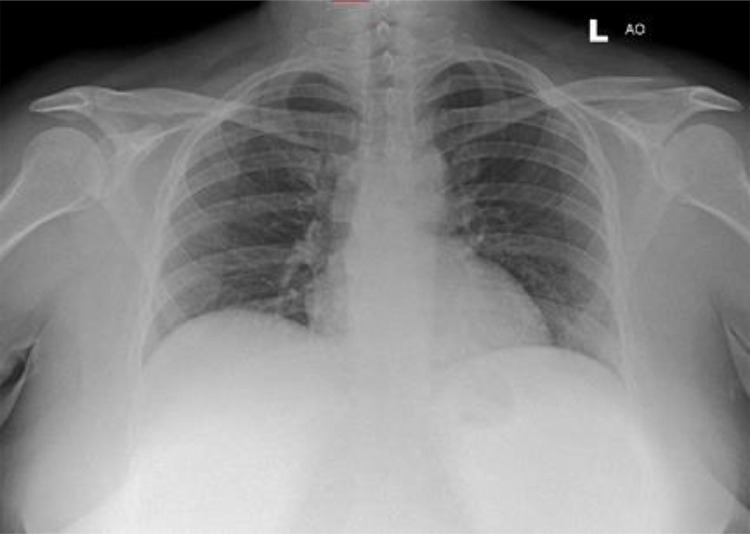
A chest radiograph was also obtained as part of the work-up (Fig. 1). Allowing for a suboptimal inspiratory effort, appearances were unchanged compared to recent studies and demonstrated clear lungs and pleural spaces with normal cardiomediastinal contours. In the absence of a definitive diagnosis to account for her persistent symptoms and abnormal serology, she was admitted under the medical team for further investigation.
Fig. 1.
AP chest radiograph acquired during the current presentation to the emergency department. Allowing for a suboptimal inspiratory effort, the cardiomediastinal contours remained within normal limits and the lungs and pleural spaces were clear. Specifically, there was no upper lobe predominant consolidation, cavitation or hilar abnormality
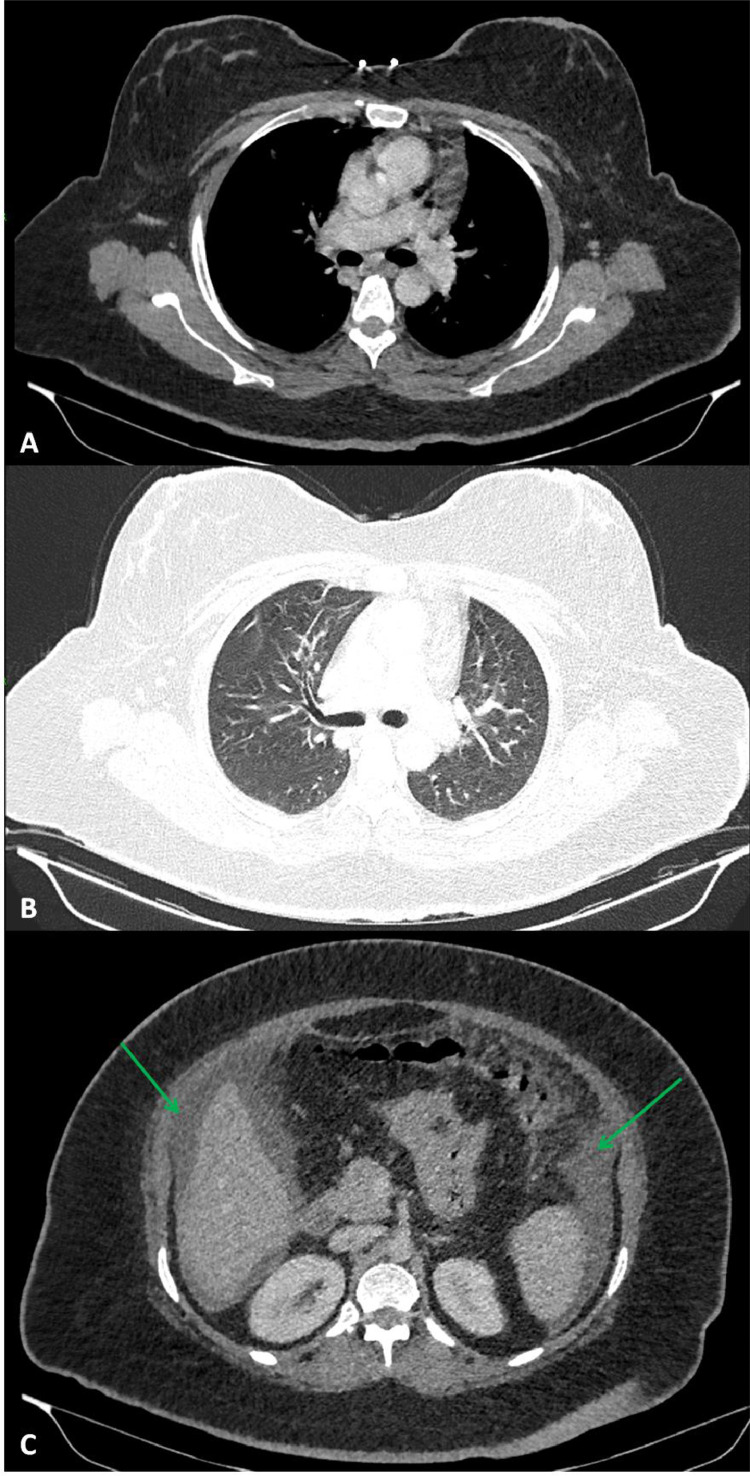
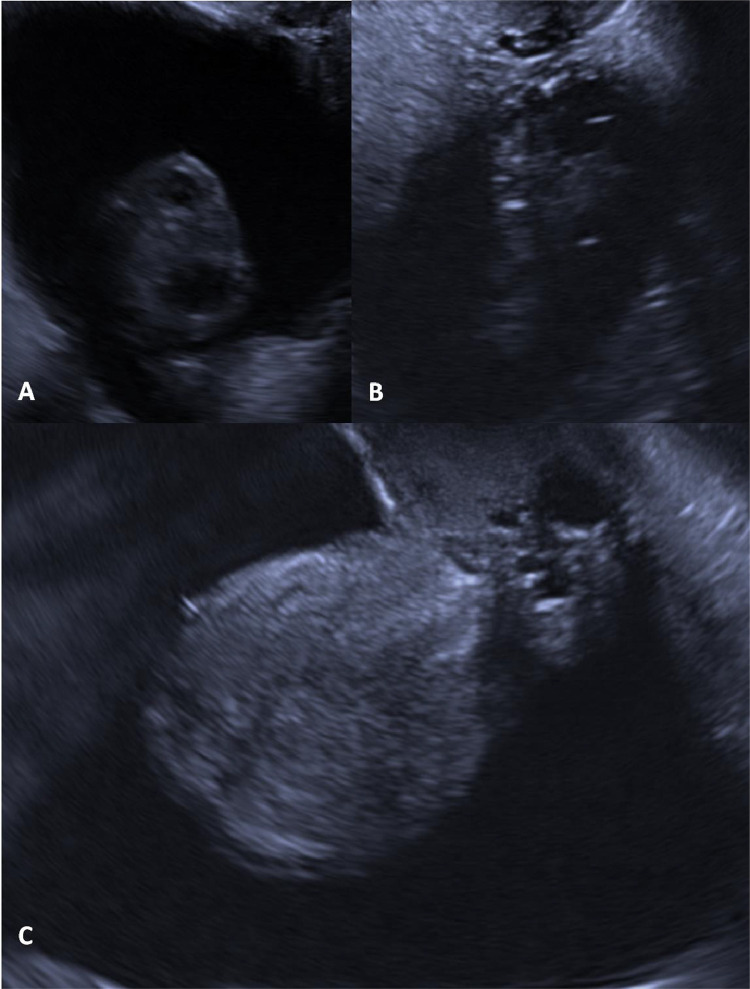
The next most appropriate investigation to determine an aetiology for the patient's symptoms was an unenhanced computed tomography (CT) scan of the thorax (Fig. 2). This demonstrated no intrathoracic pathology or enlarged lymph nodes however revealed a large volume of free fluid within the partially imaged upper abdomen. Following this unexpected finding, serological tests for tumour markers were requested as the prospect of an underlying malignant process became more likely. The results were as follows: alpha-fetoprotein level <0.8 kunits/L (0-6); CA-125 level 327 units/mL (0-35); CA19-9 level 23.5 unit/mL (0-34) and carcinoembryonic antigen level 1.5 ug/L (0-4). In this clinical context, an elevated CA-125 raised the clinical suspicion of a possible ovarian malignancy. In light of this, transvaginal and transabdominal ultrasound scans were performed which revealed a normal uterus and ovaries as well as a large volume of simple free fluid (Fig. 3).
Fig. 2.
Axial sections of the unenhanced CT thorax in A) soft-tissue windows, B) lung windows and C) soft-tissue windows of the partially imaged upper abdomen. These images demonstrate no size-significant hilar or mediastinal lymph nodes (A), normal lung parenchyma (B) and free intra-abdominal fluid within the subphrenic spaces bilaterally, indicated by green arrows (C)
Fig. 3.
Transvaginal ultrasound illustrating normal appearances of the right ovary (A), left ovary (B) and uterus (C). Note is made of the large volume of free-fluid surrounding the pelvic organs, which appears hypoechoic compared to the surrounding soft tissue
At this stage, an ultrasound-guided ascitic fluid aspirate was performed and the samples were sent for microscopy, culture and sensitivities as well as cytology. No organisms were cultured and cytology revealed lymphocytes, macrophages and reactive mesothelial cells but no malignant cells.
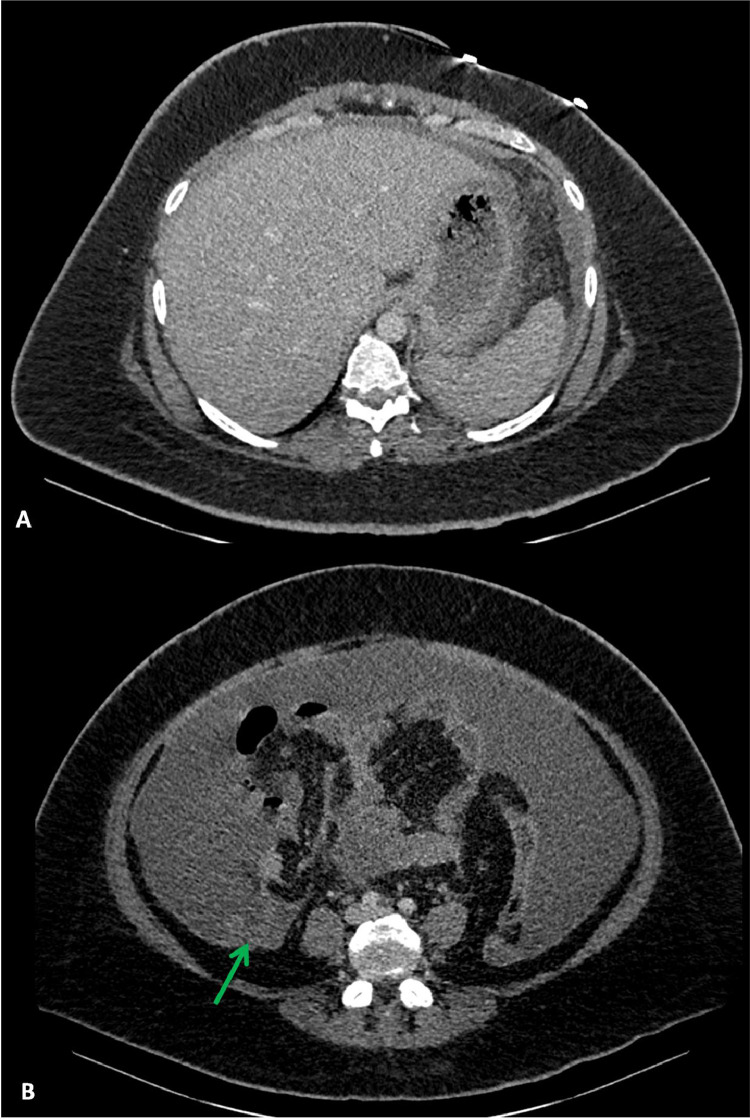
Following this, a portal-venous phase CT scan of the abdomen and pelvis was performed (Fig. 4). This revealed diffuse ascites and focal peritoneal thickening and/or nodularity in the right paracolic gutter. Further evaluation with an magnetic resonance imaging of the abdomen and pelvis revealed diffuse peritoneal and omental thickening in the absence of pathological intra-abdominal or pelvic lymph nodes. The appearance of the solid abdominal viscera and adnexa were unremarkable (Fig. 5). Whilst peritoneal TB was in the differential, the absence of pathological lymph node enlargement (with or without necrosis) favoured a diagnosis of primary peritoneal carcinoma.
Fig. 4.
Axial sections of the portal-venous phase CT abdomen and pelvis illustrating normal liver architecture and contour with no evidence of splenomegaly to suggest chronic liver disease or portal hypertension as a cause for the ascites (A) and diffuse ascites with focal peritoneal nodularity/thickening within the right paracolic gutter indicated by the green arrow (B). No intra-abdominal or pelvic lymphadenopathy was demonstrated
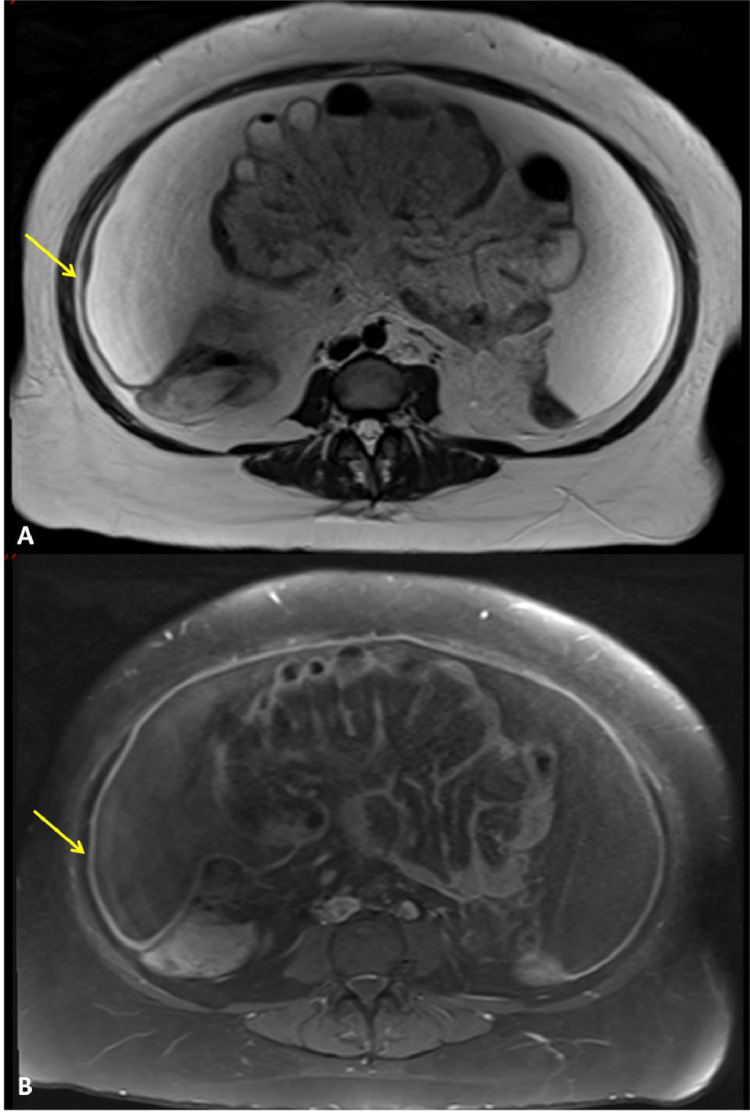
Fig. 5.
Axial magnetic resonance imaging sequences of the abdomen and pelvis that illustrate diffuse peritoneal and omental thickening which is low-signal in the T2-weighted sequence (A) and high-signal on the T1-weighted fat-saturated sequence (B), as indicated by the yellow arrows. A large volume of ascites can also be seen as large areas of increased signal intensity on (A) and low-signal on (B). No enlarged abdominal or pelvic lymph nodes were seen. Overall, findings were suspicious for peritoneal disease with both primary peritoneal carcinoma and abdominal tuberculosis in the differential
A peritoneal biopsy was subsequently performed in an attempt to reach a definitive histological diagnosis. This yielded samples of fibroadipose tissue with features of granulomatous inflammation, multinucleated giant cells and evidence of focal central necrosis. In addition, although the Ziehl-Neelsen and P-aminosalicylic acid stains were negative for microorganisms, cultures from the biopsy sample tested positive for Mycobacterium tuberculosis. This confirmed the diagnosis of peritoneal TB.
The patient was subsequently initiated on quadruple anti-tuberculous therapy containing a combination of rifampicin, isoniazid, pyrazinamide and ethambutol for 2 months. This was followed by an additional 4-month course of dual therapy with rifampicin and isoniazid, amounting to a 6-month duration of treatment in total. The patient was periodically reviewed during her treatment in the specialist TB clinic and went on to make a complete recovery.
Discussion
Abdominal TB is a common extrapulmonary site of TB infection. It accounts for between 0.1% and 0.7% of TB cases in developing countries [6]. It can involve the gastrointestinal tract, peritoneum, mesenteric lymph nodes or genitourinary system [7]. In 1 Indian retrospective study [8] with 48 cases of abdominal TB, the most common presenting complaint was abdominal pain (100%), followed by anorexia (98%) and fever (88%). Ascites was present in 40% of the cases in this series. Of note, 10 of the 48 cases also had abnormal chest radiographs with findings including cavitatory lesions, pleural effusions and hilar enlargement. Another retrospective Indian study [9] of 105 patients with abdominal TB also found the most common presenting complaint to be abdominal pain (78%) followed by fever (43%). Of note, not a single patient with abdominal TB in either of these studies presented with a cough, highlighting the atypicality of the presenting features in this case study.
Abdominal TB is often referred to as ‘the great mimicker’ due to its ability to affect single abdominal organs without chest involvement, as was the case in this case study [10]. Peritoneal TB is the most common form of abdominal TB [11]. It is thought to occur due to haematogenous spread however may also be secondary to lymph node rupture, gastrointestinal dissemination or tubal involvement [12]. Peritoneal TB is conventionally divided into wet, dry, and fibrotic-fixed types although there is considerable overlap in their CT appearances [12]. The wet type presents as free or loculated ascites associated with smooth, diffuse peritoneal thickening. The dry type has peritoneal and mesenteric thickening with caseous nodules, lymph node enlargement and fibrinous adhesions. The fibrotic-fixed type is characterised by omental thickening and entanglement of bowel loops, clinically resembling a mass, with or without ascites [11]. Whilst the radiological features of the 3 different forms can be seen in the same patient, distinguishing between the subtypes does not affect management or prognosis [13].
A 2020 systematic review and meta-analysis published by Chen et al [14] analysed 6 studies containing a total of 656 patients who either had peritoneal TB or peritoneal carcinomatosis. The analysis found that the radiological feature with the highest diagnostic discriminating value between peritoneal TB and peritoneal carcinomatosis was the presence of smooth peritoneal thickening (as opposed to the irregular and/or nodular thickening typically found in peritoneal carcinomatosis). The sensitivity of smooth peritoneal thickening as a discriminator is limited by the fact that it can also be seen in pyogenic peritonitis, primary peritoneal mesothelioma or peritoneal lymphomatosis. Lymph node necrosis or calcification and mesenteric macro nodules were found to have good specificity but limited sensitivity for differentiating peritoneal TB from peritoneal carcinomatosis [14]. The omental rim sign on CT imaging has also been shown to have a radiological role in distinguishing between peritoneal TB and peritoneal carcinomatosis. In a prospective study of 85 peritoneal TB patients and 168 peritoneal carcinomatosis patients, the sign was found to be both sensitive and specific for peritoneal TB [15].
The most common CT findings in peritoneal TB include ascites, smooth peritoneal thickening with marked contrast enhancement, densification of the mesenteric root fat planes and lymph node enlargement with areas of central necrosis or calcification [11]. Although absent in this case study, abdominal lymphadenopathy is seen in 55%-66% of patients with abdominal TB [12]. 40%-60% of these patients have enlarged nodes with hypoattenuating centres and hyperattenuating enhancing rims on CT; findings that are consistent with but not pathognomonic for caseous necrosis. The most common CT findings in peritoneal carcinomatosis include nodular and irregular peritoneal thickening, homogenous retroperitoneal lymph node enlargement and omental cake [11]. Omental cake appearance however is non-specific and can also be seen in peritoneal TB, although a “smudged” omentum is more likely [9].
CA-125 is a non-specific marker of peritoneal inflammation [5] and can be elevated to a similar degree in both peritoneal TB and metastatic ovarian cancer. The tumour marker has even been proposed as a potential parameter to evaluate treatment success for TB peritonitis [16]. In this case study, a moderately elevated CA-125 led the work-up in the direction of ovarian malignancy. This was excluded prior to the decision to perform a peritoneal biopsy.
The diagnosis of peritoneal TB can be challenging, particularly in the absence of any pulmonary symptoms [17]. Several tests have been designed to detect M tuberculosis in ascites. Polymerase chain reaction is 1 such test, however it has a sensitivity of 48% in smear-negative patients. X-pert MTP/RIF assay is a nucleic acid amplification test that is commonly used to diagnose tuberculous meningitis but has low sensitivity when diagnosing abdominal TB. Adenosine deaminase (ADA) levels above 30 IU/L in the ascitic fluid has been shown to have 100% sensitivity and 97.2% specificity for diagnosing peritoneal TB. ADA is a purine degrading enzyme that is essential for T lymphocyte proliferation and differentiation, a process that is necessary for the immune response against M tuberculosis. T-cell-based interferon gamma release assay (IGRA) is another non-invasive test that could be used as a substitute for tuberculin skin test [18],[2]. In 1 study, IGRA was found to have sensitivity between 72-90% and specificity between 68%-82% [18]. While having lower sensitivity and specificity than ADA, IGRA is sometimes used first as it doesn't require an invasive procedure to obtain ascitic fluid. The gold standard for diagnosing peritoneal TB is biopsy obtained either by laparoscopy or laparotomy [19]. Peritoneal TB is generally medically treated with rifampin, isoniazid, pyrazinamide and ethambutol. Surgical interventions are only required in bowel perforation, intestinal obstruction, abscess and haemorrhage [2].
In conclusion, this case corroborates the known difficulty in radiologically distinguishing peritoneal TB from peritoneal carcinomatosis and illustrates the need for tissue sampling to obtain a definitive diagnosis. This case also highlights that a high index of suspicion as well as a rigorous investigative process is often required to diagnose abdominal TB. The diagnosis should be considered in all patients with persistent, treatment-resistant pyrexia of undetermined aetiology and a compatible travel history, even in the absence of any localising abdominal features.
Patient consent
Informed consent obtained from patient to publish the case report and use images. We have ensured anonymity of clinical and graphical data used.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. American Society for Microbiology (ASM)[cited 2021 Mar 23]Available from: /pmc/articles/PMC164219/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz J., Ganji M., Canha C., Isache C. A challenging diagnosis of ascites: a case report of peritoneal tuberculosis. Case Rep Infect Dis. 2018;2018:1–3. doi: 10.1155/2018/8136476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri S., Gupta S., Suri R. Computed tomography in abdominal tuberculosis. Brit J Radiol. 1999;72:92–98. doi: 10.1259/bjr.72.853.10341698. https://www.birpublications.org/doi/abs/10.1259/bjr.72.853.10341698 [cited 2021 Feb 14]Available from: [DOI] [PubMed] [Google Scholar]
- 4.Chau T.N., Leung V.K.S., Wong S., Siu T.L., Wai H.C., Luk I.S.C. Diagnostic challenges of tuberculosis peritonitis in patients with and without end-stage renal failure. Clin Infect Dis. 2007;45(12):e141–e146. doi: 10.1086/523727. https://academic.oup.com/cid/article-lookup/doi/10.1086/523727 [cited 2021 Feb 14]Available from: [DOI] [PubMed] [Google Scholar]
- 5.Varghese A., Fader A., Wilbur M.A., Salimian K., Azadi J.R., Johnson P.T. Peritoneal tuberculosis: the great mimicker. Int J Gynecol Cancer. 2020;30(4):546–550. doi: 10.1136/ijgc-2020-001291. http://ijgc.bmj.com/ [cited 2021 Feb 14]Available from: [DOI] [PubMed] [Google Scholar]
- 6.Bulut Gökten D., Katipoglu B., Basara E., Ates I., Yılmaz N. A case report of peritoneal tuberculosis: a challenging diagnosis. Case Rep Infect Dis. 2018;2018:1–3. doi: 10.1155/2018/4970836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden M.P., Vikram H.R. Extrapulmonary tuberculosis: an overview - American Family Physician. Am Fam Physician. 2005;72 www.aafp.org/afpAmericanFamilyPhysician1761 [cited 2021 Feb 14]. Available from: [PubMed] [Google Scholar]
- 8.Awasthi S., Saxena M., Ahmad F., Kumar A., Dutta S. Abdominal tuberculosis: a diagnostic dilemma. J Clin Diagnostic Res. 2015;9(5):EC01–EC03. doi: 10.7860/JCDR/2015/13350.5887. [cited 2021 Mar 23]Available from: /pmc/articles/PMC4484072/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande S.S., Joshi A.R., Deshpande S.S., Phajlani S.A. Computed tomographic features of abdominal tuberculosis: unmask the impersonator! Abdom Radiol. 2019;44(1):11–21. doi: 10.1007/s00261-018-1700-3. https://link.springer.com/article/10.1007/s00261-018-1700-3 [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]
- 10.Debi U., Ravisankar V., Prasad K.K., Sinha S.K., Sharma A.K. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20:14831–14840. doi: 10.3748/wjg.v20.i40.14831. [cited 2021 Feb 14]Available from: /pmc/articles/PMC4209546/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdominal tuberculosis: a radiological review with emphasis on computed tomography and magnetic resonance imaging findings. [cited 2021 Mar 23]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4492571/ [DOI] [PMC free article] [PubMed]
- 12.Burrill J., Williams C.J., Bain G., Conder G., Hine A.L., Misra R.R. Tuberculosis: a radiologic review. Radiographics. 2007;27:1255–1273. doi: 10.1148/rg.275065176. www.rsna.org/rsnarights [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]
- 13.Ladumor H., Al-Mohannadi S., Ameerudeen F.S., Ladumor S., Fadl S. TB or not TB: a comprehensive review of imaging manifestations of abdominal tuberculosis and its mimics. Clin Imaging. 2021;76:130–143. doi: 10.1016/j.clinimag.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Liu S., Tang Y., Zhang X., Cao M., Xiao Z. Diagnostic performance of CT for differentiating peritoneal tuberculosis from peritoneal carcinomatosis: a systematic review and meta-analysis. Clin Radiol. 2020;75(5):396.e7–396.e14. doi: 10.1016/j.crad.2019.12.014. http://www.clinicalradiologyonline.net/article/S0009926020300076/fulltext [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]
- 15.Ramanan R.V., Venu V. Differentiation of peritoneal tuberculosis from peritoneal carcinomatosis by the Omental Rim sign. A new sign on contrast enhanced multidetector computed tomography. Eur J Radiol. 2019;113:124–134. doi: 10.1016/j.ejrad.2019.02.019. http://www.ejradiology.com/article/S0720048X19300725/fulltext [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]
- 16.Mas M.R., Comert B., Saglamkaya U., Yamanel L., Kuzhan O., Ateskan U. CA-125; a new marker for diagnosis and follow-up of patients with tuberculous peritonitis. Dig Liver Dis. 2000;32(7):595–597. doi: 10.1016/s1590-8658(00)80841-5. http://www.dldjournalonline.com/article/S1590865800808415/fulltext [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]
- 17.Kashyap R.S., Saha S.M., Nagdev K.J., Kelkar S.S., Purohit H.J., Taori G.M. Diagnostic markers for tuberculosis ascites: A preliminary study. Biomark Insights. 2010;2010(5):87–94. doi: 10.4137/bmi.s5196. https://pubmed.ncbi.nlm.nih.gov/20838606/ [cited 2021 Mar 23]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahmi M.N., Harti A.P. A diagnostic approach for differentiating abdominal tuberculosis from ovarian malignancy: a case series and literature review. BMC Proc. 2019;13:13. doi: 10.1186/s12919-019-0180-y. https://bmcproc.biomedcentral.com/articles/10.1186/s12919-019-0180-y [cited 2021 Mar 23]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan L., Chen Z., Hao X.-H., Hu Z.-Y., Xiao H-P. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol Med Microbiol. 2012;65(3):456–466. doi: 10.1111/j.1574-695X.2012.00972.x. https://academic.oup.com/femspd/article-lookup/doi/10.1111/j.1574-695X.2012.00972.x [cited 2021 Mar 23]Available from: [DOI] [PubMed] [Google Scholar]