Abstract
Intracranial solitary fibrous tumor/hemangiopericytoma (SFT/HPC) is a relatively rare type of tumor that originates from meningeal mesenchyme. A 30-year-old man presented leaning his body to the left and with weakness of his left lower limb. Computed tomography revealed a heterogeneous mass with multiple cystic components and hyperostosis of the right cranial convexity. Magnetic resonance imaging showed the mass was broadly attached to the dura matter with dural tail sign. In addition, the lesion had extensive cystic degeneration and a solid compartment showing low apparent diffusion coefficient values. The patient underwent gross total resection of the intracranial lesion and presented no recurrence within a 12-month follow-up period. Histopathology confirmed SFT/HPC (World Health Organization grade Ⅱ). Although there have been several useful techniques reported to differentiate SFT/HPC from meningioma, in this case the atypical findings for SFT/HPC made it difficult. We report the imaging findings of this case and some literature reviews.
Keywords: Intracranial solitary fibrous tumor/hemangiopericytoma (SFT/HPC), Meningioma, Diffusion-weighted imaging
Introduction
Intracranial solitary fibrous tumor/hemangiopericytoma (SFT/HPC) originates from meningeal mesenchyme. It is a rare type of tumor and makes up less than 1% of all intracranial neoplasms [1], [2], [3]. Because SFT/HPC and meningiomas present with similar imaging findings, they are often difficult to differentiate [4,5]. However, they do require significantly different treatment methods and surgical planning and have significantly different prognoses. Compared with meningioma, SFT/HPC has a much more aggressive biological behavior, and it often recurs after surgical resection (60%) and metastasizes to extracranial organs (20%) [2,6]. Therefore, in order to determine an appropriate treatment, it is important to differentiate between SFT/HPC and meningioma. We report a case of SFT/HPC, which was difficult to distinguish from meningioma based on imaging findings, with some literature review.
Case report
A 30-year-old man had been taking internal medicine for schizophrenia, prescribed by a local doctor, for 6 years. He presented with his body leaning to the left and weakness in the left upper and lower limbs. A head computed tomography was performed, which revealed a mass in the cranium, and he was referred to our hospital for neurosurgical treatment.
At our hospital, he presented with impaired consciousness (Japan Coma ScaleⅠ-1,Glasgow Coma Scale E4V5M5). Left facial nerve palsy and dysarthria were observed, and muscle strength of the left upper and lower limbs was reduced (Manual Muscle Test left upper limbs, 3/5; left lower limbs, 3/5). Biochemical investigations were unremarkable.
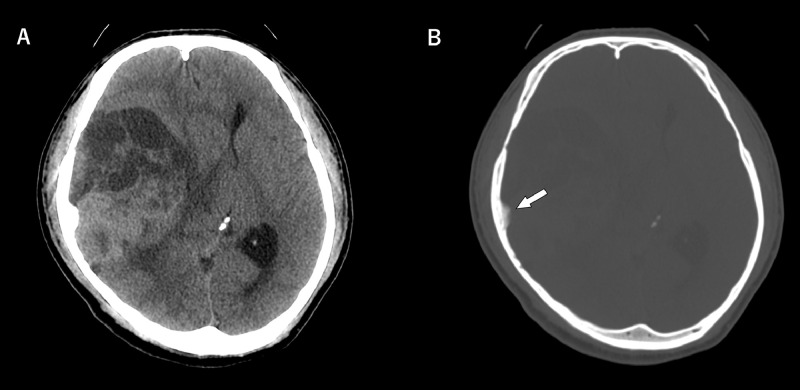
Non-enhanced computed tomography (Fig. 1) revealed a 3.5 × 9.3 × 7.4 cm solid and cystic mass adjacent to the right temporal lobe attached to the right cranial convexity. The solid part showed higher attenuation than the gray matter. The tumor showed the remarkable mass effect with the midline shift from the right side to the left, and the midbrain was also highly compressed with a risk of transtentorial hernia. The temporal bone bordering the lesion showed bony thickening, which was considered to be a finding of hyperostosis.
Fig. 1.
Preoperative non-enhanced computed tomography (NECT).
(A) Axial NECT head image showed a solid and cystic mass, compressing the right temporal lobe, adjacent to the right cranial convexity. The solid part showed higher density than the brain parenchyma. The mass effect was marked with a midline shift from right to left.
(B) Axial NECT bone image revealed hyperostosis in contact with the lesion (white arrow).
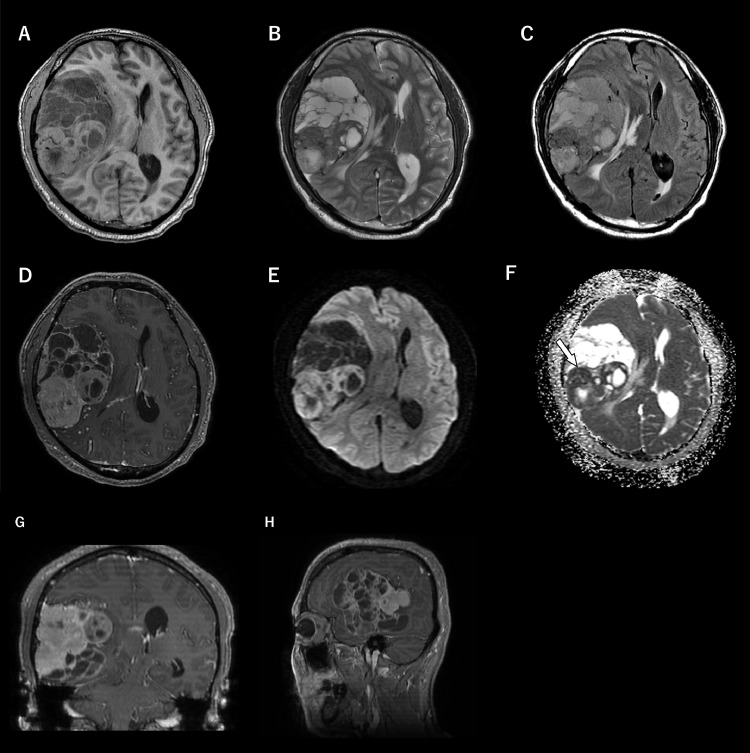
Magnetic resonance imaging (Fig. 2) demonstrated an extra axial tumor attached to the right cranial convexity with an oval shape and a well-defined margin. Mild edema was also observed in the temporal lobe, and there were extensive polycystic components in the anterior half of the lesion. The solid partitions demonstrated iso- to high intense on a T1-weighted image (T1WI) and low to slightly high on a T2-weighted image (T2WI) in comparison with cortical gray matter. Cystic areas and signal void vessels were also observed in the lesion. Postcontrast enhanced T1WI showed heterogeneous mild to well-defined enhancement with the dural tail sign. Corresponding apparent diffusion coefficient (ADC) maps showed relatively restricted diffusion (minimum ADC value = 0.79 × 10−3 mm2/s).
Fig. 2.
Preoperative magnetic resonance imaging.
(A–C) Axial images of T1WI, T2WI, and FLAIR (A, T1WI; B, T2WI; C, FLAIR) showed a heterogeneous mass with multiple cystic components adjacent to the right temporal lobe. The solid component demonstrated iso to high intense on T1WI (A) and low to slightly high intense on T2WI (B) in comparison with the cortical gray matter.
(D, G, H) Contrast-enhanced T1WI (D, axial; G, coronal; H, sagittal) showed heterogeneous and good enhancement with the dural tail sign.
(E) DWI showed the solid compartment with a high signal.
(F) The measured minimum ADC value on ADC map was 0.79 × 10-3 mm2/s (white arrow).
Because of the dural tail sign and hyperostosis, the cystic component occupying nearly half of the lesion, and the low ADC value in the solid area, we suspected a cystic meningioma. The lesion had a marked mass effect with a risk of transtentorial hernia, and it was promptly removed. The patient underwent gross total resection of the intracranial lesion, and he tolerated the procedure well; his symptoms and clinical effect improved postoperatively. A postoperative MRI confirmed gross total resection. Twelve months have passed without recurrence since the operation.
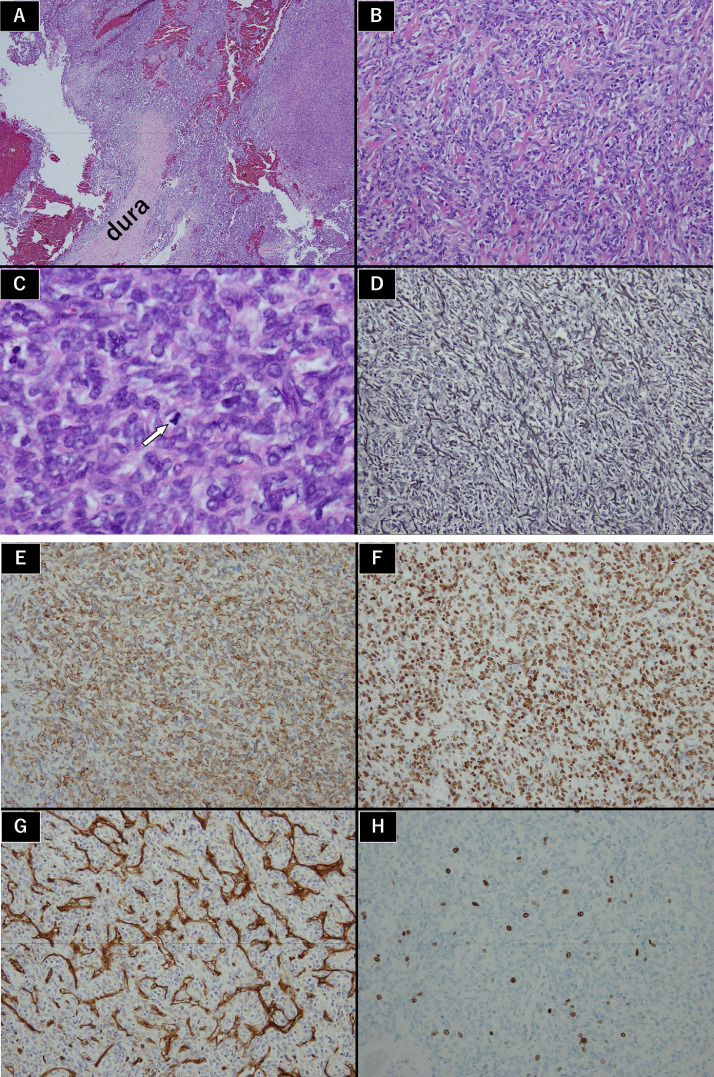
On microscopic examination (Fig. 3), his tumor cells exhibited a diffuse growth pattern with abundant slit-shaped vessels in the central area. Silver staining revealed linear structures that were thought to be collagen fibers. Mitosis exceeded 5 or less in 10 high power fields. Immunohistochemistry examination was positive for vimentin, signal transducer and activator of transcription 6 (STAT6), and CD34 and negative for epithelial membrane antigen, S-100, CD31, desmin, CKA1, A3, and glial fibrillary acidic protein. Ki-67 staining showed a proliferative index of 15%. These findings led to a diagnosis of SFT/HPC (World Health Organization [WHO] grade Ⅱ, HPC phenotype).
Fig. 3.
Histological findings
(A–C) H&E stain (A, 40 ×; B, 100 ×; C, 400 ×) showed tumor cells presenting a diffuse growth pattern with abundant slit-shaped vessels in the central area. Ten high power fields (400 ×) revealed mitosis 5 or less (white arrow).
(D) Silver staining (100 ×) revealed linear structures indicating collagen fibers.
(E–H) Immunohistochemistry examination of vimentin (E, 100 ×), STAT6 (F, 100 ×), and CD34 (G, 100 ×) was positive. Ki-67 staining (H, 100 ×) showed a proliferative index of 15%. No other immunostainings were positive.
Discussion
The 2016 Central Nervous System WHO update merged the entities of meningeal SFT/HPC into a single entity based on the presence of the nerve growth factor 1A binding protein 2- STAT6 gene fusion in these tumors. In our case, immunohistochemical staining with STAT6 resulted in a strong nuclear positivity, confirming the diagnosis [1].
SFT/HPC and meningioma exhibit similar radiological features; however, their prognoses are different. SFT/HPC is much more biologically aggressive and often recurs after surgical resection (60%) and metastasizes to extracranial organs (20%) [2,6]. In order to determine treatment and follow-up, it is important to differentiate between the 20 preoperatively.
In general, SFT/HPC appears as isointense to slightly high on T1WI and as isointense on T2WI, compared with gray matter. Conversely, meningiomas are usually hypo- to isointense compared with the cerebral cortex on T1WI and iso- to hyperintense on T2WI. There are some imaging features that can be used to differentiate SFT/HPC from meningioma. Specifically, less frequent calcification, a narrow-based attachment, lack of dural tail sign, and frequent presentation of “flow void” sign are more frequent in SFT/HPC than in meningioma. SFT/HPC tends to be aggressive, with behavior such as apparent parenchymal invasion, irregular or multi-lobulated borders, bone erosion, and heterogeneous contrast enhancement. However, these imaging features cannot be used to reliably differentiate SFT/HPC from meningioma, and previous studies have reported a low diagnostic rate accuracy [7], [8], [9], [10], [11], [12], [13], [14], [15]. In the present case, the dural tail sign and hyperostosis made it difficult to differentiate SFT/HPC from meningioma.
Meningiomas characteristically demonstrate iso- to hypointensity on T1WI and iso- to hyperintensity on T2WI compared with gray matter [16]. Some meningiomas may appear as hyperintense on T1WI. High signal intensity on T1WI in meningiomas is thought to be associated with hemorrhage, high lipid content in tumor cells, and calcification. Thus, lipomatous meningioma is a histological variant of meningiomas that shows hyperintensity on T1WI [17,18]. The present case presented hyperintensity on T1WI compared with gray matter, but it was not fat, hemorrhage, or calcification, and therefore, with respect to the T1WI signal, this finding may have been more suggestive of SFT/HPC than meningioma.
Approximately 3%-5% of adult meningiomas are so-called “cystic meningiomas,” which are meningiomas with a cystic component [19], [20], [21]. Although cases of HPC with cystic degeneration have been reported [22], there have been few reports [23,24] of cystic degeneration in nearly half of the lesions, as in this case. Widespread cystic degeneration may be 1 factor that makes the differentiation between SFT/HPC and meningioma difficult.
There are several reports comparing ADC between SFT/HPC and meningioma. Liu et al. [25] found a significant difference, with min ADC of 1.116 (±0.127) × 10−3 mm2/s for HPC and 0.875 (±0.104) × 10−3 mm2/s for meningioma. Jai et al. [26] reported that there was a significant difference between HPC and meningioma in mean ADC (1163.23 ± 134.47 mm2/s for SFT/HPC and 863.94 ± 63.55 mm2/s for meningioma) but not in min ADC.
On the other hand, some reports suggest that ADC values are variable depending on the histological system of the meningioma and the grade of the SFT/HPC. Liu et al. [27] compared the ADC levels of HPC and angiomatous and anaplastic (WHO grade Ⅲ) meningiomas. Mean ADC values were significantly different between HPC and anaplastic meningioma (1.17 ± 0.30 × 10−3 mm2/s and 0.75 ± 0.11 × 10−3 mm2/s, respectively). Mean ADC values did not differ significantly between angiomatous meningioma and HPC (the ADC value of angiomatous meningioma was 1.23 ± 0.25 × 10−3 mm2/s, P > 0.05). Furthermore, Kanazawa et al. [28] reported the mean ADC value was significantly high in angiomatous meningioma compared with SFT/HPC and other WHO grade I meningioma (1.214 ± 0.213 × 10−3 mm2/s, 0.972 ± 0.154 × 10−3 mm2/s, and 0.941 ± 0.119 × 10−3 mm2/s, respectively). Mama [29] reported that all grade II HPCs showed higher ADC values than grade III (range 1.26–1.50 × 10−3 mm2/s and 0.638–0.833 × 10−3 mm2/s, respectively).
According to previous reports, most of which were on HPC phenotype, ADC values, which vary depending on the histological type and grade, are generally higher in SFT/HPC than in meningioma. Therefore, these values might be better used only as a reference for the differentiation of SFT/HPC and meningioma.
In conclusion, differentiating SFT/HPC from meningioma is sometimes difficult. Our case of SFT/HPC with multiple findings suggesting meningioma, including dural tail sign, hyperostosis, extensive cystic degeneration, and relatively low ADC values, appears to be very rare. However, focusing on the T1WI findings might have allowed us to consider SFT/HPC as a differential disease.
Patient consent
Informed consent was obtained from the patient.
Footnotes
Competing Interests: The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Louis DN. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Ratneswaren T. Surveillance for metastatic hemangiopericytoma-solitary fibrous tumors-systematic literature review on incidence, predictors and diagnosis of extra-cranial disease. J Neurooncol. 2018;138(3):447–467. doi: 10.1007/s11060-018-2836-2. [DOI] [PubMed] [Google Scholar]
- 3.Kinslow CJ. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol. 2018;138(1):173–182. doi: 10.1007/s11060-018-2787-7. DOI: 10.1002/1097-0142(19930801)72:3<639::aid-cncr2820720304>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Meng Y. Preoperative radiologic characters to predict hemangiopericytoma from angiomatous meningioma. Clin Neurol Neurosurg. 2015;138:78–82. doi: 10.1016/j.clineuro.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Smith AB. From the radiologic pathology archives: mass lesions of the dura: beyond meningioma-radiologic-pathologic correlation. Radiographics. 2014;34(2):295–312. doi: 10.1148/rg.342130075. [DOI] [PubMed] [Google Scholar]
- 6.Chiechi MV. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996;17(7):1365–1371. PMID: 8871726. [PMC free article] [PubMed] [Google Scholar]
- 7.Huang RY. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol. 2019;21(Suppl 1):i44–i61. doi: 10.1093/neuonc/noy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosentino CM. Giant cranial hemangiopericytoma: MR and angiographic findings. AJNR Am J Neuroradiol. 1993;14(1):253–256. PMID: 8427100. [PMC free article] [PubMed] [Google Scholar]
- 9.Alén JF. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien) 2001;143(6):575–586. doi: 10.1007/s007010170062. [DOI] [PubMed] [Google Scholar]
- 10.Fountas KN. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29(2):145–153. doi: 10.1007/s10143-005-0001-9. Epub 2006 Jan 4. [DOI] [PubMed] [Google Scholar]
- 11.Wu W. Hemangiopericytomas in the central nervous system. J Clin Neurosci. 2009;16(4):519–523. doi: 10.1016/j.jocn.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Brunori A. Recent experience in the management of meningeal hemangiopericytomas. Tumori. 1997;83(5):856–861. doi: 10.1177/030089169708300516. PMID: 9428922. [DOI] [PubMed] [Google Scholar]
- 13.Younis GA. Aggressive meningeal tumors: review of a series. J Neurosurg. 1995;82(1):17–27. doi: 10.3171/jns.1995.82.1.0017. [DOI] [PubMed] [Google Scholar]
- 14.Payne BR. Gamma surgery for hemangiopericytomas. Acta Neurochir (Wien) 2000;142(5):527–536. doi: 10.1007/s007010050465. discussion 536-7. [DOI] [PubMed] [Google Scholar]
- 15.Osborne DR. Primary intracranial meningeal and spinal hemangiopericytoma: radiologic manifestations. AJNR Am J Neuroradiol. 1981;2(1):69–74. PMID: 6784553. [PMC free article] [PubMed] [Google Scholar]
- 16.Buetow M P. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11(6):1087–1106. doi: 10.1148/radiographics.11.6.1749851. [DOI] [PubMed] [Google Scholar]
- 17.Cakirer Sinan. Spontaneously T1-hyperintense lesions of the brain on MRI: a pictorial review. Curr Probl Diagn Radiol. 2003;32(5):194–217. doi: 10.1016/s0363-0188(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 18.Maiuri F. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999;31(1):69–75. doi: 10.1016/s0720-048x(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 19.De Jesús O. Cystic meningiomas: a review. Neurosurgery. 1995;36(3):489–492. doi: 10.1227/00006123-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Boukobza M. Cystic meningioma: radiological, histological, and surgical particularities in 43 patients. Acta Neurochir (Wien) 2016;158(10):1955–1964. doi: 10.1007/s00701-016-2898-x. [DOI] [PubMed] [Google Scholar]
- 21.Amin OS. Cystic meningioma. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207690. 2015 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spatola C. Recurrent intracranial hemangiopericytoma with extracranial and unusual multiple metastases: case report and review of the literature. Tumori. 2004;90(2):265–268. doi: 10.1177/030089160409000222. PMID: 15237597. [DOI] [PubMed] [Google Scholar]
- 23.Pang H. Morphologic patterns and imaging features of intracranial hemangiopericytomas: a retrospective analysis. Onco Targets Ther. 2015;8:2169–2178. doi: 10.2147/OTT.S85971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X. Magnetic resonance features of meningeal solitary fibrous tumors. Oncol Lett. 2018;15(6):8825–8832. doi: 10.3892/ol.2018.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G. Intracranial hemangiopericytoma: MR imaging findings and diagnostic usefulness of minimum ADC values. J Magn Reson Imaging. 2013;38(5):1146–1151. doi: 10.1002/jmri.24075. [DOI] [PubMed] [Google Scholar]
- 26.Shankar Jai Jai Shiva. Diffusion weighted imaging may help differentiate intracranial hemangiopericytoma from meningioma. J Neuroradiol. 2019;46(4):263–267. doi: 10.1016/j.neurad.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Liu Li. Comparison of ADC values of intracranial hemangiopericytomas and angiomatous and anaplastic meningiomas. J Neuroradiol. 2014;41(3):188–194. doi: 10.1016/j.neurad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa Tokunori. Preoperative prediction of solitary fibrous tumor/hemangiopericytoma and angiomatous meningioma using magnetic resonance imaging texture analysis. World Neurosurg. 2018;120:e1208–e1216. doi: 10.1016/j.wneu.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Mama N, Ben Abdallah A, Hasni I, Kadri K, Arifa N, Ladib M. MR imaging of intracranial hemangiopericytomas. J Neuroradiol. 2014;41(5):296–306. doi: 10.1016/j.neurad.2013.10.007. [DOI] [PubMed] [Google Scholar]