Key Points
Question
Are patient-reported outcomes, such as treatment satisfaction with medication, higher among patients treated with weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine?
Findings
In this open-label randomized clinical trial of 119 participants, the mean Treatment Satisfaction Questionnaire for Medication global score was significantly higher in the group receiving depot buprenorphine than in the group receiving sublingual buprenorphine at week 24.
Meaning
These findings suggest that treatment satisfaction and other patient-reported outcomes may serve as alternative end points to traditional markers of substance use when comparing different medications in addiction outcome studies.
This randomized clinical trial compares patient satisfaction between depot buprenorphine and sublingual buprenorphine in adult outpatients with opioid dependence.
Abstract
Importance
Patient-reported outcomes in the treatment of opioid dependence may differ between subcutaneously administered depot buprenorphine and daily sublingual buprenorphine.
Objective
To compare patient satisfaction between depot buprenorphine and sublingual buprenorphine in adult outpatients with opioid dependence.
Design, Setting, and Participants
This open-label, randomized clinical trial was conducted among adult patients with opioid dependence at 6 outpatient clinical sites in Australia from October 2018 to September 2019. Data analysis was conducted from October 2019 to May 2020.
Interventions
Participants were randomized to receive treatment with weekly or monthly depot buprenorphine or daily sublingual buprenorphine over 24 weeks.
Main Outcomes and Measures
The primary end point was the difference in global treatment satisfaction, assessed by the Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4 (range, 0-100; higher score indicates greater satisfaction) at week 24. Secondary end points included other patient-reported outcomes, including quality of life, treatment burden, and health-related outcomes, as well as measures of opioid use, retention in treatment, and safety.
Results
A total of 119 participants (70 [58.8%] men; mean [SD] age, 44.4 [10.5] years) were enrolled, randomized to, and received either depot buprenorphine (60 participants [50.4%]) or sublingual buprenorphine (59 participants [49.6%]). From the initial sample of 120, a participant (0.8%) in the sublingual buprenorphine group withdrew consent and did not receive study treatment. All participants were receiving sublingual buprenorphine when enrolled. The mean TSQM global satisfaction score was significantly higher for the depot group compared with the sublingual group at week 24 (mean [SE] score, 82.5 [2.3] vs 74.3 [2.3]; difference, 8.2; 95% CI, 1.7 to 14.6; P = .01). Improved outcomes were also observed for several secondary end points after treatment with depot buprenorphine (eg, mean [SE] treatment burden assessed by the Treatment Burden Questionnaire global score, on which lower scores indicate lower burden: 13.2 [2.6] vs 28.6 [2.5]; difference, −15.4; 95% CI, −22.6 to −8.2; P < .001). Thirty-nine participants (65.0%) in the depot buprenorphine group experienced 117 adverse drug reactions, mainly injection site reactions of mild intensity following subcutaneous administration, and 12 participants (20.3%) in the sublingual buprenorphine group experienced 21 adverse drug reactions. No participants withdrew from the trial medication or the trial due to adverse events.
Conclusions and Relevance
In this study, participants receiving depot buprenorphine reported improved treatment satisfaction compared with those receiving sublingual buprenorphine. The results highlight the application of patient-reported outcomes as alternative end points to traditional markers of substance use in addiction treatment outcome studies.
Trial Registration
anzctr.org.au Identifier: ANZCTR12618001759280
Introduction
Opioid dependence is a chronic relapsing disorder with considerable individual and global public health burden.1,2 The current standard of care for opioid dependence includes treatment with methadone or sublingual (SL) buprenorphine or buprenorphine-naloxone (hereafter, buprenorphine), combined with psychosocial and behavioral support.3,4 Both medications are associated with reductions in mortality, illicit opioid use, bloodborne viral infections, and criminal behavior as well as better cost-effectiveness than no treatment or psychosocial treatment alone.3,4,5 Buprenorphine is a partial μ-opioid receptor agonist, enabling office-based treatment for nonsupervised or take-home use of the medication.6,7 However, SL formulations of buprenorphine are prone to nonmedical use (eg, injecting, diversion), prompting models of care, particularly in the early phases of treatment, requiring regular attendance at clinics or pharmacies for administration of doses.8,9
Long-acting injectable depot buprenorphine formulations have been developed to mitigate some of the concerns of daily dosing. A monthly subcutaneous (SC) depot buprenorphine formulation has shown superiority to placebo, and a different weekly and monthly depot buprenorphine formulation with flexible doses has shown noninferiority and superiority to SL buprenorphine for illicit opioid use.10,11 These weekly and monthly SC depot buprenorphine formulations have also shown high levels of patient satisfaction in open-label follow-up and safety studies.12,13 No study has compared patient-reported outcomes (PROs) between treatment with depot buprenorphine and SL buprenorphine in a randomized clinical trial (RCT). Furthermore, the previous double-masked RCTs of depot buprenorphine required all participants to attend services to receive the active or placebo medication to maintain masking, thus obscuring potential differences in patient experiences.
There has been increased interest in using PROs as alternative end points to traditional biological markers of substance use (eg, urinalysis) for the assessment and regulatory approval of new medications.14 In opioid dependence, most RCTs have only included general PROs as exploratory end points, and a disorder-specific quality-of-life instrument for patients treated with opioid agonists has only recently been developed.15 In other chronic diseases, there is growing focus on the burden of treatment, measured for instance by the Treatment Burden Questionnaire (TBQ).16 Open-label studies examining PROs under naturalistic conditions are also important for understanding the implementation of new interventions. The aim of the present RCT was to compare depot buprenorphine vs daily SL buprenorphine using the primary end point Treatment Satisfaction Questionnaire for Medication (TSQM), a validated PRO with good psychometric properties.17,18
Methods
Study Design
The Depot Evaluation–Buprenorphine Utilization Trial (DEBUT) was an open-label, randomized parallel group active-controlled trial conducted under naturalistic conditions across 6 outpatient drug treatment centers in Australia. The trial protocol was reviewed and approved by the South Eastern Sydney Local Health Human Research Ethics Committee in accordance with the Declaration of Helsinki19 and the International Council for Harmonization Good Clinical Practice. The trial protocol is available as Supplement 1. Written informed consent was obtained from participants. Trial participants did not pay for medications or for dispensing, and all were informed that they were eligible to receive depot buprenorphine treatment after the trial. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Eligible participants were patients aged 18 years or older who met the criteria for opioid dependence in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Key exclusion criteria included (1) severe respiratory and hepatic insufficiency or any other serious medical condition, including unstable and severe pain or untreated psychiatric conditions, and (2) pregnancy, breastfeeding, or planning to become pregnant during the trial. Complete eligibility criteria are provided in the study protocol (Supplement 1). Trial participants were classified according to race and ethnicity by the investigators as part of the clinical trial protocol.
Randomization and Masking
Eligible participants were randomized 1:1 to either depot buprenorphine or SL buprenorphine using an interactive web-based randomization system. The trial was open-label with no masking of allocated treatment.
Procedures
Weekly and monthly depot buprenorphine were administered at trial sites as SC injections according to clinician and individual participant choice of dosing strength, dosing frequency, and injection site. Doses were established after initiation or switching from previous treatment with SL buprenorphine to a maximum dose of 32 mg for the weekly product or 160 mg for the monthly product, as previously described.13 SC injections were given in the buttock, thigh, abdomen, or upper arm. Participants in the SL buprenorphine group received daily doses at the discretion of investigators to the maximum daily dose of 32 mg. Most participants in the SL buprenorphine group received SL buprenorphine with naloxone in the film formulation, which is the most commonly used formulation in Australia. Dose adjustments in both groups were allowed at any time during the trial.
Mandatory scheduled visits, which included safety assessments by investigators, outcome assessments collected by independent researchers, and urinary drug screenings (UDS) took place on day 1, week 0 (baseline) and at weeks 4, 8, 12, 16, 20, and 24. Psychosocial interventions (case management, counselling) were provided during the trial according to local guidelines, independent of the scheduled visits. Participants in the SL buprenorphine group could receive dosing at the trial clinic or at a nominated community pharmacy, attending between daily to weekly (with as many as 6 unsupervised doses per week) according to local guidelines and prescriber discretion.
Outcomes
The primary efficacy outcome measure was the global treatment satisfaction score at week 24 of the TSQM version 1.4.17 TSQM comprises 14 items across 4 domains, focusing on effectiveness, side effects, convenience, and global satisfaction. Except for the presence of side effects, which is binary (ie, yes or no), all items are scored on a 5- or 7-point Likert scale. The scores for each domain are computed by adding the TSQM items and then transforming the composite score into a value ranging from 0 to 100, with a higher score indicating better satisfaction.
Key secondary efficacy end points included measures of other subdomain scores of TSQM, patient satisfaction ratings (0-100) on a visual analog scale (PS-VAS; higher scores indicate greater satisfaction), Patient Global Impression of Improvement (PGI-I; range, 1-7; lower score indicates more improvement),20 TBQ (range, 1-150; lower score indicates lower burden),16 Opioid Substitution Quality of Life (OSTQoL; 7 subdomains scored 0-4; higher scores indicate higher quality of life),15 Substance Use Recovery Evaluator (SURE; range, 21-63; higher score indicates greater recovery),21 36-item Health Survey Short Form (SF-36; physical and mental components scored 0-100; higher scores indicate better health),22 EuroQol 5-Dimensions 3-Levels (EQ-5D-3L; range, 0.217-1.00; higher score indicates better quality of life),23 Opioid-Related Behaviors in Treatment (ORBIT; range, 0-40; higher scores indicate more aberrant behavior) scale,24 and mental health symptoms measured by the Depression Anxiety Stress Scales (DASS-21; 3 subdomains scored 0-42; higher scores indicate more severe symptoms).25 Additional secondary end points presented are treatment retention, Clinical Opiate Withdrawal Scale (COWS; 0-48; higher scores indicate more severe withdrawal),26 craving VAS (range, 0-100; higher scores indicate more severe craving), and illicit opioid drug use measured by UDS, with missing values imputed as positive, and self-reports of days used by TimeLine Follow Back (TLFB).
Safety was evaluated to week 26. Assessments included treatment-emergent adverse events (TEAEs), adverse drug reactions (ADRs), and serious AEs (SAEs).
Statistical Analysis
The trial size was calculated based on the primary outcome measure. The residual standard deviation was obtained from an analysis using a mixed model for repeated measures (MMRM) method in a multiple sclerosis trial.27 The derived standard deviation at 48 weeks was approximately 26 units and assumed to be relevant also at 24 weeks. With 60 participants per treatment group and with a true treatment difference of 13.4 units, the power was expected to be approximately 80%. The analysis was based on the full analysis set for the primary outcome, ie, participants who received at least 1 dose and had at least 1 follow-up assessment of the TSQM global satisfaction. The estimated treatment effects with SEs, treatment differences, and the 2-sided 95% CIs of the treatment differences at all postbaseline points are presented. The primary comparison was the treatment difference at week 24, as estimated in a linear MMRM with treatment as factor and baseline as covariate.28 Missing data were not imputed, allowing the MMRM to adjust the outcome via preceding nonmissing data.28 This model was used for the primary outcome measure and the secondary end points, except for retention, which was analyzed by a log rank test. For the overall treatment effect across the study, an analysis of variance was used with treatment as factor and baseline as covariate. No correction was made for multiple comparisons when analyzing the secondary end points. SAS version 9.4 (SAS Institute) was used for the analyses. Statistical significance was set at P < .05, and all tests were 2-tailed. Adverse events were summarized using descriptive statistics for participants who received at least 1 dose of trial medication. No data monitoring committee was involved. A post hoc analysis of the number of participants showing a TSQM global satisfaction score of 80 or greater, which has been used in other studies as a cutoff for treatment satisfaction,29 was conducted, with the numbers needed to treat (NNT) calculated using the inverse of the Newcombe Hybrid Score interval for the difference of proportions.30 Data analysis was conducted from October 2019 to May 2020.
Results
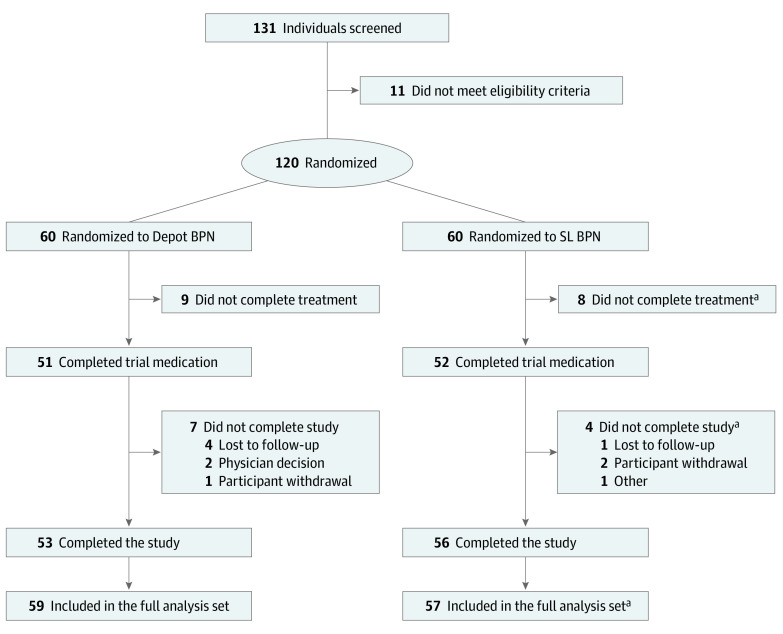
Between October 2018 and September 2019, 131 participants with opioid dependence were screened for eligibility, and 119 participants (70 [58.8%] men; mean [SD] age, 44.4 [10.5] years) were enrolled, randomized to, and received either depot buprenorphine (60 participants [50.4%]) or SL buprenorphine (59 participants [49.6%]). One participant (0.8%) who had been randomized to the SL buprenorphine group withdrew consent and did not receive study treatment. There were 53 participants (88.3%) who completed the trial in the depot buprenorphine group and 56 (93.3%) in the SL buprenorphine group; the reasons for participants discontinuing trial medication or the trial are listed in Figure 1. There were 54 participants (90.0%) in the depot buprenorphine group and 27 participants (45.8%) in the SL buprenorphine group who attended all 7 assessment visits according to the trial protocol.
Figure 1. Trial Flowchart.
aOne participant in the SL buprenorphine group was randomized and attended the day 1, week 0 (baseline) visit but discontinued from the trial without receiving treatment and was reported as lost to follow-up. This participant was not included in the largest full analysis set (ie, modified intention-to-treat analysis). Participants could remain in the study after discontinuing trial medication and complete the trial assessments until week 24 for completion of the study. The full analysis set for the primary end point comprised all randomized participants who were administered at least 1 dose of trial medication and had at least 1 postbaseline assessment of the primary outcome efficacy end point.
Participant demographic and clinical characteristics were similar between the groups (Table 1). The mean (SD) duration since diagnosis of opioid dependence before randomization was 13.0 (10.8) years, and the mean (SD) duration from first illicit use of opioids to randomization was 19.3 (12.6) years. The mean (SD) duration of the last treatment episode with SL buprenorphine before randomization was 136.3 (161.3) weeks in the depot buprenorphine group and 179.6 (280.2) weeks in the SL buprenorphine group. More than half the population in both treatment groups (76 [63.9%]) primarily used heroin (rather than prescription opioids). However, there was a chance imbalance regarding those who used heroin, which was more frequent in the depot buprenorphine group (44 [73.3%]) than the SL buprenorphine group (32 [54.2%]). Clinical characteristics and key patient reported outcomes were otherwise balanced at baseline (Table 1).
Table 1. Demographic and Baseline Clinical Characteristics.
| Characteristic | Patients, mean (SD) | |
|---|---|---|
| Depot buprenorphine group (n = 60) | SL buprenorphine group (n = 59) | |
| Age, y | 43.6 (10.4) | 45.3 (10.6) |
| Men, No. (%) | 34 (56.7) | 36 (61.0) |
| Women, No. (%) | 26 (43.3) | 23 (39.0) |
| White, No. (%) | 51 (85.0) | 51 (86.4) |
| BMI | 26.7 (6.5) | 28.2 (6.0) |
| Employed, No. (%) | 14 (23.3) | 13 (22.0) |
| Time from first illicit use of opioid to randomization, y | 19.7 (12.5) | 19.0 (12.8) |
| Heroin primary opioid used, No. (%) | 44 (73.3) | 32 (54.2) |
| Injection use, No. (%) | 41 (68.3) | 36 (61.0) |
| Age when first used heroin, y | 22.2 (7.5) | 21.9 (6.6) |
| Age at first medical treatment of opioid dependence, y | 31.7 (10.3) | 35.3 (10.7) |
| Medical history, No. (%) | ||
| Hepatitis C | 34 (56.7) | 21 (35.6) |
| Depression | 29 (48.3) | 36 (61.0) |
| Drug use by UDS with self-reports, No. (%) | ||
| Illicit opioids | 23 (38.3) | 19 (32.2) |
| Amphetamine | 24 (40.0) | 13 (22.0) |
| Cocaine | 5 (8.3) | 4 (6.8) |
| Cannabinoids | 24 (40.0) | 16 (27.1) |
| Benzodiazepines | 21 (35.0) | 19 (32.2) |
| Baseline clinical characteristics | ||
| Opioid craving VAS | 21.5 (23.7) | 30.7 (29.0) |
| COWS | 1.8 (2.9) | 2.0 (2.4) |
| TSQM | ||
| Global satisfaction score | 71.2 (18.0) | 73.8 (17.5) |
| Effectiveness score | 72.6 (15.8) | 74.8 (15.8) |
| Side effect score | 88.8 (19.9) | 87.3 (21.7) |
| Convenience score | 62.4 (18.7) | 69.8 (20.4) |
| Patient satisfaction VAS | 67.7 (25.7) | 73.8 (21.3) |
| TBQ | 36.4 (27.5) | 42.5 (33.0) |
| OSTQoL | ||
| Overall score | 2.4 (1.1) | 2.4 (1.0) |
| Personal development domain score | 2.4 (0.9) | 2.2 (0.9) |
| Mental distress domain score | 2.3 (0.9) | 2.1 (1.0) |
| Social contacts domain score | 3.0 (1.0) | 2.5 (1.2) |
| Material well-being domain score | 2.7 (1.1) | 2.5 (1.0) |
| Opioid substitution treatment domain score | 3.0 (0.9) | 2.8 (0.9) |
| Discrimination domain score | 2.3 (1.2) | 2.1 (1.3) |
| SF-36 | ||
| Physical component summary score | 49.7 (9.3) | 48.5 (10.9) |
| Mental component summary score | 43.6 (10.6) | 39.7 (12.2) |
| EQ-5D-3L | ||
| Index value | 0.76 (0.23) | 0.72 (0.23) |
| VAS | 70.8 (18.3) | 67.4 (18.0) |
| ORBIT | 2.0 (2.5) | 1.5 (2.7) |
| SURE | 53.7 (7.7) | 51.7 (9.2) |
| DASS-21 | ||
| Depression score | 11.6 (9.5) | 15.3 (12.0) |
| Anxiety score | 8.3 (7.6) | 11.7 (9.1) |
| Stress score | 12.5 (9.1) | 14.5 (9.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COWS, Clinical Opiate Withdrawal Scale; DASS, Depression Anxiety Stress Scales; EQ-5D-3L, EuroQol 5-Dimensions 3-Levels; ORBIT, Opioid-Related Behaviors in Treatment scale; OSTQoL, opioid substitution quality of life; SF-36, 36-item Short Form Health Survey; SL, sublingual; SURE, Substance Use Recovery Evaluator; TBQ, Treatment Burden Questionnaire; TSQM, Treatment Satisfaction Questionnaire for Medication; UDS, urinary drug screening; VAS, visual analog scale.
During the trial, the mean daily dose of SL buprenorphine was 15.6 (7.8) mg/d. Twenty-nine participants (48.3%) in the depot buprenorphine group received at least 1 weekly dose. The mean weekly depot buprenorphine dose (excluding 8-mg weekly supplemental doses received by 20 participants) was 21.5 (8.7) mg/week and the mean monthly depot buprenorphine dose was 107.4 (31.6) mg/month. The median weekly and monthly depot buprenorphine doses were 24 (range, 8-32) mg and 96 (range, 64-160) mg, respectively, both corresponding to a daily SL buprenorphine dose of 12 to 16 mg.13
For the primary outcome measure of TSQM Global Satisfaction Score, the mean (SD) at baseline was 71.2 (18.0) in the depot buprenorphine group and 73.8 (17.5) in the SL buprenorphine group. For the primary MMRM analysis at week 24, the mean (SE) score was 82.5 (2.3) for the depot buprenorphine group and 74.3 (2.3) for the SL buprenorphine group, corresponding to a treatment difference of 8.2 (95% CI, 1.7-14.6; P = .01). The corresponding mean (SE) scores for the overall study period were 82.4 (1.9) for the depot buprenorphine group and 73.8 (1.9) for the SL buprenorphine group (difference, 8.6; 95% CI, 3.3-13.9; P = .002). The analysis population for the primary outcome measure was 59 (98.3%) in the depot buprenorphine group and 57 (96.6%) in the SL buprenorphine group.
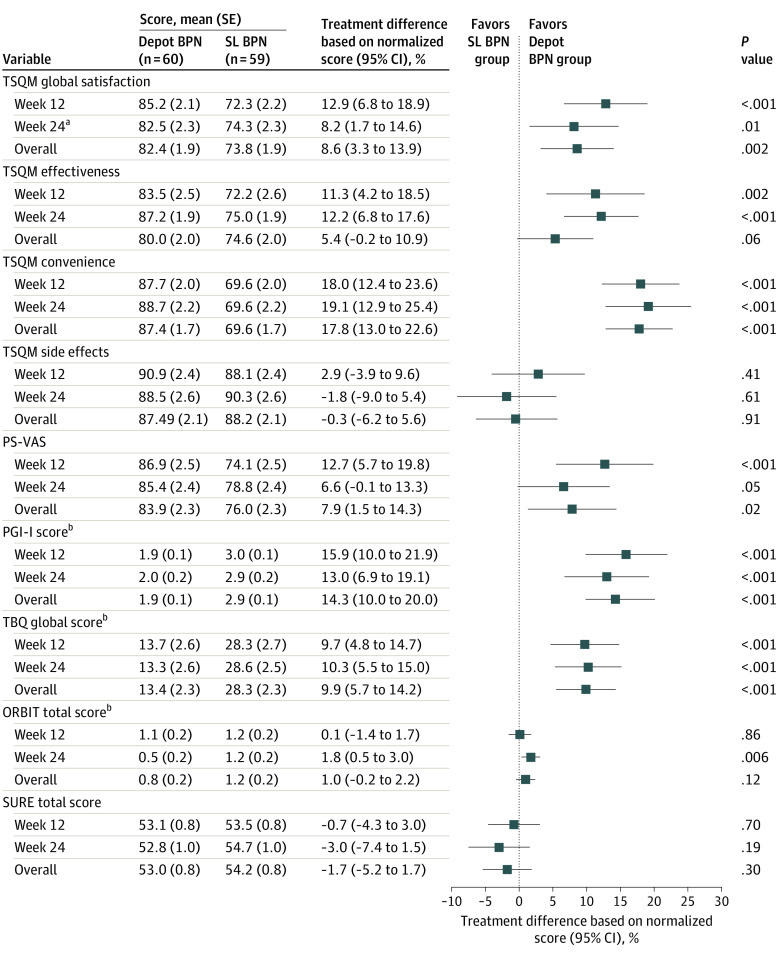
The higher treatment satisfaction and change from baseline in the depot buprenorphine group was also seen for the secondary TSQM convenience and effectiveness domain scores, while no difference was observed between the groups for the side effect domain score (Figure 2). For the TSQM convenience score, a significant improvement was seen already 4 weeks after initiation of study treatment, and the difference was maintained until week 24. In contrast, the difference in the treatment effectiveness score increased steadily during the treatment period up to week 24. Post hoc analysis of the TSQM global satisfaction score showed that the number of participants with a score of 80 or greater was higher in the depot buprenorphine group (32 participants [53.3%]) than in the SL buprenorphine group (20 participants [33.9%]) at week 24. The NNT to achieve the threshold of 80 or greater was 5.1 (95% CI, 2.8-61.1).
Figure 2. Overview of Treatment Satisfaction Questionnaire for Medication (TSQM), Patient Satisfaction–Visual Analog Scale (PS-VAS), Patient Global Impression of Improvement (PGI-I), Treatment Burden Questionnaire (TBQ), Opioid-Related Behaviors in Treatment (ORBIT), and Substance Use Recovery Evaluator (SURE) Results.
Summary of results from the mixed model for repeated measures, in which the 95% CIs in the forest plot have been normalized by the maximal possible score by instrument to give a range in percentages for ease of comparability. Analysis by week was performed by mixed model for repeated measures and overall by analysis of covariance.
aPrimary variable.
bThe 95% CIs in the forest plot graph were sign-reversed to achieve comparability.
There was no significant difference in illicit opioid use measured by UDS and self-reports by TLFB at week 24, with a mean for negative results on UDS and self-reports by TLFB of 69.9% (95% CI, 60.6%-79.3%) for the depot buprenorphine group and 73.5% (95% CI, 64.1%-82.9%) for the SL buprenorphine group. A descriptive post hoc analysis showed mean (SD) TSQM global satisfaction scores at week 24 among participants with positive and negative UDS results in the depot buprenorphine group of 84.2 (17.8) and 81.8 (23.3), respectively, and in the SL buprenorphine group of 65.4 (14.8) and 77.7 (15.6), respectively.
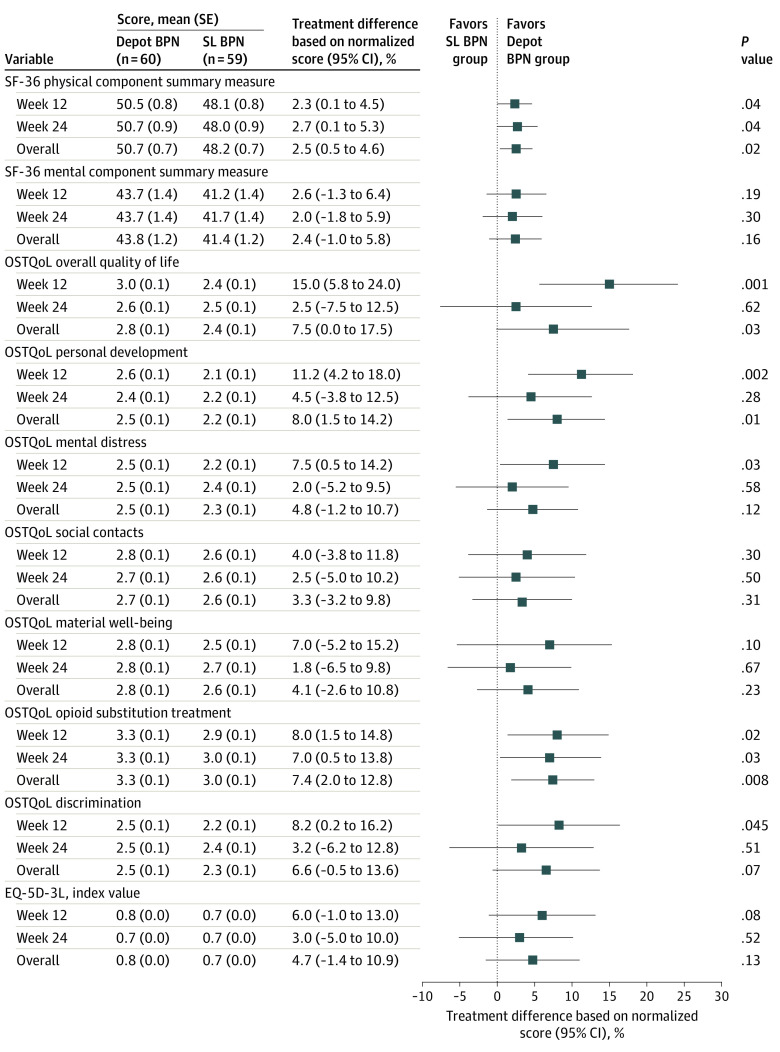
Significant differences in PROs between the treatment groups were observed for several other secondary end points, including higher patient satisfaction (measured by PS-VAS), lower treatment burden (measured by TBQ), improved treatment effectiveness (measured by PGI-I), and quality of life domain scores (measured by OSTQoL, opioid substitution treatment domain), fewer aberrant opioid medication behaviors (measured by ORBIT), and better physical functioning (measured by SF-36, physical component) in the depot buprenorphine group compared with the SL buprenorphine group (eg, mean [SE] TBQ global score: 13.3 [2.6] vs 28.6 [2.5]; difference, −15.4; 95% CI, −22.6 to −8.2; P < .001) (Figure 2 and Figure 3). For other secondary PROs (including EQ-5D-3L; SF-36, mental health component; SURE; and DASS-21), there were no statistically significant observed treatment differences (Figure 2 and Figure 3). Withdrawal and craving scores and illicit opioid use measures revealed no significant treatment differences between the groups and were generally well controlled across treatments.
Figure 3. Overview of 36-Item Health Survey Short Form (SF-36), Opioid Substitution Quality of Life (OSTQoL), and EuroQol 5-Dimensions 3-Levels (EQ-5D-3L) Results.
Summary of results from mixed model for repeated measures, in which the 95% CIs in the forest plot have been normalized by the maximal possible score by instrument to give a range in percentages for ease of comparability. Analysis by week was performed by mixed model for repeated measures and overall by analysis of covariance.
There were no deaths during the trial in either treatment group. No participants were discontinued from the trial medication and or/withdrawn from the trial due to adverse events. Thirty-nine participants (65.0%) in the depot buprenorphine group experienced 117 adverse drug reactions, mainly injection site reactions of mild intensity following subcutaneous administration, and 12 participants (20.3%) in the sublingual buprenorphine group experienced 21 adverse drug reactions. The incidence of all TEAEs reported during the trial was higher in the depot buprenorphine group (54 participants [90.0%]) than the SL buprenorphine group (49 participants [83.1%]) (Table 2), mainly related to injection site reactions of generally mild intensity in the depot buprenorphine group. Three participants (5.0%) in the depot buprenorphine group reported TEAEs of severe intensity, of which 1 event of suicidal ideation was considered possibly related to the drug. In this case, the dose was increased, and the event resolved. Four participants (6.8%) in the SL buprenorphine group reported TEAEs of severe intensity. The severe events comprised intentional overdose (2 events), pneumonia, and alcohol dependence. None of these events were considered related to the drug, and all events resolved without sequelae.
Table 2. Summary of Treatment-Emergent AEsa.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Depot buprenorphine group (n = 60) | SL buprenorphine group (n = 59) | Total (N = 119) | |
| Treatment-emergent AEs | 54 (90.0) | 49 (83.1) | 103 (86.6) |
| Drug-related ADRs | 39 (65.0) | 12 (20.3) | 51 (42.9) |
| SAEs | 9 (15.0) | 9 (15.3) | 18 (15.1) |
| Serious drug-related ADRs | 1 (1.7) | 0 | 1 (0.8) |
| AEs or SAEs leading to withdrawal of trial medication | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 |
| Drug overdoses | 0 | 4 (6.8) | 4 (3.4) |
| AEs occurring in ≥8% of participants | |||
| Injection site | |||
| Pain | 11 (18.3) | 0 | 11 (9.2) |
| Mass | 10 (16.7) | 0 | 10 (8.4) |
| Bruising | 5 (8.3) | 0 | 5 (4.2) |
| Upper respiratory tract infection | 7 (11.7) | 2 (3.4) | 9 (7.6) |
| Nausea | 5 (8.3) | 2 (3.4) | 7 (5.9) |
| Vomiting | 5 (8.3) | 2 (3.4) | 7 (5.9) |
| Toothache | 5 (8.3) | 1 (1.7) | 6 (5.0) |
| Arthralgia | 5 (8.3) | 4 (6.8) | 9 (7.6) |
Abbreviations: ADRs, adverse drug reactions; AEs, adverse events; SAEs, serious adverse events; SL, sublingual.
Summary of treatment-emergent AEs in safety analysis set. Treatment-emergent AEs occurring in more than 5 participants in any treatment group are shown.
Discussion
This open-label RCT showed significantly higher global treatment satisfaction measured with TSQM after 24 weeks with depot buprenorphine than SL buprenorphine. The depot buprenorphine group also showed significant improvements in measures of treatment convenience and effectiveness (TSQM subdomains), patient satisfaction (PS-VAS), treatment burden (TBQ), treatment effectiveness (PGI-I), quality of life (OSTQoL opioid substitution treatment domain), and physical functioning (SF-36, physical component). The magnitude of the improvements in the treatment-related PROs in the depot buprenorphine group are noteworthy, as shown by the NNT of 5.1 to achieve the TSQM threshold for satisfaction of 80% or greater between the 2 groups. This study was conducted under naturalistic open-label conditions, with few participants excluded from enrolling, suggesting generalizability of the study findings. To our knowledge, this study is the first to use many different PRO measures in individuals who use substances and adds important insights to previous studies of patient perception of treatment.31,32
There were no major improvements in treatment-related PROs in the control SL buprenorphine group during the study, indicating that the effects in PRO measures seen in the depot buprenorphine group were not a Hawthorne effect of the study-related procedures. The findings may not generalize to persons naive to opioid agonist treatment. The improvement in TSQM global satisfaction and convenience domain scores was evident early (by week 4), while the improvement in the effectiveness domain scores became evident at week 12. The persistence of the increased TSQM scores until the end of the study suggest that these effects were not merely initial perceptions (ie, a honeymoon period) that abated with time.
The low levels of illicit opioid use in both groups throughout the study reflects that all participants were already receiving SL buprenorphine treatment prior to commencing the study. This is in contrast to previous RCTs that examined patients entering treatment with more recent extensive illicit opioid use,10,11 and our study highlights that depot buprenorphine treatment is effective in patients transferring from SL buprenorphine, who will most likely represent most patients treated with depot buprenorphine products initially. Although the study was not powered for a formal correlation analysis between the outcome of the primary end point (TSQM global satisfaction score) and the traditional measure of illicit opioid use as measured by UDS, a descriptive post hoc analysis did not reveal major differences in treatment satisfaction at the end of the study in patients with and without evidence of illicit opioid use in the depot buprenorphine group. However, participants in the SL buprenorphine group with illicit opioid use did report lower TSQM global satisfaction scores. Finally, there were no improvements in either the depot buprenorphine or SL buprenorphine group of the recovery measures using SURE, in contrast to most other PRO measures used in the study. While SURE has been validated against other PROs, its sensitivity to change over time has not been previously examined, and we propose further secondary analysis of this instrument.21
Limitations
This study has limitations. It was powered for the primary outcome and, therefore, was not powered to detect minor between-group differences for some of the other outcomes (such as illicit opioid use). The sample size enrolled allowed comparisons with a statistical model with treatment as factor and baseline as covariate, thereby adjusting for imbalances between groups at the time of randomization. However, it is difficult to draw conclusions on the clinical relevance of some of the PROs based on the number of participants enrolled and the length of the study. The results of the secondary outcomes were not corrected for multiple comparisons due to the size and nonconfirmatory nature of the study. The primary outcome TSQM might not capture all principal effects of the medications used to treat addiction, eg, the effect on craving of substances.33 The study was conducted under certain treatment conditions, and a noninterventional observational study might detect different outcomes. The study was conducted under naturalistic conditions in Australia, and the translation of some of the findings, eg, regarding treatment convenience and burden, might be less straightforward in countries and regions with different frequency and grade of supervision during dispensing of medication, such as France and the United States.9,34 In contrast, satisfaction and retention in the SL buprenorphine group may have increased by payment of pharmacy dosing fees during the trial; if so, the difference in treatment satisfaction under routine clinical conditions of paid dosing might be even greater than observed. All participants included in the full analysis set of the study were treated with SL buprenorphine before randomization in the study, and the results may not be representative of those that would be achieved in patients who are new to treatment.
Conclusions
In this study, participants randomized to receive depot buprenorphine administered weekly or monthly reported significantly higher and more sustained treatment global satisfaction than those randomized to continue to receive SL buprenorphine, adding to the evidence regarding depot buprenorphine from earlier double-blind RCTs. To our knowledge, this is the first randomized study that has used a range of PROs to compare outcomes between a long-acting injection and daily dosing of buprenorphine in the treatment of opioid dependence. The study highlights the application of PROs as alternate end points to traditional markers of substance use in addiction treatment outcome studies.
Trial Protocol
Data Sharing Statement
References
- 1.Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. doi: 10.1038/s41572-019-0137-5 [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560-1579. doi: 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi: 10.1016/S0140-6736(18)33078-2 [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Blanco C. Medications for opioid use disorders: clinical and pharmacological considerations. J Clin Invest. 2020;130(1):10-13. doi: 10.1172/JCI134708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization model list of essential medicines: 21st list 2019. Published 2019. Accessed April 1, 2021. https://apps.who.int/iris/handle/10665/325771
- 6.Kyzer JL, Wenthur CJ. Classics in chemical neuroscience: buprenorphine. ACS Chem Neurosci. 2020;11(10):1385-1399. doi: 10.1021/acschemneuro.0c00100 [DOI] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration . TIP 63: medications for opioid use disorder. Published May 2020. Accessed April 1, 2021. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document/PEP20-02-01-006
- 8.Chilcoat HD, Amick HR, Sherwood MR, Dunn KE. Buprenorphine in the United States: motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Marshall BDL, Degenhardt L, et al. Global opioid agonist treatment: a review of clinical practices by country. Addiction. 2020;115(12):2243-2254. doi: 10.1111/add.15087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haight BR, Learned SM, Laffont CM, et al. ; RB-US-13-0001 Study Investigators . Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi: 10.1016/S0140-6736(18)32259-1 [DOI] [PubMed] [Google Scholar]
- 11.Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178(6):764-773. doi: 10.1001/jamainternmed.2018.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling W, Nadipelli VR, Solem CT, et al. Effects of monthly buprenorphine extended-release injections on patient-centered outcomes: a long-term study. J Subst Abuse Treat. 2020;110:1-8. doi: 10.1016/j.jsat.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Frost M, Bailey GL, Lintzeris N, et al. Long-term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult out-patients with opioid use disorder. Addiction. 2019;114(8):1416-1426. doi: 10.1111/add.14636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND. Personalizing the treatment of substance use disorders. Am J Psychiatry. 2020;177(2):113-116. doi: 10.1176/appi.ajp.2019.19121284 [DOI] [PubMed] [Google Scholar]
- 15.Strada L, Franke GH, Schulte B, Reimer J, Verthein U. Development of OSTQOL: a measure of quality of life for patients in opioid substitution treatment. Eur Addict Res. 2017;23(5):238-248. doi: 10.1159/000484239 [DOI] [PubMed] [Google Scholar]
- 16.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109. doi: 10.1186/1741-7015-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katusiime B, Corlett S, Reeve J, Krska J. Measuring medicine-related experiences from the patient perspective: a systematic review. Patient Relat Outcome Meas. 2016;7:157-171. doi: 10.2147/PROM.S102198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 20.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26-35. doi: 10.1016/j.jmpt.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Neale J, Vitoratou S, Finch E, et al. Development and validation of “SURE”: a patient reported outcome measure (PROM) for recovery from drug and alcohol dependence. Drug Alcohol Depend. 2016;165:159-167. doi: 10.1016/j.drugalcdep.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 24.Larance B, Bruno R, Lintzeris N, et al. Development of a brief tool for monitoring aberrant behaviours among patients receiving long-term opioid therapy: the Opioid-Related Behaviours In Treatment (ORBIT) scale. Drug Alcohol Depend. 2016;159:42-52. doi: 10.1016/j.drugalcdep.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 25.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343. doi: 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- 26.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259. doi: 10.1080/02791072.2003.10400007 [DOI] [PubMed] [Google Scholar]
- 27.Vermersch P, Czlonkowska A, Grimaldi LM, et al. ; TENERE Trial Group . Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20(6):705-716. doi: 10.1177/1352458513507821 [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui O, Hung HM, O’Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19(2):227-246. doi: 10.1080/10543400802609797 [DOI] [PubMed] [Google Scholar]
- 29.Radawski C, Genovese MC, Hauber B, et al. Patient perceptions of unmet medical need in rheumatoid arthritis: a cross-sectional survey in the USA. Rheumatol Ther. 2019;6(3):461-471. doi: 10.1007/s40744-019-00168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 31.Strada L, Vanderplasschen W, Buchholz A, et al. Measuring quality of life in opioid-dependent people: a systematic review of assessment instruments. Qual Life Res. 2017;26(12):3187-3200. doi: 10.1007/s11136-017-1674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solà I, Trujols J, Ribalta E, et al. Quality of life and well-being from the perspective of patients on opioid agonist maintenance treatment: study protocol for a systematic review of qualitative research and a scoping review of measures. Syst Rev. 2019;8(1):299. doi: 10.1186/s13643-019-1237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez de Los Cobos J, Trujols J, Alcaraz S, Siñol N, Lozano Ó, González-Saiz F; Buprenorphine Naloxone Survey Group . Development and validation of the scale to assess satisfaction with medications for addiction treatment—buprenorphine-naloxone for heroin addiction (SASMAT-BUNHER). Int J Drug Policy. 2018;58:126-134. doi: 10.1016/j.drugpo.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 34.Nielsen S, Dietze PM. What can Australia learn from the North American opioid crisis? the role of opioid regulation and other evidence-based responses. Drug Alcohol Rev. 2019;38(3):223-225. doi: 10.1111/dar.12916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement