Abstract
Background
Any type of seizure can be observed in Alzheimer's disease. Antiepileptic drugs seem to prevent the recurrence of epileptic seizures in most people with Alzheimer's disease. There are pharmacological and non‐pharmacological treatments for epilepsy in people with Alzheimer's disease, however there are no current systematic reviews to evaluate the efficacy and tolerability of these treatments. This review aims to investigate these different modalities.
This is an updated version of the Cochrane Review previously published in 2018.
Objectives
To assess the efficacy and tolerability of pharmacological or non‐pharmacological interventions for the treatment of epilepsy in people with Alzheimer's disease (including sporadic Alzheimer's disease and dominantly inherited Alzheimer's disease).
Search methods
For the latest update, on 3 August 2020 we searched the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid, 1946 to 31 July 2020). CRS Web includes randomized or quasi‐randomized controlled trials from PubMed, EMBASE, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups, including Cochrane Epilepsy. In an effort to identify further published, unpublished and ongoing trials, we searched ongoing trials registers, reference lists and relevant conference proceedings; we also contacted trial authors and pharmaceutical companies.
Selection criteria
We included randomized and quasi‐randomized controlled trials investigating treatment for epilepsy in people with Alzheimer's disease, with the primary outcomes of proportion of participants with seizure freedom and proportion of participants experiencing adverse events.
Data collection and analysis
Two review authors independently screened the titles and abstracts of identified records, selected studies for inclusion, extracted data, cross‐checked the data for accuracy and assessed the methodological quality. We performed no meta‐analyses due to there being limited available data.
Main results
We included one randomized controlled trial (RCT) on pharmacological interventions; the trial included 95 participants. No studies were found for non‐pharmacological interventions. Concerning the proportion of participants with seizure freedom, no significant differences were found for the comparisons of levetiracetam versus lamotrigine (RR) 1.20, 95% CI 0.53 to 2.71; 67 participants; very low‐certainty evidence), levetiracetam versus phenobarbital (RR 1.01, 95% CI 0.47 to 2.19; 66 participants; very low‐certainty evidence), or lamotrigine versus phenobarbital (RR 0.84, 95% CI 0.35 to 2.02; 57 participants; very low‐certainty evidence). It seemed that levetiracetam could improve cognition and lamotrigine could relieve depression, while phenobarbital and lamotrigine could worsen cognition, and levetiracetam and phenobarbital could worsen mood. The risk of bias relating to allocation, blinding and selective reporting was unclear. We judged the certainty of the evidence for all outcomes to be very low.
Authors' conclusions
This review does not provide sufficient evidence to support levetiracetam, phenobarbital or lamotrigine for the treatment of epilepsy in people with Alzheimer's disease. Regarding efficacy and tolerability, no significant differences were found between levetiracetam, phenobarbital and lamotrigine.
Large RCTs with a double‐blind, parallel‐group design are required to determine the efficacy and tolerability of treatment for epilepsy in people with Alzheimer's disease.
Plain language summary
Treatment of epilepsy for people with Alzheimer's disease
Background Alzheimer's disease is a risk factor for increased seizures in older people. Seizures of any type can be observed in Alzheimer's disease and are probably underestimated.
Study characteristics We searched scientific databases for clinical trials comparing medication and non‐medication‐based treatments for epilepsy in people with Alzheimer's disease. We wanted to evaluate how well the treatment worked and if it had any side effects.
Key results We included and analyzed one randomized controlled trial (a clinical study where people are randomly put into one or two (or more) treatment groups) with 95 participants. Concerning the proportion of participants with freedom from seizures, no significant differences were found between the antiepileptic drugs (levetiracetam versus lamotrigine, levetiracetam versus phenobarbital, and lamotrigine versus phenobarbital). It seemed that levetiracetam could improve cognition (thinking) and lamotrigine could relieve depression, while phenobarbital and lamotrigine could worsen cognition, and levetiracetam and phenobarbital could worsen mood.
Certainty of the evidence The certainty of the evidence for all the outcomes from the study were very low. This means that we are very uncertain about the results and they should be interpreted with caution. Large randomized controlled trials are required to determine how effective and well tolerated treatments are for epilepsy in people with Alzheimer's disease.
The evidence is current to August 2020.
Summary of findings
Background
Description of the condition
Epilepsy is a common neurological disorder and becomes more frequent with age (Brodie 2009). Meanwhile, Alzheimer's disease (AD) is the most common neurodegenerative disease in older people, and is characterized by memory loss, cognitive decline, and behavioral disorders. Although epilepsy is not the predominant symptom in sporadic AD, it is more common in autosomal dominant AD (Wu 2012). It has been estimated that AD is a risk factor for increased seizures in older people (Pandis 2012); approximately 10% to 22% of people with AD have at least one unprovoked seizure (Mendez 2003). Any type of seizure can be observed in AD (Rao 2009). The prevalence of epilepsy is probably underestimated (Tallis 2002), considering the unrecognized non‐convulsive forms. Seizures can be seen even in the early stages of AD (Palop 2007), which suggests seizures may contribute to cognitive impairment.
Description of the intervention
Antiepileptic drugs (AEDs) are the current intervention for treating epilepsy in people with AD. According to a previous study, the efficacy of AEDs in older people was proven to be better than that in the younger population (Mattson 1985). The first generation AEDs, such as valproic acid and benzodiazepines, can aggravate cognitive decline in people with AD (Fleisher 2011; Wu 2009). In contrast, the new generation AEDs, e.g. lamotrigine and gabapentin, seem to be well tolerated by older people (Rowan 2005; Saetre 2007). Drugs targeting beta amyloid may also be a rational choice for treatment of epilepsy in AD as beta amyloid accumulation can contribute to seizures, as confirmed by Down syndrome and AD mouse models (Puri 2001; Westmark 2008). Non‐pharmacological interventions, such as transcranial magnetic stimulation (TMS), deep brain stimulation and acupuncture, are potentially beneficial for people with AD or people with epilepsy (Hsu 2015; Kimiskidis 2010; Laxpati 2014; McElroy‐Cox 2009).
How the intervention might work
The common mechanisms between AD and epilepsy can probably be attributed to hippocampal sclerosis; that is, neuron loss and astrogliosis in cornu ammonis, with sparing of granule cells in the dentate nucleus (Chin 2013). Furthermore, hippocampal sclerosis may be associated with dementia and neuropsychiatric symptoms in people with AD or epilepsy. In AD mouse models, a‐beta amyloid and neurofibrillary tangle‐related tau protein have been suggested to contribute to seizures (Roberson 2011; Westmark 2008). Some studies have demonstrated that seizures occur even earlier than amyloid pathology, which possibly causes the progressive neurodegeneration in AD (Minkeviciene 2009; Palop 2007). Thus, AEDs may reduce the progression of AD by controlling epilepsy, while drugs targeting beta amyloid may reduce seizures by preventing amyloid aggregation. With regard to the mechanisms of TMS, either excitatory or inhibitory responses can be generated in cortical tissues, which improve the symptoms of both AD and epilepsy.
Why it is important to do this review
At present, AED therapy has been tested in clinical trials and seems to prevent the recurrence of epileptic seizures in most people with AD (Rao 2009). Meanwhile, drugs targeting beta amyloid, such as bapineuzumab and solanezumab, have not been widely applied in the treatment of AD. Therefore, their antiepileptic efficacy is still unknown. TMS treatment has been applied in AD and in epilepsy, but the efficacy of TMS for epilepsy in people with AD is rarely reported. In this review, we aim to evaluate the efficacy and tolerability of the treatment of epilepsy for people with AD. To our knowledge, no systematic review or meta‐analysis on this topic exists.
Objectives
To assess the efficacy and tolerability of pharmacological or non‐pharmacological interventions for the treatment of epilepsy in people with AD (including sporadic AD and dominantly inherited AD).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized controlled trials.
Types of participants
We included people diagnosed with Alzheimer's disease (AD) combined with epilepsy. We used the definitions of AD and epilepsy as provided in the original studies. We applied no limitations on sex or age.
Types of interventions
Experimental intervention: any pharmacological or non‐pharmacological intervention for epilepsy, alone or combined with other treatment.
Control intervention: no treatment; placebo alone or combined with other treatment (concomitant interventions had to be the same in each group); different doses of the experimental intervention.
Types of outcome measures
The outcomes of all the participants initially randomized were collected and subjected to intention‐to‐treat (ITT) analysis. All the outcomes were measured at the endpoint, in comparison with the data from the baseline. We followed the endpoints as provided in the original publications, and merged the data at the same time point, if possible.
Primary outcomes
Proportion of participants with seizure freedom.
Proportion of participants who experienced any adverse events (AEs).
Secondary outcomes
Proportion of participants with dominantly inherited AD with seizure freedom.
Reduction in seizure frequency of 50% or more.
Change in cognition, measured by Mini Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale‐Cognitive (ADAS‐Cog), etc.
Change in neuropsychiatric symptoms, measured by Neuropsychiatric Inventory (NPI), Geriatric Depression Scale (GDS), etc.
Improvement in quality of life, measured by Quality of Life Scale, etc.
Search methods for identification of studies
Electronic searches
Searches were run for the original version of the review on 1 February 2016. Subsequent searches were run on 10 July 2018. For the latest update, we searched the following databases on 3 August 2020:
Cochrane Register of Studies (CRS Web), using the search strategy set out in Appendix 1;
MEDLINE (Ovid, 1946 to July 31, 2020), using the search strategy set out in Appendix 2;
CRS Web includes randomized or quasi‐randomized controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups, including Cochrane Epilepsy.
Note: it is no longer necessary to search Embase, because randomized and quasi‐randomized controlled trials from Embase are now included in CENTRAL.
Searching other resources
In addition to the strategies listed above, we also:
searched reference lists of reviews and retrieved articles for additional studies;
searched conference proceedings from the last three years for relevant studies, in World Congress of Neurology, International Conference of Alzheimer's Disease, Alzheimer's Association International Conference, and International Epilepsy Congress;
contacted researchers, pharmaceutical companies, and relevant trial authors to seek information about unpublished or incomplete trials.
We did not impose any language limitations for the search, and we attempted to obtain translations of articles where necessary.
Data collection and analysis
Selection of studies
Two review authors (JL, L‐NW) independently evaluated titles and abstracts of identified trials to determine if they fulfilled the inclusion criteria. All potentially relevant studies were obtained in full text for further consideration. We listed the excluded studies and reported the reasons for exclusion. Any disagreements were resolved by discussion or by an independent party if necessary.
Data extraction and management
Two review authors (JL, L‐NW) independently extracted eligible data from the published reports onto standardized forms, and cross‐checked them for accuracy. Disagreements regarding data extraction were resolved by discussion between the review authors.
We used checklists to independently record details of the following:
study design;
total study duration;
methods of generating the randomization schedule;
method of concealment of allocation;
blinding;
use of an ITT analysis (all participants initially randomized will be included in the analyses as allocated to groups);
AEs and dropouts for all reasons;
participants (country, number of participants, age, gender, inclusion and exclusion criteria);
comparison (details of the intervention in treatment and control groups, details of co intervention(s) in both groups, duration of treatment);
outcomes and time points of measures (number of participants in each group and outcome, regardless of compliance);
factors for heterogeneity (sample size, missing participants, confidence interval (CI) and P value in measurement, subgroup analyses).
Assessment of risk of bias in included studies
Two review authors (JL, L‐NW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving an independent party. We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We assessed the risk of bias for each domain as being high, low or unclear, and provided information from the study report. We described sources of bias.
Measures of treatment effect
We expressed continuous data as mean differences (MDs) with 95% CIs. Standardized mean difference (SMD) would have been used for the outcomes when they were measuring the same thing but in different ways. For all dichotomous outcomes we calculated risk ratios (RRs), again with 95% CIs. As it is possible that some trials (or groups within a trial) had no adverse events or no dropouts, we would have calculated risk differences (RDs) instead of RRs in these specific situations, again with 95% CIs. We used ITT analysis for all the outcomes. Different control groups were analyzed separately.
Unit of analysis issues
We planned to deal with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
We planned to contact the authors of the studies for further details if any data were missing, and to establish the characteristics of unpublished trials through correspondence with the trial co‐ordinator or principal investigator. According to the ITT principle, all randomized participants should be included.
Assessment of heterogeneity
We planned to use the standard Chi² statistic and I² statistic to measure the heterogeneity (Higgins 2017), and make a judgement along with visual inspection of forest plots. For the Chi² test, we would have rejected the hypothesis of tolerability if the P value was less than 0.10, and we would have interpreted an I² greater than 50% as representing significant heterogeneity. In this case, we would have tried to explore factors for heterogeneity.
Assessment of reporting biases
We planned to assess publication bias by funnel plot if we had found more than 10 studies. However, the review included only one study.
Data synthesis
If we found neither clinical nor statistical heterogeneity, we planned to pool results using a fixed‐effect model. We would have analyzed different controls separately. In the case of statistical heterogeneity, we planned to pool the results using a random‐effects model. For heterogeneity that could not be readily explained, we would not have pooled the data but only given a description of the results.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses due to the limited available data, however we planned to analyze subgroups of studies categorized according to the subtype of AD (sporadic AD and dominantly inherited AD), stage of AD (mild, moderate, or severe AD), and the dosage and duration of interventions.
As a formal method of comparing subgroups, we planned to use the Chi² test (to test for significant differences between subgroups of participants). For all statistical analyses, we used Review Manager 5 (Review Manager 2020).
Sensitivity analysis
We were unable to perform any of these analyses due to the limited available data, however we planned to use best‐case and worst‐case scenarios for taking into account missing data. We also planned to undertake the following sensitivity analyses to investigate unexplained heterogeneity and to test the robustness of results:
exclusion of cross‐over trials from the analysis;
exclusion of studies at high risk of bias, with inadequate allocation concealment or lack of blinded outcome assessor;
comparison of fixed‐effect versus random‐effects models.
Summary of findings and assessment of the certainty of the evidence
We have presented three 'Summary of findings' tables, one for each comparison (Table 1; Table 2; Table 3). We reported all outcomes in the tables.
Summary of findings 1. Levetiracetam compared with lamotrigine for the treatment of epilepsy in people with Alzheimer's disease.
| Levetiracetam compared with lamotrigine for the treatment of epilepsy in people with Alzheimer's disease | ||||||
|
Patient or population: people with AD and epileptic seizures Settings: the community of Caltanissetta, Italy Intervention: levetiracetam Comparison: lamotrigine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lamotrigine | Levetiracetam | |||||
| Proportion with seizure freedom | 241 per 1000 | 289 per 1000 (128 to 653) | RR 1.20 (0.53 to 2.71) | 67 (1 study) | ⊕⊝⊝⊝ very low¹ | RR more than 1 favours levetiracetam |
| Proportion with adverse events | 241 per 1000 | 158 per 1000 (60 to 419) | RR 0.65 (0.25 to 1.74) | 67 (1 study) | ⊕⊝⊝⊝ very low¹ | RR less than 1 favours levetiracetam |
| Reduction in seizure frequency of 50% or more | 586 per 1000 | 711 per 1000 (492 to 1026) | RR 1.21 (0.84 to 1.75) | 67 (1 study) | ⊕⊝⊝⊝ very low¹ | RR more than 1 favours levetiracetam |
| Proportion with seizure freedom in dominantly inherited AD | Not reported | Not reported | ||||
| Change in cognition | See comment | See comment | 67 (1 study) | ⊕⊝⊝⊝ very low¹ | In the lamotrigine group, participants showed slight declines in MMSE and ADAS‐Cog scores. In the levetiracetam group, MMSE scores reflected improvement by a mean of 0.23 points as compared with baseline. Similar improvement was observed in ADAS‐Cog scores (−0.23 points). | |
| Change in neuropsychiatric symptoms | See comment | See comment | 67 (1 study) | ⊕⊝⊝⊝ very low¹ | In the lamotrigine group, score change of −0.72 was recorded on the Cornell scale. In the levetiracetam group, mood score worsened by 0.20 points. | |
| Improvement in quality of life | Not reported | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes.² The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive; CI: confidence interval; MMSE: Mini Mental State Examination; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
¹ Only one trial was included, with 67 randomized participants and potential methodological insufficiency.
² Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Summary of findings 2. Levetiracetam compared with phenobarbital for the treatment of epilepsy in people with Alzheimer's disease.
| Levetiracetam compared with phenobarbitalfor the treatment of epilepsy in people with Alzheimer's disease | ||||||
|
Patient or population: patients with AD and epileptic seizures Settings: the community of Caltanissetta, Italy Intervention: levetiracetam Comparison: phenobarbital | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenobarbital | Levetiracetam | |||||
| Proportion with seizure freedom | 286 per 1000 | 289 per 1000 (134 to 626) | RR 1.01 (0.47 to 2.19) | 66 (1 study) | ⊕⊝⊝⊝ very low¹ | RR more than 1 favours levetiracetam |
| Proportion with adverse events | 357 per 1000 | 158 per 1000 (64 to 382) | RR 0.44 (0.18 to 1.07) | 66 (1 study) | ⊕⊝⊝⊝ very low¹ | RR less than 1 favours levetiracetam |
| Reduction in seizure frequency of 50% or more | 643 per 1000 | 711 per 1000 (502 to 1003) | RR 1.11 (0.78 to 1.56) | 66 (1 study) | ⊕⊝⊝⊝ very low¹ | RR more than 1 favours levetiracetam |
| Proportion with seizure freedom in dominantly inherited AD | Not reported | Not reported | ||||
| Change in cognition | See comment | See comment | 66 (1 study) | ⊕⊝⊝⊝ very low¹ | In the phenobarbital group, significant worsening of cognitive performance was found, with lower mean scores indicating aggravation of existing cognitive impairment at both 6 and 12 months post‐randomization on MMSE and ADAS‐Cog. In the levetiracetam group, MMSE scores reflected improvement by a mean of 0.23 points as compared with baseline. Similar improvement was observed in ADAS‐Cog scores (−0.23 points). | |
| Change in neuropsychiatric symptoms | See comment | See comment | 66 (1 study) | ⊕⊝⊝⊝ very low¹ | In the phenobarbital group, mood score worsened by 1.74 points. In levetiracetam group, mood score worsened by 0.20 points. | |
| Improvement in quality of life | Not reported | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes.² The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive; CI: confidence interval; MMSE: Mini Mental State Examination; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
¹ Only one trial was included, with 66 randomized participants and potential methodological insufficiency.
² Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Summary of findings 3. Lamotrigine compared with phenobarbital for the treatment of epilepsy in people with Alzheimer's disease.
| Lamotrigine compared with phenobarbitalfor the treatment of epilepsy in people with Alzheimer's disease | ||||||
|
Patient or population: patients with AD and epileptic seizures Settings: the community of Caltanissetta, Italy Intervention: lamotrigine Comparison: phenobarbital | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenobarbital | Lamotrigine | |||||
| Proportion with seizure freedom | 286 per 1000 | 241 per 1000 (100 to 578) | RR 0.84 (0.35 to 2.02) | 57 (1 study) | ⊕⊝⊝⊝ very low¹ | RR less than 1 favours phenobarbital |
| Proportion with adverse events | 357 per 1000 | 241 per 1000 (107 to 546) | RR 0.68 (0.30 to 1.53) | 57 (1 study) | ⊕⊝⊝⊝ very low¹ | RR less than 1 favours lamotrigine |
| Reduction in seizure frequency of 50% or more | 643 per 1000 | 586 per 1000 (386 to 887) | RR 0.91 (0.60 to 1.38) | 57 (1 study) | ⊕⊝⊝⊝ very low¹ | RR less than 1 favours phenobarbital |
| Proportion with seizure freedom in dominantly inherited AD | Not reported | Not reported | ||||
| Change in cognition | See comment | See comment | 57 (1 study) | ⊕⊝⊝⊝ very low¹ | In the phenobarbital group, significant worsening of cognitive performance was found, with lower mean scores indicating aggravation of existing cognitive impairment at both 6 and 12 months post‐randomization on MMSE and ADAS‐Cog. In the lamotrigine group, participants showed slight declines in MMSE and ADAS‐Cog scores. | |
| Change in neuropsychiatric symptoms | See comment | See comment | 57 (1 study) | ⊕⊝⊝⊝ very low¹ | In the phenobarbital group, mood score worsened by 1.74 points. In the lamotrigine group, score change of −0.72 was recorded on the Cornell scale. | |
| Improvement in quality of life | Not reported | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes.² The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive; CI: confidence interval; MMSE: Mini Mental State Examination; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
¹ Only one trial was included, with 57 randomized participants and potential methodological insufficiency.
² Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
We determined the certainty of the evidence using the GRADE approach (GRADEPro 2004; Higgins 2017), and downgraded evidence in the presence of: a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, and high probability of publication bias. We downgraded the evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
The previous version of this review included one study. On re‐running the searches on 3 August 2020, we identified 153 papers after de‐duplicating the results (Figure 1). After screening the titles and abstracts, we obtained the full papers of five studies and assessed them for eligibility. We excluded four studies, two because they had non‐randomized designs (Campion 1995; Zelano 2020) and two because the participants were ineligible (Lovestone 2015; Musaeus 2017). There are two ongoing RCTs (NCT02002819; NCT03489044).
1.
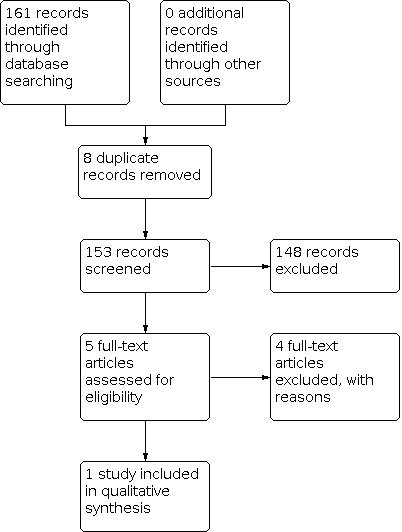
Study flow diagram.
Included studies
One RCT met the inclusion criteria (Cumbo 2010). A total of 95 participants with Alzheimer's disease (AD) and epileptic seizures (41 males, 54 females) were enrolled. The participants had a mean age of 71.75 years (range: 65 to 82 years), a mean duration of education of 6.3 years (range: 5 to 17 years), and lived in the community. They were randomly assigned with an antiepileptic drug (AED) as monotherapy: 38 were administered levetiracetam (LEV), 28 phenobarbital (PB), and 29 lamotrigine (LTG). This study comprised a four‐week dose adjustment period followed by a 12‐month dose evaluation period. All the participants had concomitant cholinesterase inhibitor therapy for AD. Other concomitant medications (including diuretics, antihypertensives, lipid‐reducing agents, and antidiabetic drugs) started prior to the baseline visit were allowed during the study. During the treatment periods, participants received LEV, PB, or LTG and were titrated to an effective dose. No participant was previously exposed to another AED. The initial target dosage of LEV was 500 mg/day, increased weekly by 500 mg/day; for PB it was 50 mg/day, increased weekly by 50 mg/day; and for LTG it was 25 mg/day, increased weekly by 25 mg/day. Thereafter, the dosage was individually adjusted. In summary, the mean daily dose of LEV monotherapy was 956 mg (range: 500 to 2000 mg/day); the mean daily dose of PB monotherapy was 90 mg/day (range: 50 to 100 mg/day); and the mean daily dose of LTG monotherapy was 57.5 mg/day (range: 25 to 100 mg/day). Further details of the included study is provided in the Characteristics of included studies.
Excluded studies
We excluded four studies after full‐text evaluation (Campion 1995; Lovestone 2015; Musaeus 2017; Zelano 2020). The reasons for exclusion were due to non‐randomized design or ineligible participants (see Characteristics of excluded studies).
Ongoing studies
We found two related ongoing studies (NCT02002819; NCT03489044). NCT02002819 was about levetiracetam for AD‐associated network hyperexcitability with randomized cross‐over assignment. The participants met the National Institute on Aging‐Alzheimer's Association Workgroups criteria for probable AD dementia, whose age were ≤ 80 years at time of screening; and MMSE score were ≥ 18 and/or CDR < 2 at the initial screening assessment. The comparison was levetiracetam and placebo, and the outcomes were changes in executive function, epileptiform activity frequency, cognitive function and behavior and level of disability. NCT03489044 was an investigation of levetiracetam in AD with double‐blind, randomized, cross‐over assignment. The participants met the National Institute of Aging‐Alzheimer's Association criteria for probable AD. The comparison was levetiracetam and placebo, and the outcomes were hippocampal function, mood, quality of life and adverse events.
Risk of bias in included studies
The information regarding risk of bias is provided in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study did not describe the method used to generate allocation sequence, or whether the methods of allocation were concealed or not. Therefore we regarded it as having an unclear risk of selection bias.
Blinding
The details of blinding were not mentioned in the included study. Thus, we evaluated it as having an unclear risk of both performance bias and detection bias.
Incomplete outcome data
Eighty‐three participants (87%) completed the study (35 (92%) on LEV, 23 (82%) on PB, and 25 (86%) on LTG). The reasons for dropouts were given. Therefore, we assessed the study as having a low risk of attrition bias.
Selective reporting
We intended to use the table of 'Outcome Reporting Bias In Trials' (ORBIT) to evaluate selective outcome reporting (Kirkham 2010). However, we could not assess reporting bias as no pre‐published protocols were available.
Other potential sources of bias
We found no other potential sources of bias. Insufficient numbers of trials were available for a funnel plot analysis.
Effects of interventions
See: Table 1; Table 2; Table 3
See: Table 1; Table 2; Table 3. An efficacy and tolerability analysis was conducted of all participants who had taken at least one dose, i.e. intention‐to‐treat (ITT) population. People who discontinued the drug for any reason were considered non‐responders with 0% difference from baseline seizure frequency and were included in the efficacy results. There was no difference between groups in demographic or baseline characteristics. All the outcomes were measured at the 12‐month endpoint.
Primary outcomes
1. Proportion of participants with seizure freedom
At 12 months, 11 of 38 participants (29%) in the LEV group had become seizure free; seven of the 11 were seizure free from the start of therapy, and the other four became seizure free after two months (with a relatively high dosage, 2000 mg/day). In the PB group, eight of 28 participants (29%) were seizure free from the start of therapy, at a dosage of 100 mg/day. In the LTG group, elimination of seizures was achieved by seven of 29 participants (24%) (Analysis 1.1; Analysis 2.1; Analysis 3.1).
1.1. Analysis.

Comparison 1: Levetiracetam versus lamotrigine, Outcome 1: Proportion with seizure freedom
2.1. Analysis.

Comparison 2: Levetiracetam versus phenobarbital, Outcome 1: Proportion with seizure freedom
3.1. Analysis.

Comparison 3: Lamotrigine versus phenobarbital, Outcome 1: Proportion with seizure freedom
2. Proportion of participants who experienced any adverse events (AEs)
In the LEV group, AEs were observed in six participants (16%). These AEs included somnolence, asthenia, headache and dizziness. No participant withdrew due to AEs. In the PB group, 10 participants (36%) experienced AEs. Somnolence and asthenia were the most frequently reported AEs. PB was discontinued by four participants due to AEs. In the LTG group, seven participants (24%) reported AEs. No participant withdrew because of AEs. There was no statistically significant difference in any of these comparisons (Analysis 1.3; Analysis 2.3; Analysis 3.3).
1.3. Analysis.

Comparison 1: Levetiracetam versus lamotrigine, Outcome 3: Proportion with adverse events
2.3. Analysis.

Comparison 2: Levetiracetam versus phenobarbital, Outcome 3: Proportion with adverse events
3.3. Analysis.

Comparison 3: Lamotrigine versus phenobarbital, Outcome 3: Proportion with adverse events
Secondary outcomes
1. Proportion of participants with dominantly inherited Alzheimer's disease with seizure freedom
No data were reported.
2. Reduction in seizure frequency of 50% or more
In the LEV group, 27 of 38 participants (71%) had a greater than 50% reduction in seizure frequency at 12 months follow‐up. In the PB group, 18 of 28 participants (64%) had a greater than 50% reduction in seizure frequency. In the LTG group, a greater than 50% reduction in seizure frequency was observed in 17 of 29 participants (59%). There was no statistically significant difference in any of these comparisons (Analysis 1.2; Analysis 2.2; Analysis 3.2).
1.2. Analysis.

Comparison 1: Levetiracetam versus lamotrigine, Outcome 2: Reduction in seizure frequency of 50% or more
2.2. Analysis.

Comparison 2: Levetiracetam versus phenobarbital, Outcome 2: Reduction in seizure frequency of 50% or more
3.2. Analysis.

Comparison 3: Lamotrigine versus phenobarbital, Outcome 2: Reduction in seizure frequency of 50% or more
3. Change in cognition
In the LEV group, Mini Mental State Examination (MMSE) scores recorded at the end point reflected improvement by a mean of 0.23 points as compared with baseline. Similar improvement was observed in Alzheimer's Disease Assessment Scale‐Cognitive (ADAS‐Cog) scores (−0.23 points). LEV was particularly associated with improved attention, short‐term memory, and oral fluency. In the PB group, significant worsening of cognitive performance was found, with lower mean scores indicating aggravation of existing cognitive impairment at both six and 12 months post‐randomization on MMSE and ADAS‐Cog. Participants in the LTG group showed slight declines in MMSE and ADAS‐Cog scores. Due to the lack of mean differences and standard deviations provided, qualitative analyses were unavailable.
4. Change in neuropsychiatric symptoms
Participants in the LTG group scored better on measures of mood. They exhibited progressive improvement on the Cornell scale for depression from six months onward. At the endpoint, a score change of −0.72 was recorded on the Cornell scale. Mood scores worsened for the LEV group (0.20) and PB group (1.74). Due to the lack of standard deviations provided, qualitative analyses were unavailable.
5. Improvement in quality of life
No data were reported.
Discussion
Summary of main results
We included one randomized controlled trial (RCT) with 95 participants. We found methodological defects in the trial, details of which are provided in the Characteristics of included studies section. We were unable to carry out a meta‐analysis and instead reported results narratively. There were no significant differences reported between levetiracetam (LEV), phenobarbital (PB) and lamotrigine (LTG), in terms of participants with seizure freedom, reduction in seizure frequency of 50% or more, and adverse events. It seemed that LEV could improve cognition and LTG could relieve depression, while PB and LTG could worsen cognition, and LEV and PB could worsen mood.
Overall completeness and applicability of evidence
The one included RCT on pharmacological interventions reported no significant differences between LEV, PB and LTG in the outcomes of efficacy and safety. There were 95 participants randomized, and there were 12 dropouts at the end point. All the participants had concomitant cholinesterase inhibitor therapy for AD. Other concomitant medications (including diuretics, antihypertensives, lipid‐reducing agents, and antidiabetic drugs) started prior to the baseline visit were allowed during the study. No studies were found for non‐pharmacological interventions. Large, well‐designed, parallel‐group RCTs on pharmacological or non‐pharmacological interventions are required.
Quality of the evidence
Only one RCT comprising 95 randomized participants was included. We identified methodological issues in the study and we are therefore uncertain about the results. The study did not provide details of allocation sequence generation, allocation concealment, or blinding of participants and personnel. Considering only one trial was included with a small sample size and potential methodological insufficiency, we judged the certainty of the evidence for the outcomes to be very low.
Potential biases in the review process
The search for trials was rigorously performed based on the strategies in different electronic databases. We found one eligible RCT and two ongoing studies. To identify unpublished or incomplete trials, we also searched for protocols, but found no eligible studies. It is possible that certain unpublished trials were not identified. In addition, due to the inclusion of only one RCT, we could not assess publication bias using funnel plots.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first review to systematically evaluate the efficacy and tolerability of treatment of epilepsy in people with AD.
Authors' conclusions
Implications for practice.
This review does not provide sufficient evidence to support levetiracetam (LEV), phenobarbital (PB), or lamotrigine (LTG) for the treatment of epilepsy in people with Alzheimer's disease (AD). Regarding efficacy and tolerability, no significant differences were found between LEV, PB and LTG.
Implications for research.
Large randomized, double‐blind, controlled, parallel‐group clinical trials are required to determine the efficacy and tolerability of treatment of epilepsy in people with AD.
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2020 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 3 August 2020 | New search has been performed | Searches updated 3 August 2020; no new studies were identified. |
History
Protocol first published: Issue 10, 2015 Review first published: Issue 11, 2016
| Date | Event | Description |
|---|---|---|
| 10 July 2018 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 10 July 2018 | New search has been performed | Searches updated 10 July 2018; no new studies were identified. |
Acknowledgements
The review authors acknowledge the help provided by the Cochrane Epilepsy Group, as well as Yu‐Ping Wang and Li‐Yong Wu for their contributions to previous versions of the review.
This review update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Epilepsy. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register
1. MESH DESCRIPTOR Alzheimer Disease EXPLODE ALL AND CENTRAL:TARGET
2. MESH DESCRIPTOR Dementia EXPLODE ALL AND CENTRAL:TARGET
3. (alzheimer* OR dement*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
4. #1 OR #2 OR #3
5. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
6. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
7. (epilep* OR seizure* OR convuls*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
8. #5 OR #6 OR #7 AND CENTRAL:TARGET
9. #4 AND #8
Appendix 2. MEDLINE (Ovid)
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2019).
1. exp Alzheimer Disease/ or exp Dementia/
2. (alzheimer$ or dement$).tw.
3. 1 or 2
4. exp Epilepsy/
5. exp Seizures/
6. (epilep$ or seizure$ or convuls$).tw.
7. 4 or 5 or 6
8. exp *Pre‐Eclampsia/ or exp *Eclampsia/
9. 7 not 8
10. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
11. clinical trials as topic.sh.
12. trial.ti.
13. 10 or 11 or 12
14. exp animals/ not humans.sh.
15. 13 not 14
16. 3 and 9 and 15
17. remove duplicates from 16
Data and analyses
Comparison 1. Levetiracetam versus lamotrigine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion with seizure freedom | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Reduction in seizure frequency of 50% or more | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Proportion with adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Levetiracetam versus phenobarbital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion with seizure freedom | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Reduction in seizure frequency of 50% or more | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Proportion with adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Lamotrigine versus phenobarbital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion with seizure freedom | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Reduction in seizure frequency of 50% or more | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Proportion with adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cumbo 2010.
| Study characteristics | ||
| Methods | A prospective, randomized, three‐arm parallel‐group clinical trial | |
| Participants | Inclusion criteria: mild to moderate disease (MMSE score: 10 to 26), age between 60 and 90 years, educational level ≥ 5 years, a diagnosis of partial epilepsy according to the International League Against Epilepsy diagnostic scheme, and a caregiver who could ensure compliance with treatment and provide the information required for psychometric assessments. Exclusion criteria: history of primary neurological or psychiatric disease other than AD, history of seizures experienced prior to the development of AD, drug or alcohol abuse, clinically significant or unstable medical or surgical disorders that could influence the outcome of the study, previous treatment for epilepsy, concomitant treatment with antidepressants or neuroleptics, use of investigational drugs, and refusal to give informed consent in writing. A total of 95 people with AD and epileptic seizures (41 males, 54 females) with a mean age of 71.75 years (range: 65 to 82 years) and a mean duration of education of 6.3 years (range: 5 to 17 years) living in the community were included in the study. Participants were randomly assigned with an AED as monotherapy: 38 were administered LEV, 28 PB, and 29 LTG. |
|
| Interventions | This study comprised a 4‐week dose adjustment period followed by a 12‐month dose evaluation period. During the treatment periods, participants received LEV, PB, or LTG and were titrated to an effective dose. No participant was previously exposed to another AED. The initial target dosage of LEV was 500 mg/day, increased weekly by 500 mg/day; for PB, it was 50 mg/day increased weekly by 50 mg/day; and for LTG, it was 25 mg/day increased weekly by 25 mg/day. Thereafter, the dosage was individually adjusted. The mean daily dose of LEV monotherapy was 956 mg (range: 500 to 2000 mg/day). The mean daily dose of PB monotherapy was 90 mg/day (range: 50 to 100 mg/day). The mean daily dose of LTG monotherapy was 57.5 mg/day (range: 25 to 100 mg/day). |
|
| Outcomes | Primary outcomes: percentage of participants achieving at least 50% and 100% (seizure‐freedom) reduction in seizure frequency; adverse events. Secondary outcomes: changes in MMSE score; changes in ADAS‐Cog score; changes in Cornell scale for depression. | |
| Notes | All participants had concomitant cholinesterase inhibitor therapy for AD. Other concomitant medications, including diuretics, antihypertensives, lipid‐reducing agents, and antidiabetic drugs, started prior to the baseline visit were allowed during the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was randomly assigned to be treated with an AED as monotherapy: 38 were administered LEV, 28 PB, and 29 LTG. Further details were not provided. |
| Allocation concealment (selection bias) | Unclear risk | The details were not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The details were not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The details were not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 participants (35 on LEV, 23 on PB, 25 on LTG) (87%) completed the study. The reasons for dropouts were given. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported in the methods and results were consistent; and all important outcomes expected were reported. |
| Other bias | High risk | Minimum necessary sample size was not calculated. |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer’s Disease Assessment Scale‐Cognitive; AED: antiepileptic drug; LEV: levetiracetam; LTG: lamotrigine; MMSE: Mini Mental State Examination; PB: phenobarbital
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Campion 1995 | Not a randomized controlled trial |
| Lovestone 2015 | The participants were not eligible. |
| Musaeus 2017 | The participants were not eligible. |
| Zelano 2020 | Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
NCT02002819.
| Study name | Levetiracetam for Alzheimer's Disease‐Associated Network Hyperexcitability |
| Methods | Randomized cross‐over assignment |
| Participants | Meets National Institute on Aging‐Alzheimer's Association Workgroups criteria for probable AD dementia; age ≤ 80 years at time of screening; willing and able caregiver who has daily contact with the subject; MMSE score ≥ 18 and/or CDR < 2 at the initial screening assessment |
| Interventions | Levetiracetam and placebo |
| Outcomes | Changes in executive function, epileptiform activity frequency, cognitive function and behavior and level of disability |
| Starting date | January 2018 |
| Contact information | Keith A Vossel, MD, MSc. University of Minnesota ‐ Clinical and Translational Science Institute |
| Notes | Estimated study completion date: December 2019 |
NCT03489044.
| Study name | An Investigation of Levetiracetam in Alzheimer's Disease |
| Methods | Double‐blind, randomized, cross‐over assignment |
| Participants | Meets the National Institute of Aging‐Alzheimer's Association criteria for probable AD |
| Interventions | Levetiracetam and placebo |
| Outcomes | Hippocampal function, mood, quality of life and adverse events |
| Starting date | April 2018 |
| Contact information | Arjune Sen, PhD, FRCPE. Nuffield Department of Clinical Neurosciences |
| Notes | Estimated Study Completion Date: December 2019 |
AD: Alzheimer's disease; CDR: Clinical Dementia Rating; MMSE: Mini Mental Status Exam
Differences between protocol and review
None.
Contributions of authors
JL and L‐NW formulated the idea and developed the basis for the review. JL and L‐NW selected the studies and assessed the risk of bias. The manuscript was completed by JL and revised by L‐NW. JL will be in charge of future updates.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research (NIHR), UK
Declarations of interest
JL: none known. L‐NW: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cumbo 2010 {published data only}
- Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer's disease. Epilepsy & Behavior 2010;17(4):461-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Campion 1995 {published data only}
- Campion D, Brice A, Hannequin D, Tardieu S, Dubois B, Calenda A, et al. A large pedigree with early-onset Alzheimer's disease: clinical, neuropathologic, and genetic characterization. Neurology 1995;45(1):80-5. [DOI] [PubMed] [Google Scholar]
Lovestone 2015 {published data only}
- Lovestone S, Boada M, Dubois B, Hüll M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer's disease. Journal of Alzheimer's Disease 2015;45(1):75-88. [DOI] [PubMed] [Google Scholar]
Musaeus 2017 {published data only}
- Musaeus CS, Shafi MM, Santarnecchi E, Herman ST, Press DZ. Levetiracetam alters oscillatory connectivity in Alzheimer's disease. Journal of Alzheimer's Disease 2017;58(4):1065-76. [DOI] [PubMed] [Google Scholar]
Zelano 2020 {published data only}
- Zelano J, Brigo F, Garcia-Patek S. Increased risk of epilepsy in patients registered in the Swedish Dementia Registry. European Journal of Neurology 2020;27(1):129-35. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02002819 {unpublished data only}
- Vossel KA. Levetiracetam for Alzheimer's disease-associated network hyperexcitability. https://clinicaltrials.gov (accessed 3 August 2020). [Google Scholar]
NCT03489044 {unpublished data only}
- Sen A. An investigation of levetiracetam in Alzheimer's disease. https://clinicaltrials.gov (accessed 3 August 2020). [Google Scholar]
Additional references
Brodie 2009
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurology 2009;8(11):1019-30. [DOI] [PubMed] [Google Scholar]
Chin 2013
- Chin J, Scharfman HE. Shared cognitive and behavioral impairments in epilepsy and Alzheimer's disease and potential underlying mechanisms. Epilepsy & Behavior 2013;26(3):343-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleisher 2011
- Fleisher AS, Truran D, Mai JT, Langbaum JB, Aisen PS, Cummings JL, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 2011;77(13):1263-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro 2004 [Computer program]
- GRADE Working Group GRADEPro Version 3.6 for Windows. Brozek J, Oxman A, Schunemann H. GRADE Working Group, 2004.
Higgins 2017
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 [updated June 2017]. The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hsu 2015
- Hsu WY, Ku Y, Zanto TP, Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer's disease: a systematic review and meta-analysis. Neurobiology of Aging 2015;36(8):2348-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimiskidis 2010
- Kimiskidis VK. Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. European Neurology 2010;63(4):205-10. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Laxpati 2014
- Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics 2014;11(3):508-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 [updated July 2019]. Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Mattson 1985
- Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado-Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. The New England Journal of Medicine 1985;313(3):145-51. [DOI] [PubMed] [Google Scholar]
McElroy‐Cox 2009
- McElroy-Cox C. Alternative approaches to epilepsy treatment. Current Neurology and Neuroscience Reports 2009;9(4):313-8. [DOI] [PubMed] [Google Scholar]
Mendez 2003
- Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs & Aging 2003;20(11):791-803. [DOI] [PubMed] [Google Scholar]
Minkeviciene 2009
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fülöp L, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. The Journal of Neuroscience 2009;29(11):3453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palop 2007
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 2007;55(5):697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandis 2012
- Pandis D, Scarmeas N. Seizures in Alzheimer disease: clinical and epidemiological data. Epilepsy Currents 2012;12(5):184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puri 2001
- Puri BK, Ho KW, Singh I. Age of seizure onset in adults with Down's syndrome. International Journal of Clinical Practice 2001;55(7):442-4. [PubMed] [Google Scholar]
Rao 2009
- Rao SC, Dove G, Cascino GD, Petersen RC. Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy & Behavior 2009;14(1):118-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roberson 2011
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. The Journal of Neuroscience 2011;31(2):700-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowan 2005
- Rowan AJ, Ramsay RE, Collins JF, Pryor F, Boardman KD, Uthman BM, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005;64(11):1868-73. [DOI] [PubMed] [Google Scholar]
Saetre 2007
- Saetre E, Perucca E, Isojarvi J, Gjerstad L, LAM 40089 Study Group. An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia 2007;48(7):1292-302. [DOI] [PubMed] [Google Scholar]
Tallis 2002
- Tallis R, Boon P, Perucca E, Stephen L. Epilepsy in elderly people: management issues. Epileptic Disorders 2002;4 Suppl 2:S33-9. [PubMed] [Google Scholar]
Westmark 2008
- Westmark CJ, Westmark PR, Beard AM, Hildebrandt SM, Malter JS. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. International Journal of Clinical and Experimental Pathology 2008;1(2):157-68. [PMC free article] [PubMed] [Google Scholar]
Wu 2009
- Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. The American Journal of Geriatric Psychiatry 2009;17(7):614-20. [DOI] [PubMed] [Google Scholar]
Wu 2012
- Wu L, Rosa-Neto P, Hsiung GY, Sadovnick AD, Masellis M, Black SE, et al. Early-onset familial Alzheimer's disease (EOFAD). Canadian Journal of Neurological Sciences 2012;39(4):436-45. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2015
- Liu J, Wang LN. Treatment of epilepsy for people with Alzheimer's disease. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD011922. [DOI: 10.1002/14651858.CD011922] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016
- Liu J, Wang LN. Treatment of epilepsy for people with Alzheimer's disease. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD011922. [DOI: 10.1002/14651858.CD011922.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


