Abstract
Introduction:
A drug–drug interaction (DDI) describes the influence of one drug upon another or the change in a drug’s effect on the body when the drug is taken together with a second drug. A DDI can delay, decrease or enhance absorption or metabolism of either drug. Several antifungal agents have a large number of potentially deleterious DDIs.
Methods:
The antifungal drug interactions database https://antifungalinteractions.org/was first launched in 2012 and is updated regularly. It is available as web and app versions to allow information on potential drug interactions with antifungals with a version for patients and another for health professionals. A new and updated database and interface with apps was created in 2019. This allows clinicians and patients to rapidly check for DDIs. The database is fully referenced to allow the user to access further information if needed. Currently DDIs for fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole, terbinafine, amphotericin B, caspofungin, micafungin and anidulafungin are cross-referenced against 2398 other licensed drugs, a total of nearly 17,000 potential DDIs.
Results:
The database records 541 potentially severe DDIs, 1129 moderate and 1015 mild DDIs, a total of 2685 (15.9%).
Conclusion:
As the online database and apps are free to use, we hope that widespread acceptance and usage will reduce medical misadventure and iatrogenic harm from unconsidered DDIs.
Keywords: antifungal, antifungal interactions, antifungal stewardship, cytochrome P450, drug–drug interaction
Introduction
Antifungal agents interact with many other drugs via many different mechanisms. Clinicians may find it difficult to access and interpret the data in a time-efficient manner when consulting patients. Rapid accurate information available in a single place, rather than having to access different sources of information, would be beneficial to both clinician and patient, and reduce harm from known drug interactions.
In this era of growing antimicrobial resistance, healthcare professionals often only have a limited number of chances to ensure correct treatment of any given infection. Ensuring correct treatment of infection is easier with the better diagnostics now available but there are hundreds of licensed drugs to select from or that may be used together. This may mean that multimorbidity can lead to polypharmacy, now a common phenomenon and readily opens the door for drug–drug interactions (DDIs). The challenge of treating infections appropriately together with ensuring minimal harm to the patient is a difficult predicament for all healthcare professionals across the world.1
Often preventable, a DDI occurs when two or more drugs interact with each other, resulting in altered drug effectiveness or toxicity. DDIs have the potential to cause serious harm to patients. However, the true extent of harm as a result of DDIs is not well established.2 A review found that 33% of general inpatients and 67% of intensive care patients experienced a DDI during their hospital stay.2 Another study found outpatient experience to be around 22%.3
Antimicrobial stewardship (AMS) embeds the principles of the responsible usage and preservation of antimicrobials.4 Increasing vigilance of antimicrobial use has focused not just on antibiotics, but antifungals alike. The World Health Organization has developed a toolkit on AMS for low- and middle-income countries, making it a priority for everyone across the globe.5 Antifungal stewardship has been brought to the forefront of this campaign to ensure appropriate use but does pose some unique challenges. Patients presenting with invasive fungal infections have greater comorbidities and high case-mortality rates, and the treatments are associated with higher costs and greater risk of toxicity. These toxicities are frequently as a result of drug interactions.4
The use of antifungals poses a daily challenge for professionals of many different specialities in modern hospitals and primary care alike, with more patients being managed in their own homes. Since the introduction of newer antifungal agents, prescribing complexity has increased, and while the newer agents have fewer potential DDIs, they still have much potential for adverse events, some associated with DDIs.
One situation where stewardship efforts can be hindered is where patients are taking many drugs and concern about possible interactions can limit the use of the best (or affordable) antifungal agent. If knowledge of these interactions was at a clinician’s fingertips, it would allow for interactions to be managed much more effectively and for patients to be treated with the most appropriate antifungal agent, while adhering to antifungal stewardship principles.
An antifungal interactions database was first launched in 2012 by the Fungal Infection Trust to support the National Aspergillosis Centre as it was felt there was a need for a single central resource to hold all interaction data for this class of drugs.6 The continuously updated database allows clinicians to access timely antifungal interaction information in an easy access format, within minutes. The database is a resource not available elsewhere in a single location and free to use. It allows immediate decision-making on whether the antifungal agent is suitable for each patient or needs a dose modification.
However as the database was updated only periodically this lead to delays of many weeks or months when newly licensed drugs were not included. The older version also did not have the ability to cite references used to compile the entry for the particular interaction listed.
Materials and methods
In 2018, a decision was made to update the database to allow real-time updating and link DDIs with pertinent published or book references. Using .net core software for the desktop version and NativeScript for the mobile app versions, there is now real-time updating. References on where the information originated are added allowing the user to check the credibility of the reference source. These can be accessed easily by a simple click on the link within the database.
Apart from using a standard internationally recognised ‘traffic light’ system to describe the severity of interactions easily, a fourth (blue), ‘unlikely’ was added. This allows the person adding and reviewing the interaction to categorise all interactions, whether that be severe, moderate, mild or unlikely. It was felt that this was necessary as in the previous version there was a blank space which could be mistaken as missing data, rather than no interaction by the end user.
Originally there were two versions of the database; one for clinicians and one for patients and carers. An easy toggle bar now allows the user to interchange easily between the two. Prior to this the easy switch between the two was not possible, as the databases were separate. The patient database uses more straightforward, non-technical language.
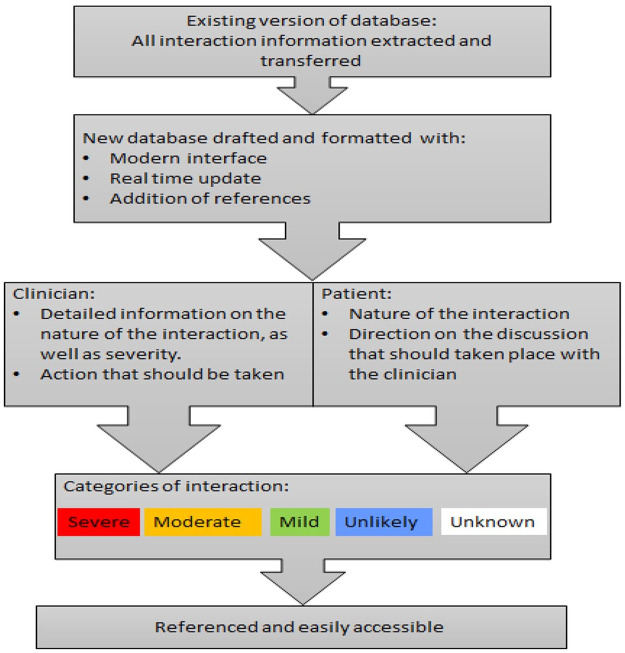
Figure 1 shows the process of how the database is structured.
Figure 1.
Sample of web view.
Searching relies on generic drug names and uses predictive text. The database is available on https://antifungalinteractions.org/ as well as being accessible via the https://www.aspergillus.org.uk/ website. Being a well-known and respected site, it gives the user confidence about the credibility of the information provided. The database is publicly available for everyone to view and access.
The interaction categories are clear immediately, giving the user a very quick answer in an instant. The text provides further assurance on the nature of the action required, if indeed any action at all.
Table 1 shows the number of interactions present, corresponding to the severity they have been allocated in the database. This number increases on a weekly basis as more drugs and interactions are added
Table 1.
The number of interactions by antifungal agent categorised as severe, moderate, mild and unlikely.
| Triazoles | Polyenes | Echinocandins | Terbinafine | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Itra | Vori | Fluco | Posa | Isavu | Am B | Ambisome | Caspo | Mica | Andidula | |||
| Severe | 143 | 154 | 45 | 99 | 52 | 19 | 18 | 10 | – | – | 1 | 541 |
| Moderate | 162 | 181 | 181 | 196 | 92 | 127 | 127 | 40 | 7 | 3 | 13 | 1129 |
| Mild | 130 | 159 | 197 | 159 | 43 | 99 | 98 | 35 | 30 | 22 | 43 | 1015 |
| Unlikely | 1101 | 1042 | 1113 | 1082 | 1349 | 1291 | 1293 | 1451 | 1499 | 1511 | 1479 | 14,211 |
| 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 1536 | 16,896 | |
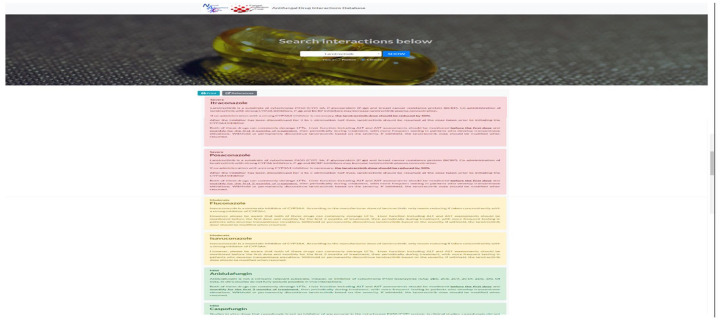
Figure 2 is an example of the web version of the antifungal interaction database. Interactions with a red-shaded background indicate a severe interaction, an amber-shaded background indicates a moderate interaction and a green-shaded background denotes mild interaction. A blue-shaded background (not shown) indicates interaction is unlikely.
Figure 2.
Sample view of web version of antifungal interaction database. Interactions with a red-shaded background indicate a severe interaction, an amber-shaded background indicates a moderate interaction and a green-shaded background denotes mild interaction. A blue-shaded background (not shown) indicates interaction is unlikely.
Figure 3 shows the icon as it appears in the Google app store. A quick response (QR) code for ease of download is also included.
Figure 3.
Sample view of the android app as seen in the Google app store.
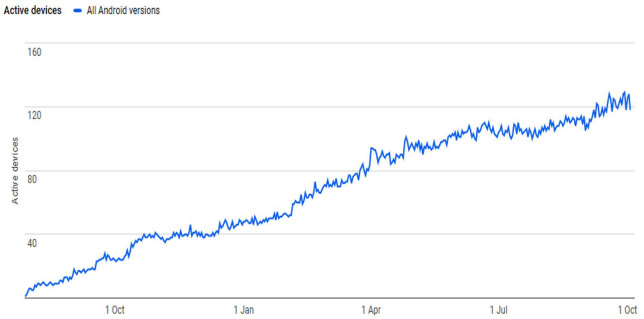
The database is available in smartphone app format. The android version has been functional since early 2019 with a steady increase in downloads with time. Figure 4 indicates the increase in downloads with easy accessibility for download from the app store and the QR code provided. Over 3300 (android) and 501 (iOS) apps have been downloaded.
Figure 4.
Smartphone app downloads on active devices (2019–2020).
Downloads over time from the launch of the newer version of the app can be seen in Figure 4. Unfortunately, Apple has refused to host the app on the iOS platform on the grounds that it is not complex enough to warrant inclusion in the Apple store. However, as the database is available in a webpage format, it allows flexibility for use in the smaller browsing formats on mobile phones and tablets. Instructions on the https://antifungalinteractions.org/ host site easily allow those using an iOS smartphone to use the database effectively.
To ensure no users are unable to use the database, Figure 5 shows the instructions available on the aspergillus website as a guide for how to optimise the website for a smaller screen.
Figure 5.
Small screen instructions. Message on the aspergillus website is a guide for users on how to optimise the website for a smaller screen.
In this day and age of fast-paced technology, simplicity is often overlooked. The database was designed to allow the user ease of access to antifungal drug interaction information in order to ensure patient safety is at the forefront.
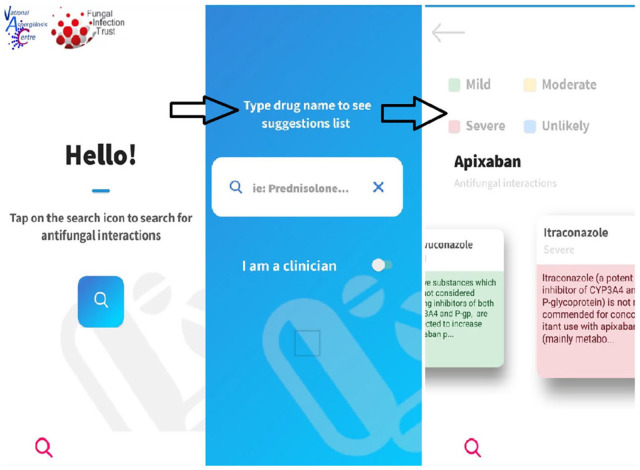
A sample view of the smartphone app can be seen in Figure 6. The smartphone app version allows the user to access the information with minimal effort. The toggle between clinician and patient versions is as easy as the web version. The visual on the severity of the interactions remains with an easy sliding of the screen to allow navigation between the different antifungal agents.
Figure 6.
Sample of smartphone app view. The smartphone app version allows the user to access the information with minimal effort. The toggle between clinician and patient versions is as easy as the web version. The visual on the severity of the interactions remains with an easy sliding of the screen to allow navigation between the different antifungal agents.
The database is updated on a weekly basis using a horizon scanning approach combined with weekly literature searches. All new drugs licensed in Europe or the USA are reviewed for potential DDIs with antifungals. A key data source is the summary of product characteristics (SPC) together with further exploration, as needed, with reviews of published papers. Cytochrome P450 (CYP P450) and P-glycoprotein (P-gp) are the major interaction pathways where antifungal drugs are involved. However other pathways, including renal dysfunction, organic anion-transporting polypeptides (OATPs) and breast cancer resistance protein (BCRP) function are also explored, as there is new and emerging information on the effect of these pathways with antifungal DDIs.
Interactions pathways
CYP P450 enzymes are essential for the metabolism of many drugs. This class has more than 50 enzymes, with CYP3A4 being one of the most significant enzymes involved in antifungal agent interactions. CYP P450 enzymes can be inhibited or induced by drugs, resulting in clinically significant DDIs that can cause adverse reactions or therapeutic failures. Interactions with warfarin, antidepressants, anti-epileptics, antifungals and statins often involve the CYP P450 enzymes.8
There is a particular polymorphism in CYP 2C19 that substantially affects voriconazole metabolism. Voriconazole undergoes extensive hepatic metabolism, which is mediated by the CYP 2C19 enzyme. The gene encoding CYP 2C19 is highly polymorphic, with many variant alleles.9 These variations or polymorphisms in this enzyme can lead to an individual with genotypes that confer the rapid metaboliser and ultra-rapid metaboliser phenotypes. This can lead to subtherapeutic levels of voriconazole due to increased enzyme activity compared with the normal metaboliser, and in certain circumstances could even lead to failure of treatment.
Equally, polymorphisms can also result in the opposite effect, whereby individuals are intermediate and poor metabolisers. This can give rise to significant reduction in enzyme activity compared with normal metabolisers, which can result in toxicity due to higher systemic levels.
P-gp is localised in the apical plasma membrane of intestinal epithelial cells, where it limits entry of substrates from the gut lumen, and at the apical surface of endothelial cells in the capillaries of the brain.10 P-gp is a multidrug efflux pump, that is, it transports substances out of cells. It is the most studied of the adenosine triphosphate (ATP)-binding cassette family transporters, and first evidence of the involvement of P-gp was demonstrated in vitro when vinblastine and docetaxel were increased 10-fold and 20-fold, respectively.11
OATP1B1 and OATP1B3 are reported to be exclusively expressed in the liver (localised on the sinusoidal membrane) and play important roles in the hepatic uptake of many therapeutic reagents. These transporters accept, as substrates, several organic anions and mediate their hepatic uptake, for example, simvastatin, valsartan and olmesartan, to name a few of the drugs involved.12
The human BCRP is an ATP-binding cassette efflux transporter. It was so named because it was initially cloned from a multidrug-resistant breast cancer cell line where it was found to confer resistance to chemotherapeutic agents such as mitoxantrone and topotecan.13
Results and Discussion
Since the antifungal drug interaction database was first launched in 2012, the number of interactions listed has steadily increased with the inclusion of the newer antifungal agent, isavuconazole, and new drugs approved for use across the world. Currently DDIs for fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole, terbinafine, amphotericin B, caspofungin, micafungin and anidulafungin are cross referenced against 2398 other licensed drugs, a total of nearly 17,000 potential DDIs. The database records 541 potentially severe DDIs, 1129 moderate and 1015 mild DDIs, a total of 2685 (15.9%).
From Figure 7, it can be seen that of the interactions listed in the antifungal interaction database 20% are severe, 42% are moderate and 38% are of a mild nature. The database not only gives the user this information within minutes, but also gives the user management strategies on how these interactions could be managed. Not using certain combinations of drugs may be one of the options, but dose reduction or increase, blood monitoring (e.g. liver function, renal function, etc.), and changing the timing of administration and formulation are some examples of the management strategies that are included. In addition to these there are also 14, 211 interactions marked as unlikely/unknown.
Figure 7.
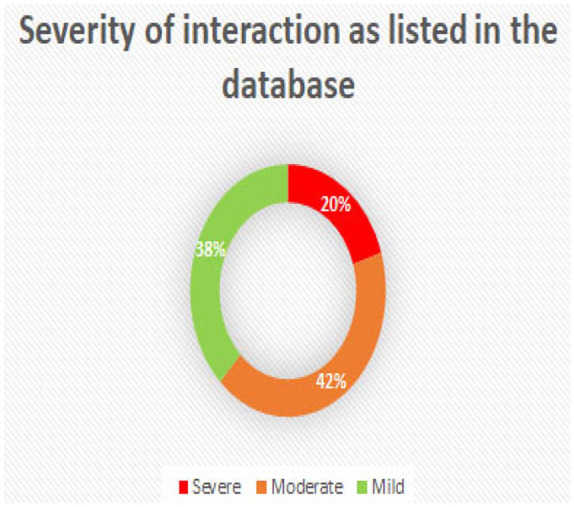
Pictogram of the proportion of different categories of deleterious interactions.
Since 2018, 245 new drugs have been added to the database. Figure 8 demonstrates the number of interactions documented in the database over time and reflects the increase in new drugs licensed and documentation of new drug interactions, including new mechanisms of resistance. This gives the end users of the database a quick and comprehensive summary of DDI information at their fingertips, without having to delve into the literature or scan the SPC looking for individual snippets of information, allowing them to interpret DDI potential for themselves. This is time consuming and many individuals, unfortunately, do not have the time to undertake such a large challenge. At the same time, interpretation of the information available, from a multitude of sources, is vital in keeping patients safe and minimising harm.
Figure 8.
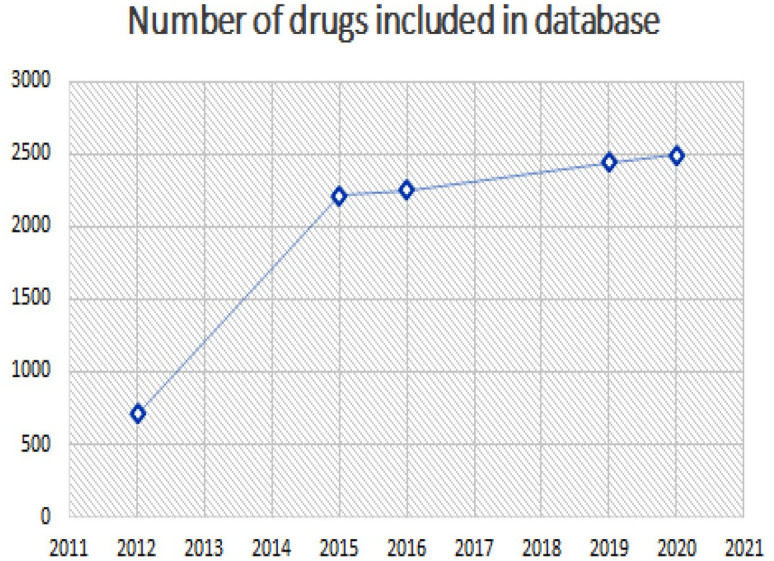
The number of interactions documented in the database over time reflects the increase in new drugs licensed and documentation of new drug interactions, including new mechanisms of resistance.
Limitations
Multimorbidity and polypharmacy
Many older adults have ‘multimorbidity’, defined as experiencing two or more long-term health conditions.14 Often these conditions interact with each other and can make the treatment of individual patients difficult. The word polypharmacy is derived from the ancient Greek ‘polús’ meaning ‘many’, and ‘pharmakeía’ meaning ‘the use of drugs’. This broad meaning from a purely linguistic perspective is reflected in the fact that there is no consensus on a clinical definition of polypharmacy.15
Polypharmacy can magnify side effects, which are generally more common in older people and in those with multiple conditions. Most formal DDI studies are carried out in healthy volunteers, so accurate assessment of the negative impact of polypharmacy and multiple DDIs is difficult. Side effects are usually not observed in relatively small clinical DDI trials.16 It is impossible to test all possible pairs of drugs, and studies of more than two together provide an insurmountable statistical and logistical challenge.
The antifungal drug interaction database gives end users the ability to ‘check’ potential interactions with ease, in a format that most people across the world are now accustomed to use. However, it cannot compensate for the need of clinical judgement to be exercised. The database is an amalgamation of various sources of information in one central place. It is not currently possible to evaluate multidrug interactions and the user is needed to access individual combinations of drugs and then clinically assess the effect this will have on the patient in question. However, this is perhaps a consideration when further developing the database in the future. It would allow the user to evaluate combinations of medications, rather than checking each drug individually.
Patients and carers
Clinicians from all backgrounds of healthcare are one group of likely end users. However, due to the existence of a patient version of the database it is vitally important that patients and their carers can interpret the data in an appropriate manner. Antifungals are often required to be taken for long durations (months) due to the intrinsic challenges of curing or suppressing fungal disease. Many patients are on long-term antifungals for infections such as aspergillosis or chronic Candida infection. It is important that users can identify interactions of any medications they are taking or asked to take while on an antifungal agent and understand it in its entirety. Patients and carers can use the database as a point of reference, but it is imperative that if this group is unsure about any of what they read, they consult with a healthcare professional.
Language
Another limitation of the database is that it is only available in English. Being a widely used language across the world it seems to be the most reasonable choice as currently having the database available in more languages would not be feasible.
Drug names
The decision to only use generic names and omit brand names seemed to be the only way to minimise confusion. This is recognised as, perhaps, a limitation. Many antifungals have multiple trade names, and these often differ, depending on the country in which the drugs are sold. Keeping to generic names allows some standardisation.
Herbal, complementary and alternative medicine
Currently the database does not have an extensive bank of herbal, complementary and alternative medicines. Drugs, such as cannabis, that have licensed products for recognised conditions, have been included but others, such as vitamin supplements, are not included. A future development plan to incorporate all these medicines would be beneficial. This would require perhaps a subcategory as well as all the current choices and toggles available. For the time being, this is a limitation. A solution currently is available within the aspergillus website as a separate entity and can be accessed via the following URL: https://aspergillosis.org/herbal-supplements-drugdrug-interactions-with-antifungal-medication/.
Patients and clinicians alike are not disadvantaged as the information is available. The advantage of having all the information available in the same format is recognised and this issue will be addressed with future updates of the database.
Antifungal market
The systemic antifungal market was thought to be around US$7.4 billion in 2013 and is growing at around 2–3% annually,17 making it worth around US$9 billion today. A paper from 201718 found the global estimates of chronic pulmonary aspergillosis to be around 3,000,000 cases, ~223,100 cases of cryptococcal meningitis (complicating HIV/AIDs), ~700,000 cases of invasive candidiasis, ~500,000 cases of Pneumocystis jirovecii pneumonia, ~250,000 cases of invasive aspergillosis, ~100,000 cases of disseminated histoplasmosis, over 10,000,000 cases of fungal asthma and ~1,000,000 cases of fungal keratitis occurring annually. These are huge numbers of patients worldwide requiring intervention with antifungal agents, who will no doubt have a number of multimorbidity and polypharmacy issues. It is important that healthcare professionals and patients alike are able to access data on drug interactions in order to optimise antifungal therapy to treat the infection whilst optimising existing drug therapies to ensure least harm for the patient.
Acknowledgments
Susan Banfield provided the DDI data for the first 6 years of the antifungal interaction database.
Footnotes
Authors contribution: SN-A undertook the database revision and improvements, including adding referencing, and write the first draft of the article.
GA has orchestrated links to the website, the app updates and dissemination and contributed to drafts.
MW built the underlying software, including the immediate updates, and contributed to drafting.
DWD conceived the project, raised funding for it and contributed to data curation and drafts of the article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The database is funded by the Fungal Infection Trust. The National Aspergillosis Centre supported the redesign and launch of the new version.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical statement: Our study did not require an ethical board approval because it did not contain human or animal trials
ORCID iD: Saarah Niazi-Ali  https://orcid.org/0000-0001-9976-4243
https://orcid.org/0000-0001-9976-4243
Contributor Information
Saarah Niazi-Ali, Fungal Infection Trust, PO Box 482, Macclesfield, Cheshire SK10 9AR.
Graham T. Atherton, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK National Aspergillosis Centre, Manchester University NHS Foundation Trust, Wythenshawe Hospital, Manchester, UK.
Marcin Walczak, McCollum Consultants, Manchester, UK.
David W. Denning, Manchester Fungal Infection Group, The University of Manchester, Manchester, UK
References
- 1. Muñoz P, Bouza E. The current treatment landscape: the need for antifungal stewardship programmes. J Antimicrob Chemother 2016; 71(Suppl. 2): ii5–ii12. [DOI] [PubMed] [Google Scholar]
- 2. Zheng WY, Richardson LC, Li L, et al. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol 2018; 74: 15–27. [DOI] [PubMed] [Google Scholar]
- 3. Ismail M, Noor S, Harram U, et al. Potential drug-drug interactions in outpatient department of a tertiary care hospital in Pakistan: a cross-sectional study. BMC Health Serv Res 2018; 18: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gohlar G, Hughes S. How to improve antifungal stewardship. Pharm J 2019; 303: 7927. [Google Scholar]
- 5. WHO. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries. A WHO practical toolkit. Geneva: World Health Organization, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atherton GT, Atherton N, Le Sueur H, et al. Antifungal drug interactions database. In: 22nd European congress of clinical microbiology and infectious diseases, London, 31 March–2 April 2012. Abstract P2104. [Google Scholar]
- 7. Gangneux J-P, Lortholary O, Cornely OA, et al. 9th trends in medical mycology held on 11–14 October 2019, Nice, France, organized under the auspices of EORTC-IDG and ECMM. J Fungi (Basel) 2019; 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician 2007; 76: 391–396. [PubMed] [Google Scholar]
- 9. Hamadeh IS, Klinker KP, Borgert SJ, et al. Impact of the CYP2C19 genotype on voriconazole exposure in adults with invasive fungal infections. Pharmacogenet Genomics 2017; 27: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melchior DL, Sharom FJ, Evers R, et al. Determining P-glycoprotein-drug interactions: evaluation of reconstituted P-glycoprotein in a liposomal system and LLC-MDR1 polarized cell monolayers. J Pharmacol Toxicol Methods 2012; 65: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin JH. Drug-drug interaction mediated by inhibition and induction of P-glycoprotein. Adv Drug Deliv Rev 2003; 55: 53–81. [DOI] [PubMed] [Google Scholar]
- 12. Shitara Y. Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab Pharmacokinet 2011; 26: 220–227. [DOI] [PubMed] [Google Scholar]
- 13. Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport – an update. AAPS J 2015; 17: 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mc Namara KP, Breken BD, Alzubaidi HT, et al. Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia. Age Ageing 2017; 46: 291–299. [DOI] [PubMed] [Google Scholar]
- 15. Payne RA. The epidemiology of polypharmacy. Clin Med (Lond) 2016; 16: 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zitnik M, Agrawal M, Leskovec J. Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics 2018; 34: i457–i466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ostrosky-Zeichner L, Casadevall A, Galgiani JN, et al. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 2010; 9: 719–727. [DOI] [PubMed] [Google Scholar]
- 18. Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]