Abstract
Background:
Although the conversion of clinically used breast cancer biomarkers such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) between primary tumors and metastatic lesions is well recognized, data on whether receptor conversion has an effect on therapy management and survival in patients with metastatic breast cancer is limited. This study aimed to investigate the clinical implications of receptor conversion throughout tumor progression.
Methods:
In total, 2450 patients diagnosed with metastatic breast cancer in Tianjin Medical University Cancer Institute and Hospital were analyzed and 426 female patients with available biopsy results from both primary and metastatic sites were included in this study. We investigated the alteration of ER, PR and HER2 during breast cancer progression and evaluated the therapy management and prognostic value of receptor conversion.
Results:
The conversion rates of ER, PR, and HER2 between primary tumors and metastasis were 21.1% (McNemar’s test p < 0.001), 33.2% (p < 0.001), and 11.6% (p = 0.868), respectively. Evaluation of ER, PR, and HER2 status in multiple consecutive metastases revealed a change in 19.1% (p > 0.05), 23.5% (p = 0.021), and 9.8% (p > 0.05) of patients, respectively. Adjuvant therapy (chemotherapy/endocrine therapy) was related to hormone receptor conversion (p < 0.05). A statistically significant differential survival associated with hormone receptor (ER/PR) conversion (log-rank p < 0.05) was observed. In the multivariate analysis, ER conversion was an independent influence factor of survival (p < 0.05). Molecular typing conversion in primary and metastatic lesions also had a significant effect on survival (p < 0.05). We found that changing treatment based on the receptor conversion could affect clinical outcomes (p < 0.05).
Conclusions:
Our findings indicated that receptor conversion during breast tumor progression had a significant effect on survival. Most importantly, our findings proved that patients with receptor conversion benefited from a change in therapy.
Keywords: breast cancer, metastasis, outcomes, primary tumor, receptor conversion, therapy
Introduction
Breast cancer is the most common cancer and the leading cause of cancer-related death among women worldwide.1 Despite significant progress in treatment, tumor metastasis and failure of therapy remain the main causes of death in patients with breast cancer.2 According to ER, PR, and HER2 status, breast cancer is classified into different subtypes, with different epidemiological risk factors and responsiveness to systemic treatments.3 The prognostic value of breast cancer markers such as ER, PR, and HER2 in primary tumors is well recognized and previous studies have already confirmed receptor conversion between primary breast tumor and metastatic sites.4–11 In addition, the American Society of Clinical Oncology/College of American Pathologists guidelines suggest that when there is a change in receptor expression, treatment should be determined according to the ER, PR, and HER2 status of metastatic lesions.12 Therefore, it is clinically significant to assess the receptor status in metastasis. Nevertheless, limited information is available on the effect of receptor conversion on long-term survival, and evidence to determine whether changing the therapy regime based on receptor conversion affects clinical outcomes is lacking. In this retrospective study, we aimed to investigate the clinical implications of receptor conversion throughout tumor progression.
Patients and methods
This study retrospectively obtained the clinical data of 2450 patients with breast cancer treated at Tianjin Medical University Cancer Institute and Hospital to investigate the clinical implications of receptor conversion throughout tumor progression. The median follow-up time was 129 months (113–144 months). All patients provided written informed consent, and the study was approved by relevant institutional ethics committee (approved by the medical ethics committee of Tianjin Medical University Cancer Institute and Hospital on 8 April 2020; Approval number: bc2020032). The study was conducted in accordance with the provisions of the Declaration of Helsinki (2013) and local laws.
To be eligible for the study, female patients must be diagnosed with metastatic breast cancer and all patients must have biopsy confirming a primary tumor and metastasis. The exclusion criteria were as follows: (1) patients with bilateral primary breast cancer; (2) de novo metastatic breast cancer (breast cancer patients with distant metastasis at the initial diagnosis); (3) patients without biopsy confirming a primary tumor or metastasis. In total, 2450 patients diagnosed with metastatic breast cancer treated at Tianjin Medical University Cancer Institute and Hospital were analyzed and 426 patients with available biopsy results from both primary and metastatic sites were included in this study (Figure A1 shows the flow chart of this cohort). The pathological biopsy of primary tumors and corresponding asynchronous metastatic lesions were reassessed by two independent pathologists.
The status of ER, PR and HER2 in primary tumor was dependent on pathology results in surgery puncture biopsy results. Hormone receptors were assessed by immunohistochemistry (IHC). ER/PR status was classified as positive with a threshold of at least 10% of tumor cell nuclei staining positively, as recommended by the European guidelines.13 Hormone receptor status positive was defined as ER positive or PR positive. HER2 positive was defined as HER2 membrane staining scored 3+ by IHC or gene amplification by fluorescent in situ hybridization (FISH). Lesions exhibiting 2+ staining by IHC without FISH results were not included in the analysis.
The McNemar’s test was performed to analyze the conversion of ER, PR, and HER2 status in primary and metastatic sites. A multivariate logistic regression model was used to investigate the correlation between receptor conversion and clinicopathological variables. We performed survival analyses of ER and PR status in primary tumor and the earliest metastasis. The survival data included disease-free survival (DFS) (follow-up from the time from excision of primary tumor to the earliest time of metastasis), progression-free survival (PFS) (follow-up from the time of the earliest metastasis diagnosis to progression or censoring) and overall survival (OS) (follow-up from primary breast cancer diagnosis to death or censoring). Patients with ER, PR, and HER2 conversion in primary tumors and metastasis were divided into four groups: primary (+)-metastasis (+), primary (+)-metastasis (−), primary (−)-metastasis (+) and primary (−)-metastasis (−). According to ER and HER2 expression status between primary and metastatic sites, patients were divided into four groups as follows: group 1: ER (+)/HER2 (+/−) to ER (+)/HER2 (+/−), group 2: ER(+)/HER2 (+/−) to ER(−)/HER2 (+/−), group 3: ER(−)/HER2 (−) to ER (+)/HER2 (+/−) and group 4: ER(−)/HER2 (−) to ER(−)/HER2 (−). To further test whether changing treatment based on hormone receptor conversion could improve prognosis of metastatic breast cancer, the patients were divided into different groups based on receptor conversion and first-line therapy: hormone receptor or ER gain, endocrine therapy (+); hormone receptor or ER gain, endocrine therapy (−); hormone receptor or ER loss, endocrine therapy (+); and hormone receptor or ER loss, endocrine therapy (−). To indirectly investigate whether changing therapy based on HER2 conversion could improve prognosis, the patients were divided into four groups based on HER2 status in metastatic tumor and first-line anti-HER2 therapy: HER2 (−), anti-HER2 therapy (−); HER2 (−), anti-HER2 therapy (+); HER2 (+), anti-HER2 therapy (−); and HER2 (+), anti-HER2 therapy (+). Kaplan Meier analysis was used to calculate the cumulative survival rates of different groups of receptor conversion between primary tumor and metastasis. The log-rank test was used to analyze the comparisons between curves. The multivariate Cox proportional hazards regression model was performed to further test the survival differences after adjusting for potential confounders on survival. A p-value < 0.05 (two-tailed) was considered to be statistically significant. SPSS 25.0 was used to perform statistical analyses. GraphPad Prism 8.0 was applied for image production.
Results
Primary tumor characteristics
Primary tumor characteristics are shown in Table S1. All patients underwent radical resection of their primary tumor. More than 80% of patients were diagnosed with invasive duct carcinomas (85.7%). The expression of ER and PR was positive in 251 (58.4%) and 213 (50.0%) patients, respectively. In total, 90 (21.1%) patients were diagnosed with HER2-positive breast tumor.
Receptor conversion between primary tumor and the earliest metastasis
The status of ER, PR, and HER2 in primary tumor and at the earliest point of metastasis was evaluated in 426, 422, and 309 patients, respectively (Table 1). The conversion rates of ER, PR, and HER2 were 21.1% (McNemar’s test p < 0.001), 33.2% (p < 0.001), and 11.6% (p = 0.868), respectively. The loss of ER and PR in metastasis was observed in 14.8% and 27.7% of patients, respectively, while the acquisition of ER and PR in metastasis was observed in 6.3% and 5.5% of patients, respectively. Loss of hormone receptor in metastasis was more common than acquisition. HER2 status was altered in 11.6% (36 of 309) of patients. With regards to molecular typing conversion, approximately 20% of breast cancer patients had different molecular subtypes between the primary and metastatic sites. A change in molecular type from ER (+)/HER2 (+/−) to ER (−)/HER2 (+/−) was observed in about 15% of patients. In addition, nearly 4% of patients with ER (−)/HER2 (−)-primary tumors were found to have ER (+)/HER2 (+/−)-metastasis.
Table 1.
ER, PR and HER2 conversion in primary and metastatic sites.
| ER, PR, and HER2 status | ER | PR | HER2 | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Primary tumor and metastasis | ||||||
| Local and distant metastasis | ||||||
| Primary positive/metastasis positive | 186 | 43.7 | 95 | 22.5 | 57 | 18.5 |
| Primary positive/metastasis negative | 63 | 14.8 | 117 | 27.7 | 17 | 5.5 |
| Primary negative/metastasis positive | 27 | 6.3 | 23 | 5.5 | 19 | 6.1 |
| Primary negative/metastasis negative | 150 | 35.2 | 187 | 44.3 | 216 | 69.9 |
| Total | 426 | 100.0 | 422 | 100.0 | 309 | 100.0 |
| Local metastasis | ||||||
| Primary positive/metastasis positive | 86 | 43.4 | 37 | 18.9 | 26 | 17.0 |
| Primary positive/metastasis negative | 19 | 9.6 | 54 | 27.6 | 7 | 4.6 |
| Primary negative/metastasis positive | 11 | 5.6 | 13 | 6.6 | 6 | 3.9 |
| Primary negative/metastasis negative | 82 | 41.4 | 92 | 46.9 | 114 | 74.5 |
| Total | 198 | 100.0 | 196 | 100.0 | 153 | 100.0 |
| Distant metastasis | ||||||
| Primary positive/metastasis positive | 100 | 43.9 | 58 | 25.7 | 31 | 19.9 |
| Primary positive/metastasis negative | 44 | 19.3 | 63 | 27.9 | 10 | 6.4 |
| Primary negative/metastasis positive | 16 | 7.0 | 10 | 4.4 | 13 | 8.3 |
| Primary negative/metastasis negative | 68 | 29.8 | 95 | 42.0 | 102 | 65.4 |
| Total | 228 | 100.0 | 226 | 100.0 | 156 | 100.0 |
| Multiple metastasis* | ||||||
| Both local and distant metastasis | ||||||
| Metastasis positive/metastasis positive | 32 | 47.1 | 11 | 16.2 | 8 | 15.7 |
| Metastasis positive/metastasis negative | 7 | 10.3 | 13 | 19.1 | 2 | 3.9 |
| Metastasis negative/metastasis positive | 6 | 8.8 | 3 | 4.4 | 3 | 5.9 |
| Metastasis negative/metastasis negative | 23 | 33.8 | 41 | 60.3 | 38 | 74.5 |
| Total | 68 | 100.0 | 68 | 100.0 | 51 | 100.0 |
The earliest metastasis and the second metastasis.
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Multiple consecutive metastases
The ER, PR, and HER-2 status of multiple (⩾2) consecutive metastases were assessed in 68, 68, and 51 patients, respectively (Table 1). In this study, we aimed to investigate the change in receptor status between the earliest and second metastases. The discordance rates of ER, PR, and HER-2 status between the earliest and second metastases were 19.1%, 23.5%, and 9.8%, respectively. Only PR conversion (p = 0.021) between different metastatic sites was statistically significant. Furthermore, PR loss in the second metastasis was the dominative pattern. Overall, the highest receptor conversion rate was observed in the PR. In addition, dynamic receptor conversion ran through the entire process of tumor progression.
Metastatic sites stratified by ER, PR, and HER2 status
Metastatic sites were divided into local (ipsilateral mammary gland, chest wall, and regional lymph nodes), and distant subgroups (contralateral breast, chest wall, and lymph nodes, bone, brain, pleura, and viscera). Table 1 showed the sites of metastasis stratified by receptor status. Specific anatomical sites stratified by receptor status are shown in Supplementary Table S2. ER conversion was significantly higher in the distant metastasis than in the local metastasis (Fisher’s exact test p = 0.006). Furthermore, compared with local lymph node metastasis, the conversion of ER was higher in the distant lymph node metastasis (p = 0.033). No statistically significant difference was observed in PR or HER2 conversion between the local and distant subgroups.
Correlation between receptor conversion and clinicopathological variables
We performed multivariate logistic regression analysis (forward stepwise procedure) to investigate possible independent influences on receptor conversion (Table S3). Analysis showed that postoperative adjuvant endocrine therapy (p = 0.002) was an independent factor of ER conversion. Patients treated with adjuvant endocrine therapy had a significantly increased risk of ER loss in metastasis compared with patients who did not receive adjuvant endocrine therapy [odds ratio (OR): 5.967, 95% confidence interval (CI): 2.861–12.445; p < 0.001]. With regards to PR conversion, the significant influence of adjuvant chemotherapy (p = 0.023) and endocrine therapy (p < 0.001) on PR loss in metastasis was noted. Since HER2 conversion between primary tumor and metastasis was an event with low probabilities (p > 0.05), we did not perform further analysis on HER2 conversion.
Survival stratified by receptor status in primary tumor and metastasis
By the end of the last follow-up, all patients had a metastatic disease and 80.5% (343/426) of them had a progressive disease after the diagnosis of the earliest metastasis. Follow-up of OS was available for 266 (62.4%) women, for whom primary tumor and metastasis could be compared. In total, 129 deaths were recorded at the point of the last follow-up. The median follow-up time was 129 months.
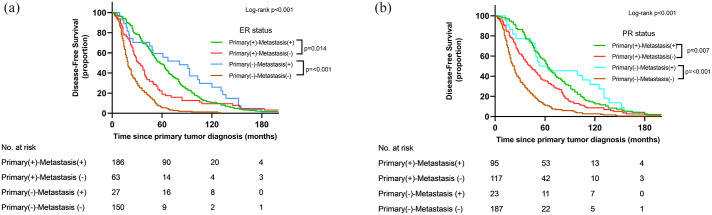
The median DFS was 36 (95% CI: 31–40) months. Univariate survival analysis (Figure 1) showed significantly differential DFS related to ER and PR conversion between primary tumor and metastasis (p < 0.05). In a pairwise comparison, the DFS was worse in ER/PR loss groups than in stable ER/PR-positive groups (p < 0.05). Compared with patients who were persistently hormone receptor-negative, patients who gained hormone receptor status in metastasis had a longer DFS (p < 0.001). Furthermore, DFS was better in the hormone receptor loss group than that in stable hormone receptor-negative group (p < 0.001). In the multivariate survival analysis (Table 2), ER conversion was an independent influence factor of DFS (p < 0.05) after adjusting for age of primary tumor diagnosis (continuous), primary tumor PR status, tumor size, lymph node metastasis, and adjuvant therapies (radiotherapy, endocrine, chemotherapy, and anti-HER2 therapy). Compared with patients with positive ER status in both primary and metastatic sites, patients with ER status that changed from positive to negative had a 49% increased risk of metastasis after operation [hazard ratio (HR): 1.498; 95% CI: 1.107–2.027; p = 0.009]. Patients with ER conversion from negative to positive had an 80% decreased risk of metastasis compared with patients with stable ER-negative disease (HR: 0.200; 95% CI: 0.128–0.312; p < 0.001). No statistically significant difference was observed in DFS between the hormone receptor acquisition and persistent hormone receptor-positive groups in either univariate or multivariate survival analyses (p > 0.05).
Figure 1.
Disease-free survival stratified by hormone receptor status in primary tumor and metastasis.
(a) Estrogen receptor status in primary and metastatic sites. (b) Progesterone receptor status in primary and metastatic sites.
Table 2.
Disease-free survival analysis stratified by ER status in primary and metastatic sites (multivariable model).
| DFS | No. of patients | No. of diseases | Adjusted HR* 95% CI | p-value |
|---|---|---|---|---|
| ER status | ||||
| Primary positive/metastasis positive | 185 | 185 | 1.000 (reference) | |
| Primary positive/metastasis negative | 62 | 62 | 1.498 (1.107–2.027) | 0.009 |
| Primary negative/metastasis positive | 26 | 26 | 0.699 (0.458–1.067) | 0.097 |
| Primary negative/metastasis negative | 150 | 150 | 3.396 (2.671–4.318) | p < 0.001 |
| Primary positive/metastasis positive | 185 | 185 | 0.295 (0.232–0.375) | p < 0.001 |
| Primary positive/metastasis negative | 62 | 62 | 0.409 (.0298–0.562) | p < 0.001 |
| Primary negative/metastasis positive | 26 | 26 | 0.200 (0.128–0.312) | p < 0.001 |
| Primary negative/metastasis negative | 150 | 150 | 1.000 (reference) | |
After adjustment for age of primary tumor diagnosis (continuous), PR status in primary tumor, tumor size, lymph node metastasis and adjuvant therapy (radiotherapy, endocrine, chemotherapy, and anti-HER2 therapy).
CI, confidential interval; DFS, disease-free survival; ER, estrogen receptor; HR, hazard ratio.
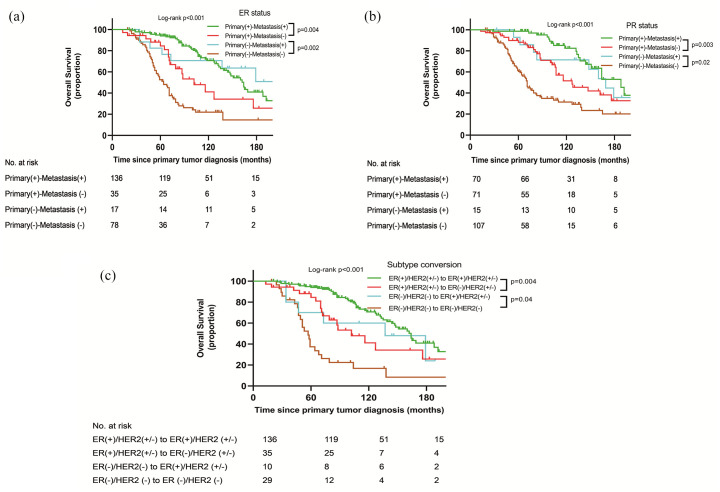
By the last follow-up, 266 patients were analyzed for OS and 129 (48.5%) deaths were observed. Univariate survival analysis is presented in Figure 2. The OS was longer in the stable hormone receptor-positive group than in the hormone receptor loss group (p < 0.05). Patients with hormone receptor status that changed from negative to positive had longer OS compared with patients with persistently hormone receptor-negative (p < 0.05) status. Furthermore, patients with hormone receptor loss had longer OS than patients with stable hormone receptor-negativity (p < 0.05). In the multivariate survival analysis (Table 3), patients with ER status that changed from positive to negative had a significantly increased risk of death compared with patients with stable ER-positive status (HR: 2.140; 95% CI: 1.236–3.708; p = 0.007). Compared with patients with persistent ER-negative status, patients with ER conversion from negative to positive had a significantly 64% decreased risk of death (HR: 0.366; 95% CI: 0.151–0.888; p = 0.026). Figure 2(c) showed the OS stratified by molecular typing conversion. A statistically significant differential OS stratified by molecular typing conversion was noted (p < 0.05). The OS was worse in group 2 than that in group 1 (p = 0.004). Compared with group 4, the OS of group 3 was longer (p = 0.04). In the multivariate survival analysis (Table 3), patients in group 2 had a significantly increased risk of death compared with group 1 (HR: 2.199; 95% CI: 1.343–3.965; p = 0.005). Patients in group 3 had a significantly decreased risk of death compared with group 4 (HR: 0.262; 95% CI: 0.103–0.668; p = 0.005). Furthermore, the risk of death of group 3 was similar to that of group 1 (HR = 1.436; 95% CI = 0.603 to 3.422; p = 0.414). Overall, molecular typing conversion from ER (+)/HER2 (+/−) to ER (−)/HER2 (+/−) was related to negative outcomes. Moreover, the conversion of molecular typing from ER (−)/HER2 (−) to ER (+)/HER2 (+/−) was associated with positive long-term survival.
Figure 2.
Overall survival analysis for hormone receptor and molecular typing conversion. (a) ER status in primary and metastatic sites. (b) PR status in primary and metastatic sites. (c) Molecular typing in primary and metastatic sites.
Table 3.
Overall survival analysis stratified by estrogen receptor status/molecular typing in primary and metastatic sites (multivariable model).
| OS (time since the primary tumor prognosis) | No. of patients | No. of deaths | Adjusted HR* 95% CI | p-value |
|---|---|---|---|---|
| ER status | ||||
| Primary positive/metastasis positive | 134 | 47 | 1.000 (reference) | |
| Primary positive/metastasis negative | 35 | 18 | 2.245 (1.292–3.899) | 0.004 |
| Primary negative/metastasis positive | 16 | 7 | 0.905 (0.403–2.032) | 0.808 |
| Primary negative/metastasis negative | 78 | 54 | 4.640 (3.081–6.989) | p < 0.001 |
| Primary positive/metastasis positive | 134 | 47 | 0.284 (0.150–0.540) | p < 0.001 |
| Primary positive/metastasis negative | 35 | 18 | 0.379 (.0188–0.764) | 0.007 |
| Primary negative/metastasis positive | 16 | 7 | 0.366 (0.151–0.888) | 0.026 |
| Primary negative/metastasis negative | 78 | 54 | 1.000 (reference) | |
| OS (time since the primary tumor prognosis) | No. of patients | No. of death | Adjusted HR† 95% CI | p-value |
| Molecular typing conversion | ||||
| ER (+)/HER2 (+/−) to ER (+)/HER2 (+/−) | 136 | 49 | 1.000 (reference) | |
| ER (+)/HER2 (+/−) to ER (−)/HER2 (+/−) | 35 | 18 | 2.199 (1.343–3.965) | 0.005 |
| ER (−)/HER2 (−) to ER (+)/HER2 (+/−) | 10 | 6 | 1.436 (0.603–3.422) | 0.414 |
| ER (−)/HER2 (−) to ER (−)/HER2 (−) | 29 | 23 | 5.475 (3.277–9.146) | p < 0.001 |
| ER (+)/HER2 (+/−) to ER (+)/HER2 (+/−) | 136 | 49 | 0.183 (0.109–0.305) | p < 0.001 |
| ER (+)/HER2 (+/−) to ER (−)/HER2 (+/−) | 35 | 18 | 0.402 (0.213–0.756) | 0.007 |
| ER (−)/HER2 (−) to ER (+)/HER2 (+/−) | 10 | 6 | 0.262 (0.103 to 0.668) | 0.005 |
| ER (−)/HER2 (−) to ER (−)/HER2 (−) | 29 | 23 | 1.000 (reference) | |
After adjustment for age of primary tumor diagnosis (continuous), age of first metastasis diagnosis (continuous), PR status in primary and metastatic sites, site of metastasis (local and distant metastasis), tumor size, lymph node metastasis, adjuvant therapy (radiotherapy, endocrine, chemotherapy, and anti-HER2 therapy), and first-line therapy (endocrine, chemotherapy, and anti-HER2 therapy).
After adjustment for age of primary tumor diagnosis (continuous), age of first metastasis diagnosis (continuous), site of metastasis (local and distant metastasis), tumor size, lymph node metastasis, adjuvant therapy (radiotherapy, endocrine, chemotherapy, and anti-HER2 therapy), and first-line therapy (endocrine, chemotherapy, and anti-HER2 therapy).
CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival.
Survival stratified by receptor conversion and therapy management
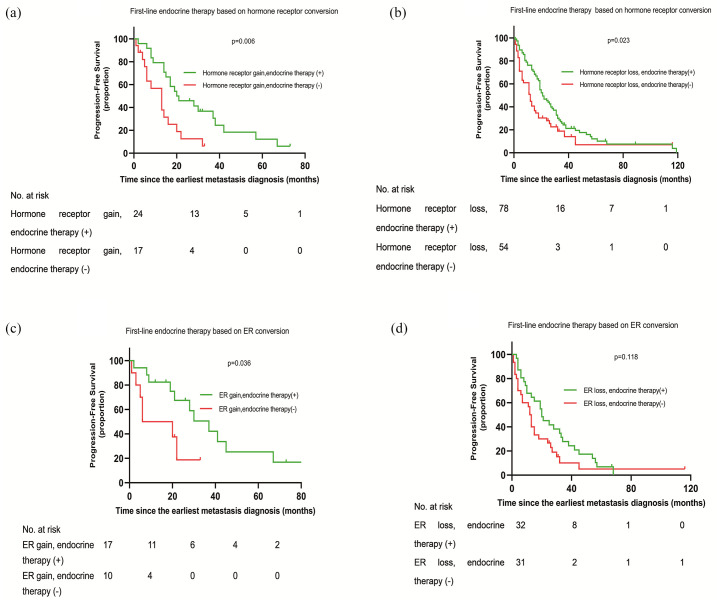
To further test whether changing treatment based on receptor conversion could improve prognosis of metastatic breast cancer, the patients were divided into different groups based on receptor conversion and first-line therapy. As shown in Figure 3(a), a statistically significantly differential PFS (follow-up from the time of the earliest metastasis diagnosis to progression or censoring) was noted (p = 0.006). The PFS was longer in the hormone receptor gain, endocrine therapy (+) group than in the hormone receptor gain, endocrine therapy (−) group. However, a significantly better PFS was observed in the hormone receptor loss, endocrine therapy (+) group compared with hormone receptor loss, endocrine therapy (−) group (Figure 3(b), p = 0.023). Considering that patients in the hormone receptor loss, endocrine (+) group might only have PR conversion between primary and metastatic sites (the ER was stable positive between primary tumor and metastasis), they might benefit from endocrine therapy. Therefore, we performed further survival analysis based on ER conversion and endocrine therapy. Univariate survival analysis showed that patients who gained ER status following metastasis and received endocrine therapy had a better PFS than patients who gained ER status in metastasis and did not received endocrine therapy (Figure 3(c), p = 0.036). However, no significant difference in PFS was observed between the ER loss, endocrine therapy (+) and ER loss, endocrine therapy (−) groups (Figure 3(d), p > 0.05). In other words, patients who lost ER in metastasis could not benefit from continued endocrine therapy. In the multivariate survival analysis, introducing endocrine therapy for patients with hormone receptor/ER status that changed from negative to positive, this was an independent influence factor of PFS (Table 4). The study cohort included 309 patients who had confirmed HER2 status in both their primary tumor and metastasis. A total of 36 (11.6%) of the 309 patients had discordant HER2 status in their primary tumor and metastasis. 17 patients with HER2-positive primary tumors had HER2-negative metastatic tumors and among these patients, only six patients received trastuzumab. In addition, 19 patients with HER2-negative primary tumors had HER2-positive metastatic tumors, and 10 patients received trastuzumab. The samples of HER2 conversion were too small to perform survival analysis on PFS stratified by HER2 conversion and therapy management. Thus, we performed survival analysis stratified by HER2 status in the metastatic tumors and therapy management to indirectly investigate whether patients with the HER2 conversion could benefit from the appropriate use of targeted therapy. The results showed that patients with HER2-positive metastatic tumors who received trastuzumab had longer PFS compared with patients with HER2-positive metastatic tumors who did not receive trastuzumab (Figure A2, p < 0.001). As only seven patients with HER2-negative metastatic tumors received trastuzumab, this group was too small to analyze. In the multivariate survival analysis (Table S4), patients with HER2-positive metastatic tumors who did not receive trastuzumab had a significantly increased risk of progression compared with patients with HER2-positive metastatic tumors who received trastuzumab (HR: 1.821, 95% CI: 1.181–2.807, p = 0.007). Collectively, patients with receptor conversion would benefit from a change in therapy.
Figure 3.
Progression-free survival stratified by hormone receptor conversion and first-line endocrine therapy.
(a), (b) First-line endocrine therapy based on hormone receptor conversion. (c), (d) First-line endocrine therapy based on ER conversion.
Table 4.
PFS analysis stratified by hormone receptor conversion and first-line therapy (multivariable model).
| PFS (time since the earliest metastasis diagnosis) | No. of patients | No. of progressions | Adjusted HR† 95% CI | p-value |
|---|---|---|---|---|
| Hormone receptor gain and first-line endocrine therapy | ||||
| Hormone receptor gain, endocrine therapy (−) | 17 | 15 | 1.000 (reference) | |
| Hormone receptor gain, endocrine therapy (+) | 24 | 20 | 0.382 (0.185–0.790) | 0.009 |
| PFS (time since the earliest metastasis diagnosis) | No. of patients | No. of progressions | Adjusted HR† 95% CI | p-value |
| ER gain and first-line endocrine therapy | ||||
| ER gain, endocrine therapy (−) | 10 | 7 | 1.000 (reference) | |
| ER gain, endocrine therapy (+) | 17 | 12 | 0.333 (0.114–0.978) | 0.04 |
After adjustment for age of first metastasis diagnosis (continuous), HER2 status in metastatic site, site of metastasis (local and distant metastasis), and first-line therapy (chemotherapy and anti-HER2 therapy).
CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PFS, progression-free survival.
Discussion
Our study indicated that ER, PR, and HER2 status are unstable throughout tumor progression. More than 30% of patients with breast cancer present with hormone receptor conversion between primary tumor and metastasis. Patients with breast cancer are less likely to have HER2 conversion during tumor progression. Moreover, to the best of the authors’ knowledge, this study is the first to confirm that changing treatment based on receptor conversion could improve prognosis.
Receptor conversion during tumor progression was observed in a considerable proportion of patients with breast cancer. PR had the highest conversion rates, followed by ER and HER2. Hormone receptor loss in metastasis was the dominative pattern, whereas the proportion of HER2 acquisition was slightly higher than that of receptor loss. Because insignificant discordant HER2 expression status between primary tumor and metastasis was noted (p > 0.05), we did not perform further analysis of HER2 conversion. Compared with local metastasis, ER conversion was more common in distant metastasis. In terms of the association between clinicopathological variables and receptor conversion, the analyses indicated that hormone receptor conversion is related to previous adjuvant endocrine therapy. More than 40% of patients receiving adjuvant endocrine therapy lost PR in metastasis and 20% of patients treated with adjuvant endocrine therapy experienced ER loss in metastasis. These statistical results were similar to that of previous reports.4,11,14,15 Nevertheless, adjuvant chemotherapy was also found to be positively associated with PR loss in relapse.
Importantly, receptor conversion has an effect on survival. In survival curves stratified by hormone receptor status in primary tumor and corresponding metastasis, significant differences in unadjusted DFS and OS were observed. Patients with hormone receptor conversion from positive to negative had a worse survival (DFS/OS) compared to patients with persistent hormone receptor-positive disease. Patients with hormone receptor that changed from negative to positive had longer survival than patients with stable hormone receptor-negative disease. Furthermore, the survival of patients with hormone receptor gain was similar to patients with stable hormone receptor-positive disease. In multivariable models, ER conversion from positive to negative was associated with worse survival, and ER acquisition in metastasis was associated with better outcomes. We also observed that molecular typing conversion caused by receptor conversion affects the outcomes of different molecular subtypes of primary breast cancer. To summarize, breast cancer outcomes were affected by both receptor status/molecular typing in primary tumor and receptor conversion. In patients with accessible metastasis, biopsy confirmation of receptor status is necessary for determining prognosis.
During breast cancer progression, a considerable proportion of patients lose or gain ER, PR, and HER2 expression. Patients with hormone receptor/HER2 status that changes from negative to positive might benefit from first-line endocrine/anti-HER2 therapy. However, loss of hormone receptor/HER2 status might confer resistance to endocrine/anti-HER2 therapy. Little information is yet available to determine whether therapy management based on receptor conversion has an effect on survival. Our findings indicated that changing the endocrine therapy regime based on ER conversion, as an independent influence factor, can prolong PFS. Patients who gained hormone receptor status following metastasis were found to benefit from endocrine therapy. Furthermore, patients with an ER status changed from positive to negative no longer benefited from continued endocrine therapy. For such patients, to avoid drug-related side effects, continued endocrine therapy should not be recommended. Because patients with breast cancer were less likely to experience HER2 conversion during tumor progression, we chose to indirectly investigate whether patients with HER2 conversion could benefit from a change in therapy. The results revealed that patients with HER2-positive metastatic tumors who received trastuzumab had improved prognosis when compared with patients with HER2-positive metastatic tumors who did not receive trastuzumab, similar to a previous study.16 Therefore, it is important to assess HER2 status in metastatic sites. In summary, patients with receptor conversion would benefit from a change in therapy.
Our study is limited by its retrospective nature and higher lost follow-up rate. Owing to the higher rate of loss to follow-up, some cases of receptor conversion could not be used for overall survival analysis. The prognostic values of receptor conversion and alterations in treatment are best assessed in prospective trials.
Several explanations might account for receptor conversion. Previous studies have shown that primary tumors have different metastatic capacities, as a result of tumor heterogeneity; therefore, metastasis might result from preexisting variant cells within the primary tumor. An early tumor stem line clone evolves independently in the primary tumor and corresponding metastases, and leads to multiple, genetically almost completely different clones in the various tumor locations in individuals.17–20 Heterogeneity and clonal selection/evolution might be the main contributors to receptor status change during the course of cancer progression and treatment. In addition, previous adjuvant therapy may affect markers conversion.21–23 It is possible that a part of the receptor conversion may be the result of material selection and detection.24–26
Our study is clinically important, despite its retrospective nature. In this study, we demonstrated that receptor conversion is not only observed in primary tumor and metastasis, but also in multiple consecutive metastases. This dynamic receptor conversion was influenced by adjuvant therapy and had statistically significant prognostic implications. In addition, we found that the molecular typing conversion induced by receptor conversion had an effect on the outcomes of different molecular subtypes of primary breast cancer. Most importantly, our findings indicated that patients with receptor conversion would benefit from a change in therapy, a novel finding. Given that in a considerable proportion of patients, receptor status is instable throughout tumor progression and this dynamic conversion is associated with prognosis, biopsies should be taken for all of new diagnosed primary, recurrent, and metastatic breast cancers whenever possible to optimize clinical management.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359211012982 for Receptor conversion impacts outcomes of different molecular subtypes of primary breast cancer by Weipeng Zhao, Linlin Sun, Guolei Dong, Xiaorui Wang, Yan Jia and Zhongsheng Tong in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors express their gratitude to all patients who participated in this study.
Footnotes
Author contributions: All authors made significant contributions in article design and conception, data acquisition, analysis and manuscript writing and revision. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Key Task Project of Tianjin Health and Family Planning Commission (16KG128), Anticancer Key Technologies R&D Program of Tianjin (12ZCDZSY16200) and Natural Science Foundation of Tianjin (18JCYBJC91600).
Compliance with ethical standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of Medical Ethics Committee of Tianjin and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
ORCID iD: Zhongsheng Tong  https://orcid.org/0000-0001-8060-5699
https://orcid.org/0000-0001-8060-5699
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Weipeng Zhao, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Ti Yuan Bei, Hexi District, Tianjin, China.
Linlin Sun, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Ti Yuan Bei, Hexi District, Tianjin, China.
Guolei Dong, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Ti Yuan Bei, Hexi District, Tianjin, China.
Xiaorui Wang, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Ti Yuan Bei, Hexi District, Tianjin, China.
Yan Jia, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Ti Yuan Bei, Hexi District, Tianjin, China.
Zhongsheng Tong, Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, West Huan-Hu Road, Ti Yuan Bei, Hexi District, Tianjin, 300060, China.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. DOI: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast 2018; 39: 131–138. [DOI] [PubMed] [Google Scholar]
- 3. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010; 7: e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Z, Li N, Li X, et al. The prognostic impact of hormonal receptor and HER-2 expression discordance in metastatic breast cancer patients. Onco Targets Ther 2020; 13: 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamanouchi K, Kuba S, Eguchi S. Hormone receptor, human epidermal growth factor receptor-2, and Ki-67 status in primary breast cancer and corresponding recurrences or synchronous axillary lymph node metastases. Surg Today. Epub ahead of print 12 June 2019. DOI: 10.1007/s00595-019-01831-8. [DOI] [PubMed] [Google Scholar]
- 6. Ibrahim T, Farolfi A, Scarpi E, et al. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact. Oncology 2013; 84: 150–157. [DOI] [PubMed] [Google Scholar]
- 7. Yang YF, Liao YY, Yang M, et al. Discordances in ER, PR and HER2 receptors between primary and recurrent/metastatic lesions and their impact on survival in breast cancer patients. Med Oncol 2014; 31: 214. [DOI] [PubMed] [Google Scholar]
- 8. Wame S, Kpm S, van Gils CH, et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst 2018; 110: 568–580. [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto A, Jinno H, Murata T, et al. Prognostic implications of receptor discordance between primary and recurrent breast cancer. Int J Clin Oncol 2015; 20: 701–708. [DOI] [PubMed] [Google Scholar]
- 10. Lower EE, Khan S, Kennedy D, et al. Discordance of the estrogen receptor and HER-2/neu in breast cancer from primary lesion to first and second metastatic site. Breast Cancer (Dove Med Press) 2017; 9: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2013; 24: 143–144. [DOI] [PubMed] [Google Scholar]
- 12. Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2015; 33: 2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol 2005; 26: 281–293. [DOI] [PubMed] [Google Scholar]
- 14. Ongaro E, Gerratana L, Cinausero M, et al. Comparison of primary breast cancer and paired metastases: biomarkers discordance influence on outcome and therapy. Future Oncol 2018; 14: 849–859. [DOI] [PubMed] [Google Scholar]
- 15. Yi ZB, Yu P, Zhang S, et al. Profile and outcome of receptor conversion in breast cancer metastases: a nation-wide multicenter epidemiological study. Int J Cancer. Epub ahead of print 19 August 2020. DOI: 10.1002/ijc.33227. [DOI] [PubMed] [Google Scholar]
- 16. Shaheenah D, Kristine B, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. Epub ahead of print 23 November 2009. DOI: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beca F, Polyak K. Intratumor heterogeneity in breast cancer. Adv Exp Med Biol 2016; 882: 169–189. [DOI] [PubMed] [Google Scholar]
- 18. Ng CKY, Bidard FC, Piscuoglio S, et al. Genetic heterogeneity in therapy-naive synchronous primary breast cancers and their metastases. Clin Cancer Res 2017; 23: 4402–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sprouffske K, Kerr G, Li C, et al. Genetic heterogeneity and clonal evolution during metastasis in breast cancer patient-derived tumor xenograft models. Comput Struct Biotechnol J 2020; 18: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977; 197: 893–895. [DOI] [PubMed] [Google Scholar]
- 21. Vogel C, Malter W, Morgenstern B, et al. The role of previous therapies and sites of metastasis as influencing factors on discordance of ER, PR and HER2 status between primary and metastasized breast cancer. Anticancer Res 2019; 39: 2647–2659. [DOI] [PubMed] [Google Scholar]
- 22. Hawkins RA, Tesdale AL, Anderson ED, et al. Does the oestrogen receptor concentration of a breast cancer change during systemic therapy? Br J Cancer 1990; 61: 877–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gross GE, Clark GM, Chamness GC, et al. Multiple progesterone receptor assays in human breast cancer. Cancer Res 1984; 44: 836–840. [PubMed] [Google Scholar]
- 24. Rhodes A, Jasani B, Barnes DM, et al. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol 2000; 53: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rüdiger T, Höfler H, Kreipe HH, et al. Quality assurance in immunohistochemistry: results of an interlaboratory trial involving 172 pathologists. Am J Surg Pathol 2002; 26: 873–882. [DOI] [PubMed] [Google Scholar]
- 26. Sighoko D, Liu JX, Hou NQ, et al. Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: biological difference or misclassification? Oncologist 2014; 19: 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359211012982 for Receptor conversion impacts outcomes of different molecular subtypes of primary breast cancer by Weipeng Zhao, Linlin Sun, Guolei Dong, Xiaorui Wang, Yan Jia and Zhongsheng Tong in Therapeutic Advances in Medical Oncology