Abstract
The rapid evolution of resistance, particularly among Gram-negative bacteria, requires appropriate identification of patients at risk followed by administration of appropriate empiric antibiotic therapy. A primary tenet of antimicrobial stewardship programs (ASPs) is the establishment of empiric antibiotic recommendations for commonly encountered infections. An important tool in providing empiric antibiotic therapy recommendations is the use of an antibiogram. While the majority of institutions use a traditional antibiogram, ASPs have an opportunity to enhance antibiogram data. The authors provide the rationale for why ASPs should implement alternative antibiograms, and the importance of incorporating an antibiogram into clinical decision support systems with the goal of providing effective empiric antibiotic therapy.
Keywords: Antimicrobial stewardship, antibiogram, antimicrobial stewardship program
Introduction
Antimicrobial resistance (AMR) is a global threat to society, with deaths attributed to resistant infections projected to exceed 10 million per year by 2050.1 Among the most commonly resistant pathogens are Gram-negative bacteria, including Pseudomonas aeruginosa, Acinetobacter baumannii, Extended-spectrum beta-lactamase (ESBL) and carbapenemase-producing organisms.2 Alarming trends in Gram-negative resistance are observed in many hospitals in the United States, where, for example, 15–20% of all P. aeruginosa isolates are categorized as multidrug resistant (MDR) because of non-susceptibility to at least one antibiotic in three or more antibiotic classes.2 The rapid evolution of resistance among Gram-negative bacteria requires identification of patients at risk for infections by these pathogens followed by administration of appropriate empiric antibiotic therapy. Appropriate initial antibiotic therapy has demonstrated improved clinical outcomes, including a reduction in mortality.3–8 However, healthcare providers must often select an antibiotic regimen before culture results are available. Studies have demonstrated that an ineffective empiric antibiotic regimen can be harmful to patients while unnecessary broad-spectrum antibiotics can lead to increased resistance.3–5
A strategy widely endorsed to promote appropriate empiric antibiotic therapy is the implementation of antimicrobial stewardship programs (ASPs). The Infectious Diseases Society of America, Society for Healthcare Epidemiology of America (SHEA) and Pediatric Infectious Diseases Society (PIDS) released a policy statement on antimicrobial stewardship, noting the following: ‘The major objectives of antimicrobial stewardship are to achieve optimal clinical outcomes related to antimicrobial use, thereby limiting the selective pressure on bacterial populations that drives the emergence of antimicrobial-resistance strains.’8 A primary tenet of ASPs is the establishment of empiric antibiotic recommendations for commonly encountered infections. An important tool in providing empiric antibiotic recommendations is the use of an antibiogram. An antibiogram represents a convenient and widely available measurement of an institution’s pathogens and susceptibilities. While the antibiogram provides a reflection of local resistance patterns, there are several limitations, including: (a) lack of syndromic-specific recommendations; (b) typically no information on organism distribution for a specific infection; (c) lack of utility for infections caused by twos or more pathogens; (d) constructed using historical data potentially not reflecting current susceptibility data.
To ensure patients receive appropriate empiric antibiotic therapy based on a suspected site of infection, hospital location, and patient characteristics, there is a need for ASPs to go beyond the traditional antibiogram. ASPs are well suited to collaborate with clinical microbiologists in creating more sophisticated antibiograms to optimize empiric antibiotic therapy. Therefore, in this article we provide the rationale for why ASPs should implement alternative antibiograms, including combination and syndromic, and the importance of incorporating an antibiogram into clinical decision support systems with the goal of providing effective empiric antibiotic therapy.
Traditional antibiograms
The most convenient and widely available antibiogram is a traditional antibiogram. A traditional antibiogram is a periodic profile of the proportion of pathogens that are susceptible to an institution’s formulary antibiotics over a given time frame, typically 1 year.9 The antibiogram has multiple uses, including providing guidance for empiric antibiotic therapy, monitoring changes in resistance over time, and assisting in formulary decisions. The traditional antibiogram is used by a variety of healthcare personnel, including ASPs, infection preventionists, epidemiologists, microbiologists, pharmacists, and prescribers.
In the United States, the Clinical and Laboratory Standards Institute (CLSI) provides recommendations for antibiogram development and reporting.10 CLSI provides several key recommendations in antibiogram development, including: (a) inclusion of isolates collected from patients for diagnostic purposes; (b) inclusion of the first isolate of a given organism per patient per analysis period; (c) inclusion of at least 30 isolates of a specific pathogen during the analysis period; (d) antibiogram analysis at least annually to ensure availability of current data.10 Importantly, there are a few notable considerations based on CLSI recommendations, primarily antibiograms constructed using first isolate per patient per year will likely underestimate the rate of resistance as resistant pathogen isolates from patients with previously positive culture with a susceptible phenotype are not included in the antibiogram. Importantly, ASPs should be aware of the several advantages and disadvantages associated with traditional antibiograms (Table 1).
Table 1.
Advantages and disadvantages of traditional, combination, syndromic, and weighted incidence syndromic combination antibiograms (WISCA).
| Advantages | Disadvantages | |
|---|---|---|
| Traditional antibiograms Example: Susceptibility of Pseudomonas aeruginosa to piperacillin/tazobactam (TZP) |
• Readily available • Easily understood by clinicians • Completed at least annually • Ability to assist in empiric antibiotic therapy recommendations • Easily incorporated into disease- state treatment guidelines |
• Require a minimum of 30 pathogens/year • Revision of antibiotic breakpoints may not be included • Lack of inclusion of infection source and/or hospital location • Binary measure of susceptibility (susceptible versus non-susceptible/resistant) • Lack of incorporation of patient variables (age, gender, and comorbidities) • Limited correlation with clinical and microbiological outcomes |
| Combination antibiograms Example: Additional susceptibility of Pseudomonas aeruginosa to TZP + tobramycin versus TZP alone |
• Ability to evaluate coverage of multiple antibiotics • Ease of completion • Useful in determining combined empiric antibiotic regimens for multidrug-resistant pathogens |
• Less easily understood by prescribers • Typically requires manual completion • Lack of CLSI guidance for completion • Require a minimum of 30 pathogens/year • Antibiotic susceptibilities derived from percentages not in vitro synergy • Lack of incorporation of patient variables (age, gender, and comorbidities) • Limited correlation with clinical and microbiological outcomes |
| Syndromic antibiograms Example: Susceptibility of Pseudomonas aeruginosa to TZP among respiratory specimens (obtained among ICU patients only) |
• Increased likelihood of providing effective empiric antibiotic therapy for a specific infectious syndrome • May be further stratified based on hospital location • Provide increased granularity for resistance awareness • May be incorporated into disease-state treatment guidelines |
• Typically requires manual completion • Less easily understood by prescribers • Lack of incorporation of patient variables (age, gender, and comorbidities) • Lack of correlation with clinical and microbiological outcomes |
| Weighted incidence syndromic antibiogram (WISCA) Example: Susceptibility of Pseudomonas aeruginosa to TZP among respiratory specimens (obtained among ICU patients only) for male patients age ⩾65 years with heart failure |
• Ability to incorporate into electronic healthcare record • Provide real-time decision support for empiric antibiotic therapy recommendations • Integration of patient variables (age, gender, and comorbidities) • Provide empiric antibiotic therapy recommendations for a specific infectious syndrome |
• Requires manual completion • Requires collaboration with information technology • Less easily understood by prescribers • Lack of patient variable standardization • Lack of correlation with clinical and microbiological outcomes |
CLSI, Clinical and Laboratory Standards Institute; ICU, intensive care unit.
Combination antibiograms
For certain types of infections such as hospital-acquired and ventilator-associated pneumonia, guidelines recommend the empiric use of two antibiotics to minimize the potential of inappropriate therapy.11,12 Frequently, empiric combination antibiotic therapy is based on knowledge from a traditional antibiogram; additional information to assist in the selection of an appropriate combination regimen is lacking. A combination antibiogram shows the likelihood that at least one drug in a regimen compromising multiple antibiotics will cover a given pathogen and provides a useful clinical tool for evaluating antimicrobial coverage.13–15 The data are particularly useful when there are significant susceptibility differences in pathogens to individual antibiotics. When interpreting a combination antibiogram, the antibiotic percentage susceptibility should clearly indicate an increase in empiric coverage with the combination compared to the individual agents alone.
Several studies have demonstrated the utility of a combination antibiogram in evaluating the extent of coverage of multiple antibiotics and in empiric combination therapy recommendations.14,15 Puzniak et al.14 evaluated single-agent susceptibility rates for 11,701 non-duplicate P. aeruginosa isolates. Susceptibility ranged from 72.7% for fluoroquinolones to 85% for piperacillin/tazobactam (TZP). Adding an aminoglycoside resulted in higher susceptibility rates than adding a fluoroquinolone; TZP plus an aminoglycoside resulted in the highest susceptibility rate (93.3%). A second single-center study showed the addition of a second antibiotic (aminoglycoside or fluoroquinolone) to ceftazidime or imipenem significantly increased the likelihood of providing appropriate empiric therapy compared to a single agent.15 In the study, susceptibility to the Gram-negative pathogen was 71.5% and 84% to ceftazidime and imipenem, respectively. Susceptibility increased to 82.9% with ceftazidime plus ciprofloxacin and 95% for imipenem plus amikacin.
There are notable limitations with the use of a combination antibiogram. While a combination antibiogram is useful when the pathogen is known, but susceptibilities are not yet available, it does not give the likelihood that the combination of antibiotics will cover all identified organisms. Second, there is a lack of guidance on the use of combination antibiograms for empiric therapy recommendations in high-risk patients. Finally, susceptibilities of the combination therapy are not derived from in vitro synergy evaluation, but solely, on percentage susceptibility.
Syndromic antibiograms
A syndromic antibiogram provides an increased likelihood of appropriate empiric antibiotic therapy for a specific infectious syndrome, considering the weighted incidence of pathogens causing the syndrome. A syndromic antibiogram may be further refined by stratifying susceptibilities based on patient location. This type of antibiogram provides an additional opportunity to enhance data and increase the likelihood of effective empiric antibiotic therapy.
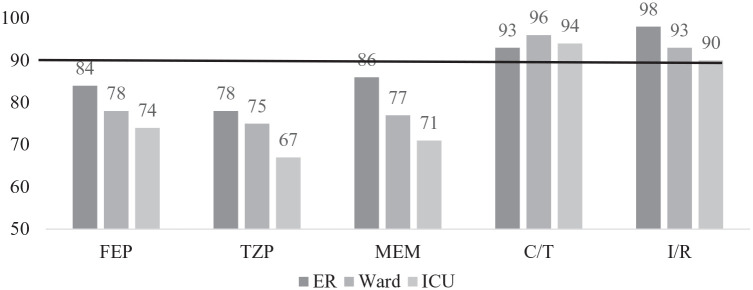
Klinker et al.16 compared antibiotic susceptibilities using a traditional versus syndromic antibiogram for common Gram-negative pathogens associated with pneumonia stratified by patient location. The traditional antibiogram included susceptibility for the three most common Gram-negative pathogens (Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa) from all sources. The syndromic antibiogram included susceptibility for the same three Gram-negative pathogens isolated from a respiratory source. A targeted empiric antibiotic susceptibility of ⩾90% was selected. A total of 17,561 Gram-negative isolates, including 6654 lower respiratory isolates were evaluated. The traditional antibiogram demonstrated that susceptibilities for cefepime (FEP), TZP, and meropenem (MEM) were near or above the 90% threshold for E. coli and Klebsiella spp. (Table 2). In contrast, antibiotic susceptibilities did not achieve this target for P. aeruginosa. When antibiotic susceptibilities were stratified by location [emergency room (ER), ward, and intensive care unit (ICU)], a 5–8% reduction in aggregate susceptibility for FEP, TZP, and MEM was observed for isolates obtained from patients in the ER versus the ICU. Upon refinement of the analysis to only P. aeruginosa respiratory isolates, a ⩾10% reduction in susceptibility for FEP, TZP, and MEM was observed in isolates collected from patients in the ICU compared to those in the ER. In contrast, ceftolozane/tazobactam and imipenem/relebactam maintained ⩾90% susceptibility regardless of isolated pathogen and/or location (Figure 1). The study concluded that the traditional antibiogram underestimated resistance patterns observed in ICU patients with respiratory infections, potentially resulting in the administration of ineffective empiric antibiotic therapy. The use of a syndromic antibiogram stratified by geographical location provided granularity to increase resistance awareness and better to inform the creation of optimized empiric therapy recommendations.
Table 2.
Traditional antibiogram evaluating susceptibility for Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa collected from all sources.
| Pathogen (n) | FEP | TZP | MEM | C/T | I/R |
|---|---|---|---|---|---|
| E. coli (6095) | 87 | 95 | 99 | 98 | 99 |
| Klebsiella spp. (4097) | 91 | 89 | 98 | 95 | 99 |
| P. aeruginosa (3649) | 78 | 78 | 77 | 95 | 93 |
C/T, ceftolozane/tazobactam; FEP, cefepime; I/R, imipenem/relebactam; MEM, meropenem; TZP, piperacillin/tazobactam.
Figure 1.
Syndromic antibiogram evaluating susceptibility of Pseudomonas aeruginosa respiratory isolates stratified by patient location.
ER, emergency room; ICU, medical or surgical ICU; Ward, medical or surgical ward.
The challenge of P. aeruginosa is as increasing frequency of resistance to first-line treatment options recommended by clinical guidelines.11 Carbapenem-resistant (CR) isolates create clinical challenges due to co-resistance among first-line agents and delays to timely effective therapy resulting in poor outcomes.17–19 Due to co-resistance among empiric first-line beta-lactams, a simple strategy for assessing risk for ineffective empiric therapy is evaluating the syndromic frequency of carbapenem-resistant P. aeruginosa (CRPA). A recent study aimed to identify beta-lactam susceptibility patterns based on CRPA frequency among lower respiratory tract specimens collected from ICU patients.20 A total of 871 P. aeruginosa isolates were collected from lower respiratory specimens obtained from ICU patients across 20 US institutions. Institutions were stratified into one of three categories based on CRPA frequency: CRPA rates ⩽20% (CR group 1); 21–40% (CR group 2); and ⩾41% (CR group 3). Beta-lactam susceptibility was evaluated relative to CRPA frequency. Resistance to TZP, FEP, and MEM was reported as 32.4%, 25.7%, and 28.4% (Table 3). In MEM-non-susceptible isolates, resistance to TZP and FEP increased to 64.8% and 55.7% of isolates reported as non-susceptible, respectively. Antibiotic susceptibility based on CR group is presented in Table 3. The authors concluded that co-resistance among first-line beta-lactams is frequently observed, limiting empiric choices for the management of hospital-acquired and ventilator-associated bacterial pneumonia. Furthermore, in hospital settings where CRPA frequency is ⩾20%, susceptibility testing of newer antipseudomonal agents or consideration for antibiotic modification is warranted.
Table 3.
Pseudomonas aeruginosa susceptibility among ICU lower respiratory tract isolates stratified by frequency of carbapenem resistance.
| Antibiotic | CR group 1 (N = 37) (n = 264, %) | CR group 2 (N = 25) (n = 363, %) | CR group 3 (N = 18) (n = 244, %) |
|---|---|---|---|
| Cefepime | 83.7 | 74.9 | 63.1 |
| Piperacillin/tazobactam | 79.6 | 68.9 | 52.9 |
| Meropenem | 91.3 | 73.6 | 47.5 |
| Levofloxacin | 68.6 | 66.1 | 48 |
| Ceftolozane/tazobactam | 96.6 | 94.2 | 90.6 |
| Imipenem/relebactam | 98.1 | 91.7 | 81.6 |
CR group 1 = CR P. aeruginosa rates ⩽20%; CR group 2 = 21–40%; CR group 3 = ⩾41%.
CR, carbapenem resistant; ICU, intensive care unit; N, number of institutions; n, number of isolates.
Antibiograms and clinical decision support systems
Clinical decision support systems (CDSSs) improve the delivery of healthcare by integrating patient information, providing targeted clinical knowledge, and establishing clinical management tools.21 By merging patient and institutional-specific information, healthcare providers can deliver personalized patient treatment and management. However, it is critical that clinicians have access to and appropriate interpretation of data at the point of prescribing. Typically, antibiograms are available to clinicians through a hospital or ASP website, pocket card, or book. This may result in clinicians not consulting the antibiogram and subsequently prescribing ineffective empiric antibiotic therapy. Therefore, integration of antibiogram data within a CDSS provides an important opportunity to ensuring the administration of appropriate empiric antibiotic therapy.
CDSS tools have been developed and implemented to improve empiric antibiotic administration through a variety of mechanisms. CDSSs typically include a patient’s health problem list populated on and throughout a hospital admission. Treatment guidelines for common infectious diseases syndromes are created and updated by the ASP with endorsement from multidisciplinary healthcare professionals and incorporated into the CDSS. Clinicians are alerted to prescribe medications based on the treatment guideline which is prompted from the health problem list. For common infectious syndromes, treatment guidelines include empiric antibiotic therapy recommendations based on institutional antibiogram data. This concept can be further advanced to include the incorporation of syndromic-specific and/or unit-specific antibiotic recommendations into treatment guidelines. For example, an institution may have a guideline for the management of pneumonia. It would be prudent for the guideline to include empiric antibiotic recommendations based on a patient’s presentation (community versus hospital), hospital location (ICU versus ward), and suspected pathogens. This concept has demonstrated improvement in guideline concordant antibiotic prescribing and reductions in the delivery of ineffective therapy. Eudaley et al.22 demonstrated that clinicians using CDSS tools increased guideline concordance for empiric therapy by 30%. Notably, integration of these tools reduced the frequency of ineffective antibiotic therapy by 40% in critically ill patients.23,24
Currently, CDSS tools use traditional antibiogram data, resulting in several limitations, including: (a) data may not be real-time in the absence of frequent updates; (b) available data may not reflect current susceptibility patterns for a specific syndrome or hospital location. To overcome these limitations, ASPs should consider an electronic antibiogram (e-antibiogram). An e-antibiogram provides a comprehensive, visual analytic report, resulting in susceptibility maps for pathogens and antibiotics.25 There is the further ability to stratify data by source of infection, infection acquisition (community versus hospital-acquired), hospital location, and patient characteristics (i.e. age). In addition, an e-antibiogram can be configured to map bug–drug combinations based on pathogen, antibiotic, and infection source. The integration of an e-antibiogram into CDSSs has been shown to be feasible and user-friendly; however, there is a lack of data demonstrating the impact on appropriate empiric antibiotic therapy.25
To advance antibiogram data and integration into CDSSs further, patient-specific variables should be incorporated.26 A weighted incidence syndromic combination antibiogram (WISCA) uses electronic healthcare data to provide real-time decision support by integrating patient characteristics and, subsequently, recommending empiric antibiotic therapy for a specific infectious syndrome.27,28 A recently published study determined the impact of WISCA use for empiric antibiotic prescription on hospital length of stay at four hospitals.27 Study participants included adult inpatients receiving empiric antibiotics for urinary tract infection, abdominal-biliary infection, pneumonia, or non-purulent cellulitis. Antimicrobial stewardship physicians used WISCA and clinical guidelines to provide empiric antibiotic recommendations. There were no overall differences in outcomes, including length of stay (LOS), 30-day mortality, and 30-day readmission among the intervention versus control groups. Guidelines-based interventions were associated with decreased LOS for cellulitis and decreased mortality for community-acquired pneumonia. Although the study failed to show a significant difference in hospital LOS, there were several notable limitations. There was a high frequency of agreement between antimicrobial stewardship physicians and primary prescribers within the intervention arm. Secondly, recommendation acceptance was low, mitigating any potential benefit. Over half of the patients had an infection amenable to source control or were receiving effective therapy initiated within 48 h of culture obtainment. Finally, approximately 90% of patients were admitted to general wards and may have been less susceptible to suboptimal outcomes associated with effective antibiotic therapy delays. The conclusions of the study demonstrate that ASPs have an opportunity to continue to develop and incorporate WISCA-guided recommendations with the goal of improving outcomes for infectious syndromes in which outcomes are closely associated with early appropriate empiric antibiotic therapy.
Antibiograms and rapid diagnostic technology
Rapid diagnostic technology (RDT) has revolutionized the management of infectious diseases, allowing antimicrobial stewardship programs the ability to recommend targeted antibiotic therapy, resulting in improved clinical and microbiological outcomes.29 Many clinical laboratories are using RDT to detect antibiotic resistance genes for diagnostic and surveillance purposes. RDT can be performed directly on clinical specimens, including respiratory and blood; however, routine antimicrobial susceptibility testing (AST) may or may not be completed as follow-up to confirm resistance determinants results. The incorporation of resistance determinant information into an antibiogram may assist in augmenting early appropriate empiric antibiotic therapy based on the presence or absence of resistance markers. For example, the identification of mecA in Staphylococcus aureus predicts methicillin-resistant S. aureus which allows prescribers to ensure targeted antibiotic therapy.30 In contrast, predicting Gram-negative resistance determinants is more complex due to heterogenous mechanisms. Therefore, RDTs may provide lower accuracies of prediction potentially not allowing the administration of targeted antibiotic therapy.
RDT provides earlier organism and key resistance determinant identification; however, information is incomplete, and ASPs may be without a clear direction for intervention in the absence of resistance determinants. Pogue et al.31 determined the ability of Verigene BC-GN organism identification and resistant determinant presence/absence to predict antibiotic susceptibility among target Gram-negative organisms in order better to direct ASPs. A total of 1046 Gram-negative bloodstream isolates that were analyzed with the Verigene BC-GN platform were assessed. Except for P. aeruginosa, the absence of resistance determinants as reported by the RDT largely predicted susceptibility to target antibiotics. Negative predict values (NPVs) for ceftriaxone susceptibility for E. coli and K. pneumoniae in the absence of either Cefotaxime-Munich (CTX-M) or a carbapenemase gene were 98% and 93–94%, respectively. The authors concluded that clinicians may be able to use a similar approach in de-escalating antibiotic therapy in the absence of resistance determinant detection.
Conclusions
Studies have demonstrated that an incorrect empiric antibiotic regimen can be harmful to patients while unnecessary broad-spectrum antibiotics can lead to increased resistance. A primary tenet of ASPs is the establishment of empiric antibiotic recommendations for commonly encountered infections. An important tool in providing empiric antibiotic therapy recommendations is the use of an antibiogram. Numerous antibiogram strategies have been evaluated and shown to improve empiric antibiotic therapy selection, but each strategy has advantages and disadvantages (Table 1). Currently, most institutions use a traditional antibiogram, ASPs have an opportunity to enhance data with the completion of syndromic and WISCA antibiograms. Further, the incorporation of antibiograms into CDSSs at the point of prescribing is imperative to ensure timely administration of appropriate empiric therapy. Future research is warranted on the impact of syndromic and WISCA antibiograms on clinical and microbiological outcomes.
Footnotes
Author contributions: All authors substantially contributed to the conception, drafting, and final approval of the manuscript.
Conflict of interest statement: All authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The study did not require an ethical board approval because it did not contain human or animal trials.
ORCID iD: Karri A. Bauer  https://orcid.org/0000-0003-1219-8133
https://orcid.org/0000-0003-1219-8133
Contributor Information
Kenneth P. Klinker, MRL, Merck & Co., Inc., Kenilworth, NJ, USA
Levita K. Hidayat, MRL, Merck & Co., Inc., Kenilworth, NJ, USA
C. Andrew DeRyke, MRL, Merck & Co., Inc., Kenilworth, NJ, USA.
Daryl D. DePestel, MRL, Merck & Co., Inc., Kenilworth, NJ, USA
Mary Motyl, MRL, Merck & Co., Inc., Kenilworth, NJ, USA.
Karri A. Bauer, MRL, Merck & Co., Inc., 2000 Galloping Hill Road, Kenilworth, NJ 07033, USA.
References
- 1. O’Neill J. Review on AMR. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. UK Government and Wellcome Trust. http://amrreview.org; AMR review paper tackling a crisis for the health and wealth of nations_1.pdf (2014, accessed 15 January 2021). [Google Scholar]
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Antibiotic Resistance Threats in the United States, (cdc.gov) (2019, accessed 15 January 2021). [Google Scholar]
- 3. Paul M, Shani V, Muchtar E, et al. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54: 4851–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care 2008; 23: 91–100. [DOI] [PubMed] [Google Scholar]
- 5. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 6. Dellinger RP, Levy MM, Rhodes A, et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for the management of severe sepsis and septic shock. Crit Care Med 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 7. The PRISM investigators. Early, goal-directed therapy for septic shock: a patient-level meta-analysis. N Engl J Med 2017; 376: 2223–2234. [DOI] [PubMed] [Google Scholar]
- 8. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007; 27: 1306–1312. [DOI] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute (CLSI). Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline, 4th ed. CLSI document M39-A4. Wayne, PA: Clinical and Laboratory Standards Institute, 2014. [Google Scholar]
- 11. Kalil A, Metersky M, Klompas M, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres A, Niederman M, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoa mericana del Tórax (ALAT). Eur Respir J 2017; 50: 1700582. [DOI] [PubMed] [Google Scholar]
- 13. Pogue JM, Alaniz C, Carver PL, et al. Role of unit-specific combination antibiograms for improving the selection of appropriate empiric therapy for gram-negative pneumonia. Infect Control Hosp Epidemiol 2011; 32: 289–292. [DOI] [PubMed] [Google Scholar]
- 14. Puzniak L, DePestel D, Srinivasan A, et al. A combination antibiogram evaluation for Pseudomonas aeruginosa in respiratory and blood sources from intensive care unit (ICU) and non-ICU settings in U.S. hospitals. Antimicrob Agents Chemother 2019; 63: e02564-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christoff J, Tolentino J, Mawdsley E, et al. Optimizing empirical antimicrobial therapy for infection due to Gram-negative pathogens in the intensive care unit: utility of a combination antibiogram. Infect Control Hosp Epidemiol 2010; 31: 256–261. [DOI] [PubMed] [Google Scholar]
- 16. Klinker KP, Bauer KA, DeRyke CA, et al. Empiric antibiotic susceptibility using a traditional vs. syndromic antibiogram – Implications for antimicrobial stewardship programs. IDWeek 2020 Annual Meeting, Virtual, October 2020. [abstract 103]. [Google Scholar]
- 17. McCann E, Sung AH, Ye G, et al. Contributing factors to the clinical and economic burden of patients with laboratory-confirmed carbapenem-nonsusceptible Gram-negative respiratory infections. Infect Drug Resist 2020; 13: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4: ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible Gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 2018; 5: ofy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klinker KP, DePestel DD, Motyl M, et al. Frequency of carbapenem-resistant Pseudomonas aeruginosa among respiratory pathogens impacts first-line beta-lactam susceptibility: potential role for ceftolozane/tazobactam and/or imipenem/relebactam. IDWeek 2020 Annual Meeting, Virtual, October 2020. [abstract 1450]. [Google Scholar]
- 21. Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eudaley ST, Mihm AE, Higdon R, et al. Development and implementation of a clinical decision support tool for treatment of uncomplicated urinary tract infections in a family medicine resident clinic. J Am Pharm Assoc 2019; 59: 579–585. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez-Maresca M, Sorlozano A, Grau M, et al. Implementation of a computerized decision support system to improve the appropriateness of antibiotic therapy using local microbiologic data. Biomed Res Int 2014; 2014: 395434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thursky KA, Buising KL, Bak N, et al. Reduction of broad-spectrum antibiotic use with computerized decision support in an intensive care unit. Int J Qual Health Care 2006; 18: 224–331. [DOI] [PubMed] [Google Scholar]
- 25. Simpao AF, Ahumada LM, Larru Martinez B, et al. Design and implementation of a visual analytics electronic antibiogram within an electronic health record system at a tertiary pediatric hospital. Appl Clin Inform 2018; 9: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacFadden DR, Coburn B, Shah N, et al. Decision-support models for empiric antibiotic selection in Gram-negative bloodstream infections. Clin Microbiol Infect 2019; 25: 108.e1–108.e7. [DOI] [PubMed] [Google Scholar]
- 27. Ridgway JP, Robicsek A, Shah N, et al. A randomized controlled trial of an electronic clinical decision support tool for inpatient antimicrobial stewardship. Clin Infect Dis. Epub ahead of print 26 July 2020. DOI: 10.1093/cid/ciaa1048 [DOI] [PubMed] [Google Scholar]
- 28. Tandogdu Z, Kakariadis ETA, Naber K, et al. Appropriate empiric antibiotic choices in health care associated urinary tract infections in urology departments in Europe from 2006 to 2015: a Bayesian analytical approach applied in a surveillance study. PLoS One 2019; 14: e0214710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bauer KA, Perez K, Forrest G, et al. Review of rapid diagnostic tests used by antimicrobial stewardship program. Clin Infect Dis 2014; 59: S134–S145. [DOI] [PubMed] [Google Scholar]
- 30. Arena F, Giani T, Pollino S, et al. Molecular antibiogram in diagnostic clinical microbiology: advantages and challenges. Future Microbiol 2017; 12: 361–364. [DOI] [PubMed] [Google Scholar]
- 31. Pogue JM, Heil EL, Lephart P, et al. An antibiotic stewardship program blueprint for optimizing Verigene BC-GN within an institution: a tale of two cities. Antimicrob Agents Chemother 2018; 62: e02538–17. [DOI] [PMC free article] [PubMed] [Google Scholar]