Abstract
Background:
A better understanding of the epidemiology of cryptococcal infection in HIV-negative individuals is an international research interest. Immune dysfunction in diabetes mellitus (DM) significantly increases the risk of acquiring and reactivation of infection due to Cryptococcus neoformans. Risk factors and outcomes of cryptococcosis in DM are not well documented.
Objective:
The objective of this study was to determine the clinical characteristics and outcomes of cryptococcal infections in persons living with DM.
Methods:
MEDLINE (via PubMed), EMBASE, and the Cochrane Library databases were searched in November 2020. The searches covered the period between 1980 and 2020.We included studies that reported confirmed cryptococcosis in patients with DM. Reference lists of included articles were also searched, and additional studies were included if appropriate. No language restriction was applied. Single case reports, case series and original articles were included whereas review articles were excluded.
Results:
A total of 28 studies (24 single case reports, 4 retrospectives) were included involving 47 unique patients from Asia (17 cases), North America (six cases), South America (three cases) and Africa (two cases). Men constituted 75% (n = 18) of the cases. Median age was 60.5 (range: 27–79) years. The majority of the patients had cryptococcal meningitis (68.1%, n = 32) followed by disseminated cryptococcosis (6.4%, n = 7), and others (isolated cutaneous disease one, peritonitis one, pleural one, thyroid one, adrenal one). Diagnosis was achieved through either culture and microscopy (38/47), cryptococcal antigen tests (9/47) or histopathology (9/47) singly or in a combination. All-cause mortality was 38.3% (n = 18). Among those with meningitis mortality was 36.2%.
Conclusion:
A wide spectrum of cryptococcal infections with varying severity occurs in DM. Mortality remains unacceptably high. There is a need for more studies to characterize better cryptococcal disease in DM.
Keywords: clinical characteristics, cryptococcosis, diabetes mellitus, mortality
Introduction
Cryptococcosis is an opportunistic, multi-systemic fungal infection with a predilection for the central nervous system (CNS). It is acquired through inhalation of airborne yeast cells (basidiospores) and subsequent pulmonary infection of mainly two etiological agents, Cryptococcus neoformans and Cryptococcus gattii.1 Soil containing pigeon droppings are the main reservoir for C. neoformans, whereas eucalyptus trees and decaying hollows in living trees are the major environmental source for C. gattii.2–4 C. neoformans is the most predominant cause of cryptococcosis and accounts for 80% of cases globally. Immune suppressive states such as HIV/AIDS, immunosuppressive drugs, and solid organ transplant recipients are key risk factors for acquisition and reactivation of the infection. Conversely, C. gattii is less frequent (20%), and a growing number of cases occur mainly in immunocompetent individuals.5–7
There is growing data on the epidemiology of cryptococcosis among HIV-negative populations.8 In a recent prospective study conducted among HIV-negative people in the United States, CNS cryptococcosis was found to be present in 50% of 145 study subjects with underlying immunosuppression, and was associated with recurrent neurological morbidities and pressure-related complications.9 In a recent review of isolated pulmonary cryptococcosis among HIV-negative patients, men were found to be more prone than women, and isolated pulmonary cryptococcosis was associated with underlying diseases and immune dysfunction.10 The key risk factors of cryptococcosis in HIV-negative individuals are systemic lupus erythematosus and other autoimmune rheumatic diseases, smoking, corticosteroid use, and chronic obstructive pulmonary disease,7 cirrhosis,11 pregnancy and diabetes mellitus (DM).12
DM is one of the most common predisposing underlying diseases for cryptococcosis in HIV-negative individuals, accounting for 10–20% of cryptococcosis patients with underlying diseases.10,13–15 Cryptococcal meningitis in patients with DM is associated with poor clinical outcomes.16,17 Hyperglycemia seen in DM patients causes defects in the host’s immune system which predisposes DM patients to opportunistic infections such as cryptococcosis.18 In mainland China, a retrospective study among Chinese individuals with type 2 DM found a hospital-based cryptococcal prevalence of 0.21%, with 62% having DM before infection.19 In a study in Taiwan, in addition to DM being associated with the occurrence of cryptococcosis in HIV-negative patients, it was also found to be associated with a 1-year and overall mortality from cryptococcosis and cryptococcal meningitis.20
There have been few data available to understand cryptococcosis clinical manifestations, management, and outcomes of cryptococcosis in people without HIV.21 In addition, the role of the different types of DM in the development of cryptococcosis in DM patients is unclear. We undertook this study to describe the epidemiology, clinical manifestations and treatment outcomes of cryptococcosis in patients with DM through a scoping review of case reports, case series and studies of the epidemiology and clinical characteristics of DM patients with cryptococcosis.
Methods
We systematically explored international medical databases: MEDLINE (via PubMed), Embase, and the Cochrane Library in November 2020. The searches covered the period between 1980 and 2020. The search terms used were ‘disseminated cryptococcosis’, ‘cryptococcal meningitis’, ‘cryptococcal pneumonia’, ‘diabetic mellitus’, ‘organ transplant’. Boolean operators AND, OR and NOT were used appropriately. No language restrictions were applied.
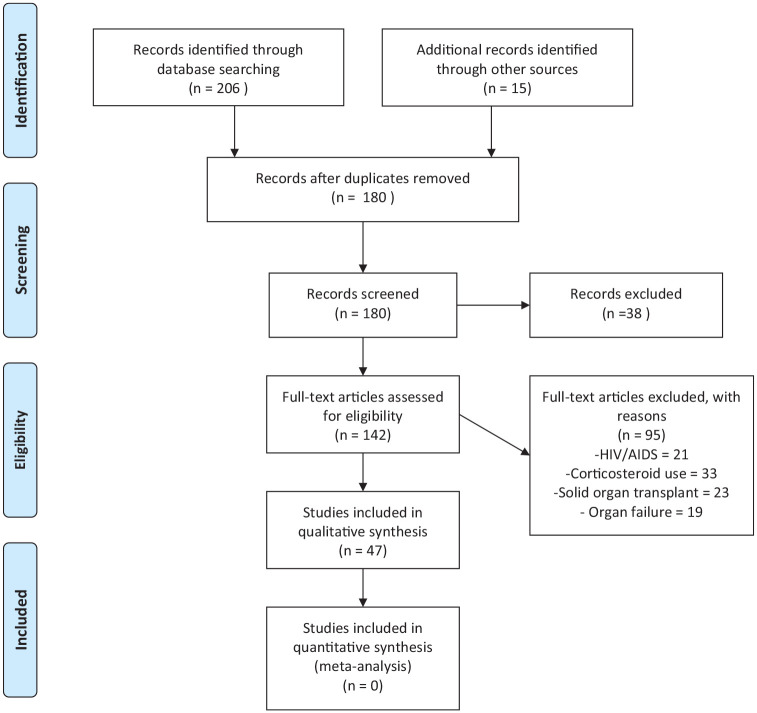
Reference lists of included articles were also searched, and additional studies were included if appropriate. We included single case reports, case series and original articles and excluded review articles and studies including DM and other known risk factors for cryptococcosis such as HIV/AIDS and solid organ transplant (Figure 1). We included studies that reported confirmed cryptococcosis in DM patients. The confirmation of cryptococcosis was based on culture and microscopy, positive Indian ink staining, histopathological examination and/or cryptococcal antigen screening (CrAg). The diagnosis of DM should have been made prior to cryptococcosis diagnosis. We collected the following data: publication data, age, gender, study type, clinical characteristics, other underlying conditions, diagnostic modalities, and disease outcomes.
Figure 1.
PRISMA flow diagram.
Data were analyzed using Microsoft Excel 2016 and descriptive statistics (mean or median for numerical data; frequency and percentages for categorical variables) were employed. This study did not require approval by the institutional review board.
Results
Epidemiology of cryptococcosis among patients with DM
We identified 28 studies, including 47 patients from across four different continents: Asia (60.7%, n = 17), North America (21.4%, n = 6), South America (10.7%, n = 3), and Africa (7.1% n = 2). Most of the studies were single case reports (85.7%, n = 24) and retrospective (14.3%, n = 4) studies. Male patients (75%, n = 18) were recorded more than female patients (25%, n = 6). The median age was 60.5 years (27–79) (Table 1).
Table 1.
Study characteristics.
| Study | Year of publication | Country of origin of cases | Study type | Total number of patients | Age | Sex | Diabetes | HbA1C | CrAg status and how diagnosis was made | Manifestation | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anto et al.22 | 2020 | India (Asia) | Case report | 1 | 64 | F | yes | Blood and urine cultures were positive and nucleotide sequencing confirmed as C. neoformans var. grubii | Meningitis | Dead | |
| Kalyango et al.23 | 2020 | Uganda (Africa) | Case report | 1 | 59 | M | Yes | 9.3% | Sputum culture | Pulmonary | Alive |
| Acharya et al.24 | 2020 | USA (North America) | Case report | 1 | 61 | F | Yes | 9 mmol/mol | Lumbar puncture | Meningitis | Alive |
| Zitun et al.25 | 2020 | USA (North America) | Case report | 1 | 66 | M | Yes | Positive serum CrAg | Pleural | Alive | |
| Wan et al.26 | 2020 | China (Asia) | Retrospective (2013–2017) | 15 | Yes 3/15 | Positive CSF CrAg, culture | Meningitis | alive | |||
| Owuor27 | 2019 | Kenya (Africa) | Case report | 1 | 27 | F | yes | India ink on 2 CSF samples | Meningitis | Alive | |
| Badami et al.28 | 2019 | USA (North America) | Case report | 1 | 68 | M | Yes | 6.0% | Positive CSF CrAg, transbronchial biopsy | Disseminated | Alive |
| Jha et al.29 | 2019 | India (Asia) | Case report | 1 | 56 | M | Yes | 6.4% | CSF gram staining, India ink and culture | Meningitis | Dead |
| Ikumi et al.30 | 2016 | Japan (Asia) | Case report | 1 | 72 | F | Yes | Blood and CSF culture | Meningitis | Alive | |
| De Oliveira Nunes et al.31 | 2016 | Brazil (South America) | Case report | 1 | 69 | M | Yes | Blood culture and BAL culture | Pulmonary | Alive | |
| Sung et al.32 | 2015 | South Korea (Asia) | Case report | 1 | 32 | M | Yes | 10.9% | Percutaneous supraclavicular lymph node core needle biopsy. | Pulmonary | Alive |
| Chen et al.33 | 2015 | China (Asia) | Case report | 1 | 71 | M | Yes | blood culture | Disseminated | Alive | |
| Ho et al.34 | 2015 | China (Asia) | Case report | 1 | 73 | M | Yes | Operative debridement | Cutaneous | Alive | |
| Koike et al.35 | 2014 | Japan (Asia) | Case report | 1 | 79 | M | Yes | CSF culture | Meningitis | Alive | |
| Messina et al.16 | 2014 | Argentina (South America) | Retrospective (2008–2011) | 182 | Yes 14/182 | CSF culture | Meningitis | 12/14 dead | |||
| Poojary et al.18 | 2014 | India (Asia) | Case report | 1 | 48 | M | Yes | Culture of tissue on Sabouraud dextrose agar | Disseminated | Alive | |
| Sugita et al.36 | 2014 | Japan (Asia) | Case report | 1 | 71 | M | Yes | Fibre optic bronchoscopy ,and biopsy | Pulmonary | Alive | |
| Raheja et al.37 | 2012 | USA (North America) | Case report | 1 | 54 | M | yes | Positive CSF CrAg | Meningitis | Alive | |
| Shoji et al.38 | 2012 | Japan (Asia) | Case report | 1 | 68 | M | Yes | India ink staining of CSF | Meningitis | Alive | |
| Araujo et al.39 | 2012 | Brazil (South America ) | Case report | 1 | 51 | M | Yes | Positive serum CrAg, incision ,biopsy and culture of biopsy | Peritonitis | Alive | |
| Kurahara et al.40 | 2011 | Japan (Asia) | Case report | 1 | 57 | M | yes | Positive CrAg, transbronchial lung biopsy | Pulmonary | Alive | |
| Juhi et al.41 | 2009 | India (Asia) | Retrospective (2004–2007) | 7 | Yes 2/7 | CSF India ink, gram stain, culture | Meningitis | ½ dead | |||
| Huang et al.42 | 2005 | China (Asia) | Case report | 1 | 69 | M | Yes | Transthoracic needle biopsy | Pulmonary | Alive | |
| Tanigawa et al.43 | 2004 | Japan (Asia) | Case report | 1 | 65 | M | yes | Transbronchial lung biopsy | Pulmonary | Alive | |
| Prasad et al.44 | 2003 | India (Asia) | Retrospective (1996–2001) | 45 | Yes 4/45 | CSF culture | Meningitis | ¼ dead | |||
| Karpinsky et al.45 | 1998 | USA (North America) | Case report | 1 | 60 | M | Yes | Lumbar puncture | Meningitis | Dead | |
| Vaidya et al.46 | 1991 | USA (North America) | Case report | 1 | 54 | F | Yes | Fine-needle aspirate and culture; blood culture | Thyroidal | Dead | |
| Liu et al.47 | 1991 | Taiwan (Asia) | Case report | 1 | 62 | F | Yes | Positive serum CrAg, biopsy | Adrenal | Alive |
BAL, bronchoalveolar lavage; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; F, female; M, male; SDA, Sabouraud dextrose agar.
Clinical manifestations of cryptococcosis among patients with DM
The majority of the patients had cryptococcal meningitis (68.1%, n = 32), followed by disseminated cryptococcosis (6.4%, n = 3), isolated pulmonary disease (14.9%, n = 7), and others (10.6%, n = 5) (Table 1). The most reported presenting complaints among cryptococcal meningitis were headache (57.1%, n = 8), fever (57.1%, n = 8), altered mental status (35.7%, n = 5), and nausea and vomiting (28.6%, n = 4). Isolated pulmonary cryptococcosis common symptoms included fever (42.9%, n = 3), shortness of breath (42.9%, n = 3), and cough (100%, n = 7). Although patients with disseminated disease presented with varying symptoms, fever was the most common among the cases.
Diagnosis of cryptococcosis among patients with DM
The diagnosis of cryptococcal meningitis was made by culture and microscopy of cerebrospinal fluid (CSF) 81.3% (n = 26), India ink 21.9% (n = 7), and CSF cryptococcal antigen 12.5% (n = 4), whereas the diagnosis of isolated pulmonary disease was made by culture 42.9% (n = 3) and histopathology 71.4% (n = 5). Disseminated disease was diagnosed by culture and microscopy culture 66.7% (n = 2), and other tests listed (Table 2).
Table 2.
Diagnostic yield stratified by sample type and clinical syndrome of cryptococcosis in patients with diabetes mellitus.
| Diagnostic test | All cases N = 47 | Disseminated N = 3 | Pulmonary N = 7 | CNS N = 32 | Cutaneous N = 1 | Peritoneal N = 1 | Pleural N = 1 | Thyroid N = 1 | Adrenal N = 1 |
|---|---|---|---|---|---|---|---|---|---|
| Culture (any site) | 38 (80.9) | 2 (66.7) | 4 (57.1) | 29 (90.6) | 1 (100) | 1 (100) | 1 (100) | ||
| Blood culture | 5 (10.6) | 1 (33.3) | 1 (14.3) | 2 (6.3) | 1 (100) | ||||
| CSF culture | 26 (55.3) | 26 (81.3) | |||||||
| BAL culture | 1 (2.1) | 1 (14.3) | |||||||
| Sputum culture | 1 (2.1) | 1 (14.3) | |||||||
| Urine culture | 1 (2.1) | 1 (3.1) | |||||||
| Skin and soft tissue culture | 4 (8.5) | 1 (33.3) | 1 (14.3) | 1 (100) | 1 (100) | ||||
| CrAg (all) | 9 (19.1) | 1 (33.3) | 1 (14.3) | 4 (12.5) | 1 (100) | 1 (100) | 1 (100) | ||
| Serum CrAg | 4 (8.5) | 1 (14.3) | 1 (100) | 1 (100) | 1 (100) | ||||
| CSF CrAg | 5 (10.6) | 1 (33.3) | 4 (12.5) | 1 (100) | |||||
| Histopathology | 9 (19.1) | 1 (33.3) | 5 (71.4) | 7 (21.9) | 1 (100) | 1 (100) |
BAL, bronchoalveolar lavage; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid.
Treatment outcomes of cryptococcosis among patients with DM
Of the 47 patients, 38.3% (n = 18) patients died on therapy. Mortality in patients with meningeal disease was 36.2% (n = 17) and thyroid disease 0.21% (n = 1), whereas patients with pulmonary disease and disseminated disease reported recoveries (Table 1).
Discussion
There is an emerging body of evidence, mainly from observational studies and case reports, to support DM as an important risk factor for cryptococcosis.39,48–50 In the present study, we sought to describe the clinical characteristics and outcomes of cryptococcosis in people living with DM. We found that the median age was 60.5 years, which is consistent with previous studies that showed a prevalence of cryptococcosis and DM in the older population.51
According to a population-based study in Taiwan reported by Lin and colleagues, 30.6% of 4587 people who were found to have cryptococcosis had DM as the underlying condition.20 In that study, it was argued that the higher prevalence of cryptococcosis in DM was attributed to the higher age of DM patients. However, after accounting for the effect of age through case–control matching, a relationship between DM and cryptococcosis was demonstrated.20
Our data showed a male predominance, which was similar to previous studies.52,53 A proposed explanation for the male gender predominance could be a difference in the host’s immune response between men and women. The immune modulating effect of testosterone makes men more susceptible to IRIS.54 Furthermore, we found that cryptococcal meningitis was the most frequent form of cryptococcal disease in patients with DM. This is in line with a multicenter study performed in the USA with cryptococcosis in people living with HIV.55 However, Baddley and colleagues found that in a study of 166 HIV-negative individuals, pulmonary cryptococcosis was the most prevalent form of cryptococcal disease, which is in contrast with our findings.56
The clinical manifestations of cryptococcal infection vary depending on the patient’s immune status and site of infection, from asymptomatic to having cough, fever, pneumonia, meningoencephalopathy, spread to multiple body sites, severe life-threatening disease, or even death. However, due to the predilection of C. neoformans for the CNS, the most common clinical presentation of cryptococcosis is meningoencephalitis whose signs include meningismus, papilledema, cranial nerve palsies, focal neurological deficits, and depressed level of consciousness.2 The severity of cryptococcosis depends on the characteristic virulence factors of the Cryptococcus and host immune system responses. A predominant T helper (Th) immune response is associated with protection from cryptococcosis whereas Th-2 responses are associated with susceptibility to cryptococcal infection.7,57 According to this study, common clinical manifestations of cryptococcal disease included: headache, fever, shortness of breath, altered mental status, nausea and vomiting in the respective sites of infection.
Furthermore, this study indicated an overall mortality of 21.3%, with the death rate in meningeal disease as high as 43.8%. No mortalities were reported among patients with disseminated or pulmonary disease. Cases have been reported of pulmonary cryptococcosis resolving without treatment in immunocompetent individuals.13 Lin et al. was able to demonstrate a higher 1-year and overall mortality among HIV-uninfected individuals with DM than those without DM in cryptococcosis and cryptococcal meningitis.20
A study in the USA identified DM as a major risk factor for cryptococcal disease in solid organ transplant and immunocompetent patients.58 Other predisposing conditions related to cryptococcosis include: recipients of immunosuppressive treatment, this was the most common in this study, HIV/AIDS, chronic renal failure, malignancy, and Cushing’s syndrome.59 Cell mediated immunity is the most important host response to cryptococcal disease, hence the high incidence of disease among immunosuppressed individuals. DM leads to decreased cell mediated immunity through neutrophilic chemotaxis impairment, abnormal phagocytosis, poor lymphocyte blast transformation, and defective opsonization. Hyperglycemia also influences the microbicidal function of macrophages. This allows for opportunistic infections and is hypothesized to be the relationship between cryptococcal infection and DM.60–62
Culture of appropriate respiratory samples is the gold standard for the diagnosis of cryptococcal infection hence it comes as no surprise that it was the most commonly favored diagnostic modality in this study. The use of CrAg detection for confirming the diagnosis was also widely favored because it is more convenient for all hospital settings and takes a shorter duration of time.63 In a large validation study in Africa by Boulware et al.,64 CrAg lateral flow assay (LFA) reported a high sensitivity (99.3%) and a high specificity (99.1%) in HIV-positive individuals and a median sensitivity and specificity of 100% [95% confidence interval (CI) 95.6–100%] and 99.5% (95% CI 95.7–100%) whereas culture, although specific, has a low sensitivity (50–80%).65
The limitations of our study were an inability to have a complete picture on diabetic control and cryptococcal infection because hemoglobin A1C was not recorded for the majority of articles and there were few data on Cryptococcus species typing. Further studies should be done to confirm the relationship between cryptococcosis and other comorbidities and also to document the commonest species of Cryptococcus encountered in DM. Studies concerning diabetic control in cryptococcal infection would be insightful.
Conclusion
There is significant evidence to support the relationship between cryptococcosis and DM. Therefore, health workers should have a higher index of suspicion when presented with elderly patients with DM complaining of headache, fever and shortness of breath. Diagnosis should be ascertained by culture and microscopy, cryptococcal antigen testing or histopathology. Healthcare providers should also give thought to the effect of hyperglycemia in the management of cryptococcal infection and DM. Additional studies on hyperglycemic control with cryptococcosis are recommended.
Footnotes
Author contributions: Felix Bongomin conceptualized the study. Lauryn Nsenga, Jonathan Kajjimu, Ronald Olum, Sandra Ninsiima, Andrew Peter Kyazze, Phillip Ssekamatte, Davis Kibirige, Joseph Baruch Baluku, Irene Andia-Biraro and Felix Bongomin contributed to the drafting of the manuscript. All authors reviewed and approved the final manuscript prior to submission.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical statement: Our study did not require ethical board approval because of its design of a literature review of published data. Our study did not require consent because no individual patient data were included.
ORCID iD: Felix Bongomin  https://orcid.org/0000-0003-4515-8517
https://orcid.org/0000-0003-4515-8517
Contributor Information
Lauryn Nsenga, School of Medicine, Kabale University, Kabale, Uganda.
Jonathan Kajjimu, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Ronald Olum, Department of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Sandra Ninsiima, Department of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Andrew Peter Kyazze, Department of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Phillip Ssekamatte, Department of Immunology and Molecular Biology, School of Biomedical Sciences, Makerere University College of Health Sciences, Kampala, Uganda.
Davis Kibirige, Department of Medicine, Uganda Martyrs Lubaga Hospital, Kampala, Uganda; Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine, Uganda Research Unit, Entebbe, Uganda.
Joseph Baruch Baluku, Division of Pulmonology, Kiruddu National Referral Hospital, Kampala, Uganda; Department of Programs, Mildmay Uganda, Wakiso, Uganda.
Irene Andia-Biraro, Department of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine, Uganda Research Unit, Entebbe, Uganda.
Felix Bongomin, Department of Medical Microbiology and Immunology, Faculty of Medicine, Gulu University, P.O. Box, 166, Gulu, Uganda.
References
- 1. Kwon-Chung KJ, Fraser JA, Doering TL, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med 2014; 4: a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li SS, Mody CH. Cryptococcus. Proc Am Thorac Soc 2010; 7: 186–196. [DOI] [PubMed] [Google Scholar]
- 3. Emmons CW. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am J Hyg 1955; 62: 227–232. [DOI] [PubMed] [Google Scholar]
- 4. Evans EE. The antigenic composition of Cryptococcus neoformans: I. A serologic classification by means of the capsular and agglutination reactions. J Immunol 1950; 64: 423–430. [PubMed] [Google Scholar]
- 5. Meyer W, Gilgado F, Ngamskulrungroj P, et al. Molecular typing of the Cryptococcus neoformans/Cryptococcus gattii species complex. In: Heitman J, Kozel TR, Kwon-Chung KJ, et al. (eds) Cryptococcus: from human pathogen to model yeast. Wiley Online Books, 2011, pp. 327–357. [Google Scholar]
- 6. Franco-Paredes C, Womack T, Bohlmeyer T, et al. Management of Cryptococcus gattii meningoencephalitis. Lancet Infect Dis 2015; 15: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pereira ABM, Rogerio AP. Chronic obstructive pulmonary disease (COPD) and asthma–chronic obstructive pulmonary disease overlap syndrome (ACOS) are risk factors for cryptococcosis. Open Allergy J 2020; 11: 1–4. [Google Scholar]
- 8. O’Halloran JA, Powderly WG, Spec A. Cryptococcosis today: it is not all about HIV infection. Curr Clin Microbiol Rep 2017; 4: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marr KA, Sun Y, Spec A, et al. A multicenter, longitudinal cohort study of cryptococcosis in human immunodeficiency virus-negative people in the United States. Clin Infect Dis 2020; 70: 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu H-H, Chen Y-X, Fang S-Y. Clinicopathological features of isolated pulmonary cryptococcosis in HIV-negative patients. J Int Med Res 2020; 48: 0300060520927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh N, Husain S, De Vera M, et al. Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine 2004; 83: 188–192. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura S, Izumikawa K, Seki M, et al. Pulmonary cryptococcosis in late pregnancy and review of published literature. Mycopathologia 2008; 167: 125. [DOI] [PubMed] [Google Scholar]
- 13. Kohno S, Kakeya H, Izumikawa K, et al. Clinical features of pulmonary cryptococcosis in non-HIV patients in Japan. J Infect Chemother 2015; 21: 23–30. [DOI] [PubMed] [Google Scholar]
- 14. Tsai WC, Lien CY, Lee JJ, et al. The clinical characteristics and therapeutic outcomes of cryptococcal meningitis in elderly patients: a hospital-based study. BMC Geriatr 2019; 19: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, et al. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis 2006; 10: 72–78. [DOI] [PubMed] [Google Scholar]
- 16. Messina FA, Negroni R, Maiolo EI, et al. Criptococosis meníngea en pacientes con diabetes y sida. Enferm Infecc Microbiol Clin 2014; 32: 643–646. [DOI] [PubMed] [Google Scholar]
- 17. Lao M, Li C, Li J, et al. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: clinical features and associated factors. J Diabetes Investig 2020; 11: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poojary S, Khatu SP. Disseminated cryptococcosis in a diabetic patient. Cutis 2014; 94: 91–95. [PubMed] [Google Scholar]
- 19. Li Y, Fang W, Jiang W, et al. Cryptococcosis in patients with diabetes mellitus II in mainland China: 1993–2015. Mycoses 2017; 60: 706–713. [DOI] [PubMed] [Google Scholar]
- 20. Lin KH, Chen CM, Chen TL, et al. Diabetes mellitus is associated with acquisition and increased mortality in HIV-uninfected patients with cryptococcosis: a population-based study. J Infect 2016; 72: 608–614. [DOI] [PubMed] [Google Scholar]
- 21. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis 2010; 10: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anto PV, Reddy NM, Krishnan V, et al. Abstracts from the International Science Symposium on HIV and Infectious Diseases (ISSHID 2019): infectious diseases: Chennai, India. 12–14 October 2019. BMC Infect Dis 2020; 20: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalyango N, Kwizera R, Baluku JB, et al. Intra-cavitary pulmonary cryptococcoma in poorly controlled diabetes mellitus. Med Mycol Case Rep 2020; 28: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acharya R, Khanal K, Upadhyaya P, et al. Diabetes mellitus as a risk factor for cryptococcal meningitis in immunocompetent. IDCases 2020; 22: e00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zitun M, Elmassry M, El Nawaa SE, et al. A rare case of cryptococcal empyema in an immunocompetent patient. Chest 2020; 158: A1257. [Google Scholar]
- 26. Wan Y, Li X, Wang Y, et al. Clinical characteristic of 15 cases of cryptococcal meningitis treated with Ommaya reservoir. Acta Neurol Belg 2019; 1–7. [DOI] [PubMed] [Google Scholar]
- 27. Owuor OH, Chege P. Cryptococcal meningitis in a HIV negative newly diagnosed diabetic patient: a case report. BMC Infect Dis 2019; 19: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Badami V, Zulfikar R. Cryptococcosis in an immunocompetent well-controlled diabetic. D48 critical care case reports: infection and sepsis II. Am Thorac Soc 2019; 201: A6605. [Google Scholar]
- 29. Jha MK, Mohanty A, Gupta P. Cryptococcus gattii meningitis in a diabetic adult in South India. J Family Med Prim Care 2019; 8: 1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ikuma K, Yokoi K, Ando T. Cryptococcal meningoencephalitis presenting with treatable cognitive impairment and involuntary movement. Clin Neurol 2016; 56: 27–31. [DOI] [PubMed] [Google Scholar]
- 31. de Oliveira Nunes J, Pillon KRAP, Bizerra PL, et al. Erratum to: The simultaneous occurrence of histoplasmosis and cryptococcal fungemia: a case report and review of the literature. Mycopathologia 2016, 181: 899. [DOI] [PubMed] [Google Scholar]
- 32. Sung JH, Do Hoon Kim MJ, Lee KJ, et al. A case of pulmonary cryptococcosis in an immunocompetent male patient diagnosed by a percutaneous supraclavicular lymph node biopsy. Tuberc Respir Dis 2015; 78: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen CM, Yu WL. Complicated cryptococcal meningitis, spinal myelitis and probably tracheobronchitis: a rare case report. J Microbiol Immunol Infect 2015; 48: S167. [Google Scholar]
- 34. Ho SW, Ang CL, Ding CS, et al. Necrotizing fasciitis caused by Cryptococcus gattii. Am J Orthop 2015; 44: E517–E522. [PubMed] [Google Scholar]
- 35. Koike Y, Ishihara T, Ozawa T, et al. Cryptococcal meningitis successfully treated with voriconazole and low-dose amphotericin B. Neurol Clin Neurosci 2014; 2: 166–168. [Google Scholar]
- 36. Sugita C, Tanaka S, Takahashi T. Endobronchial cryptococcosis in a non-HIV immunocompromised patient. J Exp Clin Med 2014; 6: 105–106. [Google Scholar]
- 37. Raheja H, Sinha A, Irukulla PK, et al. Cryptococcal meningitis masquerading as normal pressure hydrocephalus in an immune-competent adult. J Glob Infect Dis 2017; 9: 157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shoji H, Takuma T, Ohbayashi H, et al. Measurement of antifungal drug levels in cerebrospinal fluid for cryptococcal meningoencephalitis. J Infect Chemother 2012; 18: 775–779. [DOI] [PubMed] [Google Scholar]
- 39. Araújo BS, Bay M, Reichert R, et al. Intra-abdominal cryptococcosis by Cryptococcus gattii: case report and review. Mycopathologia 2012; 174: 81–85. [DOI] [PubMed] [Google Scholar]
- 40. Kurahara Y, Tachibana K, Katsura H, et al. A case of cryptococcal empyema successfully treated by debridement by medical thoracoscopy with local anesthesia. Nihon Kokyuki Gakkai Zasshi 2011; 49: 142–147. [PubMed] [Google Scholar]
- 41. Juhi T, BibhaBati M, Aradhana B, et al. Cryptococcal meningitis in a tertiary care hospital. Nihon Ishinkin Gakkai Zasshi 2009; 50: 95–99. [DOI] [PubMed] [Google Scholar]
- 42. Huang CJ, Yang MC, Ueng SH. Large cryptococcoma mimicking lung cancer in an HIV-negative, type 2 diabetic patient. J Thorac Imaging 2005; 20: 115–117. [DOI] [PubMed] [Google Scholar]
- 43. Tanigawa M, Kimura M, Ichioka M, et al. A case of secondary pulmonary cryptococcosis complicating diabetes mellitus. Nihon Kokyuki Gakkai Zasshi 2004; 42: 272–276. [PubMed] [Google Scholar]
- 44. Prasad KN, Agarwal J, Nag VL, et al. Cryptococcal infection in patients with clinically diagnosed meningitis in a tertiary care center. Neurol India 2003; 51: 364–366. [PubMed] [Google Scholar]
- 45. Karpinsky NC, Powell H. Case of the month: July 1997 – Diabetic male with transient ischemic attacks. Brain Pathol 1998; 8: 235–236. [PubMed] [Google Scholar]
- 46. Vaidya KP, Lomvardias S. Cryptococcal thyroiditis: report of a case diagnosed by fine-needle aspiration cytology. Diagn Cytopathol 1991; 7: 415–416. [DOI] [PubMed] [Google Scholar]
- 47. Liu YC, Cheng DL, Liu CY, et al. Isolated cryptococcosis of the adrenal gland. J Intern Med 1991; 230: 285–287. [DOI] [PubMed] [Google Scholar]
- 48. Sah R, Jaiswal A, Singla S, et al. Pulmonary cryptococcosis: report of the first confirmed autochthonous case in Nepal. Acta Trop 2019; 105205. [DOI] [PubMed] [Google Scholar]
- 49. Indira P, Kumar PM, Shalini S, et al. Opportunistic infections among people living with HIV (PLHIV) with diabetes mellitus (DM) attending a tertiary care hospital in coastal city of South India. PLoS One 2015; 10: e0136280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chuang YM, Ho YC, Chang HT, et al. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis 2008; 27: 307–310. [DOI] [PubMed] [Google Scholar]
- 51. Lee YC, Wang JT, Sun HY, et al. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect 2011; 44: 338–345. [DOI] [PubMed] [Google Scholar]
- 52. Tsai WC, Lien CY, Lee JJ, et al. The prognostic factors of HIV-negative adult cryptococcal meningitis with a focus on cranial MRI-based neuroimaging findings. J Clin Neurosci 2018; 55: 57–61. [DOI] [PubMed] [Google Scholar]
- 53. Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 2001; 33: 690–699. [DOI] [PubMed] [Google Scholar]
- 54. Casadevall A, Perfect J. Cryptococcus neoformans. Washington, DC: American Society for Microbiology Press, 1998, pp. 351–369. [Google Scholar]
- 55. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013; 124: 61–79. [PMC free article] [PubMed] [Google Scholar]
- 56. Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis 2008; 27: 937–943. [DOI] [PubMed] [Google Scholar]
- 57. Wormley FL, Perfect JR. Immunology of infection caused by Cryptococcus neoformans. Methods Mol Med 2005; 118: 193–198. [DOI] [PubMed] [Google Scholar]
- 58. George IA, Spec A, Powderly WG, et al. Comparative epidemiology and outcomes of human immunodeficiency virus (HIV), non-HIV non-transplant, and solid organ transplant associated cryptococcosis: a population-based study. Clin Infect Dis 2018; 66: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shih CC, Chen YC, Chang SC, et al. Cryptococcal meningitis in non-HIV-infected patients. QJM 2000; 93: 245–251. [DOI] [PubMed] [Google Scholar]
- 60. May RC, Stone NR, Wiesner DL, et al. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 2016; 14: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huffnagle GB, Lipscomb MF, Lovchik JA, et al. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol 1994; 55: 35–42. [DOI] [PubMed] [Google Scholar]
- 62. Kakeya H, Izumikawa K, Yamada K, et al. Concurrent subcutaneous candidal abscesses and pulmonary cryptococcosis in a patient with diabetes mellitus and a history of corticosteroid therapy. Intern Med 2014; 53: 1385–1390. [DOI] [PubMed] [Google Scholar]
- 63. Meya D, Manabe Y, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count ⩽100 cells/µl who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51 :448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Snow RM, Dismukes WE. Cryptococcal meningitis diagnostic value of cryptococcal antigen in cerebrospinal fluid. Arch Intern Med 1975; 135: 1155–1157. [DOI] [PubMed] [Google Scholar]