Abstract
Conventional antibody-based targeted cancer therapy is one of the most promising avenues of successful cancer treatment, with the potential to reduce toxic side effects to healthy cells surrounding tumor cells. However, the full potential of antibodies is severely limited due to their large size, low stability, slow clearance, and high immunogenicity. Alternatively, recently discovered nanobodies, which are the smallest naturally occurring antigen-binding format, have shown great potential for addressing these limitations. Bioconjugation of nanobodies to functional groups such as toxins, enzymes, radionucleotides, and fluorophores can improve the efficacy and potency of nanobodies, enhance their in vivo pharmacokinetics, and expand the range of potential applications. Herein, we review the superior characteristics of nanobodies in comparison to conventional antibodies and provide insight into recent developments in nanobody conjugates for targeted cancer therapy and imaging.
Keywords: targeted caner treatment, antibody fragments, nanobodies, bioconjugations, cancer imaging, therapeutic nanobodies
Introduction
Due to the side effects of traditional chemotherapy and radiotherapy on healthy cells surrounding tumor sites, the development of strategies for successful cancer treatment remains challenging. Much effort has been made to achieve the specific delivery of drugs or toxins to diseased cells rather than healthy tissues. Since the first administration of a monoclonal antibody (mAb) to a patient with lymphoma in 1980,1 antibodies with exquisite specificity have become potent tools for targeted cancer therapy and precise diagnosis.2 Antibodies (Abs) or immunoglobulins (Igs) are naturally derived therapeutic compounds that are produced by the vertebrate immune system for the identification and elimination of foreign pathogens.3 Abs are 150 kDa proteins consisting of 2 identical heavy chains and 2 identical light chains that are linked together by disulfide bonds and non-covalent interactions (Figure 1A). One antibody comprises 2 antigen-binding sites, and each antigen-binding site consists of 2 variable domains, referred to as VH and VL.
Figure 1.
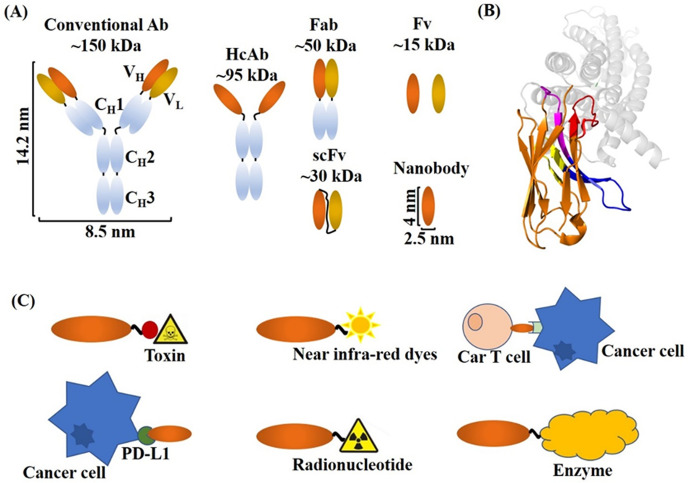
Depiction of Nb structure and their applications in cancer therapy and diagnosis. (A) Schematic representation of different Ab formats. Conventional Abs consist of two light chains and two heavy chains. HcAbs consist of two identical heavy chains only. Nb is the smallest naturally occurring antigen binding fragment. (B) A crystal structure of an Nb binding its antigen G protein-coupled receptor (GPCR). GPCR is shown in gray, the FR regions (except FR2) are in orange, the FR2 region consisting of featured hydrophilic amino acids, CDR1, CDR2, and the prolonged CDR3 regions are shown in blue, magenta, yellow, and red, respectively (PDB ID: 4XT1). (C) Schematic representations of the applications of Nb conjugates in cancer therapy.
Antibodies have been applied in the clinical treatment of cancer for several decades.4 To date, 79 therapeutic mAbs have been approved by the United States Food and Drug Administration, including 30 mAbs for the treatment of cancer.5 Abs can bind to transmembrane receptors or soluble ligands directly, thereby interfering with the corresponding signal pathways in tumor cells. In addition, intact Abs can evoke antibody-dependent cell-mediated cytotoxicity (ADCC) through the Fc functional domain by attracting effector cells such as NK cells and macrophages. Moreover, Abs have been used as vehicles for targeted delivery of cytotoxic drugs or nanoparticles containing therapeutic molecules. Besides targeting therapeutic agents, Abs are also used in the clinic in positron emission tomography (PET), single-photon emission computerized tomography (SPECT) or optical imaging, in which Abs can direct radioactive or fluorescent reagents to diseased sites. The application of Abs, however, has been limited, in part due to their relatively large sizes (14.2 nm × 8.5 nm × 3.8 nm), as shown in Figure 1A, which has been suggested as the main reason for their suboptimal pharmacokinetic profiles and limited tumor penetration.6 Another complication of Abs derives from their complex structures, including posttranslational glycan modifications and inter- and intramolecular disulfide bonds, resulting in high costs during large-scale production of antibodies.7 Moreover, the antigen-binding sites of conventional antibodies have evolved to concave or flat patterns, which can not recognize antigens with cryptic or hidden epitopes. Another important concern of utilizing Abs is that they may induce unwanted immunogenic responses.8
To address these limitations, much attention has been paid to the development of smaller antibody formats that are either derived from IgGs or synthetic binders (e.g., single-chain variable fragment, scFV: ∼30 kDa; FV: ∼28 kDa; antigen-binding fragment, Fab: ∼50 kDa, Figure 1A).9,10 Many of these emerging antigen binders show improved pharmacokinetic properties both in vitro and in vivo. New strategies for cancer therapy based on these new formats have also generated promising results. However, these recombinant proteins are still suboptimal and often suffer from poor solubility, low stability, and reduced affinity.11,12
During the early 1990s, an exceptional Ab isotype was discovered serendipitously in the sera of the Camelidae family, referred to as heavy-chain-only antibodies (HcAbs).13 In addition to Camelidae, HcAbs have also been found in much cartilaginous fish.14 As indicated by the name, one distinguishable feature of this new antibody isotype is the absence of the VH domain and the first constant CH1 domain within the heavy chain. Although the antigen-binding unit of HcAb comprises only 1 single variable domain (VHH), the antigen-binding site can recognize and bind to its cognate antigen with similar affinity to that of conventional Abs. Moreover, the 10 times smaller VHH domain of HcAb alone preserves all binding capacity, and VHH could be considered as the smallest naturally occurring antigen-binding fragment.8,15,16 Given its small size (12-14 kDa) with dimensions of 4 nm × 2.5 nm × 3 nm in the nanometer range, the recombinant VHH is also referred to as a nanobody (Nb, Figure 1A).
Given the rapid development of Nb technology and the large number of publications, reviewing all the aspects of this burgeoning field is not practical. In this review, we focus on Nb conjugates for targeted cancer therapy and imaging. The characteristic features and structure of Nbs will be described in comparison with other conventional Ab formats. The bioconjugation strategies for Nbs are briefly discussed. Then, the applications of Nb conjugates in cancer therapy are reviewed. Following this, Nb-based imaging reagents for cancer diagnosis are presented. Nb-nanomedicine conjugates were not included in this review, although these conjugates showed great potential for targeted delivery of therapeutic nanoparticles. The interested reader is referred to the excellent literature review by Patra.17
Figure 1 Depiction of Nb structure and their applications in cancer therapy and diagnosis. (a) Schematic representation of different Ab formats. Conventional Abs consist of 2 light chains and 2 heavy chains. HcAbs consist of 2 identical heavy chains only. Nb is the smallest naturally occurring antigen-binding fragment. (b) A crystal structure of an Nb binding its antigen G protein-coupled receptor (GPCR). GPCR is shown in gray, the FR regions (except FR2) are in orange, the FR2 region consisting of featured hydrophilic amino acids, CDR1, CDR2, and the prolonged CDR3 regions are shown in blue, magenta, yellow, and red, respectively (PDB ID: 4XT1).18 (c) Schematic representations of the applications of Nb conjugates in cancer therapy.
Structure and Characteristics of Nbs
Nbs exhibit high architectural similarities in comparison with the VH domains of Abs. More specifically, as shown in Figure 1A, Nbs are composed of 3 highly variable loops (complementary determining regions, CDR1/2/3) residing at the tip of the Nb and 4 conserved sequence regions (framework region, FR1/2/3/4). The 3 CDR loops form the antigen-binding site of the Nb, referred to as the paratope, in which CDR3 contributes most of the antigen-binding specificity, while CDR1 and CDR2 are responsible for the enhancement of the binding strength.19,20
One distinctive feature of Nbs is the longer CDR3 loop than that of conventional Abs, as shown in Figure 1B. Most of the finger-like CDR3 structures of Nbs contain approximately 18 amino acids, whereas CDR3 loops of Abs only comprise 12 or 14 amino acids.21 The enlarged CDR3 regions have the potential to provide more interactions between the Nb and the cognate antigen than that of the CDR3 loops of convectional Abs, counterbalancing in part the missing VL-domain.16 The protruding CDR3 makes Nbs well suited for recognizing and interacting with unique epitopes, which are inaccessible to conventional Abs.22 The finger-like CDR3 and prolate shape together expand the potential targets of Nbs.
One of the other noteworthy hallmarks of Nbs is their significantly reduced size compared to conventional Abs. This small size allows Nbs to achieve quick extravasation from the vein, rapid diffusion throughout the body, and deep penetration into the tumor tissues, making them suitable for molecular imaging and solid tumor treatment. Although the small size of Nbs is often considered as an advantage, especially in non-invasive imaging, it is fair to state that it might also be a limitation. Since the molecular weight of Nbs is below the threshold (50–60 kDa) of kidney glomerular filtration, a potential problem is the rapid clearance of Nbs from the bloodstream by the kidney.23 Consequently, only a marginal fraction of the administrated Nbs accumulates at the diseased sites. Frequent administration is essential to achieve optimal efficacy. To address this limitation, massive efforts have been made to increase the half-life of Nbs. It has been shown that albumin can be used as a versatile platform to prolong the half-life of drugs.24 Albumin-based strategies have also been applied to Nb technology. In 2011, one study was published in which a bispecific trivalent Nb was developed consisting of a bivalent EGFR-targeting Nb and an albumin-targeting Nb.25 The involvement of albumin in the trivalent Nbs resulted in an extended half-life of 2–3 days in mice. Alternatively, Harmsen et al reported that the attachment of porcine IgG (pIgG) to Nbs led to a 100-fold extended in vivo residence time in comparison to control Nbs that lack the ability to bind to pIgG.26
The other characteristic of Nbs is their extraordinary robustness. It has been demonstrated that 4 hydrophobic amino acids residing in the FR2 region of conventional Abs are substituted by 4 hydrophilic amino acids in Nbs. In conventional Abs, these hydrophobic amino acids lying at the surface of the VH domain have been suggested to provide the binding surface for VL domains. The distinctive substitution of hydrophobic amino acids with hydrophilic amino acids in Nbs has been thought to be the main contributor to the increased solubility and stability of Nbs.16 Due to their hydrophilicity, lack of posttranslational modification, fewer disulfide bonds, and a monomeric structure, Nbs can be expressed in high yields using low-cost production systems such as E. coli and S. cerevisiae. In sharp contrast, the production of scFV often suffers from low yield because of its decreased stability and solubility.27
According to published sequence information, VHH shows a high degree of sequence identity with the human type 3 VH domain.28 The high sequence identity is the main contributor to Nbs’ low immunogenic profiles. This notion was also supported by a study investigating the ability of Nbs to target transgenic tumors expressing lysozymes.29 Phase I clinical trials carried out by Ablynx have also proven the inherent low immunogenicity of Nbs. The low immunogenicity of Nbs makes them suitable for prolonged and repeated administration. In addition, strategies for Nb humanization have been developed and are now routine before clinical trials,30,31 further alleviating unwanted immune responses. However, it should be noted that additional engineering might be essential after the humanization to restore the sufficient binding capacity of Nbs for their targets.
Nbs Bioconjugation Strategies
The attachment of effector groups such as small molecular drugs, toxins, enzymes, and imaging agents to Nbs will generate so-called Nb conjugates, which can substantially expand the applicability of Nbs in cancer treatment and diagnosis (Figure 1C). In this context, Nbs are utilized as a means of vehicle that can specifically target effector groups to or into target sites with low systemic toxicity. Nb conjugates combine the advantages of both molecules, such as high tissue penetration, high specificity, potent anti-tumor effect, and/or imageable properties. There is a compelling need to establish strategies for Nb bioconjugation.
Because of their modular and monomeric nature, Nbs are amenable to both chemical modification and molecular manipulation. Taking advantage of modularity, multivalent Nbs, bispecific Nbs, and biparatopic Nbs can be constructed simply through gene fusion, leading to increased affinity, specificity, and efficacy.25,32 Fc domains, enzymes, fluorescent proteins, and protein ligands and toxins have also been successfully attached to Nbs through gene fusion.33,34,35,36
With respect to the chemical functionalization of Nbs, traditionally, nucleophilic cysteine and lysine are most commonly used. Lysin bioconjugation is a popular strategy because of the very high prevalence of lysin in almost all protein surfaces, including Nbs. However, lysin-based bioconjugation methods lack the ability to control the number and location of the functionalization, leading to a mixture of Nb conjugates. Moreover, with the heterogeneity, the decrease or loss of Nbs’ binding capacities if addressable lysin was present at CDR loops.37
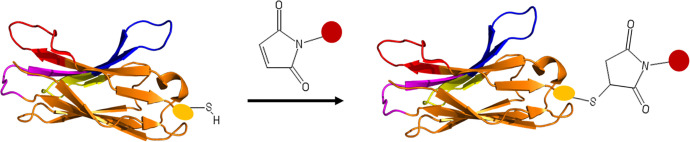
Site-specific functionalization of Nbs is more likely to be obtained through addressable cysteine because of its low abundance in most protein sequences.38 Foreign cysteine can be readily introduced to the C-terminus via gene manipulation. In most conditions, this newly introduced cysteine is incorporated into the C-terminus of Nbs, most distal from the CDR loops, which ensures minimum interference with the Nb antigen recognition capacity.39 However, it was reported that the introduction of foreign cysteine caused a low yield of Nbs.40
Figure 2 Site-specific functionalization of nanobody via engineered cysteine. Cysteine is introduced into nanobody through genetic modification. Maleimide is one of the most widely used sulfhydryl-reactive chemical groups. Yellow oval indicates backbone of foreign cysteine. Red sphere denotes functional group (e.g. toxin or fluorescence) attached to maleimide.
Figure 2.
Site-specific functionalization of nanobody via engineered cysteine. Cysteine is introduced into nanobody through genetic modification. Maleimide is one of the most widely used sulfhydryl-reactive chemical groups. Yellow oval indicates backbone of foreign cysteine. Red sphere denotes functional group (e.g. toxin or fluorescence) attached to maleimide.
Amber suppression is an alternative technology to achieve Nbs bioconjugation.41 The highlight of this method is the introduction of unique unnatural amino acids carrying reactive chemical entities such as ketones, azides, and alkenes into protein sequences, which enables exquisite selectivity. These reactive groups can then be employed to link desired payloads onto the protein site specifically by using biorthogonal-coupling reactions. However, the introduction of unnatural amino acids may cause lower expression of the Nb.42 This drawback limits the use of amber suppression in Nb modification.
Site-specific chemoenzymatic protein functionalization is probably the most successful protein conjugation strategy.43 In principle, a specific reactive peptide tag is introduced to the C terminal of Nbs, rendering it a substrate for a variety of enzymes, including Sortase A,44 lipoic acid ligase,45 transglutaminases,46 bacterial biotin ligase,47 and tubulin tyrosine ligase.48 These enzymes can recognize the incorporated peptide tags and initiate site-specific chemoenzymatic protein functionalization. The payload of interests, such as fluorophores and drugs, can be attached to the activated peptide tag through well-established biorthogonal reactions.
Expressed protein ligation (EPL) has also been involved in the site-specific modification of Nbs. To attach effectors to Nbs (equivalent to N extein) by EPL, the mutated intein lacking splicing ability is fused to the terminus of the Nb. Addition of excess thiol can activate the intein and generate a desired protein thioester that can react with a peptide (equivalent to C extein) carrying an N-terminal free cysteine, leading to the formation of the Cys thioester by a thiol-thioester exchange. The payload of interest could be readily appended to the ‘C extin peptide’ by solid-phase peptide synthesis (SPPS). EPL has been applied to ligate fluorophores, and biotin to Nbs.49,50 However, the introduction of intein to protein may cause misfolding of the resultant fusion protein. A refolding procedure is often required in most artificial intein-based techniques.51
Therapeutic Nb Conjugates
Nbs possess great potential as therapeutics for cancer treatment and many of them are under clinical trial (Table 1). In oncology, Nbs serving as antagonists have been investigated extensively, such as Nbs targeting epidermal growth factor receptor (EGFR),52 human epidermal growth factor (HER2), and hepatocyte growth factor receptor (HGFR). These antagonistic Nbs can bind to extracellular proteins on tumor cells and interfere with their corresponding signaling pathways, leading to inhibition of the growth and proliferation of tumor cells. However, due to the lack of the Fc domain, the anti-tumor efficacy of Nbs alone is inferior to that of traditional Abs. An ideal anti-tumor therapeutic is very potent and specific, which means it can cause cell death and tumor regression rather than inhibition of cancer cell proliferation, and this toxic effect should be strictly restricted at tumor sites avoiding healthy tissues. Conjugation of Nbs to therapeutic agents holds promise for the development of specific anti-cancer therapeutics with improved potency.
Table 1.
Nanobodies in Cancer Clinical Trial.
| Product name | Disease | Target | Phase | Clinical trial identifier | References |
|---|---|---|---|---|---|
| ALX-0651 | Non-Hodgkin’s lymphoma and multiple myeloma | CXCR4 | I | NCT01374503 | 53 |
| DR5Nb1 | Colon and pancreatic cancers | DR5 | I | 32 | |
| [131I]-SGMIB anti-HER2 VHH1 | Breast cancer | HER2 | I | NCT02683083 | 54 |
| TAS266 | Solid tumors | DR5 | I | NCT01529307 | 55 |
| 68GaNOTA-Anti-HER2 VHH1 | Breast cancer | HER2 | I | 56 | |
| 99mTc-NM-02 | Breast cancer | HER2 | I | NCT04040686 | |
| KN035 | Solid tumors | PD-L1 | I | NCT03248843 | 57 |
| KN044 | Advanced solid tumors | CTLA-4 | I | NCT04126590 | |
| CD19/CD20 bispecific CAR T cells | B-cell lymphoma | CD19/CD20 | I | NCT03881761 | 58 |
| BCMA CAR T cells | Myeloma | BCMA | I | NCT03664661 | 59 |
| 68 GaNOTA-Anti-MMR VHH2 | Malignant solid tumor | MMR | I/IIa | NCT04168528 | 60 |
Abbreviations: BCMA, B-cell maturation antigen; CD19/CD20, cluster of differentiation 19/20; CTLA-4, cytotoxic T lymphocyte antigen-4; CXCR4, chemokine receptor type 4; DR5, death receptor-5; MMR, macrophage mannose receptor.
Nbs-Based Immunotoxins
Promising results were obtained with Pseudomonas exotoxin A (PE38)-decorated VEGFR2-specific Nbs.34 PE38 is a potent toxin comprising 3 domains, which can enter the cell in an endocytic vesicle and bind to ADP-ribosylating elongation factor II, destroying cancer cells. An in vitro study showed that the immunotoxin Nb-PE38 conjugates could specifically target cells expressing VEGFR2 and inhibit the proliferation of VEGFR2-expressing cells. Although the bacterial origin of the PE38 toxin might be a concern in clinical applications, this study is an excellent example demonstrating the potential of Nbs in targeted delivery of cytotoxic effectors.
Besides the direct attachment of potent toxins to Nbs, Nbs have also been utilized to site-selectively convert non- or low-toxic pro-drugs into their active forms.35 Cortez-Retamozo et al reported on an anti-carcinoembryonic antigen (CEA) Nb fused with beta-lactamase. This in vitro study showed that Nb specifically directed beta-lactamase to the surface of CEA-expressing LS174 T cancer cells, resulting in the activation of the co-administrated cephalosporin nitrogen mustard prodrug in the close vicinity of diseased cells. In addition, the Nb-beta lactamase conjugates had a preferred biodistribution profile. Remission and cure of mice bearing xenografts were shown. Although beta-lactamase is derived from Enterobacter cloacae and may cause adverse immune responses in patients, this study provides an excellent example of site-specific prodrug activation and shows its potential in cancer treatment.
Nbs Attached to Effector Moieties
In a unique study, Van de Water and co-workers developed a combined strategy for glioblastoma multiform (GBM) therapy, in which both the specificity of Nbs and the pathotropic property of neural stem cells (NSCs) were utilized. Both NSCs and mesenchymal stem cells (MSCs) are characterized by tumor-targeting properties,61,62 and this advantageous feature has been applied to direct therapeutic proteins in a mouse model. Van de Water et al used NSCs as vehicles to transport therapeutic Nbs to GBM cancer cells. NSCs were transduced with lentivirus virions, which encoded a recombinant protein of proapoptotic tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) fused to bivalent and bispecific Nbs (ENB2).36 In vivo and in vitro, on-site and sustainable secretion of Nbs and ENB2-TRAIL from NSCs were observed, and the secreted proteins were shown to specifically home in on GBM tumor tissues. The secreted Nbs were shown to inhibit EGFR signaling significantly in vitro, resulting in decreased tumor growth. In comparison, immunoconjugate ENb2-TRAIL, which could target both tumor cell proliferation and death pathways, exhibited a profound effect on the GBMs. This study indicates that the addition of cytotoxic molecules to Nbs is a promising strategy to improve their therapeutic efficacy.
Nbs are also involved in photodynamic therapy (PDT) to actively deliver photosensitizer (PS) to tumor tissues. During PDT, following the administration of PS, light of a particular wavelength can activate the PS and generate potent singlet oxygen, which can cause damage to cancer cells. In 2014, an Nb-PS conjugate-based PDT was presented in which a near-infrared fluorophore (IRDye700DX) labeled monovalent or biparatopic anti-EGFR nanobody was developed.63 This in vitro study showed that the monovalent Nbs conjugated to PS specifically bound to cells overexpressing EGFR and caused cell death, whereas free PS or Nb-PS without exposure to light did not affect cell viability. Moreover, a more potent PDT efficacy was discovered in PS conjugated to biparatopic anti-EGFR Nb. The results presented in this study are very promising, although the in vivo evaluation of these Nb-PS conjugates will further determine the value of this approach for clinical applications. Recently, the same group evaluated the therapeutic potential of Nb-PS conjugates for targeted PDT in a pre-clinical model of head and neck cancer.64 It was demonstrated that the EGFR specific Nb-PS conjugates specifically accumulated in tumors in vivo, leading to pronounced tumor necrosis and the infiltration of immune cells. Likewise, GPCR-targeting Nb-PS conjugates and HER2-targeting Nb-PS conjugates have been developed for targeted PDT for glioblastoma and breast cancer, respectively.65,66 These studies together pave the way for clinical trials of Nb-PS-based PDT.
Nbs in CAR T Cell Therapy
The T lymphocytes used in chimeric antigen receptor (CAR) T cell therapy have also been armed with Nbs. Adoptive transfer of engineered T cells expressing recombinant receptors originating from antibodies provides a great opportunity for modern cancer treatment. The scFV fragments are the most commonly used antigen recognition modules in recombinant receptors. However, the administration of scFV-based therapeutic T cells often causes a severe immune response and poor in vivo persistence of CAR-redirected T cells in Phase I clinical trials.67,68,69 In addition, due to the paucity of cancer-specific targets of solid tumors, Car T cell therapy is relatively less successful in the treatment of solid tumors, although this therapy has proven its efficacy in treating hematological cancers.70 Owing to their inherent low immunogenicity, Nbs are an ideal alternative recombinant CAR. Xie et al developed Nb-based CAR T cells that targeted the tumor microenvironment, leading to the inhibition of solid tumor growth in immunocompetent mice.71 It was shown that both PD-L1-specific Nbs as well as stroma and extracellular matrix marker-specific Nbs could specifically target engineered T cells to the tumor microenvironment, resulting in an improved survival rate in different tumor models. These promising results demonstrate the feasibility and efficacy of Nb-based CAR T cells in treating solid tumors by targeting the tumor microenvironment.
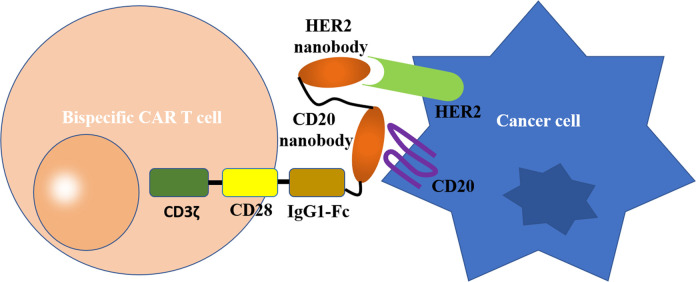
In addition, Nbs have also proven their potential in overcoming the antigen escape of cancer cells, which is the main cause of the failure of Car T cell therapy. To circumvent this limitation, Munter and coworkers recently presented a study in which engineered T cells expressing the bispecific Nbs-based CARs were able to kill tumor cells expressing CD20, HER2, or both in vitro.72 It was demonstrated that the bispecific CARs consisting of 2 different Nbs were stably expressed at high levels in the membrane of the engineered T cells. Moreover, the bispecific CARs retained similar binding affinities for antigens in comparison to the original Nbs. However, no additive or synergistic effects of these bispecific CARs were discovered for the treatment of cancer. This result indicates that further optimization is essential to achieve full utilization of bivalent and bispecific Nbs-based Car T cell therapy.
Figure 3 Bispecific CAR T cells targeting cancer cells. The traditionally used antigen recognition module ScFv in CAR T cell is replaced by a tandem of 2 nanobodies, which can target HER2 and CD20 respectively. CD3Ζ, CD3Ζ intracellular signaling domain; CD28, CD28 transmembrane domain; IgG1-Fc, human IgG1 CH2CH3 (Fc) spacer.
Figure 3.
Bispecific CAR T cells targeting cancer cells. The traditionally used antigen recognition module ScFv in CAR T cell is replaced by a tandem of two nanobodies, which can target HER2 and CD20 respectively. CD3ζ, CD3ζ intracellular signaling domain; CD28, CD28 transmembrane domain; IgG1-Fc, human IgG1 CH2CH3 (Fc) spacer.
Nbs Conjugates in Cancer Imaging
Early diagnosis is important in reducing cancer mortality rates. A variety of techniques have been developed and applied in the clinic for cancer diagnosis, such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), computed tomography (CT), and optical imaging. Owing to their small size, robustness, high stability, and fast clearance from the body, Nb conjugate based molecular imaging modalities have drawn increased attention, which enables the circumvention of the limitations of mAb-based tracers.
Nbs in Nuclear Imaging
Several radionuclides such as (99 m)Tc, (89)Zr, (68)Ga, (18)F, and (64)Cu have been used to label antigen-specific Nbs. Huang et al demonstrated the use of anti-EGFR Nbs (8B6) as targeting moieties for non-invasive imaging through pinhole SPECT.73 The (99 m)Tc-labeled Nb tracers exhibited a relatively earlier imaging time point (at 3 h post-injection) compared to imaging probes using mAbs as targeting moieties. This improvement was attributed to the favorable biodistribution and rapid blood clearance (half-life = 1.5 h) of such Nb conjugates. In addition, in vivo pinhole SPECT imaging showed that (99 m)Tc-8B6 conjugates could differentiate tumors with high and moderate EGFR overexpression. Likewise, Gainkam compared the tumor uptake and biodistribution of 2 different monovalent anti-EGFR Nbs using SPECT/micro-CT imaging. Both radionuclide-labeled Nbs were found to be receptor-specific.74
Furthermore, (99 m)Tc and (68)Ga have also been employed to label Nbs. Xavier et al developed a (68)Ga-labeled anti-HER2 (Nb, 2Rs15d) probe for PET imaging.75 In vivo biodistribution assays demonstrated specific accumulation of radiolabeled tracers in HER2-positive tumor cells. High tumor-to-blood and high tumor-to-muscle ratios were found 1 h after injection of Nbs conjugates, leading to high contrast PET/CT images. This so-called same-day imaging is especially preferable in preclinical or clinical imaging, since rehospitalization and extra radiation exposure can be avoided. In contrast, sufficiently clear images can only be obtained after several hours or days with intact mAbs and most antibody fragment-based tracers.76,77 Interestingly, (68)Ga-nanobody showed reduced kidney uptake than that of the same Nb labeled with (99 m)Tc. Further reduction of kidney uptake was obtained by removal of the C-terminal his6 tag of the Nb. Taken together, the author suggested that (68)Ga-Nb can be regarded as safe for clinical diagnostic translation. Recently, a Phase I clinical trial of this Nb-based radiotracer was completed in 20 women with breast cancer.56 The result was encouraging, and a Phase II study is ongoing.
There is also increased interest in developing (18)F-labeled Nb probes. The advantageous features of (18)F-based tracers include a longer half-life and no need for premodification of Nbs. (18)F-labeled Nb for PET imaging of HER2 overexpressing tumors was recently reported.78 These (18)F-Nb probes showed favorable biodistribution and lower kidney retention in comparison with (68)Ga-based probes. In addition, it was demonstrated that this probe did not compete with therapeutic trastuzumab for binding to the HER2 receptor. Thus, this Nb tracer has the potential not only for diagnosis but also for staging of patients subjected to targeted therapy.
In addition to nuclear imaging, radionuclide-labeled Nbs have also proven their potential in targeted radionuclide therapy (RIP). Ideally, the targeting moieties can target most of the injected cytotoxic radionuclides to diseased sites rather than healthy tissues, thus enabling irradiation in a confined space surrounding the tumor cells during RIP. However, accompanied by high tumor uptake, relatively high normal tissue uptake was also observed in mAb-based RIPs. Because of their rapid washout from blood, high target specificity, and robustness, Nbs are promising candidates for addressing the limitations found in mAb-based RIPs. Nb-based RIP was realized by D’Huyvetter et al, who reported a (177)Lu labeled Nb (2Rs15d) for HER2-overexpression cancer.79 The attachment of (177)Lu to Nb was achieved either by marocyclic chelators or acyclic chelators, and the resultant Lu-Nb conjugates showed high stability. Moreover, compared with radionuclide-labeled Abs, the (177)Lu labeled 2Rs15 showed significantly lower accumulation in healthy tissues, with the exception of high uptake in the kidney. The authors reasoned that the undesirable kidney retention was partly due to the interaction between charged amino acids of Nbs and the charged lumen of kidney glomeruli. The unfavored accumulation may result in cell toxicity and potential renal failure, which is a major issue to be addressed during the development of Nb-based therapy. To address this limitation, the same author developed RIP later based on using untagged Nbs and confusion with Gelofusin.80 Consequently, a sharp decrease in kidney retention was observed, and an almost complete blockade of tumor growth and an improved survival rate were observed in xenograft mice.
Radionuclide-Labeled Nbs Assisted Cancer Immunotherapy
Nb-based radiotracers have also been applied to the assessment of immune checkpoint status during cancer immunotherapy. Hijacking immune checkpoints (e.g., programmed death-1, PD-1; programmed death-ligand 1, PD-L1) is an important mechanism of cancer cells for their immune escape. Thus, inhibition of the inhibitory immune checkpoint holds great promise for successful cancer treatment. The development of Ab-based immune checkpoint inhibitors has revolutionized cancer treatment, and until now, 7 mAb-based immune checkpoint inhibitors targeting PD-1/PDL-1 have been approved by the FDA.81 Despite great success, this emerging therapy only benefits a fraction of patients, and a substantial number of patients do not respond. This limited efficacy is at least partially attributed to the high heterogeneous and dynamic nature of PD-1/PD-L1 expression within primary tumors and metastases. Thus, accurate assessment of PD-L1 expression levels is of immense importance for successful immunotherapy of cancer. However, invasive biopsy-based immunohistochemistry (IHC) rarely provides a complete picture of PD-1/PD-L1 status and thus is not an ideal technique to accomplish this task.82,83
Alternatively, radiolabeled mAbs show great potential in the imaging of PD-L1/PD-1 expression.84,85 However, radiolabeled mAb tracers have several inherent drawbacks, such as slow washout and low tissue penetration capacity. To address these challenges, Nbs-based radiotracers have been developed. Broos and co-workers achieved non-invasive SPECT/PET imaging of murine PD-L1 expression quantitatively using (99 m)Tc-labeled Nbs as tracers in syngeneic tumor models. As expected, the Nb-based tracers showed superior imaging characteristics and improved pharmacokinetics in comparison with tracers derived from Abs. It is noteworthy that the radiolabeled Nb probes had the ability to differentiate PD-L1 expression tumors from PD-L1 non-expressing tumors, even when PD-L1 expression level was low. Later, the same author further demonstrated the use of radiolabeled anti-human PD-L1 Nbs as probes in non-invasive SPECT/CT imaging of mice bearing xenografts. The Nb-based tracer allowed good signal-to-background ratios and strong ability to specifically detect PD-L1 in melanoma and breast tumors.86 More recently, an encouraging result was obtained from a Phase I clinical trial of (99 m)Tc-labeled anti-PD-L1 Nbs on 16 patients with non-small cell lung cancer.87 Taken together, these results indicate that Nb-radionuclides are promising probes in nuclear imaging of PD-1/PDL-1.
Nbs in Optical Imaging
Compared to other imaging modalities, optical imaging possesses many advantageous features. First because no radiation exposure is needed, this imaging technique is believed to be much safer. Second, the probes involved in optical imaging are relatively easy to produce, and the equipment used for imaging is relatively inexpensive. Third, owing to the much shorter time required to obtain optical imaging, real-time information from imaging is possible. One of the important criteria during the development of optical imaging probes is rapid tumor uptake and high tumor to background ratio. Due to their rapid distribution and fast clearance, Nbs are an ideal candidate to fulfill these requirements. Oliveira et al established an anti-EGFR Nb (7D12)-based NIR IRDye800CW probe and compared it with mAb cetuximab-based IR in optical molecular imaging of human tumor xenografts.88 In vivo, 7D12-IR permitted rapid visualization of the tumor (30 min after administration), whereas the cetuximab-NIR probe provided no signal above background at the tumor site. Higher tumor uptake was also observed with 7D12-NIR probes than with the NIR and other radionuclide-labeled 7D12. The author suggested that this improved tumor uptake efficiency resulted from conjugation efficiency, the nature of the tracer, and detection time. This study is the first report in which a direct comparison was made between Nbs-NIR and mAb-NIR. The promising results of this study show the great potential of NIR fluorophores in rapid preclinical optical imaging.
Taking advantage of the “real-time nature” of the NIR fluorophore, Nb-NIR probe-guided surgery has also been developed. IRDye 800CW-labelled anti-HER-2 Nbs (11A4) showed rapid optical imaging of human breast cancer xenografts with high tumor to background ratio. Moreover, assisted by a near-infrared multispectral fluorescence camera system, the fluorescent images with high contrast allowed successful resection of a HER-2-positive xenograft from a mouse.89 Later, Kijanka et al developed an optical imaging platform based on 2 different Nbs (Nb B9 against carbonic anhydrase IX[CAIX] and Nb 11A4 against HER2).90 The same fluorophore labeled B9 and 11A4 resulted in an increase in the tumor to background ratio. Moreover, labeling of 2 Nbs with different NIRs allowed imaging of expression levels of both CAIX and HER2 in vivo, under conditions mimicking surgical conditions.
Summary
Given that cancer is a growing global threat, there is a compelling need for the establishment of effective cancer therapies and accurate diagnostic approaches. Since the approval of the first mAbs, rituximab, for cancer treatment 23 years ago,91 owing to their exceptional specificity, conventional Ab-based techniques have revolutionized cancer treatments and paved the way for personalized cancer therapy. However, the inherent drawbacks such as large size and high immunogenicity hamper the full utilization of Abs in cancer therapy. Because of their compact size, antigen-binding site, excellent stability, and low immunogenicity, Camelidae-derived Nbs hold great promise for addressing these limitations. First, their small size ensures improved tumor penetration ability, rapid diffusion, and fast clearance from the body. Second, the finger-like CDR3 regions allow Nbs to bind to cryptic, concave, or hidden epitopes that are not accessible to Ab antigen-binding sites. Third, the low immunogenicity, which can be further reduced by humanization, makes Nbs safe for human administration. Last but not least, the extraordinary robustness combined with their simple structure and fewer posttranslational modifications enable the production of Nbs at low expense. Taken together, nanobodies are versatile and potent binders for a variety of applications in cancer therapy and diagnosis.
Although “naked” Nbs have been exploited as antagonists against tumor angiogenesis, metabolism, and metastasis, decoration of Nbs with functional modules such as small molecular drugs, toxins, imaging agents, and enzymes can greatly broaden the applications of Nbs for cancer treatment and diagnosis. Owing to the advancement of bioconjugation technology, conjugation of Nbs to other modules can be readily achieved either chemically or biologically by choosing an appropriate bioconjugation technique from the available toolbox. However, due to the fragility and complexity of proteins, even the most predictable conjugation technique may generate unexpected protein conjugates. Thus, it should be noted that careful examination of the binding property of the resultant Nb conjugates is a prerequisite for further preclinical and clinical applications. Conjugation of Nbs to toxins is probably the most straightforward way to combine the advantages of Nbs and functional modules. Alternatively, Nb-enzyme conjugates have been developed to activate administered prodrugs in situ. In addition because of their small size, fast diffusion, and rapid washout from the body, Nb-based imaging probes allow high-contrast imaging soon after administration. Moreover, given the fact that each cancer therapy has its own limitations, successful treatment of cancer is most likely to be established by a rational combination of these techniques. Thus, Nb-based therapeutics and imaging tracers have also been used in conjunction with CAR T cell therapy, PDT, RIP, and monitoring of PD-1/PD-L1 levels during immunotherapy.
As the studies referred to in this review have reported, Nb conjugates have demonstrated their potential in cancer diagnosis and therapy with striking properties, with some of the most successful ones entering preclinical or clinical trials. However, there are still some problems to be addressed to achieve the full utilization of these extraordinary binders. First, the monomeric nature of Nbs is not always a preferred property; low binding affinity is observed in some of the Nbs derived from phage display due to mono valency. A high binding affinity is required for sufficient accumulation of Nbs at diseased sites before rapid clearance of Nbs from the kidney. This limitation could be partially circumvented by the construction of multivalent Nbs. However, this strategy may comprise the compact size of the Nbs. Alternatively, affinity maturation processes using error-prone PCR or DNA shuffling techniques hold promise for improving the binding affinity.92,93 Second, the cost of Camelidae immunization is relatively higher than that of small animal immunization, although the expense of expression and production of Nbs in microbial hosts is low. Moreover, some antigens of interest are toxic, lethal, have low immunogenicity, or are transmittable, further complicating the immunization process. Thus, much effort has been put into developing alternative strategies for Nb selection such as synthetic VHH libraries and naïve library generation of transgenic small animals harboring the genes of Camelidae VHH domains.94,95,96 Finally, just as for the spread of antibiotic contamination, the biosecurity issues of Nbs should be carefully evaluated.97
Acknowledgments
The authors thank Prof. Baizhu Chen for proofreading this article.
Abbreviations
- Ab
Antibody
- ADCC
Antibody-dependent Cell-mediated Cytotoxicity
- CAR
Chimeric Antigen Receptor
- CDR
Complementary Determining Region
- CT
Computed Tomography
- EPL
Expressed Protein Ligation
- EGFR
Epidermal Growth Factor Receptor
- HER2
Human Epidermal Growth Factor
- HGFR
Hepatocyte Growth Factor Receptor
- Fab
Antigen-binding Fragment
- FV
Variable Fragment
- FR
Framework Region
- GPC
G protein-coupled receptor
- HcAb
Heavy-chain-only Antibody
- Ig
Immunoglobulin
- mAb
Monoclonal Antibody
- PET
Positron Emission Tomography
- MSC
Mesenchymal Stem Cell
- Nb
Nanobody
- NSC
Neural Stem Cells
- RIP
Radionuclide Therapy
- ScFV
Single-chain variable Fragment
- SPECT
Single-photon Emission Computerized Tomography
- VH
Heavy Chain Variable Domain
- VL
Light Chain Variable Domain
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Author Contribution: Wei Kang and Chuanfeng Ding contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this work was provided via Youth Program of National Natural Science Foundation of China (21807043) and the Fundamental Research Funds for the Central Universities (DUT20RC(3)069) to WK, the National Natural Science Foundation of China (81902622) and the Shanghai “Science and Technology Innovation Action Plan” Hong Kong, Macao, and Taiwan Science and Technology Cooperation Project (20430760100) to YY, and the National Natural Science Foundation of China (81771659) to QZ.
ORCID iD: Wei Kang, PhD  https://orcid.org/0000-0002-2288-3540
https://orcid.org/0000-0002-2288-3540
Yongsheng Yu, PhD  https://orcid.org/0000-0001-5139-3439
https://orcid.org/0000-0001-5139-3439
References
- 1. Nadler LM, Stashenko P, Hardy R, et al. Serotherapy of a patient with a monoclonal-antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40(9):3147–3154. [PubMed] [Google Scholar]
- 2. Brekke OH, Loset GA. New technologies in therapeutic antibody development. Curr Opin Pharmacol. 2003;3(5):544–550. [DOI] [PubMed] [Google Scholar]
- 3. Chiu ML, Goulet DR, Teplyakov A, Gilliland GL. Antibody structure and function: the basis for engineering therapeutics. Antibodies. 2019;8(4):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu YZ, Liu CX, Muyldermans S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front Immunol. 2017;8:1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez I, Bott SW, Patel AS, et al. Pricing of monoclonal antibody therapies: higher if used for cancer? Am J Manag Care. 2018;24(2):109–112. [PubMed] [Google Scholar]
- 8. Oliveira S, Heukers R, Sornkom J, Kok RJ, Henegouwen PMPVE. Targeting tumors with nanobodies for cancer imaging and therapy. J Control Release. 2013;172(3):607–617. [DOI] [PubMed] [Google Scholar]
- 9. Tsumura R, Sato R, Furuya F, et al. Feasibility study of the fab fragment of a monoclonal antibody against tissue factor as a diagnostic tool. Int J Oncol 2015;47(6):2107–2114. [DOI] [PubMed] [Google Scholar]
- 10. Kelly MP, Lee FT, Tahtis K, et al. Tumor targeting by a multivalent single-chain Fv (scFv) anti-lewis Y antibody construct. Cancer Biother Radio. 2008;23(4):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helma J, Cardoso MC, Muyldermans S, Leonhardt H. Nanobodies and recombinant binders in cell biology. J Cell Biol. 2015;209(5):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson AL. Antibody fragments hope and hype. Mabs-Austin. 2010;2(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamerscasterman C, Atarhouch T, Muyldermans S, et al. Naturally-occurring antibodies devoid of light-chains. Nature. 1993;363(6428):446–448. [DOI] [PubMed] [Google Scholar]
- 14. Greenberg AS, Avila D, Hughes M, Hughes A, Mckinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374(6518):168–173. [DOI] [PubMed] [Google Scholar]
- 15. Jovcevska I, Muyldermans S. The therapeutic potential of nanobodies. Biodrugs. 2020;34(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. [DOI] [PubMed] [Google Scholar]
- 17. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burg JS, Ingram JR, Venkatakrishnan AJ, et al. Structural biology. structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347(6226):1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zavrtanik U, Luken J, Loris R, Lah J, Hadzi S. Structural basis of epitope recognition by heavy-chain camelid antibodies. J Mol Biol. 2018;430(21):4369–4386. [DOI] [PubMed] [Google Scholar]
- 20. De Genst E, Silence K, Decanniere K, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. P Natl Acad Sci USA. 2006;103(12):4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muyldermans S, Baral TN, Retarnozzo VC, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunop. 2009;128(1-3):178–183. [DOI] [PubMed] [Google Scholar]
- 22. Desmyter A, Transue TR, Ghahroudi MA, et al. Crystal structure of a camel single-domain V-H antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3(9):803–811. [DOI] [PubMed] [Google Scholar]
- 23. Papandrianos N, Savvopoulos C, Barla P, et al. SPECT/CT imaging with 99mTc-depreotide in lymphoma. comparison to Ga-67 scintigraphy. Eur J Nucl Med Mol I. 2008;35. [Google Scholar]
- 24. Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830(12):5526–5534. [DOI] [PubMed] [Google Scholar]
- 25. Roovers RC, Vosjan MJWD, Laeremans T, et al. A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int J Cancer. 2011;129(8):2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harmsen MM, Van Solt CB, Fijten HPD, Van Setten MC. Prolonged in vivo residence times of llama single-domain antibody fragments in pigs by binding to porcine immunoglobulins. Vaccine. 2005;23(41):4926–4934. [DOI] [PubMed] [Google Scholar]
- 27. Sarker A, Rathore AS, Gupta RD. Evaluation of scFv protein recovery from E-coli by in vitro refolding and mild solubilization process. Microb Cell Fact. 2019;18(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muyldermans S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7(9):1129–1135. [DOI] [PubMed] [Google Scholar]
- 29. Cortez-Retamozo V, Lauwereys M, Gh GH, et al. Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer. 2002;98(3):456–462. [DOI] [PubMed] [Google Scholar]
- 30. Bartunek J, Barbato E, Heyndrickx G, Vanderheyden M, Wijns W, Holz JB. Novel antiplatelet agents: ALX-0081, a nanobody directed towards von willebrand factor. J Cardiovasc Transl. 2013;6(3):355–363. [DOI] [PubMed] [Google Scholar]
- 31. Steeland S, Vandenbroucke RE, Libert C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 2016;21(7):1076–1113. [DOI] [PubMed] [Google Scholar]
- 32. Huet HA, Growney JD, Johnson JA, et al. Multivalent nanobodies targeting death receptor 5 elicit superior tumor cell killing through efficient caspase induction. Mabs-Austin. 2014;6(6):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothbauer U, Zolghadr K, Tillib S, et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods. 2006;3(11):887–889. [DOI] [PubMed] [Google Scholar]
- 34. Behdani M, Zeinali S, Karimipour M, et al. Development of VEGFR2-specific nanobody pseudomonas exotoxin a conjugated to provide efficient inhibition of tumor cell growth. N Biotechnol. 2013;30(2):205–209. [DOI] [PubMed] [Google Scholar]
- 35. Cortez-Retamozo V, Backmann N, Senter PD, et al. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64(8):2853–2857. [DOI] [PubMed] [Google Scholar]
- 36. van de Water JAJM, Bagci-Onder T, et al. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. P Natl Acad Sci USA. 2012;109(41):16642–16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pleiner T, Bates M, Trakhanov S, et al. Nanobodies: site-specific labeling for super-resolution imaging, rapid epitope-mapping and native protein complex isolation. Elife. 2015;4:e11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brooks DJ, Fresco JR, Lesk AM, Singh M. Evolution of amino acid frequencies in proteins over deep time: Inferred order of introduction of amino acids into the genetic code. Mol Biol Evol. 2002;19(10):1645–1655. [DOI] [PubMed] [Google Scholar]
- 39. Massa S, Xavier C, De Vos J, et al. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjugate Chem. 2014;25(5):979–988. [DOI] [PubMed] [Google Scholar]
- 40. Massa S, Xavier C, Muyldermans S, Devoogdt N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin Drug Deliv. 2016;13(8):1149–1163. [DOI] [PubMed] [Google Scholar]
- 41. Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of escherichia coli. Science. 2001;292(5516):498–500. [DOI] [PubMed] [Google Scholar]
- 42. Yamaguchi A, Matsuda T, Ohtake K, et al. Incorporation of a doubly functionalized synthetic amino acid into proteins for creating chemical and light-induced conjugates. Bioconjug Chem. 2016;27(1):198–206. [DOI] [PubMed] [Google Scholar]
- 43. Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol. 2011;7(12):876–884. [DOI] [PubMed] [Google Scholar]
- 44. Rashidian M, Wang L, Edens JG, et al. Enzyme-mediated modification of single-domain antibodies for imaging modalities with different characteristics. Angew Chem Int Edit. 2016;55(2):528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gray MA, Tao RN, DePorter SM, Spiegel DA, McNaughton BR. A Nanobody activation immunotherapeutic that selectively destroys HER2-positive breast cancer cells. Chembiochem. 2016;17(2):155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dennler P, Bailey LK, Spycher PR, Schibli R, Fischer E. Microbial transglutaminase and c-myc-tag: a strong couple for the functionalization of antibody-like protein scaffolds from discovery platforms. Chembiochem. 2015;16(5):861–867. [DOI] [PubMed] [Google Scholar]
- 47. Zhu M, Gong X, Hu YH, Ou WJ, Wan YK. Streptavidin-biotin-based directional double Nanobody sandwich ELISA for clinical rapid and sensitive detection of influenza H5N1. J Transl Med 2014;12:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schumacher D, Lemke O, Helma J, et al. Broad substrate tolerance of tubulin tyrosine ligase enables one-step site-specific enzymatic protein labeling. Chem Sci. 2017;8(5):3471–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ta DT, Redeker ES, Billen B, et al. An efficient protocol towards site-specifically clickable nanobodies in high yield: cytoplasmic expression in escherichia coli combined with intein-mediated protein ligation. Protein Eng Des Sel. 2015;28(10):351–363. [DOI] [PubMed] [Google Scholar]
- 50. Bachmann AL, Mootz HD. N-terminal chemical protein labeling using the naturally split GOS-TerL intein. J Pept Sci. 2017;23(7-8):624–630. [DOI] [PubMed] [Google Scholar]
- 51. Muralidharan V, Muir TW. Protein ligation: an enabling technology for the biophysical analysis of proteins. Nat Methods. 2006;3(6):429–438. [DOI] [PubMed] [Google Scholar]
- 52. Tintelnot J, Baum N, Schultheiss C, et al. Nanobody targeting of epidermal growth factor receptor (EGFR) ectodomain variants overcomes resistance to therapeutic EGFR antibodies. Mol Cancer Ther. 2019;18(4):823–833. [DOI] [PubMed] [Google Scholar]
- 53. Peled A, Wald O, Burger J. Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs. 2012;21(3):341–353. [DOI] [PubMed] [Google Scholar]
- 54. D’Huyvetter M, De Vos J, Caveliers V, et al. Phase I trial of (131)I-GMIB-Anti-HER2-VHH1, a new promising candidate for HER2-targeted radionuclide therapy in breast cancer patients. J Nucl Med. 2020. doi:10.2967/jnumed.120.255679 [DOI] [PubMed] [Google Scholar]
- 55. Papadopoulos KP, Isaacs R, Bilic S, et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic nanobody(R) targeting the DR5 receptor. Cancer Chemother Pharmacol. 2015;75(5):887–895. [DOI] [PubMed] [Google Scholar]
- 56. Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57(1):27–33. [DOI] [PubMed] [Google Scholar]
- 57. Zhang F, Wei H, Wang X, et al. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov. 2017;3:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B Cells. Cancer Immunol Res. 2016;4(6):498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bathula NV, Bommadevara H, Hayes JM. Nanobodies: the future of antibody-based immune therapeutics. Cancer Biother Radiopharm. 2020;36(2):109–122. [DOI] [PubMed] [Google Scholar]
- 61. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas (vol 97, pg 12846, 2000). P Natl Acad Sci USA. 2001;98(2):777–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14(13):1247–1254. [DOI] [PubMed] [Google Scholar]
- 63. Heukers R, Henegouwen PMPVE, Oliveira S. Nanobody-photosensitizer conjugates for targeted photodynamic therapy. Nanomed-Nanotechnol. 2014;10(7):1441–1451. [DOI] [PubMed] [Google Scholar]
- 64. van Driel PBAA, Boonstra MC, Slooter MD, et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J Control Release. 2016;229:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Groof TWM, Mashayekhi V, Fan TS, et al. Nanobody-targeted photodynamic therapy selectively kills viral GPCR-expressing glioblastoma cells. Mol Pharmaceut. 2019;16(7):3145–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Deken MM, Kijanka MM, Hernandez IB, et al. Nanobody-targeted photodynamic therapy induces significant tumor regression of trastuzumab-resistant HER2-positive breast cancer, after a single treatment session. J Control Release. 2020;323:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14(6):405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Russo V, Bondanza A, Ciceri F, et al. A dual role for genetically modified lymphocytes in cancer immunotherapy. Trends Mol Med. 2012;18(4):193–200. [DOI] [PubMed] [Google Scholar]
- 69. Gattenlohner S, Marx A, Markfort B, et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody- based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006;66(1):24–28. [DOI] [PubMed] [Google Scholar]
- 70. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xie YJ, Dougan M, Jailkhani N, et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice (vol 116, pg 7624, 2019). P Natl Acad Sci USA. 2019;116(33):16656–16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Munter S, Ingels J, Goetgeluk G, et al. Nanobody based dual specific CARs. Int J Mol Sci 2018;19(2):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huang L, Gainkam LO, Caveliers V, et al. SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Mol Imaging Biol. 2008;10(3):167–175. [DOI] [PubMed] [Google Scholar]
- 74. Gainkam LO, Huang L, Caveliers V, et al. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J Nucl Med. 2008;49(5):788–795. [DOI] [PubMed] [Google Scholar]
- 75. Xavier C, Vaneycken I, D’Huyvetter M, et al. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013;54(5):776–784. [DOI] [PubMed] [Google Scholar]
- 76. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 77. Dijkers EC, Kosterink JG, Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50(6):974–981. [DOI] [PubMed] [Google Scholar]
- 78. Xavier C, Blykers A, Vaneycken I, et al. F-18-nanobody for PET imaging of HER2 overexpressing tumors. Nucl Med Biol. 2016;43(4):247–252. [DOI] [PubMed] [Google Scholar]
- 79. D’Huyvetter M, Aerts A, Xavier C, et al. Development of 177Lu-nanobodies for radioimmunotherapy of HER2-positive breast cancer: evaluation of different bifunctional chelators. Contrast Media Mol Imaging. 2012;7(2):254–264. [DOI] [PubMed] [Google Scholar]
- 80. D’Huyvetter M, Vincke C, Xavier C, et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4(7):708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alderton GK. Epigenetic and genetic heterogeneity in metastasis. Nat Rev Cancer. 2017;17(3):141–141. [DOI] [PubMed] [Google Scholar]
- 83. Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 2018;20(12):1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bensch F, van der Veen EL, Lub-de Hooge MN, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 85. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9(1):4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Broos K, Lecocq Q, Xavier C, et al. Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent. Cancers (Basel). 2019;11(6):872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xing Y, Chand G, Liu C, Cook GJR, O’Doherty J, Zhao L, Wong NCL, Meszaros LK, Ting HH, Zhao J. Early phase I study of a (99 m)Tc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J Nucl Med. 2019;60(9):1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Oliveira S, van Dongen GAMS, van Walsum MS, et al. Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol Imaging. 2012;11(1):33–46. [PubMed] [Google Scholar]
- 89. Kijanka M, Warnders FJ, El Khattabi M, et al. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med Mol Imaging. 2013;40(11):1718–1729. [DOI] [PubMed] [Google Scholar]
- 90. Kijanka MM, van Brussel ASA, van der Wall E, et al. Optical imaging of pre-invasive breast cancer with a combination of VHHs targeting CAIX and HER2 increases contrast and facilitates tumour characterization. Ejnmmi Res. 2016;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grillo-Lopez AJ. The first antibody therapy for cancer: a personal experience. Expert Rev Anticancer Ther. 2013;13(4):399–406. [DOI] [PubMed] [Google Scholar]
- 92. Harmsen MM, van Solt CB, van Bemmel AMV, Niewold TA, van Zijderveld FG. Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Appl Microbiol Biot. 2006;72(3):544–551. [DOI] [PubMed] [Google Scholar]
- 93. Stijlemans B, Caljon G, Natesan SKA, et al. High affinity nanobodies against the trypanosome brucei VSG are potent trypanolytic agents that block endocytosis. Plos Pathog. 2011;7(6):e1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yan JR, Wang PY, Zhu M, et al. Characterization and applications of nanobodies against human procalcitonin selected from a novel naive nanobody phage display library. J Nanobiotechnol. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koide A, Tereshko V, Uysal S, Margalef K, Kossiakoff AA, Koide S. Exploring the capacity of minimalist protein interfaces: interface energetics and affinity maturation to picomolar KD of a single-domain antibody with a flat paratope. J Mol Biol. 2007;373(4):941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zou X, Smith JA, Nguyen VK, et al. Expression of a dromedary heavy chain-only antibody and B cell development in the mouse. J Immunol. 2005;175(6):3769–3779. [DOI] [PubMed] [Google Scholar]
- 97. Dolk E, van der Vaart M, Lutje Hulsik D, et al. Isolation of llama antibody fragments for prevention of dandruff by phage display in shampoo. Appl Environ Microbiol. 2005;71(1):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]