Key Points
Question
Is fortification of expressed breast milk by preterm formula powder noninferior, in terms of weight gain, to fortification by a commercially available human milk fortifier?
Findings
In this noninferiority trial enrolling 123 neonates at less than 34 weeks of gestation, the mean weight gain in the preterm formula group was 15.7 g/kg/d and in the human milk fortifier group was 16.3 g/kg/d. The difference was not significant and did not reach the prespecified noninferiority margin, and the incidence of feed intolerance was significantly lower in the preterm formula group.
Meaning
Preterm formula powder might be a better alternative for breast milk fortification in resource-constrained settings.
Abstract
Importance
Fortification of expressed breast milk (EBM) using commercially available human milk fortifiers (HMF) increases short-term weight and length in preterm very low-birth-weight (VLBW) neonates. However, the high cost and increased risk of feed intolerance limit their widespread use. Preterm formula powder fortification (PTF) might be a better alternative in resource-limited settings.
Objective
To demonstrate that fortification of EBM by preterm formula powder is noninferior to fortification by HMF, in terms of short-term weight gain, in VLBW neonates.
Design, Setting, and Participants
Open-label, noninferiority, randomized trial conducted from December 2017 to June 2019 at a level 3 neonatal unit in India. The trial enrolled preterm (born at or before 34 weeks of gestation) VLBW neonates receiving at least 100 mL/kg/d of feeds and consuming 75% of milk or more as EBM.
Interventions
Neonates were randomly assigned to receive fortification by either PTF or HMF. Calcium, phosphorus, iron, vitamin D, and multivitamins were supplemented in PTF and only vitamin D in the HMF group to meet the recommended dietary allowances.
Main Outcomes and Measures
The primary outcome was the weight gain until discharge from the hospital or 40 weeks’ postmenstrual age, whichever was earlier; the prespecified noninferiority margin was 2 g/kg/d. Secondary outcomes included morbidities such as necrotizing enterocolitis, feed intolerance, and extrauterine growth restriction (<10th percentile on the Fenton chart at 40 weeks’ postmenstrual age).
Results
Of the 123 neonates enrolled, 60 and 63 were randomized to the PTF and HMF groups, respectively. The mean gestation (30.5 vs 29.9 weeks) and birth weight (1161 vs 1119 g) were comparable between the groups. There was no difference in the mean (SD) weight gain between the PTF and HMF groups (15.7 [3.9] vs 16.3 [4.0] g/kg/d; mean difference, −0.5 g/kg/d; 95% CI, −1.9 to 0.7). The lower bound of 95% CI did not cross the noninferiority margin. The incidence of feed intolerance was lower in the PTF group (1.4 vs 6.8 per 1000 patient-days; incidence rate ratio 0.19; 95% CI, 0.04 to 0.95), and fewer neonates required withholding of fortification for 24 hours or more (5% vs 22%; risk ratio, 0.22; 95% CI, 0.07 to 0.75). The incidence of necrotizing enterocolitis stage II or more (0 vs 5%) and extrauterine growth restriction (73% vs 81%) was comparable between the groups.
Conclusions and Relevance
Fortification with preterm formula powder is not inferior to fortification with human milk fortifiers in preterm neonates. Given the possible reduction in feed intolerance and lower costs, preterm formula might be a better option for fortification, especially in resource-restricted settings.
Trial Registration
Clinical Trial Registry, India Identifier: CTRI/2017/11/010593
This randomized clinical trial investigates whether fortification of expressed breast milk by preterm formula powder is noninferior to fortification by human milk fortifier, in terms of short-term weight gain, in very low-birth-weight neonates.
Introduction
With the increasing survival of preterm neonates, postnatal growth restriction has come to the forefront as significant morbidity, especially in low- and middle-income countries (LMICs).1 Human milk, a key component of enteral feeding strategy, is not enough to meet the high nutrient requirements of preterm neonates.2 A simple comparison of the nutrient content of preterm human milk and recommended dietary allowances (RDA) of preterm neonates as per the recommendations from European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) 2010 shows that the nutrient needs of preterm neonates cannot be met with exclusive breast milk feeding alone without any additional supplements.3 To address this pertinent issue, most units fortify expressed breast milk (EBM) with commercially available human milk fortifiers (HMF).
A 2020 Cochrane review4 showed an increase in short-term weight, length, and head circumference but no effect on long-term neurodevelopment and anthropometry in neonates receiving multinutrient fortification. Subgroup analysis of the trials conducted in LMICs also showed similar benefits. Consequently, many units in LMICs use HMF powder for the fortification of EBM.5,6
The high cost of HMF and the potential risk of adverse effects such as NEC, feed intolerance, sepsis, and late metabolic acidosis (LMA) limit the widespread use of HMF, particularly in LMICs.7,8 In our unit, over 6 months in 2016, we observed a high incidence of feed intolerance (39.2%) and LMA (10.8%) in neonates receiving fortified milk feeds with a commercial milk fortifier (written communication). The findings could have been owing to the high osmolarity of fortified milk. An earlier study from our unit showed that the fortified milk’s osmolarity increased to 393 mOsm/kg compared with the osmolarity of unfortified milk (302 mOsm/kg).9 We hypothesized that the fortification of EBM with the preterm formula (PTF) would be a safer, more economical, and possibly equally effective option. Although PTF fortification is done in some LMICs,10 there is a paucity of evidence to recommend its routine use. Therefore, we planned this trial to demonstrate whether fortification of EBM using PTF is noninferior to fortification by using commercially available HMF.
Methods
Study Design
This open-label, parallel-group, noninferiority randomized trial was conducted in a level-3 neonatal unit in Delhi, India, from December 2017 to June 2019. The Institute Ethics Committee approved the trial protocol, and the trial was registered with the Clinical Trial Registry of India (CTRI/2017/11/010593). The formal trial protocol can be found in Supplement 1. We did not receive any external funding for the conduct of the trial.
Study Population
Neonates born at or before 34 weeks of gestation and having a birth weight less than 1500 g (very low birth weight; VLBW) were eligible for enrollment. Those receiving 100 mL/kg per day of enteral feeds and consuming at least 75% of feeds as EBM were enrolled after obtaining written informed consent from one of the parents. We excluded neonates with major congenital anomalies.
Randomization and Allocation Concealment
Enrolled neonates were randomly allocated to receive fortification with either PTF powder (Dexolac Special Care; DANONE India) or commercially available HMF – PreNAN (Nestle and Co). The nutrient contents of the 2 preparations and the assumed values for the EBM are provided in eTable 1 in Supplement 2. Randomization was done separately for the 2 strata, namely, appropriate-for-gestational-age (AGA) and small-for-gestational-age (SGA) neonates. Computer-generated random numbers with variable block sizes were generated for each stratum. The investigator who generated the random numbers did not participate in the enrollment of neonates or data collection. We ensured concealment of allocation using serially numbered, opaque, sealed envelopes that contained a slip of paper with the name of the allocation group. The duty resident opened the envelopes at the time of enrollment and randomly allocated the neonates to 1 of 2 groups. The 2 fortifiers’ different physical characteristics and packaging precluded us from blinding the caregivers from the group allocation.
Intervention
Baseline maternal, neonatal, and anthropometry details were recorded, and the neonates began receiving fortification as per the standard operating procedures (SOP) of the study. For fortification with PTF, 1 g of formula powder was added for every 25 mL of EBM.11 Sachets of the specified quantity of PTF were made by the pharmacy for each neonate randomized to PTF fortification. The HMF fortification was done as per the manufacturers’ recommendation (1 sachet added to 25 mL EBM). A uniform feed volume of 180 mL/kg/d was ensured to all enrolled neonates. Before the start of the study, a nutrition audit was done to assess the need for additional supplements required after fortification, assuming a daily breast milk intake of 180 mL/kg in both the PTF and HMF groups. Based on the audit findings, iron drops, calcium and phosphate, vitamin D3, and multivitamin syrup were supplemented in the PTF group, and only vitamin D3 in the HMF group (eTable 2 in Supplement 2). Supplementation of additional nutrients ensured that both the groups received the recommended dietary allowance of micronutrients as per the ESPGHAN 2010 guidelines3,11 (eTable 3 in Supplement 2).
Weekly nutrition and anthropometric audits were done as per the unit policy. If the calculated weight gain was less than 10 g/kg/d during the weekly audit, the neonates were assessed for any systemic causes of poor weight gain such as anemia and LMA. If no systemic causes were identified, feed volume was increased to 200 mL/kg/d. If the weight gain was less than 10 g/kg/d for 2 consecutive weeks, then the neonate was intended to be crossed over to the other fortifier (eFigure 1 in Supplement 2).
At discharge, parents were counseled regarding the importance of adding fortification to EBM even after discharge from the hospital. They were advised to add fortification until the neonate reached a weight of 2 kg or until 40 weeks of postmenstrual age (PMA), whichever was later. We ensured compliance with the intervention during fortnightly visits for retinopathy of prematurity (ROP) follow-up.
Outcomes
The primary outcome was the rate of in-hospital weight gain measured as gram per kilogram per day from the day of randomization to the day of discharge or 40 weeks’ PMA, whichever was earlier. The secondary outcomes were mortality, rate of increase in head circumference and length at discharge, feed intolerance, necrotizing enterocolitis (NEC) stage 2 or more, intraventricular hemorrhage (IVH), metabolic bone disease (MBD), anemia requiring transfusion, late metabolic acidosis (LMA), bronchopulmonary dysplasia (BPD), and ROP. Extrauterine growth restriction (EUGR) was assessed at 40 weeks’ PMA.
Outcome Assessment
The weekly weight gain in grams per kilograms per day was calculated by dividing absolute weight gain during the week by 7 and midweek weight (average of day 1 and day 7 weight). At the time of discharge or 40 weeks’ PMA, whichever was earlier, an average of the cumulative weekly weight gain was done to calculate the weight gain over the intervention period. The principal investigator (PI) collected the weight gain data prospectively. A standard digital weighing machine with an accuracy of 1 g (ADE M1 18600 electronic baby weighing scale) was used for weighing the neonates.
Anthropometric measurements were done weekly until discharge/death. Length and head circumference gain were calculated as centimeters per week from fortification till discharge. Length and head circumference were measured by an infantometer and a nonstretchable tape, respectively. Incidence of sepsis, LMA, NEC, IVH, BPD, ROP, MBD, and PDA was recorded as per standard definitions.11 The clinical team monitored the abdominal girth before each feed and documented the vomiting episodes throughout the hospital stay. The prefeed aspirate was checked if the abdominal girth increased by more than 2 cm from the baseline in 24 hours. Feed intolerance was defined if at least 2 of the following were present: (1) more than 1 vomit with altered milk; (2) more than 2 cm increase in abdominal girth over baseline within 24 hours; (3) prefeed aspirate greater than 50% of the feed volume; or (4) bilious or blood-stained aspirate.12 A uniform protocol for managing feed intolerance was practiced during the study period.11,12
Follow-up was done until 40 ± 1 weeks’ PMA, and weight, length, and head circumference were measured. Fenton growth chart was used for monitoring the growth of the neonates during hospital admission and at term corrected age. Extrauterine growth restriction was defined as weight less than 10th percentile at 40 weeks’ PMA.
Quality Assurance
Processes for outcome assessment were standardized for uniformity by preparing SOPs. The weighing machine used for measuring the weight was periodically calibrated. The weight of the pharmacy-prepared PTF sachets was rechecked periodically. After discharge, compliance with fortification was ensured using regular telephone calls to parents and during their follow-up visits in the high-risk clinic. They were also encouraged to maintain a nutrition diary, which was regularly checked by the PI.
Statistical Analysis
The mean (SD) weight gain of neonates receiving fortification with commercially available human milk fortifiers in our unit was 13.5 (3.8) g/kg/d (written communication). Assuming a noninferiority margin of 2 g/kg/d, an α error of 5%, and a power of 90%, we had to recruit 62 neonates in each group. Previous studies comparing HMF fortified breast milk with unfortified milk have shown a difference in mean weight up to 3 g/kg/d.13,14 The weight gain reported in the studies on HMF in LMICs varies from 9.4 to 19.4 g/kg/d.4 We considered a noninferiority margin of 2 g/kg/d to be clinically meaningful based on the significant difference in long-term growth and rehospitalization outcomes between 2 quartiles with a mean difference in in-hospital weight gain of 2.2 g/kg/d in the study by Ehrenkranz et al.15
Data were collected in a predesigned proforma and entered in Microsoft Access 2016 (Microsoft Corp) and analyzed using Stata, version 15.1 (StataCorp). Categorical variables were compared using the χ2/Fisher exact test. The Student t test and Wilcoxon rank sum test were used to analyze the continuous variables with normal distribution and skewed distribution, respectively. Both per-protocol and intention-to-treat analyses were conducted for the primary outcome. The 95% CI was obtained by independent-sample t test. Noninferiority was tested by hypothesis testing and 95% CI approach.16 If the lower margin of the 95% CI for the primary outcome was more than −2 g/kg/d, the intervention would be considered as noninferior to HMF fortification. The negative binomial regression was used to calculate the incidence rate ratio of feed intolerance. The trial’s statistical analysis plan can be found in Supplement 3.
Results
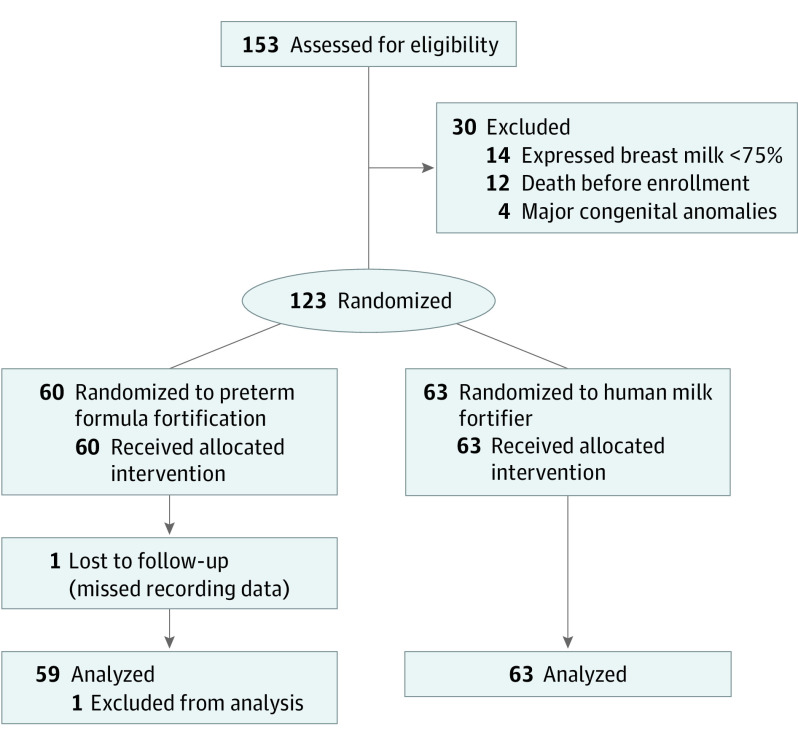
Of 447 neonates born at a gestation of 34 weeks or less during the study period, 153 had a birth weight of less than 1500 g (Figure). After excluding 30 infants based on prespecified exclusion criteria, the remaining 123 infants were randomized to either PTF (n = 60) or HMF (n = 63) group. Data for the primary outcome were available for 122 neonates. No study participant fulfilled the criteria for crossover to the other group.
Figure. Study Flow.
The baseline characteristics, including gestation, birth weight, intrauterine growth restriction, the proportion of neonates receiving parenteral nutrition, the duration of parenteral nutrition, and the day of reaching 100 mL/kg enteral feeds were comparable between the 2 groups. The median age at enrollment was 7 and 8 days, and the mean weight at enrollment was 1038 g and 1074 g in the PTF and HMF groups, respectively. The maternal characteristics were also similar between the 2 groups (Table 1).
Table 1. Comparison of Baseline Characteristics.
| Variable | No. (%) | |
|---|---|---|
| Preterm formula fortification (n = 59) | Human milk fortifier (n = 63) | |
| Maternal characteristics | ||
| Gestational hypertension | 2 (3) | 4 (6) |
| Preeclampsia/eclampsia | 11 (19) | 7 (11) |
| Umbilical artery doppler changes | ||
| AEDF | 6 (10) | 7 (11) |
| REDF | 9 (15) | 9 (14) |
| Multiple pregnancies | 21 (36) | 25 (40) |
| Received antenatal corticosteroids | ||
| 1 Course | 18 (31) | 22 (35) |
| 2 Course | 6 (10) | 8 (13) |
| Incomplete | 31 (53) | 31 (49) |
| Not given | 5 (9) | 2 (3) |
| Neonatal characteristics | ||
| Gestation, mean (SD), wk | 30.5 (2.2) | 29.9 (2.2) |
| Birth weight, mean (SD), g | 1161 (251) | 1119 (265) |
| Intrauterine growth | ||
| SGA | 22 (37) | 22 (35) |
| AGA | 38 (64) | 40 (64) |
| Infant sex | ||
| Male | 29 (49) | 39 (62) |
| IUGR | 33 (56) | 30 (47.6) |
| Apgar, median (IQR) | ||
| At 1 min | 7 (3-7) | 7 (3-7) |
| At 5 min | 8 (7-8) | 8 (7-8) |
| Received PN | 27 (45) | 30 (47) |
| Age at starting PN, median (IQR), da | 1 (1-2) | 1 (1-2) |
| Age at stopping PN, median (IQR), da | 8 (6-11) | 9 (7-11) |
| Total duration of PN, median (IQR), da | 7 (5-10) | 7 (6-10) |
| Age at initiation of enteral feeds, median (IQR), d | 1 (1-3) | 2 (1-3) |
| Age of attainment of enteral intake 100 mL/kg/d, median (IQR), d | 4 (2-6.5) | 4 (2-9) |
| Age at enrollment, median (IQR), d | 7 (5-10) | 8 (4-11) |
| Weight at enrollment, mean (SD), g | 1038 (350) | 1074 (323) |
| Length at enrollment, mean (SD), cm | 37.5 (3.8) | 36.4 (3.5) |
| HC at enrollment, mean (SD), cm | 26.6 (1.7) | 26.2 (2.3) |
| Mean fluid volume at enrollment, mean (SD), mL/kg/d | 144 (17.6) | 146 (16) |
Abbreviations: AGA, appropriate for gestational age; AEDF, absent end diastolic flow; HC, head circumference; IQR, interquartile range; IUGR, intrauterine growth restriction; LGA, large for gestational age; PN, parenteral nutrition; REDF, reversed end diastolic flow; SGA, small for gestational age.
Data shown for neonates who received any parenteral nutrition.
Primary Outcome
The mean (SD) weight gain from the time of fortification until hospital discharge was 15.7 (3.9) and 16.3 (4) g/kg/d in the PTF and HMF groups, respectively (mean difference, −0.5 g/kg/d; 95% CI, −1.9 to 0.7; Table 2). The lower bound of 95% CI did not cross the prespecified noninferiority margin of 2 g/kg/d (eFigure 2 in Supplement 2). Given that there were no protocol deviations or any crossover between the groups, the per-protocol analysis was the same as the intention-to-treat analysis.
Table 2. Primary and Secondary Outcomes of the Study Groups.
| Variables | No. (%) | ||
|---|---|---|---|
| Preterm formula fortification (n = 59) | Human milk fortifier (n = 63) | Mean difference/relative risk (95% CI) | |
| Primary outcome | |||
| In-hospital weight gain, mean (SD), g/kg/d | 15.7 (3.9) | 16.3 (4.0) | −0.5 (−1.9 to 0.7) |
| Secondary outcomes | |||
| Anthropometry | |||
| Length gain, mean (SD), cm/wk | 0.83 (0.4) | 0.86 (0.4) | −0.03 (−0.05 to 0.12) |
| Head circumference gain, mean (SD), cm/wk | 0.64 (0.3) | 0.69 (0.4) | −0.05 (−0.07 to 0.09) |
| EUGR at 40 wk PMA, No./total No. (%) | 29/40 (73) | 42/52 (81) | 0.89 (0.71 to 1.13) |
| No. of episodes of feed intolerance, per 1000 patient-days | 2/1442 (1.4) | 12/1771 (6.8) | 0.19 (0.04 to 0.95)a |
| Age at regaining birth weight, median (IQR), d | 11 (8 to 14) | 11 (8 to 14) | NA |
| Neonates requiring increase of feed volume >180 mL/kg | 4 (7) | 8 (13) | 0.53 (0.17 to 1.68) |
| NEC stage 2 or more | 0 | 3 (5) | NA |
| Late-onset sepsis | 9 (15) | 10 (16) | 0.96 (0.42 to 2.2) |
| Duration of hospital stay, median (IQR), d | 24 (10 to 43) | 34 (15 to 47) | NA |
| Metabolic bone disease | 3 (5) | 4 (6) | 0.8 (0.19 to 3.43) |
| Cholestasis | 3 (5) | 5 (8) | 0.64 (0.16 to 2.56) |
| Anemia of prematurity | 8 (14) | 16 (25) | 0.53 (0.25 to 1.15) |
| PDA | 15 (25) | 16 (25) | 1.0 (0.54 to 1.84) |
| ROP requiring laser | 5 (8) | 4 (6) | 1.33 (0.38 to 4.73) |
| Moderate to severe BPD | 24 (41) | 20 (32) | 1.28 (0.80 to 2.06) |
| IVH grade >2, No./total No. (%) | 2/47 (4) | 2/52 (4) | 1.11 (0.16 to 7.54) |
| Death | 1 (2) | 6 (10) | 0.18 (0.02 to 1.43) |
Abbreviations: BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; IQR, interquartile range; NA, not applicable; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PMA, postmenstrual age; ROP, retinopathy of prematurity.
Incidence rate ratio (with 95% confidence interval) calculated using the negative binomial regression model.
Secondary Outcomes
The gain in length and head circumference from the time of fortification until discharge was comparable in both the groups (Table 2). The incidence of feed intolerance was significantly lower in the PTF group (1.4 vs 6.8 per 1000 patient-days; incidence rate ratio [IRR], 0.19; 95% CI, 0.04 to 0.95). While none of the neonates in the PTF group developed NEC, 3 (5%) in the HMF group developed NEC stage II or more; however, the difference was not significant (Table 2). The feed volume had to be increased to more than 180 mL/kg/d (because of inadequate weight gain, as per the protocol) in 4 and 8 neonates in the PTF and HMF groups, respectively. The proportion in whom the fortification had to be withheld for 24 hours or more was also lower in the PTF group (5% vs 22%; risk ratio [RR], 0.22; 95% CI, 0.07 to 0.75) (eTable 4 in Supplement 2).
There were no differences in the incidence of mortality, clinical sepsis or culture-positive sepsis, MBD, IVH, PDA, ROP, or BPD between the 2 groups (Table 2). At 40 weeks’ PMA, 73% of neonates in the PTF group and 81% of neonates in the HMF group were noted to have extrauterine growth restriction. A total of 7 neonates, 1 in the PTF and 6 in the HMF group, died during the study period. The weight gain of these neonates until death was included in the analysis.
Discussion
This study’s findings demonstrate that PTF is noninferior to commercial human milk fortifiers in terms of short-term weight gain until discharge from birth hospitalization. The weight gain observed in the commercial HMF group (16.3 g/kg/d) was in accord with the weight gain reported in previous studies using HMF fortification (ranging from 15.1 to 16.7 g/kg/d).13,14,17 Therefore, the lack of difference in the weight gain between the 2 groups cannot be attributed to a suboptimal outcome in the control (HMF) group. There was not much difference in the energy content of the milk fortified with PTF from that fortified with HMF, but the latter did have a higher protein content. However, the higher protein content did not translate into gains in length or weight. A possible reason for the comparable gains in short-term anthropometry is the increased risk of feed intolerance in the HMF group.
The previous 2 studies with PTF fortification had recorded a higher weight gain (18.3 g/kg/d18 and 18.9 g/kg/d19) than the weight gain observed in the PTF group in this study. This could possibly be owing to the differences in the calorie and protein content of the preterm formula powder used in the 3 studies. We used Dexolac Special care, designed as a standalone formula for preterm neonates, in the PTF fortification group. The choice of both the PTF and HMF was guided by the availability of products in the unit. The composition of the formulations used in the study was similar to other products available in the country (eTable 5 in Supplement 2). In contrast to the weight gain, the weekly length gain and head circumference growth in this study were comparable with that in the previous studies.10,20,21
Neonates in the PTF group had a significantly lower incidence of feed intolerance during the hospital stay (eTable 4 in Supplement 2). The proportion of neonates having 1 or more episodes of feed intolerance was also lower in the PTF group: 3% (n = 2) vs 14% (n = 9) (eTable 4 in Supplement 2). Previous studies have reported an incidence of feed intolerance of 10% to 30% following HMF fortification.13,22 The higher incidence in the HMF group could be because of the higher osmolality of feeds following fortification with HMF than with preterm formula (464 mOsm/kg vs 357 mOsm/kg).23 Fortification with PTF also reduced the need for withholding fortification for 24 hours or more (eTable 4 in Supplement 2). There were 3 cases of NEC stage 2 or more during the study period, all in the HMF group; 2 of these neonates died due to the illness. This increased incidence of NEC in the HMF group was also observed in the previous study by Willeitner et al.18
The risk of other comorbidities, including MBD, PDA, ROP, IVH, and BPD, was comparable between the 2 groups. Feeding fortified breast milk has been shown to be associated with a higher incidence of metabolic acidosis than feeding unfortified milk in preterm neonates.7,24,25 Our unit follows a policy to evaluate for LMA, after ruling out anemia and other organic causes.11 None of the neonates in either of the groups satisfied the criteria for LMA.
A remarkably high proportion of neonates had postnatal growth restriction at 40 weeks’ PMA. Although nearly half of the study population was growth restricted at birth itself, the almost universal incidence of EUGR at 40 weeks is still not acceptable. The proportion of neonates with EUGR was higher than that reported in the previous studies from high-income countries.26,27,28,29,30,31 Almost a similar magnitude of postnatal growth restriction was reported in VLBW infants in a study from another unit in India.32 In a review of 3-year data before initiation of current study in our unit, the incidence of EUGR at the time of discharge (mean PMA of 36 weeks) was found to be 68.4%, consistent with the pattern of poor “in-hospital growth” seen in our study (fortification by HMF was being practiced as a unit policy in these 3 years as well). The possible reasons for such high EUGR rates could be (1) a high incidence of IUGR; (2) poor nutritional status and high sickness levels of the mothers resulting in breast milk deficient in calories and proteins; (3) suboptimal postdischarge nutrition; and (4) possibly inherent genetic differences in the assimilation of nutrients in the Indian neonates.
One of the key reasons for the conduct of the study was the high cost of fortification associated with the commercially available HMFs, which could limit its use in most LMIC settings. The estimated cost of fortification based on the market rates of commercial HMF, PTF powder, and the supplements added for a 1-kg infant would be $4 and $0.27 per day, respectively (Table 3). Thus, for a month of fortification, the cost incurred would be $120 with HMF and about $8 with PTF fortification. A simple comparison of the costs associated with breast milk fortification for a neonatal intensive care unit with 10 000 deliveries per year with an assumption of 2.5% neonates needing fortification is presented in Table 3. It shows a staggering difference of $39 165 annually between HMF and PTF fortification, a significant saving for any hospital, more so in facilities from the LMICs. The money potentially saved can be better used in strengthening the health care infrastructure in these countries to reduce preventable neonatal and infant deaths.
Table 3. Comparison of Cost of Fortification With Preterm Formula Powder and HMF.
| Study cohort | Preterm formula fortification, $ | Human milk fortifier fortification, $ |
|---|---|---|
| Estimated cost of fortification for the study cohorta | ||
| Cost of fortification for 1 d for a neonate with fluid intake of 180 mL/kg/d | 0.27 | 4 |
| Estimated costs for the whole cohort | 382 | 8568 |
| Estimated costs for a community health facility for a typical neonate of weight 1.25 kgb | ||
| Cost of fortification for 1 infant for a period of 42 d (average duration of hospital stay) | 11.3 | 168 |
| Cost for fortification for 1 y in a NICU with 10 000 deliveries per year, assuming neonates needing fortification to be 250/y (2.5%) | 2835 | 42 000 |
Abbreviation: HMF, human milk fortifiers.
Assuming a uniform feed volume of 180 mL/kg/d throughout the intervention period, ie, the median duration of in-hospital fortification of 24 and 34 days in the PTF and HMF groups, respectively, in the study cohort.
Assumptions: average weight of neonate needing fortification is 1.25 kg. Average duration of fortification required is 42 days (7 weeks); proportion of preterm neonates requiring fortification is 2.5%.
Strengths and Limitations
The study had high internal validity; random allocation and allocation concealment were ensured, the processes for measurement of outcomes were standardized by SOPs, and there were no dropouts from the study and no protocol deviations. The study also had a few limitations. The study intervention could not be blinded because of practical reasons, but the primary outcome, weight gain, is an “objective” outcome. Although performance and other biases may still not be ruled out, their likelihood is very low given the uniform adherence to unit policies and SOPs for clinical management in both the groups. We considered a noninferiority margin of 2 g/kg/d. A smaller noninferiority margin, for example, 1 g/kg/d, could have been ideal given that we compared 2 methods of fortification and not fortification vs no fortification, but this would have required a large sample size. The Cochrane review33 comparing individualized (targeted or adjustable) fortification with standard fortification showed a mean difference of 1.9 g/kg/d between the 2 groups, slightly more than the difference observed with fortification vs no fortification. Therefore, comparison of 2 different modalities of fortification does not necessarily mandate an assumption for a smaller effect size of 1 g/kg/d.
Conclusions
Fortification with PTF powder (along with additional supplements to meet the recommended dietary allowances of different nutrients) is not inferior, in terms of short-term weight gain, to fortification with HMFs in preterm VLBW neonates. Moreover, it reduces the incidence of feed intolerance and the need to withhold fortification for 24 hours or more. Given that fortification with preterm formula is also less expensive, it seems to be a better option for fortifying breast milk, particularly in neonates from resource-constrained settings.
Trial Protocol
eTable 1. Composition of breast milk and the two fortifiers used in the study
eTable 2. Additional nutrients added with fortification in the two groups
eTable 3. Nutritional content of the fortified milk preparations considering enteral intake of 180 ml/kg with standardized fortification in the two groups
eTable 4. Comparison of outcomes related to individual components of feed intolerance in the two groups
eTable 5. Composition of preterm formula powders and human milk fortifiers available in India other than those used in the study
eFigure 1. Intervention overview
eFigure 2. Non inferiority margin and the mean difference in weight gain between two fortification strategies
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Mukhopadhyay K, Louis D, Mahajan G, Mahajan R. Longitudinal growth and post-discharge mortality and morbidity among extremely low birth weight neonates. Indian Pediatr. 2014;51(9):723-726. doi: 10.1007/s13312-014-0489-6 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Ann Nutr Metab. 2011;58(suppl 1):8-18. doi: 10.1159/000323381 [DOI] [PubMed] [Google Scholar]
- 3.Agostoni C, Buonocore G, Carnielli VP, et al. ; ESPGHAN Committee on Nutrition . Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85-91. doi: 10.1097/MPG.0b013e3181adaee0 [DOI] [PubMed] [Google Scholar]
- 4.Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;(5):CD000343.. doi: 10.1002/14651858.CD000343.pub4 [DOI] [PubMed] [Google Scholar]
- 5.Kler N, Thakur A, Modi M, et al. Human milk fortification in India. Nestle Nutr Inst Workshop Ser. 2015;81:145-151. doi: 10.1159/000365904 [DOI] [PubMed] [Google Scholar]
- 6.Banait N, Basu S, Desai P, et al. Clinical Practice Guidelines: Feeding of Low Birth Weight Neonates. J Neonatol. 2020;34(1). [Google Scholar]
- 7.Rochow N, Jochum F, Redlich A, et al. Fortification of breast milk in VLBW infants: metabolic acidosis is linked to the composition of fortifiers and alters weight gain and bone mineralization. Clin Nutr. 2011;30(1):99-105. doi: 10.1016/j.clnu.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 8.Thoene M, Hanson C, Lyden E, Dugick L, Ruybal L, Anderson-Berry A. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients. 2014;6(1):261-275. doi: 10.3390/nu6010261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Singal A, Aggarwal R, Deorari AK, Paul VK. Effect of fortification with human milk fortifier (HMF) and other fortifying agents on the osmolality of preterm breast milk. Indian Pediatr. 2004;41(1):63-67. [PubMed] [Google Scholar]
- 10.El Sakka A, El Shimi MS, Salama K, Fayez H. Post discharge formula fortification of maternal human milk of very low birth weight preterm infants: an introduction of a feeding protocol in a University Hospital. Pediatr Rep. 2016;8(3):6632. doi: 10.4081/pr.2016.6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R, Deorari AK, Paul VK, Sankar MJ, Sachdeva A. AIIMS Protocols in Neonatology, 2/e 2019 2 Vol. Set. Second edition. Noble; 2019. [Google Scholar]
- 12.Tewari VV, Dubey SK, Kumar R, Vardhan S, Sreedhar CM, Gupta G. Early versus late enteral feeding in preterm intrauterine growth restricted neonates with antenatal doppler abnormalities: an open-label randomized trial. J Trop Pediatr. 2018;64(1):4-14. doi: 10.1093/tropej/fmx018 [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286-290. [PubMed] [Google Scholar]
- 14.Gross SJ. Bone mineralization in preterm infants fed human milk with and without mineral supplementation. J Pediatr. 1987;111(3):450-458. doi: 10.1016/S0022-3476(87)80478-X [DOI] [PubMed] [Google Scholar]
- 15.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253-1261. doi: 10.1542/peds.2005-1368 [DOI] [PubMed] [Google Scholar]
- 16.Kaul S, A. Diamond G. Good enough: a primer on the analysis and interpretation of noninferiority trials. Annals of Internal Medicine. Published online July 4, 2006. Accessed January 18, 2021. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-145-1-200607040-00011 [DOI] [PubMed]
- 17.Lucas A, Hudson GJ. Preterm milk as a source of protein for low birthweight infants. Arch Dis Child. 1984;59(9):831-836. doi: 10.1136/adc.59.9.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willeitner A, Anderson M, Lewis J. Highly concentrated preterm formula as an alternative to powdered human milk fortifier: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2017;65(5):574-578. doi: 10.1097/MPG.0000000000001638 [DOI] [PubMed] [Google Scholar]
- 19.Khorana M, Jiamsajjamongkhon C. Pilot study on growth parameters and nutritional biochemical markers in very low birth weight preterm infants fed human milk fortified with either human milk fortifier or post discharge formula. J Med Assoc Thai. 2014;97(6):164-175. [PubMed] [Google Scholar]
- 20.Pillai A, Albersheim S, Matheson J, et al. Evaluation of A Concentrated Preterm Formula as a Liquid Human Milk Fortifier in Preterm Babies at Increased Risk of Feed Intolerance. Nutrients. 2018;10(10):1433. doi: 10.3390/nu10101433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta V, Rebekah G, Sudhakar Y, Santhanam S, Kumar M, Thomas N. A randomized controlled trial comparing the effect of fortification of human milk with an infant formula powder versus unfortified human milk on the growth of preterm very low birth weight infants. J Matern. 2019:1-9. doi: 10.1080/14767058.2018.1554046 [DOI] [PubMed]
- 22.Wauben IP, Atkinson SA, Grad TL, Shah JK, Paes B. Moderate nutrient supplementation of mother’s milk for preterm infants supports adequate bone mass and short-term growth: a randomized, controlled trial. Am J Clin Nutr. 1998;67(3):465-472. doi: 10.1093/ajcn/67.3.465 [DOI] [PubMed] [Google Scholar]
- 23.Siripattanapipong P, Yangthara B, Ngerncham S. Effect of fortifiers on the osmolality of preterm human milk. Paediatr Int Child Health. 2019;1-4. doi: 10.1080/20469047.2019.1575537 [DOI] [PubMed] [Google Scholar]
- 24.Cibulskis CC, Armbrecht ES. Association of metabolic acidosis with bovine milk-based human milk fortifiers. J Perinatol. 2015;35(2):115-119. doi: 10.1038/jp.2014.143 [DOI] [PubMed] [Google Scholar]
- 25.Moya F, Sisk PM, Walsh KR, Berseth CL. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics. 2012;130(4):e928-e935. doi: 10.1542/peds.2011-3120 [DOI] [PubMed] [Google Scholar]
- 26.Raturi S, Zheng Q, Daniel LM, Shi L, Rajadurai VS, Agarwal PK. Nutritional intake and growth velocity in preterm extremely low-birthweight infants in Asia: Are we doing enough? J Paediatr Child Health. 2017;53(12):1199-1207. doi: 10.1111/jpc.13630 [DOI] [PubMed] [Google Scholar]
- 27.Sakurai M, Itabashi K, Sato Y, Hibino S, Mizuno K. Extrauterine growth restriction in preterm infants of gestational age < or =32 weeks. Pediatr Int. 2008;50(1):70-75. doi: 10.1111/j.1442-200X.2007.02530.x [DOI] [PubMed] [Google Scholar]
- 28.Pages A-S, Tandonnet O, Renesme L. [Evaluation of a modification of the nutrition policy on the frequency of extrauterine growth retardation in premature newborns between 2012 and 2014]. Arch Pediatr. 2017;24(10):925-933. doi: 10.1016/j.arcped.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 29.Iacobelli S, Viaud M, Lapillonne A, Robillard PY, Gouyon JB, Bonsante F; NUTRIQUAL Group . Nutrition practice, compliance to guidelines and postnatal growth in moderately premature babies: the NUTRIQUAL French survey. BMC Pediatr. 2015;15:110. doi: 10.1186/s12887-015-0426-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofek Shlomai N, Reichman B, Lerner-Geva L, Boyko V, Bar-Oz B. Population-based study shows improved postnatal growth in preterm very-low-birthweight infants between 1995 and 2010. Acta Paediatr. 2014;103(5):498-503. doi: 10.1111/apa.12569 [DOI] [PubMed] [Google Scholar]
- 31.Avila-Alvarez A, Solar Boga A, Bermúdez-Hormigo C, Fuentes Carballal J. Extrauterine growth restriction among neonates with a birthweight less than 1,500 grams. An Pediatr (Barc). 2018;89(6):325-332. doi: 10.1016/j.anpedi.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Modi M, Saluja S, Kler N, et al. Growth and neurodevelopmental outcome of VLBW infants at 1 year corrected age. Indian Pediatr. 2013;50(6):573-577. doi: 10.1007/s13312-013-0170-5 [DOI] [PubMed] [Google Scholar]
- 33.Fabrizio V, Trzaski JM, Brownell EA, et al. Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database Syst Rev. 2020;11(11):CD013465. doi: 10.1002/14651858.CD013465.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Composition of breast milk and the two fortifiers used in the study
eTable 2. Additional nutrients added with fortification in the two groups
eTable 3. Nutritional content of the fortified milk preparations considering enteral intake of 180 ml/kg with standardized fortification in the two groups
eTable 4. Comparison of outcomes related to individual components of feed intolerance in the two groups
eTable 5. Composition of preterm formula powders and human milk fortifiers available in India other than those used in the study
eFigure 1. Intervention overview
eFigure 2. Non inferiority margin and the mean difference in weight gain between two fortification strategies
Statistical Analysis Plan
Data Sharing Statement