Key Points
Question
What is the 2-year risk of recurrent stroke in patients with a symptomatic carotid web (CW)?
Findings
In this cohort study among 3439 patients with large vessel occlusion stroke, during 2 years’ follow-up, 17% of patients with an ipsilateral CW had a recurrent stroke compared with 3% of patients without CW. Ninety-three percent of patients with a CW received medical management after the index stroke.
Meaning
In this study, 1 of 6 patients with a symptomatic CW had a recurrent stroke within 2 years, suggesting that medical management alone may not provide sufficient protection for patients with CW.
Abstract
Importance
A carotid web (CW) is a shelf-like lesion along the posterior wall of the internal carotid artery bulb and an underrecognized cause of young stroke. Several studies suggest that patients with symptomatic CW have a high risk of recurrent stroke, but high-quality data are lacking.
Objective
To assess the 2-year risk of recurrent stroke in patients with a symptomatic CW.
Design, Setting, and Participants
A comparative cohort study used data from the MR CLEAN trial (from 2010-2014) and MR CLEAN Registry (from 2014-2017). Data were analyzed in September 2020. The MR CLEAN trial and MR CLEAN Registry were nationwide prospective multicenter studies on endovascular treatment (EVT) of large vessel occlusion (LVO) stroke in the Netherlands. Baseline data were from 3439 consecutive adult patients with anterior circulation LVO stroke and available computed tomography (CT)–angiography of the carotid bulb. Two neuroradiologists reevaluated CT-angiography images for presence or absence of CW and identified 30 patients with CW ipsilateral to the index stroke. For these 30 eligible CW participants, detailed follow-up data regarding stroke recurrence within 2 years were acquired. These 30 patients with CW ipsilateral to the index stroke were compared with 168 patients without CW who participated in the MR CLEAN extended follow-up trial and who were randomized to the EVT arm.
Main Outcomes and Measures
The primary outcome was recurrent stroke occurring within 2 years after the index stroke. Cox proportional hazards regression models were used to compare recurrent stroke rates within 2 years for patients with and without CW, adjusted for age and sex. The research question was formulated prior to data collection.
Results
Of 3439 patients with baseline CT-angiography assessed, the median age was 72 years (interquartile range, 61-80 years) and 1813 (53%) were men. Patients with CW were younger (median age, 57 [interquartile range, 46-66] years vs 66 [interquartile range, 56-77] years; P = .02 and more often women (22 of 30 [73%] vs 67 of 168 [40%]; P = .001) than patients without CW. Twenty-eight of 30 patients (93%) received medical management after the index stroke (23 with antiplatelet therapy and 5 with anticoagulant therapy). During 2 years of follow-up, 5 of 30 patients (17%) with CW had a recurrent stroke compared with 5 of 168 patients (3%) without CW (adjusted hazard ratio, 4.9; 95% CI, 1.4-18.1).
Conclusions and Relevance
In this study, 1 of 6 patients with a symptomatic CW had a recurrent stroke within 2 years, suggesting that medical management alone may not provide sufficient protection for patients with CW.
This cohort study assesses the recurrent stroke risk in a population of patients with a large vessel occlusion stroke of the anterior circulation and ipsilateral carotid web.
Introduction
A carotid web (CW) is a shelf-like lesion located along the posterior wall of the internal carotid artery bulb. Imaging and pathologic analyses suggest CW is an intimal variant of fibromuscular dysplasia (FMD).1 Computed tomography angiography (CTA) imaging is a common noninvasive method for identification of CW.1,2,3,4,5 Because CWs protrude into the lumen of the carotid artery, flow disruption and blood stasis can occur, resulting in thrombus formation and subsequent ischemic stroke.2,6 Case-control studies have found that CWs are present in 9% to 37% of patients younger than 60 years with cryptogenic stroke, and that a CW increases the risk of ischemic stroke approximately 10- to 20-fold.3,7,8 Although data are limited, patients with CW with ischemic stroke are more often than usual women and of Black racial identity.2,4,7,9,10
It is unclear how patients with a symptomatic CW (those who have had an ipsilateral ischemic stroke) are best treated to prevent recurrent stroke.1 Most patients are treated with antiplatelet therapy, but some physicians advocate using anticoagulation therapy as a better choice because of focal blood stasis in the carotid artery caused by CW.1 Carotid artery stenting and surgical removal of the CW have also been reported, especially in those with recurrent ischemic stroke.9,10
One of the major knowledge gaps in deciding the optimal treatment is the lack of studies on the risk of recurrent stroke in patients with a symptomatic CW.1,3 A 2018 systematic review reported an ischemic stroke recurrence rate of 56% (with a median of 12 months to the recurrent stroke, range, 0-97 months) in patients with CW receiving medical management, but these data come from case reports and uncontrolled, retrospective, single center studies.10 Owing to publication and selection bias, the true recurrent stroke risk in patients with a symptomatic CW remains unknown. The aim of the current study was to assess the recurrent stroke risk in a population of patients with a large vessel occlusion (LVO) stroke of the anterior circulation and ipsilateral CW.
Methods
Study Design and Patient Selection
We conducted a comparative cohort study using data from the MR CLEAN trial11 (December 2010-March 2014) and the MR CLEAN Registry12 (March 2014-November 2017). The MR CLEAN trial was a randomized clinical trial conducted at 16 centers in the Netherlands in which adult patients with ischemic stroke due to an anterior circulation LVO were allocated to endovascular treatment (EVT) or no EVT.11 The MR CLEAN Registry was a nationwide observational cohort study in which data were collected from consecutive patients with LVO stroke treated with EVT in the Netherlands.12 All Dutch EVT centers participated in this registry, and enrollment started directly after the last patient was randomized in the MR CLEAN trial. Details of both studies have been published.11,12 From both studies, we selected adult patients with an anterior circulation LVO stroke (index stroke) and with available baseline CTA of the carotid bulb. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
CW Assessment
All CTA images from the MR CLEAN trial and Registry were reassessed by an experienced neuroradiologist (B.J.E. or A.C.G.M.v.E.) to determine whether patients had a CW ipsilateral to the index stroke. Carotid bulbs were evaluated in multiplanar reconstruction view. We defined CW on CTA as a thin linear filling defect arising from the posterior wall of the proximal internal carotid bulb, with a typically smooth border and without atherosclerosis.2,13 We distinguished CW from arterial dissection by defining the latter as a linear filling defect beyond the distal internal carotid bulb, with typically irregular borders.1,14
Data Collection and Ethical Approval
To determine recurrent stroke risk, we obtained extended follow-up data from patients with CW. In addition to interviewing patients, we acquired follow-up data from medical records by contacting their treating physicians. We collected information on the use of anti-thrombotic therapy and inquired whether patients had undergone any carotid artery intervention (stenting or surgical resection). The medical ethics committee of the Erasmus University Medical Center in Rotterdam approved the study and waived the need for written informed consent because the study did not fall within the scope of the Dutch Medical Research Involving Human Subjects Act (WMO).
To compare the risk of recurrent stroke of patients with a CW ipsilateral to the index stroke with patients without a CW, we used data of patients who participated in the MR CLEAN extended follow-up trial, details of which have been published.15 In this extension study of the original MR CLEAN trial, detailed follow-up data, including stroke recurrences were assessed 2 years after randomization.15 The investigators of the MR CLEAN extended follow-up trial used the same methods for data collection on stroke recurrences as used for patients with CW (telephone interviews and medical records). From 391 patients who participated in the MR CLEAN extended follow-up trial,15 we selected the 168 patients without a CW and who were randomized to the EVT arm as a comparative cohort (Figure 1). The rationale for excluding patients who were randomized to the no EVT arm was to avoid large disparities between patients with a CW ipsilateral to the index stroke and the comparative cohort in terms of proportion of patients who received reperfusion treatment for the index stroke.
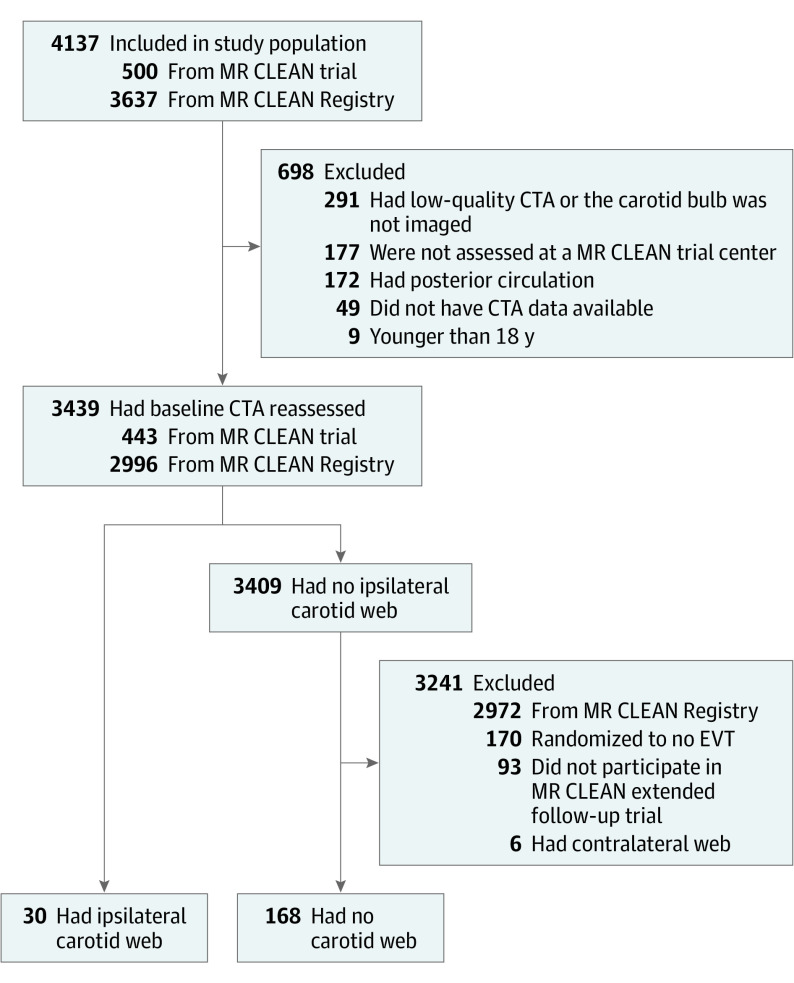
Figure 1. Flow Diagram of Patient Selection.
CTA indicates computed tomography angiography; EVT, endovascular treatment.
Statistical Analysis
The primary outcome was recurrent stroke occurring within 2 years after the index stroke. Strokes occurring within 24 hours of EVT for the index stroke were considered periprocedural and were excluded.
We compared patients with an ipsilateral CW from the MR CLEAN trial and Registry with patients from the MR CLEAN extended follow-up trial without a CW. For comparison of continuous data, we used a 2-sided independent t test and for proportions, we used a χ2 test or Fisher exact test, as appropriate. Two-sided P values of ≤.05 were considered to indicate statistical significance. To compare cumulative incidences of recurrent stroke within 2 years between groups, we used Kaplan-Meier survival analysis techniques with a log-rank test. In addition, we used Cox proportional hazards regression models to estimate a hazard ratio (HR) for recurrent stroke risk within 2 years for patients with an ipsilateral CW compared with patients without a CW. We used univariable Cox regression to calculate an unadjusted HR and multivariable Cox regression to calculate an HR adjusted for age and sex. We censored patients who died of another cause than a recurrent stroke or who were lost to follow-up before 2 years. Using the same methods, we performed an additional analysis for recurrent ischemic stroke ipsilateral to the index stroke within 2 years after the index stroke. For both 2-year outcomes. there was no indication of violation of the proportional hazards assumption by visual inspection of the Schoenfeld residuals. Furthermore, we provided an incidence rate for recurrent stroke in patients with CW ipsilateral index stroke, throughout the total available follow-up duration. We calculated recurrent stroke incidence per 100 person-years, with 95% CIs, using Mid-P exact test. Statistical analyses were performed with SPSS software, version 26.0 (IBM) and R software, version 3.6.1 (R Foundation for Statistical Computing).
Results
Of 4137 patients included in the MR CLEAN trial (n = 500) or Registry (n = 3637), 698 were excluded (Figure 1). Of the remaining 3439 patients of whom the CTA was reassessed (median age, 72 years [interquartile range, 61-80 years]; 1813 [53%] were men and 1626 [47%] were women), we identified 30 patients (0.9%) with a CW ipsilateral to the side of the index stroke. The CWs were located in the right internal carotid artery in 24 of 30 (80%) patients. There were 2 patients with a bilateral CW. In addition, 6 of 3439 patients (0.2%) had a CW contralateral to the index stroke. None of the patients with CW had a stenosis greater than 50% according to North American Symptomatic Carotid Endarterectomy Trial criteria owing to their CW.16
Patients with an ipsilateral CW were younger (median age, 57 years [interquartile range, 46-66 years] vs 66 years [interquartile range,56-77 years]; P = .01) and more often women (73% vs 40%; P = .001) compared with patients without a CW (Table 1). Three patients with an ipsilateral CW had had an ischemic stroke prior to the index stroke, 2 of which were in the same vascular territory as the CW. In 24 of 30 patients (80%) with an ipsilateral CW, no other cause for the index stroke was found, whereas 6 of 30 patients (20%) also had a potential cardioembolic cause (atrial fibrillation). Among patients without a CW, the cause of the index stroke was large-artery atherosclerosis in 48 of 168 (29%), cardioembolic in 44 of 168 (26%), other determined in 11 of 168 (7%), and undetermined in 65 of 168 (39%).17
Table 1. Baseline Characteristics at Index Stroke.
| Characteristic | No./total No. (%) | P value | |
|---|---|---|---|
| Carotid web (n = 30) | No carotid web (n = 168) | ||
| Age, median (IQR), y | 57 (46-66) | 66 (56-77) | .02 |
| Women | 22/30 (73) | 67/168 (40) | .001 |
| Medical history | |||
| Ischemic stroke | 3/30 (10) | 20/168 (12) | >.99 |
| Hypertension | 10/30 (33) | 73/168 (44) | .30 |
| Hypercholesterolemia | 5/29 (17) | 41/168 (24) | .40 |
| Smoking | 5/28 (18) | 47/168 (28) | .26 |
| Diabetes | 2/30 (7) | 24/168 (14) | .38 |
| Atrial fibrillation | 6/30 (20)a | 43/168 (26) | .51 |
| Myocardial infarction | 0/30 (0) | 22/168 (13) | .03 |
| Prestroke mRS score | |||
| 0 | 23/29 (79) | 136/168 (81) | .44 |
| 1 | 1/29 (3) | 15/168 (9) | |
| ≥2 | 5/29 (17) | 17/168 (10) | |
| Right hemisphere stroke | 24/30 (80) | 82/168 (49) | .002 |
| Median NIHSSb | 15 (12-19) | 17 (14-21) | .02 |
| Systolic BP, median (IQR), mm Hgc | 137 (119-150) | 145 (130-160) | .03 |
| ASPECTS, median (IQR)d | 9 (8-10) | 9 (7-10) | .68 |
| Occlusion location | |||
| ICA-T | 6/29 (21) | 42/168 (25) | .50 |
| M1 | 19/29 (66) | 114/168 (68) | |
| M2 | 4/29 (14) | 10/168 (6) | |
| Other | 0/29 (-) | 2/168 (1) | |
Abbreviations: ASPECTS, Alberta stroke program early CT score; BP, blood pressure; ICA-T, internal carotid artery terminal; IQR, interquartile range; M1, middle cerebral artery segment 1; M2, middle cerebral artery segment 2; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Diagnosed at admission in 3 of 6 patients.
Number of patients with missing data in carotid web group vs no carotid web group, 1 vs 0.
Number of patients with missing data in carotid web group vs no carotid web group, 1 vs 0.
Number of patients with missing data in carotid web group vs no carotid web group, 0 vs 1.
Twenty-eight of 30 patients (93%) received medical management to prevent recurrent stroke after the index stroke, 1 of 30 (3%) underwent carotid endarterectomy 19 days after the index stroke and 1 of 30 (3%) died 1 day after admission for the index stroke prior to start of secondary prevention therapy. Of the patients receiving medical management, 23 were prescribed antiplatelet agents (15 single and 8 double) and 5 anticoagulants (4 vitamin K antagonists and 1 direct oral anticoagulant). One of the patients who received antiplatelet therapy had a recurrent ischemic stroke 6 months after the index stroke and underwent carotid endarterectomy 8 days thereafter. Both patients remained free of stroke after carotid endarterectomy. Histopathologic samples of CW were not available. None of the patients with CW underwent carotid stenting.
Recurrent Stroke
We obtained 2-year follow-up data for 28 of 30 patients (93%) with an ipsilateral CW. Four patients with an ipsilateral CW were censored before the 2-year follow-up was completed: 2 owing to death (1 patient died 1 day after the index stroke, and the other died following palliative sedation for chronic refractory pain 18 months after the index stroke), 1 patient declined to participate in the follow-up telephone interview resulting in missing data beyond 3 months, and the last patient was lost to follow-up after emigrating 20 months after the index stroke.
In the comparator cohort, 61 of 168 patients (36%) were censored before the 2-year follow-up was completed, 48 (29%) because of death due to other causes than a recurrent stroke and 13 patients (7%) did not complete the 2-year follow-up. Censoring was unrelated to a recurrent stroke in all cases.
During the 2 years after the index stroke, 5 of 30 patients (17%) with an ipsilateral CW had a recurrent stroke, compared with 5 of 168 patients (3%) without a CW (adjusted HR, 4.9; 95% CI, 1.4-18.1) (Figure 2). Median time to recurrent stroke was 6 months (interquartile range, 1-10 months) in patients with a CW and 1 month (interquartile range, 0-7 months) in patients without a CW. Incidence of recurrent stroke per treatment strategy in patients with CW is presented in eTable 1 in Supplement 1. The 2-year risk of patients with recurrent stroke with CW receiving medical management excluding patients who were censored was 5 of 25 (20%).
Figure 2. Kaplan-Meier Estimates of Recurrent Stroke and Recurrent Ipsilateral Ischemic Stroke During 2-Year Follow-up After the Index Stroke.
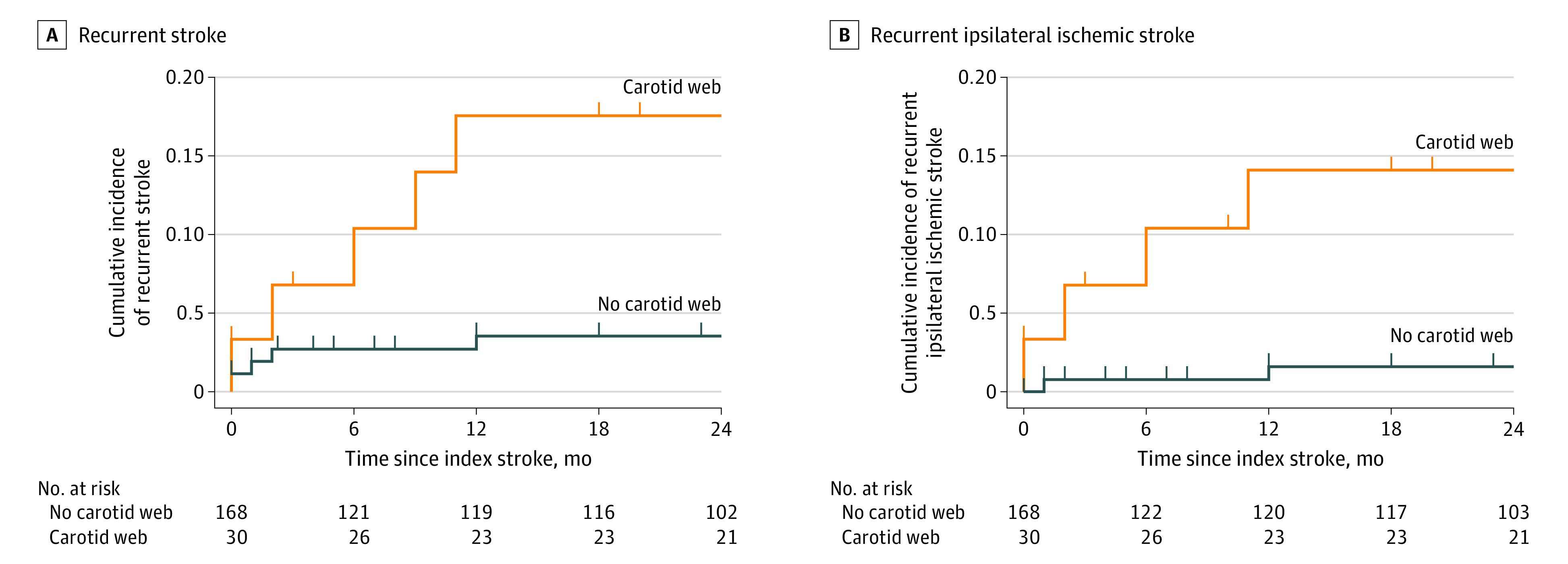
A., During 2-year follow-up, 5 of 30 patients (17%) with a carotid web ipsilateral to the index stroke had a recurrent stroke compared with 5 of 168 patients (3%) without carotid web (hazard ratio, 5.0; 95% CI, 1.4-17.3; adjusted hazard ratio, 4.9; 95% CI, 1.4-18.1). B, During 2-year follow-up, 4 of 30 patients (13%) with a carotid web ipsilateral to the index stroke had a recurrent ischemic stroke ipsilateral to the index stroke compared with 2 of 168 patients (1%) without carotid web (hazard ratio, 9.9; 95% CI, 1.8-54.2; adjusted hazard ratio, 8.1; 95% CI, 1.4-46.8).
Type and location of the recurrent stroke could be ascertained in 4 of 5 patients with CW, and these 4 recurrences were ischemic strokes in the same vascular territory as the CW (Table 2). For the last case, the details of the recurrent stroke could not be determined because the patient died shortly thereafter in a nursing home and no imaging or autopsy was performed (Table 2). Figure 3 presents the CTA images (acquired at the time of the index stroke) of the patients with CW who had recurrent stroke.
Table 2. Patients With Carotid Web With Recurrent Stroke During 2-Year Follow-up.
| Patient | Agea | Sexa | Cardiovascular risk factorsa | Index strokea | Recurrent stroke (time since index stroke), details on type and acute treatment | Treatment at time of recurrent stroke | Treatment after recurrent stroke | Alive at 2-y follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Early 40s | F | HT | Right M1 | (6 mo) Ipsilateral ischemic LVO treated with IVT | AP | CEA, AP | Yes |
| 2 | Early 70s | F | HT, AF, D, HC | Right M1 | (9 mo) Ipsilateral ischemic, no cerebral artery imaging performed, no RT | AP, ACb | Palliative care | Noc |
| 3 | Early 90s | F | HT, AF | Right M1 | (2 mo) Undetermined type, no imaging performed, no RT | ACd | Palliative care | Noe |
| 4 | Early 90s | F | NR | Right M2 | (6 d) Ipsilateral ischemic LVO treated with EVT | AP | AP | Yes |
| 5 | Early 60s | F | NR | Right M1 | (11 mo) Ipsilateral ischemic LVO treated with IVT and EVT | AP | AP | Yes |
Abbreviations: AC, anticoagulation; AF, atrial fibrillation; AP, antiplatelet; CEA, carotid endarterectomy; D, diabetes; EVT, endovascular treatment; F, female; HC, hypercholesterolemia; HT, hypertension; IVT, intravenous thrombolysis; LVO, large vessel occlusion; M1, middle cerebral artery segment 1; M2, middle cerebral artery segment 2; NR, none reported; RT, reperfusion therapy.
All patients received IVT and EVT for index stroke.
Vitamin K antagonist. The patient had a therapeutic international normalized ratio (2.0) at the time of the recurrent stroke.
Hemorrhagic transformation occurred after recurrent ischemic stroke; patient was discharged from hospital with palliative care and died of aspiration pneumonia.
Vitamin K antagonist. Data on international normalized ratio at the time of the recurrent stroke were not available.
Died in a nursing home; no imaging or autopsy was performed.
Figure 3. Carotid Webs on Computed Tomography Angiography Imaging at the Index Stroke in Patients With Recurrent Stroke During 2-Year Follow-up.
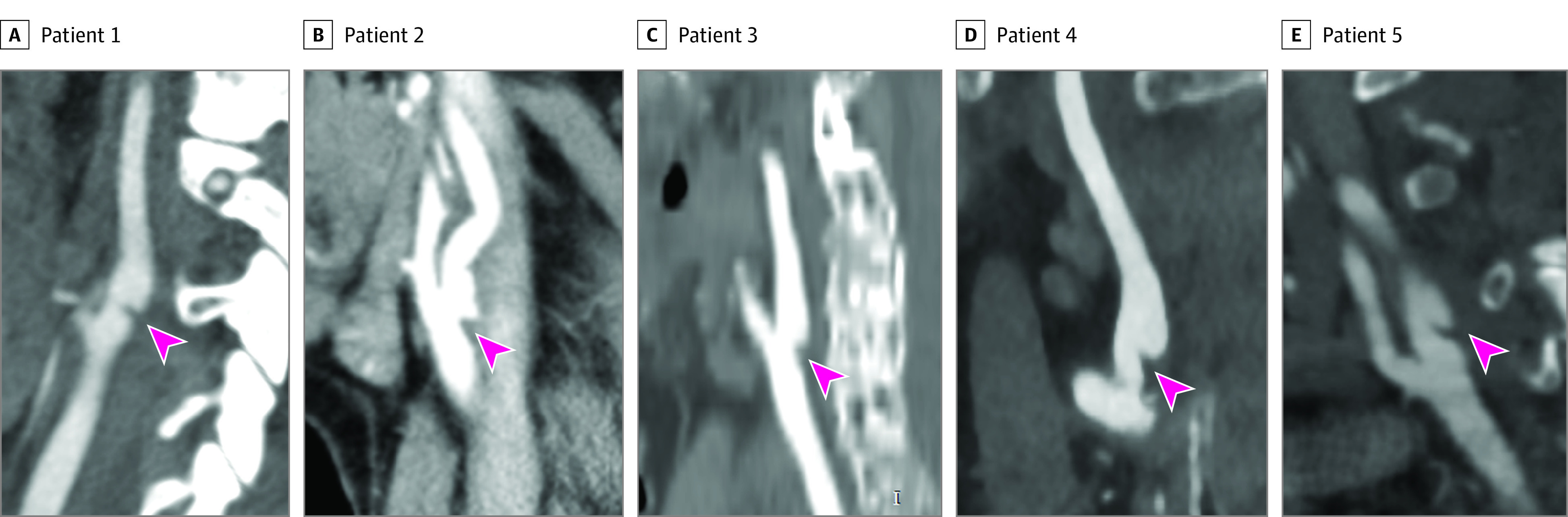
Carotid webs are indicated by arrowheads. Detailed patient data are provided in Table 2.
All 5 recurrent strokes in the group of patients without a CW were ischemic, 2 of which were in the same vascular territory as the index stroke. When restricting the analysis to those with recurrent ischemic stroke ipsilateral to the index stroke, the risk was increased 8-fold in patients with an ipsilateral CW as compared with patients without a CW (adjusted HR, 8.1; 95% CI, 1.4-46.8) (Figure 2B). None of the 6 patients with a CW contralateral to the index stroke had a recurrent stroke.
During the total follow-up period (median 39 months, interquartile range, 16-74 months) of patients with an ipsilateral CW, 2 additional recurrent strokes beyond 2 years occurred. One patient who received antiplatelet therapy had a recurrent ischemic stroke in the same vascular territory as the CW at 30 months. The other patient ( Table 2) had a second recurrent stroke at 28 months and died 6 days later in a nursing home; no imaging or autopsy was performed. The incidence rate of recurrent stroke in the 30 patients with ipsilateral CW throughout the total follow-up period was 5.5 per 100 person-years (95% CI, 2.2-11.4).
Discussion
This cohort study found that patients with a CW ipsilateral to the index stroke had a 5 times higher risk of recurrent stroke compared with patients without a CW. In absolute terms, 1 of 6 patients with a CW ipsilateral to the index stroke had a recurrent stroke within 2 years. Moreover, the recurrent ischemic strokes were in the same vascular territory as the CW, and the risk of recurrent stroke appeared to persist after the 2-year follow-up period.
In this study, 93% of patients with an ipsilateral CW received medical management after the index stroke, most with antiplatelet therapy. We observed a relatively higher incidence of recurrent stroke in patients receiving anticoagulant therapy similar to the systematic review by Zhang et al.10 This finding may be unexpected, considering the imaging evidence of flow disruption and blood stasis in CW.6,9 However, interpretation is limited by the small sample in both studies. Notably, all patients receiving anticoagulant therapy experienced concomitant atrial fibrillation, which may further compound the stroke risk. Also, information on whether the international normalized ratio was in the therapeutic range was not available for 1 of the 2 patients who had a recurrent stroke receiving anticoagulation therapy. While we cannot determine from our data what the optimal therapy for these patients should be, the high recurrent stroke risk we observed indicates that standard medical management alone may not provide sufficient protection. Endovascular stenting may be a better secondary stroke prevention strategy, particularly in patients already receiving endovascular treatment for LVO, but further study is warranted.9 Carotid surgery may also be an option, although such an invasive strategy should be deliberated with caution.18
Despite the high recurrent stroke risk in our study, the risk is lower than reported in a systematic review of patients with CW receiving medical management (56%, with a median time to recurrent stroke of 12 months, range 0-97 months).10 This difference may result from publication and reporting bias inherent to case reports and case series.
The prevalence of a CW ipsilateral to the index ischemic stroke was approximately 1% in our study, which is consistent with a previous report2 that used data from a general acute ischemic stroke population (0.7% ipsilateral), but lower than reported in a smaller study by Compagne et al13 in a population with acute large vessel occlusion stroke from the MR CLEAN trial (2.5%). Other studies have shown that CW is more prevalent in selected populations of young (<60 years) patients with ischemic stroke with no other apparent cause.3,7,8,19,20 Patients in our sample were older than in the study by Compagne et al13 (eTable 2 in Supplement 1), and because older age comes with an increase in traditional cardiovascular risk factors, it is possible stroke was less often the result of a CW. Moreover, as the prevalence of atherosclerosis increases with age, and we used a narrow definition of carotid web whereby patients with carotid atherosclerosis were not scored as CW, it is possible we excluded some cases with CW who had superimposed carotid atherosclerosis.
The exact pathophysiologic nature of CW is unknown. The most widely propagated hypothesis is that CW represents an intimal variant of FMD.1 Alternatively, CW may be a remnant of aberrant embryologic development of the carotid bifurcation and internal carotid artery from the third aortic arch.21,22 Acquired etiologic theories for CW have also been proposed.23 Our observation that patients with CW were more often women is consistent with previous CW studies9,10,18 and in line with the predominance of women in FMD.24,25 FMD has been diagnosed across all ethnic groups, but is most often reported in White populations, a potential result of referral bias.26 In contrast, limited CW data on racial identities suggest that Black populations may be more often affected than other ethnic populations.2,4,7,9 In our study, data on ethnicity were not available.
Notably, the CW was located in the right internal carotid artery in 80% of patients. We could not find a similar skewed distribution in any of the other studies on CW, but location was often not reported.9,18 A right-sided predominance has been observed in some studies on renal FMD,27 but not in others.24,25
Limitations
Our study has several limitations. Ideally, we would have collected 2-year follow-up data for all 3439 patients, but this was not feasible. Importantly, we applied the same methodology for acquiring follow-up data of patients with CW as was used in the comparative cohort. Second, secondary prevention was not standardized. The majority of patients with CW were treated with antiplatelets, but approximately 25% of patients received anticoagulant therapy. Third, despite data being derived from a large cohort, the number of patients with a CW was limited, and therefore our estimate of the recurrent stroke risk has a wide confidence interval. Fourth, we did not correct for multiple testing in the statistical analyses. Fifth, the study populations included in the MR CLEAN trial and MR CLEAN Registry are not entirely the same.12 We attempted to minimize differences by selecting patients with anterior circulation LVO stroke treated with EVT from the total study population. The broad inclusion criteria of the MR CLEAN trial and the MR CLEAN Registry permit a wide generalizability of our findings to other patients with LVO stroke. However, our results may not be fully representative for patients who had a stroke with an ipsilateral CW but without LVO or with a TIA. Finally, 20% of patients with an ipsilateral CW in our sample had atrial fibrillation. We do not know whether CW and/or atrial fibrillation contributed to stroke etiology in these cases.
Conclusions
In this cohort study, patients with a symptomatic CW have a high risk of recurrent stroke. Our data suggest that medical management alone may not provide sufficient protection for recurrent stroke. Prospective studies on targeted secondary prevention measures for patients with a CW are warranted.
eTable 1. Incidence of Recurrent Stroke Within 2 Years in Patients With a Symptomatic Carotid Web, per Management Strategy
eTable 2. Baseline Characteristics at Index Stroke of Study Populations Screened for Prevalence of Carotid Web
Nonauthor Collaborators. The MR CLEAN trial and MR CLEAN Registry Investigators
References
- 1.Kim SJ, Nogueira RG, Haussen DC. Current understanding and gaps in research of carotid webs in ischemic strokes: a review. JAMA Neurol. 2019;76(3):355-361. doi: 10.1001/jamaneurol.2018.3366 [DOI] [PubMed] [Google Scholar]
- 2.Choi PM, Singh D, Trivedi A, et al. Carotid webs and recurrent ischemic strokes in the era of CT angiography. AJNR Am J Neuroradiol. 2015;36(11):2134-2139. doi: 10.3174/ajnr.A4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho JM, Derkatch S, Potvin AR, et al. Carotid artery web and ischemic stroke: a case-control study. Neurology. 2017;88(1):65-69. doi: 10.1212/WNL.0000000000003464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sajedi PI, Gonzalez JN, Cronin CA, et al. Carotid bulb webs as a cause of “cryptogenic” ischemic stroke. AJNR Am J Neuroradiol. 2017;38(7):1399-1404. doi: 10.3174/ajnr.A5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H, Zhang X, Zhao J, Li Y, Zhao Y. Transient ischemic attack and carotid web. AJNR Am J Neuroradiol. 2019;40(2):313-318. doi: 10.3174/ajnr.A5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compagne KCJ, Dilba K, Postema EJ, et al. ; MR CLEAN investigators . Flow patterns in carotid webs: a patient-based computational fluid dynamics study. AJNR Am J Neuroradiol. 2019;40(4):703-708. doi: 10.3174/ajnr.A6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joux J, Boulanger M, Jeannin S, et al. Association between carotid bulb diaphragm and ischemic stroke in young Afro-Caribbean patients: a population-based case-control study. Stroke. 2016;47(10):2641-2644. doi: 10.1161/STROKEAHA.116.013918 [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Allen JW, Bouslama M, et al. Carotid webs in cryptogenic ischemic strokes: a matched case-control study. J Stroke Cerebrovasc Dis. 2019;28(12):104402. doi: 10.1016/j.jstrokecerebrovasdis.2019.104402 [DOI] [PubMed] [Google Scholar]
- 9.Haussen DC, Grossberg JA, Bouslama M, et al. Carotid web (intimal fibromuscular dysplasia) has high stroke recurrence risk and is amenable to stenting. Stroke. 2017;48(11):3134-3137. doi: 10.1161/STROKEAHA.117.019020 [DOI] [PubMed] [Google Scholar]
- 10.Zhang AJ, Dhruv P, Choi P, et al. A systematic literature review of patients with carotid web and acute ischemic stroke. Stroke. 2018;49(12):2872-2876. doi: 10.1161/STROKEAHA.118.021907 [DOI] [PubMed] [Google Scholar]
- 11.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 12.Jansen IGH, Mulder MJHL, Goldhoorn RB; MR CLEAN Registry investigators . Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compagne KCJ, van Es ACGM, Berkhemer OA, et al. ; MR CLEAN Trial Investigators . Prevalence of carotid web in patients with acute intracranial stroke due to intracranial large vessel occlusion. Radiology. 2018;286(3):1000-1007. doi: 10.1148/radiol.2017170094 [DOI] [PubMed] [Google Scholar]
- 14.Guglielmi V, Mandell DM. Letter to the editor: classic radiological appearance of a carotid web. J Neurosurg. 2019;1-2. doi: 10.3171/2018.12.JNS183522 [DOI] [PubMed] [Google Scholar]
- 15.van den Berg LA, Roos YB. Two-year outcome after endovascular treatment for stroke. N Engl J Med. 2017;376(26):2597. doi: 10.1056/NEJMc1705673 [DOI] [PubMed] [Google Scholar]
- 16.Barnett HJ, Taylor DW, Eliasziw M, et al. ; North American Symptomatic Carotid Endarterectomy Trial Collaborators . Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339(20):1415-1425. doi: 10.1056/NEJM199811123392002 [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 18.Joux J, Chausson N, Jeannin S, et al. Carotid-bulb atypical fibromuscular dysplasia in young Afro-Caribbean patients with stroke. Stroke. 2014;45(12):3711-3713. doi: 10.1161/STROKEAHA.114.007313 [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Wang B, Zheng S, Kou J, Gu X, Liu T. Carotid web and ischemic stroke: a CT angiography study. Clin Imaging. 2020;67:86-90. doi: 10.1016/j.clinimag.2020.05.033 [DOI] [PubMed] [Google Scholar]
- 20.Labeyrie MA, Serrano F, Civelli V, et al. Carotid artery webs in embolic stroke of undetermined source with large intracranial vessel occlusion. Int J Stroke. 2020;1747493020929945. doi: 10.1177/1747493020929945 [DOI] [PubMed] [Google Scholar]
- 21.McNamara MF. The carotid web: a developmental anomaly of the brachiocephalic system. Ann Vasc Surg. 1987;1(5):595-597. doi: 10.1016/S0890-5096(06)61448-9 [DOI] [PubMed] [Google Scholar]
- 22.Dungan DH, Heiserman JE. The carotid artery: embryology, normal anatomy, and physiology. Neuroimaging Clin N Am. 1996;6(4):789-799. [PubMed] [Google Scholar]
- 23.Hassani S, Nogueira RG, Al-Bayati AR, Kala S, Philbrook B, Haussen DC. Carotid webs in pediatric acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(12):105333. doi: 10.1016/j.jstrokecerebrovasdis.2020.105333 [DOI] [PubMed] [Google Scholar]
- 24.Narula N, Kadian-Dodov D, Olin JW. Fibromuscular dysplasia: contemporary concepts and future directions. Prog Cardiovasc Dis. 2018;60(6):580-585. doi: 10.1016/j.pcad.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 25.Kadian-Dodov D, Goldfinger JZ, Gustavson S, Olin JW. Natural history of cervical artery fibromuscular dysplasia and associated neurovascular events. Cerebrovasc Dis. 2018;46(1-2):33-39. doi: 10.1159/000491437 [DOI] [PubMed] [Google Scholar]
- 26.Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125(25):3182-3190. doi: 10.1161/CIRCULATIONAHA.112.091223 [DOI] [PubMed] [Google Scholar]
- 27.Varennes L, Tahon F, Kastler A, et al. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights Imaging. 2015;6(3):295-307. doi: 10.1007/s13244-015-0382-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Incidence of Recurrent Stroke Within 2 Years in Patients With a Symptomatic Carotid Web, per Management Strategy
eTable 2. Baseline Characteristics at Index Stroke of Study Populations Screened for Prevalence of Carotid Web
Nonauthor Collaborators. The MR CLEAN trial and MR CLEAN Registry Investigators