Abstract
Objective
To investigate whether the results of a rhythm control strategy differ according to the duration between diagnosis of atrial fibrillation and treatment initiation.
Design
Longitudinal observational cohort study.
Setting
Population based cohort from the Korean National Health Insurance Service database.
Participants
22 635 adults with atrial fibrillation and cardiovascular conditions, newly treated with rhythm control (antiarrhythmic drugs or ablation) or rate control strategies between 28 July 2011 and 31 December 2015.
Main outcome measure
A composite outcome of death from cardiovascular causes, ischaemic stroke, admission to hospital for heart failure, or acute myocardial infarction.
Results
Of the study population, 12 200 (53.9%) were male, the median age was 70, and the median follow-up duration was 2.1 years. Among patients with early treatment for atrial fibrillation (initiated within one year since diagnosis), compared with rate control, rhythm control was associated with a lower risk of the primary composite outcome (weighted incidence rate per 100 person years 7.42 in rhythm control v 9.25 in rate control; hazard ratio 0.81, 95% confidence interval 0.71 to 0.93; P=0.002). No difference in the risk of the primary composite outcome was found between rhythm and rate control (weighted incidence rate per 100 person years 8.67 in rhythm control v 8.99 in rate control; 0.97, 0.78 to 1.20; P=0.76) in patients with late treatment for atrial fibrillation (initiated after one year since diagnosis). No significant differences in safety outcomes were found between the rhythm and rate control strategies across different treatment timings. Earlier initiation of treatment was linearly associated with more favourable cardiovascular outcomes for rhythm control compared with rate control.
Conclusions
Early initiation of rhythm control treatment was associated with a lower risk of adverse cardiovascular outcomes than rate control treatment in patients with recently diagnosed atrial fibrillation. This association was not found in patients who had had atrial fibrillation for more than one year.
Introduction
Atrial fibrillation increases the risk of mortality and morbidity from stroke and congestive heart failure, and impaired quality of life, even for patients with optimal anticoagulation and rate control treatment.1 2 3 4 Rate control is integral to the management of atrial fibrillation, and is often sufficient to improve related symptoms.1 Rhythm control refers to attempts to restore and maintain sinus rhythm using antiarrhythmic drug treatment, cardioversion, and atrial fibrillation ablation together with adequate rate control. Such treatment improves symptoms and the quality of life of patients.1 5 6 Several randomised trials have compared rhythm control with rate control, including the landmark Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) trial, and shown no significant differences between these treatment strategies in their effect on mortality and stroke.7 8 9 10 It has been suggested that ablation improves outcomes in selected patients with atrial fibrillation and heart failure, in comparison with antiarrhythmic drug treatment combined with rate control.11 One trial showed, that in comparison with a placebo, the antiarrhythmic drug dronedarone reduced the composite outcome of death from any causes or the first admission to hospital for cardiovascular events in patients with atrial fibrillation.12 Data on the prognostic effect of rhythm control treatment are inconclusive, with no clear indication of benefit or harm.13
Recently, the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) showed that rhythm control treatment was associated with a lower risk of adverse cardiovascular outcomes than usual care among patients who had recently (within one year) been diagnosed with atrial fibrillation.14 It is unclear whether the results can be generalised to patients in whom rhythm control is initiated later, or to the non-trial clinical setting without the structured protocol based follow-up of randomised trials.
To investigate whether the results of a rhythm control strategy are related to the duration between atrial fibrillation diagnosis and treatment initiation, we examined the association between rhythm control and clinical outcomes in comparison with rate control, stratified by timing of treatment initiation.
Methods
Data sources
This study is a retrospective analysis based on the national health claims database established by the National Health Insurance Service (NHIS) of Korea. A majority (97.1%) of the Korean population mandatorily subscribe to the NHIS, which is a single insurer managed by the Korean government, with the remaining 3% categorised as people requiring medical aid. The database also includes information on the medical aid population, and thus represents the entire Korean population. Sociodemographic, inpatient and outpatient service, prescription, and mortality data are included in the database. All data and materials of the NHIS are accessible to the public on the National Health Insurance Data Sharing Service homepage of the NHIS (http://nhiss.nhis.or.kr). Applications to use NHIS data are reviewed by the inquiry committee of research support and once approved, raw data are provided, on payment of a fee, to the authorised researcher at several permitted sites. This study was approved by the institutional review board of the Yonsei University Health System (4-2016-0179). The requirement for informed consent was waived because personal identification information was removed after cohort generation, in accordance with strict confidentiality guidelines.
Cohort design and study population
This study emulated a randomised controlled trial, comparing the effect of rhythm control and rate control treatment for atrial fibrillation on the risk of cardiovascular outcomes. Details of the trial protocol are presented in supplementary table 1. We identified adults with atrial fibrillation who were treated with rhythm control or rate control strategies between 28 July 2011 and 31 December 2015, and who were older than 75, had a history of a transient ischaemic attack or stroke, or met two of the following criteria: age >65, female sex, heart failure, hypertension, diabetes mellitus, previous myocardial infarction, or chronic kidney disease, using a similar inclusion period and criteria as the EAST-AFNET 4 trial.14
Atrial fibrillation was defined according to the ICD-10 (international classification of diseases, 10th revision) code I48. The diagnosis of atrial fibrillation has been previously validated in the NHIS database with a positive predictive value of 94.1%.15 We used a new user and intention to treat design for rhythm or rate control treatments. New use was defined as having no previous records of prescription or procedure of interest in the database. The NHIS database includes information on drug prescription of the entire Korean population from 1 January 2002, which enables a minimum of 9.5 year look back period before each person’s date of inclusion (the earliest date of inclusion was 28 July 2011).
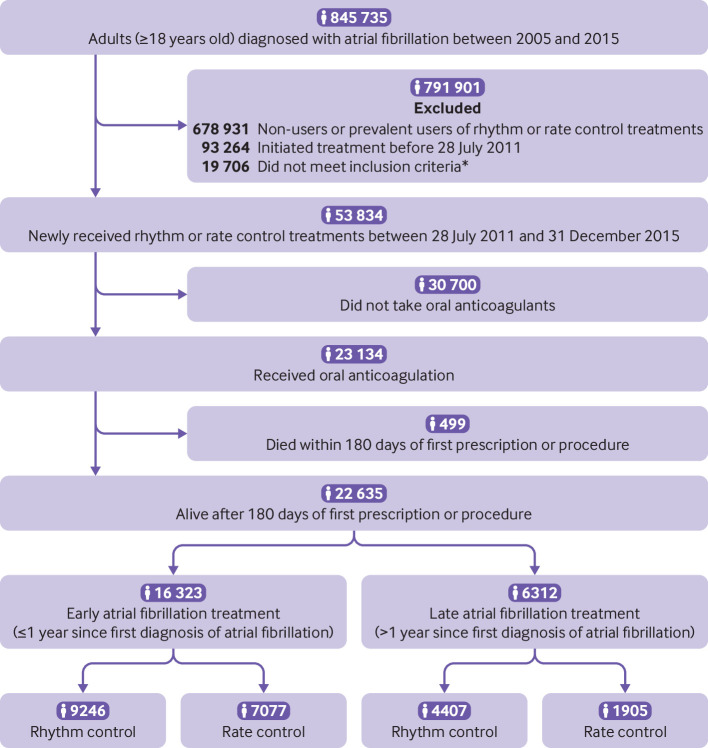
Intention to treat with rhythm control was defined as a prescription of more than 90 days’ supply of any rhythm control drugs in the 180 day period since the first prescription or the performance of an ablation procedure for atrial fibrillation. Intention to treat with rate control was defined as a prescription of more than 90 days’ supply of any rate control drugs in the 180 day period since the first prescription and with no prescription of a rhythm control drug and no ablation within this period. Patients prescribed rhythm control drugs for more than 90 days or those who underwent ablation within the 180 day period since the initiation of rate control drugs were classified as intention to treat with rhythm control. Details of rhythm control and rate control drugs and claim codes for ablation procedures are presented in supplementary table 2. This study excluded those who did not receive a prescription of more than 90 days’ supply of warfarin or a non-vitamin K antagonist oral anticoagulant within the 180 day period since the initiation of rhythm control or rate control drugs or the performance of an ablation procedure for atrial fibrillation, and those who died within 180 days of their first record of a prescription or procedure (fig 1).
Fig 1.
Flow chart of enrolment and analysis of the study population, *Aged >75, had a previous transient ischaemic attack or stroke, or met two of the following criteria: age >65, female, heart failure, hypertension, diabetes mellitus, previous myocardial infarction, or chronic kidney disease
Early treatment of atrial fibrillation was defined as initiation of rhythm or rate control treatments within one year after its diagnosis. Late treatment of atrial fibrillation was defined as initiation of treatment one year after its diagnosis. We used a 2×2 design to compare rhythm control and rate control for both early and late treatment of atrial fibrillation (fig 1).
Outcome and covariates
The primary outcome was a composite of death from cardiovascular causes, ischaemic stroke, admission to hospital due to heart failure, or acute myocardial infarction. We investigated the individual components of the primary composite outcome and the number of nights spent in hospital each year during the individual follow-up—that is, the same endpoints as the EAST-AFNET 4 trial. The composite safety outcome comprised a combination of death from any cause, bleeding (intracranial or gastrointestinal) requiring hospital admission, or prespecified serious adverse events of special interest indicating complications of the rhythm control treatment. Detailed definitions of the outcomes are presented in supplementary table 3. Follow-up of the study outcomes was started 180 days after the first recorded prescription or procedure and lasted until the end of follow-up of the database (31 December 2016) or death.
We obtained information about selected baseline comorbid conditions using inpatient and outpatient hospital diagnoses, and pharmacy claims within the look back period from 1 January 2002 before the start of treatment (supplementary table 2). Baseline relative economic status was determined on the basis of health insurance premiums in the index year. Concurrent use of medication was verified by identifying NHIS database claims and defined as prescription of more than a 90 day supply within the 180 day period since the initiation of rhythm control or rate control treatment.
Statistical analysis
Descriptive statistics were used to characterise baseline characteristics. We used propensity overlap weighting to account for the differences in baseline characteristics between patients who received rhythm control or rate control. A propensity score—that is, the probability of receiving rhythm control, was estimated using logistic regression based on sociodemographics, time since diagnosis of atrial fibrillation, year of treatment initiation, level of care at which the initial prescription was provided, clinical risk scores, medical history, and concurrent medication use (variables in table 1). Continuous variables were modelled as cubic spline functions. The distribution of propensity scores before and after overlap weighting is presented in supplementary figure 1. The overlap weight was calculated as 1 minus the propensity score for patients receiving rhythm control, and as the propensity score for patients receiving rate control, to obtain estimates representing population average treatment effects with a minimised asymptotic variance of the treatment effect and a desirable exact balance property.16 The balance between the treatment groups was evaluated by standardised differences of all baseline covariates using a threshold of 0.1 to indicate imbalance. Weighted incidence rates were calculated as the weighted number of clinical events during the follow-up period divided by 100 person years at risk. We compared the incidence of outcomes using the weighted log rank test and plotted the weighted failure curves. Competing risk regression, according to Fine and Gray, was used to consider all cause death as a competing event when estimating the relative hazards of clinical outcomes.17 Cofactors that had not been balanced by weighting were included as covariates in the competing risk regression. The proportional hazards assumption was tested based on Schoenfeld residuals.18
Table 1.
Baseline characteristics before overlap weighting. Values are presented as median (interquartile range) or number (%)
| Variables | Before overlap weighting | ||||||
|---|---|---|---|---|---|---|---|
| Early atrial fibrillation treatment (≤1 year since diagnosis) | Late atrial fibrillation treatment (>1 year since diagnosis) | ||||||
| Rhythm control (n=9246) | Rate control (n=7077) | ASD (%) | Rhythm control (n=4407) | Rate control (n=1905) | ASD (%) | ||
| Age (years) | 69 (61-75) | 72 (64-78) | 23.0 | 68 (60-74) | 72 (65-78) | 32.1 | |
| Age groups: | |||||||
| <65 | 3143 (34.0) | 1867 (26.4) | 16.6 | 1652 (37.5) | 467 (24.5) | 28.3 | |
| 65-74 | 3585 (38.8) | 2465 (34.8) | 8.2 | 1694 (38.4) | 695 (36.5) | 4.0 | |
| ≥75 | 2518 (27.2) | 2745 (38.8) | 24.8 | 1061 (24.1) | 743 (39.0) | 32.5 | |
| Male | 4892 (52.9) | 3675 (51.9) | 2.0 | 2472 (56.1) | 1161 (60.9) | 9.9 | |
| AF duration (months) | 0.2 (0.0-1.4) | 0.0 (0.0-0.2) | 28.5 | 66.1 (33.6-109.0) | 55.1 (27.7-91.0) | 22.7 | |
| Enrolment year: | |||||||
| 2011 | 580 (6.3) | 448 (6.3) | 0.2 | 361 (8.2) | 133 (7.0) | 4.6 | |
| 2012 | 1514 (16.4) | 1318 (18.6) | 5.9 | 838 (19.0) | 379 (19.9) | 2.2 | |
| 2013 | 1908 (20.6) | 1553 (21.9) | 3.2 | 951 (21.6) | 421 (22.1) | 1.3 | |
| 2014 | 2260 (24.4) | 1589 (22.5) | 4.7 | 1028 (23.3) | 443 (23.3) | 0.2 | |
| 2015 | 2984 (32.3) | 2169 (30.6) | 3.5 | 1229 (27.9) | 529 (27.8) | 0.3 | |
| High tertile of income | 4429 (47.9) | 2981 (42.1) | 11.6 | 2134 (48.4) | 859 (45.1) | 6.7 | |
| No of OPD visits ≥12/year | 7938 (85.9) | 5425 (76.7) | 23.7 | 3874 (87.9) | 1543 (81.0) | 19.2 | |
| Living in metropolitan areas | 4422 (47.8) | 3003 (42.4) | 10.9 | 2051 (46.5) | 775 (40.7) | 11.8 | |
| Level of care initiating treatment: | |||||||
| Tertiary | 5637 (61.0) | 2849 (40.3) | 42.3 | 2933 (66.6) | 784 (41.2) | 52.7 | |
| Secondary | 3304 (35.7) | 3680 (52.0) | 33.2 | 1357 (30.8) | 924 (48.5) | 36.8 | |
| Primary | 305 (3.3) | 548 (7.7) | 19.6 | 117 (2.7) | 197 (10.3) | 31.6 | |
| Risk scores: | |||||||
| CHA2DS2-VASc score | 4 (3-5) | 4 (3-5) | 3.1 | 4 (3-6) | 4 (3-6) | 3.3 | |
| mHAS-BLED score* | 2 (2-3) | 2 (2-3) | 21.4 | 3 (2-4) | 3 (2-3) | 3.7 | |
| Charlson comorbidity index | 4 (2-6) | 3 (1-5) | 34.3 | 5 (3-7) | 4 (3-6) | 18.1 | |
| Hospital frailty risk score | 2.4 (0.0-6.2) | 2.5 (0.0-6.5) | 4.1 | 3.6 (0.8-8.0) | 4.0 (0.8-9.2) | 8.5 | |
| Medical history: | |||||||
| Heart failure | 4534 (49.0) | 3885 (54.9) | 11.7 | 2897 (65.7) | 1048 (55.0) | 22.1 | |
| Previous admission to hospital for heart failure | 1083 (11.7) | 1167 (16.5) | 13.8 | 752 (17.1) | 201 (10.6) | 19.0 | |
| Hypertension | 7796 (84.3) | 4539 (64.1) | 47.4 | 4127 (93.6) | 1555 (81.6) | 37.1 | |
| Diabetes | 2893 (31.3) | 1780 (25.2) | 13.7 | 1443 (32.7) | 530 (27.8) | 10.7 | |
| Dyslipidaemia | 7937 (85.8) | 5320 (75.2) | 27.2 | 4053 (92.0) | 1614 (84.7) | 22.7 | |
| Ischaemic stroke | 2841 (30.7) | 2400 (33.9) | 6.8 | 1582 (35.9) | 895 (47.0) | 22.6 | |
| Transient ischaemic attack | 1040 (11.2) | 570 (8.1) | 10.8 | 603 (13.7) | 215 (11.3) | 7.3 | |
| Intracranial bleeding | 203 (2.2) | 163 (2.3) | 0.7 | 184 (4.2) | 86 (4.5) | 1.7 | |
| Myocardial infarction | 809 (8.7) | 408 (5.8) | 11.5 | 701 (15.9) | 197 (10.3) | 16.5 | |
| Peripheral arterial disease | 1464 (15.8) | 758 (10.7) | 15.1 | 899 (20.4) | 318 (16.7) | 9.5 | |
| Valvular heart disease | 795 (8.6) | 725 (10.2) | 5.6 | 773 (17.5) | 322 (16.9) | 1.7 | |
| Chronic kidney disease | 689 (7.5) | 312 (4.4) | 12.9 | 424 (9.6) | 116 (6.1) | 13.2 | |
| Proteinuria | 673 (7.3) | 452 (6.4) | 3.5 | 368 (8.4) | 161 (8.5) | 0.4 | |
| Hyperthyroidism | 1147 (12.4) | 552 (7.8) | 15.3 | 927 (21.0) | 199 (10.4) | 29.4 | |
| Hypothyroidism | 1311 (14.2) | 667 (9.4) | 14.8 | 866 (19.7) | 238 (12.5) | 19.6 | |
| Malignancy | 2251 (24.3) | 1510 (21.3) | 7.2 | 1216 (27.6) | 557 (29.2) | 3.7 | |
| COPD | 2804 (30.3) | 2108 (29.8) | 1.2 | 1667 (37.8) | 668 (35.1) | 5.7 | |
| Chronic liver disease | 4074 (44.1) | 2578 (36.4) | 15.6 | 2256 (51.2) | 810 (42.5) | 17.4 | |
| Hypertrophic cardiomyopathy | 185 (2.0) | 77 (1.1) | 7.4 | 126 (2.9) | 17 (0.9) | 14.5 | |
| Osteoporosis | 3341 (36.1) | 2469 (34.9) | 2.6 | 1589 (36.1) | 685 (36.0) | 0.2 | |
| Sleep apnoea | 57 (0.6) | 26 (0.4) | 3.6 | 42 (1.0) | 8 (0.4) | 6.5 | |
| Concurrent drugs:† | |||||||
| Oral anticoagulant | 9246 (100.0) | 7077 (100.0) | <0.1 | 4407 (100.0) | 1905 (100.0) | <0.1 | |
| Warfarin | 7312 (79.1) | 5881 (83.1) | 10.3 | 3638 (82.6) | 1644 (86.3) | 10.4 | |
| NOAC | 2471 (26.7) | 1594 (22.5) | 9.8 | 993 (22.5) | 361 (19.0) | 8.8 | |
| β blocker | 4498 (48.6) | 5148 (72.7) | 50.9 | 2026 (46.0) | 1333 (70.0) | 50.1 | |
| Non-DHP CCB | 1191 (12.9) | 1117 (15.8) | 8.3 | 568 (12.9) | 260 (13.6) | 2.2 | |
| Digoxin | 675 (7.3) | 2401 (33.9) | 69.7 | 431 (9.8) | 526 (27.6) | 47.0 | |
| Aspirin | 2033 (22.0) | 1311 (18.5) | 8.6 | 982 (22.3) | 351 (18.4) | 9.6 | |
| P2Y12 inhibitor | 873 (9.4) | 602 (8.5) | 3.3 | 406 (9.2) | 157 (8.2) | 3.4 | |
| Statin | 4191 (45.3) | 3090 (43.7) | 3.4 | 2022 (45.9) | 862 (45.2) | 1.3 | |
| DHP CCB | 2080 (22.5) | 869 (12.3) | 27.2 | 817 (18.5) | 301 (15.8) | 7.3 | |
| ACEI/ARB | 5063 (54.8) | 3747 (52.9) | 3.6 | 2266 (51.4) | 1020 (53.5) | 4.3 | |
| Loop/thiazide diuretics | 3626 (39.2) | 3779 (53.4) | 28.7 | 1910 (43.3) | 936 (49.1) | 11.6 | |
| K+ sparing diuretics | 1255 (13.6) | 1719 (24.3) | 27.6 | 715 (16.2) | 386 (20.3) | 10.5 | |
| α blocker | 193 (2.1) | 124 (1.8) | 2.4 | 97 (2.2) | 45 (2.4) | 1.1 | |
ACEI=angiotensin converting enzyme inhibitor; AF=atrial fibrillation; ARB=angiotensin receptor blocker; ASD=absolute standardised difference; CCB=calcium channel blockers; COPD=chronic obstructive pulmonary disease; DHP=dihydropyridine; NOAC=non-vitamin K antagonist oral anticoagulant.
Modified HAS-BLED=hypertension, 1 point; age >65 years, 1 point; history of stroke, 1 point; history of bleeding or predisposition, 1 point; liable international normalised ratio, not assessed; ethanol or drug abuse, 1 point; drug predisposing to bleeding, 1 point.
Defined as a prescription fill of >90 days within the 180 day after the first prescription for rhythm or rate control drugs or an ablation procedure for atrial fibrillation.
We developed a predictive model to determine the benefit to harm ratio of rhythm control versus rate control considering that rhythm control might be accompanied by additional, clinically significant, serious adverse events, using a method similar to that of Phillips et al.19 In each weighted cohort receiving early or late treatment, we used prespecified serious adverse events (supplementary table 3) as a robust measure of harm and employed Poisson regression to determine the average number of predicted events from four models: rhythm control using primary composite outcome and person days; rhythm control using prespecified serious adverse events and person days; rate control using primary composite outcome and person days; and rate control using prespecified serious adverse events and person days. The analysis accounted for the primary composite outcome and the first serious adverse event experienced by a participant; recurrent events were not included in the analysis. For each of the four models, the predicted probability of a primary composite outcome and the predicted probability of a prespecified serious adverse event were determined for each participant. The benefit to harm ratio was then based on the ratio of the difference in the average predicted primary composite outcome to the difference in the average predicted prespecified serious adverse events in the rhythm control and rate control groups. We simulated 1000 random variates of the benefit to harm ratio to calculate standard errors.
To explore the timing dependent effect of rhythm control on primary composite outcome and composite safety outcome, a Cox proportional hazards model was fitted to the entire weighted study population using an interaction term for treatment timing after diagnosis of atrial fibrillation (modelled as a cubic spline) and treatment (rhythm control or rate control strategy). Standard errors were computed using 1000 bootstrap replicates.
Two sided P values of <0.05 were considered significant. Because of the potential for type 1 error due to multiple comparisons, findings for the analyses of secondary outcomes should be interpreted as exploratory. Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) and R version 3.6.0 (The R Foundation, www.R-project.org).
Sensitivity analyses
Firstly, we performed subgroup analyses for the primary composite outcome stratified by sex, age, previous histories of the components of primary composite outcome, hypertension, diabetes, chronic kidney disease, and CHA2DS2-VASc score. Interaction tests were performed for all subgroups. We used the test parameter from the weighting procedure to recreate the overlap weighting. The Cox proportional hazard model was fitted with new weights, and the interaction term was added for testing. Furthermore, two measures were calculated to examine biological interaction (or additive interaction), including relative excess risk due to interaction and attributable proportion due to interaction.20 The relative excess risk is the excess risk due to interactions relative to risk without exposure, while the attributable proportion is the disease due to interactions among individuals with both exposures. Values of the relative risk due to interaction greater than zero and attributable proportion greater than zero indicated biological interactions.
Secondly, we performed analyses in line with the on treatment principle by censoring patients who switched to another treatment or who discontinued their treatments (censored at the time of switch or discontinuation).
Thirdly, we performed a time varying regression in which treatment (rhythm versus rate) was treated as a time dependent variable considering switches between treatments (supplementary figure 2).
Fourthly, we used one to one propensity score matching (without replacement with a calliper of 0.01) instead of propensity overlap weighting. The balance of covariates after matching is presented in supplementary table 4.
Fifthly, we excluded those with prevalent heart failure, hypertension, or previous myocardial infarction considering the possibility of using β blockers with no intention for rate control.
Sixthly, we defined intention to treat as a prescription of more than a 20 day supply of the drugs in the 30 day period since the first prescription, instead of the 180 day period in the main analyses because such a 180 day observational period necessarily ignores the outcome incidence in the high risk period early in treatment (that is, torsades de pointes a few weeks after initiating antiarrhythmic agents). Follow-up began 30 days after the first recorded prescription or procedure to avoid immortal time bias. Concurrent medication use in the analyses was defined as prescription of more than a 20 day supply within the 30 day period after the initiation of rhythm control or rate control treatment.
Seventhly, the sensitivity of administrative data for the diagnosis of heart failure is reported to be low (≥69% in ≥50% of studies).21 Considering the possibility of a higher prevalence of underdiagnosed heart failure in rate controlled patients than in rhythm controlled patients and its effect on the primary composite outcome, we investigated the risk of a composite outcome not including admission to hospital for heart failure.
Eighthly, we performed “falsification analysis” to measure systematic bias of this study using 35 prespecified falsification endpoints, with true hazard ratios of 1.22 23 Detailed definitions of the falsification endpoints are presented in supplementary table 5.
Ninthly, we conducted a duration analysis, in which cumulative exposure was defined as the total number of days of rhythm control drug supply up to the last day of the prescription. To deal with immortal time bias, T0 in this analysis was set as the end of the last prescription.
Patient and public involvement
The authors had no direct contact information of the study participants because anonymised nationwide data were used in accordance with strict confidentiality guidelines. No patient was involved in developing the hypothesis, specific aims, research questions, or plans for the study’s design or implementation. No patient was involved in the interpretation or writing of the results. There are no plans to disseminate the results of this study to the individual study participants.
Results
Patient characteristics
This study identified 16 323 and 6312 patients undergoing early and late treatment for atrial fibrillation, respectively. Patients treated early were likely to have fewer comorbidities, including heart failure, hypertension, diabetes, vascular disease, and stroke, at the time of treatment initiation than patients treated late (supplementary table 6). Since the first diagnosis of atrial fibrillation, treatment was started after a mean of 1.0 months for those receiving early treatment and a mean of 69.5 months for the group receiving late treatment. The most commonly used rhythm control strategy was the class III drug amiodarone (3692 (39.9%) of 9246 patients in early rhythm control and 1825 (41.4%) of 4407 patients in late rhythm control groups), followed by class Ic drugs (fig 2). Ablation was an initial rhythm control strategy in 145 (1.6%) and 639 (14.5%) patients in early and late rhythm control groups, and was eventually performed during follow-up in 639 (6.9%) and 863 (19.6%) patients in these groups, respectively.
Fig 2.
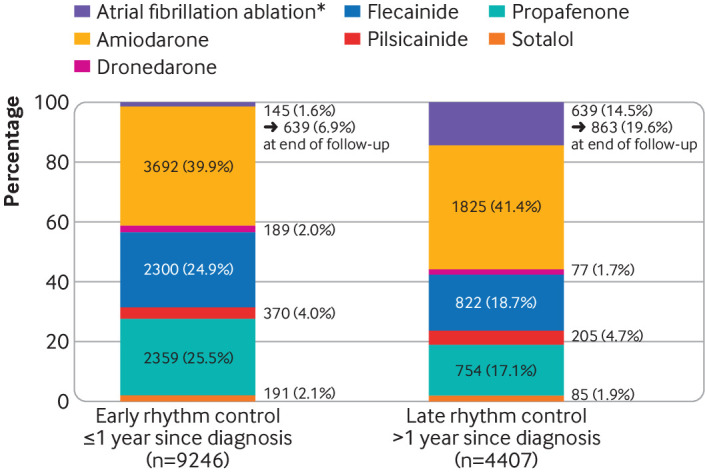
Initial choice of rhythm control treatments. *Catheter ablations performed within 180 days after the initial prescription of rhythm control drugs were classified as initial choices for rhythm control
Early treatment of atrial fibrillation
Rhythm control and rate control treatments were initiated in 9246 and 7077 patients, respectively, within one year after diagnosis of atrial fibrillation. Patients undergoing rhythm control tended to be younger, live in metropolitan areas, have longer duration of atrial fibrillation, and higher incomes and comorbidity indices than rate controlled patients (table 1). After overlap weighting, sociodemographic and clinical characteristics were well balanced between the groups (supplementary table 7).
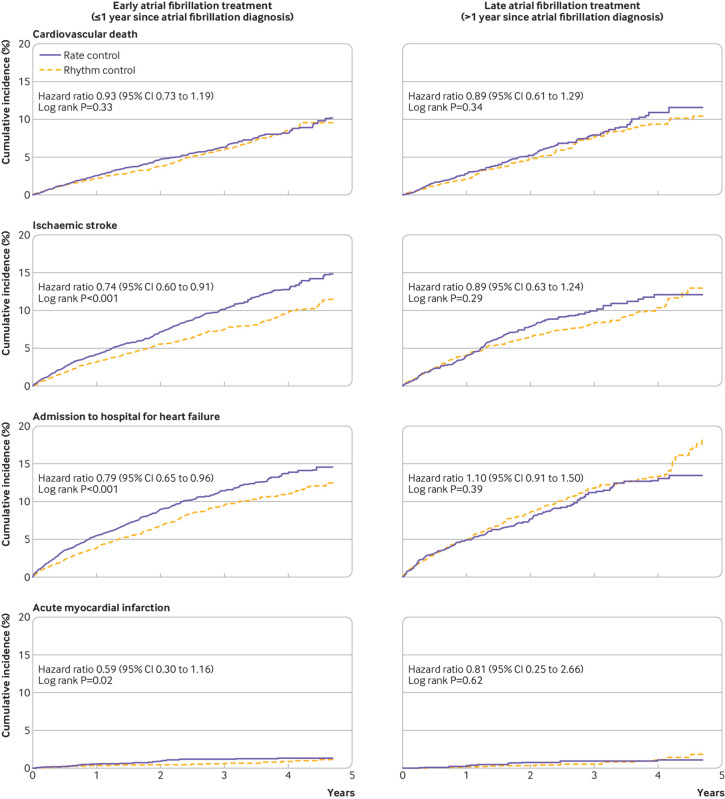
Patients with rhythm control were followed up for a median of 2.1 (interquartile range 1.1-3.3) years and those with rate control for 2.1 (1.1-3.2) years. Early rhythm control was associated with a reduction in the primary composite outcome compared with early rate control (weighted incidence rate 7.42 v 9.25 events per 100 person years; hazard ratio 0.81, 95% confidence interval 0.71 to 0.93; P=0.002; table 2 and fig 3). Among individual components of the primary composite outcome, early rhythm control was associated with lower risks of ischaemic stroke (0.74, 0.60 to 0.91) and admission owing to heart failure (0.79, 0.65 to 0.96; table 2 and fig 4). The mean number of nights spent in the hospital each year was lower in the early rhythm control group than in the early rate control group (26.1 v 30.4 nights per year; P<0.001; table 2).
Table 2.
Efficacy outcomes in patients undergoing rhythm or rate control
| Outcome | Rhythm control | Rate control | Absolute rate difference per 100 person years* (95% CI) |
Weighted hazard ratio (95% CI) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of events | Person years | Event rate* | No of events | Person years | Event rate* | |||||
| Early treatment of atrial fibrillation (≤1 year since the first diagnosis; rhythm control n=9246, rate control n=7077): | ||||||||||
| Primary composite outcome | 1187 | 19 461 | 7.42 | 1366 | 14 354 | 9.25 | −1.82 (−2.91 to −0.73) | 0.81 (0.71 to 0.93) | 0.002 | |
| Components of primary outcome | ||||||||||
| Cardiovascular death | 327 | 20 742 | 2.12 | 404 | 15 905 | 2.30 | −0.18 (−0.72 to 0.36) | 0.93 (0.73 to 1.19) | 0.56 | |
| Ischaemic stroke | 469 | 20 165 | 2.70 | 543 | 15 234 | 3.69 | −1.00 (−1.65 to −0.34) | 0.74 (0.60 to 0.91) | 0.004 | |
| Admission to hospital for heart failure | 541 | 20 030 | 3.33 | 689 | 14 976 | 4.27 | −0.94 (−1.66 to −0.22) | 0.79 (0.65 to 0.96) | 0.02 | |
| Acute myocardial infarction | 52 | 20 685 | 0.23 | 51 | 15 841 | 0.39 | −0.16 (−0.36 to 0.04) | 0.59 (0.30 to 1.16) | 0.13 | |
| Nights spent in hospital/year† | 26.1 (66.2) | 30.4 (72.7) | −4.2 (−6.4 to −2.1) | <0.001 | ||||||
| Late treatment of atrial fibrillation (>1 year since the first diagnosis; rhythm control n=4407, rate control n=1905): | ||||||||||
| Primary composite outcome | 691 | 9602 | 8.67 | 373 | 3926 | 8.99 | −0.32 (−2.19 to 1.54) | 0.97 (0.78 to 1.20) | 0.76 | |
| Components of primary outcome | ||||||||||
| Cardiovascular death | 196 | 10 407 | 2.46 | 128 | 4348 | 2.77 | −0.31 (−1.28 to 0.66) | 0.89 (0.61 to 1.29) | 0.53 | |
| Ischaemic stroke | 246 | 10 063 | 3.13 | 161 | 4144 | 3.54 | −0.41 (−1.53 to 0.71) | 0.89 (0.63 to 1.24) | 0.49 | |
| Admission to hospital for heart failure | 380 | 9926 | 4.24 | 167 | 4122 | 3.89 | 0.36 (−0.88 to 1.60) | 1.10 (0.91 to 1.50) | 0.53 | |
| Acute myocardial infarction | 26 | 10 382 | 0.23 | 13 | 4332 | 0.28 | −0.06 (−0.36 to 0.25) | 0.81 (0.25 to 2.66) | 0.73 | |
| Nights spent in hospital/year† | 29.3 (70.9) | 30.3 (72.4) | −1.0 (−4.9 to 2.8) | 0.60 | ||||||
Weighted incidence rate (per 100 person years) comparing rhythm and rate controlled patients after overlap weighting was applied.
Results are reported as mean (standard deviation) and the difference between the treatment groups was estimated using a two sample weighted t test.
Fig 3.
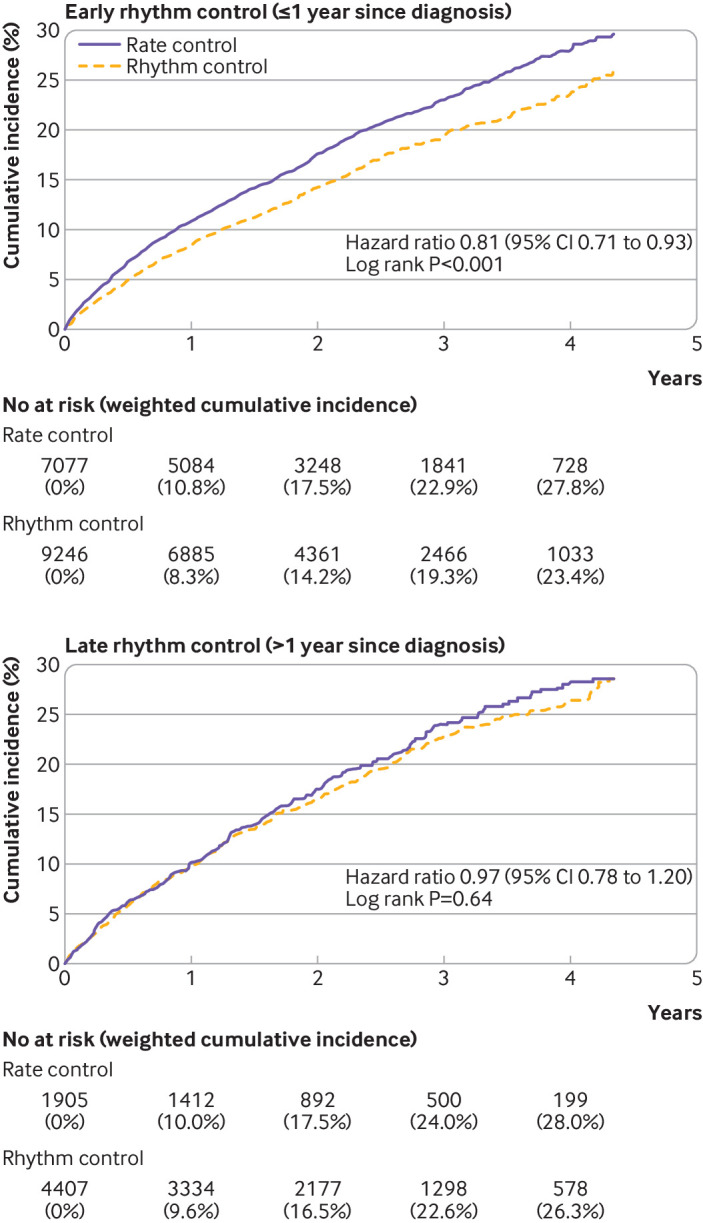
Weighted cumulative incidence curves for the primary composite outcome in early and late treatments for atrial fibrillation. CI=confidence interval
Fig 4.
Weighted cumulative incidence curves for individual components of the primary composite outcome in early and late atrial fibrillation treatments. CI=confidence interval
No significant difference was found in the risk of the composite safety outcome between early rhythm control and rate control (weighted incident rate 9.56 v 9.54 events per 100 person years; hazard ratio 1.00, 95% confidence interval 0.88 to 1.23; table 3).
Table 3.
Safety outcomes (presented as rates per 100 person years after application of overlap weighting) in patients undergoing rhythm or rate control
| Outcome | Early AF treatment (≤1 year since diagnosis) | Late AF treatment (>1 year since diagnosis) | |||||
|---|---|---|---|---|---|---|---|
| Rhythm control (n=9246) | Rate control (n=7077) | Absolute rate difference per 100 person years (95% CI) | Rhythm control (n=4407) | Rate control (n=1905) | Absolute rate difference per 100 person years (95% CI) | ||
| Composite safety outcome | 9.56 | 9.54 | 0.01 (−1.14 to 1.17)* | 11.13 | 9.83 | 1.30 (−0.72 to 3.32)† | |
| All cause death | 4.64 | 5.43 | −0.79 (−1.60 to 0.02) | 5.57 | 5.99 | −0.42 (−1.86 to 1.02) | |
| Intracranial bleeding | 0.86 | 0.90 | −0.04 (−0.38 to 0.29) | 0.61 | 1.06 | −0.45 (−1.00 to 0.10) | |
| Gastrointestinal bleeding | 1.78 | 2.30 | −0.52 (−1.04 to 0.00) | 2.45 | 2.78 | −0.33 (−1.32 to 0.66) | |
| Serious adverse event related to rhythm control: | |||||||
| Cardiac tamponade | 0.15 | 0.07 | 0.07 (−0.05 to 0.19) | 0.24 | 0.07 | 0.17 (−0.07 to 0.41) | |
| Syncope | 1.69 | 1.41 | 0.28 (−0.17 to 0.74) | 2.00 | 1.07 | 0.93 (0.17 to 1.68) | |
| Sick sinus syndrome | 1.29 | 0.47 | 0.82 (0.47 to 1.17) | 1.48 | 0.35 | 1.12 (0.54 to 1.71) | |
| Atrioventricular block | 0.50 | 0.24 | 0.25 (0.03 to 0.48) | 0.33 | 0.24 | 0.09 (−0.24 to 0.41) | |
| Pacemaker implantation | 0.54 | 0.29 | 0.25 (0.01 to 0.49) | 0.53 | 0.22 | 0.31 (−0.06 to 0.69) | |
| Sudden cardiac arrest | 0.69 | 0.61 | 0.08 (−0.21 to 0.37) | 0.56 | 0.72 | −0.15 (−0.63 to 0.33) | |
AF=atrial fibrillation.
The risk of composite safety outcome did not significantly differ according to treatment strategies with a weighted hazard ratio of 1.00 (95% confidence interval 0.88 to 1.23).
The risk of composite safety outcome did not significantly differ according to treatment strategies with a weighted hazard ratio of 1.13 (95% confidence interval 0.93 to 1.37).
Late treatment of atrial fibrillation
Rhythm control and rate control treatments were initiated in 4407 and 1905 patients with atrial fibrillation, respectively, more than one year after their diagnosis. Similar to the observations in the group receiving early treatment, rhythm controlled patients were likely to be younger, have longer duration of atrial fibrillation, and higher comorbidity indices than those receiving rate control (table 1). After weighting, all variables were balanced between the groups (supplementary table 7).
Follow-up was a median of 2.2 (interquartile range 1.2-3.3) years for the rhythm controlled patients and a median of 2.2 (1.2-3.4) years for the rate controlled patients. No difference in the risk of the primary composite outcome was seen between late rhythm control and late rate control (weighted incidence rate 8.67 v 8.99 events per 100 person years; hazard ratio 0.97, 95% confidence interval 0.78 to 1.20; P=0.76; table 2 and fig 3). We found no differences in the risks of individual components of the primary composite outcome between the two treatment strategies (table 2 and fig 4). The mean number of nights spent in the hospital each year were similar between the treatment groups (29.3 v 30.3 nights per year; P=0.60; table 2).
We found no significant difference in the risk of the composite safety outcome between the late rhythm control and rate control groups (11.13 v 9.83 events per 100 person years; weighted hazard ratio 1.13, 95% confidence interval 0.93 to 1.37; table 3).
Sensitivity analyses
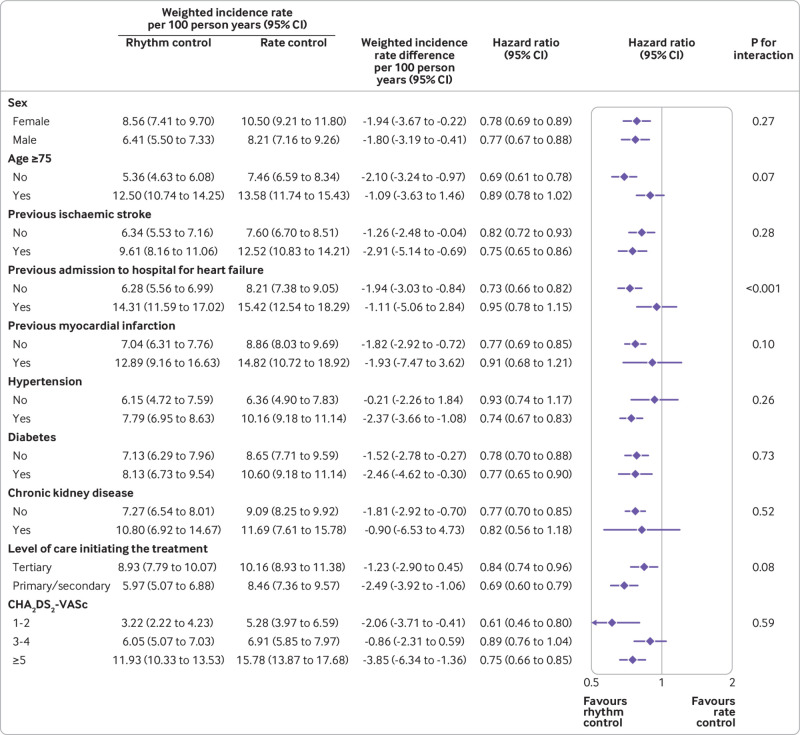
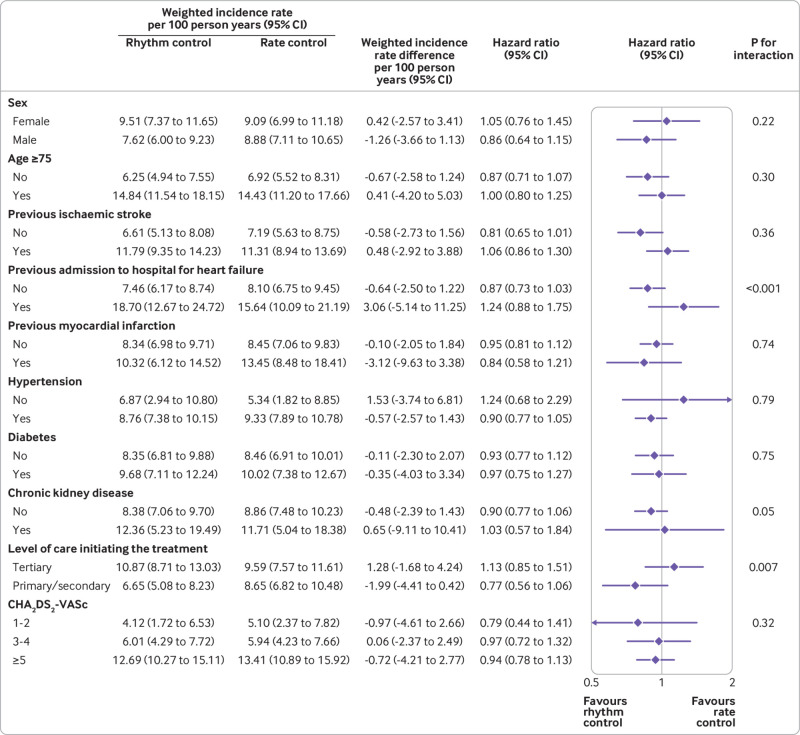
Subgroup analyses showed that there were no significant interactions between the protective associations of early rhythm control with the primary composite outcome and sex, age, histories of stroke or myocardial infarction, comorbid diseases, and estimated stroke risk (fig 5 and fig 6). The lower risk associated with early rhythm control versus rate control was more pronounced in those who had never been admitted for heart failure (P for interaction <0.001). Biological interactions are presented in supplementary table 8.
Fig 5.
Subgroup analyses for the primary composite outcome in early treatments of atrial fibrillation. CI=confidence interval
Fig 6.
Subgroup analyses for the primary composite outcome in late treatments of atrial fibrillation. CI=confidence interval
Some patients switched between treatment strategies; in early treatment, 557 (7.9%) of 7077 patients from rate control switched to rhythm control treatment, whereas 4078 (44.1%) of 9246 patients switched from rhythm control to rate control. In the late treatment group, 119 (6.2%) of 1905 patients switched from rate control to rhythm control, whereas 1993 (45.2%) of 4407 patients switched from rhythm control to rate control (supplementary table 9). The results from the on treatment analyses (supplementary table 10) and the time varying regression analyses (supplementary table 11) were consistent with the main findings.
Performing one to one propensity matching (supplementary table 12), excluding those with prevalent heart failure, hypertension, or myocardial infarction (supplementary table 13), using the 30 day enrolment period instead of the 180 day point after initiation of treatments (supplementary table 14), and investigating composite outcomes not including admission to hospital for heart failure (supplementary table 15) generated results that were similar to the main findings.
In the analyses of 35 falsification endpoints, the 95% confidence intervals of the associations of rhythm control with each endpoint covered one in 32 (91.4) endpoints in early treatment and 32 (91.4%) endpoints in late treatment groups, respectively (supplementary figure 3 and supplementary table 16). A graded association was found between duration of exposure to rhythm control drugs and lower risks of the primary composite outcome (supplementary tables 17 and 18).
Net benefit of rhythm control according to timing of treatment initiation
The benefit to harm ratio of rhythm control relative to rate control was greater than one in the groups receiving early treatment, suggesting that patients undergoing early rhythm control treatment would receive greater benefit than harm (fig 7). The 95% confidence interval of the ratio in groups with late treatment included one, suggesting that there was no significant difference in the benefit and harm of late rhythm control treatments relative to late rate control.
Fig 7.
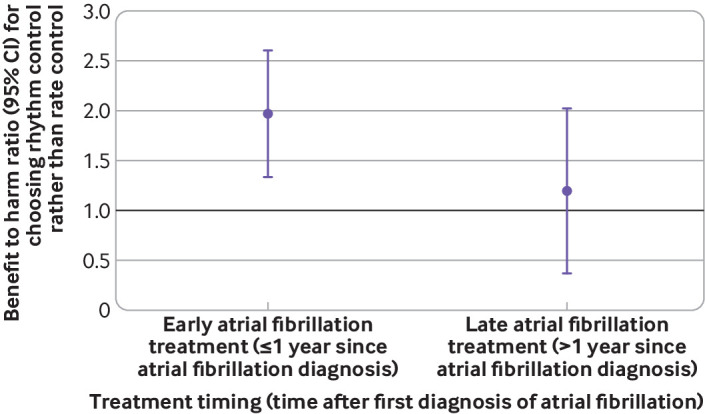
Benefit to harm ratios of rhythm control compared with rate control according to treatment timing. The ratios >1 indicate positive net benefit. CI=confidence interval
Treatment timing and outcomes
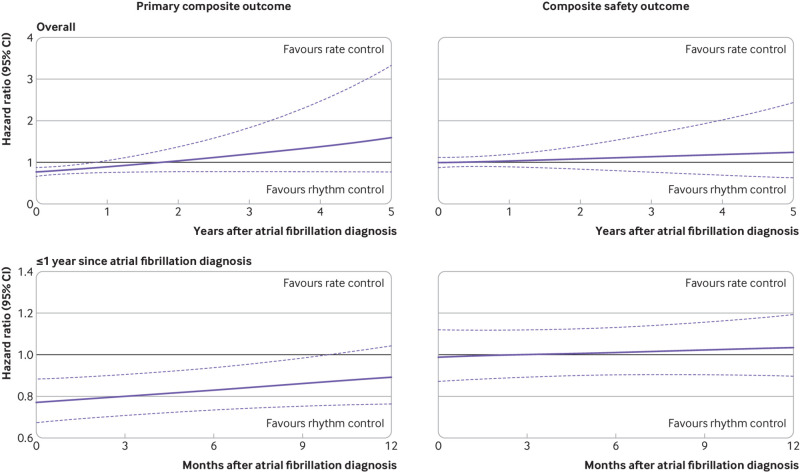
With later timing of treatment initiation, a linearly increasing association was found between rhythm control and adverse outcomes in comparison with rate control. Rhythm control, compared with rate control, was associated with a lower risk of the primary composite outcome within one year after the first diagnosis of atrial fibrillation, and the point estimate exceeded one between the timing of treatment initiation of one and two years after diagnosis (fig 8). Within one year after diagnosis of atrial fibrillation, earlier initiation of treatment was associated with more favourable outcome with rhythm control than with rate control (fig 8). The risk of composite safety outcome did not differ between rhythm and rate control strategies regardless of the timing of treatment initiation.
Fig 8.
Relation between treatment timing and risk of clinical outcomes for rhythm control or rate control in the overall period and within one year after the first diagnosis of atrial fibrillation. The y axis shows hazard ratios associated with rhythm control compared with rate control. The black horizontal lines indicate a hazard ratio of 1, corresponding to an equal risk of outcomes in patients treated with rhythm and rate control. Dashed purple lines show the 95% confidence interval (CI)
Discussion
In this study, the strategy of initiating rhythm control treatment in patients with early atrial fibrillation (within one year after the diagnosis) was associated with a decreased risk of death from cardiovascular causes, ischaemic stroke, admission to hospital for heart failure, or acute myocardial infarction in comparison with rate control (absolute decrease in risk 1.82 events per 100 person years), consistent with the findings of the EAST-AFNET 4 trial. Secondly, a rhythm control strategy initiated one year after diagnosis of atrial fibrillation was not significantly associated with the risk of outcomes. Thirdly, earlier timing of initiating rhythm control was linearly associated with more favourable cardiovascular outcomes in comparison with rate control. Fourthly, for safety outcomes, no differences were found between the rhythm control and rate control strategies, regardless of the timing of treatment
Comparison with other studies
In the meta-analyses of previous randomised trials comparing rhythm control with rate control strategies, no significant difference was seen in all cause mortality or thromboembolic outcomes between the two strategies, although a non-significant trend in favour of a rate control approach was found.24 25 26 27 In our study, early rhythm control was associated with less frequent cardiovascular events, which might be largely attributable to a lower risk of stroke in the rhythm control group (absolute decrease in risk, 1.00 events per 100 person years). This result is in line with a post hoc analysis of the ATHENA trial, which showed that dronedarone was associated with a significant reduction in the risk of ischaemic and haemorrhagic stroke.28 In addition, using a population based observational cohort, Tsadok et al reported that rhythm control treatment started within one week after discharge of patients from hospital was associated with lower rates of stroke/transient ischaemic attack than with rate control treatment.29
The association between early rhythm control and lower mortality in this study was less prominent than that shown in EAST-AFNET 4, which might be explained by a relatively shorter follow-up (median 2.1 v 5.1 years in EAST-AFNET 4). It is worth noting that Ionescu-Ittu et al found little difference in mortality within four years of initiating treatment between patients starting rhythm control treatment and those starting rate control treatment; however, the reduction in mortality reached 23% with rhythm control after eight years of follow-up compared with rate control.30
The overall rates of cardiovascular events and the nights spent in hospital during follow-up were much higher in this study than in the EAST-AFNET 4 trial. Although eligible criteria similar to those of EAST-AFNET 4 were used in this study, the CHA2DS2-VASc score of participants was higher in this study (early rhythm control: mean 4.4 (median 4), early rate control: mean 4.5 (median 4)) than in the EAST-AFNET 4 trial (rhythm control: mean 3.4, rate control: mean 3.3). Furthermore, the prevalence of heart failure was much higher in this study (early rhythm control: 49.0%, early rate control: 54.9%) than in the EAST-AFNET 4 trial (rhythm control: 28.4%, rate control: 28.8%). These results might suggest a substantial level of comorbidities in patients with atrial fibrillation from routine clinical practice.
The results of the Permanent Atrial fibriLLAtion outcome Study (PALLAS) using dronedarone in addition to standard treatment indicated that dronedarone increased the rates of heart failure, stroke, and death from cardiovascular causes in patients with permanent atrial fibrillation who were at risk for major vascular events.31 Consistently, we observed non-significant trends in favour of a rate control approach when the treatments were started late (about after two years since the first diagnosis of atrial fibrillation).
Meaning of the study
The precise mechanisms by which early rhythm control confers benefits are not assessed by this clinical observational study, but might reflect an early effect on electrical and substrate remodelling.32 Also, patients undergoing rhythm control might have had a more careful structured follow-up; however, in that case, we would have seen benefits in both early and late rhythm control subgroups. Contemporary drug management for rhythm control also uses antiarrhythmic drugs that are better tolerated and safer than those used (that is, class Ia agents) in the rate versus rhythm control trials of two to three decades ago.10 Although a rhythm control strategy in this study included all major antiarrhythmic drugs and ablation, both dronedarone and ablation were not popular choices in both early (dronedarone: 189 (2.0%); ablation: 145 (1.6%) of 9246 patients) and late rhythm control strategies (dronedarone: 77 (1.7%); ablation: 639 (14.5%) of 4407 patients; fig 2). These findings suggest that favourable outcomes of rhythm control, which were seen only in patients with atrial fibrillation starting treatment shortly after diagnosis, could not be fully explained by the use of a promising drug or ablation, neither of which were available in previous trials, and the important factor might be timing of the start of treatment. Furthermore, the effect of early treatment on better outcomes might support initiatives to implement screening for atrial fibrillation among asymptomatic individuals in clinical practice.
Early or late rhythm control was not associated with any alteration in composite safety outcomes compared with rate control. Early rhythm control was associated with fewer nights spent in the hospital, and no difference was found between the late rhythm and rate control treatment groups. These results might provide reassurance in view of the excess admissions to hospital associated with rhythm control treatment reported in two previous large trials.8 10
Strength and limitations of this study
This study has several strengths, including the use of nationwide large scale data, which were captured during routine clinical practice. Thus, selection bias was minimised. We applied a new user design with an active comparator control to emulate a head to head randomised clinical trial,33 performed propensity score overlap weighting, and tested the falsification endpoints to estimate the risk of cardiovascular outcomes more accurately.
The study has several limitations. In this claims based database, the burden of atrial fibrillation (rhythm status) was not evaluated; thus its role as a contributor to outcomes remains unknown. We defined diagnosis of atrial fibrillation and the use of ablation using only ICD-10 or claim codes, and thus data for types or symptoms of atrial fibrillation (paroxysmal v non-paroxysmal; symptomatic v asymptomatic) were not available. Our observational study findings cannot be used to establish causal associations, and residual confounding might persist even after propensity score weighting or matching. Furthermore, consistency in the results across various sensitivity analyses was identified, despite treatment options being heterogeneous (typical of “real world” observational studies).
We were unable to determine the exact reasons for choosing rhythm control rather than rate control, which could introduce potential bias, and unmeasured confounders might have influenced the findings (eg, quality of anticoagulation). Nonetheless, the results from the falsification analysis showed that significant systematic bias is less likely. We identified sufficient overlaps of propensity scores between the groups, which represents the existence of equipoise between the two treatment strategies.34
The proportions of ablation as initial choice for rhythm control were low. Ablation is permitted and reimbursed by the national health insurance only in patients with documented atrial fibrillation after undergoing antiarrhythmic drug treatment for more than six weeks. As first line treatment, ablation is reimbursed only in those who cannot tolerate antiarrhythmic drugs owing to tachycardia-bradycardia syndrome or other conditions. Thus the proportion of patients treated with catheter ablation at baseline (within 180 days after the initiation of rhythm control) is low (1.6% in the early treatment group and 14.5% in the late treatment group). The proportions were increased, however, to 6.9% and 19.6% at the end of follow-up in the groups, respectively, which were comparable to the 7% (as an initial choice) and 19.4% (at two years after randomisation) in the EAST-AFNET 4 trial.14
We were unable to assess the effects of lifestyle factors, such as obesity, alcohol intake, and physical activity. A continuum of unhealthy lifestyle factors might contribute to the progression of disease and worse cardiovascular outcomes among patients diagnosed with atrial fibrillation.1
We were also unable to investigate the association between treatment strategies and patients’ quality of life, which was often included as a secondary outcome in most trials, including the EAST-AFNET 4 trial.
Owing to the active comparator design of our study, asymptomatic patients with atrial fibrillation who did not require treatment might have been excluded. In addition, owing to the new user design, in which prevalent drug users at the time of atrial fibrillation diagnosis were excluded, the proportion of treatment strategies chosen among patients with atrial fibrillation in this study cannot fully reflect preferences in real world clinical practice.
Lastly, although administrative databases are increasingly used for clinical research, such studies are potentially susceptible to inaccuracies arising from coding errors. To minimise this problem, we applied the definition that we had validated in previous studies using the Korean NHIS database.4 15 35 36
Conclusions
In this nationwide population based study, early initiation of a rhythm control strategy was associated with less frequent cardiovascular events than rate control in patients with early atrial fibrillation (within one year after the diagnosis), but the association was not seen in patients who had had atrial fibrillation for more than one year. Within one year since the diagnosis of atrial fibrillation, initiating rhythm control as early as possible was associated with more favourable cardiovascular outcomes than with rate control. These results suggest that the favourable effects of rhythm control over rate control seen in the EAST-AFNET 4 trial might be attributable to the inclusion of patients with early atrial fibrillation, and initiation of rhythm control treatment earlier is needed to maximise the efficacy.
What is already known on this topic
Data on the prognostic effect of rhythm control in comparison with rate control treatment in patients with atrial fibrillation are inconclusive, with no clear indication of benefit or harm
The recently published EAST-AFNET 4 trial showed that rhythm control treatment was associated with a lower risk of adverse cardiovascular outcomes than usual care among patients who had recently (within one year) been diagnosed with atrial fibrillation
What this study adds
Early initiation of a rhythm control strategy was associated with less frequent cardiovascular events than rate control in patients who had recently been diagnosed with atrial fibrillation (within one year), but the association was not seen in patients who had had atrial fibrillation for more than one year
Within one year since the diagnosis of atrial fibrillation, initiating rhythm control as early as possible was associated with more favourable cardiovascular outcomes compared with rate control
Acknowledgments
We thank the National Health Insurance Service of Korea for providing invaluable data.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-18 and figures 1-3
Contributors: DK and P-SY contributed equally to this work. BJ and GYHL are joint senior authors and contributed to the conception and design of the work and critical revision of the manuscript. DK contributed to the conception and design of the work, interpretation of data, and drafting of the manuscript. P-SY and EJ contributed to the acquisition and analysis of data. SCY, J-HS, HTY, T-HK, H-NP, and M-HL contributed to the conception and design of the work and revision of the manuscript. All authors approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by grants from the Korean Healthcare Technology research and development project funded by the Korean Ministry of Health and Welfare (HI15C1200, HC19C0130), and by a CMB-Yuhan research grant from the Yonsei University College of Medicine (6-2019-0124). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; GYHL has served as a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo and as a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees have been received directly or personally. BJ has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi-Sankyo and received research funds from Medtronic and Abbott. No fees have been received directly or personally. The remaining authors have no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the institutional review board of Yonsei University Health System (No 4-2016-0179), which waived the requirement for informed consent as only deidentified data were used in this study.
Data sharing: Data sharing is not possible because of legislation from the Korean government. Additional data are available through approval and oversight by the Korean National Health Insurance Service.
The lead author (BJ) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: There are no plans to disseminate the results to the individual study participants or the relevant patient community. Since we used deidentified data in this study, we had no direct contact information of the individual study participants for disseminating the study results.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hindricks G, Potpara T, Dagres N, et al. ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373-498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2. Marijon E, Le Heuzey JY, Connolly S, et al. RE-LY Investigators . Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192-201. 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 3. Camm AJ, Amarenco P, Haas S, et al. XANTUS Investigators . XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016;37:1145-53. 10.1093/eurheartj/ehv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim D, Yang PS, Jang E, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart 2018;104:2010-7. 10.1136/heartjnl-2017-312930 [DOI] [PubMed] [Google Scholar]
- 5. Roy D, Talajic M, Dorian P, et al. Canadian Trial of Atrial Fibrillation Investigators . Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med 2000;342:913-20. 10.1056/NEJM200003303421302 [DOI] [PubMed] [Google Scholar]
- 6. Mark DB, Anstrom KJ, Sheng S, et al. CABANA Investigators . Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275-85. 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlsson J, Miketic S, Windeler J, et al. STAF Investigators . Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003;41:1690-6. 10.1016/S0735-1097(03)00332-2 [DOI] [PubMed] [Google Scholar]
- 8. Roy D, Talajic M, Nattel S, et al. Atrial Fibrillation and Congestive Heart Failure Investigators . Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667-77. 10.1056/NEJMoa0708789 [DOI] [PubMed] [Google Scholar]
- 9. Van Gelder IC, Hagens VE, Bosker HA, et al. Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group . A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-40. 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 10. Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-33. 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 11. Marrouche NF, Brachmann J, Andresen D, et al. CASTLE-AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417-27. 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 12. Hohnloser SH, Crijns HJ, van Eickels M, et al. ATHENA Investigators . Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668-78. 10.1056/NEJMoa0803778 [DOI] [PubMed] [Google Scholar]
- 13. Willems S, Meyer C, de Bono J, et al. Cabins, castles, and constant hearts: rhythm control therapy in patients with atrial fibrillation. Eur Heart J 2019;40:3793-3799c. 10.1093/eurheartj/ehz782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhof P, Camm AJ, Goette A, et al. EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305-16. 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 15. Lee SS, Ae Kong K, Kim D, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J 2017;38:2599-607. 10.1093/eurheartj/ehx316 [DOI] [PubMed] [Google Scholar]
- 16. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc 2018;113:390-400. 10.1080/01621459.2016.1260466 . [DOI] [Google Scholar]
- 17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. 10.1080/01621459.1999.10474144 . [DOI] [Google Scholar]
- 18. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515-26. 10.1093/biomet/81.3.515 . [DOI] [Google Scholar]
- 19. Phillips RA, Xu J, Peterson LE, Arnold RM, Diamond JA, Schussheim AE. Impact of cardiovascular risk on the relative benefit and harm of intensive treatment of hypertension. J Am Coll Cardiol 2018;71:1601-10. 10.1016/j.jacc.2018.01.074 [DOI] [PubMed] [Google Scholar]
- 20. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575-9. 10.1007/s10654-005-7835-x [DOI] [PubMed] [Google Scholar]
- 21. McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One 2014;9:e104519. 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383-8. 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. You SC, Rho Y, Bikdeli B, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA 2020;324:1640-50. 10.1001/jama.2020.16167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Denus S, Sanoski CA, Carlsson J, Opolski G, Spinler SA. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med 2005;165:258-62. 10.1001/archinte.165.3.258 [DOI] [PubMed] [Google Scholar]
- 25. Kumana CR, Cheung BM, Cheung GT, Ovedal T, Pederson B, Lauder IJ. Rhythm vs. rate control of atrial fibrillation meta-analysed by number needed to treat. Br J Clin Pharmacol 2005;60:347-54. 10.1111/j.1365-2125.2005.02449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Testa L, Biondi-Zoccai GG, Dello Russo A, Bellocci F, Andreotti F, Crea F. Rate-control vs. rhythm-control in patients with atrial fibrillation: a meta-analysis. Eur Heart J 2005;26:2000-6. 10.1093/eurheartj/ehi306 [DOI] [PubMed] [Google Scholar]
- 27. Caldeira D, David C, Sampaio C. Rate vs rhythm control in patients with atrial fibrillation and heart failure: a systematic review and meta-analysis of randomised controlled trials. Eur J Intern Med 2011;22:448-55. 10.1016/j.ejim.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 28. Connolly SJ, Crijns HJ, Torp-Pedersen C, et al. ATHENA Investigators . Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation 2009;120:1174-80. 10.1161/CIRCULATIONAHA.109.875252. [DOI] [PubMed] [Google Scholar]
- 29. Tsadok MA, Jackevicius CA, Essebag V, et al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation 2012;126:2680-7. 10.1161/CIRCULATIONAHA.112.092494 [DOI] [PubMed] [Google Scholar]
- 30. Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med 2012;172:997-1004. 10.1001/archinternmed.2012.2266 [DOI] [PubMed] [Google Scholar]
- 31. Connolly SJ, Camm AJ, Halperin JL, et al. PALLAS Investigators . Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268-76. 10.1056/NEJMoa1109867 [DOI] [PubMed] [Google Scholar]
- 32. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62-73. 10.1161/CIRCEP.107.754564 [DOI] [PubMed] [Google Scholar]
- 33. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221-8. 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 35. Kim D, Yang PS, Kim TH, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol 2018;72:1233-45. 10.1016/j.jacc.2018.05.076 [DOI] [PubMed] [Google Scholar]
- 36. Kim D, Yang PS, Sung JH, et al. Less dementia after catheter ablation for atrial fibrillation: a nationwide cohort study. Eur Heart J 2020;41:4483-93. 10.1093/eurheartj/ehaa726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-18 and figures 1-3