Abstract
Spinocerebellar ataxias (SCAs) are a group of dominantly-inherited cerebellar ataxias, among which CAG expansion-related SCAs are most common. These diseases have very high penetrance with defined disease progression, and emerging therapies are being developed to provide either symptomatic or disease-modifying benefits. In clinical trial design, it is crucial to incorporate biomarkers to test target engagement or track disease progression in response to therapies, especially in rare diseases such as SCAs. In this article, we review the available rating scales and recent advances of biomarkers in CAG-repeat SCAs. We divided biomarkers into neuroimaging, body fluid, and physiological studies. Understanding the utility of each biomarker will facilitate the design of robust clinical trials to advance therapies for SCAs.
1. Introduction
Spinocerebellar ataxias (SCA) are a group of dominantly-inherited ataxias. The core clinical features for SCAs are progressive cerebellar ataxia, involving ocular movements, speech, hand dexterity, gait, and balance. The most common presenting symptom for SCAs is gait abnormality [1], while other symptoms are involved later. In addition, SCA patients often have manifestations other than cerebellar ataxia, including dystonia, tremor, myoclonus, and parkinsonian features [2]. The motor dysfunction invariably leads to impaired daily activities and oftentimes premature death in individuals living with SCAs [3].
To date, 48 SCA subtypes have been identified. The most common SCA subtypes are caused by exonic CAG trinucleotide repeat expansions that encode abnormally long polyglutamine (poly-Q). These CAG-repeat SCAs are SCA1, 2, 3, 6, 7, 17, and dentatorubral-pallidoluysian atrophy (DRPLA), constituting over 50% of SCA patients [4–8]. CAG-repeat SCAs have a very high disease penetrance, implying that the disease process is mostly driven by the genetic mutation. In addition, the clinical progression of SCAs has been characterized in the natural history studies in the United States [9], Europe [10], Japan [11, 12], Brazil [13–15], Portugal [16], Taiwan [17], and China [18], and a linear disease progression has been identified. Given the high disease penetrance and the well-characterized disease progression, CAG-repeat SCAs present a unique opportunity to develop clinical trials for gene therapies or anti-sense oligonucleotides (ASOs). However, several challenges exist for successful clinical trials for SCAs. First, SCAs are rare diseases with a prevalence of 1–6 per 100,000 [19], posing challenges to patient recruitment. Second, despite cerebellar ataxia as the core clinical feature for SCAs, there is still a significant variability of clinical presentations for individuals with different SCAs. Therefore, the development of biomarkers for progression and target engagement, as well as the validation of clinical rating scales for diverse clinical features, are essential to ensure scientific rigor in clinical trials. Specifically, rating scales reflect the disease severity and responsiveness to therapy, capture motor and non-motor features of SCAs, and measure the impact on the activities of daily living. Biomarker development in SCAs can be divided into categories of neuroimaging, fluid, and physiology (Fig. 1). Each biomarker serves a unique purpose. For instance, some neuroimaging findings can track the disease progression, while certain fluid biomarkers are essential to test target engagement. We aim to provide a comprehensive review of existing clinical rating scales and recent advances of biomarker development for SCAs.
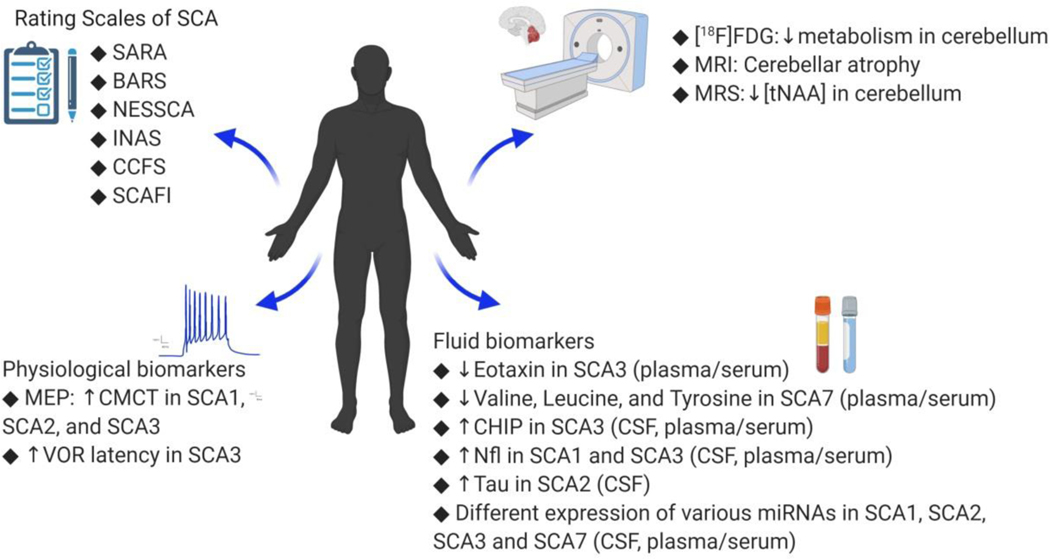
Fig. 1.
The summary of biomarkers of SCAs, including clinical rating scales, neuroimaging, and biofluid biomarkers (see text for abbreviations).
2. Rating Scales
Several clinical rating scales for SCAs have been developed. Among these, the measurement of ataxia severity is critical and often serves as the primary endpoint for clinical trials for SCAs. In addition, rating scales for neurological symptoms other than ataxia are important to capture full motor symptoms of SCAs. Finally, cognitive impairment associated with cerebellar dysfunction has been recently identified and can be tracked using a rating scale. We listed the commonly used rating scales in Table 1.
Table 1.
Summary of Clinical Scales of Spinocerebellar Ataxias
| Methods | Comment | Reference |
|---|---|---|
| International Cooperative Ataxia Rating Scale (ICARS) Scale for the assessment and rating of ataxia (SARA) Brief Ataxia Rating Scale (BARS) Neurological Examination Score for the Assessment of Spinocerebellar Ataxia (NESSCA) The Inventory of Non-Ataxia Symptoms (INAS) Composite Cerebellar Functional Severity Score (CCFS) and CCFS with writing test SCA Functional Index (SCAFI) Cerebellar Cognitive Affective Syndrome Scale (CCAS) UHDRS IV EQ-5D Patient Health Questionnaire-9 (PHQ-9) |
Evaluate various body part of ataxia motor symptoms, including oculomotor examination (0–100) Most extensively used ataxia scale with only 8 domains assessment without oculomotor evaluation. (0–40) Only evaluate 5 domains, including oculomotor evaluation (0–30) Originally developed for SCA3 but later validated in SCA2. Including items to assess cerebellar ataxia neuropathy, parkinsonism, and pyramidal signs. (0–40) Evaluate extra-cerebellar symptoms associated with SCA patients. A part of the scale is subjective, patient reported outcomes. (0–16) Consisted of two performance-based tasks: nine-hole pegboard test and click test Consists of three functional measures: timed 8-meter walk, 9-hole peg test, and PATA repetition. Assess the cognition of ataxia patients Patient reported functional capacity Patient reported functional capacity and overall health A self-administered questionnaire to assess the severity of depression |
[20] [21] [22] [23, 24] [28] [29] [30] [31] [38] [29] [36] |
2.1. Scales for motor dysfunction
International Cooperative Ataxia Rating Scale (ICARS) is a commonly used rating scale to measure the severity of ataxia [20]. ICARS has 19 items and a total score of 100, divided into 4 subscales: posture and gait disturbances, limb ataxia (kinetic functions), dysarthria (speech disturbances), and oculomotor disorders [20]. Another commonly used rating scale for ataxia severity is the Scale for the Assessment and Rating of Ataxia (SARA) [21], which has 8 rating items (gait, stance, sitting, speech, finger chase, nose-finger test, fast alternating hand movements, and heel-shin slide) with a total score of 40. Brief Ataxia Rating Scale (BARS) is derived from a modified version of ICARS. It is a five-item scale with a total score of 30, including gait, kinetic function of legs and arms, speech, and eye movements [22]. While BARS is more concise than ICARS, BARS has finer granularity in each item. Another rating scale for ataxia assessment is the Neurological Examination Score for the Assessment of Spinocerebellar Ataxia (NESSCA), which was originally developed to assess individuals with SCA2 [23] and SCA3 [24]. In addition to ataxia symptoms, NESSCA includes non-ataxia motor symptoms: eyelid retraction, fasciculations, sensory loss, blepharospasm, rigidity, bradykinesia, distal amyotrophy, sphincter dysfunction, vertigo, and optic atrophy. Among these rating scales, SARA is most commonly used and has been extensively validated in the natural history study of SCA1, 2, 3, and 6 in the cohorts in Europe and the United States [3, 9, 10, 25–27], showing linear progression in these diseases. SARA also has excellent inter-rater reliability (interclass coefficient = 0.98) and test-retest reliability (interclass coefficient = 0.90) [21], making it the most widely used scale in the SCA research field.
Apart from the core symptoms of cerebellar ataxia, SCA patients often have additional neurological symptoms. The Inventory of Non-Ataxia Symptoms (INAS) is used to assess spasticity, fasciculations, myoclonus, tremor, dystonia, and vibratory sense, while the oculomotor findings are partially overlapping with typical cerebellar signs such as nystagmus and hypo- or hyper-metric saccades [28]. INAS thus is a useful tool to monitor whether a particular therapy improves the non-ataxia motor symptoms in SCA patients.
Both SARA and INAS have been adopted for tracking disease progression in patients with SCA1, 2, 3, 6, and 17, demonstrating that the rates of disease progression differ between SCAs [9, 10, 12, 15, 17]. Interestingly, even within the same type of SCA, the rate of progression measured by SARA showed a geographical difference (summarized in Supplementary Table 1).
2.2. Performance scales
There are two performance-based rating scales for SCAs: Composite Cerebellar Functional Severity Score (CCFS) [29] and SCA Functional Index (SCAFI) [30]. CCFS contains two different functional tests. One is the 9-peg board test, which measures the time for a patient to place 9 pegs into holes. The other is the click test, which measures the time required for a patient to press two buttons alternatively for 10 times. CCFS assesses the accuracy of hand movements, which is linked to the core symptoms of cerebellar ataxia; however, these measurements are restricted to the upper limbs. SCAFI is similar to CCFS but with the addition of a timed 8-meter walk to assess a composite of lower limb function and balance. However, the timed 8-meter walk can only be measured by patients who can still ambulate either with or without assistive devices, and it is unclear how to adjust for the use of different assistive devices in data analysis. Overall, CCFS and SCAFI may provide better information on ataxia as it reflects real-world performance in the activities involving coordination.
2.3. Scales for non-motor symptoms
The cerebellum has diverse connections with the cerebral cortex to modulate cerebral function; therefore, SCA patients, not surprisingly, can have a variety of cognitive symptoms in addition to motor impairments. Recently, the Cerebellar Cognitive Affective Syndrome Scale (CCAS) was developed to capture the cognitive dysfunction in ataxia patients, including SCAs [31]. Cognitive dysfunction related to ataxia is assessed in several domains, including semantic fluency, phonemic fluency, category switching, verbal registration, digit span, cube drawing/copying, recalls, similarities, go-no-go, and affect. Because these cognitive dysfunctions can have a major impact on the quality of life in SCA patients, CCAS can be used to monitor cognitive responses to therapies.
Depression is one of the most common non-motor symptoms among SCAs [32], and it has been frequently reported in SCA3 [33–35]. Patient Health Questionnaire-9 (PHQ-9), a self-administered questionnaire to assess the severity of depression [36], has been adopted by clinical studies in both Europe [32] and the United States [37], such as the EUROSCA and CRC-SCA natural history study [32, 37]. PHQ-9 has 9 items, each with scores ranging from 0 (no depression at all) to 3 (depressed nearly every day) [36] with a maximal total score of 27. Cutoffs at 5, 10, 15, and 20 correspond to mild, moderate, moderately severe, and severe depression. PHQ-9 can monitor the temporal progression of depression in SCA patients.
2.4. Scales for functional capacity and quality of life
Two scales are frequently used in ataxia research to measure the functional status of a patient. The first is Part IV of the Unified Huntington’s Disease Rating Scale. This scale (UHDRS IV) measures the functional capacity, including 25 questions to gauge the patient’s ability to accomplish activities of daily living, handle financial matters, and perform at work [38]. Another commonly used scale in ataxia research for the functional outcome is the EQ-5D [29]. This scale has been widely used in clinical trials for various diseases. In addition to measuring the functional level of a patient, EQ-5D also rates the overall health of a patient. EQ-5D-3L includes five 3-level questions to query the patient’s mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, as well as a self-reported score between 0 and 100 to represent the patient’s health state [39]. UHDRS IV and EQ-5D are important because they can reflect the clinical meaningfulness of improvements in response to treatment. In fact, a growing concern is that patients may not necessarily perceive a change in their daily activities, despite an improvement in the rating scales based on neurological examinations, such as SARA, ICARS, or INAS. The Food and Drug Administration also requests the inclusion of outcome measurements in clinical trials that reflect functional improvements reported by patients [40, 41].
3. Neuroimaging Biomarkers
The main pathological feature of SCAs is cerebellar degeneration, which can be measured by structural imaging such as magnetic resonance imaging (MRI). Preceding the actual structural alterations, the metabolism of the cerebellum and related brainstem areas is often found to be altered and can be assessed by positron emission tomography (PET) or magnetic resonance spectroscopy (MRS). Finally, single-photon emission computed tomography (SPECT) is used to assess dopaminergic neurons, GABAergic binding, and cerebral perfusion. We will review each neuroimaging modality in SCAs (Table 2) and also summarize the utility of each imaging tool in designing clinical trials (Table 3). It is important to note that neuroimaging studies have focused on the CAG-repeat SCAs, which are most commonly encountered. It has been challenging to recruit enough patients for such investigations in less common SCAs; therefore, the neuroimaging findings could be SCA subtype-specific and may not be generalizable.
Table 2.
Summary of Neuroimaging Findings in Ataxia
| Modality | Major Finding |
|---|---|
| MRI |
SCA1 ROI: ↓cerebellum [42], brainstem [42] VBM: ↓cerebellum [54], brainstem (including midbrain, pons, and medulla) [54], caudate [54], putamen [54], and temporal lobe [54] ↓WM in cerebellar hemispheres [51] ↓GM in cerebellar hemispheres [51], left anterior and posterior cerebellum [48], vermis [51], brainstem [48], right putamen, and pallidum [48] SCA2 ROI: ↓cerebellum [42, 43], brainstem [42, 43] (mesencephalon [43], pons [43], anteroposterior diameter of the pons [43]) VBM: ↓WM in pons [51], middle cerebellar peduncle [51], cerebellar hemispheres [51] ↓GM in cerebellar hemispheres and vermis [51] DTI: ↓fractional anisotropy and mode of anisotropy in the brain stem, cerebellar peduncles, cerebellum, cerebral hemisphere WM, corpus callosum, and thalami [56] SCA3 ROI: ↓cerebellum [46, 47], cerebellar nuclei [44], brainstem [46], vermis [47] volume, spinal core area [60], and spinal cord eccentricity [60] VBM: ↓cerebellum [48, 54], basal ganglia [48], brainstem (including midbrain, pons, and medulla) [48, 54], caudate [54], putamen [54], striatal [48], and temporal lobe [54] ↓GM in cerebellum [52], cerebellar hemispheres [49, 51], brainstem [49, 52], vermis [51], bilateral thalamus [49] ↓GMD in cerebellum [50], brainstem [50], frontal [50], occipital lobes [50], parietal [50], subcortical GM [50], temporal lobes [50] ↓WM in cerebellum [52], cerebellar hemispheres [49], brainstem [52], bilateral thalamus [49] DTI: ↓fractional anisotropy in cerebellum [52], brainstem [52];↑radial diffusivity in cerebellum [52], brainstem [52], frontal lobes [52], temporal lobes [52], thalamus [52] ↓cerebellum [124], anterior lobe [124], left posterior lobe [124], right posterior upper lobe [124] SCA6 ROI: ↓cerebellum [44, 46], cerebellar nuclei [44], brainstem [46], superior vermis [65] VBM: ↓cerebellum [48, 54], basal ganglia [48], brainstem [48, 54], caudate [48] SCA17 ROI: ↓cerebellum [45], vermis, caudate nucleus [45] VBM: ↓cerebellum, limbic system (parahippocampus, cingulate) and parietal precuneus [53] DRPLA ROI: ↓midbrain [55], pontine tegmentum [55], basis pontis [55], cerebellar vermis [55] SIR: ↑cerebral WM [57], thalamus [57], midbrain [57], pontine tegmentum [57], basis pontis [57], inferior olive [57], and cerebellar WM [57] Atrophy of the cerebrum [57], midbrain tegmentum [57], pontine tegmentum [57], basis pontis [57], superior cerebellar peduncle [57], and cerebellum [57] |
| MRS |
SCA1 ↓[Glu] in cerebellar hemisphere [125, 126], pons [125–127], vermis [125, 126] ↓[NAA] in cerebellar hemisphere [42], cerebellar WM [127], pons [42, 127], vermis [125, 127] ↓[NAAG] in cerebellar WM [127], pons [127] ↓[tNAA] in cerebellum [128], cerebellar hemisphere [125, 126], cerebellar WM [127], parietofrontal lobe WM [128], pons [125–127], sensory cortex [128], vermis [125–127], visual cortex [128] ↓Cho/Cr ratio in cerebellar hemisphere [62], basis pontis [61] ↓Glu/Gln ratio in vermis [125] ↓NAA/Cho ratio in cerebellar hemisphere [62] ↓NAA/Cr ratio in cerebellar hemisphere [61, 62], basis pontis [61], vermis [62] ↑[Asc] in vermis [127] ↑[Glc] in vermis [127] ↑[Gln] in cerebellar white matter [127], vermis [125, 126] ↑[mI] in cerebellar hemisphere [125, 126], pons [125–127] ↑[Tau] in cerebellar WM [127] ↑[tCr] in cerebellar hemisphere [125, 126], cerebellar WM [127] ↑[Glc + Tau] in cerebellar hemisphere [125, 126], cerebellar WM [127], vermis [127] SCA2 ↓[Cho] in cerebellum [129], vermis [129] ↓[Glu] in cerebellar hemisphere [126], pons [126], vermis [126] ↓[NAA] in cerebellar hemisphere [42, 63], cerebellum [129], pons [42], vermis [129] ↓[tNAA] in cerebellar hemisphere [126], pons [126], vermis [126] ↓Cho/Cr ratio in cerebellar hemisphere [62], vermis [62] ↓NAA/Cho ratio in cerebellar hemisphere [62], vermis [62] ↓NAA/Cr ratio in cerebellar hemisphere [62], basal ganglia [63], frontal lobes [63], pons [63], vermis [62, 63] ↑[Gln] in cerebellar hemisphere [126], cerebellar WM [127], vermis [126] ↑[GSH] in cerebellar WM [127], vermis [126] ↑[mI] in cerebellar hemisphere [63], cerebellar WM [127], pons [63, 127], vermis [126, 127] ↑[Tau] in cerebellar WM [127], vermis [126, 127] ↑[tCr] in cerebellar hemisphere [126], cerebellar WM [127], pons [126], vermis [126, 127] ↑[Glc + Tau] in cerebellar hemisphere [126], cerebellar WM [127], pons [127], vermis [127] ↑mI/Cr ratio in cerebellar hemispheres [63], basal ganglia [63], frontal lobes [63], pons [63], vermis [63] SCA3 ↓[NAA] in cerebellum [124, 129], pons [127], vermis [124, 129] ↓[NAAG] in cerebellar WM [127] ↓[tNAA] in cerebellar WM [127], vermis [127] ↓NAA/Cho ratio in cerebellar hemisphere [62], dentate nucleus [64], vermis [62, 64] ↓NAA/Cr ratio in cerebellar hemisphere [62], cortex [64], dentate nucleus [64], middle cerebellar peduncle [64], vermis [62, 64] ↑[mI] in pons [127] ↑[Tau] in cerebellar WM [127] ↑[tCr] in cerebellar WM [127] ↑[Glc + Tau] in vermis [127] SCA6 ↓[GABA] in cerebellar WM [127], vermis [127] ↓[NAA] in cerebellum [63, 129], vermis [127, 129] ↓[tNAA] in vermis [127] ↓NAA/Cho ratio in cerebellar hemisphere [62, 65], vermis [62, 65] ↓NAA/Cr ratio in cerebellar hemisphere [62], vermis [62] ↑[Lac] in vermis [126] ↑[mI] in cerebellar hemisphere [126], vermis [126] ↑[Glc + Tau] in cerebellar hemisphere [126], vermis [127] SCA17 ↓NAA/Cho ratio in vermis [62] ↓NAA/Cr ratio in cerebellar hemisphere [62], vermis [62] |
| fMRI | ↓cerebellar cortex [44], cerebellar nuclei [44] |
| PET |
SCA1 [18F]FDG:↓metabolism in cerebellum [68], cerebral cortex [67], cerebellar hemispheres [67], vermis [67], brainstem [67, 68], caudate nucleus [67], putamen [67], thalamus [67], whole brain cortex [67] SCA2 [18F]FDG:↓metabolism in cerebellum [58, 68, 69], anterior-posterior lobe ratio [69], brainstem [68], parietal cortex [68], pons [58], parahippocampal gyrus [58], frontal cortex [58] [11C]dMP:↓Dopamine transporter levels in putamen [68], caudate nucleus [68] SCA3 [18F]FDG:↓metabolism in cerebellum [58, 68], cerebellar hemispheres [70, 71], cerebellar vermis [70], occipital cortex [70, 71], brainstem [68, 70, 71], putamen [68], thalamus [68], parahippocampal gyrus of the limbic system [58], lentiform nucleus [58];↑metabolism in parietal and temporal cortices preclinically [71] [11C]dMP:↓Dopamine transporter levels in putamen [68], caudate nucleus [68] [11C]MP4P:↓thalamus [130] SCA6 [18F]FDG:↓metabolism in cerebellum [58, 68, 69], cerebellar hemispheres [72], anterior-posterior lobe ratio [69], basal ganglia [72], brainstem [72], caudate [72], putamen [68], frontal cortex [58, 72], prefrontal cortex [58], occipital cortex [72], temporal cortex [72];↑temporal cortex [68] SCA17 [18F]FDG:↓metabolism in caudate nucleus [45], putamen [45] [11C]dMP:↓Dopamine transporter levels in caudate nucleus and putamen [45] [11C]Raclopride:↓D2 receptor levels in caudate nucleus and putamen [45] |
| SPECT |
SCA2 [99mTc]TRODAT-1 SPECT:↓striatal DAT binding [73] [123I]β-CTT SPECT:↓striato-cerebellar ratio [131] [123I]IBZM SPECT:↓striato-frontal IBZM binding ratio [131] [123I]FP-CIT SPECT:↓uptake in caudate, putamen [132] SCA3 [99mTc]TRODAT-1 SPECT:↓nigrostriatal ratio [74] [99mTc]HMPAO SPECT:↓perfusion in cerebellar hemispheres [47], inferior [47] and superior [47] frontal lobe [47], lateral temporal lobe [47], parietal lobe [47], vermis [47] [99mTc]ECD SPECT:↓perfusion in bilateral cerebellum [75], vermis [75] [123I]iomazenil SPECT:↓binding in cerebellum [133], cerebral cortex [133], thalamus [133], striatum [133] SCA6 [99mTc]ECD SPECT:↓perfusion in cerebellar hemisphere[76], cerebral vermis[76] SCA17 [99mTc]TRODAT-1 SPECT:↓striatal DAT binding [73] |
Asc: ascorbate; Cho: Choline; Cr: Creatine; [123I]β-CIT :
[123I]2β-carbomethoxy-3b-(4-iodophenyl)tropane; [11C]dMP:
[11C]D-threo-methylphenidate; DAT: dopamine transporters; DTI: diffusion tensor imaging; [99mTc]ECD: technetium-99m N,N-1,2-ethylene diylbis-L-cysteine diethyl ester dihydrochloride; [18F]FDG: [18F]fluorodeoxyglucose; 3D-FD: three-dimensional fractal dimension; fMRI: functional magnetic resonance imaging; [123I]FP-CIT:
[123I]N-fluoropropyl-2b-carbomethoxy-3b-(4-iodophenyl) nortropane ([123I]ioflupane);
GABA: gamma-aminobutyric acid; Glc: glucose; Gln: glutamine; Glu: glutamate; GM: gray matter; GMD: gray matter density; GSH: glutathione; [99mTc]HMPAO: technetium-99m hexamethylpropylene amine oxime; [123I]IBZM SPECT:
[123I](S-)-2-hydroxy-3-iodo-6-methoxy-N[(l-ethyl-2-pyrrolidinyl) methyl]-benzamide; [123I]IMZ: [123I]iomazenil; Lac: lactate; mI: myo-Inositol; [11C]MP4P: N-[11C]-methyl piperidine-4-propionate; MRI: magnetic resonance imaging; MRS: magnetic resonance spectroscopy; NAA: N-acetyl aspartate; NAAG: N-acetylaspartylglutamate; PET: positron emission tomography; [11C]RAC: [11C]Raclopride; ROI: region of interest; SIR: signal intensity ratio; SPECT: single photon emission computed tomography; Tau: taurine; tCr: total creatine; tNAA: total N-acetyl aspartate; VBM: voxel-based morphometry; WM: white matter.
Table 3.
The utility of each imaging technique for SCAs
| Utility | Method |
|---|---|
| Differentiating symptomatic SCA patients from healthy controls | MRI [42–50, 52–54, 56, 60, 61] MRS [42, 61–65, 124–129, 134–137] PET [45, 58, 67–72] SPECT [47, 74–76, 131–133] |
| Differentiating presymptomatic SCA patients from healthy controls | MRI: SCA2 [43], SCA17 [45] PET: SCA3 [71], SCA17 [45] |
| Differentiating presymptomatic SCA patients from symptomatic SCA patients | MRI: SCA2 [43], SCA17 [45] PET: SCA3 [71], SCA17 [45] |
| Differentiating different SCA subtypes | MRI [42, 44, 48, 54, 58] MRS [42, 62, 126, 127, 129, 134, 136, 138] PET [48, 51, 58, 68, 69, 130] |
| Differentiating SCA patients with mild ataxia from severe ataxia | MRI [46, 49, 54, 59, 60] PET [51] |
3.1. MRI
Cerebellar atrophy is the hallmark of the neuroimaging finding of SCAs. The reduction of the cerebellum volume has been found to closely correlate with the ataxia severity, either using region of interest (ROI)-based analysis [42–47] or voxel-based morphometry (VBM) [48–54], in SCA1 [42, 48, 51, 54], SCA2 [42, 43, 51], SCA3 [44, 46–52, 54], SCA6 [44, 46, 48, 54], SCA17 [45, 53], and DRPLA [55]. In addition, white matter alterations are identified in SCA2 using diffusion tensor imaging (DTI) [56]. On the other hand, high signal intensity ratio in T2-weighted image was observed in the cerebral white matter, thalamus, midbrain, pontine tegmentum, basis pontis, inferior olive, and cerebellar white matter in patients with DRPLA [57]. While the cerebellum is the predominantly affected brain region in SCAs, other brain areas may be involved. Studies using MRI have demonstrated volume changes in the brainstem [42, 43, 46, 48, 49, 52, 54], basal ganglia [48], and pons [43, 51, 54]. MRI techniques can also be used to detect structural changes before the onset of symptoms in SCA2 [43] and SCA17 [45], as well as the progression of the cerebellar volume reduction from the presymptomatic stage to the symptomatic stage [43]. These findings indicate that MRI is more sensitive than clinical rating scales and can be useful to predict the symptom onset and to test disease-modifying therapies in presymptomatic stages. Interestingly, each SCA studied seems to have its own pattern of cerebellar degeneration (i.e., lobule-specific) [42, 44, 48, 58]. Therefore, a detailed analysis of each lobule will yield additional information regarding the degenerative mapping of each SCA.
Several imaging parameters are shown to correlate with clinical rating scales. For example, SARA correlates with the volume of the cerebellum [54], brainstem [52, 54, 59], caudate [54], and spinal cord [60], as well as the cerebellar white matters [49]. These parameters can be useful markers to track disease progression.
3.2. MRS
MRS is a sensitive imaging modality to detect chemical changes, which may precede the structural alterations seen in MRI. The commonly studied metabolites in MRS are N-acetylaspartate (NAA, a marker of neuronal density and function), creatine/phosphocreatine (Cr, a metabolism marker), choline compounds (Cho, a marker of synthesis and degradation of cell membranes), and myoinositol (mI, a marker for gliosis). In SCA1 [61, 62], SCA2 [62, 63], SCA3 [62, 64], SCA6 [62, 65], and SCA17 [62] cerebellum, a reduction in NAA/Cr and NAA/Cho ratios is identified as a marker for neurodegeneration. An interesting aspect of MRS as a biomarker is its translatability. A SCA1 mouse model has been shown to have biochemical changes in the cerebellum spanning the presymptomatic and symptomatic stages similar to SCA1 patients [66]. Therefore, therapies that can ameliorate motor symptoms of the SCA1 mouse model with corresponding MRS signal improvement can be studied in clinical trials in SCA1 patients using MRS as a neuroimaging biomarker.
3.3. Functional MRI (fMRI)
Resting-state fMRI can be used to assess the oxygen consumption of the cerebellum. While this technique has not been studied extensively in SCAs, the reduction of fMRI signals in the cerebellar cortex and cerebellar nuclei have been observed in SCA6 [44].
3.4. PET
The most commonly used PET tracer in SCAs is [18F]fluorodeoxyglucose ([18F]FDG), which reflects the overall metabolism. Reduction of the metabolism of the cerebellum can be seen in SCA1 [67, 68], SCA2 [58, 68, 69], SCA3 [58, 68, 70, 71], and SCA6 [58, 68, 69, 72]. Such a reduction can also be observed in the presymptomatic SCA patients [71]. PET can thus be used to track the disease progression in SCA patients [51]. As patients with SCA2, SCA3, SCA17 can present with parkinsonian symptoms, several PET ligands, such as [11C]D-threo-methylphenidate ([11C]dMP) or [11C]raclopride ([11C]RAC), can be useful to interrogate the involvement of the dopaminergic axis. Overall, PET provides important information in metabolic changes, which may be sensitive neuroimaging biomarkers for SCAs.
3.5. SPECT
SPECT is an imaging technique that integrates radioactive tracers to provide functional and metabolic information. SPECT studies in ataxia studies focus on the dopamine axis and overall brain perfusion. Using SPECT techniques, dopaminergic dysfunction is identified in SCA2 [73], SCA3 [74], and SCA17 [73]. [99mTc]ECD SPECT (technetium-99m N,N-1,2-ethylene diylbis-L-cysteine diethyl ester dihydrochloride or ethyl cysteinate dimer) is used to assess brain perfusion and has demonstrated a perfusion reduction in SCA3 [75] and SCA6 [76]. However, it is unclear whether SPECT is more sensitive to PET in identifying perfusion changes in the brain.
4. Fluid biomarkers
Genetic mutations of SCA patients can cause various biological alterations that can be traced either in blood or in cerebrospinal fluid (CSF) (Table 4). These fluid biomarkers can be used to either track disease progression and/or test target engagement in future clinical trials.
Table 4.
Summary of SCA fluid biomarkers
| Biomarker | SCA cases vs. controls | Presymptomatic SCA cases vs. controls | Presymptomatic SCA cases vs. symptomatic SCA cases | Adult-onset SCA cases vs. early-onset SCA cases* |
|---|---|---|---|---|
| Poly-Q expanded ataxin-3 | ↑ in PBMC of SCA3 [80] ↑ in plasma and CSF of SCA3 [82] |
↑ in PBMC of presymptomatic SCA3 [80] ↑ in plasma and CSF of presymptomatic SCA3 [82] |
↑ in CSF of symptomatic SCA3 vs. presymptomatic SCA3 [82] |
|
| Catalase activity | ↑ in serum of SCA3 [91] | |||
| CHIP | ↑ in serum of SCA3 [83] ↑ in CSF of SCA3 [83] |
|||
| Oxidation of DCFH-DA | ↑ in serum of SCA3 [92] | ↑ in serum of presymptomatic SCA3 [92] |
↑ in serum of symptomatic SCA3 vs. presymptomatic SCA3 [92] |
|
| Eotaxin | ↑ in serum of symptomatic SCA3 vs. presymptomatic SCA3 [84] |
|||
| GFAP | ↑ in serum of SCA3 [139] | |||
| Glutathione peroxidase activity | ↓ in serum of symptomatic SCA3 [92] |
↓ in plasma of symptomatic SCA3 vs. presymptomatic SCA3 [92] |
||
| IGFBP-1 | ↑ in serum of SCA3 [94] | |||
| IGFBP-3 | ↓ in serum of SCA3 [94] | |||
| IGF-1/IGFBP-3 molar ratio | ↑ in serum of SCA3 [94] | |||
| Insulin | ↓ in serum of SCA3 [94] | |||
| miRNA | ↑ miR-34b [85] in serum of SCA3 |
Alterations of miRs in plasma of early onset SCA7 vs. adult onset SCA7 [97] |
||
| ↑miR-7014 in CSF of SCA1, SCA2, and SCA3 [96] ↑71 miRs in plasma of SCA7 [97] ↓miR-25 [85], miR-29a [85], miR-125b [85] in serum of SCA3 ↓ miR-7014 [96] in plasma of SCA3 Different expression of various exosomal miRs in plasma and CSF of SCA3 [96] |
||||
| Neurofilament light chain | ↑ in serum of SCA1 [78] and SCA3 [78–80] ↑ in CSF of SCA3 [79] |
↑ in serum of presymptomatic SCA3 [79, 80] |
||
| NSE | ↑ in serum of SCA3 [86, 87] | |||
| Phosphorylated neurofilament heavy chain | ↑ in serum of SCA3 [80] | |||
| S100B | ↑ in serum of SCA3 [86] | |||
| Superoxide dismutase activity | ↓ in serum of symptomatic SCA3 vs. presymptomatic SCA3 [92] |
|||
| Tau | ↑ in CSF of SCA2 [77] | |||
| Valine, leucine, and tyrosine | ↓ in plasma of SCA7 [95] |
CHIP, carboxyl terminus of the Hsp70-interacting protein; DCFH-DA, 2’,7’-dichlorofluorescein diacetate; GFAP, glial fibrillary acidic protein; GSH-Px, glutathione peroxidase; IGFBP, insulin-like growth factor-binding protein; IGF, insulin-like growth factor; miRNA, microRNA; NSE, neuron-specific enolase; S100B, protein S 100 B; SOD, superoxide dismutase.
Adult-onset ≧ 20 years old, early-onset < 20 years old
Axonal degeneration is commonly seen in neurodegenerative disorders, and SCAs are no exception. Therefore, markers of axonal degeneration have been studied. Specifically, tau protein level is reduced in the CSF of SCA2 patients compared to that in controls [77]. Another axonal maker is the neurofilament light chain, which is increased in the serum of SCA1 [78] and SCA3 [78, 79] patients and in the CSF of SCA3 patients [79]. In addition, the neurofilament light chain level in the CSF of SCA3 patients highly correlates with the level in the serum [79], indicating a peripheral source can be a reliable indicator for the neurodegenerative process. The increased level of neurofilament light chain in CSF can also be observed in the presymptomatic SCA3 patients [79], indicating this biomarker precedes the symptom onset of SCA3. Another translational study investigated neurofilament levels in SCA3 patients and SCA3 mouse models [80]. This study found a step-wise increase in the serum levels of neurofilament light chain and phosphorylated neurofilament heavy chain in controls, presymptomatic and symptomatic SCA3 patients. Among these markers, serum neurofilament light chain level can reflect disease severity and clinical progression, highlighting its role as a disease progression biomarker. Interestingly, it is estimated that the elevation of serum neurofilament light chain can precede the clinical symptoms by 7.5 years. Similar changes in neurofilament light chain level can be found in the blood and brain of a mouse model of SCA3, providing additional evidence that these changes are the results of a degenerative process driven by mutant ATXN3 [80]. While the alterations of neurofilament light chain level can be found in other neurodegenerative disorders, SCA patients are often in their thirties to fifties; therefore, they are less likely to have other co-existing late-onset neurodegenerative disorders such as Parkinson disease or Alzheimer disease, which may confound the interpretation of the level of neurofilament light chain.
Since CAG-repeat SCAs are caused by abnormal poly-Q proteins, knocking down such protein expression has been the main goal for gene therapies or ASO-based therapies. To ensure the sufficient efficacy of reducing the Poly-Q protein production, assay development to detect such Poly-Q proteins is essential. By utilizing time-resolved fluorescence energy transfer-based immunoassays, ataxin-3 with abnormally expanded poly-Q can be distinguished from normal ataxin-3 and reliably detected in the peripheral blood mononuclear cells from both presymptomatic and symptomatic SCA3 patients [81]. However, this assay cannot detect ataxin-3 protein in serum or CSF. On the other hand, another group has recently developed an immunoassay to sensitively detect the level of abnormally expanded poly-Q ataxin-3 in the CSF and plasma, which is elevated in both presymptomatic and symptomatic SCA3 patients [82]. Therefore, this new assay may be used to measure expanded poly-Q ataxin-3 level as a fluid biomarker to test target engagement for SCA3.
Besides the direct measurement of ataxin-3 level, endogenous binding partners of mutant ataxin-3 have been investigated as potential biomarkers. The carboxyl terminus of Hsp-70 interacting protein (CHIP) is a co-chaperone protein that can bind to mutant ataxin-3. CHIP protein level is increased in both serum and CSF of SCA3 patients [83].
Neuroinflammation is a common process in neurodegenerative disorders. Along this line, the protein level of eotaxin, a cytokine involved in the chemotaxis of eosinophil, is increased in the serum of symptomatic SCA3 patients compared to presymptomatic SCA3 patients [84], suggesting that eotaxin may be useful to study the phenoconversion from presymptomatic to symptomatic stages of SCA3.
Biomarkers related to glial cells, such as activation of astrocyte and glial loss, have been reported in SCA3. Glial fibrillary acidic protein (GFAP), a type III intermediate filament protein, is increased in the serum of SCA3 patients [85], indicating astrogliosis may occur in response to the degenerative mechanism. Similarly, the serum level of the astrocytic marker S100B is increased in SCA3 patients [86]. Neuron-specific enolase (NSE) is a peripheral marker of neuronal disruption, and an increased level of NSE has been observed in SCA3 patient serum [86, 87].
Oxidative stress has been reported to be linked to the pathogenesis of SCA3 [88–90]. The activity of catalase [91] (an antioxidant enzyme) and the oxidation of DCFH-DA [92] (an artificial substrate to assess the degree of oxidation) are increased in the serum of SCA3 patients. The oxidation of DCFH-DA is also increased in presymptomatic SCA3 patients compared to controls [92]. Interestingly, serums from symptomatic SCA3 patients oxidize DCFH-DA more than serums from presymptomatic SCA3 patients [92], indicating the oxidative burden increases as the disease progresses. The activities of glutathione peroxidase [92] and superoxide dismutase [92] are decreased in the serum of SCA3 patients. Similar to the oxidation of DCFH-DA, symptomatic SCA3 patients have a more severe reduction in the activities of glutathione peroxidase [92] and superoxide dismutase [92] compared to presymptomatic SCA3 patients [92], further supporting the role of oxidative stress in the disease progression.
Insulin resistance has been observed in patients with poly-Q disorders. The mechanism is still unclear, but it has been hypothesized that poly-Q peptides may interfere with the expression of insulin-related genes, such as insulin-like growth factor 1 (IGF-1) [93]. Therefore, levels of IGF-1 and its binding partners, IGFBP1 and IGFBP3, have been studied in SCAs. Specifically, SCA3 patients have a higher serum IGFBP1 level and a higher serum IGF-1/IGFBP-3 ratio than those in healthy controls [94]. On the other hand, IGFBP-3 and insulin levels are reduced in SCA3 patients [94].
The biochemical composition of biofluids from SCA patients has also been studied. Levels of amino acids, such as valine, leucine, and tyrosine, are reduced in the plasma of SCA7 patients [95]. Moreover, alterations of various microRNA (miRNA) levels have been identified in serum, plasma, or CSF in SCA1 [96], SCA2 [96], SCA3 [85, 96], and SCA7 [97] patients. However, the sample sizes in these miRNA studies remain small.
To date, most studies on fluid biomarkers have been conducted in SCA3 as it is the most common SCA worldwide, and few studies were done in patients with SCA1, 2, and 7. There is a need to expand the search of fluid biomarkers in other types of SCAs.
5. Physiology biomarkers
SCA pathology involves the cerebellum and associated brainstem areas, leading to brain circuitry re-organization. These defects may be detected using physiological tools such as vestibulo-oculography (VOG) and evoked potentials, including visual-evoked potential (VEP), brainstem auditory-evoked potential (BAEP), somatosensory-evoked potential (SSEP), and motor-evoked potential (MEP). The findings are summarized in Table 5.
Table 5.
Physiological biomarkers of SCAs
| Biomarker | SCA vs. controls | Presymptomatic SCA vs. controls | Presymptomatic SCA vs. Symptomatic SCA | Comparisons between different SCA subtypes |
|---|---|---|---|---|
| Auditory evoked potential | Reduced suppression of P50 in SCA3 [140] | |||
| BAEP | prolonged absolute III and V latencies and interpeak I–III latency in SCA2 [101] prolonged I and III latency in SCA6 [102] |
|||
| EEG | ↓ late bereitschaftspotential with dominant (right) hand movements in SCA3 [141] |
|||
| F wave amplitudes of ulnar nerve | ↑ in SCA1, SCA2, SCA3 [106] |
|||
| MEP | ↑ CMCT in SCA1 [104–106], SCA2 [104], SCA3 [104, 107] |
↓ intracortical facilitation in presymptomatic SCA3 [107] |
↑ CMCT in SCA1 vs. SCA2 [104] ↑ RMT in SCA1 vs. |
|
| ↑ RMT in SCA1 [104–106], SCA3 [104] ↑ amplitude in SCA3 [105] ↓ intracortical facilitation in SCA2 [106], SCA3 [106, 107] ↓ SICI in SCA3 [107] |
↓ SICI in presymptomatic SCA3 [107] |
SCA2 [104] Prolonged latency more commonly seen in SCA1 than SCA2 or SCA3 [103] |
||
| SSEP | Loss of cortical SEP in SCA1, SCA2, and SCA3 [103] Prolonged latency of P40 in SCA2 [101] |
Delayed P40 seen in SCA3 (69%) and SCA2 (23%) but not in SCA1 [103] |
||
| VEP | Prolonged P100 more commonly seen in SCA1 than SCA3 [103] | |||
| VOG | Gaze-evoked nystagmus and dysmetric saccade ↑ in SCA1 [99], SCA3 [99, 100], and SCA6 [99] SWJ/SWO ↑ in SCA3 [99, 100] Downward nystagmus in SCA6 [99] pHSN in SCA6 [99] ↑ VOR latency in SCA3 [98] ↓ VORr, VOR40, VOR60, and VOR80 in SCA3 [98] |
SWJ and impaired smooth pursuit ↑ in presymptomatic SCA3 [100] |
Gaze-evoked nystagmus ↑ in symptomatic SCA3 vs. presymptomatic SCA3 [100] |
BAEP: brainstem auditory evoked potentials; CMCT: central motor conduction time; EEG: electroencephalography; MEPs: motor evoked potentials; pHSN: perverted head shaking nystagmus or vertical nystagmus after horizontal head shaking; RMT: resting motor threshold; SICI: short-interval intracortical inhibition; SSEPs: somatosensory evoked potentials; VOG: video-oculography; VOR: vestibulo-ocular reflex; VOR40, VOR60, and VOR80: the median of the eye and head velocity ratio during 35–45, 55–65, and 75–85 ms after head-turning impulse starts.
The vestibulo-ocular system is often affected in SCAs. SCA3 patients have abnormal vestibulo-ocular reflex with prolonged reflex latency [98]. VOG has been implemented to detect quantitative oculomotor dysfunction in SCAs. Increased gaze-evoked eye movements (nystagmus, dysmetric saccade, and square-wave jerks) can be seen in SCA1 [99], SCA3 [99, 100], and SCA6 [99] patients when compared to healthy controls. Gaze-evoked eye movements occur more frequently in symptomatic SCA3 patients compared to presymptomatic SCA3 patients [100].
BAEP can be used to study brainstem involvement, and abnormalities have been demonstrated in SCA2 and SCA6 [101, 102]. SSEP has been applied to study the integrity of the posterior column. Specifically, SCA1, 2, and 3 patients have delayed or loss of P40 cortical SEP (from tibial nerve stimulation). Delayed P40 latency was seen in SCA3 (69%) and SCA2 (23%) but not seen in SCA1 patients [103]. Prolonged P100 latency in VEP was more commonly seen in SCA1 than SCA3 patients (78% vs. 25%) [103].
MEP is commonly used to monitor the dysfunction of the descending motor pathway by transcranial magnetic stimulation. Central motor conduction time (CMCT) is prolonged in SCA1 [104–106], SCA2 [104], SCA3 [104, 107], and SCA6 [108], suggesting damages to the descending motor pathways in these SCAs. The resting motor threshold at the motor cortex is also increased in SCA1 [104–106] and SCA3 [104].
These physiological measurements are helpful to probe the abnormalities of the brain circuit. However, whether these sensitive physiological parameters can be implemented in SCA trials to interrogate the relevant neurological symptoms remains undetermined. Specifically, whether the improvement of these measures correlates with clinical improvement remains to be answered. If such correlation is confirmed, incorporating these physiological biomarkers into SCA clinical trial design will be valuable.
A gap still exists between neuroscientists and clinicians. The cerebellum is crucial for motor prediction, error correction, and motor learning, and neuroscientists have been studying these aspects in patients with ataxia versus controls. A commonly used test is the hand-reach perturbation, which implements an error in the visual input to subjects when they try to reach a target and measures the rate subjects make the correction [109–113]. Assessments for such error-based learning can potentially be useful in tracking the disease progression but have not been applied in the clinical setting. Similarly, quantitative kinematic-based measurements of limb movements and gait in patients with ataxia have been widely studied [112, 114–122] but not implemented as a standard clinical practice. Such techniques can provide objective and accurate scoring of clinical rating scales, therefore, precisely monitoring the disease progression and treatment effects in clinical trials.
6. Conclusion
This review summarizes the recent development of rating scales and biomarkers in CAG-repeat SCAs. While rating scales provide important information for therapeutic responses and often serve as the primary endpoint for large-scale clinical trials, the integration of imaging and fluid biomarkers can provide additional information for target engagement, disease mechanism, and patient selection. As each biomarker has its distinct characteristic, the combination of biomarkers is likely to yield useful information. Of note, we found a lack of fluid biomarkers for DRPLA, likely owing to the rarity of the disease. Nevertheless, efforts to find markers for DRPLA, such as neurofilament, is ongoing [7] and will add to our knowledge of the CAG-repeat SCAs.
The understanding of the natural history of SCAs in both the United States [9] and Europe [10] is leading the way towards clinical trial readiness for SCAs [123]. In addition, the advances in the knowledge of the disease pathomechanism of SCAs have identified several therapeutic targets. Finally, the identification of multimodal biomarkers will ensure a rigorous clinical trial design. We are now at the forefront of therapy development for SCAs to eventually bring hope to patients and their families to combat these relentless disorders.
Supplementary Material
Acknowledgments
Funding
Dr. Rosenthal has received support from the NIH (U01NS097049, U01NS102035, and P50NS038377), Parkinson’s Foundation, Michael J. Fox Foundation, the Gordon and Marilyn Macklin Foundation, the Daniel B and Florence E Green Foundation, the National Ataxia Foundation, and support for clinical trials from Biohaven Pharmaceuticals. Dr. Opal currently receives support from the NIH (R01NS062051, R01NS082351, and R56NS108639) and the following sources for clinical trials: Biohaven Pharmaceuticals, U01, and the National Ataxia Foundation (CRC-SCA natural history study). Dr. Kuo has received funding from the NIH (R01NS104423, R01NS118179, R03NS114871, and R13NS117005), Brain Research Foundation, National Ataxia Foundation, Parkinson’s Foundation, and International Essential Tremor Foundation.
Footnotes
Declaration of Interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Luo L, Wang J, Lo RY, Figueroa KP, Pulst SM, Kuo PH, Perlman S, Wilmot G, Gomez CM, Schmahmann J, Paulson H, Shakkottai VG, Ying SH, Zesiewicz T, Bushara K, Geschwind M, Xia G, Subramony SH, Ashizawa T, Kuo SH, The Initial Symptom and Motor Progression in Spinocerebellar Ataxias, Cerebellum 16(3) (2017) 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rossi M, Perez-Lloret S, Cerquetti D, Merello M, Movement Disorders in Autosomal Dominant Cerebellar Ataxias: A Systematic Review, Mov Disord Clin Pract 1(3) (2014) 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Diallo A, Jacobi H, Cook A, Labrum R, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Panzeri M, Rakowicz M, Sobanska A, Sulek A, Schmitz-Hubsch T, Schols L, Hengel H, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Boesch S, Pandolfo M, Schulz JB, Bauer P, Giunti P, Kang JS, Klockgether T, Tezenas du Montcel S, Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): a longitudinal cohort study, Lancet Neurol 17(4) (2018) 327–334. [DOI] [PubMed] [Google Scholar]
- [4].Takano H, Cancel G, Ikeuchi T, Lorenzetti D, Mawad R, Stevanin G, Didierjean O, Durr A, Oyake M, Shimohata T, Sasaki R, Koide R, Igarashi S, Hayashi S, Takiyama Y, Nishizawa M, Tanaka H, Zoghbi H, Brice A, Tsuji S, Close associations between prevalences of dominantly inherited spinocerebellar ataxias with CAG-repeat expansions and frequencies of large normal CAG alleles in Japanese and Caucasian populations, Am J Hum Genet 63(4) (1998) 1060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maruyama H, Izumi Y, Morino H, Oda M, Toji H, Nakamura S, Kawakami H, Difference in disease-free survival curve and regional distribution according to subtype of spinocerebellar ataxia: a study of 1,286 Japanese patients, Am J Med Genet 114(5) (2002) 578–83. [DOI] [PubMed] [Google Scholar]
- [6].Tsuji S, Onodera O, Goto J, Nishizawa M, Study D. Group on Ataxic, Sporadic ataxias in Japan--a population-based epidemiological study, Cerebellum 7(2) (2008) 189–97. [DOI] [PubMed] [Google Scholar]
- [7].Chaudhry A, Anthanasiou-Fragkouli A, Houlden H, DRPLA: understanding the natural history and developing biomarkers to accelerate therapeutic trials in a globally rare repeat expansion disorder, J Neurol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Durr A, Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond, Lancet Neurol 9(9) (2010) 885–94. [DOI] [PubMed] [Google Scholar]
- [9].Ashizawa T, Figueroa KP, Perlman SL, Gomez CM, Wilmot GR, Schmahmann JD, Ying SH, Zesiewicz TA, Paulson HL, Shakkottai VG, Bushara KO, Kuo SH, Geschwind MD, Xia G, Mazzoni P, Krischer JP, Cuthbertson D, Holbert AR, Ferguson JH, Pulst SM, Subramony SH, Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study, Orphanet J Rare Dis 8 (2013) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, Durr A, Marelli C, Globas C, Linnemann C, Schols L, Rakowicz M, Rola R, Zdzienicka E, Schmitz-Hubsch T, Fancellu R, Mariotti C, Tomasello C, Baliko L, Melegh B, Filla A, Rinaldi C, van de Warrenburg BP, Verstappen CC, Szymanski S, Berciano J, Infante J, Timmann D, Boesch S, Hering S, Depondt C, Pandolfo M, Kang JS, Ratzka S, Schulz J, Tezenas du Montcel S, Klockgether T, The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study, Neurology 77(11) (2011) 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sasaki H, Fukazawa T, Yanagihara T, Hamada T, Shima K, Matsumoto A, Hashimoto K, Ito N, Wakisaka A, Tashiro K, Clinical features and natural history of spinocerebellar ataxia type 1, Acta Neurol Scand 93(1) (1996) 64–71. [DOI] [PubMed] [Google Scholar]
- [12].Yasui K, Yabe I, Yoshida K, Kanai K, Arai K, Ito M, Onodera O, Koyano S, Isozaki E, Sawai S, Adachi Y, Sasaki H, Kuwabara S, Hattori T, Sobue G, Mizusawa H, Tsuji S, Nishizawa M, Nakashima K, A 3-year cohort study of the natural history of spinocerebellar ataxia type 6 in Japan, Orphanet J Rare Dis 9 (2014) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Franca MC Jr., D’Abreu A, Nucci A, Cendes F, Lopes-Cendes I, Progression of ataxia in patients with Machado-Joseph disease, Mov Disord 24(9) (2009) 1387–90. [DOI] [PubMed] [Google Scholar]
- [14].Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, Cendes F, Franca MC Jr., Structural signature of SCA3: From presymptomatic to late disease stages, Ann Neurol 84(3) (2018) 401–408. [DOI] [PubMed] [Google Scholar]
- [15].Piccinin CC, Rezende TJR, de Paiva JLR, Moyses PC, Martinez ARM, Cendes F, Franca MC Jr., A 5-Year Longitudinal Clinical and Magnetic Resonance Imaging Study in Spinocerebellar Ataxia Type 3, Mov Disord 35(9) (2020) 1679–1684. [DOI] [PubMed] [Google Scholar]
- [16].Mendonca N, Franca MC Jr., Goncalves AF, Januario C, Clinical Features of Machado-Joseph Disease, Adv Exp Med Biol 1049 (2018) 255–273. [DOI] [PubMed] [Google Scholar]
- [17].Lee YC, Liao YC, Wang PS, Lee IH, Lin KP, Soong BW, Comparison of cerebellar ataxias: A three-year prospective longitudinal assessment, Mov Disord 26(11) (2011) 2081–7. [DOI] [PubMed] [Google Scholar]
- [18].Guo J, Chen H, Biswal BB, Guo X, Zhang H, Dai L, Zhang Y, Li L, Fan Y, Han S, Liu J, Feng L, Wang Q, Wang J, Liu C, Chen H, Gray matter atrophy patterns within the cerebellum-neostriatum-cortical network in SCA3, Neurology 95(22) (2020) e3036–e3044. [DOI] [PubMed] [Google Scholar]
- [19].Ashizawa T, Oz G, Paulson HL, Spinocerebellar ataxias: prospects and challenges for therapy development, Nat Rev Neurol 14(10) (2018) 590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B, International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology, J Neurol Sci 145(2) (1997) 205–11. [DOI] [PubMed] [Google Scholar]
- [21].Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R, Scale for the assessment and rating of ataxia: development of a new clinical scale, Neurology 66(11) (2006) 1717–20. [DOI] [PubMed] [Google Scholar]
- [22].Schmahmann JD, Gardner R, MacMore J, Vangel MG, Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS, Mov Disord 24(12) (2009) 1820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Monte TL, Reckziegel ER, Augustin MC, Silva ASP, Locks-Coelho LD, Barsottini O, Pedroso JL, Vargas FR, Saraiva-Pereira ML, Leotti VB, Jardim LB, Rede N, NESSCA Validation and Responsiveness of Several Rating Scales in Spinocerebellar Ataxia Type 2, Cerebellum 16(4) (2017) 852–858. [DOI] [PubMed] [Google Scholar]
- [24].Kieling C, Rieder CR, Silva AC, Saute JA, Cecchin CR, Monte TL, Jardim LB, A neurological examination score for the assessment of spinocerebellar ataxia 3 (SCA3), Eur J Neurol 15(4) (2008) 371–6. [DOI] [PubMed] [Google Scholar]
- [25].Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P, A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7, Orphanet J Rare Dis 11(1) (2016) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, Parkinson MH, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Panzeri M, Rakowicz M, Sulek A, Sobanska A, Schmitz-Hubsch T, Schols L, Hengel H, Baliko L, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Szymanski S, Boesch S, Kang JS, Pandolfo M, Schulz JB, Molho S, Diallo A, Klockgether T, Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study, Lancet Neurol 14(11) (2015) 1101–8. [DOI] [PubMed] [Google Scholar]
- [27].Schmitz-Hubsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, Filla A, Mariotti C, Rakowicz M, Charles P, Ribai P, Szymanski S, Infante J, van de Warrenburg BP, Durr A, Timmann D, Boesch S, Fancellu R, Rola R, Depondt C, Schols L, Zdienicka E, Kang JS, Dohlinger S, Kremer B, Stephenson DA, Melegh B, Pandolfo M, di Donato S, du Montcel ST, Klockgether T, Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms, Neurology 71(13) (2008) 982–9. [DOI] [PubMed] [Google Scholar]
- [28].Jacobi H, Rakowicz M, Rola R, Fancellu R, Mariotti C, Charles P, Durr A, Kuper M, Timmann D, Linnemann C, Schols L, Kaut O, Schaub C, Filla A, Baliko L, Melegh B, Kang JS, Giunti P, van de Warrenburg BP, Fimmers R, Klockgether T , Inventory of Non-Ataxia Signs (INAS): validation of a new clinical assessment instrument, Cerebellum 12(3) (2013) 418–28. [DOI] [PubMed] [Google Scholar]
- [29].du Montcel ST, Charles P, Ribai P, Goizet C, Le Bayon A, Labauge P, Guyant-Marechal L, Forlani S, Jauffret C, Vandenberghe N, N’Guyen K, Le Ber I, Devos D, Vincitorio CM, Manto MU, Tison F, Hannequin D, Ruberg M, Brice A, Durr A, Composite cerebellar functional severity score: validation of a quantitative score of cerebellar impairment, Brain 131(Pt 5) (2008) 1352–61. [DOI] [PubMed] [Google Scholar]
- [30].Schmitz-Hubsch T, Giunti P, Stephenson DA, Globas C, Baliko L, Sacca F, Mariotti C, Rakowicz M, Szymanski S, Infante J, van de Warrenburg BP, Timmann D, Fancellu R, Rola R, Depondt C, Schols L, Zdzienicka E, Kang JS, Dohlinger S, Kremer B, Melegh B, Filla A, Klockgether T, SCA Functional Index: a useful compound performance measure for spinocerebellar ataxia, Neurology 71(7) (2008) 486–92. [DOI] [PubMed] [Google Scholar]
- [31].Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD, The cerebellar cognitive affective/Schmahmann syndrome scale, Brain 141(1) (2018) 248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schmitz-Hubsch T, Coudert M, Tezenas du Montcel S, Giunti P, Labrum R, Durr A, Ribai P, Charles P, Linnemann C, Schols L, Rakowicz M, Rola R, Zdzienicka E, Fancellu R, Mariotti C, Baliko L, Melegh B, Filla A, Salvatore E, van de Warrenburg BP, Szymanski S, Infante J, Timmann D, Boesch S, Depondt C, Kang JS, Schulz JB, Klopstock T, Lossnitzer N, Lowe B, Frick C, Rottlander D, Schlaepfer TE, Klockgether T, Depression comorbidity in spinocerebellar ataxia, Mov Disord 26(5) (2011) 870–6. [DOI] [PubMed] [Google Scholar]
- [33].Kawai Y, Takeda A, Abe Y, Washimi Y, Tanaka F, Sobue G, Cognitive impairments in Machado-Joseph disease, Arch Neurol 61(11) (2004) 1757–60. [DOI] [PubMed] [Google Scholar]
- [34].McMurtray AM, Clark DG, Flood MK, Perlman S, Mendez MF, Depressive and memory symptoms as presenting features of spinocerebellar ataxia, J Neuropsychiatry Clin Neurosci 18(3) (2006) 420–2. [DOI] [PubMed] [Google Scholar]
- [35].Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felicio AC, Minett T, Weisman P, Santos-Galduroz RF, Bertolucci PH, Gabbai AA, Barsottini OG, Cerebellar cognitive affective syndrome in Machado Joseph disease: core clinical features, Cerebellum 11(2) (2012) 549–56. [DOI] [PubMed] [Google Scholar]
- [36].Kroenke K, Spitzer RL, Williams JB, The PHQ-9: validity of a brief depression severity measure, J Gen Intern Med 16(9) (2001) 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lo RY, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez C, Schmahmann J, Paulson H, Shakkottai VG, Ying S, Zesiewicz T, Bushara K, Geschwind M, Xia G, Yu JT, Lee LE, Ashizawa T, Subramony SH, Kuo SH, Depression and clinical progression in spinocerebellar ataxias, Parkinsonism Relat Disord 22 (2016) 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group, Mov Disord 11(2) (1996) 136–42. [DOI] [PubMed] [Google Scholar]
- [39].Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A, Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D), Br J Rheumatol 36(5) (1997) 551–9. [DOI] [PubMed] [Google Scholar]
- [40].U.S.D.o. Health, F.D.A.C.f.D.E. Human Services, Research, U.S.D.o. Health, F.D.A.C.f.B.E. Human Services, Research, U.S.D.o. Health, F.D.A.C.f.D. Human Services, H. Radiological, Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance, Health Qual Life Outcomes 4 (2006) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M, The importance of patient-reported outcomes in clinical trials and strategies for future optimization, Patient Relat Outcome Meas 9 (2018) 353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guerrini L, Lolli F, Ginestroni A, Belli G, Della Nave R, Tessa C, Foresti S, Cosottini M, Piacentini S, Salvi F, Plasmati R, De Grandis D, Siciliano G, Filla A, Mascalchi M, Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study, Brain 127(Pt 8) (2004) 1785–95. [DOI] [PubMed] [Google Scholar]
- [43].Reetz K, Rodriguez-Labrada R, Dogan I, Mirzazade S, Romanzetti S, Schulz JB, Cruz-Rivas EM, Alvarez-Cuesta JA, Aguilera Rodriguez R, Gonzalez Zaldivar Y, Auburger G, Velazquez-Perez L, Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2, Ann Clin Transl Neurol 5(2) (2018) 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stefanescu MR, Dohnalek M, Maderwald S, Thurling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D, Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia, Brain 138(Pt 5) (2015) 1182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brockmann K, Reimold M, Globas C, Hauser TK, Walter U, Machulla HJ, Rolfs A, Schols L, PET and MRI reveal early evidence of neurodegeneration in spinocerebellar ataxia type 17, J Nucl Med 53(7) (2012) 1074–80. [DOI] [PubMed] [Google Scholar]
- [46].Eichler L, Bellenberg B, Hahn HK, Koster O, Schols L, Lukas C, Quantitative assessment of brain stem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status, AJNR Am J Neuroradiol 32(5) (2011) 890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Etchebehere EC, Cendes F, Lopes-Cendes I, Pereira JA, Lima MC, Sansana CR, Silva CA, Camargo MF, Santos AO, Ramos CD, Camargo EE, Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease, Arch Neurol 58(8) (2001) 1257–63. [DOI] [PubMed] [Google Scholar]
- [48].Reetz K, Costa AS, Mirzazade S, Lehmann A, Juzek A, Rakowicz M, Boguslawska R, Schols L, Linnemann C, Mariotti C, Grisoli M, Durr A, van de Warrenburg BP, Timmann D, Pandolfo M, Bauer P, Jacobi H, Hauser TK, Klockgether T, Schulz JB, I. axia Study Group, Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6, Brain 136(Pt 3) (2013) 905–17. [DOI] [PubMed] [Google Scholar]
- [49].Kang JS, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R, White matter damage is related to ataxia severity in SCA3, J Neurol 261(2) (2014) 291–9. [DOI] [PubMed] [Google Scholar]
- [50].D’Abreu A, Franca MC Jr., Yasuda CL, Campos BA, Lopes-Cendes I, Cendes F, Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study, J Neuroimaging 22(3) (2012) 285–91. [DOI] [PubMed] [Google Scholar]
- [51].Goel G, Pal PK, Ravishankar S, Venkatasubramanian G, Jayakumar PN, Krishna N, Purushottam M, Saini J, Faruq M, Mukherji M, Jain S, Gray matter volume deficits in spinocerebellar ataxia: an optimized voxel based morphometric study, Parkinsonism Relat Disord 17(7) (2011) 521–7. [DOI] [PubMed] [Google Scholar]
- [52].Guimaraes RP, D’Abreu A, Yasuda CL, Franca MC Jr., Silva BH, Cappabianco FA, Bergo FP, Lopes-Cendes IT, Cendes F, A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3, Mov Disord 28(8) (2013) 1125–32. [DOI] [PubMed] [Google Scholar]
- [53].Reetz K, Lencer R, Hagenah JM, Gaser C, Tadic V, Walter U, Wolters A, Steinlechner S, Zuhlke C, Brockmann K, Klein C, Rolfs A, Binkofski F, Structural changes associated with progression of motor deficits in spinocerebellar ataxia 17, Cerebellum 9(2) (2010) 210–7. [DOI] [PubMed] [Google Scholar]
- [54].Schulz JB, Borkert J, Wolf S, Schmitz-Hubsch T, Rakowicz M, Mariotti C, Schols L, Timmann D, van de Warrenburg B, Durr A, Pandolfo M, Kang JS, Mandly AG, Nagele T, Grisoli M, Boguslawska R, Bauer P, Klockgether T, Hauser TK, Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6, Neuroimage 49(1) (2010) 158–68. [DOI] [PubMed] [Google Scholar]
- [55].Koide R, Onodera O, Ikeuchi T, Kondo R, Tanaka H, Tokiguchi S, Tomoda A, Miike T, Isa F, Beppu H, Shimizu N, Watanabe Y, Horikawa Y, Shimohata T, Hirota K, Ishikawa A, Tsuji S, Atrophy of the cerebellum and brainstem in dentatorubral pallidoluysian atrophy. Influence of CAG repeat size on MRI findings, Neurology 49(6) (1997) 1605–12. [DOI] [PubMed] [Google Scholar]
- [56].Mascalchi M, Toschi N, Giannelli M, Ginestroni A, Della Nave R, Nicolai E, Bianchi A, Tessa C, Salvatore E, Aiello M, Soricelli A, Diciotti S, Progression of microstructural damage in spinocerebellar ataxia type 2: a longitudinal DTI study, AJNR Am J Neuroradiol 36(6) (2015) 1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sugiyama A, Sato N, Nakata Y, Kimura Y, Enokizono M, Maekawa T, Kondo M, Takahashi Y, Kuwabara S, Matsuda H, Clinical and magnetic resonance imaging features of elderly onset dentatorubral-pallidoluysian atrophy, J Neurol 265(2) (2018) 322–329. [DOI] [PubMed] [Google Scholar]
- [58].Wang PS, Liu RS, Yang BH, Soong BW, Regional patterns of cerebral glucose metabolism in spinocerebellar ataxia type 2, 3 and 6 : a voxel-based FDG-positron emission tomography analysis, J Neurol 254(7) (2007) 838–45. [DOI] [PubMed] [Google Scholar]
- [59].de Rezende TJ, D’Abreu A, Guimaraes RP, Lopes TM, Lopes-Cendes I, Cendes F, Castellano G, Franca MC Jr., Cerebral cortex involvement in Machado-Joseph disease, Eur J Neurol 22(2) (2015) 277–83, e23–4. [DOI] [PubMed] [Google Scholar]
- [60].Fahl CN, Branco LM, Bergo FP, D’Abreu A, Lopes-Cendes I, Franca MC Jr., Spinal cord damage in Machado-Joseph disease, Cerebellum 14(2) (2015) 128–32. [DOI] [PubMed] [Google Scholar]
- [61].Mascalchi M, Tosetti M, Plasmati R, Bianchi MC, Tessa C, Salvi F, Frontali M, Valzania F, Bartolozzi C, Tassinari CA, Proton magnetic resonance spectroscopy in an Italian family with spinocerebellar ataxia type 1, Ann Neurol 43(2) (1998) 244–52. [DOI] [PubMed] [Google Scholar]
- [62].Lirng JF, Wang PS, Chen HC, Soong BW, Guo WY, Wu HM, Chang CY, Differences between spinocerebellar ataxias and multiple system atrophy-cerebellar type on proton magnetic resonance spectroscopy, PLoS One 7(10) (2012) e47925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Viau M, Marchand L, Bard C, Boulanger Y, (1)H magnetic resonance spectroscopy of autosomal ataxias, Brain Res 1049(2) (2005) 191–202. [DOI] [PubMed] [Google Scholar]
- [64].Lei L, Liao Y, Liao W, Zhou J, Yuan Y, Wang J, Jiang H, Shen L, Tang B, Magnetic resonance spectroscopy of the cerebellum in patients with spinocerebellar ataxia type 3/Machado-Joseph disease, Zhong Nan Da Xue Xue Bao Yi Xue Ban 36(6) (2011) 511–9. [DOI] [PubMed] [Google Scholar]
- [65].Hadjivassiliou M, Wallis LI, Hoggard N, Grunewald RA, Griffiths PD, Wilkinson ID, MR spectroscopy and atrophy in Gluten, Friedreich’s and SCA6 ataxias, Acta Neurol Scand 126(2) (2012) 138–43. [DOI] [PubMed] [Google Scholar]
- [66].Friedrich J, Kordasiewicz HB, O’Callaghan B, Handler HP, Wagener C, Duvick L, Swayze EE, Rainwater O, Hofstra B, Benneyworth M, Nichols-Meade T, Yang P, Chen Z, Ortiz JP, Clark HB, Oz G, Larson S, Zoghbi HY, Henzler C, Orr HT, Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles, JCI Insight 3(21) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gilman S, Sima AA, Junck L, Kluin KJ, Koeppe RA, Lohman ME, Little R, Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions, Ann Neurol 39(2) (1996) 241–55. [DOI] [PubMed] [Google Scholar]
- [68].Wullner U, Reimold M, Abele M, Burk K, Minnerop M, Dohmen BM, Machulla HJ, Bares R, Klockgether T, Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6, Arch Neurol 62(8) (2005) 1280–5. [DOI] [PubMed] [Google Scholar]
- [69].Oh M, Kim JS, Oh JS, Lee CS, Chung SJ, Different subregional metabolism patterns in patients with cerebellar ataxia by 18F-fluorodeoxyglucose positron emission tomography, PLoS One 12(3) (2017) e0173275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Soong B, Cheng C, Liu R, Shan D, Machado-Joseph disease: clinical, molecular, and metabolic characterization in Chinese kindreds, Ann Neurol 41(4) (1997) 446–52. [DOI] [PubMed] [Google Scholar]
- [71].Soong BW, Liu RS, Positron emission tomography in asymptomatic gene carriers of Machado-Joseph disease, J Neurol Neurosurg Psychiatry 64(4) (1998) 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Soong B, Liu R, Wu L, Lu Y, Lee H, Metabolic characterization of spinocerebellar ataxia type 6, Arch Neurol 58(2) (2001) 300–4. [DOI] [PubMed] [Google Scholar]
- [73].Yun JY, Lee WW, Kim HJ, Kim JS, Kim JM, Kim HJ, Kim SY, Kim JY, Park SS, Kim YK, Kim SE, Jeon BS, Relative contribution of SCA2, SCA3 and SCA17 in Korean patients with parkinsonism and ataxia, Parkinsonism Relat Disord 17(5) (2011) 338–42. [DOI] [PubMed] [Google Scholar]
- [74].Yen TC, Lu CS, Tzen KY, Wey SP, Chou YH, Weng YH, Kao PF, Ting G, Decreased dopamine transporter binding in Machado-Joseph disease, J Nucl Med 41(6) (2000) 994–8. [PubMed] [Google Scholar]
- [75].Braga-Neto P, Pedroso JL, Gadelha A, Laureano MR, de Souza Noto C, Garrido GJ, Barsottini OG, Psychosis in Machado-Joseph Disease: Clinical Correlates, Pathophysiological Discussion, and Functional Brain Imaging. Expanding the Cerebellar Cognitive Affective Syndrome, Cerebellum 15(4) (2016) 483–90. [DOI] [PubMed] [Google Scholar]
- [76].Honjo K, Ohshita T, Kawakami H, Naka H, Imon Y, Maruyama H, Mimori Y, Matsumoto M, Quantitative assessment of cerebral blood flow in genetically confirmed spinocerebellar ataxia type 6, Arch Neurol 61(6) (2004) 933–7. [DOI] [PubMed] [Google Scholar]
- [77].Brouillette AM, Oz G, Gomez CM, Cerebrospinal Fluid Biomarkers in Spinocerebellar Ataxia: A Pilot Study, Dis Markers 2015 (2015) 413098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wilke C, Bender F, Hayer SN, Brockmann K, Schols L, Kuhle J, Synofzik M, Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: a pilot study, J Neurol 265(7) (2018) 1618–1624. [DOI] [PubMed] [Google Scholar]
- [79].Li QF, Dong Y, Yang L, Xie JJ, Ma Y, Du YC, Cheng HL, Ni W, Wu ZY, Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3, Mol Neurodegener 14(1) (2019) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wilke C, Haas E, Reetz K, Faber J, Garcia-Moreno H, Santana MM, van de Warrenburg B, Hengel H, Lima M, Filla A, Durr A, Melegh B, Masciullo M, Infante J, Giunti P, Neumann M, de Vries J, Pereira de Almeida L, Rakowicz M, Jacobi H, Schule R, Kaeser SA, Kuhle J, Klockgether T, Schols L, S.C.A.n.s. group, Barro C, Hubener-Schmid J, Synofzik M, Neurofilaments in spinocerebellar ataxia type 3: blood biomarkers at the preataxic and ataxic stage in humans and mice, EMBO Mol Med 12(7) (2020) e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gonsior K, Kaucher GA, Pelz P, Schumann D, Gansel M, Kuhs S, Klockgether T, Forlani S, Durr A, Hauser S, Rattay TW, Synofzik M, Hengel H, Schols L, Riess OH, Hubener-Schmid J, PolyQ-expanded ataxin-3 protein levels in peripheral blood mononuclear cells correlate with clinical parameters in SCA3: a pilot study, J Neurol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Prudencio M, Garcia-Moreno H, Jansen-West KR, Al-Shaikh RH, Gendron TF, Heckman MG, Spiegel MR, Carlomagno Y, Daughrity LM, Song Y, Dunmore JA, Byron N, Oskarsson B, Nicholson KA, Staff NP, Gorcenco S, Puschmann A, Lemos J, Januario C, LeDoux MS, Friedman JH, Polke J, Labrum R, Shakkottai V, McLoughlin HS, Paulson HL, Konno T, Onodera O, Ikeuchi T, Tada M, Kakita A, Fryer JD, Karremo C, Gomes I, Caviness JN, Pittelkow MR, Aasly J, Pfeiffer RF, Veerappan V, Eggenberger ER, Freeman WD, Huang JF, Uitti RJ, Wierenga KJ, Marin Collazo IV, Tipton PW, van Gerpen JA, van Blitterswijk M, Bu G, Wszolek ZK, Giunti P, Petrucelli L, Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3, Sci Transl Med 12(566) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hu ZW, Yang ZH, Zhang S, Liu YT, Yang J, Wang YL, Mao CY, Zhang QM, Shi CH, Xu YM, Carboxyl Terminus of Hsp70-Interacting Protein Is Increased in Serum and Cerebrospinal Fluid of Patients With Spinocerebellar Ataxia Type 3, Front Neurol 10 (2019) 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].da Silva Carvalho G, Saute JA, Haas CB, Torrez VR, Brochier AW, Souza GN, Furtado GV, Gheno T, Russo A, Monte TL, Schumacher-Schuh A, D’Avila R, Donis KC, Castilhos RM, Souza DO, Saraiva-Pereira ML, Torman VL, Camey S, Portela LV, Jardim LB, Cytokines in Machado Joseph Disease/Spinocerebellar Ataxia 3, Cerebellum 15(4) (2016) 518–25. [DOI] [PubMed] [Google Scholar]
- [85].Shi Y, Huang F, Tang B, Li J, Wang J, Shen L, Xia K, Jiang H, MicroRNA profiling in the serums of SCA3/MJD patients, Int J Neurosci 124(2) (2014) 97–101. [DOI] [PubMed] [Google Scholar]
- [86].Zhou J, Lei L, Shi Y, Wang J, Jiang H, Shen L, Tang B, Serum concentrations of NSE and S100B in spinocerebellar ataxia type 3/Machado-Joseph disease, Zhong Nan Da Xue Xue Bao Yi Xue Ban 36(6) (2011) 504–10. [DOI] [PubMed] [Google Scholar]
- [87].Tort AB, Portela LV, Rockenbach IC, Monte TL, Pereira ML, Souza DO, Rieder CR, Jardim LB, S100B and NSE serum concentrations in Machado Joseph disease, Clin Chim Acta 351(1–2) (2005) 143–8. [DOI] [PubMed] [Google Scholar]
- [88].Araujo J, Breuer P, Dieringer S, Krauss S, Dorn S, Zimmermann K, Pfeifer A, Klockgether T, Wuellner U, Evert BO, FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3, Hum Mol Genet 20(15) (2011) 2928–41. [DOI] [PubMed] [Google Scholar]
- [89].Weber JJ, Sowa AS, Binder T, Hubener J, From pathways to targets: understanding the mechanisms behind polyglutamine disease, Biomed Res Int 2014 (2014) 701758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yu YC, Kuo CL, Cheng WL, Liu CS, Hsieh M, Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado-Joseph disease, J Neurosci Res 87(8) (2009) 1884–91. [DOI] [PubMed] [Google Scholar]
- [91].Pacheco LS, da Silveira AF, Trott A, Houenou LJ, Algarve TD, Bello C, Lenz AF, Manica-Cattani MF, da Cruz IB, Association between Machado-Joseph disease and oxidative stress biomarkers, Mutat Res 757(2) (2013) 99–103. [DOI] [PubMed] [Google Scholar]
- [92].de Assis AM, Saute JAM, Longoni A, Haas CB, Torrez VR, Brochier AW, Souza GN, Furtado GV, Gheno TC, Russo A, Monte TL, Castilhos RM, Schumacher-Schuh A, D’Avila R, Donis KC, de Mello Rieder CR, Souza DO, Camey S, Leotti VB, Jardim LB, Portela LV, Peripheral Oxidative Stress Biomarkers in Spinocerebellar Ataxia Type 3/Machado-Joseph Disease, Front Neurol 8 (2017) 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Craft S, Watson GS, Insulin and neurodegenerative disease: shared and specific mechanisms, Lancet Neurol 3(3) (2004) 169–78. [DOI] [PubMed] [Google Scholar]
- [94].Saute JA, da Silva AC, Muller AP, Hansel G, de Mello AS, Maeda F, Vedolin L, Saraiva-Pereira ML, Souza DO, Arpa J, Torres-Aleman I, Portela LV, Jardim LB, Serum insulin-like system alterations in patients with spinocerebellar ataxia type 3, Mov Disord 26(4) (2011) 731–5. [DOI] [PubMed] [Google Scholar]
- [95].Nambo-Venegas R, Valdez-Vargas C, Cisneros B, Palacios-Gonzalez B, Vela-Amieva M, Ibarra-Gonzalez I, Cerecedo-Zapata CM, Martinez-Cruz E, Cortes H, Reyes-Grajeda JP, Magana JJ, Altered Plasma Acylcarnitines and Amino Acids Profile in Spinocerebellar Ataxia Type 7, Biomolecules 10(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hou X, Gong X, Zhang L, Li T, Yuan H, Xie Y, Peng Y, Qiu R, Xia K, Tang B, Jiang H , Identification of a potential exosomal biomarker in spinocerebellar ataxia Type 3/Machado-Joseph disease, Epigenomics 11(9) (2019) 1037–1056. [DOI] [PubMed] [Google Scholar]
- [97].Borgonio-Cuadra VM, Valdez-Vargas C, Romero-Cordoba S, Hidalgo-Miranda A, Tapia-Guerrero Y, Cerecedo-Zapata CM, Hernandez-Hernandez O, Cisneros B, Magana JJ, Wide Profiling of Circulating MicroRNAs in Spinocerebellar Ataxia Type 7, Mol Neurobiol 56(9) (2019) 6106–6120. [DOI] [PubMed] [Google Scholar]
- [98].Luis L, Costa J, Munoz E, de Carvalho M, Carmona S, Schneider E, Gordon CR, Valls-Sole J, Vestibulo-ocular reflex dynamics with head-impulses discriminates spinocerebellar ataxias types 1, 2 and 3 and Friedreich ataxia, J Vestib Res 26(3) (2016) 327–34. [DOI] [PubMed] [Google Scholar]
- [99].Kim JS, Kim JS, Youn J, Seo DW, Jeong Y, Kang JH, Park JH, Cho JW, Ocular motor characteristics of different subtypes of spinocerebellar ataxia: distinguishing features, Mov Disord 28(9) (2013) 1271–7. [DOI] [PubMed] [Google Scholar]
- [100].Wu C, Chen DB, Feng L, Zhou XX, Zhang JW, You HJ, Liang XL, Pei Z, Li XH, Oculomotor deficits in spinocerebellar ataxia type 3: Potential biomarkers of preclinical detection and disease progression, CNS Neurosci Ther 23(4) (2017) 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Velazquez Perez L, Sanchez Cruz G, Canales Ochoa N, Rodriguez Labrada R, Rodriguez Diaz J, Almaguer Mederos L, Laffita Mesa J, Electrophysiological features in patients and presymptomatic relatives with spinocerebellar ataxia type 2, J Neurol Sci 263(1–2) (2007) 158–64. [DOI] [PubMed] [Google Scholar]
- [102].Kumagai R, Kaseda Y, Kawakami H, Nakamura S, Electrophysiological studies in spinocerebellar ataxia type 6: a statistical approach, Neuroreport 11(5) (2000) 969–72. [DOI] [PubMed] [Google Scholar]
- [103].Abele M, Burk K, Andres F, Topka H, Laccone F, Bosch S, Brice A, Cancel G, Dichgans J, Klockgether T, Autosomal dominant cerebellar ataxia type I. Nerve conduction and evoked potential studies in families with SCA1, SCA2 and SCA3, Brain 120 ( Pt 12) (1997) 2141–8. [DOI] [PubMed] [Google Scholar]
- [104].Jhunjhunwala K, Prashanth DK, Netravathi M, Jain S, Purushottam M, Pal PK, Alterations in cortical excitability and central motor conduction time in spinocerebellar ataxias 1, 2 and 3: a comparative study, Parkinsonism Relat Disord 19(3) (2013) 306–11. [DOI] [PubMed] [Google Scholar]
- [105].Yokota T, Sasaki H, Iwabuchi K, Shiojiri T, Yoshino A, Otagiri A, Inaba A, Yuasa T, Electrophysiological features of central motor conduction in spinocerebellar atrophy type 1, type 2, and Machado-Joseph disease, J Neurol Neurosurg Psychiatry 65(4) (1998) 530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schwenkreis P, Tegenthoff M, Witscher K, Bornke C, Przuntek H, Malin JP, Schols L, Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect, Brain 125(Pt 2) (2002) 301–9. [DOI] [PubMed] [Google Scholar]
- [107].Farrar MA, Vucic S, Nicholson G, Kiernan MC, Motor cortical dysfunction develops in spinocerebellar ataxia type 3, Clin Neurophysiol 127(11) (2016) 3418–3424. [DOI] [PubMed] [Google Scholar]
- [108].Lee YC, Chen JT, Liao KK, Wu ZA, Soong BW, Prolonged cortical relay time of long latency reflex and central motor conduction in patients with spinocerebellar ataxia type 6, Clin Neurophysiol 114(3) (2003) 458–62. [DOI] [PubMed] [Google Scholar]
- [109].Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ, Cerebellar motor learning: are environment dynamics more important than error size?, J Neurophysiol 110(2) (2013) 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R, Size of error affects cerebellar contributions to motor learning, J Neurophysiol 103(4) (2010) 2275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Butcher PA, Ivry RB, Kuo SH, Rydz D, Krakauer JW, Taylor JA, The cerebellum does more than sensory prediction error-based learning in sensorimotor adaptation tasks, J Neurophysiol 118(3) (2017) 1622–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Honda T, Nagao S, Hashimoto Y, Ishikawa K, Yokota T, Mizusawa H, Ito M, Tandem internal models execute motor learning in the cerebellum, Proc Natl Acad Sci U S A 115(28) (2018) 7428–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Honda T, Mitoma H, Yoshida H, Bando K, Terashi H, Taguchi T, Miyata Y, Kumada S, Hanakawa T, Aizawa H, Yano S, Kondo T, Mizusawa H, Manto M, Kakei S, Assessment and Rating of Motor Cerebellar Ataxias With the Kinect v2 Depth Sensor: Extending Our Appraisal, Front Neurol 11 (2020) 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lee J, Kagamihara Y, Kakei S, A New Method for Functional Evaluation of Motor Commands in Patients with Cerebellar Ataxia, PLoS One 10(7) (2015) e0132983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bhanpuri NH, Okamura AM, Bastian AJ, Predicting and correcting ataxia using a model of cerebellar function, Brain 137(Pt 7) (2014) 1931–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Aprigliano F, Martelli D, Kang J, Kuo SH, Kang UJ, Monaco V, Micera S, Agrawal SK , Effects of repeated waist-pull perturbations on gait stability in subjects with cerebellar ataxia, J Neuroeng Rehabil 16(1) (2019) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bakhti KKA, Laffont I, Muthalib M, Froger J, Mottet D, Kinect-based assessment of proximal arm non-use after a stroke, J Neuroeng Rehabil 15(1) (2018) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Earhart GM, Bastian AJ, Selection and coordination of human locomotor forms following cerebellar damage, J Neurophysiol 85(2) (2001) 759–69. [DOI] [PubMed] [Google Scholar]
- [119].Hashimoto Y, Honda T, Matsumura K, Nakao M, Soga K, Katano K, Yokota T, Mizusawa H, Nagao S, Ishikawa K, Quantitative evaluation of human cerebellum-dependent motor learning through prism adaptation of hand-reaching movement, PLoS One 10(3) (2015) e0119376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Matsuda S, Matsumoto H, Furubayashi T, Hanajima R, Tsuji S, Ugawa Y, Terao Y, The 3-second rule in hereditary pure cerebellar ataxia: a synchronized tapping study, PLoS One 10(2) (2015) e0118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Morton SM, Bastian AJ, Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking, J Neurosci 26(36) (2006) 9107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tran H, Pathirana PN, Horne M, Power L, Szmulewicz DJ, Quantitative Evaluation of Cerebellar Ataxia Through Automated Assessment of Upper Limb Movements, IEEE Trans Neural Syst Rehabil Eng 27(5) (2019) 1081–1091. [DOI] [PubMed] [Google Scholar]
- [123].Lin CC, Ashizawa T, Kuo SH, Collaborative Efforts for Spinocerebellar Ataxia Research in the United States: CRC-SCA and READISCA, Front Neurol 11 (2020) 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Huang SR, Wu YT, Jao CW, Soong BW, Lirng JF, Wu HM, Wang PS, CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3, Neuroimage Clin 13 (2017) 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Oz G, Hutter D, Tkac I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM, Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status, Mov Disord 25(9) (2010) 1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Oz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM, Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy, Cerebellum 10(2) (2011) 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Joers JM, Deelchand DK, Lyu T, Emir UE, Hutter D, Gomez CM, Bushara KO, Eberly LE, Oz G, Neurochemical abnormalities in premanifest and early spinocerebellar ataxias, Ann Neurol 83(4) (2018) 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Doss S, Brandt AU, Oberwahrenbrock T, Endres M, Paul F, Rinnenthal JL, Metabolic evidence for cerebral neurodegeneration in spinocerebellar ataxia type 1, Cerebellum 13(2) (2014) 199–206. [DOI] [PubMed] [Google Scholar]
- [129].Wang PS, Chen HC, Wu HM, Lirng JF, Wu YT, Soong BW, Association between proton magnetic resonance spectroscopy measurements and CAG repeat number in patients with spinocerebellar ataxias 2, 3, or 6, PLoS One 7(10) (2012) e47479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hirano S, Shinotoh H, Arai K, Aotsuka A, Yasuno F, Tanaka N, Ota T, Sato K, Fukushi K, Tanada S, Hattori T, Irie T, PET study of brain acetylcholinesterase in cerebellar degenerative disorders, Mov Disord 23(8) (2008) 1154–60. [DOI] [PubMed] [Google Scholar]
- [131].Boesch SM, Donnemiller E, Muller J, Seppi K, Weirich-Schwaiger H, Poewe W, Wenning GK, Abnormalities of dopaminergic neurotransmission in SCA2: a combined 123I-betaCIT and 123I-IBZM SPECT study, Mov Disord 19(11) (2004) 1320–5. [DOI] [PubMed] [Google Scholar]
- [132].Varrone A, Salvatore E, De Michele G, Barone P, Sansone V, Pellecchia MT, Castaldo I, Coppola G, Brunetti A, Salvatore M, Pappata S, Filla A, Reduced striatal [123 I]FP-CIT binding in SCA2 patients without parkinsonism, Ann Neurol 55(3) (2004) 426–30. [DOI] [PubMed] [Google Scholar]
- [133].Ishibashi M, Sakai T, Matsuishi T, Yonekura Y, Yamashita Y, Abe T, Ohnishi Y, Hayabuchi N, Decreased benzodiazepine receptor binding in Machado-Joseph disease, J Nucl Med 39(9) (1998) 1518–20. [PubMed] [Google Scholar]
- [134].Chen HC, Lirng JF, Soong BW, Guo WY, Wu HM, Chen CC, Chang CY, The merit of proton magnetic resonance spectroscopy in the longitudinal assessment of spinocerebellar ataxias and multiple system atrophy-cerebellar type, Cerebellum Ataxias 1 (2014) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Boesch SM, Wolf C, Seppi K, Felber S, Wenning GK, Schocke M, Differentiation of SCA2 from MSA-C using proton magnetic resonance spectroscopic imaging, J Magn Reson Imaging 25(3) (2007) 564–9. [DOI] [PubMed] [Google Scholar]
- [136].Adanyeguh IM, Henry PG, Nguyen TM, Rinaldi D, Jauffret C, Valabregue R, Emir UE, Deelchand DK, Brice A, Eberly LE, Oz G, Durr A, Mochel F, In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7, Mov Disord 30(5) (2015) 662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].D’Abreu A, Franca M Jr., Appenzeller S, Lopes-Cendes I, Cendes F, Axonal dysfunction in the deep white matter in Machado-Joseph disease, J Neuroimaging 19(1) (2009) 9–12. [DOI] [PubMed] [Google Scholar]
- [138].Boesch SM, Schocke M, Burk K, Hollosi P, Fornai F, Aichner FT, Poewe W, Felber S, Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6, J Magn Reson Imaging 13(4) (2001) 553–9. [DOI] [PubMed] [Google Scholar]
- [139].Shi Y, Wang C, Huang F, Chen Z, Sun Z, Wang J, Tang B, Ashizawa T, Klockgether T, Jiang H, High Serum GFAP Levels in SCA3/MJD May Not Correlate with Disease Progression, Cerebellum 14(6) (2015) 677–81. [DOI] [PubMed] [Google Scholar]
- [140].Ghisolfi ES, Maegawa GH, Becker J, Zanardo AP, Strimitzer IM Jr., Prokopiuk AS, Pereira ML, Carvalho T, Jardim LB, Lara DR, Impaired P50 sensory gating in Machado-Joseph disease, Clin Neurophysiol 115(10) (2004) 2231–5. [DOI] [PubMed] [Google Scholar]
- [141].Lu MK, Shih HT, Huang KJ, Ziemann U, Tsai CH, Chang FC, Chen YC, Lin YT, Huang WS, Lee CC, Liu CS, Movement-related cortical potentials in patients with Machado-Joseph disease, Clin Neurophysiol 119(5) (2008) 1010–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.