Abstract
Background
Metastatic small bowel adenocarcinoma (SBA) has a poor prognosis. Due to its rarity, high-quality data are lacking to guide treatment. This retrospective analysis was conducted to help characterize the treatment options for patients with metastatic SBA while providing clinically meaningful prognostic information.
Patients and methods
In total, 437 patients who initially presented with or developed metastatic SBA between September 1977 and September 2019 were identified from the MD Anderson Tumor Registry. Clinical data were collected from review of the medical record. Overall response rates (ORR), time to progression (TTP), and overall survival (OS) were assessed across various treatments and treatment lines.
Results
The median OS from diagnosis of metastatic disease was 15.9 months [95% confidence interval (CI): 14.3-17.9]. Seventy-five patients (17.1%) underwent metastasectomy, which was associated with a median OS of 34.5 versus 17.1 months among patients who received chemotherapy alone (P < 0.001). Fluoropyrimidine plus platinum (n = 164) was the most common first-line chemotherapy, associated with an ORR of 59% and TTP of 8.1 months. Irinotecan with 5-FU (n = 101) was the most common second-line therapy associated with an ORR of 31% and TTP of 4.0 months. Twenty-two patients received immunotherapy; 5 of 6 patients with deficient mismatch repair (dMMR) responded, while 0 of 16 with proficient mismatch repair (pMMR) responded. Taxane-based chemotherapy was given to 34 patients with an ORR of 21% and a median TTP of 2.4 months. Among 11 patients who received anti-epidermal-growth-factor-receptor (EGFR) monotherapy, the best response was stable disease (SD) in 1 patient.
Conclusions
In well-selected patients with SBA, metastasectomy appears to be associated with improved OS. This improvement was seen across metastasectomy sites, including liver, lung and peritoneal. Anti-programmed cell death protein 1 (PD-1) based immunotherapy was active for dMMR SBA but not pMMR SBA. While taxane-based chemotherapy demonstrates therapeutic activity, the activity of anti-EGFR therapy was limited.
Key words: small bowel adenocarcinoma, metastasectomy, chemotherapy
Highlights
-
•
Metastasectomy for well-selected metastatic SBA patients was associated with improved OS.
-
•
Anti-PD1-based immunotherapy was active for dMMR SBA but not pMMR SBA.
-
•
Taxane-based chemotherapy demonstrated clinical activity in refractory SBA.
-
•
Anti-EGFR therapy demonstrated minimal activity in SBA.
Introduction
Small bowel adenocarcinoma (SBA) is a rare cancer that commonly presents at an advanced stage. Patients with metastatic disease have a poor prognosis, with reported median overall survival (OS) of <20 months among patients who receive chemotherapy.1,2 Though no randomized clinical trials have been conducted in this disease, multiple prospective phase II studies and retrospective studies have determined fluoropyrimidine and oxaliplatin as the standard frontline systemic chemotherapy approach.3, 4, 5, 6, 7 However, guidance for subsequent systemic treatment options is largely limited to retrospective studies, small single-arm prospective studies, and extrapolation from experience with colorectal cancer treatment.
Recently, the National Comprehensive Cancer Network (NCCN) developed treatment algorithms for the management of SBA.8 Recommended second- and third-line therapies include irinotecan with or without 5-FU (FOLFIRI) or taxane-based chemotherapy, with immune checkpoint inhibitors reserved for those with deficiency in mismatch repair (dMMR). Notably, certain therapies such as TAS-102 (trifluridine–tipiracil), regorafenib, cetuximab, and panitumumab, which are utilized in colorectal cancer (CRC) management, were not recommend for SBA. In part, this reflects the limited nature of clinical data for systemic therapies in SBA.
In addition to systemic therapy for metastatic SBA, the role and benefit derived from metastasectomy is uncertain. Though small retrospective studies have suggested encouraging outcomes for SBA that underwent metastasectomies, these reports are limited by small sample size. The largest report included 34 metastatic SBA patients undergoing various metastasectomies with a median OS of 28.6 months.9
Through analysis of a large institutional database, this present study aims to help characterize the treatment options for patients with metastatic SBA, while providing clinically meaningful prognostic information for various subsets of SBA.
Patients and methods
Patients and data collection
The Institutional Review Board of The University of Texas MD Anderson Cancer Center (UTMDACC) approved this retrospective analysis with informed consent waiver. All patients from the MD Anderson Tumor Registry who presented for metastatic SBA at the UTMDACC between September 1977 and September 2019 were identified. Patients were included in this study if they had documented metastatic disease, either at presentation or after initially presenting with localized disease. Patients who had tumors that involved the ampulla of Vater were excluded to avoid inclusion of patients with primary ampullary tumors. In total, 675 patients with SBA were identified, of which 437 patients met criteria.
Information regarding patient demographics, pathology, treatments administered, and treatment response were obtained from review of the medical record. Designation of tumor stage was in accordance with the American Joint Cancer Committee on Cancer staging system and histologic grade was determined according to the World Health Organization standard grading system.10 dMMR was determined by either immunohistochemical testing or PCR-based microsatellite instability testing.
For the analysis of metastasectomy outcomes, only patients who underwent R0 or R1 resections (microscopic residual tumor) were included, while R2 resections (macroscopic residual tumor) were excluded. Based on institutional practice, all patients who underwent peritoneal cytoreductive surgeries had stable or responding disease to systemic chemotherapy before surgical resection. Complete cytoreductive surgeries included both completeness of cytoreduction (CC) score of CC-0 and CC-1.
First-line treatment was defined as the initial chemotherapy and/or targeted agents administered for distant metastatic disease. Second-line therapy was defined as chemotherapy and/or targeted agents administered after discontinuing first-line therapy. If adjuvant therapy was completed within 12 months, then the initial therapy for metastatic disease was considered as second-line treatment. Third-line therapies were therapies administered after discontinuation of second-line therapies. Re-challenge of chemotherapeutic agents without the addition of an additional agent were excluded from treatment response analyses.
Time to progression (TTP) was defined as time from initiation of therapy to evidence of disease progression on serial radiographic imaging; patients were censored if no progression was seen on last scan. For TTP analyses, patients were required to have at least two doses of the respective chemotherapy regimen and a radiographic imaging evaluation. Radiographic response assessment was based upon the treating physician's response assessment. Relapse-free survival (RFS) was defined as the time for metastasectomy to disease recurrence or death. OS was defined as the time from diagnosis of distant metastatic or initiation of treatment line until death of any cause; patients were censored if alive at last follow-up.
Statistical analysis
Demographic and clinical variables were summarized using descriptive statistics, frequency (%) for categorical variables, and median (min, max) for continuous variables, grouped by tumor locations. Comparisons between continuous variables were conducted using the Mann–Whitney U test, while categorical groups were conducted using the nonparametric Fisher's exact test when applicable. The Kaplan–Meier method was used to visualize and estimate survival curves. For the landmark analysis of metastasectomy outcomes, a cut-off was arrived at by determining the 75th percentile of time to metastasectomy and adding 3 months; this was chosen to ensure an adequate number of patients were included in the analysis. Cox proportional hazards regression models were applied to assess the association between patient characteristics and time-to-event outcomes while controlling for covariates. Backwards elimination technique was used to build multivariable models for certain outcomes wherein factors that showed univariate associations at P < 0.1 were passed to a second stage for multivariable modeling. In the second stage, all variables were placed in the model and the least significant factors were removed until only factors with P < 0.05 were left in the model. Our study was intended to be descriptive in nature as opposed to hypothesis-testing, and P values are presented without correction. For all analyses, a P value of ≤0.05 was considered statistically significant. All statistical analyses were conducted using R version 3.6.1 (www.r-project.org).
Results
Patient characteristics
Table 1 shows the clinical characteristics of 437 SBA patients who met our study's inclusion criteria. The median age was 58 (range, 18-85) years and 57% of patients were male. Two hundred and eighty-nine patients (66%) initially presented with stage IV disease. The duodenum was the most common primary site (n = 215, 49%), followed by jejunum (n = 142, 33%), and ileum (n = 70, 16%). Median follow-up time from diagnosis of metastatic disease was 14.5 months.
Table 1.
Patient and tumor characteristics
| Characteristic | All | Duodenum | Jejunum | Ileum | Not otherwise specified |
|---|---|---|---|---|---|
| Total patients, n (%) | 437 | 215 (49) | 142 (33) | 70 (16) | 10 (2) |
| Age, years | |||||
| Median (range) | 58 (18-85) | 62 (23-85) | 52 (27-82) | 57 (18-80) | 52 (42-70) |
| Sex, n (%) | |||||
| Male | 251 (57) | 130 (60) | 75 (53) | 41 (59) | 5 (50) |
| Female | 186 (43) | 85 (40) | 67 (47) | 29 (41) | 5 (50) |
| Race, n (%) | |||||
| White | 339 (77) | 161 (75) | 111 (78) | 59 (84) | 8 (80) |
| Black | 54 (12) | 26 (12) | 20 (14) | 6 (9) | 2 (20) |
| Hispanic | 24 (6) | 14 (7) | 8 (6) | 2 (3) | 0 (0) |
| Other | 20 (5) | 14 (7) | 3 (2) | 3 (4) | 0 (0) |
| Histologic grade, n (%) | |||||
| Grade 1 | 7 (2) | 2 (1) | 5 (3) | 0 (0) | 0 (0) |
| Grade 2 | 207 (47) | 100 (47) | 76 (54) | 27 (39) | 4 (40) |
| Grade 3 | 177 (41) | 91 (42) | 47 (33) | 35 (50) | 4 (40) |
| Unspecified | 46 (10) | 22 (10) | 14 (10) | 8 (11) | 2 (20) |
| Stage, n (%) | |||||
| Stage 1-3 | 148 (34) | 73 (34) | 42 (30) | 33 (47) | 0 (0) |
| Stage 4 | 289 (66) | 142 (66) | 100 (70) | 37 (53) | 10 (100) |
| MSI status, n (%) | |||||
| dMMR | 23 (5) | 16 (7) | 4 (3) | 3 (4) | 0 (0) |
| pMMR | 157 (36) | 73 (34) | 49 (34) | 31 (44) | 4 (40) |
| Unknown | 257 (59) | 126 (59) | 89 (63) | 36 (52) | 6 (60) |
| KRAS, n (%) | |||||
| Wild type | 84 (19) | 43 (20) | 27 (19) | 12 (17) | 2 (20) |
| Mutated | 112 (26) | 53 (25) | 33 (23) | 22 (31) | 4 (40) |
| Unknown | 241 (55) | 119 (55) | 82 (58) | 36 (52) | 4 (40) |
| Risk factors, n (%) | |||||
| IBD | 32 (7) | 1 (0) | 7 (5) | 23 (33) | 0 (0) |
| FAP | 5 (1) | 1 (0) | 2 (3) | 2 (3) | 0 (0) |
| Celiac disease | 12 (3) | 7 (3) | 5 (1) | 0 (0) | 0 (0) |
| Peutz–Jeghers syndrome | 1 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Presenting organ involvement, n (%) | |||||
| Single | 336 (77) | 160 (74) | 112 (79) | 54 (52) | 10 (100) |
| Multiple | 101 (23) | 55 (26) | 30 (21) | 16 (52) | 0 (0) |
| Presenting metastatic sites, n (%) | |||||
| Liver | 198 (45) | 127 (59) | 45 (32) | 26 (37) | 0 (0) |
| Peritoneum | 215 (49) | 66 (31) | 99 (70) | 40 (57) | 10 (100) |
| Lung | 47 (11) | 30 (14) | 10 (7) | 7 (10) | 0 (0) |
| Distant LN | 65 (15) | 37 (17) | 19 (13) | 9 (13) | 0 (0) |
| Other | 35 (8) | 18 (8) | 8 (6) | 9 (13) | 0 (0) |
Distant LN, distant lymph node; dMMR, deficient mismatch repair; FAP, familial adenomatous polyposis; IBD, inflammatory bowel disease; MSI, microsatellite instability; pMMR, proficient mismatch repair.
One hundred and eighty-three patients (42%) underwent surgical resection for their primary tumor, with 95 (22%) receiving chemotherapy in the adjuvant or neoadjuvant setting. Additionally, 14 patients (3%) received adjuvant radiation.
For patients presenting with metastatic disease, a single organ site of metastasis was noted in 336 cases (77%), with the remaining (23%) having multiple organs involved with metastatic disease. The liver and peritoneum were the most common metastatic sites. The most common metastatic site for duodenal adenocarcinoma was the liver, whereas the peritoneum was the most common metastatic site for jejunal and ileal adenocarcinomas. The distribution of metastases by small bowel subsite is shown in Supplementary Material, Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Survival and prognostic factors
Among the patients included in the study, median OS was 15.9 months [95% confidence interval (CI): 14.3-17.9] from the diagnosis of metastatic disease. On univariate analysis, non-duodenal primary site, dMMR, chemotherapy, celiac disease (CD), single metastatic site, and metastasectomy were associated with improved OS (P < 0.05 for each variable), while stage at diagnosis and year at diagnosis were not (P = 0.32 and P = 0.66, respectively). On multivariate analysis, CD, chemotherapy, metastasectomy, and dMMR status were associated with prolonged OS (P = 0.04, P < 0.001, P < 0.001, and P = 0.03, respectively). Of the celiac patients, three of eight with mismatch repair (MMR) status testing (38%) were dMMR. The coefficients, hazard ratios, and 95% CIs for each model are shown in Supplementary Material, Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Metastasectomy
Two hundred and ninety-six (67.7%) patients had received at least one line of chemotherapy after diagnosis of metastatic disease of which 75 patients (17.1%) underwent metastasectomy with a median follow-up time of 28.9 months. Metastasectomy patients were significantly younger, less likely to have poor differentiation, and less likely to have multiorgan metastasis compared with non-metastasectomy counterparts (Supplementary Material, Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100132). Those who underwent metastasectomy had a median OS of 34.5 months (95% CI: 27.7-46.0) from diagnosis of metastatic disease versus 17.1 months (95% CI: 15.2-19.9) for patients who received chemotherapy alone (P < 0.001). A landmark analysis using a cut-off of 9 months from diagnosis of metastatic disease was conducted; for 261 patients who remained after excluding patients dead or lost to follow-up at 9 months, median OS was 40.7 months (95% CI: 32.0-61.2) among metastasectomy patients versus 20.1 months (95% CI: 18.1-22.6) among non-metastasectomy patients (P < 0.001). Kaplan–Meier plots for the landmark analysis are shown in Figure 1.
Figure 1.
A Kaplan–Meier plot for the landmark analysis, including only patients who survived at least 9 months after diagnosis of metastatic disease, is shown in A.
A Kaplan–Meier plots for OS and RFS by resection site are shown in B and C, respectively.
LN, lymph node.
The sites of resection were peritoneal (n = 49, 65.3%), liver (n = 16, 21.3%), distant lymph nodes (n = 7, 9.3%), and lung (n = 3, 0.4%). OS according to site of resection was also assessed. Median OS from diagnosis of metastatic disease was 36.1 months (CI: 27.7-53.2), 43.6 months [CI: 15.5 to not reached (NR)], NR, and 31.9 months (CI: 3.2 to NR) for peritoneal, liver, distant lymph node, and lung resection, respectively, (P = 0.397). The median RFS for all metastasectomy patients was 13.4 months (CI: 11.5-18.8); median RFS by site was 12.4 months (CI: 11.5-18.8),10.9 months (CI: 4.7 to NR), and 6.3 months (2.0- to NR) for peritoneal, liver, distant lymph node, and lung resection, respectively. The number of patients alive 3 years and 5 years after undergoing a metastasectomy were 33 patients (44.0%) and 15 patients (20.0%), respectively.
First-line chemotherapy
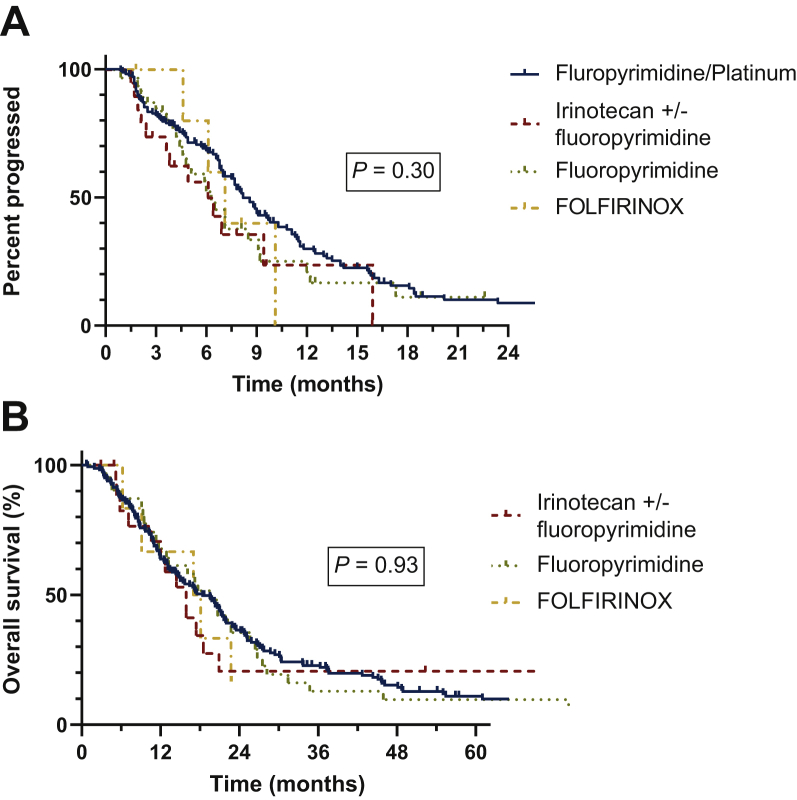
Two hundred and forty patients received chemotherapy following diagnosis of metastatic disease with imaging response data available, of which 164 (68.3%) received fluoropyrimidine plus a platinum agent (oxaliplatin in 144 and other platinum agent in 20), 30 (12.5%) received fluoropyrimidine alone, 19 (7.9%) received FOLFIRI, and 6 (2.5%) received FOLFIRINOX (Table 2). Bevacizumab was given with first-line chemotherapy for 51 patients (21%). For all first-line treated patients, the overall response rate (ORR) was 54%, median TTP was 7.0 months (95% CI: 6.7-8.3), and median OS was 16.3 months (95% CI: 13.6-19.3). The ORRs and median TTP were similar across chemotherapy groups except for the remaining patients who received other chemotherapy regimens described in the Supplementary Data, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Table 2.
Treatment response, TTP, and OS by regimen and treatment line
| First-line regimen | N | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) | Median TTP (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|
| Total | 240 | 11 (5) | 119 (50) | 53 (22) | 57 (24) | 7.0 | 16.3 |
| Fluoropyrimidine-platinum | 164 | 8 (5) | 89 (54) | 35 (21) | 32 (20) | 8.1 | 19.3 |
| FOLFIRI | 19 | 2 (11) | 7 (37) | 5 (26) | 5 (26) | 6.0 | 15.6 |
| Fluoropyrimidine | 30 | 1 (3) | 13 (43) | 9 (30) | 7 (23) | 6.4 | 19.7 |
| FOLFIRINOX | 6 | 0 (0) | 5 (83) | 1 (17) | 0 (0) | 7.0 | 17.3 |
| Othera | 21 | 0 (0) | 5 (24) | 3 (14) | 13 (62) | 2.9 | 8.8 |
| ≥Second-line therapy | N | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) | TTP (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| Total | 267 | 3 (1) | 48 (18) | 63 (24) | 153 (57) | 2.9 | 11.0 |
| FOLFIRI | 122 | 1 (1) | 32 (26) | 33 (27) | 56 (46) | 3.9 | 13.0 |
| Taxane based (mono/combo)b | 34 | 0 (0) | 7 (21) | 9 (26) | 18 (53) | 2.4 | 8.8 |
| Fluoropyrimidine-platinum | 9 | 0 (0) | 1 (13) | 5 (56) | 3 (38) | 6.4 | 19.8 |
| Anti-EGFR monotherapy | 11 | 0 (0) | 0 (0) | 1 (9) | 10 (91) | 2.6 | 8.5 |
| Anti-PD-1 | 22 | 2 (9) | 3 (14) | 4 (18) | 13 (59) | 2.9 | 15.4 |
| Gemcitabine (mono/combo)c | 19 | 0 (0) | 2 (11) | 4 (21) | 13 (68) | 2.3 | 8.4 |
| TAS-120 or regorafenib | 5 | 0 (0) | 0 (0) | 1 (20) | 4 (80) | 1.9 | 4.8 |
| Othera | 45 | 0 (0) | 2 (4) | 6 (13) | 37 (80) | 2.1 | 9.1 |
Other agents are described in the Supplementary Data, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Taxane-based combinations with gemcitabine (n = 11), capecitabine (n = 2).
Gemcitabine-based combinations with oxaliplatin (n = 1), capecitabine (n = 2), irinotecan (n = 2); and cisplatin (n = 1).
Univariate and multivariate analyses were conducted to assess which factors were associated with TTP and OS (Supplementary Material, Table S3 and S4, available at https://doi.org/10.1016/j.esmoop.2021.100132). In the multivariable model, only two factors were associated with improved TTP: fluoropyrimidine-based regimens compared with non-fluoropyrimidine-based regimens [P < 0.001; hazard ratio (HR): 0.25; 95% CI: 0.15-0.42] and the use of bevacizumab (P = 0.02; HR: 0.62; 95% CI: 0.42-0.91) were associated with improved TTP. In the multivariate model for OS, fluoropyrimidine-based regimens were superior to non-fluoropyrimidine regimens (P < 0.001; HR: 0.33; 95% CI: 0.2-0.54), however, bevacizumab use was not significantly associated with improved OS (P = 0.05; HR 0.69; 95% CI: 0.47-1.0). Kaplan–Meier plots for OS by chemotherapy regimen are shown in Figure 2 and TTP and OS stratified by bevacizumab is shown in Supplementary Material, Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Figure 2.
Kaplan–Meier plot for TTP (A) and OS (B) after receiving first-line chemotherapy.
OS, overall survival; TTP, time to progression.
Second- and third-line chemotherapy
A total of 148 patients received second-line therapy. The most common regimens were irinotecan with or without 5-FU (n = 101, 68%), taxane-based chemotherapy (n = 7, 5%), and fluoropyrimidine with a platinum agent (n = 8, 5%). ORR with second-line therapies was 25.0%, median TTP was 3.78 months (95% CI: 2.86-6.08), and median OS from initiation of second-line therapy was 12.6 months (95% CI: 10.4-14.0).
A total of 119 patients received chemotherapy after progression on second-line therapy. Twenty-seven patients (23%) received taxane-based chemotherapy, 16 patients (13%) received gemcitabine-based chemotherapy, with 21 (18%) receiving irinotecan with or without 5-FU, or other regimens. ORR was 12%, median TTP was 2.43 months (95% CI: 2.14-2.96), and median OS from initiation of third-line therapy was 9.17 months (95% CI: 8.38-11.66).
Outcomes by first-, second-, and third-line therapy are shown in Supplementary Material, Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100132 and Table 2.
Taxane-based chemotherapy, regorafenib, and TAS-102
Thirty-four patients received taxane-based chemotherapy in the second line or beyond. TAS-102 or regorafenib were given to two and three patients, respectively, in third-line therapy or beyond. Seven patients (20.5%) achieved partial response (PR) with taxane-based chemotherapy, with 9 (26.5%) achieving SD and 18 (52.9%) progressing on treatment. Regorafenib was associated with SD in one patient and progressive disease (PD) in the remaining four patients who received either TAS-102 or regorafenib. Median TTP with taxane-based chemotherapy, TAS-102, and regorafenib was 2.43 months (95% CI: 2.14-4.76), 0.7 months (95% CI: 0.7-NR), and 2.0 months (95% CI: 2.0-NR), respectively.
Immunotherapy
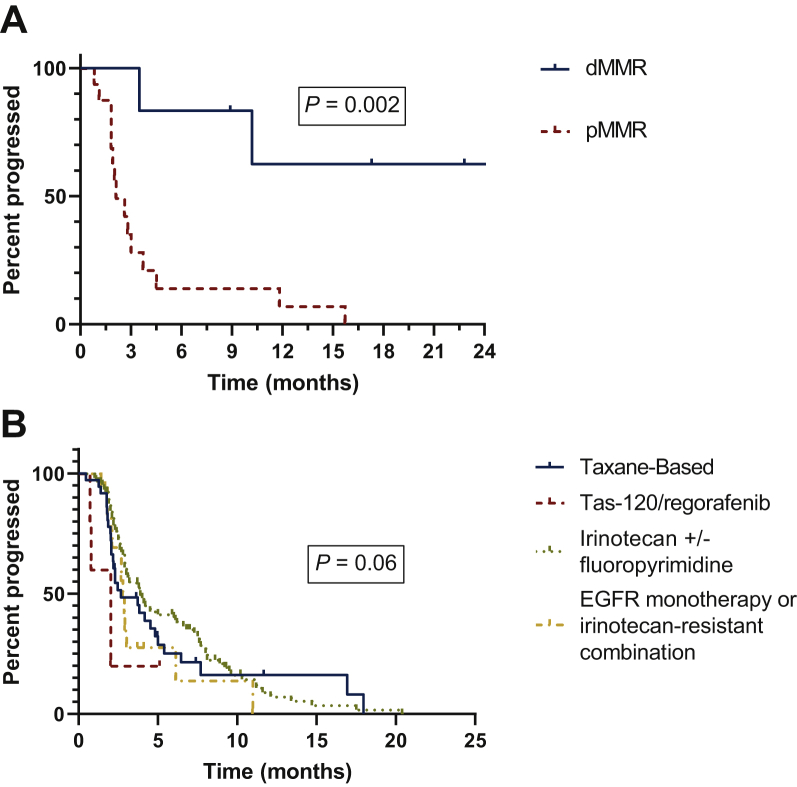
Anti-PD-1-based therapy was used in a total of 22 patients after progression on first- or second-line therapies. Anti-PD1-based agents were pembrolizumab in eight, atezolizumab in nine, nivolumab in one, and durvalumab and other anti-PD1 agents in three patients. Two patients (9%) experienced complete response, 3 (14%) achieved PR, 4 patients (18%) had stable disease (SD), and 13 patients (59%) had PD with a median TTP of 2.9 months. All responses were achieved in dMMR patients. When stratified by MMR status, patients with dMMR tumors (n = 6) had a significantly longer TTP compared with patients with proficient mismatch repair (pMMR) tumors (n = 16) (NR versus 2.1 months, P = 0.002). A Kaplan–Meier plot of TTP stratified by MMR status is shown in Figure 3A.
Figure 3.
Kaplan–Meier plot for TTP in patients with dMMR/MSI-H and pMMR/MSS tumors receiving immunotherapy is shown in A.
Kaplan–Meier plot for TTP of various second and third-line therapies is shown in B.
dMMR, deficient mismatch repair; EGFR, epidermal growth factor receptor; MSI-H, microsatellite instability-high; MSS, microsatellite stable; pMMR, proficient mismatch repair; TTP, time to progression.
Anti-epidermal-growth-factor-receptor therapy
Anti-epidermal-growth-factor-receptor (EGFR) therapy was given to 34 patients either alone (n = 11) or in combination with irinotecan (n = 23). Of the patients treated with combination therapy, these were divided into those patients who were irinotecan sensitive (n = 7) or irinotecan resistant (n = 16) at the time of anti-EGFR treatment. Mutational testing for RAS status was available for KRAS in 28 patients and NRAS in 16 patients, with all patients having a wild-type RAS status. Seven patients (21%) had PR, 6 (18%) had SD, and 21 patients (62%) had PD. Median TTP with anti-EGFR therapy was 2.9 months (95% CI: 2.7-10.0). When stratified into anti-EGFR monotherapy and irinotecan-resistant combination therapy, median TTP was 2.8 (95% CI: 2.7-NR) and response rate (RR) was 11%. Among patients who received anti-EGFR monotherapy, the best response was SD in one patient. Patients who received anti-EGFR combination therapy without prior progression on irinotecan (irinotecan sensitive) had a median TTP of 6.3 months (95% CI: 2.8-NR) and RR of 31%. Though numerically different, there were no differences between these two groups with regard to TTP (P = 0.30) or RR (P = 0.21). A Kaplan–Meier plot is shown in Supplementary Material, Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100132.
Discussion
In this analysis of a large metastatic SBA cohort, we demonstrate similar outcomes with fluoropyrimidine-based therapies as frontline therapy. Moreover, we demonstrate an association between metastasectomy and improved OS in the management of metastatic SBA among well-selected patients. Aside from the activity seen with anti-PD1 therapy for dMMR SBA, limited differences were seen between a variety of agents used in subsequent lines of therapy. However, anti-EGFR therapy demonstrated limited activity suggesting parallels between the midgut derivation of both the small bowel and right colon and anti-EGFR responsiveness.
The median OS among SBA patients diagnosed with metastatic disease was poor at 15.9 months; however, CD and dMMR status were independently associated with improved prognosis. CD-related SBA has previously been shown to be associated with improved outcomes when compared with Crohn's-related or spontaneous SBA.11,12 Molecular analyses of this subset of patients have identified a high rate of microsatellite instability (MSI) and tumor-infiltrating lymphocytes11,13 that may be associated with a favorable disease phenotype.14 Additionally, MSI status predicted response to immunotherapy, with a number of patients in our cohort achieving prolonged disease response. This is consistent with the benefits of immunotherapy seen in the prospective trial assessing pembrolizumab for dMMR non-CRCs where 19 SBA patients were enrolled with an ORR of 42.1% and median TTP of 9.2 months.15 Given the demonstrated efficacy of immunotherapy in the dMMR population, routine testing for microsatellite instability should be instituted for metastatic SBA patients.
The liver and peritoneum were the most common site of metastatic disease among SBA patients. In patients who underwent metastasectomy in our cohort, the median OS (34.5 months) was approximately double the median OS of patients who underwent chemotherapy alone (17.1 months). Notably, patients who underwent metastasectomy were younger and had less aggressive and extensive disease than their nonsurgical counterparts, suggesting that patient selection likely played a role in the outcomes after metastasectomy. While metastasectomy has a more well-defined role for CRC patients with oligometastatic disease,16 the existing data supporting this approach among SBA patients is limited to a few small reports.2,17, 18, 19 In the largest series of patients undergoing metastasectomy for SBA, 152 patients who underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastases had a median OS of 32 months. Interestingly, in our cohort, the resection site did not appear to significantly influence outcome, with long-term survivors present among patients who underwent peritoneal cytoreductive surgeries.
The majority of patients received fluoropyrimidine-based therapy with a platinum agent for metastatic disease in the first line, which was associated with an ORR of 59.1%, median OS of 19.3 months, and TTP of 8.1 months, consistent with prior reports on this regimen.3,4,20 Moreover, the use of fluoropyrimidine in the first line was associated with significant survival advantage compared with non-fluoropyrimidine-based regimens. A number of patients also received concomitant bevacizumab therapy. This combination for metastatic SBA has been described in several small reports however, with mixed results.21, 22, 23 In the largest of these, Legué et al.22 described outcomes of 25 patients who received bevacizumab with chemotherapy and did not identify a significant difference in median OS among this group compared with patients who received chemotherapy alone. However, in our larger cohort we observe that bevacizumab is associated with improved TTP and OS when given with first- and second-line therapy.
The most frequently administered second-line therapy, irinotecan, was associated with the best ORR of approximately 30%; similar outcomes were described in a clinical trial assessing second-line FOLFIRI in patients with advanced SBA after failure of first-line platinum-based chemotherapy.24 However, TTP with second- and third-line therapies was overall poor. In the absence of more effective therapies, these findings support the selection of irinotecan-based regimens such as FOLFIRI in the second line. Taxane-based chemotherapy was also offered in the second-line and beyond and demonstrated antitumor activity. This finding, which has been identified in a few small reports, supports the inclusion of taxane-based chemotherapy as an option beyond the first line in the NCCN guidelines.8,25, 26, 27 Though used in a limited number of patients, neither TAS-102 nor regorafenib appeared to demonstrate meaningful activity with best response of PD seen in four of the five patients.
Interestingly, anti-EGFR therapy demonstrated limited activity, despite the majority of patients having KRAS testing and demonstrating wild-type RAS status. Similar findings were described in a small clinical trial by Gulhati et al.28 The lack of efficacy of anti-EGFR therapy for SBA compared with CRC may be explained by the varied embryonic origins of the small and large intestines and, consequently, the distinct molecular profile of both tumor types.29 Findings suggest that right-sided colon cancers (midgut derivation) benefit less from anti-EGFR therapy compared with left-sided colon cancers (hindgut derivation).30 As an extrapolation, the midgut derivation of the small bowel may reflect the reason for the limited activity seen in SBA from anti-EGFR therapy. Notably, the use of anti-EGFR therapy is absent from the NCCN guidelines, and, though limited by patient numbers, our data support the lack of inclusion of anti-EGFR for SBA patients.
Although a sizable cohort was used for this analysis, there were several limitations to the present study, largely attributed to the retrospective nature of the analysis. The long time period over which patients were included increases the possibility of non-treatment-related factors, such as overall advances in medical care, influencing outcomes, though an attempt was made to account for this in the analysis by adding a variable pertaining to year of treatment. Additionally, small sample sizes for certain treatment groups and heterogeneous treatments administered limited analyses.
In conclusion, metastasectomy appears to be associated with improved outcomes and should be considered for well-selected patients. In addition, the activity of anti-PD-1 therapy in dMMR SBA supports the testing of all SBA for MMR. While taxane-based therapy appears to have activity in SBA, the role of anti-EGFR therapy is uncertain with limited activity. Further prospective studies are warranted, and these findings support the ongoing randomized clinical trial SWOG 1922 (NCT04205968) that compares FOLFIRI and ramucirumab and paclitaxel (Taxol) for second-line metastatic SBA.
Acknowledgments
Funding
This work was supported by the Kavanagh Family Foundation and Kevin T. Doner Memorial Fund (no grant number, philinthronpic).
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Nishikawa Y., Hoshino N., Horimatsu T. Chemotherapy for patients with unresectable or metastatic small bowel adenocarcinoma: a systematic review. Int J Clin Oncol. 2020;25(8):1441–1449. doi: 10.1007/s10147-020-01703-z. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio T., Henriques J., Manfredi S. Small bowel adenocarcinoma: results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int J Cancer. 2020;147(4):967–977. doi: 10.1002/ijc.32860. [DOI] [PubMed] [Google Scholar]
- 3.Xiang X.J., Liu Y.W., Zhang L. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23(5):561–566. doi: 10.1097/CAD.0b013e328350dd0d. [DOI] [PubMed] [Google Scholar]
- 4.Horimatsu T., Nakayama N., Moriwaki T. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol. 2017;22(5):905–912. doi: 10.1007/s10147-017-1138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulhati P., Raghav K., Shroff R.T. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: a single-center, open-label, phase 2 study. Cancer. 2017;123(6):1011–1017. doi: 10.1002/cncr.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overman M.J., Kopetz S., Wen S. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113(8):2038–2045. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 7.Zaanan A., Costes L., Gauthier M. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol. 2010;21(9):1786–1793. doi: 10.1093/annonc/mdq038. [DOI] [PubMed] [Google Scholar]
- 8.Benson A.B., Venook A.P., Al-Hawary M.M. Small bowel adenocarcinoma, version 1.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17(9):1109–1133. doi: 10.6004/jnccn.2019.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rompteaux P., Gagnière J., Gornet J.-M. Resection of small bowel adenocarcinoma metastases: results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol. 2019;45(3):331–335. doi: 10.1016/j.ejso.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Amin M.B., Edge S., Greene F., editors. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; Accessed August 30, 2020. https://www.springer.com/gp/book/9783319406176 Available at: [Google Scholar]
- 11.Vanoli A., Grillo F., Guerini C. Prognostic role of mismatch repair status, histotype and high-risk pathologic features in stage II small bowel adenocarcinomas. Ann Surg Oncol. 2021;28:1167–1177. doi: 10.1245/s10434-020-08926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter D.D., Murray J.A., Donohue J.H. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64(19):7073–7077. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann F., Singh S., Michel S. Small bowel adenocarcinomas in celiac disease follow the CIM-MSI pathway. Oncol Rep. 2010;24(6):1535–1539. doi: 10.3892/or_00001015. [DOI] [PubMed] [Google Scholar]
- 14.Latham A., Shia J., Patel Z. Characterization and clinical outcomes of DNA mismatch repair deficient (MMR-D) small bowel adenocarcinoma. Clin Cancer. 2021;27:1429–1437. doi: 10.1158/1078-0432.CCR-20-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marabelle A., Le D.T., Ascierto P.A. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kow A.W.C. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10(6):1274–1298. doi: 10.21037/jgo.2019.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam R., Chiche L., Aloia T. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244(4):524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ercolani G., Grazi G.L., Ravaioli M. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12(6):459–466. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Yonemura Y., Levine E.A. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal metastases from a small bowel adenocarcinoma: multi-institutional experience. Ann Surg Oncol. 2018;25(5):1184–1192. doi: 10.1245/s10434-018-6369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overman M.J., Varadhachary G.R., Kopetz S. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27(16):2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 21.Aydin D., Sendur M.A., Kefeli U. Evaluation of bevacizumab in advanced small bowel adenocarcinoma. Clin Colorectal Cancer. 2017;16(1):78–83. doi: 10.1016/j.clcc.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Legué L.M., van Erning F.N., Bernards N., Lemmens V.E.P.P., de Hingh I.H.J.T., Creemers G.-J. Addition of bevacizumab to first-line palliative chemotherapy in patients with metastatic small bowel adenocarcinoma: a population-based study. Target Oncol. 2019;14(6):699–705. doi: 10.1007/s11523-019-00681-1. [DOI] [PubMed] [Google Scholar]
- 23.Takayoshi K., Kusaba H., Uenomachi M. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother Pharmacol. 2017;80(2):333–342. doi: 10.1007/s00280-017-3371-0. [DOI] [PubMed] [Google Scholar]
- 24.Zaanan A., Gauthier M., Malka D. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer. 2011;117(7):1422–1428. doi: 10.1002/cncr.25614. [DOI] [PubMed] [Google Scholar]
- 25.Aldrich J.D., Raghav K.P.S., Varadhachary G.R., Wolff R.A., Overman M.J. Retrospective analysis of taxane-based therapy in small bowel adenocarcinoma. Oncologist. 2019;24(6):e384–e386. doi: 10.1634/theoncologist.2018-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overman M.J., Adam L., Raghav K. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol. 2018;29(1):139–144. doi: 10.1093/annonc/mdx688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cen P., Wray C.J., Zhang S. Durable response for ampullary and duodenal adenocarcinoma with a nab-paclitaxel plus gemcitabine ± cisplatin combination. Cancer Med. 2019;8(7):3464–3470. doi: 10.1002/cam4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulhati P., Raghav K., Shroff R. Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of Vater. Oncologist. 2018;23(3):277–e26. doi: 10.1634/theoncologist.2017-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrock A.B., Devoe C.E., McWilliams R. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol. 2017;3(11):1546–1553. doi: 10.1001/jamaoncol.2017.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venook A.P. Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol. 2017;15(1):22–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.