Abstract
Silver nanoparticles have high potential for application in food industry, as they have the ability to inhibit a wide range of bacteria of pathogenic and spoilage origin. They can be obtained from different methods classified in physical and chemical and which are aggressive with the environment since they produce toxic waste. Nowadays, environmentally friendly methods such as green synthesis can be used, through the use of agri-food waste. The use of these wastes is a more sustainable method, because it reduces the environmental pollution, at the same time that silver nanoparticles are obtained. The aim of the present study is the green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) aqueous extract from waste and its antibacterial activity on Staphylococcus aureus (Gram positive) and Pseudomonas fluorescens (Gram negative). The analyses by TEM showed that the as-synthesized silver nanoparticles were uniform and spherical particles with an average diameter of 8.67 ± 4.7 nm and confirmed by SEM. The electron diffraction and TEM analyses showed the characteristic crystallinity of silver nanoparticles. FTIR spectroscopy confirmed that various functional groups were responsible for reducing and stabilizing during the biosynthesis process. Nanoparticles inhibited the growth of both types of bacteria from the lowest concentration evaluated (0.9 μg/mL). We conclude that silver nanoparticles synthesized in the present study have potential application as antibacterial agents in food and medicine industry.
Keywords: Green synthesis, Silver nanoparticles, Antibacterial activity, Aquoeus extract, Carthamus tinctorius L.
Green synthesis, Silver nanoparticles, Antibacterial activity, Aquoeus extract, Carthamus tinctorius L.
1. Introduction
Nanotechnology is an emerging science, which is based on the design and application of nanostructures or nanomaterials that are usually in the range of 1–100 nm (nm) [1]. The interest in the use of nanoparticles or nanomaterials is due to their different properties, such as morphology, size and their area-volume relationship which are used in different fields as medical, food, health, among others [2]. Therefore, in the last decades, numerous techniques have been created for their synthesis. These techniques are mainly divided into two the "top-down" and "bottom-up", which include chemical and physical procedures. The top-down begins with a large material of interest that is reduced in size by physical and chemical processes to obtain a nanostructure [3]. On the other hand, the bottom-up technique, is based on the use of materials on atomic scale, such as groups of atoms and molecules that act as building blocks to form larger particles, thin films and nanostructured materials [4].
Some commonly used synthesis methods are ultrasonic radiation, laser ablation, chemical vapor deposition, microwave and electrochemical synthesis. However, these methodologies are characterized by having high production cost, as well as the use of toxic and dangerous chemicals, which have potentially dangerous effects for the environment and health [5]. An alternative to avoid handling toxic chemicals in the production of metallic nanoparticles, is to use green synthesis, mainly from plant extracts, as an environmentally friendly alternative. In these protocols, toxic substances are replaced by the use of molecules from plant extracts (flavonoids, polyphenols, proteins, sugars and saponins) that function as reducing and stabilizing agents [6].
More recently, the use of agri-food waste is a more sustainable alternative for the production of silver nanoparticles, than the use of plant extracts. The use of these residues has the concern of being used to reduce the environmental pollution that they generate, giving them added value, at the same time that they synthesize the nanoparticles. This has been raised in recent publications for the synthesis of silver nanoparticles, where they have used fruit peel (orange, banana, pomegranate peel) [7, 8, 9], oil cake (sesame oil cake) [10], straws of agricultural crops (wheat, corn and rice straw) [11, 12, 13], among others. All these studies agree that biomolecules present in them prove the reduction of silver ion (Ag+) to zerovalent silver (Ag0). This method from agri-food waste is called sustainable green synthesis.
Safflower (Carthamus tinctorius L.) is a plant belonging to the Asteraceae family [14]. This crop is of industrial importance due to the extraction of commercial oil, in addition, the compounds present in the petals are natural pigments for the food area [15]. However, more than 80% of this crop (leaf-stem) is considered a agri-food waste, after obtaining the seed. Compound families that have been isolated from safflower are flavonoids, quinochalcones, polyacetylenes, alkaloids, fatty acids, steroids, lignans, proteins, polysaccharides, among other; were the main active compounds are quinochalcones and flavonoids [16, 17].
It is estimated that approximately five hundred tons of silver nanoparticles are produced per year, thus becoming one of the most commercialized nanomaterials. These have different properties and application in the areas of food, medicine, chemistry, biochemistry, among others. This noble metal has been recognized to have strong inhibitory and bactericidal effects, as well as antifungal, anti-inflammatory and antiangiogenic activities [18]. Considering the importance of silver nanoparticles, the aim of this study was to synthesize silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract, and evaluate its physico-chemicals properties and its antibacterial activity on the growth of important toxigenic pathogens in food industry as Staphylococcus aureus and Pseudomonas fluorescens.
2. Materials and methods
2.1. Obtaining of aqueous extract of safflower (Carthamus tinctorius L.) waste
2.1.1. Safflower extract
The safflower (Carthamus tinctorius L.) waste used in the present investigation consisted of a mixture of stem and leaf obtained after the seed collection process. The sample was collected 150 days after sowing. The collection location was Ejido San Miguel de Horcasitas, Sonora, Mexico with coordinates: 29° 29′00 ″N 110° 45′00″ W.
2.1.2. Cleaning and reduction of raw material
The safflower (Carthamus tinctorius L.) waste was cleaned of impurities as seeds, flowers and damage parts. For the reduction of the particle size of the samples obtained, a Krups Gray brand mill model GX410011 was used, then placed on a 40 mesh sieve (425 μm), brand Newark model TS8323S77, to obtain uniform particles with a greater phenol-solvent contact area. Samples were placed in airtight bags and stored in a cold room (-5 °C) until use.
2.1.3. Extraction
Distilled water was used as the extraction solvent, 3 g of sample were weighed on an OHAUS Pioneer analytical balance and 20 mL of solvent were added. The sample was mixed for 1 min at 25 °C in a VWR Vortex 2, G-560. Then, it was sonicated for 15 min in a Branson equipment, M3800H, and then, dimensions of 230 × 140 × 100 mm (9.5L x 5.5W x 4D ″), control of 99 min of time and frequency of 40 kHz. Finally, it was centrifuged at 4400 rpm at 4 °C for 15 min in an eppendorf 5804 R kit. The supernatant was recovered and filtered on Whatman #4 paper, and stored at 4 °C until use [19].
2.2. Green synthesis of silver nanoparticles
The silver nanoparticles were synthesized by adding 30 mL of 0.1 M silver nitrate (AgNO3) solution in 10 mL of safflower waste extract. The reaction was carried out under dark conditions to avoid photoactivation of silver nitrate. The color change was indicative of the silver nanoparticles formation (12 h of reaction). The silver nanoparticle solution was centrifuged at 4400 rpm for 15 min at 25 °C. The precipitate was recovered and dried at room temperature. Finally, the fine dry powder was stored until analysis [20].
2.3. Morphology and particle size by HR-TEM
Through the JEOL transmission electron microscope (JEOL, Ltd, Tokyo, Japan), the shape and size of the silver nanoparticles obtained from the aqueous extract of safflower (Carthamus tinctorius L.) waste was analyzed. The operating voltage was 200 kV with a field emission filament. A 100 μL aliquot (100 μg in 500 μL of distilled water) of a nanoparticle suspension was placed on a copper rack and allowed to dry prior to analysis.
2.4. Fourier transform infrared (FT-IR) spectroscopy
Infrared spectrum were obtained in an FT-IR Spectrometer (Frontier, Perkin Elmer, Waltham, USA). The spectra were recorded employing the attenuated total reflectance (ATR) technique in mode of transmittance. A spectrum scan from 4000 to 500 cm−1 was utilized and average of 32 scans were recorded. The determinations were made in triplicate.
2.5. Determination of antibacterial activity
2.5.1. Bacterial strains
The bacterial strains used in the present study were Staphylococcus aureus ATCC 25923 (American Type Culture Collection) and Pseudomonas fluorescens ATCC 13867.
2.5.2. Inoculum concentration standardization
Silver nanoparticles were evaluated in a concentration range of 0.98–250 μg/mL (serial dilutions). Water was used as a solvent. The strains were propagated in Mueller-Hinton medium at 37 °C until obtain a final concentration of 106 CFU/mL.
2.5.3. Determination of bacterial growth inhibition (%)
Inhibition of bacterial growth was evaluated by spectrophotometric analysis. 210 μL of previously standardized inoculum (106 CFU/mL) was placed in 96-well microplate, then, 90 μL of sample was incorporated. Two controls were used. The first contained 210 μL of inoculum and 90 μL of sterile distilled water, the second contained 300 μL of inoculum. The microplates were shaken for 1 min before and after incubation using a microplate reader (Veloskan™ LUX, Thermo Scientific, EUA). The absorbance (630 nm) of the microplates before incubation (T0) was determined and subsequently, were incubated at 37 °C for 24 h. After the incubation time, the absorbance of the samples (TF) was determined. The results were reported as growth inhibition (%), and the calculation was carried out based on equation 1 (Eq. 1). Sterile MH culture medium was used as a blank [21].
| (Eq. 1) |
where, T0 sample and TF sample are the absorbance at 630 nm of the inoculum in the presence of the samples before (T0) and after (TF) incubation; T0 blank and TF blank corresponds to the inoculum with the samples before and after incubation and T0 control and TF control are bacterial growth in the absence of particles.
2.5.4. Minimum inhibitory concentration (MIC)
The MIC of the samples was determined according to the methodology of Moreno-Vasquez et al. (2017). The MIC is defined as the lowest concentration of silver nanoparticles at which bacteria growth is not detected. From the microplate used to determine the inhibition of bacterial growth (%), the concentration of silver nanoparticles was established as MIC, which shows a difference in absorbance [22].
2.5.5. Minimum lethal concentration (MLC)
Aliquots (100 μL) of the concentrations higher than the MIC were taken from the microplate used to determine the inhibition of bacterial growth (%) and were subsequently inoculated on plates with Mueller-Hinton agar and were incubated for 24 h at 37 °C. The lowest concentration evaluated (no growth) was established as MLC. Triplicates of each concentration evaluated were performed [23].
2.6. Experimental desing and statistical analysis
An analysis of variance was used by general linear models at a significance level of 0.05 error (p < 0.05). Comparisons of means were made by Tukey's multiple range test, at a 95% confidence interval. For all the analysis, means and standard deviations (SD) were performed.
3. Results and discussion
3.1. Aqueous extract of safflower (Carthamus tintorious L.) waste
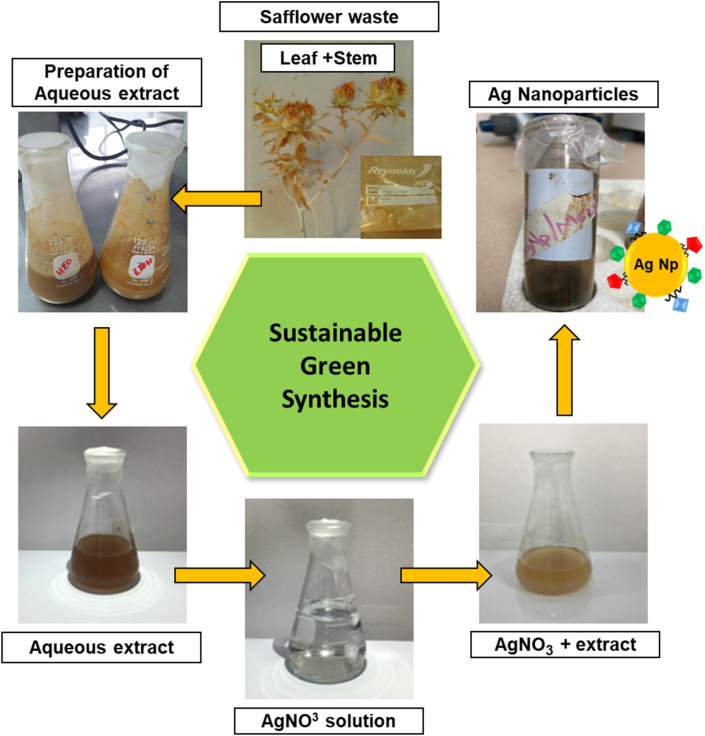
The aqueous extract of safflower was obtained using the methodology previously described. As shown in Figure 1, the initial color of the safflower mixture plus distilled water as solvent, presented a brown/yellowish tonality, which is distictive of the safflower leaf/stem mixture. After extraction, an amber coloration was obtained.
Figure 1.
Schematic representation of obtaining silver nanoparticles from aqueous extract of safflower waste.
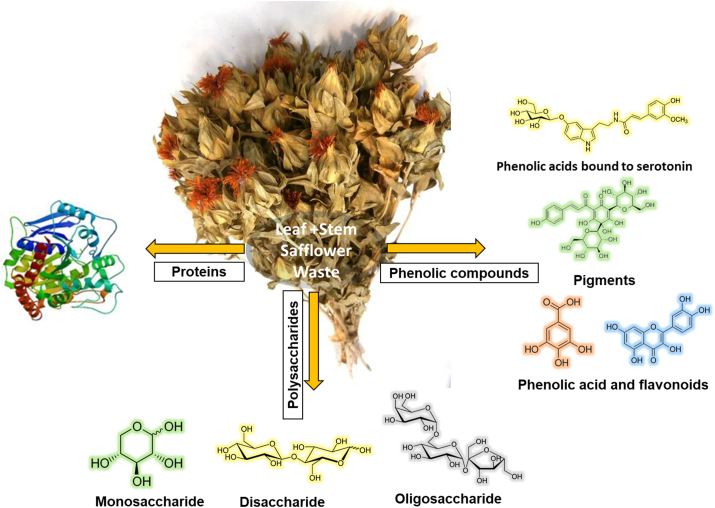
Previously, the extraction of around 200 safflower compounds had been reported using different types of solvents, such as methanol, ethanol and water [24]. The identified compounds are mainly derived from flavonoids, tannins, proteins, polysaccharides, steroids, fatty acids, phenolic acids, anthocyanins, gallic acid derivatives. Authors have reported that compounds such as flavonoids and their derivatives have been found in the safflower flower, as well as in leaves and in the whole plant [25]. Specifically, in the aqueous extract of safflower, high content of polyphenols and flavonoids has been reported, with 87.20 and 36.32 mg GAE/g, respectively [26]. Figure 2 shows the possible compounds that can be found in the aqueous extract. In this sense, the aqueous extract of safflower used in the present investigation may contain the same compounds.
Figure 2.
Possible macromolecules and phenolic compounds present in the safflower waste aqueous extract.
3.2. Green synthesis of silver nanoparticles
The formation of silver nanoparticles from the aqueous safflower leaf/stem extract was monitored by a color change in the aqueous solution. Before adding the AgNO3 solution Figure 1, the extract had a yellowish-brown color. However, when the AgNO3 solution was added and allowed to stand 12 h in the dark, the extract turned from yellowish-brown to a dark brown color. Some authors point out that the generation of silver nanoparticles is responsible for the color change in the solution, due to the reduction of silver ions to silver nanoparticles by the reducing molecules present in the extract [27]. Therefore, the formation of silver nanoparticles induces a change in the color of the aqueous safflower extract.
Plant extracts are capable of synthesizing silver nanoparticles by adding a silver salt to the solution. However, there are no studies that report the green synthesis of nanoparticles using an aqueous extract of safflower waste. Despite this, it is possible that the synthesis mechanism is similar to the synthesis of nanoparticles with aqueous extracts of plants.
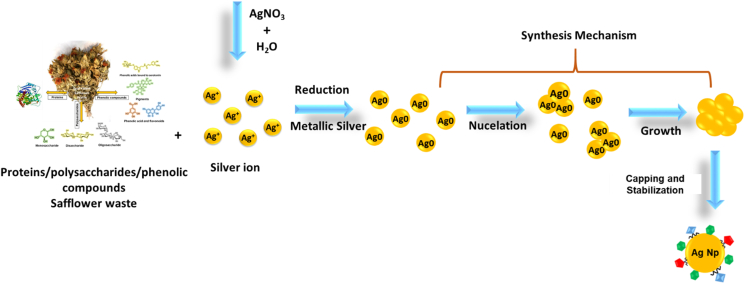
In Figure 3 the synthesis pathway for silver nanoparticles is proposed. Macromolecules and phytochemical compounds such as proteins, polysaccharides, flavonoids and polyphenols present in the aqueous extract of safflower by-prod, reduce silver ions by donating electrons, transforming them into zero-valent silver atoms. Subsequently, these atoms collide with other silver atoms in the solution, resulting in the appearance of a stable core composed of several atoms. These atoms will act as nucleation centers and will form clusters that will continue to grow as long as the supply of atoms remains active, and as a result, silver nanoparticles will be formed [28]. Some authors mention that molecules of extracts contain functional groups, which have the ability to reduce metallic ions. For example, the reactive hydrogen atom is released due to tautomeric transformations into flavonoids causing the enol form to convert to the keto form. This process is carried out by reducing metal ions into metal nanoparticles [29].
Figure 3.
Synthesis mechanism of silver nanoparticles from safflower waste aqueous extract.
3.3. Analysis by HR-TEM
3.3.1. Particle size and morphology
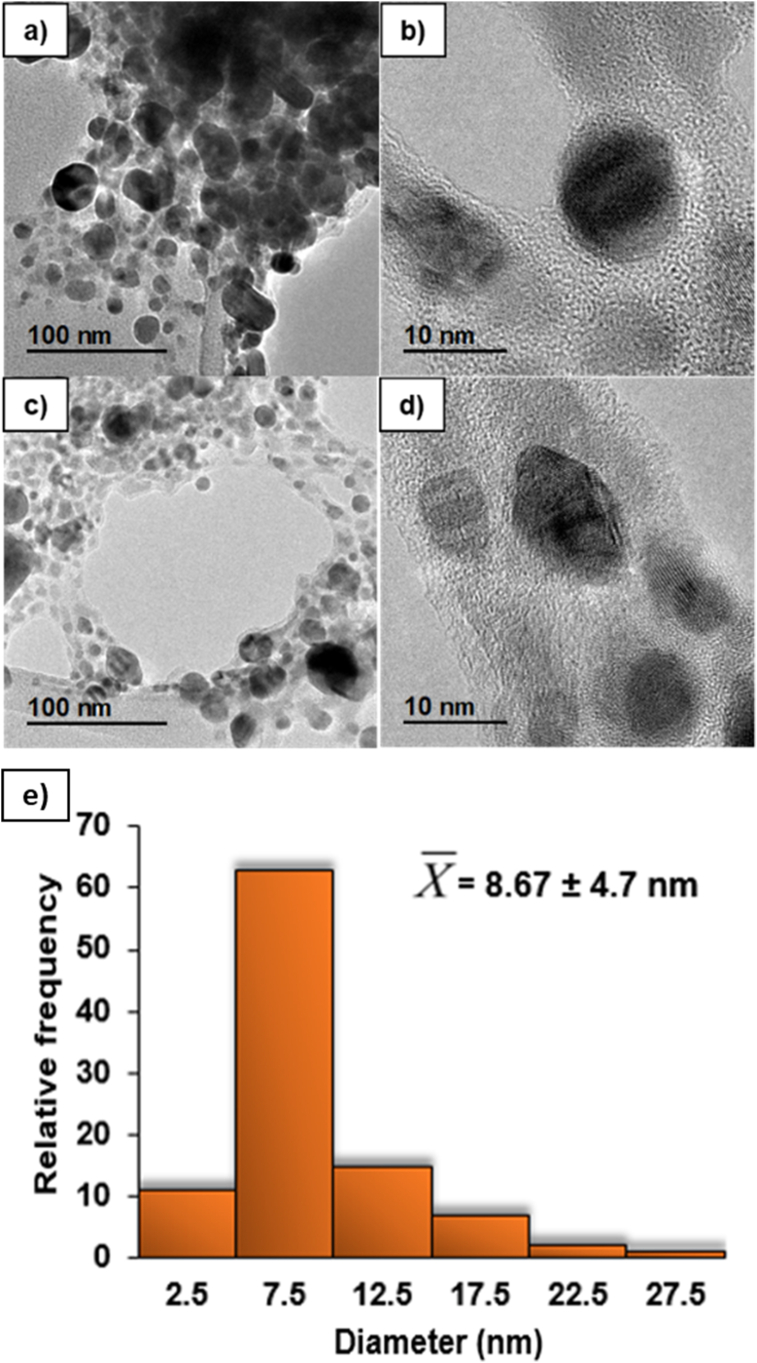
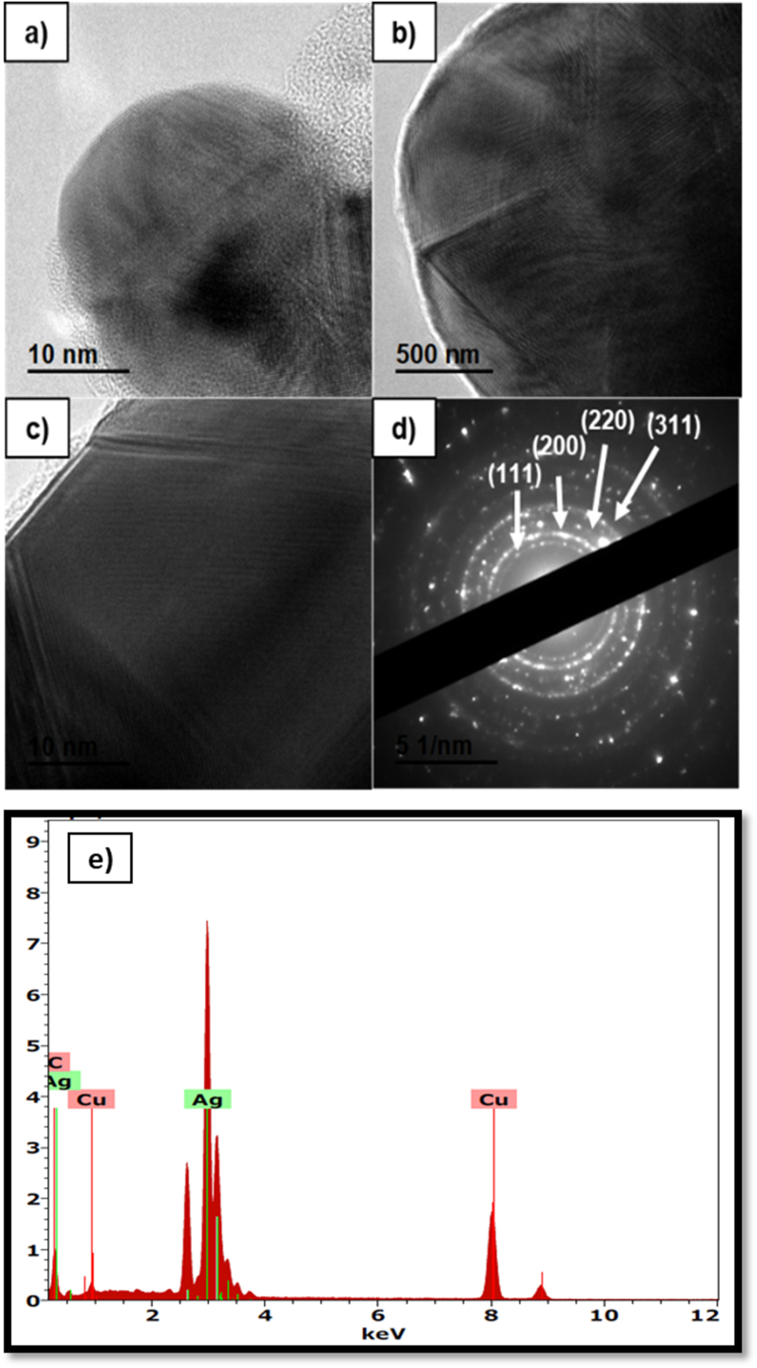
HR-TEM is a variant of TEM, which is used for studies where it is required to increase the visualization of nanomaterials [30]. Figure 4 shows the morphology and particle size distribution of silver nanoparticles synthesized by safflower residue extract. Firstly, Figure 4a and c show a set of silver nanoparticles, observed at 100 nm scale. Figure 4b and d show the silver nanoparticles at 10 nm scale, where are showed nanoparticles with spherical shape. Different authors have reported similar results using aqueous extract of olive leaves to synthesize silver nanoparticles. Khalil et al. [31] indicated the formation of spherical and homogeneous nanoparticles with a size range of 7–15 nm, by means of UV-Visible and surface plasmon resonance analysis. In other studies, Jha & Prasad [32] observed through a TEM analysis of a film coated with a Cycas leaf extract solution, that the shape of the nanoparticles is spherical, with diameters of 2–6 nm.
Figure 4.
TEM micrographs of silver nanoparticles obtained by green synthesis from safflower waste aqueous extract. The micrographs (a) and (c) depict set of nanoparticles, (b) and (d) depict individual nanoparticles and (e) particle size distribution and average diameter.
The particle size distribution (PSD) and average diameter were determined from the micrographs by HR-TEM. Specifically, PSD is a fundamental measure to investigate the repeatability and efficiency of various processes aimed at the synthesis of nanoparticles. Although the PSD size of nanomaterials has been identified as a critical point to establish the potential area of application [33]. In this sense, the PSD of silver nanoparticles synthesized in the present study showed a range between 3.8 and 30 nm. However, 63% of these are between 5 and 10 nm as indicated in Figure 4e. Furthermore, the average diameter was 8.67 ± 4.7 nm. Similar values of PSD of silver nanoparticles synthesized from aqueous extracts of plants have previously been reported, using Fritillaria flower extract where the PSD values were in a range between 5 and 10 nm [34].
3.3.2. Crystallinity and elemental analysis of silver nanoparticles
The results about the crystallinity and elemental analysis of the silver nanoparticles obtained by green synthesis using aqueous extract of safflower waste can be seen in Figure 5. Firstly, Figure 5a, b y and 5c show the crystallinity of silver nanoparticles from HR-TEM micrographs. The trend lines of crystallinity of the silver nanoparticles are observed. The crystallinity of the nanoparticles was proved by diffraction patterns SAED. Figure 5d show that the crystalline structure of the nanoparticles is evidenced with clear circular points and diffraction rings, corresponding to the cubic planes of silver (111), (200), (220) and (311) [35]. Some researchers suggest that the presence of circular diffraction rings, as well as the trend lines and the SAED pattern is due to the crystalline nature of silver nanoparticles. These types of results represent the crystalline nature of nanoparticles [36].
Figure 5.
Crystallinity (a–c), electron diffraction (d) and elemental analysis (e) of silver nanoparticles obtained by green synthesis from safflower waste aqueous extract.
The Elemental analysis (EDS) profile showed strong silver signals, along with weak peaks of carbon and copper, as shown in Figure 5e. The carbon peaks may have originated from biomolecules attached to the surface of silver nanoparticles. As for the copper peaks, these can be derived from the copper grid used for the analysis. It has been previously reported that silver nanoparticles synthesized from aqueous plant extracts are surrounded by a thin layer of some chain-terminating compound present in the extract, generating stabilization in the solution for at least 4 weeks after synthesis [37].
3.4. Scanning electron microscopy
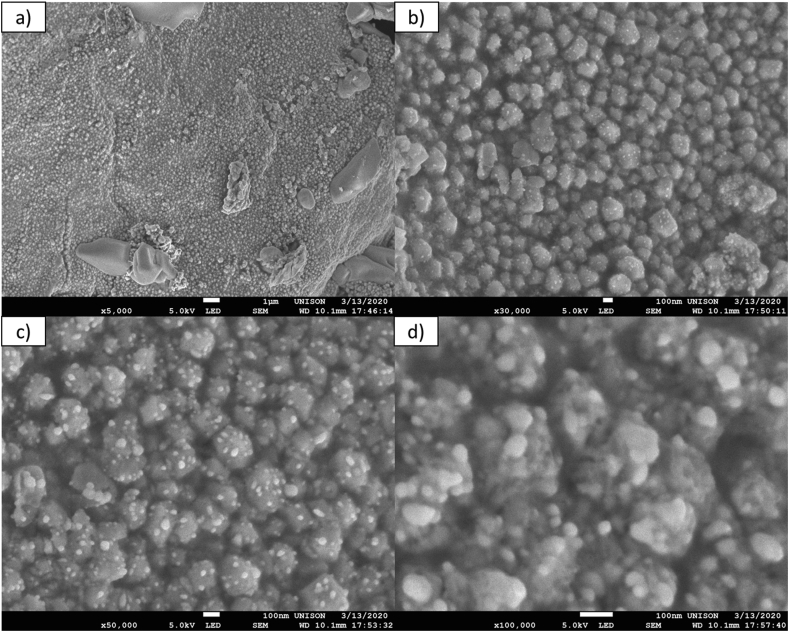
Figure 6 shows the SEM micrographs of the silver nanoparticles synthesized from the safflower waste extract at different magnifications. Firstly, slightly agglomerated, spherical shaped particles are observed. The range of the nanoparticles from 2 to 50 nm, agrees with the particle size distribution reported by TEM. The capping and stabilization of the nanoparticles were demonstrated by the inclusion on the surface of the nanoparticles, of molecules such as proteins, polysaccharides and flavonoids present in the aqueous extract of the safflower waste, mainly through amino, hydroxyl and aromatic ring groups. Similar results were obtained in a study where, from an aqueous extract of safflower flowers, they synthesized metallic nanoparticles with quasi-spherical morphology [38]. In another study was reported that the nanoparticles synthesized from aqueous extract of safflower flowers were covered by the components present in the extract, this confirmed by SEM and TEM [39]. Therefore, safflower waste extract is feasible as a precursor for the synthesis of silver nanoparticles.
Figure 6.
SEM micrographs of silver nanoparticles obtained by green synthesis from safflower waste aqueous extract. The micrographs were at different magnifications: (a) 5,000x, (b) 30,000x (c) 50,000x and (d) 100,000x.
3.5. Structural analysis of silver nanoparticles by FT-IR
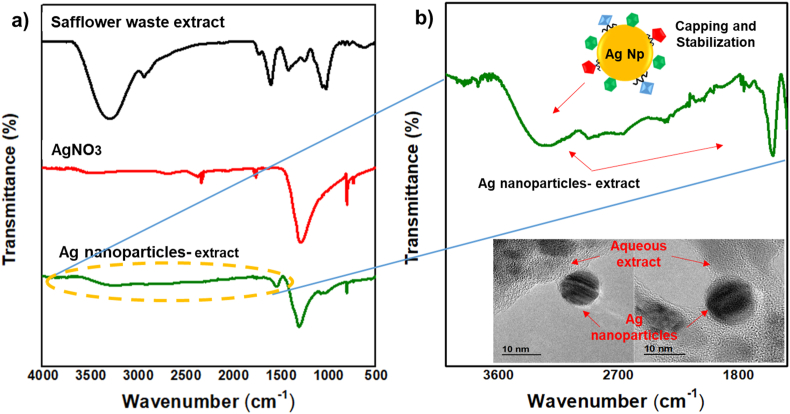
Figure 7 shows the infrared spectra of nanoparticles synthesized from the aqueous extract of safflower waste and its precursors. First, Figure 7a shows the characteristic bands of the extract, a band at 3293 cm−1 corresponding to the bond of –OH and N–H, the band at 2932 cm−1 of the C–H bond, band at the 1725 cm−1 of the C=O (carbonyl) bond, the band at 1599 cm−1 of the C=C bond, the band at 1414 cm−1 of the possible C=C aromatic and C–O–H bonds, the band at 1053 cm−1 of the C–O bond, and bands at 860, 818 and 776 cm−1 are of the C–H/ = C–H/N–H bonds. These characteristic bands predict that in the extract there are structures as proteins, polysaccharides/sugars and phenolic compounds, mainly flavonoids. Furthermore, Figure 7a shows the infrared spectra of silver nitrate and nanoparticles obtained by green synthesis, where silver ions (Ag+) can be seen in both spectra, but with less intensity and displacement at higher energy of the band present in the spectrum of silver nanoparticles, from 1288 cm−1 to 1299 cm-1, respectively, due to a change to metallic silver (Ag0) and formation of nanoparticles.
Figure 7.
Infrared spectra of silver nanoparticles obtained by green synthesis from safflower waste aqueous extract. (a) Shows the infrared spectra of the precursors used and synthesized silver nanoparticles, (b) shows the infrared spectrum for silver nanoparticles from 3600 to 1500 cm−1.
On the other hand, Figure 7b shows the infrared spectrum for silver nanoparticles from 3600 to 1500 cm−1, where previously identified characteristic bands can be seen in the spectrum of the extract of safflower waste, the band at 323 a cm−1 and the band at 1533 cm−1 corresponding to the OH/NH and C=C bonds, respectively. This predicts that the compounds present in the extract adhere to the surface of the nanoparticle, promoting capping and stabilization. This phenomenon was also observed and corroborated in the micrographs obtained by TEM of the silver nanoparticles. Other authors have also mentioned the facility of agri-food waste extracts for the synthesis of silver nanoparticles, and their subsequent capping and stabilization by molecules present to extract [40, 41, 42].
3.6. Determination of antibacterial activity
3.6.1. Inhibition of bacterial growth in liquid medium
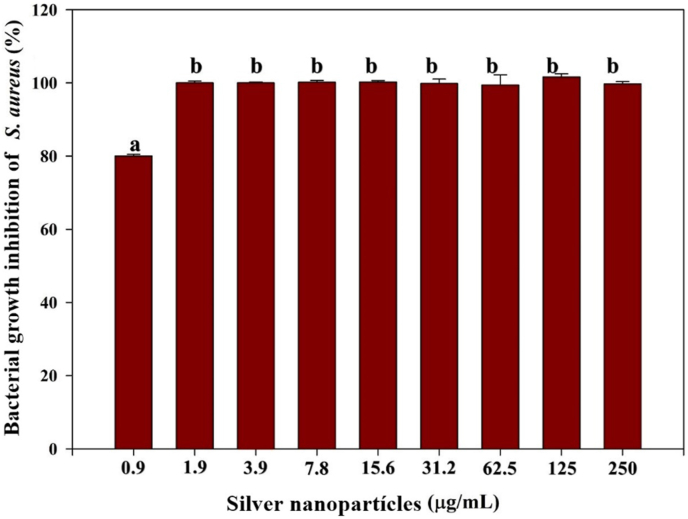
In Figure 8, the percentage of inhibition of different concentrations of silver nanoparticles on the growth of Staphylococcus aureus is observed. As the concentration increases, the effect that is exerted on the bacteria is greater and therefore the percentage of inhibition increases (p < 0.05). The results showed that from a concentration of 1.9 μg/mL of silver nanoparticles, the bacteria were inhibited almost 100%, a figure that remained stable even with the highest concentrations of nanoparticles. In contrast, the concentration that had the least significant effect (p < 0.05) on S. aureus was 0.9 μg/mL, reaching to inhibit only 80% of the bacterial cells. The results are similar to those reported in previous studies, where they observed a trend similar to that of the present study, using nanoparticle solutions from 1.25 to 10 μg/mL. They noted that as the concentration increases, the inhibitory effect on S. aureus cells is greater [43].
Figure 8.
Growth inhibition percentages of S. aureus at different concentrations of silver nanoparticles.
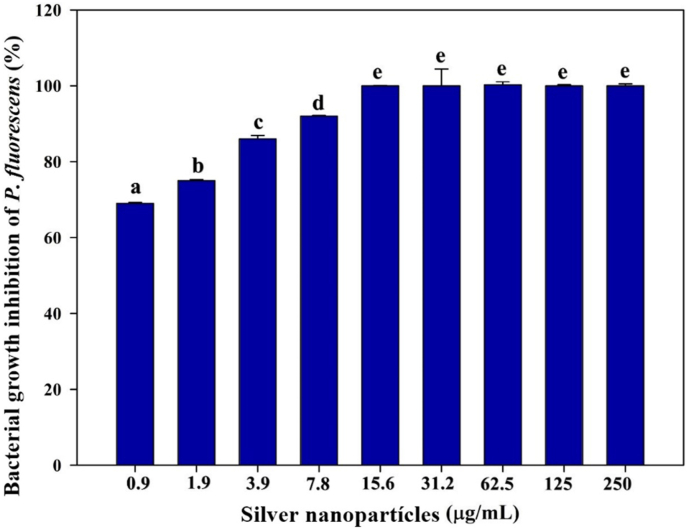
In the case of Pseudomonas fluorescens, the percentage of inhibition of silver nanoparticles at different concentrations is shown in Figure 9. It was observed that at the lowest concentration (0.9 μg/mL), bacteria growth was inhibited by approximately 70%. On the other hand, the increase in the concentration of nanoparticles produced an elevation in the percentage of inhibition of bacterial growth (p < 0.05). Likewise, from the 15.6 μg/ml solution, 100% inhibition was observed. Other studies carried out with Gram negative bacteria indicate that the increase in the percentage of inhibition in bacterial growth is dependent on the dose of AgNPs [44]. Some authors that evaluated the effect of silver nanoparticles in Salmonella spp, found out that at a concentration of 50 μg/mL, the silver nanoparticle solution had a bactericidal effect, which means that the bacterial growth was inhibited a 100% [45].
Figure 9.
Percentages of inhibition of P. fluorescens at different concentrations of silver nanoparticles.
It's possible that the antibacterial activity of silver nanoparticles synthetized by using safflower (Carthamus tinctorius L.) waste extract on Gram positive (S. aureus) and Gram negative (P. fluorescens) cells could be due to different mechanisms. One of those mechanism is the generation of reactive oxygen species (ROS), such as radicals OH, hydrogen peroxide, among others [46]. Another mechanism is the effect of silver ions on the cell membrane of bacteria, which contains sulfur amino acids. Silver can interact with these components inside and outside the cell membrane, resulting in bacterial inactivation. Furthermore, the silver ion released from AgNPs interacts with the phosphorus present in DNA and with sulfur-containing proteins, causing the inhibition of enzymatic activities. It is important to mention that the size and shape of the particles are parameters to determine antibacterial activity [47]. It has previously been reported that nanoparticles with a size smaller than 20 nm can interact more easily with membrane proteins, causing maximum permeability, which leads to the cell death of bacteria [48]. AgNPs alter the permeability of the cell by penetrating the membrane, which produces the exit of intracellular material and therefore cell death [49].
Specifically, in the case of Gram negative bacteria, the possible mechanism of action is due to the excessive generation of ROS, which can produce oxidative stress in the bacteria and attack the lipids of the outer membrane, causing lipid oxidation, as well as damage to proteins, RNA and DNA, and subsequently causing the death of the cell. Oxidative deterioration of lipids typically induces malonaldehyde formation during the last stage of the breakdown of endoperoxidase formed during intramolecular rearrangements of the unsaturated fatty acid structure [50]. For Gram positive bacteria, no studies were found that spoke specifically of the interaction between ROS and the thick layer of peptidoglycan in this type of bacteria, however, it is mentioned that the possible mechanism of action, both for Gram positive and Gram negative bacteria, is the generation of reactive oxygen species [51]. The difference between the percentage of inhibition of silver nanoparticles on the growth of S. aureus and P. fluorescens could possibly be due to differences in bacterial morphology between the bacteria strains mentioned above.
It's been previously reported that the antibacterial activity of silver nanoparticles on Gram positive and Gram negative bacteria is different. The morphology of the cell wall is partly responsible for the effect exerted by nanoparticles on bacteria. This may be due to the fact that, as Gram positive bacteria lack a protective membrane around the peptidoglycan layers in the cell wall. This allows the nanoparticles to interact more easily with the outer membrane until it ruptures it and thus to inhibit with greater efficiency the growth of Gram positive bacteria. This is similar to the results obtained in the present study, since S. aureus presented greater sensitivity to silver nanoparticles than P. fluorescens [52]. It was found, that Gram negative bacteria are more sensitive to AgNPs compared to Gram positive ones. However, researchers reported opposite results arguing that Gram positive bacteria are more sensitive to the effect of nanoparticles. Possibly, the variation in the results is due to differences in the structural and molecular composition of the bacteria, as well as the concentration of inoculum and the size and shape of the nanoparticles [53].
3.6.2. Minimum inhibitory concentration (MIC)
Table 1 presents the results obtained from the evaluation of the minimum inhibitory concentration. The MIC value obtained for S. aureus was 1.9 μg/mL, which means that at this concentration of nanoparticles there was no apparent growth of the bacteria in 24 h. P. fluorescens presented a MIC value of 7.8 μg/mL. Compared to other studies [27], in this present study, growth inhibition was achieved in S. aureus, using a concentration of 1.9 μg/mL, which is lower than the established by other authors. For P. fluorescens, no studies were found that evaluated the antibacterial effect of silver nanoparticles in this bacteria. However, some authors evaluated Gram negative bacteria strains such as E. coli and P. aureginosa where the values obtained for the MIC were lower than the obtained in this study for P. fluorescens. Comparing these results, it was observed that higher concentrations of nanoparticles are needed to be able to ensure that the bacteria don't present any growth at 24 h of incubation.
Table 1.
Evaluation of the minimum inhibitory (MIC) and minimum lethal (MLC) concentration of silver nanoparticles on Staphylococcus aureus and Pseudomonas fluorescens.
| Bacteria | Antibacterial Evaluation |
|
|---|---|---|
| MIC (μg/mL) | MLC (μg/mL) | |
| Staphylococcus aureus | 1.9 | 3.9 |
| Pseudomonas fluorescens | 7.8 | 15.6 |
3.6.3. Minimum lethal concentration (MLC)
Table 1 shows the minimum lethal concentrations of both S. aureus and P. fluorescens. The results indicated that the MLC value for S. aureus was 3.9 μg/mL, while for P. fluorescens was 15.6 μg/mL. When comparing the results of MLC obtained in the present investigation with the results of other authors [54, 55], there is a difference in the values for both bacteria, is important to highlight that there are no studies in which the authors evaluated the MLC of silver nanoparticles on P. fluorescens, so a direct comparison between results cannot be made. However, there are reports where Gram negative bacteria were evaluated, such as P. aeruginosa and E. coli.
Regarding S. aureus, the literature define that the MLC values were higher than those reported for Gram negative bacteria, which indicates that the effect of silver nanoparticles is lower in S. aureus. However, this result differs from the ones obtained in the present study, since S. aureus is more sensitive to silver nanoparticles than P. fluorescens. Previous studies indicate that this variation in results may be due to differences in experimental conditions, such as, bacterial strain, the size and shape of the nanoparticles, the concentration of inoculum, the synthesis method as well as the type of extract used to obtain the nanostructured material [56].
4. Conclusion
The structural and morphological analysis confirmed the synthesis of silver nanoparticles through the use of aqueous extract of safflower residue. The size of the nanoparticles was in the nanometric range with a spherical shape. In addition, the presence of molecules such as proteins, polysaccharides and phenolic compounds present in the extract in silver nanoparticles was demonstrated, promoting capping and stabilization. Likewise, these nanoparticles had the ability to inhibit the growth of Staphylococcus aureus as a pathogen and Pseudomonas fluorescens as a food spoilage. Based on the results, we conclude that silver nanoparticles have a potential application in a broad range of industries, mainly food and medicine.
Declarations
Author contribution statement
Francisco Rodríguez-Félix: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Astrid Guadalupe López-Cota, José Agustín Tapia-Hernández: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
María Jesús Moreno-Vásquez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Abril Zoraida Graciano-Verdugo, Carmen Lizette Del-Toro-Sánchez: Contributed reagents, materials, analysis tools or data.
Idania Emedith Quintero-Reyes: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145(1-2):83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S., Saifullah, Ahmad M., Swami B.L., Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Rad. Res. Appli. Sci. 2016;9(1):1–7. [Google Scholar]
- 3.Meyers M.A., Mishra A., Benson D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006;51(4):427–556. [Google Scholar]
- 4.Sánchez G.V., Rosales I.B., Vargas O.S. Escalamiento del proceso de síntesis de nanopartículas de Ag vía reducción química. Avances y casos en innovación tecnológica. 2019;100 [Google Scholar]
- 5.Behravan M., Panahi A.H., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 6.Kumar B., Smita K., Cumbal L., Debut A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017;24(1):45–50. doi: 10.1016/j.sjbs.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagheri S., Moradi P., Nematollahi F., Zarinnia V., Abdossi V. Investigating green synthesis of silver nanoparticles from orange peel (Citrus sinensis) and the effects of Chitosan, Sylamol, Nanosilver on Rhizopus stolanifer in Tomato (Solanum lycopersicum) Indian J. Ecol. 2020;47(1):82–86. [Google Scholar]
- 8.Pratikno H., Anggya P.B., Fadhila F., Chafidz A., Wara D.P.R. Vol. 872. Trans Tech Publications Ltd; 2021. Biosynthesis of silver nanoparticles using banana Raja (musa paradisiaca var. raja) peel extract: effect of different concentrations of the AgNO3 solution; pp. 61–66. (Key Engineering Materials). [Google Scholar]
- 9.Rozykulyyeva L., Astuti S.D., Zaidan A.H., Pradhana A.A.S., Puspita P.S. Vol. 2314. AIP Publishing LLC; 2020, December. Antibacterial activities of green synthesized silver nanoparticles from Punica granatum peel extract; p. 60012. (AIP Conference Proceedings). No. 1. [Google Scholar]
- 10.Alfuraydi A.A., Devanesan S., Al-Ansari M., AlSalhi M.S., Ranjitsingh A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019;192:83–89. doi: 10.1016/j.jphotobiol.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Saratale R.G., Saratale G.D., Ghodake G., Cho S.K., Kadam A., Kumar G., Jeon B. Pant, D., Bhatnagar A., Shin H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019;128:391–400. doi: 10.1016/j.ijbiomac.2019.01.120. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q., Liu G., Chen G., Chen T., Mi T. Green synthesis of silver nanoparticles with corn straw for the preparation of antibacterial paper. BioResources. 2017;12(4):9063–9074. [Google Scholar]
- 13.Li J., Ma Q., Shao H., Zhou X., Xia H., Xie J. Biosynthesis, characterization, and antibacterial activity of silver nanoparticles produced from rice straw biomass. BioResources. 2017;12(3):4897–4911. [Google Scholar]
- 14.Delshad E., Yousefi M., Sasannezhad P., Rakhshandeh H., Ayati Z. Medical uses of Carthamus tinctorius L. (Safflower): a comprehensive review from traditional medicine to modern medicine. Electron. Physician. 2018;10(4):6672–6681. doi: 10.19082/6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emongor V., Oagile O., Phuduhudu D., Oarabile P. Botswana: the Regional Universities Forum for Capacity Building in Agriculture–RUFORUM. 2017. Safflower production. [Google Scholar]
- 16.Zhou X., Tang L., Xu Y., Zhou G., Wang Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 2014;151(1):27–43. doi: 10.1016/j.jep.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 17.Guo D., Xue Y., Li D., He B., Jia X., Dong X., Guo M. Overexpression of CtCHS1 increases accumulation of quinochalcone in safflower. Front. Plant Sci. 2017;8:1409. doi: 10.3389/fpls.2017.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales-Del-Rio J.A., Gutiérrez-Lomelí M., Robles-García M.A., Aguilar J.A., Lugo-Cervantes E., Guerrero-Medina P.J., Ruiz-Cruz S., Cinco-Moroyoqui F.J., Wong-Corral F.J., Del-Toro-Sánchez C.L. Anti-inflammatory activity and changes in antioxidant properties of leaf and stem extracts from Vitex mollis kunth during in vitro digestion. Evid. Base Compl. Alternative Med. 2015 doi: 10.1155/2015/349235. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jini D., Sharmila S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today: Proceed. 2019 [Google Scholar]
- 21.Aljawish A., Chevalot I., Jasniewski J., Revol-Junelles A.M., Scher J., Muniglia L. Laccase-catalysed functionalisation of chitosan by ferulic acid and ethyl ferulate: evaluation of physicochemical and biofunctional properties. Food Chem. 2014;161:279–287. doi: 10.1016/j.foodchem.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Vásquez M.J., Valenzuela-Buitimea E.L., Plascencia-Jatomea M., Encinas-Encinas J.C., Rodríguez-Félix F., Sánchez-Valdes S., Graciano-Verdugo A.Z. Functionalization of chitosan by a free radical reaction: characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017;155:117–127. doi: 10.1016/j.carbpol.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Chen F., Shi Z., Neoh K.G., Kang E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009;104(1):30–39. doi: 10.1002/bit.22363. [DOI] [PubMed] [Google Scholar]
- 24.Gautam S., Bhagyawant S.S., Srivastava ∗Nidhi. Detailed studies on therapeutic properties uses and pharmacological applications of safflower (Carthamus tinctorius L.) Int. J. Ayurveda Pharma Res. 2015;2(3) [Google Scholar]
- 25.Asgarpanah J., Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013;19(2):153–159. doi: 10.1007/s11655-013-1354-5. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim F.Y., El-Khateeb A.Y., Mohamed A.H. Rhus and safflower extracts as potential novel food antioxidant, anticancer, and antimicrobial agents using nanotechnology. Foods. 2019;8(4):139. doi: 10.3390/foods8040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Rad. Res. Appl. Sci. 2015;8(3):265–275. [Google Scholar]
- 28.Ponce M. Centro de Investigación en Tecnología Avanzada, Instituto Politécnico Nacional-Unidad Altamira; 2011. Síntesis y caracterización de nanopartículas de Ni y NiMo en medio acuoso”. Tesis de Posgrado. Altamira, Tamaulipas, México. [Google Scholar]
- 29.Singh J., Dutta T., Kim K.H., Rawat M., Samddar P., Kumar P. 'Green' synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol. 2018;16(1):84. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar C.G., Pombala S., Poornachandra Y., Agarwal S.V. Nanobiomaterials in Antimicrobial Therapy. William Andrew Publishing; 2016. Synthesis, characterization, and applications of nanobiomaterials for antimicrobial therapy; pp. 103–152. [Google Scholar]
- 31.Khalil M.M., Ismail E.H., El-Baghdady K.Z., Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014;7(6):1131–1139. [Google Scholar]
- 32.Jha A.K., Prasad K. Green synthesis of silver nanoparticles using Cycas leaf. Int. J. Green Nanotechnol. Phys. Chem. 2010;1(2):P110–P117. [Google Scholar]
- 33.Varenne F., Makky A., Gaucher-Delmas M., Violleau F., Vauthier C. Multimodal dispersion of nanoparticles: a comprehensive evaluation of size distribution with 9 size measurement methods. Pharmaceut. Res. 2016;33(5):1220–1234. doi: 10.1007/s11095-016-1867-7. [DOI] [PubMed] [Google Scholar]
- 34.Hemmati S., Rashtiani A., Zangeneh M.M., Mohammadi P., Zangeneh A., Veisi H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. [Google Scholar]
- 35.Jagtap U.B., Bapat V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013;46:132–137. [Google Scholar]
- 36.Velammal S.P., Devi T.A., Amaladhas T.P. Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostruc. Chem. 2016;6(3):247–260. [Google Scholar]
- 37.Banerjee P., Satapathy M., Mukhopahayay A., Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Biores. Bioprocess. 2014;1(1):3. [Google Scholar]
- 38.Mohammadi P., Sheibani H. Green synthesis of Fe3O4@ SiO2-Ag magnetic nanocatalyst using safflower extract and its application as recoverable catalyst for reduction of dye pollutants in water. Appl. Organomet. Chem. 2018;32(4):e4249. [Google Scholar]
- 39.Aboutorabi S.N., Nasiriboroumand M., Mohammadi P., Sheibani H., Barani H. Biosynthesis of silver nanoparticles using safflower flower: structural characterization, and its antibacterial activity on applied wool fabric. J. Inorg. Organomet. Polym. Mater. 2018;28(6):2525–2532. [Google Scholar]
- 40.Soto K.M., Quezada-Cervantes C.T., Hernández-Iturriaga M., Luna-Bárcenas G., Vazquez-Duhalt R., Mendoza S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT (Lebensm.-Wiss. & Technol.) 2019;103:293–300. [Google Scholar]
- 41.Saratale G.D., Saratale R.G., Kim D.S., Kim D.Y., Shin H.S. Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens: a novel approach. Nanomaterials. 2020;10(8):1457. doi: 10.3390/nano10081457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadam J., Dhawal P., Barve S., Kakodkar S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg 2+ biosensing. SN Applied Sciences. 2020;2(4):1–16. [Google Scholar]
- 43.Li W.R., Xie X.B., Shi Q.S., Duan S.S., Ouyang Y.S., Chen Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals. 2011;24(1):135–141. doi: 10.1007/s10534-010-9381-6. [DOI] [PubMed] [Google Scholar]
- 44.Kora A.J., Arunachalam J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J. Microbiol. Biotechnol. 2011;27(5):1209–1216. [Google Scholar]
- 45.Vatandost E., Chekin F., Yasaghi S.A.S. Green synthesis of silver nanoparticles by pepper extracts reduction and its electocatalytic and antibacterial activity. Russ. J. Electrochem. 2016;52(10):960–965. [Google Scholar]
- 46.Le Ouay B., Stellacci F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10(3):339–354. [Google Scholar]
- 47.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qing Y., Cheng L., Li R., Liu G., Zhang Y., Tang X., Wang J., Liu H., Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S., Guo Y., Zhong H., Chen S., Li J., Ge Z., Tang J. Synergistic antibacterial mechanism and coating application of copper/titanium dioxide nanoparticles. Chem. Eng. J. 2014;256:238–246. [Google Scholar]
- 51.Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011;7(2):184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Hajipour M.J., Fromm K.M., Ashkarran A.A., de Aberasturi D.J., de Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Patil M.P., Kim G.D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017;101(1):79–92. doi: 10.1007/s00253-016-8012-8. [DOI] [PubMed] [Google Scholar]
- 54.Bryaskova R., Pencheva D., Kyulavska M., Bozukova D., Debuigne A., Detrembleur C. Antibacterial activity of poly (vinyl alcohol)-b-poly (acrylonitrile) based micelles loaded with silver nanoparticles. J. Colloid Interface Sci. 2010;344(2):424–428. doi: 10.1016/j.jcis.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 55.Das B., Dash S.K., Mandal D., Ghosh T., Chattopadhyay S., Tripathy S.…Roy S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chemis. 2017;10(6):862–876. [Google Scholar]
- 56.Ruparelia J.P., Chatterjee A.K., Duttagupta S.P., Mukherjia S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.