Abstract
This study examines the use of cement treatment in improving the lead immobilization properties of a compacted clay liner for sanitary landfill applications. The compaction and strength characteristics of the cement treated clay at cement contents of 0%, 3%, 6%, 9%, 12%, 15% and 18% by weight of dry soil are studied via standard compaction and unconfined compressive strength tests. The lead immobilization characteristics of the cement treated clay are also investigated using atomic absorption spectroscopy. The cement contents of 6% and 9% significantly affect the permeability coefficient and lead absorption of the clay. The permeability coefficient of the cement treated clay meets the requirement for a waterproof material in landfill, i.e., <1.49 × 10−11 m/s. Lead immobilization is shown to increase with increasing cement content. When the lead nitrate solution in the form of Pb2+ ions seeps through the cement treated clay, the hydrolysis reaction results in the formation of Ca2+ and OH− ions. The solution with high alkalinity from this reaction dissolves SiO2 and Al2O3 in the clay. The Pb2+ ions are therefore absorbed by SiO2 and Al2O3 and Pb3SiO5 is formed. As a result, the lead content in the effluent from the cement treated clay is significantly lower than that from untreated clay. The results from this research can be translated into a regulatory framework for managing the contamination dissipation of industrial waste from landfill.
Keywords: Clay-liner, Landfill, Lead, Unconfined compressive strength, Immobilization, Soil cement, Ground improvement
Clay-liner, Landfill, Lead, Unconfined compressive strength, Immobilization, Soil cement, Ground improvement.
1. Introduction
The population growth rate coupled with the rapid economic and industrial growth in Thailand in recent decades has resulted in the expansion of urban prosperity to rural areas. Moreover, the demands of land use for the production of consumer goods, as well as housing, have also increased. The inevitable consequence of this development is solid waste, which has negative environmental impacts and can affect human health. Significant efforts from communities are required to manage the amount of hazardous wastes and toxins that are contaminated in water, soil and air. Toxins and heavy metals can also be left in food, thereby representing a significant risk to human health through diseases such as cancer and genetic disorders.
The Pollution Control Department of Thailand (2016) reported that there was 14.3 million tons of solid waste or 38,221 tons/day in 2005 in Thailand and in 2015, the solid waste increased to 49,680 tons/day while the waste recycling was only ~3.1 million tons or 22% of the total amount of solid waste. The most popular method for solid waste disposal is to bury it directly underneath soil. This method has been used for hundreds of years because of its cost effectiveness and reduced negative environmental impact (Pollution Control Department, 2016). However, heavy metal leaching from the waste into groundwater sources will inevitably affect communities.
In order to avoid environmental problems due to various contaminations in surface water and groundwater, and the outbreak of various diseases, the United States changed the method of waste disposal from burning and pouring into outdoor areas into hygienic waste disposal (sanitary landfill) in 1940. Since the 1950s, the waste has been divided into two categories: general waste and hazardous waste, which contains heavy metal contaminants. Solid general waste is generally buried in a landfill area above a well compacted bottom layer. Previous studies have found that the bottom layer cannot prevent the leachate, even when the thickness of the compacted floor is increased (Rowe, 2005; Chen et al., 2018). This method is therefore prohibited in some countries.
Heavy metals are elements with specific gravity of greater than four and have a relatively slow decomposition rate. These metals mainly include zinc, lead, copper, tin, arsenic and mercury and can accumulate in the environment over long periods of time. Heavy metal elements are typically in the transition metal group and are toxic to living organisms. They cannot be decomposed via natural processes and can cause precipitation that accumulates in soil, water and aquatic animals. The heavy metals that accumulate in food, such as in animal and plant tissues, affect the health of consumers. Therefore, because of the accumulation of heavy metals in the human body, it is necessary to have a good control and management system in the food chain.
At present, the management of toxic contaminated waste in landfill consists of high-density polyethylene on top of a compacted soil layer or geosynthetic clay liner. This type of landfill is widely used to store liquid and solid waste. An important element in landfill is the floor mat, which must have a very low permeability. Previous research (Laine et al., 1988; Giroud and Bonaparte, 1989; Giroud et al., 1989; Brennecke and Corser, 1998; Rollin et al., 1999) indicated that leakage from a small hole in the rubber mat can cause heavy metals to leach into groundwater.
Chemical treatment using lime, cement, geopolymers and so on can improve the physical and mechanical properties of soil (Horpibulsuk et al., 2012; Kampala et al., 2013; Kua et al., 2016; Hassan et al., 2019; Sukmak et al., 2019; Poltue et al., 2019). In addition to improving its strength, these alkaline substances can improve the heavy metal immobilization properties of soil. Even though research works on the immobilization of heavy metals by soil/chemical treatment are available (Basta and McGowen, 2004; Hisel and Apak, 2006; Zhang et al., 2008; Lwin et al., 2018; Changjutturas et al., 2019), they mainly focus on the role of the clay mineral and cementation bond on the immobilization of heavy metals to develop encapsulated materials for disposal in landfill. Studies of the role of cement treatment on the immobilization of heavy metals in compacted low-swelling soil used as clay liner materials remain limited. Such research is innovative as clay liners are typically made of compacted high-swelling clay without cement treatment.
This work studies the capability of a compacted clay-cement layer in encapsulating heavy metals due to the improvement of its soil waterproofing properties. The cement treated soil sample is compacted under standard compaction energy to the maximum dry density and then its heavy metal absorption is measured by passing a lead solution to the sample. An extensive suite of mechanical and microstructural tests is conducted in this study. To obtain information regarding the surface topography and composition, microstructural tests including scanning electron microscopy (SEM), X-ray diffraction (XRD) and X-ray fluorescence spectroscopy (XRF) are utilized. The results of this study will lead to the development of guidelines for controlling heavy metal contamination in compacted clay for landfill applications.
2. Materials
2.1. Soft Bangkok clay
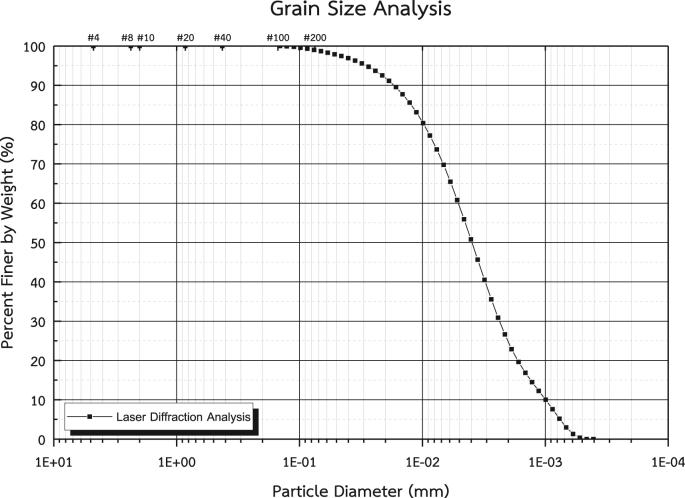
Soft Bangkok clay samples were collected at a depth of 4–6 m from the ground in Pathumthani province (14°00′48″ N, 100°31′49″ E). The natural water content, wet unit weight and specific gravity were 79.8%, 15.62 kN/m2 and 2.66, respectively. The liquid and plastic limits (ASTM D4318) were 62% and 37%, respectively. The particle size analysis of clay using a laser diffraction technique (ASTM D4464) showed 2% sand, 71% silt and 27% clay. The particle size was in the range of 0.46–127 μm, as shown in Figure 1. Based on the AASHTO classification system (AASHTO M 145, 2012), the soil was classified as group A-7-5 (0), with the appearance of fine granular clay with a high liquid. Based on the Unified Soil Classification System (ASTM D2487), the soil was classified as inorganic clay with high plasticity (CH). The Bangkok clay sample at this site is classified as low-swelling soil based on a free swell test and the dominant clay mineralogy types are likely to be kaolinite and montmorillonite (Horpibulsuk et al., 2007, Horpibulsuk et al., 2011).
Figure 1.
Grain size curve distribution.
The clay sample was slightly acidic with pH 6.6. The chemical composition of the clay sample was obtained from XRF with 57.8% SiO2, 19.9% Al2O3, 5.99% Fe2O3, 2.06% K2O, 0.96% TiO2, 0.935% MgO, 0.770% CaO and 0.318% Na2O. The major element was SiO2, making the majority of the clay surface silanol group dominating, thereby allowing it to absorb polar molecules (Hisel and Apak, 2006). The clay sample had a plate-like structure with rough surfaces and a horizontal size wider than the vertical size. It had sharp corners and unequal shapes, as shown in Figure 2a.
Figure 2.
SEM of materials used for testing: a) clay particles, b) Portland Cement type I and c) lead nitrate particles.
2.2. Portland cement type I
Portland cement type I was used to improve the waterproofing layer of the Bangkok clay sample. The main compounds were calcium silicate, calcium aluminate and calcium sulfate. The secondary compounds were alkaline and lime, with some chloride. The quantitative analysis using XRF reveals that the chemical composition of the Portland cement included 62% CaO as the main component and 15.9% silicon oxide (SiO2), as shown in Table 1. The SEM image shows that the cement particles had an irregular shape with rough surfaces (Figure 2b).
Table 1.
Chemical composition of Portland cement type I.
| Unit | CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | K2O | TiO2 | Na2O | P2O5 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KCps | 1396.7 | 155.1 | 33.9 | 63.8 | 152.8 | 10.9 | 21.4 | 2.5 | 0.7 | 0.8 | 1.8 |
| % | 62.00 | 15.90 | 3.53 | 3.33 | 3.03 | 0.92 | 0.70 | 0.24 | 0.15 | 0.0676 | 0.0452 |
2.3. Lead
According to the Agency for Toxic Substances and Disease Registry (ATSDR 2019), lead was ranked second in the toxic list after arsenic. Moreover, lead is a heavy metal that is commonly found in landfill environments. Other heavy metals, such as mercury and cadmium, are highly toxic and have small particle sizes, which make them difficult to detect. Even though copper and zinc are heavy metals, they are only weakly toxic substances. Manganese can also be commonly found in landfill in Thailand (Pattanamahakul, 2017). As such, lead was selected as a studied heavy metal in this research.
Lead was prepared from a nitrate solution form in order to reduce the harmful effects of this heavy metal. Pb(NO3)2 (0.16065 g) was dissolved in distilled water and adjusted to 1000 mL in a volumetric flask. A lead solution with a concentration of 100 mg/L was then obtained.
Lead is the main component of Pb(NO3)2 with an amount of 61.9%, as shown in Table 2. The SEM image shows that the lead particles had round and smooth surfaces of different sizes (Figure 2c).
Table 2.
Chemical composition of lead nitrate.
| Pb | Hg | Si | Cu |
|---|---|---|---|
| 61.9 % | 0.054% | 0.0263% | 0.0171% |
3. Methodology
The lead immobilization of the cement treated clay layer was studied. The clay was mixed with Portland cement at various contents of 0%, 3%, 6%, 9%, 12%, 15% and 18% by weight of dry soil. The cement treated soil sample was first compacted in three layers inside a standard Proctor compaction mold (100 mm in diameter and 114.6 mm in height) under a standard Proctor energy of 592.5 kJ/m3 (ASTM D698) to obtain the optimum moisture content (OMC) and maximum dry density (ρd,max) for each cement content. The samples for the strength, permeability and lead immobilization tests were subsequently prepared by a static compression method in cylindrical molds with a diameter of 55 mm and a height of 110 mm at OMC to obtain ρd,max from the standard Proctor compaction test. The compressed samples were dismantled, tightly wrapped within vinyl sheet and then cured in a humidity desiccator at room temperature (25–30 °C). The unconfined compression strength of the cement treated clay was measured after curing periods of 7 and 28 d (ASTM D2166).
After a curing period of 7 d, a permeability coefficient test was conducted on the untreated sample after 7 d of curing and on the 6% and 9% cement treated samples after 7 and 28 d of curing. To examine the heavy metal absorbability of the untreated and cement treated samples, a flexible wall triaxial permeability test (ASTM D5084-16a) was conducted. The test began by allowing the Pb(NO3)2 solution with a concentration of 100 mg/L to flow through the sample. The heavy metal concentration was also detected using atomic absorption spectrophotometry (AAS) referring to EPA SW-846: Test Method 3051A at the surface and layers 2, 4 and 7, as shown in Figure 3.
Figure 3.
Sample stratification for testing.
In order to prepare the samples for the triaxial permeability test (Figure 4), the soil samples were prepared using a mini compaction tool with a mold diameter of 5.5 cm. The base and top caps had the same diameter as the soil samples (Figure 5). After the test was completed, the sample was removed from the tested cell, cut and divided into seven layers. The soil samples at each layer were oven-dried at 105 °C and then ground thoroughly and packed into a plastic bag. The samples were kept in a humidity control cabinet to measure the lead concentrations at various soil layers.
Figure 4.
Triaxial permeability test.
Figure 5.
New invented base cap and top cap.
SEM imaging was used to study the physical structure, size, grains and characteristics of heavy metal bonding on the sample surface in a three-dimensional image. XRD was used to analyze the crystal structures, compounds and minerals. This technique can distinguish material types in crystal form in nature or can be used to classify mineral types by measuring the intensity of radiation reflected at various angles compared to the standard data. Each compound has different crystal structures and arrangement between planes of atoms, depending on its size and atomic charge, and therefore a unique XRD pattern.
The AAS sample preparation involved rendering liquid or solid samples into a state where the device could proceed with the elemental analysis. The sample was atomized to create a fine mist dispersion, which was then fed into the flame to break the remaining molecular bonds. In the graphite furnace AAS, the liquid sample was brought directly into the cuvette to transform it into the fine mist. The specimen was then exposed to a radiation source that typically originates from a light source. This light source was set to a specified wavelength where the metal atom in the sample absorbed it. When the absorption occurred, the light spectrum has reduced light intensity in one or more of its areas. This reduced intensity is characteristic of a given element and helps to identify it, as well as determining its concentration.
4. Results
4.1. Compaction characteristics
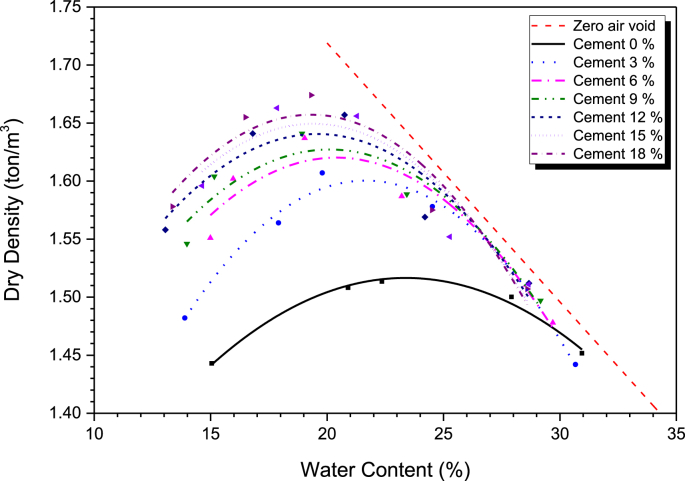
Figure 6 shows the relationship between the dry density versus water content of the cement treated clay compared with that of untreated clay. For a particular cement content, the dry density increased as the water content increased to the OMC where the ρdmax was obtained. The dry density then decreased as the water content increased because the voids between the soil grains were filled with water and hence the prevention of soil particles into the voids (Horpibulsuk et al., 2004). The ρd,max was 1.52 t/m3 and the OMC was 23.38%. As the cement content increased to 3%, 6%, 9%, 12%, 15% and 18% by weight of dry soil, the OMC decreased to 21.66%, 20.39%, 20.10%, 19.58%, 19.41% and 19.31%, respectively, while the ρd,max increased to 1.600, 1.620, 1.627, 1.641, 1.649 and 1.657 t/m3, respectively. This behavior is consistent with that of coarse-grained soil treated with cement (Lambe et al., 1959; Moh, 1965). The OMC decreased with increasing cement content because some water in the mix is used for cement hydration. With the higher specific gravity of cement, the ρd,max of the cement treated clay increases with cement content (Donrak et al., 2018; Sudla et al., 2019).
Figure 6.
Dry density and water content with different cement content.
4.2. Unconfined compression strength
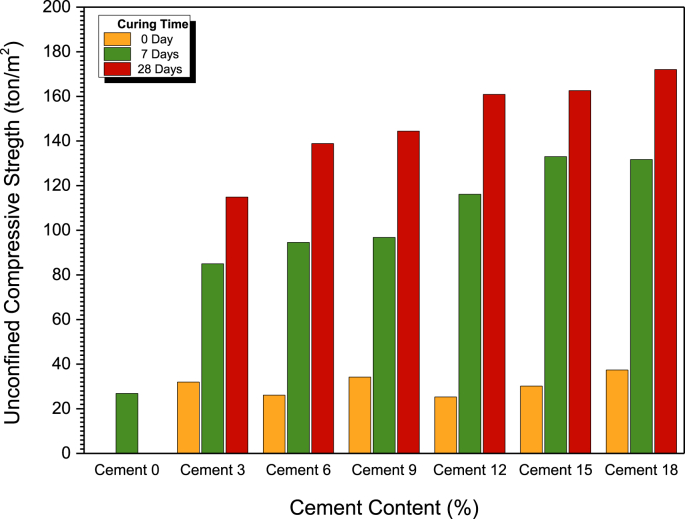
Figure 7 shows the unconfined compressive strength, qu, development over the curing period for both the untreated and treated samples. The qu values of the untreated clay samples at various curing times (0, 7 and 14 d) were essentially the same at ~26.88 t/m2 with a failure strain (εf) of 2.9%. Their average unit weight was 1.91 t/m3.
Figure 7.
Relationship between uncompressed compressive strength and cement content.
The qu of the cement treated clay increased as the cement content increased. The qu of the cement treated clay just after being prepared was close to that of the untreated clay sample due to the low degree of cement hydration. The 7-d qu values of the cement treated clays at 3%, 6%, 9%, 12%, 15% and 18% were 3.58, 3.98, 4.08, 4.89, 5.60 and 5.55 times higher than the 7-d qu value of the untreated clay, respectively. This strength increase is due to the cementitious products (calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH)), which bond the clay particles and fill the pore space.
The cement treated clay at 3%–9% cement contents possessed a 7-d qu of less than 100 t/m2. While the cement treated clay at 12%–15% cement contents had a 7-d qu higher than 120 t/m2. The qu during the first 7 d increased rapidly due to the rapid hydration reaction, resulting from CSH and CAH. After 7 d, calcium hydroxide reacts with the silica and alumina in clay and causes the secondary reaction, with CSH and CAH developing slowly and continuously with time (Sasanian and Newson, 2014).
A minimum 28-d strength requirement for a vertical barrier to control groundwater flow and to contain contaminants is 10 t/m2 (Opdyke and Evans, 2005). It was evident that the 28-d qu of the cement treated clay with cement contents ranging from 12% to 18% was not significantly different. Therefore, the 6% cement content provided a 28-d qu of >140 t/m2, which is appropriate for a waterproofing layer in landfills.
4.3. Permeability
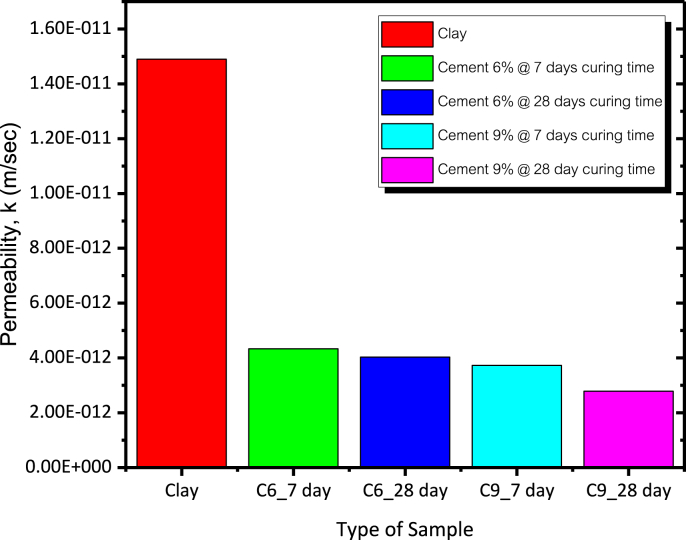
The permeability coefficients of the untreated clay and cement treated clays at 6% and 9% cement contents and at 7 and 28 d of curing are presented in Figure 8. The permeability coefficient of the cement treated clay was lower than that of untreated clay due to the growth of cementitious products filling up the pore space. The permeability coefficients of the 6% and 9% cement treated clay samples cured at 7 and 28 d under saturated conditions were 4.33 × 10−12 and 3.73 × 10−12 m/s, respectively, at 7 d of curing and 4.03 × 10−12 and 2.78 × 10−12 m/s, respectively, at 28 d of curing (Table 3).
Figure 8.
Permeability coefficients of clay and cement treated clay with different curing period.
Table 3.
Water permeability coefficient of compacted cement treated clay.
| Soil | Testing Time (day) | γdmax (ton/m3) | OMC (%) | Permeability, k (m/s) |
|---|---|---|---|---|
| Cement 0% | 7 | 1.517 | 23.38 | 1.49E-11 |
| Cement 6% | 7 | 1.600 | 21.66 | 4.33E-12 |
| Cement 6% | 28 | 1.620 | 20.39 | 4.03E-12 |
| Cement 9% | 7 | 1.627 | 20.10 | 3.73E-12 |
| Cement 9% | 28 | 1.657 | 19.31 | 2.78E-12 |
The permeability coefficient of a compacted clay required for a waterproofing material is 1.49 × 10−11 m/s (Opdyke and Evans, 2005). The permeability coefficients of both the untreated and cement treated clays were lower than 1 × 10−9 m/s; therefore, these materials can be adapted as waterproofing materials in landfill.
4.4. Lead immobilization
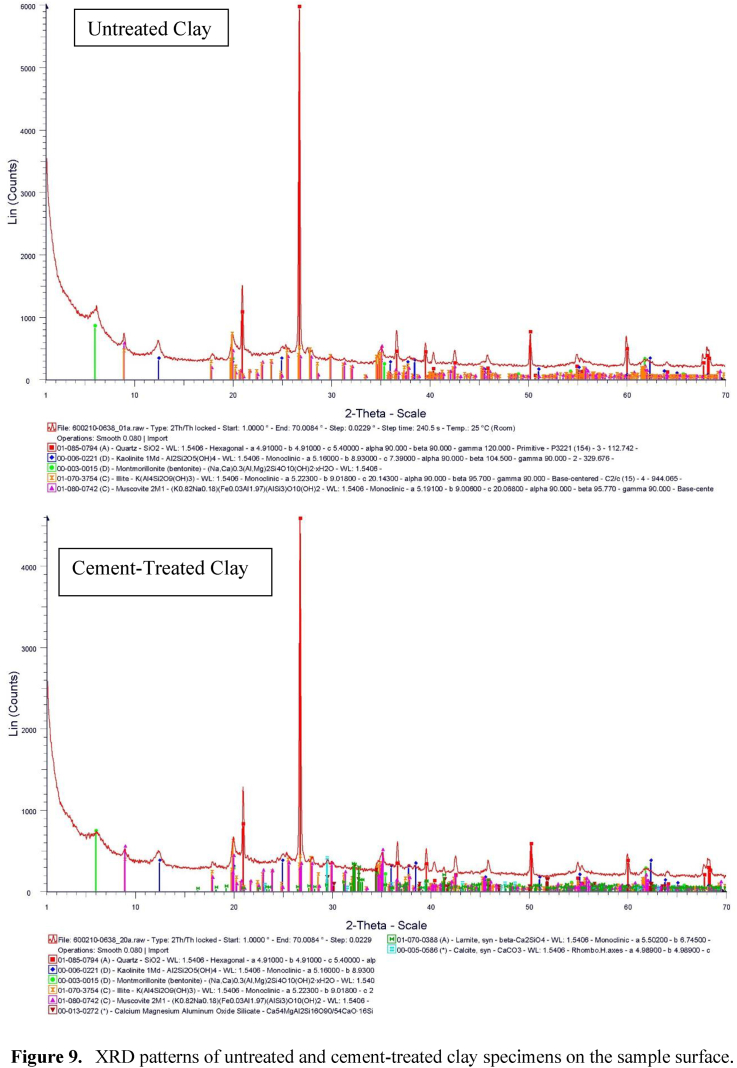
To determine the appropriate amount of cement to inhibit lead, lead immobilization ability tests in untreated clay and 6% and 9% cement treated clays at 7 and 28 d were conducted. The lead immobilization of the untreated and cement treated clays was measured using residual lead content analysis and the values at different layers (Figure 3) were compared for both the untreated and treated samples. In addition, the presence of lead in the permeable solution was also analyzed. In the flexible wall triaxial permeability test, 2 L of lead nitrate solution were applied through the top cap of the triaxial cell. Due to the low permeability of the sample, only 50 mL of lead nitrate solution seeped through the sample. A small amount of lead was found, which was hardly detected by XRD, as shown in Figure 9. Therefore, AAS was used to detect the lead content.
Figure 9.
XRD patterns of untreated and cement-treated clay specimens on the sample surface.
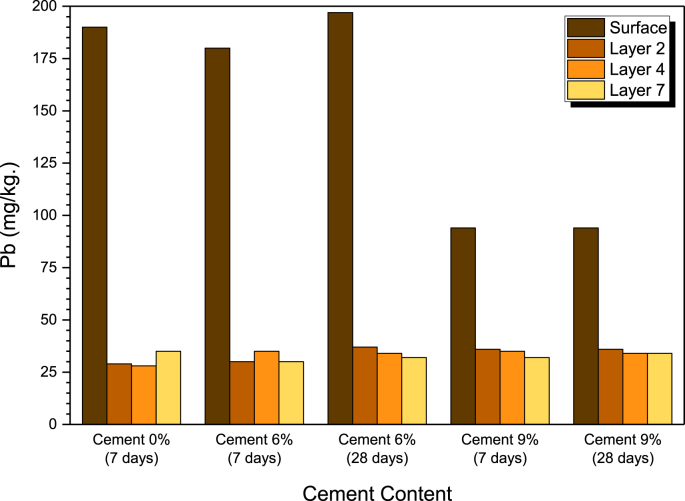
The amount of absorbed lead solution at the top, middle and bottom parts of the sample was determined by AAS, as presented in Figure 10. The untreated clay samples exhibited lead concentrations of 190, 29, 28 and 35 mg/kg, on the surface and layers 2, 4 and 7, respectively. For the 6% cement treated clay cured at 7 d, the lead concentrations were 180 (surface), 35 (layer 2), 30 (layer 4) and 30 (layer 7) mg/kg, while at 28 d, they were 94 (surface), 35 (layer 2), 36 (layer 4) and 32 (layer 7) mg/kg. For the 9% cement treated clay cured at 7 d, the lead concentrations were 197 (surface), 37 (layer 2), 34 (layer 4) and 32 (layer 7) mg/kg, while at 28 days, they were 94 (surface), 36 (layer 2), 34 (layer 4) and 34 (layer 7) mg/kg. The surface area of the sample had the highest lead concentration, whereas at the top (layer 2) through bottom (layer 7), the lead concentrations were slightly different. This might be because the clay particles on the soil surface absorbed the nitrate solution until they were saturated and the remaining nitrate solution was contaminated to the lower layers.
Figure 10.
Lead inhibition ability of samples at various curing period.
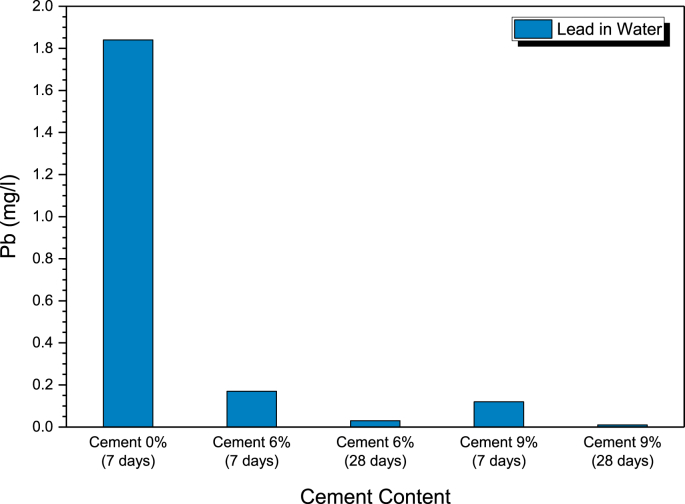
Figure 11 shows the amount of lead that can be detected from the solution. The detected amount of lead in the untreated specimen was 1.85 mg/L. For the 6% cement, the detected lead in the solution was 0.76 and 0.18 mg/L for the 7 and 28 d cured samples, respectively. While for the 9% cement, the detected lead from the solution was 0.60 and 0.09 mg/L for the 7 and 28 d cured samples, respectively. The higher the cement content and curing time, the lower the amount of lead detected. In other words, the 6% and 9% cement treatments can significantly reduce the lead content in the effluent compared with the untreated clay.
Figure 11.
The amount of lead left in the solution.
Clay is an alkaline soil with a high pH of >8.5. When improving clay with cement, its pH will increase due to the high concentration of Ca2 + and OH− and it can absorb more heavy metals than the untreated clay with a lower pH value. As a result, a solution with a pH greater than 12 causes lead to be deposited. In addition, SiO2 and Al2O3 were broken down to react with water and cause a hydrolysis reaction. Therefore, Pb2+ ions were absorbed by SiO2 and Al2O3 and some reacted with water.
This hydrolysis reaction can be developed in normal conditions and if there is sufficient moisture, the reaction will continue to develop. In contrast, the retention ability was improved due to the continuous hydrolysis reaction. Where there is sufficient moisture, CaO and SiO2 can be broken down to capture lead and hence causes lead to be deposited. The increment of cement content causes SiO2 to decompose, react and bond with lead in the form of Pb3SiO5 (Zhang et al., 2008). Because cement contains other substances, such as Al2O3, CaO3 and Fe2O3, it causes additional chemical processes, such as metal and lead ion exchange, resulting in interpolar capture or strong binding by van der Waals forces corresponding to the amount of lead remaining in the sample solution.
Apart from the clay mineral, pH and heavy metal solution, the organic matter and iron oxides in clay also play an important combination role in immobilization. To increase the efficiency of absorbing heavy metals in the soil by considering only the amount of organic matter and iron oxide quantity, one should consider the role of organic substances and oxide compounds on the immobilization of clay. However, the type of clay minerals affects the heavy metal absorption efficiency. Montmorillonite can best absorb heavy metals, followed by muscovite and kaolinite clays (non-to low swelling soil). To enhance the immobilization of heavy metals of kaolinite clay, bentonite or muscovite should be added in appropriate amounts (Hisel and Apak, 2006).
Due to the high silt content and low-swelling characteristics (Horpibulsuk et al., 2007), the OMC of the studied soil was found to be lower than that of kaolin (29.3%) and bentonite (33.8%) reported by Horpibulsuk et al. (2008). The permeability and swelling potential of the compacted clay sample are expected to be higher and lower than those of a high swelling clay, such as bentonite, respectively. These unfavorable properties must be improved to meet the national and international requirements for clay liner materials. The cement treatment is proved to be an effective means for improving the immobilization of heavy metals and the mechanical strength of low-swelling Bangkok clay in this research.
4.5. Microstructural analysis
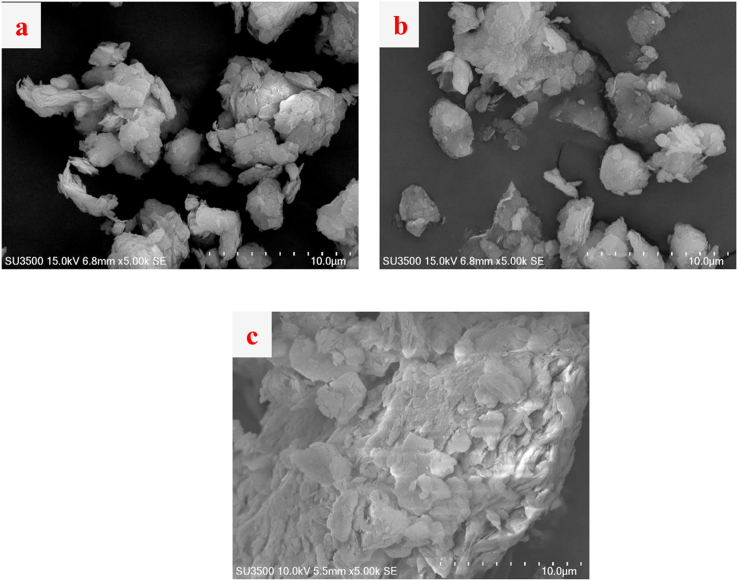
SEM was employed to investigate the lead immobilization of untreated clay samples and the 6% and 9% cement treated clays at 7 and 28 d of curing. Pb(NO3)2 can easily be dissolved in water. The lead solution is in the form of Pb2+ ions at the nanoscale, making them impossible to observed directly by SEM. The clay containing lead contamination can be observed by SEM, where Pb2+ was fixed with other substances and transforms into an insoluble substance and then settles on the surface of the clay.
4.6. Untreated clay
For compacted clay with a high degree of saturation, the soil particle arrangement influences the compressive strength and settlement resistance, which is controlled by the compaction energy and moisture content (Horpibulsuk et al., 2009). The compaction energy causes the soil particles to move into voids and reduces the pore space. Figure 12 shows that the soil particles at the surface were loosely packed compared to the other layers. The soil particle arrangement at the top layer (layer 2) was similar to that on the surface but was different from that at the lower part (layer 6), which received more compaction energy. As such, the soil particles at the lower part gathered into a larger lump with small voids.
Figure 12.
SEM of compacted clay particles under standard compaction energy: a) clay surface after 7 days, b) top part after 7 days and c) bottom part after 7 days.
4.7. Cement treated clay
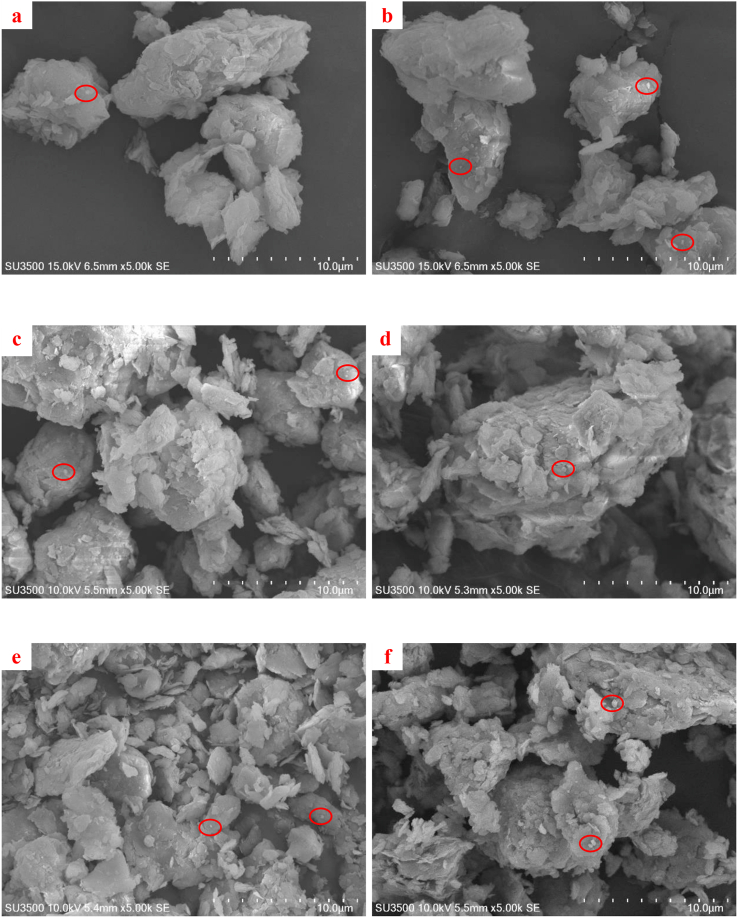
As the amount of cement increased, the hydration products in the voids also significantly increased. The hydration products not only increased the bonding strength between soil particles, but also reduced the volume of voids (Horpibulsuk et al., 2010). Figure 13 (6% cement) and Figure 14 (9% cement) show a reduction in the specific surface area of the clay with an increase in cement content due to a reduction in the water content caused by hydration.
Figure 13.
SEM images of 6% cement treated clay: a) clay surface after 7 days, b) clay surface after 28 days, c) top part after 7 days, d) top part after 28 days, e) bottom part after 7 days and f) bottom part after 28 days.
Figure 14.
SEM images of 9% cement treated clay: a) clay surface after 7 days, b) clay surface after 28 days, c) top part after 7 days, d) top part after 28 days, e) bottom part after 7 days and f) bottom part after 28 days.
Figures 13a and b show that the soil structure on the surface was disturbed due to compaction, which caused large soil particles to be broken down to smaller particles. Figures 13c and d show loose soil particles on the upper part (layer 2) of the compacted clay. Figures 13e and f show the bottom layer (layer 6) of the clay particles, where it is clearly seen that the clay particles were bonded together and became large lumps with small voids. The circles in the figures show the image of the detected lead.
The Pb(NO3)2 solution reacted with other compounds to become insoluble substances and finally settled on the surface of the clay particles. These settled compounds were round in shape and are clearly different from the plate-like clay particles. In accordance with the experimental results from AAS (Figure 11), most of lead was absorbed on the surface of the sample and was significantly reduced at the upper, middle and lower parts of the sample.
Based on the measured residual lead concentration in the samples, the lead residue on the surface of the 9% cement treated clay sample was less than that on the surface of the 6% cement treated clay sample (Figure 13). This implies that the increment of cement content had a significant effect on lead adsorption because the lead solution reacted with cement to form a new compound. Moreover, the curing period did not significantly affected lead adsorption. Voids in the cement treated soil were filled by the cementation products, resulting in an impervious soil structure. When the lead nitrate solution in the form of Pb2+ ions seeped through the cement treated clay, the hydrolysis reaction resulted in the formation of Ca2+ and OH− ions. The solution with high alkalinity occurring from this reaction caused SiO2 and Al2O3 to break down for the hydrolysis reaction (Zhang et al., 2008). Pb2+ ions can be absorbed by SiO2 and Al2O3 and broken down into Pb3SiO5. However, some Pb2+ ions react with other substances and become insoluble substances, causing the lead-absorbing substance to precipitate and adhere onto clay particles.
5. Conclusions
This study investigated the lead immobilization of cement treated Bangkok clay. The clay sample was mixed with Portland cement type 1 at cement contents of 3%, 6%, 9%, 12%, 15% and 18% by weight of dry soil. The properties of the compacted and cement treated clays were studied, including physical and engineering properties and lead immobilization. The results can be summarized as follows.
-
1)
The density of the cement treated clay increased with increasing cement content. The maximum dry densities of the compacted and compacted cement treated clays with a cement content of 18% were equal to 1.52 and 1.66 ton/m3, respectively. The cement treatment enhances the bonding between soil particles and reduces voids in the soil mass. In contrast, the optimal moisture content is inversely proportional to the cement content. Furthermore, the developed bonding enhances the unconfined compressive strength of the cement treated clay.
-
2)
The permeability coefficients of the cement treated clay were lower than the permeability coefficient of the compacted clay of 1.49 × 10−11 m/s. This is because the voids between clay particles were filled with the cementitious products. However, the permeability coefficients insignificantly changed with the cement content and curing time.
-
3)
The curing period affects the lead immobilization of the cement treated clay. The more cement that is added, the greater the immobilization of lead. When the lead nitrate solution in the form of Pb2+ ions seeps through the cement treated clay, the hydrolysis reaction resulted in the formation of Ca2+ and OH− ions. The solution with high alkalinity from this reaction dissolves SiO2 and Al2O3 in clay. Pb2+ ions are therefore absorbed by SiO2 and Al2O3 and form Pb3SiO5.
Declarations
Author contribution statement
Chairat Teerawattanasuk: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Panich Voottipruex: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Suksun Horpibulsuk: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by King Mongkut's University of Technology North Bangkok (TH) (KMUTNB-60-GOV-025).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- AASHTO M 145 . American Association of State Highway and Transportation Officials (AASHTO); 2012. Standard Specification for Classification of Soils and Soil-Aggregate Mixtures for Highway Construction Purposes. [Google Scholar]
- ATSDR . U.S. Department of Health and Human Services, Public Health Service, The Agency for Toxic Substances and Disease Registry (ATSDR); GA: 2019. ATSDR’s Substance Priority List. [Google Scholar]
- ASTM D2166/D2166M−16 . ASTM International; 2016. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. [Google Scholar]
- ASTM D2487 . ASTM International; 2017. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System) [Google Scholar]
- ASTM D4318 . ASTM International; 2017. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. [Google Scholar]
- ASTM D4464 . ASTM International; 2015. Standard Test Method for Particle Size Distribution of Catalytic Materials by Laser Light Scattering. [Google Scholar]
- ASTM D5084-16a . ASTM International; 2016. Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter. [Google Scholar]
- ASTM D698 . ASTM International; 2012. Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort (12,400 ft-lbf/ft3 (600 kN-m/m3)) [Google Scholar]
- Basta N.T., McGowen S.L. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 2004;127:73–82. doi: 10.1016/s0269-7491(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Brennecke D., Corser P. Proceedings of Tailings and Mine Waste ‘98. Balkema Publishers; Rotterdam: 1998. Performance issues related to geosynthetic liners at Monticello, Utah; pp. 351–360. [Google Scholar]
- Changjutturas K., Hoy M., Horpibulsuk S., Arulrajah A., Rashid A.S.A. Solidification and stabilization of metal plating sludge with fly ash geopolymers. Environ. Geotech. 2019 [Google Scholar]
- Chen J., Benson C., Edil T., Likos W. Hydraulic conductivity of geosynthetic clay liners with sodium bentonite to coal combustion product leachates. J. Geotech. Geoenviron. Eng. 2018 [Google Scholar]
- Donrak J., Horpibulsuk S., Arulrajah A., Kao H., Chinkulkijniwat A., Hoy M. Wetting-drying cycles durability of cement stabilized marginal lateritic soil/melamine debris blends for pavement applications. Road Mater. Pavement Des. 2018 [Google Scholar]
- EPA SW-846 . 2007. Test Method 3051A. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. [Google Scholar]
- Giroud J.P., Bonaparte R. Leakage through liners constructed with geomembranes-Parts I and II. Geotext. Geomembranes. 1989;8(1):71–111. 27–67. [Google Scholar]
- Giroud J.P., Khatami A., Badu-Tweneboah K. Evaluation of the rate of leakage through composite liners. Geotext. Geomembranes. 1989;8(4):241–271. [Google Scholar]
- Hassan H., Hassan W.H.W., Rashid A.S.A., Latifi N., Yunus N.Z.M., Horpibulsuk S. Microstructural characteristics of organic soils treated with biomass silica stabilizer. Environ. Earth Sci. 2019;78:367. [Google Scholar]
- Hisel J.H., Apak R. Modeling of copper(II) and lead(II) adsorption on kaolinite-based clay minerals individually and in the presence of humic acid. J. Colloid Interface Sci. 2006;295(1):1–13. doi: 10.1016/j.jcis.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Horpibulsuk S., Miura N., Bergado D.T. Undrained shear behavior of cement admixed clay at high water content. J. Geotech. Geoenviron. Eng. ASCE. 2004;30(10):1096–1105. [Google Scholar]
- Horpibulsuk S., Shibuya S., Fuenkajorn K., Katkan W. Assessment of engineering properties of Bangkok clay. Can. Geotech. J. 2007;44(2):173–187. [Google Scholar]
- Horpibulsuk S., Katkan W., Apchatvullop A. An approach for assessment of compaction curves of fine-grained soils at various energies using a one point test. Soils Found. 2008;48(1):115–126. [Google Scholar]
- Horpibulsuk S., Katkan W., Naramitkornburee A. Modified Ohio’s curves: a rapid estimation of compaction curves for coarse- and fine-grained soils. Geotech. Testing J., ASTM. 2009;32(1):64–75. [Google Scholar]
- Horpibulsuk S., Rachan R., Chinkulkijniwat A., Raksachon Y., Suddeepong A. Analysis of strength development in cement-stabilized silty clay based on microstructural considerations. Construct. Build. Mater. 2010;24:2011–2021. [Google Scholar]
- Horpibulsuk S., Yangsukaseam N., Chinkulkijniwat A., Du Y.J. Compressibility and permeability of Bangkok clay compared with kaolinite and bentonite. Appl. Clay Sci. 2011;52:150–159. [Google Scholar]
- Horpibulsuk S., Phochan W., Suddeepong A., Chinkulkijniwat A., Liu M.D. Strength development in blended cement admixed saline clay. Appl. Clay Sci. 2012;55(1):44–52. [Google Scholar]
- Kampala A., Horpibulsuk S., Chinkulkijniwat A., Shen S.L. Engineering properties of recycled calcium carbide residue stabilized clay as fill and pavement materials. Construct. Build. Mater. 2013;46:203–210. [Google Scholar]
- Kua T.A., Arulrajah A., Horpibulsuk S., Du Y.J., Shen S.L. Strength assessment of spent coffee grounds-geopolymer cement utilizing slag and fly ash precursors. Construct. Build. Mater. 2016;115:565–575. [Google Scholar]
- Laine D.L., Miklas M.P., Parr C.H. Environmental Protection Agency; Washington D.C.: 1988. Loading point Puncture Ability Analysis of Geosynthetic Liner Material. EPA/600/S2-88/040. [Google Scholar]
- Lambe T.W., Michaels A.S., Moh Z.C. Improvements of soil-cement with alkali metal compounds. Highway Res. Board. 1959;241:67–108. [Google Scholar]
- Lwin C.S., Seo B.H., Kim H.U., Owens G., Kim K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality-a critical review. Soil Sci. Plant Nutr. 2018;64:156–167. [Google Scholar]
- Moh Z.C. Reaction of soil minerals with cement and chemicals. Highw. Res. Rec. 1965;86:39–61. [Google Scholar]
- Opdyke S.M., Evans J.C. Slag-cement-bentonite slurry walls. J. Geotech. Geoenviron. Eng. 2018;131:673–681. [Google Scholar]
- Pattanamahakul P. Heavy metal concentration and risk assessment of soil and rice in and around an open dumpsite in Thailand. EnvironmentAsia. 2017;10(2):53–64. [Google Scholar]
- Pollution Control Department . 2016. A Pollution Situation Reports in Thailand.http://www.pcd.go.th/public/Publications/print_report.cfm Retrieved from. [Google Scholar]
- Poltue T., Suddeepong A., Horpibulsuk S., Samingthong W., Arulrajah A., Rashid A.S.A. Strength development of recycled concrete aggregate stabilized with fly ash-rice husk ash based geopolymer as pavement base material. Road Mater. Pavement Des. 2019 [Google Scholar]
- Rollin A.L. Proceedings of Geosynthetics ‘99 Conference. Industrial Fabrics Association International; Minneapolis: 1999. Leak location in exposed geomembrane liners using an electrical leak detection techniques; pp. 615–626. [Google Scholar]
- Rowe R.K. Long-term performance of contaminant barrier systems. 45th Rankine Lecture, Geotechnique. 2005;55(9):631–678. [Google Scholar]
- Sasanian S., Newson T. Basic parameters governing the behaviour of cement-treated clays. Soils Found. 2014;54(2):209–224. [Google Scholar]
- Sukmak P., Kunchariyakun K., Sukmak G., Horpibulsuk S., Kassawat S., Arulrajah A. Strength and microstructure of palm oil fuel ash-fly ash-soft soil geopolymer masonry units. J. Mater. Civ. Eng. 2019;31(8):1–13. 04019164. [Google Scholar]
- Sudla P., Donrak J., Hoy M., Horpibulsuk S., Arulrajah A., Rashid A.S.A., Nazir R., Samingthong W. Laboratory investigation of cement stabilized marginal lateritic soil by crushed/fly ash replacement for pavement applications. J. Mater. Civ. Eng. 2019 [Google Scholar]
- Zhang J., Provis J., Feng D., Van Deventer J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+ J. Hazard Mater. 2008;157(2-3):587–598. doi: 10.1016/j.jhazmat.2008.01.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.