Abstract
Background
Microthrombosis is a hallmark of COVID-19. We previously described von willebrand factor (VWF) and their high molecular weight multimers (HMWMs) as potential trigger of microthrombosis.
Objectives
Investigate VWF activity with collagen-binding assay and ADAMTS13 in COVID-19.
Methods and results
Our study enrolled 77 hospitalized COVID-19 patients including 37 suffering from a non-critical form and 40 with critical form. Plasma levels of VWF collagen-binding ability (VWF:CB) and ADAMTS13 activity (ADAMTS13:Act) were measured in the first 48 hours following admission. VWF:CB was increased in critical (631% IQR [460–704]) patients compared to non-critical patients (259% [235–330], p < 0.005). VWF:CB was significantly associated (r = 0.564, p < 0.001) with HMWMs. Moreover, median ADAMTS13:Act was lower in critical (64.8 IU/dL IQR 50.0–77.7) than non-critical patients (85.0 IU/dL IQR 75.8–94.7, p < 0.001), even if no patients displayed majors deficits. VWF:Ag-to-ADAMTS13:Act ratio was highly associated with VWF:CB (r = 0.916, p < 0.001). Moreover, VWF:CB level was highly predictive of COVID-19 in-hospital mortality as shown by the ROC curve analysis (AUC = 0.92, p < 0.0001) in which we identified a VWF:CB cut-off of 446% as providing the best predictor sensitivity–specificity balance. We confirmed this cut-off thanks to a Kaplan–Meier estimator analysis (log-rank p < 0.001) and a Cox-proportional Hazard model (HR = 49.1, 95% CI 1.81–1328.2, p = 0.021) adjusted on, BMI, C-reactive protein, and D-dimer levels.
Conclusion
VWF:CB levels could summarize both VWF increased levels and hyper-reactivity subsequent to ADAMTS13 overflow and, therefore, be a valuable and easy to perform clinical biomarker of microthrombosis and COVID-19 severity.
Keywords: COVID-19, Microthrombosis, Von Willebrand factor, Collagen-binding, Multimers, ADAMTS13, Mortality
Dear Editor,
We would like to thank Bhogal et al. [1] for their insightful response to our study demonstrated the importance of von Willebrand factor (VWF) and in particular their high molecular weight multimers (HMWMs) [2]. They propose perspectives in COVID-19 treatment according to VWF/ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) axis involvement. VWF and ADAMTS13 form a system known to play a key role in microthrombosis. Indeed, VWF is paramount for both platelet adhesion to subendothelial collagen fibers and platelet aggregation under high shear [3]: VWF is released into the circulation from endothelial cells as hyperactive ultralarge VWF multimers (ULVWFMs). ADAMTS13 controls the breakdown and regulation of ULVWFMs resulting in a balanced distribution between VWF low-, intermediate-, and HMWMs [3]. Microthrombosis, in particular in lungs, is now a hallmark of COVID-19. Indeed, significant pulmonary microthrombosis could trigger major gas exchanges defects triggering acute respiratory distress syndrome [4]. As a matter of fact, we previously demonstrated that pulmonary obstruction could lead to right heart ventricle dysfunction linked to COVID-19 severity [5]. This microthrombosis is associated to endothelial dysfunction, reflected by increased levels of circulating endothelial cells, plasma angiopoietin-2, and VWF in severe COVID-19 patients [2, 5–7]. Moreover, data on ADAMTS13 activity (ADAMTS13:Act) in severe COVID-19 patients are conflicting, some authors reporting strictly normal [8], slightly decreased [9], or even greatly diminished values [10].
In the present study, we intend to highlight VWF hyperactivity through a rapid and easy-to-perform VWF collagen-binding (VWF:CB) assay. We further explored the cause of the abovementioned VWF hyperactivity in COVID-19, which still remains elusive, through ADAMTS13:Act assessment. In this monocentric cross-sectional study, we explored 77 COVID-19 patients hospitalized including 37 suffering from a non-critical form and 40 with critical form. Patients were all included and COVID-19 severity stratified as previously described [2]. All laboratory assays were performed on samples collected less than 48 h following patients’ hospitalization. Determination of D-dimer and C-reactive protein (CRP) levels, platelet count, VWF:antigen (VWF:Ag), and VWF multimeric profile were achieved as previously described [2]. The VWF:CB was determined using the Asserachrom VWF:CB ELISA kit (Stago, Asnières-sur-Seine, France). ADAMTS13:Act was assessed using the Technozyme ADAMTS13 activity ELISA (Technoclone, Vienna, Austria). Both ELISA kits were used according to the manufacturer’s instructions.
Among the 77 COVID-19 patients hospitalized and studied here, 33 (83%) critical patients and 25 (68%) non-critical patients were male. Critical patients were not significantly older than non-critical patients (critical: median age of 62 years, interquartile range [IQR] 53–72; non-critical: 63 years IQR 52–72, p = 0.85). Conversely, critical patients had significantly higher median body mass index (BMI) than non-critical patients (critical: 28.9 kg/m2, IQR 26.9–34.9; non-critical: 25.1 kg/m2 IQR 23.6–29.5, p = 0.005). There was no significant difference in median platelet count between critical (307 G/L, IQR 199–375) and non-critical patients (275 G/L IQR 199–375). It is to be noted that we previously described that critical patients displayed higher levels of plasma VWF:Ag associated with a higher proportion of HMWMs compared with non-critical COVID-19 patients [2].
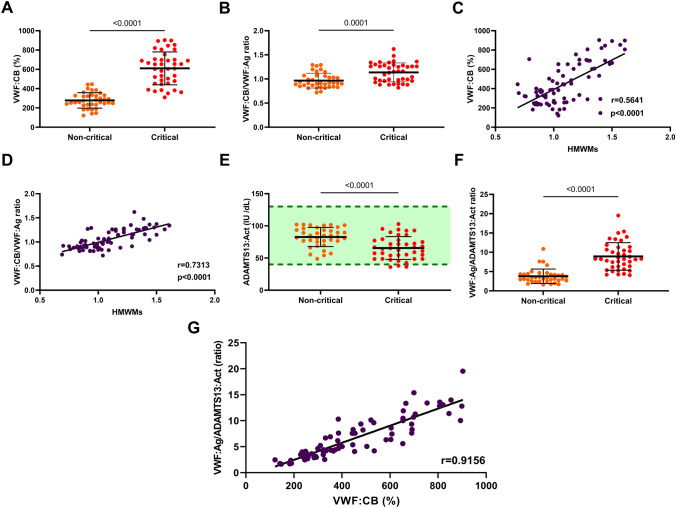
VWF:CB levels on admission were significantly higher in critical (median 631% IQR [460–704]) than in non-critical (259% [235–330], p < 0.005) COVID-19 patients (Fig. 1a). Moreover, the VWF:CB-to-VWF:Ag ratio, an indirect and quantitative way to measure VWF multimers size, was still significantly higher in critical (median 1.16 IQR 0.96–1.28) than in non-critical (0.91, IQR 0.85–1.07, p = 0.0001) COVID-19 patients, therefore, in favor of VWF hyperactivity in critical patients (Fig. 1b). Consistently, both VWF:CB and the VWF:CB-to-VWF:Ag ratio were strongly positively associated with HMWMs of VWF in our cohort (Spearman correlation coefficient r = 0.564 and r = 0.731, respectively, p < 0.0001 for both) (Fig. 1c and d). Therefore, the VWF:CB assay appears to be an adequate surrogate method to appreciate VWF multimers levels.
Fig. 1.
Levels and association of von Willebrand factor—ADAMTS13 system-related biomarkers according to critical and non-critical COVID-19 patients. Data points indicate individual measurements, whereas horizontal bars represent the means with standard deviations. Green-shaded area indicate the normal ranges of values. For comparison between severity groups, p value comes from the Mann–Whitney test. For association between two variables in the whole cohort, p value and correlation coefficient (r) comes from the Spearman correlation and the solid black line was obtained through simple linear regression. Plasma levels of von Willebrand factor:collagen binding (VWF:CB) (a) and VWF:CB-to-VWF:antigen (VWF:Ag) ratio (b). Association between VWF high molecular weight multimers (HMWMs) proportion and VWF:CB (c) or VWF HMWMs and VWF:CB-to-VWF:Ag ratio (d). Plasma levels of ADAMTS13 activity (ADAMTS13:Act) (e) and VWF:Ag-to-ADAMTS13 ratio (f). Association between VWF:Ag-to-ADAMTS13 ratio and VWF:CB (g)
Plasma ADAMTS13:Act levels at admission were significantly lower in critical (median 64.8 IU/dL IQR 50.0–77.7) than in non-critical COVID-19 patients (85.0 IU/dL IQR 75.8–94.7, p < 0.001) (Fig. 1e). Nevertheless, only 3 (7.5%) critical patients displayed ADAMTS13:Act levels under normal values (i.e., 40–130 UI/dL), while all non-critical patients showed ADAMTS13:Act levels in the normal range. Moreover, VWF:Ag-to-ADAMTS13:Act ratio is higher in critical (median 8.27 IQR 6.20–11.1) than non-critical patients, (3.46 IQR 2.61–4.30, p < 0.001) supporting a potential saturation of ADAMTS13 in critical COVID-19 patients (Fig. 1f). This result means that for a single unit of plasma ADAMTS13, there was approximately three times more and eight times more circulating VWF:Ag, in non-critical and critical COVID-19 patients, respectively. Notably, the VWF:Ag-to-ADAMTS13:Act ratio was strongly associated with VWF:CB (Spearman correlation coefficient r = 0.916, p < 0.001), suggesting that VWF:CB summarize both quantitative (i.e., plasma VWF:Ag increase) and qualitative VWF abnormalities (VWF excess of HMWMs consequential to ADAMTS13 overflow, Fig. 1g).
Finally, we evaluated the discriminatory ability between survivors and non-survivors of VWF:CB using a ROC curve (AUC = 0.92, p < 0.0001, Fig. 2a). Therefore, we estimated a cut-off value to predict in-hospital mortality. Upon admission, a VWF:CB level of 446% provided an optimal sensitivity–specificity balance, with an excellent sensitivity of 95.2% (95% confidence interval [CI] 73.3–99.8%) associated with an acceptable specificity of 76.8% (95% CI 64.2–85.9%) regarding COVID-19-related mortality. The ability of this VWF:CB cut-off to predict in-hospital mortality was further validated in a Kaplan–Meier estimator (p < 0.001, Fig. 2b) and a Cox-proportional hazard analysis adjusted for BMI, D-dimer, and CRP (Hazard ratio [HR] 49.1, 95% CI 1.81–1328.2, p = 0.021, Fig. 2c).
Fig. 2.
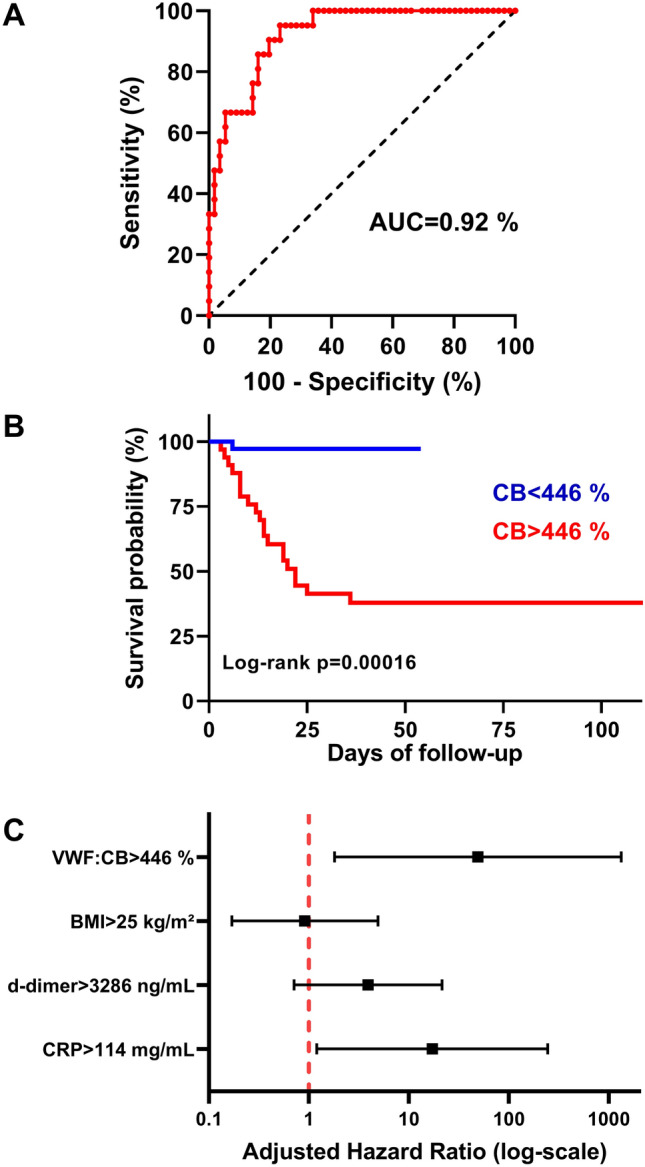
Association between von Willebrand factor collagen-binding admission level and in-hospital mortality in COVID-19 patients. a Receiver operating curves evaluating von Willebrand factor collagen-binding (VWF: CB) ability to predict in-hospital-mortality estimated by the area under the curve (AUC) value. The diagonal black-dotted segment is the reference line. b Survival curves according to VWF:CB level using a Kaplan–Meier estimator. Data are shown for patients with low VWF:CB (< 446%) and high von VWF:CB (> 446%). Survival curves are compared using the log-rank test. c Forest plot showing the Cox-proportional hazards model for VWF:CB adjusted for age, body mass index (BMI), D-dimer, and C-reactive protein (CRP). For each variable, black squares represent hazard ratios (HR) and solid black lines represent HR 95% confidence intervals
Our study highlights increased circulating hyperactive VWF and ADAMTS13 overflow in COVID-19 severity. This imbalance could be at the origin of microthrombosis. Moreover, VWF:CB appears to sum up VWF global thrombogenic impact and is a powerful predictor of COVID-19-related mortality which reinforces this hypothesis.
VWF:CB assay is initially a diagnostic test that quantifies the binding capacity of VWF-A1 and -A3 domain to collagen binding, the main subendothelial matrix component in order to improve the diagnosis and differentiation of qualitative variant of von Willebrand disease, the most common inherited bleeding disorder [3]. VWF activity is commonly assessed using VWF ristocetin cofactor assays, which evaluate the binding between the VWF-A1 domains to platelet membrane receptor GPIb. VWF:CB activity is a more sensitive test exploring HMWMs of VWF abnormalities [11]. Consistently, we observed a marked increase of VWF:CB in critical COVID-19 patients, even after normalization to VWF:Ag levels, confirming an intrinsic VWF hyperactivity, previously described using HMWMs evaluation [2]. VWF multimers quantification is a complex and time-consuming method. Thus, VWF:CB could represent a valuable, easy–to-perform assay to highlight VWF hyperactivity in COVID-19.
In addition, we found lower ADAMTS13:Act in critical COVID-19 patients than in non-critical ones even if only few patients present values above normal, a finding consistent with some previous reports [9]. Severe deficiency in ADAMTS13 (activity < 10 IU/dL) is a signature for thrombotic thrombocytopenic purpura (TTP) diagnosis, a life-threatening thrombotic condition caused by the subsequent accumulation of hyperactive HMWMs causing disseminated thrombi formation occluding arterioles and capillaries [12]. Reports of small platelets-rich thrombi within the microvasculature of SARS-CoV-2-infected patients [13] makes it tempting to draw a parallel between TTP and COVID-19 associated coagulopathy. However, the normal platelet count in nearly all COVID-19 patients in our cohort associated with the scarcity of ADAMTS13:Act true deficits make this hypothesis difficult to accept. Thus, more than a decrease in ADAMTS13:Act levels, we observed a major discrepancy between ADAMTS13:Act and VWF:Ag levels in critical patients, suggesting an overflow of ADAMTS13 capacity. Although TTP arises from severe decreased in ADAMTS13:Act, thrombotic events have been associated to slight decrease if associated with a major increase of circulating VWF level, in particular in myocardial infarction, ischemic stroke, and cancer-associated venous thrombosis [14]. Moreover, Warlo et al. demonstrated a correlation between increased VWF:Ag-to-ADAMTS13:Act ratio and high residual platelet reactivity despite aspirin treatment, suggesting that imbalance in VWF-ADAMTS13 system could also promote arise of hyper-reactive platelets [14]. The mechanisms underlying altered VWF:Ag-to-ADAMTS13 ratio in COVID-19 is still unknown. ADAMTS13 could be consumed at a higher rate due to increased VWF processing, and/or inflammatory cytokines could suppress ADAMTS13 biosynthesis in the liver [15].
All in all, VWF:CB is significantly associated to VWF:Ag-to-ADAMTS13 ratio and HMWMs of VWF. VWF:CB could summarize both VWF qualitative and quantitative anomalies in COVID-19 and therefore be a valuable, easy-to-perform, mirror of VWF global thrombotic involvement in microthrombosis formation. Our results not only highlight the potential for plasma VWF:CB to discriminate COVID-19 patient's severity and/or in-hospital mortality but also may help targeting patients for new therapeutic approaches such as (i) recombinant ADAMTS13, (ii) caplacizumab (an anti–von Willebrand factor humanized single-variable-domain immunoglobulin targeting the A1 domain of von Willebrand factor), (iii) N-acetyl cysteine known to reduce the size of VWF multimers.
Funding
This work has been funded with grants from French national agency for research ANR SARCODO (Fondation de France) and Mécénat Covid AP-HP. The study was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment (CPP 2020-04-048/2020-A01048-31/20.04.21.49318). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All author declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhogal P, Paul G, Collins O. Letter in response to: circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021 doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philippe A, Chocron R, Gendron N, et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021 doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillicrap D. Von Willebrand disease: advances in pathogenetic understanding, diagnosis, and therapy. Hematol Am Soc Hematol Educ Program. 2013;2013:254–260. doi: 10.1182/asheducation-2013.1.254. [DOI] [PubMed] [Google Scholar]
- 4.Diehl J-L, Peron N, Chocron R, et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: a hypothesis-generating study. Ann Intensive Care. 2020;10:95. doi: 10.1186/s13613-020-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goudot G, Chocron R, Augy JL, et al. Predictive factor for COVID-19 worsening: insights for high-sensitivity troponin and D-Dimer and correlation with right ventricular afterload. Front Med Front. 2020 doi: 10.3389/fmed.2020.586307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khider L, Gendron N, Goudot G, et al. Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost JTH. 2020;18(9):2391–2399. doi: 10.1111/jth.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escher R, Breakey N, Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost JTH. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinelli N, Montagnana M, Pizzolo F, et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favaloro EJ, Pasalic L, Curnow J. Laboratory tests used to help diagnose von Willebrand disease: an update. Pathology (Phila) 2016;48:303–318. doi: 10.1016/j.pathol.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost JTH. 2017;15:312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 13.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warlo EMK, Pettersen AÅR, Arnesen H, et al. vWF/ADAMTS13 is associated with on-aspirin residual platelet reactivity and clinical outcome in patients with stable coronary artery disease. Thromb J. 2017;15:28. doi: 10.1186/s12959-017-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389–394. doi: 10.1097/00062752-200209000-00001. [DOI] [PubMed] [Google Scholar]