Abstract
In nature, enzymes conventionally operate under aqueous conditions. Because of this, aqueous buffers are often the choice for reaction media when enzymes are applied in chemical synthesis. However, to meet the demands of an industrial application, due to the poor water solubility of many industrially relevant compounds, an aqueous reaction system will often not be able to provide sufficient substrate loadings. A switch to a non-aqueous solvent system can provide a solution, which is already common for lipases, but more challenging for biocatalysts from other enzyme classes. The choices in solvent types and systems, however, can be overwhelming. Furthermore, some engineering of the protein structure of biocatalyst formulation is required. In this review, a guide for those working with biocatalysts, who look for a way to increase their reaction productivity, is presented. Examples reported clearly show that bulk water is not necessarily required for biocatalytic reactions and that clever solvent systems design can support increased product concentrations thereby decreasing waste formation. Additionally, under these conditions, enzymes can also be combined in cascades with other, water-sensitive, chemical catalysts. Finally, we show that the application of non-aqueous solvents in biocatalysis can actually lead to more sustainable processes. At the hand of flowcharts, following simple questions, one can quickly find what solvent systems are viable.
It's not only lipases which can be applied in alternative solvent systems to meet industrial and environmental demands. At the hand of case studies and flowcharts this review quickly shows what solvent systems are viable.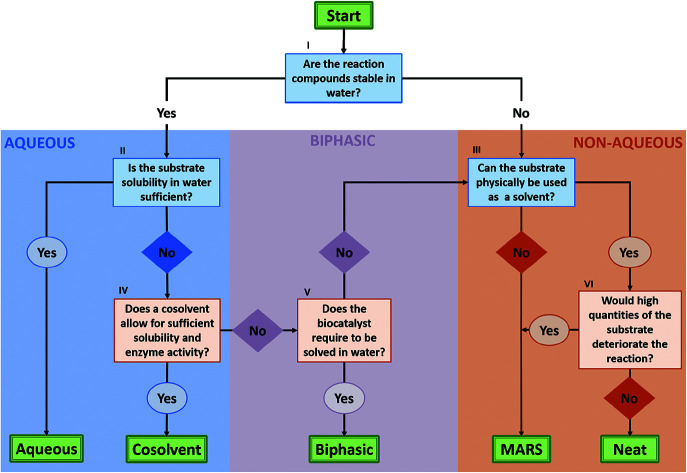
1. Introduction
Biocatalysis has emerged as a powerful strategy to efficiently perform synthetic reactions under mild conditions, also at industrial scales.1–3 In a nutshell, biocatalysis exploits nature's ability to perform chemical reactions with extraordinary selectivity and rates at ambient temperatures and pressures. While first examples of biocatalysis relied on natural reactions in buffered aqueous solutions, over the years the ability to shape these proteins for reactions of (industrial) interest has emerged.4–8 To cope with tight industrial economics, reactions need to afford high space–time yields, high catalytic turnover numbers and high product concentrations.9,10
In nature, (cytosolic) enzymes are evolved to function optimally in aqueous environments, and aqueous buffers have been thus traditionally the first choice of reaction medium when characterizing a biocatalytic reaction.11 Similarly, a significant proportion of the natural substrates for enzymes, the cell metabolites, have been biochemically decorated to become highly water soluble. However, at industrial scale – where enzymes catalyze reactions with non-natural substrates –, the use of water may be a hurdle. Many compounds of industrial interest typically exert low water solubility, which restricts product concentrations and productivity of the enzymatic process when conducted in aqueous media. Furthermore, when chemo-enzymatic cascades are considered, the non-enzymatic catalysts typically require non-aqueous conditions, which makes it challenging to find a system suitable for both, biocatalysts and chemocatalysts.12
To counter these challenges, the application of enzymes in non-aqueous reaction systems is considered. Within the different enzyme classes, several hydrolases like lipases have been naturally evolved to function in hydrophobic environments and are conventionally applied under these conditions,13–15 with first examples stemming from the thirties.16,17 Especially in the eighties and nineties, pioneering work by Zaks and Klibanov showed these enzyme's remarkable stability in non-aqueous solvents and other extreme conditions.18,19 The application of enzymes from other classes in non-aqueous reaction systems, however, has proven to be less straightforward. When it is observed that already a few volume percentages of organic solvent can be enough to inactivate a biocatalyst, researchers may become reluctant to take their biocatalysts outside their familiar aqueous environment. Furthermore, the great variety in solvents systems (e.g. cosolvent, biphasic, neat, etc.) (Table 1), and solvent types (e.g. organic, neoteric, etc.), can make the palette of options overwhelming at first sight. Ultimately, from a green chemistry perspective, the use of non-aqueous solvents often bears the stigma of making the whole process less sustainable. This may be true in some cases, but, especially when more sustainable solvents are considered, the application of these solvents might even make the whole process more environmentally benign.
Nomenclature of the principles described in this work.
| Solvent system | Describes the reaction medium; which solvents are used, in which ratios and how many phases are present |
| Cosolvent | Compound added to a reaction medium to improve reaction performance. Cosolvents are added up to saturation, so no second phase is formed |
| Dual function solvent | A cosolvent which also has a second function as a reactant. For instance to act as a regeneration reagent for cofactors |
| Biphasic solvent system | Solvent system that consists of an aqueous phase and a second, generally liquid, immiscible phase |
| Aqueous-neat | Biphasic system where the second phase is pure substrate. The substrate can be in any physical state |
| MARS | Acronym of micro aqueous reaction system. Monophasic non-aqueous solvent system. Some (non-bulk) water may be present to optimize enzyme activity, but only up to saturation |
| Neat conditions | Solvent system where the pure substrate also acts as a solvent. In other words, no other solvents to dilute the substrate are added. Some water (up to saturation) may be added to optimize enzyme activity |
In this manuscript, we show how biocatalysts from all classes, not only lipases, can be used in alternative solvent systems, albeit extra investments in protein or formulation engineering might be required. As a matter of fact, it might be a necessary step to make an industrial application viable. Furthermore, we want to show how the use of alternative solvents in biocatalytic reactions will in many cases actually increase the sustainability of a biocatalytic reaction. When a whole process is assessed in a holistic manner, it can be noted that even syntheses with excellent conversions and selectivity in aqueous media can lose their efficiency – and their inherent sustainability –, if the product cannot be isolated in an economic way. Especially when the use of large amounts of water for synthetic procedures is considered, which implies the formation of wastewater, which needs to be treated accordingly to claim the sustainability of a given synthesis.20 Also, the operation of biocatalytic reactions under non-aqueous conditions opens up the possibility of performing cascades with other, non-biological catalysts which might be sensitive to water, without the need for intermediate purification steps.12,21 Finally, due to current research on “green solvents” and the increasing availability in solvents which have a lower impact on the environment, more sustainable non-aqueous processes can be developed.
This paper explores the different options that synthetic chemists have for using enzymes, related to reaction media. Special emphasis is given on the application of biocatalytic reaction for the production of relevant products and optimization thereof. Based on the discussion, practical guidelines to choose a proper reaction medium are provided in the form of a flow chart to determine what systems have a high likelihood to work best. Furthermore, we will address the options one has in more sustainable solvents. Albeit some wet-lab work will surely still be needed to validate the proposed options, the presented guidelines are expected to reduce the experimental work needed to set-up and optimize biocatalytic reactions.
Throughout the review, examples are given in green boxes, where research groups continuously improved a biocatalytic process by using unconventional solvent systems. The aim of the optimization processes were to increase product concentration and, in some cases, catalyst or product stabilization. Both factors aim towards a more sustainable process, since higher product concentrations and a prolonged lifetime of the catalyst results in reduced waste formation and will thus decrease the environmental burden.
2. Solvent systems for biocatalysis: from purely aqueous to neat media
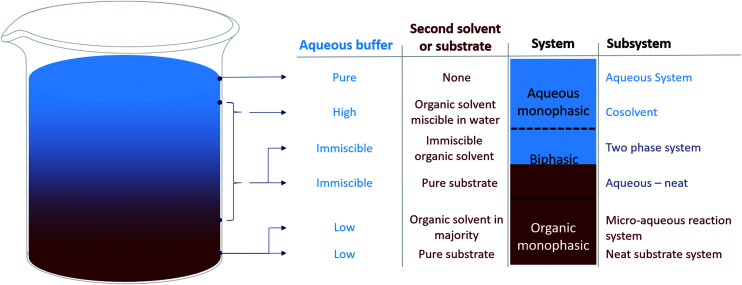
Depending on the solvent and water content, different solvent systems are formed. Herein, the three main solvent systems – monophasic aqueous, biphasic, and monophasic organic – are briefly discussed (Fig. 1) (Table 2). More information can be found in several reviews22–24 and the extensive information given in the ESI†.
Fig. 1. Scheme of different solvent systems sorted by relative water content.
Overview of the different solvent systems with their perks and challenges. MARS: micro aqueous reaction system; Neat: pure substrate system; DSP: downstream processing.
| Subsystem | Substrate | Advantages | Challenges |
|---|---|---|---|
| Aqueous system | Polar | • Straightforward application | • Restricted to polar reagents or low substrate concentrations of apolar substrates |
| • No interphase | |||
| • Most enzymes retain stability | |||
| Cosolvent | (Semi) apolar | • Straightforward in application | • Unpredictable effects on enzyme activity, selectivity and stability |
| • No interphase | • Possible DSP challenges | ||
| • Increased substrate solubility | |||
| Two (liquid) phase system | Apolar | • High substrate loading | • Interphase challenges |
| • Some control over concentrations in aqueous phase | • Enzyme instability possible | ||
| • Straightforward DSP | • Distribution issues | ||
| Aqueous-neat | Apolar | • High substrate loading | • Enzyme instability |
| • Possible advantages for DSP | • Interphase issues | ||
| • No second solvent | • Little control over substrate concentration in the aqueous phase | ||
| MARS | Apolar | • High substrate loading | • Enzyme instability |
| • No interphase | • Restriction in enzyme formulation | ||
| • Possible advantages for DSP | |||
| Neat substrate system | Liquid | • Highest substrate concentration possible | • Restrictions in use cases |
| • No interphase | • Full conversion is challenging | ||
| • No solvent | • No control over substrate concentration | ||
| • Straightforward DSP | • Enzyme instability |
2.1. Aqueous monophasic systems
Buffered solutions, with or without additives, are the most straightforward approach, and thus commonly used when a novel enzyme reaction is characterized. The lack of a second phase keeps complexity low and enables smooth application to continuous systems. However, environmental factors should be taken into consideration when using water as main and benign solvent, since the wastewater treatment and a cumbersome DSP may become major factors.
Application of enzymes in purely aqueous solutions is viable if reagents are soluble enough to reach on-spec industrial conditions. Relevant examples for enzymes in monophasic aqueous systems are the cases of most ions and sugars, or for some extremely high-valuable products for which no high product concentrations are required. If the substrate can be fed, the need for additives can also be overcome, provided that product accumulation does not hamper the reaction performance. For instance, (i) if the biocatalyst is not inhibited or deactivated by the product, (ii) if the thermodynamic driving forces are sufficient, or (iii) if the product can be easily removed in situ, e.g. via further conversion, precipitation,25,26 evaporation, extraction etc.27 As a downside of this approach, the use of large volumes of water requires economic and environmental downstream processing alternatives, as well as an adequate wastewater treatment unit.28
Most industrially relevant reagents, however, are poorly soluble in aqueous media. Here, hydrophilic water-miscible compounds can increase their solubility (cosolvents). Amongst others, these additives can be organic solvents, ionic liquids, deep eutectic solvents or surfactants.29 In cases where certain cosubstrates are required, for instance for the regeneration of cofactors in oxidoreductase-catalyzed reaction, the cosubstrate can also act as dual-function solvent which will also prove beneficial in pushing the thermodynamic equilibrium.30–34 As no second phase is formed, mass transfer limitations or protein deactivation at interphase surfaces are normally avoided (see ESI, chapter 4†). Furthermore, biocatalyst properties like stability, activity and even chemo-/stereoselectivity or substrate specificity can all be surprisingly altered with the introduction of these additives.35 It can therefore be beneficial to test them even in absence of any solubility issues.
Synthesis of lactones by a redox-neutral converging cascade of CHMO and ADH
This work, by the group of Kara, combined a reductive reaction on cyclohexanone by a Baeyer Villiger monooxygenase (BVMO), here the cyclohexanone monooxygenase (CHMO) with two oxidative reactions on a diol by the alcohol dehydrogenase from Thermoanaerobacter ethanolicus (TeSADH) in a redox-neutral process forming a lactone product. BVMO are known to be inhibited by high concentrations of organic compounds.36 In an initial study, where both enzymes were applied in an aqueous system as (partly purified) cell free extracts, up to 20 mM of the lactone was obtained.34 As the reaction was hampered by product instability (both via hydrolysis and polymerization) and reaction selectivity was an issue (cyclohexanone reduction by the ADH) a biphasic system was applied. Out of the solvents tested, the hydrophobic dodecane showed the best enzyme stability and was therefore chosen over solvents like 2-Me-THF and toluene. Though product concentrations went up to 53 mM, the required increase in protein concentration resulted in similar turnover numbers for the biocatalysts.34 In a similar study,37 the two enzymes (though other variants) were fused and used in a MARS system in lyophilized cells. The fusion aimed to shorten the required “cofactor travel distance”. The apolar MTBE and cyclopentyl methyl ether (CPME) gave the best results,38 though only 8 mM of product was obtained in this initial study.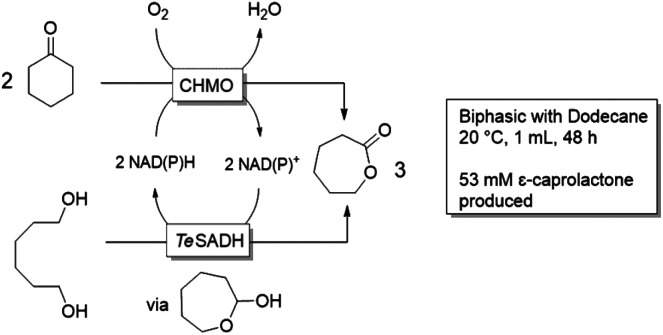
2.2. Biphasic (solvent) systems
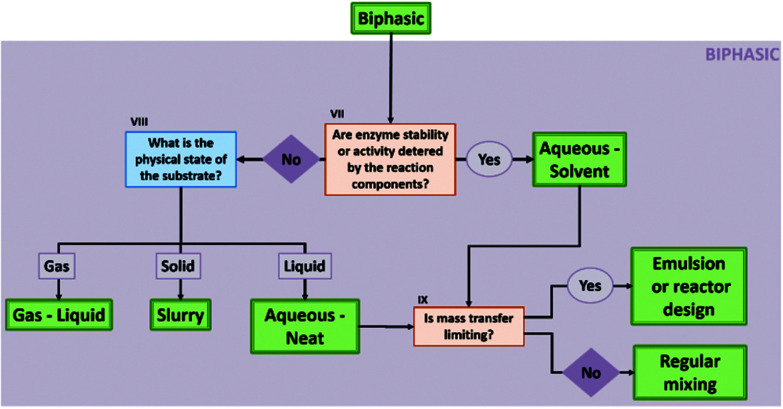
Here, we mainly regard two-liquid phase systems, though, in the case of aqueous-neat systems the second phase can also have a different aggregation state (see also Fig. 4 and chapter 1.2.2 in the ESI†). In biphasic solvent systems, a water-immiscible phase is added to the aqueous media. In some cases, if the reaction allows it (see ESI, chapter 1†), this can be purely the reactant itself, resulting in a so-called aqueous-neat system.39 However, if the biocatalyst performance is impaired in such systems – e.g. due to the high soluble substrate fraction in the aqueous phase – the substrate needs to be diluted in a second, water-immiscible phase. The biocatalyst generally resides in the aqueous reaction phase, while the second layer acts as a reservoir for the substrates and products. Assuming that the product is mainly in the non-aqueous phase, DSP is in most cases straightforward. In the case of aqueous-neat reactions, different systems are formed depending on the physical state of the substrate – gaseous, liquid, or solid – with each system bearing its own advantages and challenges. Overall biphasic systems combine high activity of the catalyst from aqueous systems with good solubility of apolar substrates and convenient DSP from organic systems. General information on biphasic systems and challenges concerning the interphase, cross-solubility and mass-transfer limitations are factors discussed in the ESI† and in the following reviews.22,40
Fig. 4. Decision flow chart on which biphasic solvent system is suitable for a biocatalytic reaction. VII: This can happen if the substrate or product concentrations will accumulate in the aqueous phase to a point they will act as an inhibitor or will negatively influence the protein structure. If this is the case, a second phase is required to act as reservoir for the compounds and to decrease aqueous concentrations of the compounds. VIII: Also in the case of aqueous-neat reaction conditions, some cosolvents can be added to increase the substrate concentration in the aqueous phase, if beneficial for the reaction. IX: If the required biocatalytic rates are significantly higher than the mass transfer rates, the reaction is not working optimally and effort should be put in increasing these transfer rates. A convenient way to test if transfer rates are limiting, is to increase the biocatalyst concentration and check if the reaction rate is increasing accordingly.
2.3. Monophasic non-aqueous systems (micro-aqueous reaction system (MARS))
The solvent system with the lowest water content presented here is the so-called micro-aqueous reaction system (MARS). Here, the non-aqueous solvent is the main solvent in the system in which the substrate is dissolved. The application of MARS typically requires the use of hydrophobic organic solvents, which at first sight seem to compromise the sustainability of the approach. However, MARS typically leads to less solvent waste formation, because of high substrate loadings, and a more straightforward DSP (less work up and possibly less wastewater). Additionally, if the solvent can be recycled even lower waste formation occurs. Thus, when considering the overall process, the application of MARS can actually lead to ecologically and economically more favourable processes. In some cases, even increased enzyme stability or changes in (enantio-)selectivity are observed.15,41
Only low amounts of (non-bulk) water, up to saturation levels, are added to retain enzyme activity, which results in a system with a single liquid phase. The amount of water (water activity) to gain optimal activity ultimately depends on the enzyme, the formulation of the catalyst, and the solvent characteristics. Lipases, for example, generally need far less water than oxidoreductases.42 This water can provide a hydration shell around the biocatalyst to ensure activity.43 When oxidoreductases are used in pure organic solvents, classical cofactor recycling systems with a second enzyme for the regeneration of the cofactor do not work anymore. Since apolar cofactors such as NAD(P)(H) cannot be transferred between two enzymes, they have to be regenerated using the same enzyme. Alternatively, the problem can be addressed by an appropriate formulation, for example, by producing and using the target enzyme and the cofactor-regenerating enzyme in the same (lyophilized) cell. In both cases, mostly sacrificial cosubstrates such as isopropanol or smart cosubstrates can be used.44 Since (lyophilized) whole cells are often used for MARS applications, the required expensive cofactors are already available. The approach of MARS greatly increases the possible substrate concentrations of apolar compounds and, in absence of an interphase, avoids mass issues with cross-solubility, transfer limitations and enzyme inactivation at interphases. Furthermore, the prevention of emulsion formation simplifies DSP.45 Finally, MARS are useful when water is unwanted in the reaction. For instance, in cases where the reaction compounds can be hydrolysed or reaction equilibria shifted,46,47 or in cascade reactions with water-sensitive chemical catalysts.12
Concluding, while MARS are commonly applied in hydrolase catalyzed reaction, recent examples also show possibilities to use other enzyme classes in this system. The overall environmental impact of MARS will be dependent on the solvent choice, but due to increased compound concentrations and simplified DSP, promising environmental-values can be reached.30,48–51 Its uses are still limited by the stability of the enzyme under these harsh conditions, though this can be improved by the engineering the biocatalyst structure and/or the formulation (see section 4).
Synthesis of aliphatic nitriles by aldoxime dehydratase
The enzyme aldoxime dehydratase from Bacillus sp. OxB-1 (OxdB) converts aliphatic aldoximes into nitriles, which in turn are useful as solvents or intermediates in the production of surfactants or pharmaceuticals.52 In a work by the groups of Asano and Gröger, reactions of the solid substrate to a liquid product, both poorly soluble in water, have been scaled up to molar concentrations. Initially, for the production of dinitriles by whole cells, DMSO was added as a cosolvent to improve solubility, but without apparent effect.53 Nevertheless, full conversion of 50 g L−1 substrate was achieved in an aqueous slurry reaction. The product formed a second liquid layer in the reaction. When the same reaction was performed on an aliphatic aldoxime, stepwise addition of n-octanaloxime resulted in full conversion of impressive 1.4 kg of substrate per litre of the aqueous medium.54 10% ethanol was used as colsolvent. Finally, this reaction was envisioned to take place in non-aqueous conditions.55 However, when whole cells or lyophilized cells were added directly to the organic medium, quick catalyst deactivation was observed. A bi-phasic system gave poor results, too. Only after entrapping the cells in a water-containing gel, full conversion of 500 mM n-octanaloxime in cyclohexanone was observed. With the immobilized cells as stationary phase, the reaction could be applied in a flow system.55 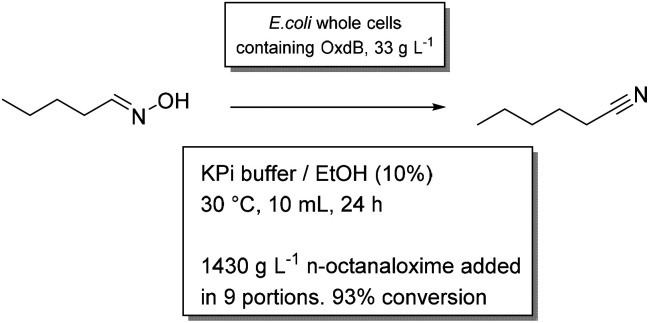
2.4. Neat substrate system
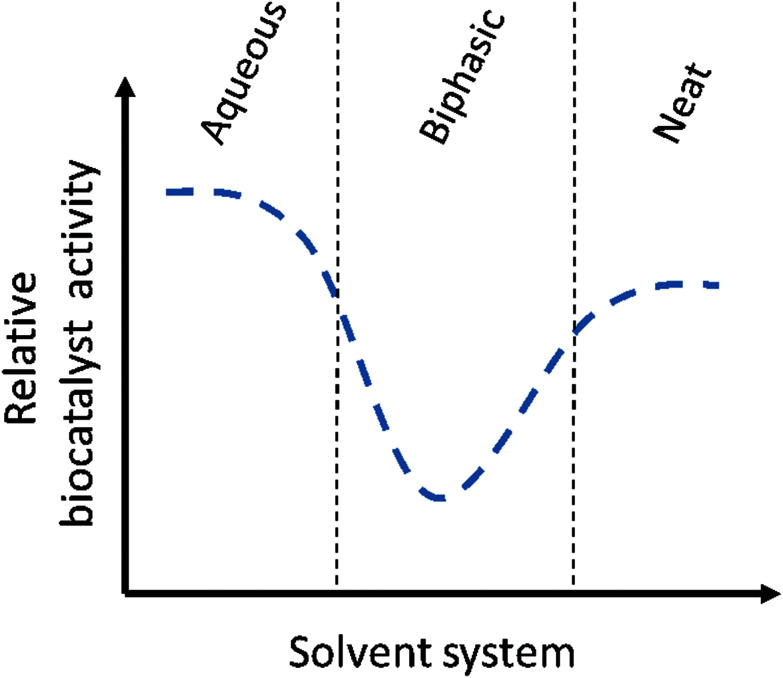
The ultimate variant of MARS is the neat substrate system. Here, no solvent is present at all, but the biocatalyst is dispersed in the substrate itself with, optionally, some dissolved water for activity. Neat substrate systems contain the maximal amount of substrate, and thus theoretically the highest product concentrations can be achieved, which can lead to impressive productivities56 However, so far, except for lipase catalyzed reactions, only few examples of enzymatic neat substrate systems have been published. This could be explained with some probability that the biocatalyst will show a decrease in stability, activity and selectivity under these conditions. In two recent examples, peroxygenases were applied in neat conditions, with the enzyme immobilized either on carriers or in alginate beads.57,58 A variant of this approach is when the combination of two substrates create deep eutectic systems, as Pätzold and co-workers have shown for the esterification of menthol with lauric acid with a conversion of up to 95%.59 Finally, in an industrial example by Enzymaster, an engineered transaminase60 in whole cells was able to catalyze the formation of (R)-phenyl ethylamine from a solution of pure acetophenone and isopropylamine.62 As elaborated in the case study below, the biocatalyst lost most of its activity when applied in a biphasic aqueous-solvent system, but showed good conversion in a neat substrate system with only small amounts of water (2% of an aqueous solution of PLP and wet cells). Though this is an outstanding example on how a solvent system can influence a biocatalytic reaction system, more research is required to determine for what other biocatalytic reactions this trend holds true. In either case, this example shows that, when a significant loss in biocatalyst activity is observed when shifting from an aqueous to a biphasic system, one should not be discouraged. In a further shift to a non-aqueous system, the biocatalyst could show good activity again (Fig. 2).62
Fig. 2. Graphical representation of the activity of the transaminase on acetophenone and isopropylamine in different solvent systems from the example by Enzymaster. While the biocatalyst showed good activity in an aqueous buffer with cosolvent, only low conversions were observed when the pure substrate was added as a second phase. When the biocatalysts was introduced in a neat system however, an enhanced activity was determined again.
Industrial example of the non-linear behaviour of a transaminase when increasing substrate loading
Chiral amines are important compounds and widely used in chemical industries. To obtain these products in high chiral purity, cumbersome resolution or recrystallization steps are required, which generates a large amount of waste, resulting in high cost and environmental impact.63 For these reasons, Enzymaster engineered a transaminase (TA) for the preparation of (R)-phenyl ethylamine from acetophenone and isopropylamine on an industrial scale. In an aqueous system, with 10% methanol as cosolvent, this catalyst was able to convert 80% of a 50 g L−1 solution of acetophenone with an enantiomeric excess of over 99% in 24 h.60 To increase the productivity of this reaction, an biphasic solvent system was suggested, using whole cells containing the engineered TA. However, in a reaction solution containing 400 g L−1 of acetophenone as a second phase, only 5% of the substrate was converted within 24 h, resulting in a product concentration of roughly 20 g L−1.61 Despite this decrease in product concentration, the reaction was taken one step further and the biocatalyst was applied in a neat solvent system (with 2% aqueous solution from PLP and wet cells) in collaboration with the Rother group. Surprisingly, the biocatalyst showed a significantly improved performance, reaching a product concentration of almost 100 g L−1 in 24 h. To further improve the system, a continuous reactor setup was designed where the biocatalyst and remaining acetophenone could be separated from the product and reused. This not only decreased the costs and waste production of the process, but also made it possible to achieve very high product concentrations of up to 168 g L−1 of (R)-phenyl ethylamine per day.62 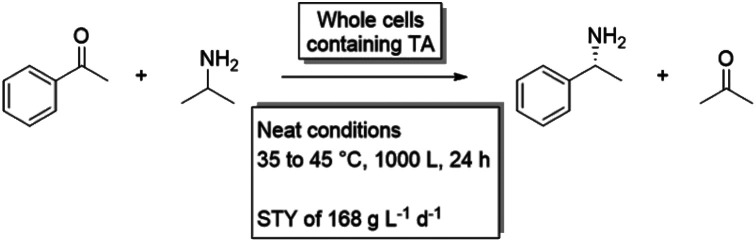
3. Decision flow chart: what solvent system is suitable for my reaction?
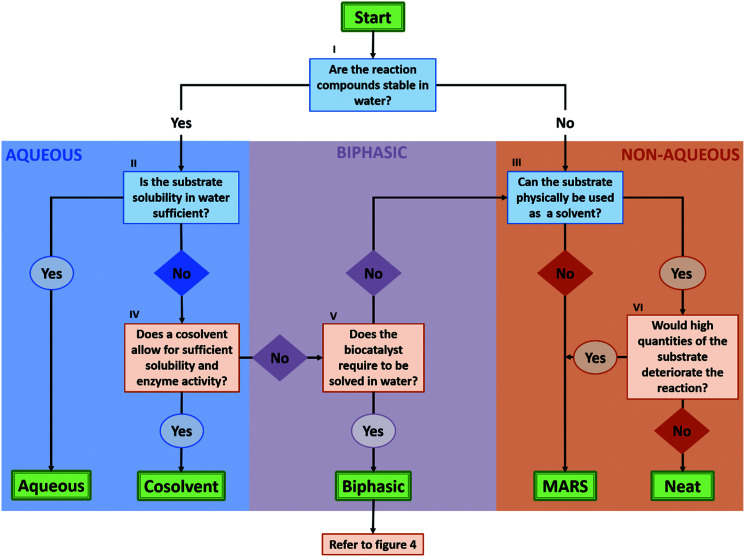
As described in the previous section, biocatalysis offers a broad range of options to perform organic synthesis, not only restricted to the classic aqueous media. To aid the decision process on what solvent system would be best for a certain reaction, a flow chart to consult has been built in this section (Fig. 3). The flow chart mainly takes into account the reaction engineering process, and puts forth the most promising approach depending on the substrate, the enzyme stability, and the DSP strategy.
Fig. 3. Decision flow chart on which solvent system is suitable for a biocatalytic reaction. I: Some compounds, like lactones and esters, can hydrolyse in presence of water and require to be synthesized under non-aqueous conditions. II: This depends on what product concentrations are required to make the system economically viable and whether a fed-batch approach is a suitable reaction mode. III: This requires the substrate to be a liquid under reaction conditions with an appropriate viscosity. IV: In other words, will the addition of the required amount of cosolvents enable sufficient solubility of the substrate, while not hampering the enzyme performance and/or downstream processing significantly? As the amount and choice in cosolvent can significantly influence enzyme behaviour, it is advised to test different conditions in solvent type and concentration. V: Most free enzymes require to be solved in water to remain sufficiently active. If this is not the case, excluding water as the solvent might improve the system in respect to productivity and convenience in DSP. If the enzyme in question is not stable or active in an alternative solvent, a change in formulation can still enable the application of non-aqueous solvent systems. VI: Here, one should take into account the influences of the substrate on the biocatalyst, the reaction and DSP. For the biocatalyst, neat conditions can hamper the reaction if the compounds can act as strong inhibitors, or if the integrity of the protein or the whole cell catalyst is deteriorated. For the reaction, as for any other system, the actual (in this case high) concentration of compounds should not induce any side reactions. Finally, from a DSP point of view, the reaction components should conveniently be separable at the end of the reaction. This, as under neat conditions, the substrate concentration cannot be conveniently adjusted by substrate loading. In the likely case that full conversion is not reached, one ends up with a mixture of substrates and products.
In the flow chart, questions in blue boxes are those who can, in most cases, be answered by consulting literature. Questions in orange boxes might require some experimental work. Specifications of the questions are noted in Roman numerals and can be found below the figure. Furthermore, the aspect of using a two-liquid phase system for in situ product removal has been left out of this scheme, as this might disregard other product removal strategies like evaporation, crystallization or cascade reactions. Finally, if the outcome shows that the application of a biphasic system might be most beneficial, one can refer to Fig. 4 for more specifications on the best solvent system.
4. Biocatalyst stability improvement
One of the main reasons why the addition of alternative solvents is considered unconventional in biocatalysis, is due to the belief that proteins are unstable in environments other than aqueous solutions. Though this is often true for many native free enzymes, there are ways to improve the resilience of the biocatalyst: alter the protein structure by protein engineering or the changing the catalyst formulation. Using these techniques, research groups applied enzymes from various different classes, not only in aqueous monophasic but also in biphasic or non-aqueous monophasic media (Table S1†).
Proteins can be modified to become more resilient towards different kinds of stress introduced in industrial processes, like increased temperatures, sheer stress, and solvents.63 These resistances often go hand in hand, a more thermostable protein often also shows a higher solvent tolerance. Several example to increase this resistance have been reported. The Janssen group used computational framework method “FRESCO” to increase the melting temperature of a dehalogenase, thereby also increasing its stability against commonly used cosolvents.64 The Damborsky group focused on modifying the access tunnel within the enzymés active site to increase its stability.65 The Gröger group developed a high throughput method to quickly assess protein stability in (co)solvents via determining melting temperatures in crude extracts.66 One can also use a semi-rational approach, as done with a largely evolved transaminase in the industrial production of sitagliptin.67 Aside from protein engineering, focus on the modification of the protein surface can also improve enzyme stability. Instead of improving an established enzyme, another approach is to search for more solvent tolerant enzymes performing similar reactions. As a matter of fact, it is advised to incorporate solvent stability in the screening of novel enzymes (e.g. through metagenomic assessment), as powerful synergies can be generated.
Decreasing cost and environmental impact using MARS
1-Phenylpropane-1,2-diol (PPD) is an optically active vicinal diol, which can serve as an interesting building block for asymmetric syntheses. In work by the group of Liese, all four stereoisomers of this product were obtained in a cascade of a lyase and an alcohol dehydrogenase.68 These reactions were performed in an aqueous media with substrate concentrations up to 26 mM. When the process was transferred to a micro-aqueous reaction system by the Rother group, with methyl-tert-butyl-ether as the main organic solvent, substrate concentrations up to 440 mM became possible, exceeding the solubility limit in water by ten-fold.49,69 Here, a benzaldehyde lyase by Pseudomonas fluorescens (PfBAL) and the alcohol dehydrogenase by Ralstonia sp. (RADH) were used. The costs of the system were further reduced by the application of lyophilized whole cells, which decreased catalyst expenses by approximately 90% (ref. 9) while the expensive cofactors ThDP and NADH were supplied with the cells. Overall, the cascade in MARS produced more than 1600 fold the product in comparison to the aqueous system.49 Later, Oeggl et al. used the same enzymes with similar substrates (4-methoxy-benzaldehyde and acetaldehyde) to yield 4-methoxyphenyl-1,2-propandiol.48 The cascade was further optimized using 1,4-butandiol as a smart cosubstrate70 or 4-methoxybenzyl alcohol to achieve a self-sufficient cascade with an integrated hydrogen-borrowing system. This eliminated the use of large amounts of isopropanol as a cosubstrate, which, in combination with the “green solvent” CPME, deemed the cascade much more environmentally friendly.48 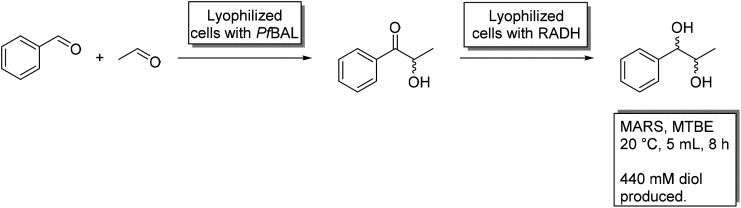
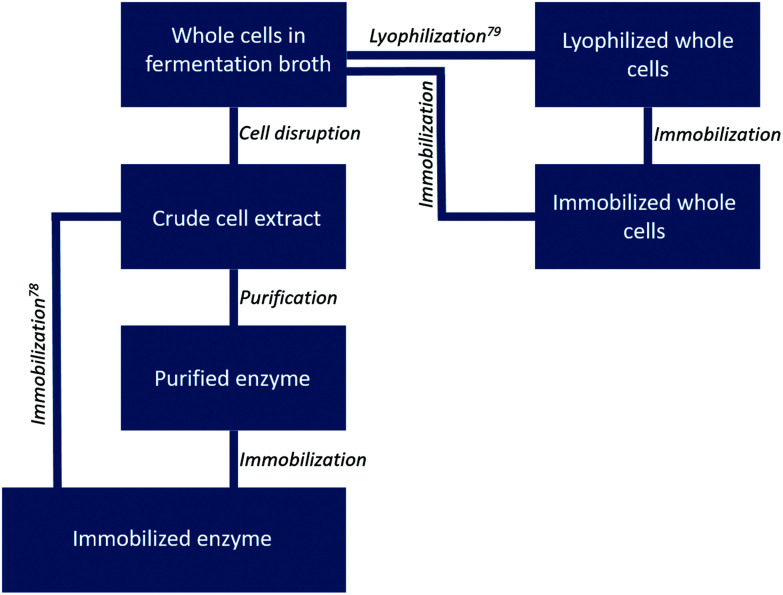
Changing the enzyme formulation is another powerful tool to influence biocatalyst activity, stability and re-usability. Here we differentiate between the following formulations: free enzymes as cell free extract, purified free enzymes, immobilized enzymes, whole cells in solution containing the biocatalyst and immobilized whole cells containing the biocatalyst (Fig. 5). Whether a certain formulation is beneficial for the reaction process depends on the type of enzyme, the target reaction and the solvent system, including all reaction compounds and conditions. Furthermore, the costs and effort required for the different formulations vary widely.9 A “best” formulation is therefore hard to point out. Not surprisingly, all combinations of enzyme formulation and solvent systems can be found in literature (Table S1†). The only restriction appears to be the combination of free enzymes in pure organic solvents. Outside the class of hydrolases, examples are scarce.30 As most enzymes have hydrophilic surfaces, they rarely retain their structure, let alone activity, in non-aqueous solution.71,72 If so, immobilization of the biocatalyst by encapsulation/entrapment, or their application in whole cells could retain the desired stability and activity. Nevertheless, we would like to encourage the reader to at least try to apply lyophilized free enzymes in a non-aqueous reaction solution. Though a positive outcome is not guaranteed, investments in such a “quick and easy” experiment are minimal, while a positive outcome would lead to a system which is significantly more convenient in application.
Fig. 5. Possible enzyme formulations and how they can be prepared from cultivated cells. Whole cells can be used directly as the catalyst, or be lyophilized or immobilized. Otherwise, cells can be disrupted to obtain cell free extract, which can be purified or (directly) immobilized.
Finally, an emerging trend in whole cell biocatalysis is to use viable, solvent-tolerant bacteria like certain Pseudomonas strains, and apply them in fermentations with exceedingly high (aromatic) substrate or solvent conditions.73–76 This becomes particularly interesting when Pseudomonas is used as a host for the production of aromatic compounds from second-generation lignocellulosic raw materials or for the upcycling of aromatic monomers from plastic waste streams (Table 3).77
Enzyme formulations, their advantages, challenges, and some examples in different solvent systems. GMO: genetically modified organism. More information, examples and literature is shown in the ESI†.
| Enzyme formulation | Advantages | Challenges | Selected examples in the different reaction systems |
|---|---|---|---|
| Crude cell extract | • High activity | • Likely unstable in organic solvents | Cosolvent67 |

|
• Relatively easy and cheap to produce | • Hard to retain in continuous systems | Biphasic80 |
| • Cofactors potentially already present in extract | • Hard to recycle for reuse | ||
| • Possible interfering native background reactions | |||
| Soluble purified enzyme | • No native background reactions | • Laborious and expensive to produce | Cosolvent67 |

|
• High activity | • Likely unstable in organic solvents | Biphasic33,81 |
| • No GMO license needed | • Hard to recycle for reuse | MARS30 | |
| • Hard to separate from reaction mixture | Neat58 | ||
| Immobilized enzyme | • No native background reactions | • Laborious and expensive to produce | Cosolvent82 |

|
• No GMO licence needed | • Additional material needed | Biphasic83 |
| • Easy to retain and reuse | • Not always biodegradable | MARS33,83,84 | |
| • Possible enhanced stability in organic solvents | • Unpredictable effect on enzyme activity and stability possible | Neat56,57,83 | |
| Whole cells | • Easy and cheap to produce | • Possible interfering native background reactions | Cosolvent85,86 |

|
• Possible enhanced stability in organic solvents | • Viable cells can be susceptible to reaction components | Biphasic55,87 |
| • Easy to separate from reaction mix | • Often GMO licence needed | MARS48,88 | |
| Neat45 | |||
| Immobilized whole cells | • Relatively cheap to produce | • Possible interfering native background reactions | Biphasic89 |
|
|
• Potentially stable in organic media | • Viable cells can be susceptible to reaction components | MARS55,90,91 |
| • Easy to retain in continuous systems | • Possibly laborious to produce | Neat76 | |
| • Easy to separate from reaction mixture | • Often GMO licence needed |
5. Solvent choices for non-conventional media
After a decision has been made on what solvent system would be optimal for a specific enzymatic conversion, the solvent itself has to be picked. At the moment, organic solvents are the most conventional to be added to, or to replace aqueous reaction media, though an increase in use of ionic liquids (IL) and especially deep eutectic solvents (DES) is also observed. Furthermore, supercritical fluid (SCF) form a niche solvent system seen in some applications (Table 4).
The discussed solvents, relative use cases in biocatalysis as reported in literature, general characteristics and common examples. Color codes: Brown: plenty of use cases reported in literature (>50). Orange: some use cases in reported in literature (5 to 50). Light orange: little to no use cases reported in literature (<5). Co: cosolvent. Bi: biphasic solvent systems. MARS: micro aqueous reaction systems. DMSO: dimethyl sulfoxide. CPME: cyclopentane methyl ether. MBTE: methyl-tert-butyl ether. [BMim]: 1-Butyl-3-methylimidazolium. [Bpy]: N-butylpyridinium. ChCl: choline chloride. [EACl]: ethyl ammonium chloride.
As organic solvents are most conventional, these will be the focus in this part. An overview of the uses of the other solvents can be found in the ESI† (chapter 3) and in reviews on ILs,92–95 DESs96–98 and SCFs.99–101
The question “what solvent is best?” has no singular answer, as many application parameters will influence this decision. Amongst others, the solvent choice will influence:
• Enzyme stability, activity and selectivity;
• Reagent solubility and, in case of multiple phases, partitioning of reagents;
• Downstream processing;
• Impact on safety and environment;
• Solvent price and availability.
The overall question will be divided in two parts: “What solvents are most beneficial for the performance of the reaction?” and “What solvents are most sustainable?”.
5.1. What solvent aspects are most beneficial for the performance of the reaction?
From a reaction standpoint, the interaction of the solvent with the biocatalyst is important. Enzyme stability, activity and selectivity can all shift drastically when in contact with polar solvents.35 Also, in case of biphasic solvent systems, the solvent properties determine the partition of the reaction compounds over the two phases and thus the concentration of the reaction components in the aqueous phase. Finally, the solvent's stability and inertness is paramount for the reaction. What type of solvent to choose for a certain reaction also depends on its role in the system, be it as cosolvent, pure solvent or second solvent. An elaboration on what consideration to make can be found in the ESI† (chapter 3).
From a holistic point of view, not only the production of a target compound should be optimal, but also its isolation afterwards should be kept in mind. Therefore, factors that influence DSP should be considered as well. A remarkable example is DMSO, a cosolvent often used in academic literature due to its frequently reported beneficial effect on reagent solubility and enzyme properties. The extraction of DMSO from a reaction mixture, however, is cumbersome. This can be considered to be beneficial in respect to retrieving the product.102 However, it also decreases the extraction efficiency of other compounds in the aqueous phase,103,104 and a more tedious wastewater treatment is required.105 Finally, the compound can also be hard to separate from the product. Even though DMSO is used in a number of pharmaceutical products,106 it does not have a GRAS (generally recognized as safe) status by the FDA107 and thus cannot be used in food products. When evaluating the holistic impact of a reaction, these factors should also be accounted for.
Retain enantioselectivity in the synthesis of cyanohydrins
Hydroxynitrile lyases (HNL) catalyze the stereoselective C–C bond formation between nucleophilic cyanide and carbonyl compounds, which can form the basis in the synthesis of several fine and bulk chemicals. One of the challenges of this reaction is the competing base-catalyzed chemical reaction, resulting in a racemic product.47,108 To retain high enantiomeric excess, this reaction needs to be repressed. Furthermore, the reactants of interest are often hydrophobic and are able to inhibit the enzyme. The reaction can be performed in an aqueous buffer, but repressing the background reaction and retaining enzyme stability requires careful balancing of the pH of the buffer.109 Ethanol could be added as a cosolvent, which had beneficial effects on both substrate solubility as reaction equilibrium, however also decreased enzyme stability.110 Application of a biphasic solvent system was beneficial,111,112 as it would limit the concentrations in the aqueous phase, further minimizing the background reaction and limit enzyme inhibition. Also an aqueous-neat reaction setup was tested, though the enzyme was found to quickly deactivate under these conditions.110 The best results were obtained in buffer saturated organic solvents, as, here, the chemical background reaction is bypassed. As the catalyst formulation, the enzyme is most conventionally immobilized on carriers.113 In a recent example, the HNL from Manihot esculenta (MeHNL) was immobilized on siliceous monolithic microreactor in a continuous setup. With buffer saturated MTBE as solvent, impressive space–time yields up to 1229 g L−1 h−1 were obtained.114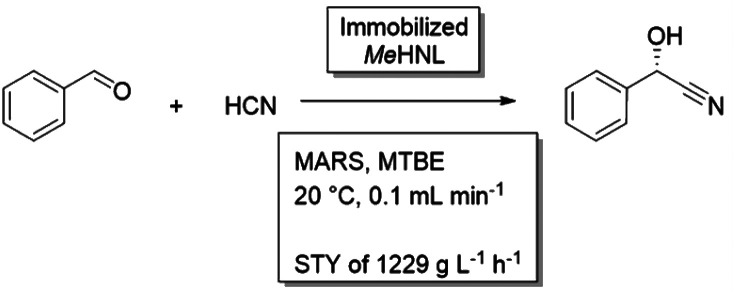
5.2. What solvents appear best for the environment?
Though organic solvents are generally classified as non-sustainable, there is a large variation in environmental impact between different solvents.115,116 If a “green solvent” can significantly reduce the total reaction volume required, implementation of these solvents can result in more environmentally friendly systems in comparison to systems that apply in aqueous media. A “green solvent” has a life cycle with a low environmental impact. This entails the following:
• It can be produced in an environmentally benign manner, ideally from renewable resources.
• It should easily be reused or recycled.
• It must have a minimal impact on the environment when released.
Solvent parameters beneficial for DSP can contradict those that benefit the environment. A volatile solvent, for instance, is conveniently distilled off during DSP, but can be hazardous for the environment and workers alike.117 Similarly, the application of apolar solvents can be beneficial for the biocatalytic process, but these same solvents are often considered problematic from an environmental point of view.118 For DSP, parameters as solvent viscosity, volatility, stability and azeotrope formation should be regarded. From a health and safety standpoint, properties on volatility, stability, flash point, explosiveness, toxicity and likelihood to form organic peroxides are important.
The properties of most conventional solvents are reported in several reviews,22,115 amongst others by pharmaceutical companies.119–121 A convenient overview of these surveys is found in the work by Prat et al.122 Another report worthwhile to consult is the one by the consortium of CHEM21,118 which gives an overview on commonly used solvents and scores them with respect to safety, health and environment and classifies them as “recommended”, “problematic” or “hazardous”. Convenient online tools to estimate the ecological effect of an organic solvent are the “Persistence, Bioaccumulation, and Toxicity Profiler”123 and the “Estimation Program Interface Suite”.124 Other factors for the life cycle assessment would be optimal but are up to now not always available.
The environmental impact of a solvent, in addition to its hazardousness to the environment, is greatly influenced by how easy the product can be obtained and the solvent can be recovered and reused.125 Water, for example, is generally considered one of the more “green” and abundant solvents. However, the process of product extraction might require large volumes of extraction material. Furthermore, an aqueous solvent cannot simply be released into the environment afterwards, as it will be contaminated. Thorough wastewater treatment is often required, which can be energy and material demanding.28,126 This is further amplified by the larger volumes of water needed to solve a similar amount of compound in cases of poor solubility. Conclusively, although water is indeed not toxic, when looking at a process from cradle to grave, it might not always be the most optimal choice in terms of sustainability.
Most organic solvents are still derived from petroleum-based sources, though increased effort is put in synthesizing these chemicals from biobased resources.127 Short-chain, mostly apolar solvents may be obtained via fermentation. These solvents may be further converted into longer, more hydrophobic, solvents. This might require dehydration and/or C–C formation steps, which may rely on noble metal catalysts or elevated temperatures and increase the burden on the environment. Other e.g. biocatalytical methods to create these compounds are currently under investigation. Some hydrophobic solvents can be obtained from dehydration of sugars128 or citrus waste.129,130 Despite the growing amount of more sustainable options, all combinations with proton donating or accepting abilities and polarity are not yet available.131 Especially if high log P solvents are required, one, for now, might need to resort to less “green” options. In the search of alterative solvents with similar properties, one can make use of solvent maps, like those designed by Murray et al.132 In the table below, a list of solvents is shown that should be considered as more benign choices (Table 5).
Examples of alternative solvents for biocatalysis which can be bio-derived. The safety rank of the solvent is as noted in the CHEM 21 report118 or, if not listed in the report, by a review of the Woodley group22.
| Solvent | log P | Safety rank | Starting material and process |
|---|---|---|---|
| Cyrene133 | −1.52 | Not classified | Pyrolysis and hydrolysis of cellulose |
| Ethanol127 | −0.18 | Recomm. | Fermentation on sugars |
| 2-Me-THF105,134 | 1.1 | Problematic | Based on the hydrogenation and dehydration of sugars |
| CPME135 | 1.6 | Problematic | Dehydration of pentoses, via cyclopentanol |
| 1-Octanola 127 | 3.5 | Recomm. | Various aldol condensation, hydrogenation and dehydration steps on sugars based intermediates |
| p-Cymene130 | 4.1 | Problematic | Extraction from citrus waste. Subsequent isomerization and dehydrogenation |
| Limonene130 | 4.4 | Problematic | Extraction from citrus waste |
1-Octanol is used here as an example of a long chain, apolar solvent which could be synthesized from biorenewables, but at the expense of relatively long synthesis routes. More examples are found in the following review:127
We encourage any group working on biocatalyst applications to always keep an eye on emerging biobased solvents and adapt them in the screening process for applicable solvents. If a biocatalytic process is developed in academia utilizing a more sustainable solvent, the incentive for industry to also use this solvent might be increased. Part of the responsibility therefore also lies at the early researchers. Finally, from an industrial point of view, the cost and availability of a solvent are vital, as are the regulations around individual solvents. For these reasons, industries may prefer to work with more established solvents. If the scientific community could introduce these less established solvents in synthetic reactions, it would provide additional motivation to use them on an industrial scale as well.
Reducing the waste formation in a neat substrate system
The research groups of Liese and Gröger developed a chemo-enzymatic cascade for the production of the pharmaceutical building block (S)-3-(benzylamino)-butanoate, consisting of a chemical aza-Michael addition with a subsequent enzymatic resolution using a Candida antarctica lipase B (CaLB). Both, the chemical and enzymatic step were performed under neat conditions.136 Later, to decrease environmental impact, the isolation of the intermediates was simplified and chromatographic workup was avoided, which decreased the Environmental factor (E-factor) from 359 to 41.137 In order to further optimise the process, reaction kinetics were simulated138 to design an optimal scale-up with an improved reaction rate and increased stability of the catalyst. This resulted in a combination of a plug-flow reactor in which the aza-Michael addition takes place with a subsequent packed bed reactor, were the immobilized CaLB performs the enzymatic resolution via aminolysis. This continuous flow system with neat substrate conditions could run for up to four days without productivity loss and final impressive space–time-yields of 128 g L−1 h−1.139 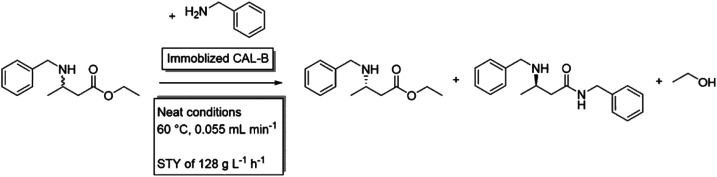
6. Final remarks and outlook
Enzyme reactions should not, by definition, be purely aqueous reactions. This statement goes beyond just lipase catalyzed reactions. In fact, a change in solvent system is in many cases a necessity to reach the productivities required for a process to be viable for industrial application and to close the gap between academia and industry. This especially holds true when not only the transformation, but also the sustainability of the overall process, including product separation, is taken into account. The utilization and recycling of “green” solvents based on renewable resources further decreases the environmental impact of biocatalytic syntheses.
Changing the solvent system, however, will influence nearly every parameter of the reaction: from compound solubility, stability and partitioning to enzyme activity, stability and selectivity. For now, what solvent and solvent system are best for a target reaction is hard to predict beforehand. Extra wet lab work will thus in most cases be required. New modelling and simulation tools currently under development may also facilitate solvent selection in the near future.140
Also non-scientific aspects come into play. When the reaction target is a high-value compound, increased product concentrations and decreased catalyst costs may have a relative small impact on the economic viability of a reaction.9 A general relevant question, regardless of what the flowchart will suggest, is therefore: “Even though a switch to this system might improve my reaction, will the extra effort be worth it?” Nevertheless, this review shows that there is more than water for enzyme applications and when applied in a suitable way, it bears a huge potential.
We therefore encourage those working in the field of biocatalysis to take a leap of faith and to introduce the work with solvents in their lab routine. Especially a switch from free enzymes to immobilized biocatalysts or lyophilized whole cells will likely show benefits. Furthermore, solvent resistance should be included in the enzyme screening, (rational) protein engineering, optimization and selection work, to reduce the effort required to enable application of biocatalysts under these conditions. We see that especially high throughput screening can play a pivotal role here. Also, even when a loss in biocatalyst activity is observed upon addition of a cosolvent or a second phase, it could still be worthwhile to test the application in a non-aqueous solvent system, as the biocatalyst might regain some if its activity under these conditions (Fig. 2).60
Though the main focus of this review was on enzyme applications, we also believe that a combination with chemical catalysts will become highly relevant for multi-step syntheses.12 First examples have already been published in which enzymatic and/or chemical synthesis steps are combined with whole living microorganisms.12,141 It is very likely and desirable that the number of examples will increase. This bears some significant potential, as these microbial cell factories can, for example, supply educts from renewable raw materials and become particularly interesting when green and/or CO2-neutral processes are considered. Especially nowadays, with the application of solvent-resistant microorganisms. Though examples are still scarce, it is clear, that the combination of catalysts across disciplinary boundaries in a suitable environment will be an effective instrument for environmentally friendly and economically feasible processes.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
MvS and DR acknowledge funding from the European Research Council (ERC) as part of the European Union's Horizon 2020 research and innovation programme (grant agreement no. 757320) within the scope of the LightCas (light-controlled synthetic enzyme cascades) project. JS and DR acknowledge funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – Cluster of Excellence 2186 “The Fuel Science Center” – ID: 390919832.
Biographies
Biography
Morten M. C. H. van Schie.

Morten van Schie received his Master of Science degree in biotechnology from the Technical University of Delft, The Netherlands. Here, he also obtained his PhD, focussing on alternative regeneration methods for oxidoreductases (2019). Now, he is a Postdoctoral fellow at the Forschungszentrum in Jülich where he works on the photo-regulation of enzymes in synthetic biocatalytic cascade reactions.
Biography
Jan-Dirk Spöring.

Jan-Dirk Spöring is a PhD student at the Forschungszentrum in Jülich and the RWTH Aachen University in Germany in the field of synthetic enzyme cascades. His research interest consists of the enzymatic production of aliphatic diols in organic media for the use as fine chemicals or potential synthetic fuels. Since he combines enzymatically and chemically catalyzed steps for the synthesis of chiral acetals, he evaluates MARS as an interesting alternative for hybrid reactions.
Biography
Marco Bocola.

Dr Marco Bocola obtained a diploma degree in Chemistry 1997 and finished his doctoral studies on structural biology and enzyme design with Prof. B. Hauer and Prof. G. Klebe from Philipps University of Marburg in collaboration with BASF in 2002. He moved then to MPI für Kohlenforschung under joined supervision of Prof. W. Thiel and M. T. Reetz focusing on computational enzyme evolution. Afterwards he was group leader for computational biotechnology at University of Regensburg and RWTH Aachen. Since 2017 he is CEO of Protedes consulting and 2018 director of Bioinformatics and Data Science at Enzymaster. He published more than 100 publications and is (co)inventor of several patents on improved biocatalysts.
Biography
Pablo Domínguez de María.

Pablo Domínguez de María holds BSc's in Pharmacy and Chemistry, and a Ph.D. in Biocatalysis (2002). He worked in industry for 6.5 years (Evonik AG from 2003–2004 and AkzoNobel BV from 2005–2009), being involved in projects related to sustainable chemistry, organocatalysis, biorefineries and white biotechnology. In 2009 he joined the RWTH Aachen University (ITMC) as group leader, defending his Habilitation in 2015. Since 2014 he is the CEO of Sustainable Momentum, SL, a consultancy firm providing technical support in areas related to biorefineries and sustainable chemistry. He has delivered several technical books, is (co)inventor of several patents, and has published more than 120 scientific publications, collecting >5800 citations (Google scholar).
Biography
Dörte Rother.

Dörte Rother is head of the group ‘biocatalysis’ at the IBG-1: Biotechnology, Forschungszentrum Jülich GmbH and full professor for ‘synthetic enzyme cascades’ at RWTH Aachen University. She received the DECHEMA-Prize 2018 and the Biotrans Junior Award 2019 for her research activities in multi-step biocatalysis and sustainable process development. In her group bio(techno)logists, chemists and engineers work on solutions to intensify biocatalytic processes and integrate downstream processing into enzyme cascades. They see the use of unconventional media as one very potent tool to increase economic and ecologic efficiency of one-step and multi-step enzymatic syntheses.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc00561h
References
- Domínguez de María P. de Gonzalo G. Alcántara A. R. Catalysts. 2019;9:802. doi: 10.3390/catal9100802. [DOI] [Google Scholar]
- Wu S. Snajdrova R. Moore J. C. Baldenius K. Bornscheuer U. Angew. Chem. 2020;60:88–119. doi: 10.1002/anie.202006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo G. and Domínguez de María P., Biocatalysis: An industrial perspective, Royal Society of Chemistry, 2017 [Google Scholar]
- Bornscheuer U. T. Philos. Trans. R. Soc., A. 2018;376:20170063. doi: 10.1098/rsta.2017.0063. [DOI] [PubMed] [Google Scholar]
- Bornscheuer U. T. Huisman G. W. Kazlauskas R. J. Lutz S. Moore J. C. Robins K. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- Reetz M. T. Acc. Chem. Res. 2019;52:336–344. doi: 10.1021/acs.accounts.8b00582. [DOI] [PubMed] [Google Scholar]
- Arnold F. H. Angew. Chem., Int. Ed. 2019;58:14420–14426. doi: 10.1002/anie.201907729. [DOI] [PubMed] [Google Scholar]
- Chen K. Arnold F. H. Nat. Catal. 2020;3:203–213. doi: 10.1038/s41929-019-0385-5. [DOI] [Google Scholar]
- Tufvesson P. R. Lima-Ramos J. Nordblad M. Woodley J. M. Org. Process Res. Dev. 2011;15:266–274. doi: 10.1021/op1002165. [DOI] [Google Scholar]
- Dias Gomes M. Woodley J. M. Molecules. 2019;24:3573. doi: 10.3390/molecules24193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romney D. K. Arnold F. H. Lipshutz B. H. Li C.-J. J. Org. Chem. 2018;83:7319–7322. doi: 10.1021/acs.joc.8b01412. [DOI] [PubMed] [Google Scholar]
- Kourist R. González-Sabín J. ChemCatChem. 2020;12:1903–1912. doi: 10.1002/cctc.201902192. [DOI] [Google Scholar]
- Ogino H. Ishikawa H. J. Biosci. Bioeng. 2001;91:109–116. doi: 10.1016/S1389-1723(01)80051-7. [DOI] [PubMed] [Google Scholar]
- Kumar A. Dhar K. Kanwar S. S. Arora P. K. Biol. Proced. Online. 2016;18:2. doi: 10.1186/s12575-016-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. M. Nature. 2001;409:241–246. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- Halling P. Kvittingen L. Trends Biotechnol. 1999;17:343–344. doi: 10.1016/S0167-7799(99)01331-1. [DOI] [PubMed] [Google Scholar]
- Sym E. Biochem. Z. 1933;258:304–324. [Google Scholar]
- Zaks A. Klibanov A. M. Science. 1984;224:1249–1251. doi: 10.1126/science.6729453. [DOI] [PubMed] [Google Scholar]
- Zaks A. Klibanov A. M. J. Biol. Chem. 1988;263:3194–3201. doi: 10.1016/S0021-9258(18)69054-4. [DOI] [PubMed] [Google Scholar]
- Domínguez de María P. Hollmann F. Front. Microbiol. 2015;6:1257. doi: 10.3389/fmicb.2015.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff F. Mihovilovic M. D. Gröger H. Snajdrova R. Iding H. Bornscheuer U. T. Nat. Catal. 2018;1:12–22. doi: 10.1038/s41929-017-0010-4. [DOI] [Google Scholar]
- Grundtvig I. P. R. Heintz S. Krühne U. Gernaey K. V. Adlercreutz P. Hayler J. D. Wells A. S. Woodley J. M. Biotechnol. Adv. 2018;36:1801–1814. doi: 10.1016/j.biotechadv.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Wachtmeister J. Rother D. Curr. Opin. Biotechnol. 2016;42:169–177. doi: 10.1016/j.copbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Guajardo N. Domínguez de María P. ChemCatChem. 2019;11:3128–3137. doi: 10.1002/cctc.201900773. [DOI] [Google Scholar]
- Schmidt S. de Almeida T. P. Rother D. Hollmann F. Green Chem. 2017;19:1226–1229. doi: 10.1039/C7GC00020K. [DOI] [Google Scholar]
- Hülsewede D. Tänzler M. Süss P. Mildner A. Menyes U. von Langermann J. Eur. J. Org. Chem. 2018:2130–2133. doi: 10.1002/ejoc.201800323. [DOI] [Google Scholar]
- Fellechner O. Blatkiewicz M. Smirnova I. Chem. Ing. Tech. 2019;91:1522–1543. doi: 10.1002/cite.201900027. [DOI] [Google Scholar]
- Blackmond D. G. Armstrong A. Coombe V. Wells A. Angew. Chem., Int. Ed. 2007;46:3798–3800. doi: 10.1002/anie.200604952. [DOI] [PubMed] [Google Scholar]
- Roura Padrosa D. De Vitis V. Contente M. L. Molinari F. Paradisi F. Catalysts. 2019;9:232. doi: 10.3390/catal9030232. [DOI] [Google Scholar]
- de Gonzalo G. Lavandera I. Faber K. Kroutil W. Org. Lett. 2007;9:2163–2166. doi: 10.1021/ol070679c. [DOI] [PubMed] [Google Scholar]
- Toda H. Imae R. Itoh N. Adv. Synth. Catal. 2014;356:3443–3450. doi: 10.1002/adsc.201400383. [DOI] [Google Scholar]
- Liang J. Lalonde J. Borup B. Mitchell V. Mundorff E. Trinh N. Kochrekar D. Nair Cherat R. Pai G. G. Org. Process Res. Dev. 2010;14:193–198. doi: 10.1021/op900272d. [DOI] [Google Scholar]
- Heidlindemann M. Rulli G. Berkessel A. Hummel W. Gröger H. ACS Catal. 2014;4:1099–1103. doi: 10.1021/cs4010387. [DOI] [Google Scholar]
- Bornadel A. Hatti-Kaul R. Hollmann F. Kara S. ChemCatChem. 2015;7:2442–2445. doi: 10.1002/cctc.201500511. [DOI] [Google Scholar]
- Gerhards T. Mackfeld U. Bocola M. von Lieres E. Wiechert W. Pohl M. Rother D. Adv. Synth. Catal. 2012;354:2805–2820. doi: 10.1002/adsc.201200284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt S. Bornscheuer U. T. Menyes U. Hummel W. Gröger H. Enzyme Microb. Technol. 2013;53:288–292. doi: 10.1016/j.enzmictec.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Huang L. Romero E. Ressmann A. K. Rudroff F. Hollmann F. Fraaije M. W. Kara S. Adv. Synth. Catal. 2017;359:2142–2148. doi: 10.1002/adsc.201700401. [DOI] [Google Scholar]
- Huang L. Aalbers F. S. Tang W. Röllig R. Fraaije M. W. Kara S. ChemBioChem. 2019;20:1653–1658. doi: 10.1002/cbic.201800814. [DOI] [PubMed] [Google Scholar]
- Kühl S. Zehentgruber D. Pohl M. Müller M. Lütz S. Chem. Eng. Sci. 2007;62:5201–5205. doi: 10.1016/j.ces.2007.02.029. [DOI] [Google Scholar]
- Huang L. Domínguez de María P. Kara S. Chim. Oggi - Chem. Today. 2018;36:48–56. [Google Scholar]
- Mutti F. G. Kroutil W. Adv. Synth. Catal. 2012;354:3409–3413. doi: 10.1002/adsc.201200900. [DOI] [Google Scholar]
- Valivety R. H. Halling P. J. Macrae A. R. FEBS Lett. 1992;301:258–260. doi: 10.1016/0014-5793(92)80252-C. [DOI] [PubMed] [Google Scholar]
- Halling P. J. Philos. Trans. R. Soc., B. 2004;359:1287–1297. doi: 10.1098/rstb.2004.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara S. Spickermann D. Weckbecker A. Leggewie C. Arends I. W. Hollmann F. ChemCatChem. 2014;6:973–976. doi: 10.1002/cctc.201300841. [DOI] [Google Scholar]
- Jakoblinnert A. Mladenov R. Paul A. Sibilla F. Schwaneberg U. Ansorge-Schumacher M. B. Domínguez de María P. Chem. Commun. 2011;47:12230–12232. doi: 10.1039/C1CC14097C. [DOI] [PubMed] [Google Scholar]
- Maugeri Z. Rother D. Adv. Synth. Catal. 2016;358:2745–2750. doi: 10.1002/adsc.201501154. [DOI] [Google Scholar]
- Bracco P. Busch H. von Langermann J. Hanefeld U. Org. Biomol. Chem. 2016;14:6375–6389. doi: 10.1039/C6OB00934D. [DOI] [PubMed] [Google Scholar]
- Oeggl R. Maßmann T. Jupke A. Rother D. ACS Sustainable Chem. Eng. 2018;6:11819–11826. doi: 10.1021/acssuschemeng.8b02107. [DOI] [Google Scholar]
- Jakoblinnert A. Rother D. Green Chem. 2014;16:3472–3482. doi: 10.1039/C4GC00010B. [DOI] [Google Scholar]
- Scholz K. E. Okrob D. Kopka B. Grünberger A. Pohl M. Jaeger K.-E. Krauss U. Appl. Environ. Microbiol. 2012;78:5025–5027. doi: 10.1128/AEM.00582-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay D. Winkler C. K. Tasnádi G. Faber K. Biotechnol. Lett. 2014;36:1329–1333. doi: 10.1007/s10529-014-1494-5. [DOI] [PubMed] [Google Scholar]
- Betke T. Higuchi J. Rommelmann P. Oike K. Nomura T. Kato Y. Asano Y. Gröger H. ChemBioChem. 2018;19:768–779. doi: 10.1002/cbic.201700571. [DOI] [PubMed] [Google Scholar]
- Betke T. Maier M. Gruber-Wölfler H. Gröger H. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzmann A. Glinski S. Worm M. Gröger H. J. Org. Chem. 2019;84:4867–4872. doi: 10.1021/acs.joc.9b00184. [DOI] [PubMed] [Google Scholar]
- Hinzmann A. Adebar N. Betke T. Leppin M. Gröger H. Eur. J. Org. Chem. 2019:6911–6916. doi: 10.1002/ejoc.201901168. [DOI] [Google Scholar]
- Erdmann V. Mackfeld U. Rother D. Jakoblinnert A. J. Biotechnol. 2014;191:106–112. doi: 10.1016/j.jbiotec.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Rauch M. C. Tieves F. Paul C. E. Arends I. W. Alcalde M. Hollmann F. ChemCatChem. 2019;11:4519–4523. doi: 10.1002/cctc.201901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobisch M. van Schie M. M. C. H. Kim J. Andersen K. R. Alcalde M. Kourist R. Park C. B. Hollmann F. Kara S. ChemCatChem. 2020;12:4009–4013. doi: 10.1002/cctc.202000512. [DOI] [Google Scholar]
- Pätzold M. Weimer A. Liese A. Holtmann D. Biotechnol. Rep. 2019;22:e00333. doi: 10.1016/j.btre.2019.e00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Bong Y. K., Wang J., Baoqin C., Shang C. and Bocola M., Engineered transaminase polypeptides and uses thereof, CN111349666A, 2020
- Zhuhong W., Kun W. Z., Huang Y., Wang J., Baoqin C., Ying Z., Jianmin L., Zhipeng M., Baohua Z., Huaihao Z., Bocora M., Yuming L., Baoguo S. and Zhenlin L., Production method and equipment of phenylethylamine, US20200332321A1, 2020
- Sehl T. Maugeri Z. Rother D. J. Mol. Catal. B: Enzym. 2015;114:65–71. doi: 10.1016/j.molcatb.2014.12.005. [DOI] [Google Scholar]
- Lehmann C. Sibilla F. Maugeri Z. Streit W. R. Domínguez de María P. Martinez R. Schwaneberg U. Green Chem. 2012;14:2719–2726. doi: 10.1039/C2GC35790A. [DOI] [Google Scholar]
- Arabnejad H. Dal Lago M. Jekel P. A. Floor R. J. Thunnissen A.-M. W. Terwisscha van Scheltinga A. C. Wijma H. J. Janssen D. B. Protein Eng., Des. Sel. 2017;30:175–189. doi: 10.1093/protein/gzw068. [DOI] [PubMed] [Google Scholar]
- Stepankova V. Damborsky J. Chaloupkova R. Biotechnol. J. 2013;8:719–729. doi: 10.1002/biot.201200378. [DOI] [PubMed] [Google Scholar]
- Wedde S. Kleusch C. Bakonyi D. Gröger H. ChemBioChem. 2017;18:2399–2403. doi: 10.1002/cbic.201700526. [DOI] [PubMed] [Google Scholar]
- Savile C. K. Janey J. M. Mundorff E. C. Moore J. C. Tam S. Jarvis W. R. Colbeck J. C. Krebber A. Fleitz F. J. Brands J. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- Kihumbu D. Stillger T. Hummel W. Liese A. Tetrahedron: Asymmetry. 2002;13:1069–1072. doi: 10.1016/S0957-4166(02)00247-1. [DOI] [Google Scholar]
- Wachtmeister J. Jakoblinnert A. Rother D. Org. Process Res. Dev. 2016;20:1744–1753. doi: 10.1021/acs.oprd.6b00232. [DOI] [Google Scholar]
- Kara S. Spickermann D. Schrittwieser J. H. Leggewie C. van Berkel W. J. Arends I. W. Hollmann F. Green Chem. 2013;15:330–335. doi: 10.1039/C2GC36797A. [DOI] [Google Scholar]
- Grunwald J. Wirz B. Scollar M. P. Klibanov A. M. J. Am. Chem. Soc. 1986;108:6732–6734. doi: 10.1021/ja00281a044. [DOI] [Google Scholar]
- Truppo M. D. Strotman H. Hughes G. ChemCatChem. 2012;4:1071–1074. doi: 10.1002/cctc.201200228. [DOI] [Google Scholar]
- Heipieper H. J. Neumann G. Cornelissen S. Meinhardt F. Appl. Microbiol. Biotechnol. 2007;74:961–973. doi: 10.1007/s00253-006-0833-4. [DOI] [PubMed] [Google Scholar]
- Volmer J. Lindmeyer M. Seipp J. Schmid A. Bühler B. Biotechnol. Bioeng. 2019;116:1089–1101. doi: 10.1002/bit.26924. [DOI] [PubMed] [Google Scholar]
- Schäfer L. Karande R. Bühler B. Front. Bioeng. Biotechnol. 2020;8:140. doi: 10.3389/fbioe.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halan B. Karande R. Bühler K. Schmid A. J. Flow Chem. 2016;6:39–42. doi: 10.1556/1846.2015.00037. [DOI] [Google Scholar]
- Schwanemann T. Otto M. Wierckx N. Wynands B. Biotechnol. J. 2020;15:1900569. doi: 10.1002/biot.201900569. [DOI] [PubMed] [Google Scholar]
- Döbber J. Pohl M. Ley S. V. Musio B. React. Chem. Eng. 2018;3:8–12. doi: 10.1039/C7RE00173H. [DOI] [Google Scholar]
- Wachtmeister J. Mennicken P. Hunold A. Rother D. ChemCatChem. 2016;8:607–614. doi: 10.1002/cctc.201501099. [DOI] [Google Scholar]
- Wedde S. Rommelmann P. Scherkus C. Schmidt S. Bornscheuer U. T. Liese A. Gröger H. Green Chem. 2017;19:1286–1290. doi: 10.1039/C6GC02529C. [DOI] [Google Scholar]
- Pedroso de Almeida T. van Schie M. M. C. H. Ma A. Tieves F. Younes S. H. H. Fernández-Fueyo E. Arends I. W. C. E. Riul Jr. A. Hollmann F. Adv. Synth. Catal. 2019;361:2668–2672. doi: 10.1002/adsc.201801312. [DOI] [Google Scholar]
- Carucci C. Bruen L. Gascón V. Paradisi F. Magner E. Langmuir. 2018;34:8274–8280. doi: 10.1021/acs.langmuir.8b01037. [DOI] [PubMed] [Google Scholar]
- Lopresto C. G. Calabro V. Woodley J. M. Tufvesson P. J. Mol. Catal. B: Enzym. 2014;110:64–71. doi: 10.1016/j.molcatb.2014.09.011. [DOI] [Google Scholar]
- Secundo F. Carrea G. Soregaroli C. Varinelli D. Morrone R. Biotechnol. Bioeng. 2001;73:157–163. doi: 10.1002/bit.1047. [DOI] [PubMed] [Google Scholar]
- Sánchez-Castañeda A. K. Moussa M. Ngansop L. Trelea I. C. Athès V. J. Chem. Technol. Biotechnol. 2020;95:1046–1056. [Google Scholar]
- Detry J. Rosenbaum T. Lütz S. Hahn D. Jaeger K.-E. Müller M. Eggert T. Appl. Microbiol. Biotechnol. 2006;72:1107–1116. doi: 10.1007/s00253-006-0391-9. [DOI] [PubMed] [Google Scholar]
- Li L. Long L. Ding S. Front. Microbiol. 2019;10:1798. doi: 10.3389/fmicb.2019.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popłoński J. Reiter T. Kroutil W. ChemCatChem. 2018;10:763–768. doi: 10.1002/cctc.201701395. [DOI] [Google Scholar]
- Xin J.-Y. Cui J.-R. Chen J.-B. Li S.-B. Xia C.-G. Zhu L.-M. Process Biochem. 2003;38:1739–1746. doi: 10.1016/S0032-9592(02)00262-5. [DOI] [Google Scholar]
- Wachtmeister J. Jakoblinnert A. Kulig J. Offermann H. Rother D. ChemCatChem. 2014;6:1051–1058. doi: 10.1002/cctc.201300880. [DOI] [Google Scholar]
- Hoschek A. Heuschkel I. Schmid A. Bühler B. Karande R. Bühler K. Bioresour. Technol. 2019;282:171–178. doi: 10.1016/j.biortech.2019.02.093. [DOI] [PubMed] [Google Scholar]
- Meyer L.-E. von Langermann J. Kragl U. Biophys. Rev. 2018;10:901–910. doi: 10.1007/s12551-018-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez de María P. Maugeri Z. Curr. Opin. Chem. Biol. 2011;15:220–225. doi: 10.1016/j.cbpa.2010.11.008. [DOI] [PubMed] [Google Scholar]
- van Rantwijk F. Sheldon R. A. Chem. Rev. 2007;107:2757–2785. doi: 10.1021/cr050946x. [DOI] [PubMed] [Google Scholar]
- Naushad M. Alothman Z. A. Khan A. B. Ali M. Int. J. Biol. Macromol. 2012;51:555–560. doi: 10.1016/j.ijbiomac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Guajardo N. Müller C. R. Schrebler R. Carlesi C. Domínguez de María P. ChemCatChem. 2016;8:1020–1027. doi: 10.1002/cctc.201501133. [DOI] [Google Scholar]
- Domínguez de María P., Guajardo N. and Kara S., Deep Eutectic Solvents: Synthesis, Properties, and Applications, 2019, 257–271 [Google Scholar]
- Gotor-Fernández V. Paul C. E. J. Biotechnol. 2019;293:24–35. doi: 10.1016/j.jbiotec.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Medina-Gonzalez Y. Camy S. Condoret J.-S. ACS Sustainable Chem. Eng. 2014;2:2623–2636. doi: 10.1021/sc5004314. [DOI] [Google Scholar]
- Matsuda T. J. Biosci. Bioeng. 2013;115:233–241. doi: 10.1016/j.jbiosc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Darani K. K. Mozafari M. R. J. Biochem. Technol. 2010;2:144–152. [Google Scholar]
- Delhaye L. Ceccato A. Jacobs P. Köttgen C. Merschaert A. Org. Process Res. Dev. 2007;11:160–164. doi: 10.1021/op060154k. [DOI] [Google Scholar]
- Singh M. Singh S. Deshaboina S. Krishnen H. Lloyd R. Holt-Tiffin K. Bhattacharya A. Bandichhor R. Catal. Sci. Technol. 2012;2:1602–1605. doi: 10.1039/C2CY00537A. [DOI] [Google Scholar]
- Domínguez de María P. Stillger T. Pohl M. Wallert S. Drauz K. Gröger H. Trauthwein H. Liese A. J. Mol. Catal. B: Enzym. 2006;38:43–47. doi: 10.1016/j.molcatb.2005.11.002. [DOI] [Google Scholar]
- Shanmuganathan S. Natalia D. van den Wittenboer A. Kohlmann C. Greiner L. Domínguez de María P. Green Chem. 2010;12:2240–2245. doi: 10.1039/C0GC00590H. [DOI] [Google Scholar]
- Strub R. McKim A. S. Pharm. Technol. 2016;2:30–35. [Google Scholar]
- Food and Drug Administration, https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras
- Andexer J. N. Langermann J. V. Kragl U. Pohl M. Trends Biotechnol. 2009;27:599–607. doi: 10.1016/j.tibtech.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Guterl J.-K. Andexer J. N. Sehl T. von Langermann J. Frindi-Wosch I. Rosenkranz T. Fitter J. Gruber K. Kragl U. Eggert T. J. Biotechnol. 2009;141:166–173. doi: 10.1016/j.jbiotec.2009.03.010. [DOI] [PubMed] [Google Scholar]
- von Langermann J. Mell A. Paetzold E. Daußmann T. Kragl U. Adv. Synth. Catal. 2007;349:1418–1424. doi: 10.1002/adsc.200700016. [DOI] [Google Scholar]
- Bauer M. Griengl H. Steiner W. Enzyme Microb. Technol. 1999;24:514–522. doi: 10.1016/S0141-0229(98)00147-1. [DOI] [Google Scholar]
- Willeman W. F. Gerrits P. J. Hanefeld U. Brussee J. Straathof A. J. van der Gen A. Heijnen J. J. Biotechnol. Bioeng. 2002;77:239–247. doi: 10.1002/bit.10002. [DOI] [PubMed] [Google Scholar]
- Hanefeld U. Chem. Soc. Rev. 2013;42:6308–6321. doi: 10.1039/C3CS35491A. [DOI] [PubMed] [Google Scholar]
- van der Helm M. P. Bracco P. Busch H. Szymańska K. Jarzębski A. B. Hanefeld U. Catal. Sci. Technol. 2019;9:1189–1200. doi: 10.1039/C8CY02192A. [DOI] [Google Scholar]
- Byrne F. P. Jin S. Paggiola G. Petchey T. H. Clark J. H. Farmer T. J. Hunt A. J. McElroy C. R. Sherwood J. Sustainable Chem. Processes. 2016;4:7. doi: 10.1186/s40508-016-0051-z. [DOI] [Google Scholar]
- Sheldon R. A. Curr. Opin. Green Sustainable Chem. 2019;18:13–19. doi: 10.1016/j.cogsc.2018.11.006. [DOI] [Google Scholar]
- Hernáiz M. J. Alcantara A. R. Garcia J. I. Sinisterra J. V. Chem. – Eur. J. 2010;16:9422–9437. doi: 10.1002/chem.201000798. [DOI] [PubMed] [Google Scholar]
- Prat D. Wells A. Hayler J. Sneddon H. McElroy C. R. Abou-Shehada S. Dunn P. J. Green Chem. 2015;18:288–296. doi: 10.1039/C5GC01008J. [DOI] [Google Scholar]
- Henderson R. K. Jiménez-González C. Constable D. J. Alston S. R. Inglis G. G. Fisher G. Sherwood J. Binks S. P. Curzons A. D. Green Chem. 2011;13:854–862. doi: 10.1039/C0GC00918K. [DOI] [Google Scholar]
- Alfonsi K. Colberg J. Dunn P. J. Fevig T. Jennings S. Johnson T. A. Kleine H. P. Knight C. Nagy M. A. Perry D. A. Green Chem. 2008;10:31–36. doi: 10.1039/B711717E. [DOI] [Google Scholar]
- Prat D. Pardigon O. Flemming H.-W. Letestu S. Ducandas V. R. Isnard P. Guntrum E. Senac T. Ruisseau S. P. Cruciani P. Org. Process Res. Dev. 2013;17:1517–1525. doi: 10.1021/op4002565. [DOI] [Google Scholar]
- Prat D. Hayler J. Wells A. Green Chem. 2014;16:4546–4551. doi: 10.1039/C4GC01149J. [DOI] [Google Scholar]
- Persistence, Bioaccumulation, and Toxicity Profiler, http://www.pbtprofiler.net/
- Estimation Program Interface Suite, https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface
- Cseri L., Razali M., Pogany P. and Szekely G., in Green Chem, Elsevier, 2018, pp. 513–553 [Google Scholar]
- Ni Y. Holtmann D. Hollmann F. ChemCatChem. 2014;6:930–943. doi: 10.1002/cctc.201300976. [DOI] [Google Scholar]
- Li H. Riisager A. Saravanamurugan S. Pandey A. Sangwan R. S. Yang S. Luque R. ACS Catal. 2018;8:148–187. doi: 10.1021/acscatal.7b02577. [DOI] [Google Scholar]
- Esteban J. Vorholt A. J. Leitner W. Green Chem. 2020;22:2097–2128. doi: 10.1039/C9GC04208C. [DOI] [Google Scholar]
- Martin-Luengo M. A. Yates M. Rojo E. S. Huerta Arribas D. Aguilar D. Ruiz Hitzky E. Appl. Catal., A. 2010;387:141–146. doi: 10.1016/j.apcata.2010.08.016. [DOI] [Google Scholar]
- Clark J. H. Macquarrie D. J. Sherwood J. Green Chem. 2012;14:90–93. doi: 10.1039/C1GC16299C. [DOI] [Google Scholar]
- Jessop P. G. Green Chem. 2011;13:1391–1398. doi: 10.1039/C0GC00797H. [DOI] [Google Scholar]
- Murray P. M. Bellany F. Benhamou L. Bučar D.-K. Tabor A. B. Sheppard T. D. Org. Biomol. Chem. 2016;14:2373–2384. doi: 10.1039/C5OB01892G. [DOI] [PubMed] [Google Scholar]
- Guajardo N. Domínguez de María P. Mol. Catal. 2020;485:110813. doi: 10.1016/j.mcat.2020.110813. [DOI] [Google Scholar]
- Pace V. Hoyos P. Castoldi L. Domínguez de María P. Alcantara A. R. ChemSusChem. 2012;5:1369–1379. doi: 10.1002/cssc.201100780. [DOI] [PubMed] [Google Scholar]
- de Gonzalo G. Alcántara A. R. Domínguez de María P. ChemSusChem. 2019;12:2083–2097. doi: 10.1002/cssc.201900079. [DOI] [PubMed] [Google Scholar]
- Weiss M. Gröger H. Synlett. 2009:1251–1254. [Google Scholar]
- Weiß M. Brinkmann T. Gröger H. Green Chem. 2010;12:1580–1588. doi: 10.1039/C002721A. [DOI] [Google Scholar]
- Strompen S. Weiss M. Gröger H. Hilterhaus L. Liese A. Adv. Synth. Catal. 2013;355:2391–2399. doi: 10.1002/adsc.201300236. [DOI] [Google Scholar]
- Strompen S. Weiß M. Ingram T. Smirnova I. Gröger H. Hilterhaus L. Liese A. Biotechnol. Bioeng. 2012;109:1479–1489. doi: 10.1002/bit.24422. [DOI] [PubMed] [Google Scholar]
- Ferrario V. Pleiss J. Eur. Phys. J. 2019;227:1631–1638. [Google Scholar]
- Schmidt S. Castiglione K. Kourist R. Chem. – Eur. J. 2018;24:1755–1768. doi: 10.1002/chem.201703353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.