Abstract
Qu-Yu-Jie-Du decoction (QYJD) is a commercially available traditional Chinese medicine (TCM). It is an aqueous extract of a Chinese herbal formula primarily consisting of eight TCM herbs: Taraxacum campylodes G.E. Haglund, Coix lacryma-jobi L., Smilax glabra Roxb., Sanguisorba officinalis L, Styphnolobium japonicum (L.) Schott, Prunus persica (L.) Batsch, Sophora flavescens Aiton, and Eupolyphaga sinensis Walker. Matrine and oxymatrine are two of the major phytochemical constituents of QYJD. Inflammation and oxidative stress are strongly associated with colon carcinogenesis. Colorectal cancer (CRC) is the third most common type of cancer. Therefore, cancer chemopreventive agents targeting CRC are urgently needed. This study was conducted to investigate the potential anticancer effects and the underlying mechanisms of QYJD and its active constituents, matrine and oxymatrine, in human colon cancer HT29 cells and in a dextran sulfate sodium (DSS)-induced colitis mouse model. QYJD and matrine effectively inhibited the proliferation and anchorage-independent growth of HT29 cells in a dose-dependent manner. QYJD and matrine also induced an Nrf2-mediated anti-oxidant response element-luciferase activity and upregulated the Nrf2-mediated anti-oxidative stress genes HO-1 and NQO1 at both the mRNA and protein levels. In the DSS-induced colitis mouse model, QYJD reduced the disease activity index (DAI) and alleviated colonic shortening. Elevated Nrf2 and HO-1 mRNA levels were also observed in QYJD-treated mice. These findings showed that QYJD could elicit anti-inflammatory and anti-oxidative stress response in vitro in a cell line and in vivo in a DSS-induced colitis mouse model. These responses may contribute to the overall anticolon cancer effect of QYJD.
Keywords: Qu-Yu-Jie-Du Decoction, Colitis, Colon Cancer, Matrine, Oxymatrine, Nrf2
Introduction
Colorectal cancer (CRC) is the third most common malignancy in males and the second most common cancer worldwide (Arnold et al., 2017). Inflammatory bowel disease (IBD), a chronic and relapsing inflammatory disease of the intestinal tract, is associated with an increased risk of CRC (Tanaka, 2012). Given the progressive nature and poor prognosis of CRC, new therapeutic strategies, such as prevention, are urgently needed (Kummar and Doroshow, 2011).
Traditional Chinese medicine (TCM) has received worldwide attention because these treatments show remarkable potency and few side effects. Thus, TCM therapeutics can be used in chemoprevention strategies and as potential alternatives for various types of diseases, including cancers.
Qu-Yu-Jie-Du decoction (QYJD) is an aqueous extract of a Chinese herbal mixture consisting of eight Chinese medicinal herbs: 20.8% of Taraxacum campylodes G.E. Haglund (Herba Taraxaci; Pu Gong Ying), 20.8% of Coix lacryma-jobi L. (Semen coicis; coix seed), 16.7% of Smilax glabra Roxb., 10.4% of Sanguisorba officinalis L. (garden burnet root; Radix Sanguisorbae), 10.4% of Styphnolobium japonicum (L.) Schott (flos sophorae immaturus), 8.3% of Prunus persica (L.) Batsch (Tao Ren; Semen Persicae), 8.3% of Sophora flavescens Aiton (Ku Shen; Sophora Flavescens) and 4.2% of Eupolyphaga sinensis Walker (Tu Bie Chong; Eupolyphaga seu steleophaga). It is a modified form of Xia Yu Xue decoction, which was first described in the Synopsis of Prescriptions of The Golden Chamber 200 AD. The classic recipe includes three mixed herbs, Rheum palmatum L., Prunus persica (L.) Batsch and Eupolyphaga sinensis Walker and has been used as an effective therapy for abdominal pain and oligomenorrhea caused by blood stasis. Studies have shown that it can ameliorate intestinal epithelial damage through targeting the inflammatory and anti-oxidant signaling pathways (Liu et al., 2015; Ma et al., 2017).
The optimized formula, QYJD, has been commonly used to treat many diseases, including cancers, in China for many years with few adverse effects. Lian et al. found that QYJD could improve the micro-environment of ova in patients with endometriosis by reducing the expression of TNF and IL-6 (Li et al., 2008; Lian et al., 2009). QYJD, in combination with endocrine therapy, has also been demonstrated to be able to (i) improve life quality of advanced prostate cancer patients; (ii) reduce adverse reactions of Western medicine treatment; and (iii) improve immune system functions (Jia et al., 2013). A previously published clinical study has revealed that QYJD significantly improved the survival outcomes and reduced the side effects of chemotherapy in hepatocellular carcinoma (HCC) patients (Zhao et al., 2017). In recent years, many studies have been conducted to investigate the beneficial effects of QYJD, individually or in combination with other therapeutic drugs, in CRC patients. Two clinical studies have demonstrated that QYJD can reduce adverse effects of chemotherapy with FOLFOX4 and 5-fluorouracil and improve the quality of life in CRC patients (Lai et al., 2012; Zhou, 2013). Another clinical study by Zhang compared the prognostic benefits between QYJD, radiofrequency ablation (RFA) and the combination of these two and found that combination therapy of QYJD and RFA had more potential in improving the quality of life in CRC patients, compared to the other two therapies (Zhang, 2012). A clinical study by Zhou et al. also reported that QYJD in combination with capecitabine could improve quality of life and reduce the adverse effects of chemotherapy in CRC patients (Zhou et al., 2016). In addition, cell line and animal xenograft studies have shown that QYJD can inhibit proliferation and induce apoptosis in Human cancer HT29 cells (Liu et al., 2016; Zhao et al., 2017). However, the underlying mechanisms of QYJD remain unclear. Matrine and oxymatrine are two major alkaloid components of QYJD. Matrine and oxymatrine have been widely used in China due to their extensive range of pharmacological effects, including anti-inflammatory and anti-oxidative properties. Thus, the antitumor activity of matrine and oxymatrine has attracted increasing attention (Li et al., 2010; Liu et al., 2010; Taylor et al., 2010; Yu et al., 2011). However, whether matrine and oxymatrine can inhibit the proliferation of human colon cancer HT29 cells and the underlying molecular mechanisms are unclear. Therefore, we aimed to investigate the antitumor effect of QYJD, matrine and oxymatrine in HT29 cells and to further elucidate the molecular mechanisms involved in its antineoplastic activities.
Anti-oxidant response elements (AREs) are present in the promoter region of genes activated by Nuclear Factor, Erythroid 2 Like 2 (NFE2L2 or Nrf2), which include phase II detoxification/anti-oxidant enzymes, such as heme oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1) and glutathione S-transferase; these enzymes maintain the normal redox state of the body and cells. Therefore, Nrf2 expression can reduce the organ damage induced by electrophilic reagents. In a relaxed state, Nrf2 is present in the cyto-plasm in association with Kelch-like ECH-associated protein 1 (Keap1). Disturbance of the interaction between Nrf2 and Keap1, including covalent or oxidative modification of cysteine thiols in Keap1 by electrophiles or oxidative stress, results in Nrf2 release and its translocation into the nucleus (Cheng et al., 2016). Many phase II enzymes as well as some detoxifying enzymes, such as glutathione S-transferase, peroxiredoxin1, γ-glutamate cysteine ligase, HO-1 and NQO1, are inducible and activated by Nrf2 (Darley-Usmar et al., 1996; Giudice and Montella, 2006; Kim et al., 2007; Park et al., 2013). Nrf2 binding to the ARE sequence in genes encoding phase II/anti-oxidant enzymes causes transcriptional activation of these genes, resulting in removal of reactive oxygen species or toxic chemicals.
Exposure to dextran sulfate sodium (DSS) leads to intestinal permeability and mucosal changes in the ileum and colon, which may also contribute to induction of oxidative stress (Dawson et al., 2010). Oxidative stress is closely linked to inflammation, which promotes many diseases, including cancers (Mantovani et al., 2008; Cheng et al., 2016). Therefore, we studied the anti-inflammatory activity of QYJD in DSS-induced colitis models and investigated whether its anti-oxidant effect was associated with the Nrf2 pathway.
Materials and Methods
Reagents
QYJD is a commercially available TCM decoction. It was kindly provided by Kangmei Pharmaceutical (Puning, China). The formula was extracted with water and freeze-dried into powder. Quality and quantity analyses of the aqueous extract were performed with LC/MS. Dimethyl sulfoxide (DMSO), matrine (M5319) and oxymatrine (O0891) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sulforaphane (SFN) was purchased from LKT Laboratories (St. Paul, MN). DSS (36–50 kDa) was purchased from MP Bio-medicals (Solon, OH, USA). Testosterone (> 98% purity) was purchased from Nacalai Tesque (Kyoto, Japan) and was used as an internal standard (IS). Acetonitrile, dichlor-omethane and formic acid were HPLC grade. Ultrapure water was used for all analyses. All the other chemicals and reagents commercially available were of the highest analytical grade. Primary and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Primers for qPCR were purchased from Integrated DNA Technologies (Coralville, IA).
Identification of Matrine and Oxymatrine in QYJD by LC/MS
The following conditions were used to analyze matrine and oxymatrine: system, Agilent 1290 Infinity LC system (Agilent Technologies, USA), which consists of a solvent degasser, a binary pump, an auto-sampler and a column oven; column, Agilent ZORBAX RRHD SB-C18 (100 × 3 mm, 1.8 μm); mobile phase A, 0.1% formic acid in water; mobile phase B, 100% acetonitrile; flow rate, 0.3 mL/min; wavelengths, 210 nm for matrine and oxymatrine and 243 nm for testosterone; injection volume, 10 μL; MS/MS detector, Agilent 6540 quadrupole-time of flight mass spectrometer, used in combination with an Agilent 1290 Infinity ultra-performance liquid chromatography system. Samples were analyzed using Dual AJSESI (Agilent Technologies) in the positive model. Data were collected and analyzed by Quantitative Analysis Software (version B.06.00, Agilent Technologies).
Cell Culture and Cell Viability Assay
HT29 cells were purchased from the American Type Culture Collection (ATCC). A stably transfected single clone HepG2-ARE-C8 (HepG2-C8) cell line was previously established in our laboratory using the pARE-TI-luciferase reporter gene (Yu et al., 2000). Both cell lines were routinely maintained in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco) at 37°C in a humidified 5% CO2 atmosphere. Cells were seeded in 96-well plates at a density of 1 × 104 cells/well. Twenty-four hours later, cells were treated with different doses of QYJD, matrine and oxymatrine.
After treatment for 48 h, cell viability was tested using the Cell Titer 96 Aqueous Nonradioactive Cell Proliferation Assay kit (Promega Corp., Madison, WI). The absorbance was read at 490 nm on an Infinite 200 PRO microplate reader (Tecan Systems, San Jose, CA).
Colony Formation Assay
HT29 cells (5 × 103 cells/well) were transferred to 1 mL of basal medium Eagle (BME) containing 0.33% agar over 3 mL of BME containing 0.5% agar with 10% FBS in 6-well plates. Both the top and the bottom layers contained different concentrations of QYJD in each well. The cells were maintained in soft agar at 37°C in a humidified 5% CO2 atmosphere for 14 days. The colonies were photographed using a computerized microscope system (Nikon Eclipse E600) with Nikon ACT-1 software (Version 2.20) and counted using ImageJ Software (Version 1.47k).
ARE-Luciferase Reporter Assay
The HepG2-C8 cell line was established as described previously (Yu et al., 2000). HepG2-C8 cells were seeded in 6-well plates at a density of 1 × 105 cells/well. After overnight incubation, cells were cultured in DMEM containing 10% FBS and different concentrations of compounds for 12 h. The luciferase activity was measured according to the manufacturer’s instructions. Briefly, after drug treatment, cells were washed twice with ice-cold PBS and harvested in reporter lysis buffer. The homogenates were centrifuged at 13, 000× g for 5 min at 4°C. A 10-μl supernatant was assayed for luciferase activity using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Luciferase activity was normalized to protein concentration. The data are presented as fold change relative to the control group (no treatment).
RNA Isolation and Reverse Transcription Quantitative PCR (RT-qPCR)
HT29 cells were seeded in 6-well plates overnight and treated with compounds for 24 h. After treatment, the cells were washed with ice-cold PBS and harvested. Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Reverse transcription was carried out using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). qPCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) on an ABI7900HT system (Applied Biosystems). Relative mRNA expression levels were determined using the ΔΔCt method.
Preparation of Protein Lysate and Western Blot Analysis
After being treated for 24 h, cells were washed with ice-cold PBS and harvested with 200 μL of a lysis buffer containing 10 mM Tris-HCl (pH 7.4), 50 mM sodium chloride, 30 mM Na-PPi, 50 mM sodium fluoride, 100 μM sodium orthovanadate, 2 mM iodoacetic acid, 5 mM ZnCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.5% Triton X-100. Cell lysates were homogenized by passing through a 23-gauge needle three times and incubated on ice for 30 min. The homogenate was centrifuged at 13, 000× g for 15 min at 4°C. Then, 25 μg of total protein, as determined by a Bio-Rad protein assay, was mixed with 4× loading buffer and preheated at 95°C for 3 min. The samples were then loaded on a 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and run at 200 V. The proteins were transferred onto a polyvinylidene difluoride membrane using a semidry transfer system (Fisher, Pittsburgh, PA). The membrane was blocked with 5% BSA solution for 1 h at room temperature and then incubated overnight at 4°C with primary antibody (1:1,000 dilution). After hybridization with primary antibody, the membrane was washed with TBST (Tris-buffered saline and Tween 20) three times, then incubated with secondary antibody (1:5,000 dilution) for 45 min at room temperature and washed with TBST three times. The protein bands were visualized with the SuperSignal chemi-luminescent substrate (Thermo Fisher Scientific) in a Bio-Rad ChemiDoc XRS system and semiquantified using ImageJ Software.
Animals and Experimental Procedures
Four-week-old male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and were maintained in a controlled environment (20–22°C, 12-h light and 12-h dark cycles, and 45–55% relative humidity) at the Rutgers Animal Care Facility. The AIN-93M diet was purchased from ResearchDiet Inc. (New Brunswick, NJ, USA). The diet and water were provided ad libitum. All animal experiments were performed under the relevant animal protocol (01–016), which was approved by the Institutional Animal Care and Use Committee (IACUC) of Rutgers University. Upon arrival, mice were randomly divided into five groups (n = 10): control, DSS and three DSS+QYJD groups (3 QYJD doses: 1, 2 or 4 g per kg body weight) as shown in Fig. 5A. Mice were administered different doses of QYJD solution or saline by oral gavage three times per week from weeks 4 through 8. For induction of acute colitis, mice were exposed to 1.2% DSS (Affymetrix, USA) in drinking water for one week, starting at 7 weeks of age. Disease activity index (DAI) and body weight were measured weekly. At the end of the experiment, mice were sacrificed, the colons were removed and the length of the colons was measured. All the colons were flushed with ice-cold saline to remove feces and were opened longitudinally on filter paper. Colon epithelial cells were isolated using a previously described method. Briefly, tissues were scraped using a glass slide on ice, and the mucosal scrapings were resuspended in ice-cold PBS. Cells were collected by centrifuging and used for molecular analyses.
Figure 5.
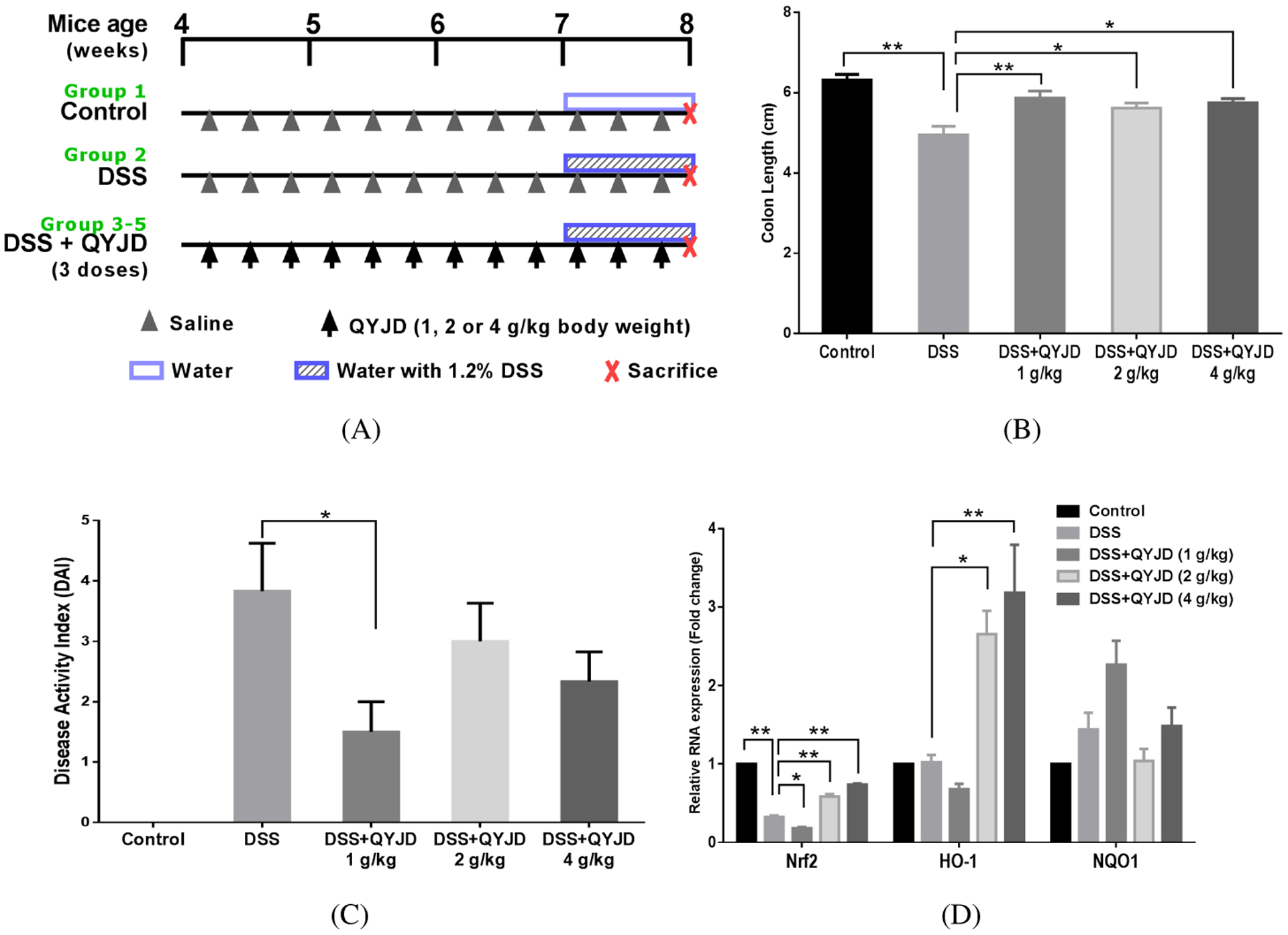
QYJD attenuates colitis in DSS-induced mice. (A) The experimental design of the animal study. Three doses of QYJD (1, 2 and 4 g/kg body weight) were used in this study. n = 10. (B, C) Average colon length and disease activity index (DAI) in each group. (*P < 0:05, **P < 0:01, one-way ANOVA; compared to the DSS group.) (D) Relative mRNA expression of Nrf2, HO-1 and NQO1 in colon epithelial cells. Data are shown as an average level of all samples in each group, and experiments were performed three times for each sample. *P < 0:05, **P < 0:01, one-way ANOVA; compared to the DSS group.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), Version 22. Data are presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA. P values less than 0.05 were considered statistically significant.
Results
Matrine and Oxymatrine Contents in the QYJD Formula
Matrine and oxymatrine are the major constituents of aqueous extract of Sophora flavescens Aiton and Kushen. The molecular structures and molecular weights are shown in Fig. 1A. The LC/MS chromatogram of QYJD is shown in Fig. 1B. We identified two peaks corresponding to matrine and oxymatrine at elution times of approximately 5 and 6 min based on the chromatograms of pure matrine and oxymatrine run in the same condition. The mass spectra of matrine and oxymatrine are shown in Fig. 1C. The mass-to-charge ratios (m/z) 249.2 and 265.2 were observed in previous peaks, confirming that they were protonated forms of matrine and oxymatrine, respectively. The concentrations of matrine and oxymatrine in 200 mg/mL of QYJD were 339 μM and 231 μM, respectively, as determined by HPLC (data not shown).
Figure 1.
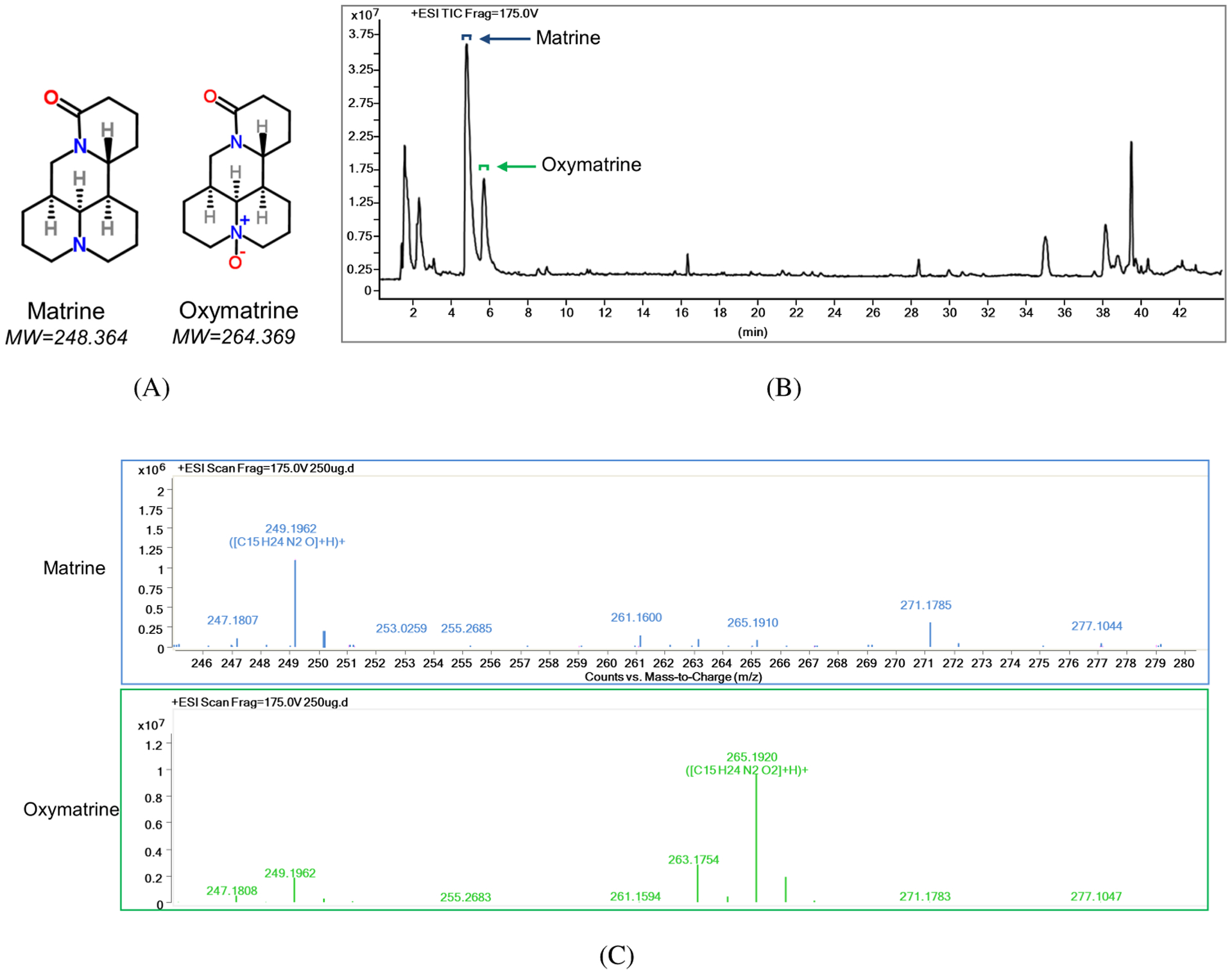
The matrine and oxymatrine contents in QYJD. (A) The molecular structure and molecular weight of matrine and oxymatrine. (B) LC/MS chromatogram of QYJD. The peaks for matrine and oxymatrine are indicated by an arrow. Top square brackets indicate the range of peaks for displaying the mass spectra. (C) Mass spectra of matrine (upper) and oxymatrine (lower). The mass-to-charge (m/z) ratios of all detected peaks are shown above each peak.
QYJD Inhibited HT29 Cell Proliferation and Anchorage-Independent Growth
HT-29 cells were incubated with different concentrations of QYJD, matrine and oxymatrine for 48 h. A dose-dependent decrease in cell viability was observed in the QYJD- and matrine-treated groups. Cell viability was decreased to approximately 50% by 5 mg/mL QYJD or 3 mM matrine treatment. In contrast, no inhibitory effect on cell viability was observed in HT29 cells treated with oxymatrine (Fig. 2A).
Figure 2.
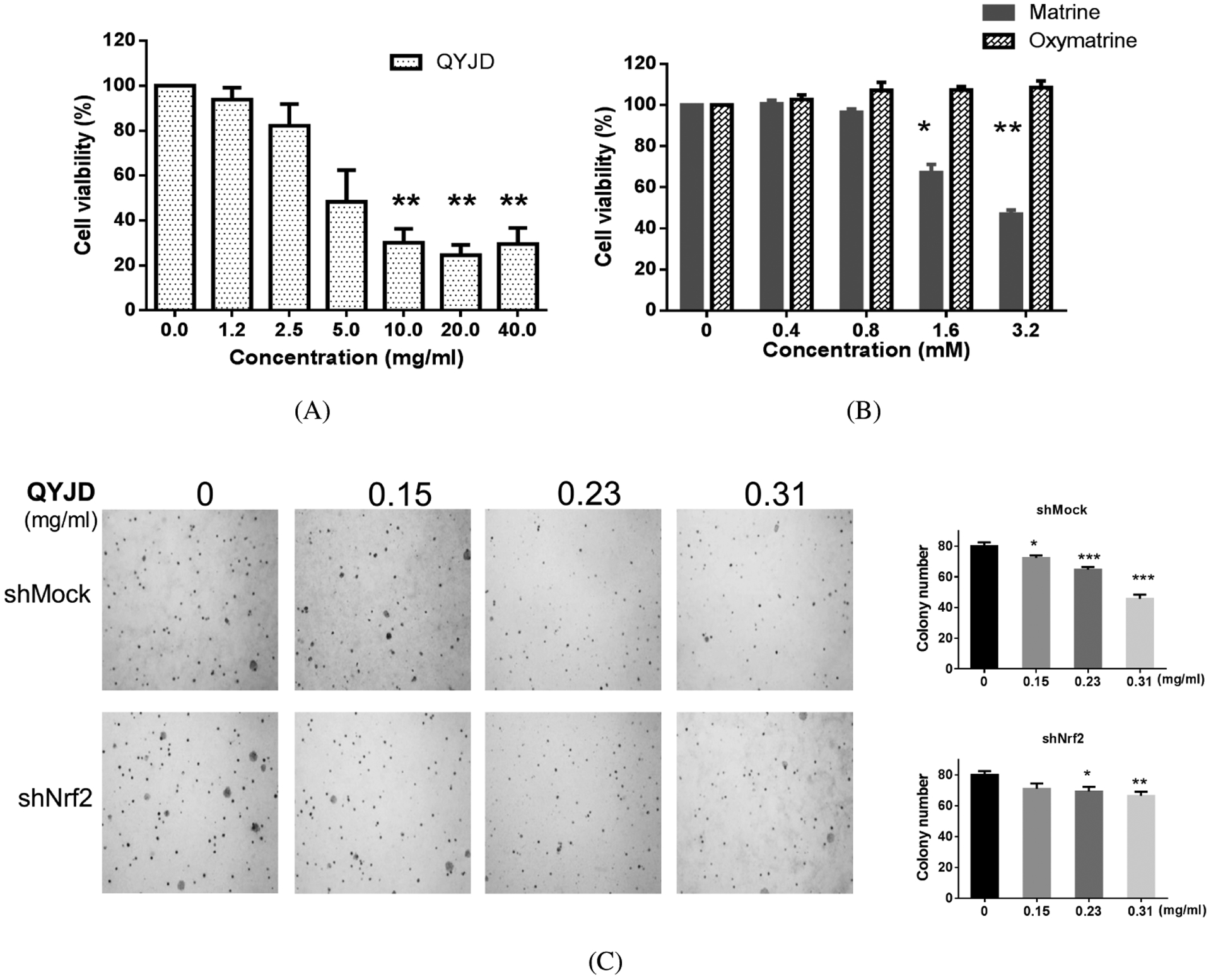
Cell viability and anchorage-independent growth of HT29 cells are inhibited by QYJD treatment. (A, B) Bar graphs showing cell viability of HT29 cells after treatment with different concentrations of QYJD, matrine and oxymatrine for 24 h. Note that the concentrations are in mg/mL for QYJD and mM for matrine and oxymatrine. (C) Colony formation of wild-type and Nrf2 knockout HT29 cells. Representative colony images of wild-type (shMock) and Nrf2 knockout (shNrf2) HT29 cells treated with different concentrations of QYJD (left) and data from three independent experiments (right). All experiments were performed three times. *P < 0:05, **P < 0:01, ***P < 0:001, one-way ANOVA; compared to the vehicle-treated group.
For analysis of the effect of QYJD on anchorage-independent growth of HT29 cells and the potential role of Nrf2 in inhibiting anchorage-independent growth, wild-type (shMock) and Nrf2 knockdown (shNrf2) HT29 cells were treated with QYJD at three concentrations — 0.15, 0.23 and 0.31 mg/mL. Based on the previously determined matrine and oxymatrine contents in QYJD, as described in Sec. 3.1, the equivalent concentrations of matrine were 0.26, 0.40 and 0.50 μM, respectively. In addition, the equivalent concentrations of oxymatrine were 0.18, 0.27, and 0.36 μM, respectively. QYJD significantly inhibited the colony formation of wild-type HT29 cells in a dose-dependent manner. However, the inhibitory effect of QYJD on Nrf2 knockout HT29 cells was not as dramatic as in wild-type cells (Fig. 2B).
Induction of ARE-Luciferase Activity by QYJD, Matrine and Oxymatrine
To test the transcriptional activation of AREs by QYJD, matrine and oxymatrine, were treated HepG2-C8 cells with QYJD, matrine and oxymatrine for 12 h, and then, the ARE-luciferase reporter activities were measured (Chen et al., 2000). Figure 3A shows that QYJD and matrine induced ARE-luciferase activity in HepG2-C8 cells with different potencies, while oxymatrine did not induce this activity. QYJD at 30 mg/mL strongly increased ARE-luciferase activity by 8 times compared to vehicle (0.1% DMSO) treatment. Matrine at a concentration of 1.6 mM modestly increased the activity approximately 4 times higher than vehicle treatment.
Figure 3.
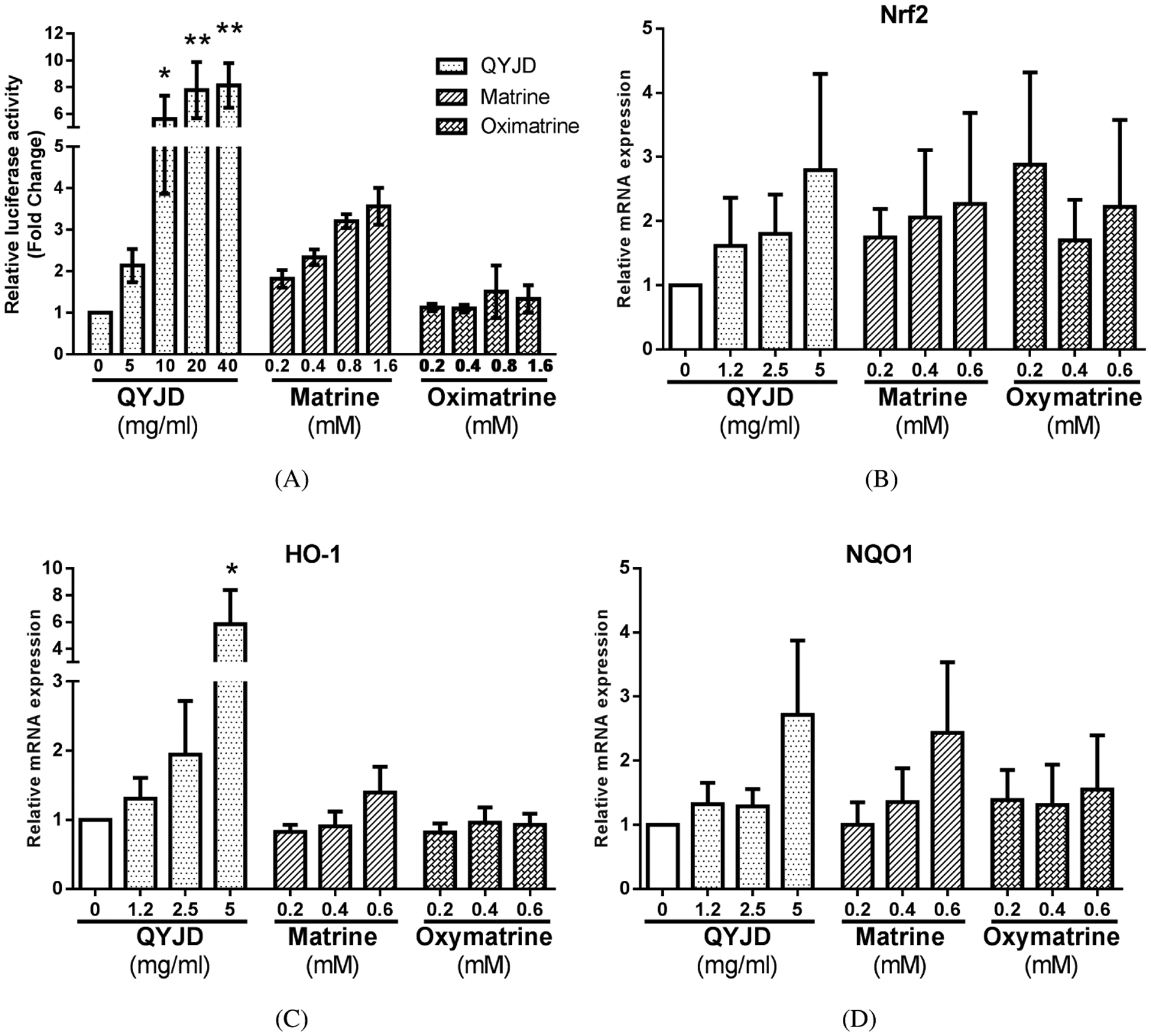
QYJD induces ARE-luciferase activity in HepG2-C8 cells and activates mRNA expression of Nrf2 and its downstream genes in HT29 cells. (A) Relative luciferase activity induced by QYJD, matrine and oxymatrine in HepG2-C8 cells. Data are presented as a fold change compared to the vehicle (0.1% DMSO)-treated group. (B–D) Relative mRNA expression of Nrf2, HO-1 and NQO1 induced by QYJD, matrine and oxymatrine in HT29 cells. All experiments were performed three times. *P < 0:05, **P < 0:01, one-way ANOVA; compared to the vehicle-treated group.
mRNA Expression of Nrf2 and Its Downstream Genes Induced by QYJD, Matrine and Oxymatrine
To confirm that QYJD, matrine and oxymatrine can induce endogenous Nrf2 and its downstream genes in HT29 cells, we conducted qPCR to quantify the mRNA expression levels of these genes (Figs. 3B–3D). The mRNA expression of Nrf2 was slightly increased by QYJD and matrine treatment. However, the differences were not statistically significant. The mRNA expression of HO-1 was significantly increased by QYJD at 5 mg/mL, while other treatments did not significantly change the mRNA level. Both QYJD at 5 mg/mL and matrine at 0.6 mM increased the mRNA expression of NQO1, but the differences were not statistically significant.
Protein Expression of NRF2 and Nrf2 Target Genes Induced by QYJD, Matrine and Oxymatrine
We then tested the protein level of NRF2 and its target enzymes in HT29 cells treated with QYJD, matrine and oxymatrine. Blots in Fig. 4A are representative of three independent experiments. We used SFN, a well-known Nrf2 activator, as a positive control (Lin et al., 2008; Kong et al., 2013). As shown in Fig. 4B, QYJD and matrine significantly increased the NRF2 protein levels, while oxymatrine had no effect. The inductions by QYJD and matrine were comparable to that by SFN. Figure 4C shows that only QYJD and SFN significantly increased the protein level of HO1, and they had similar potencies. Matrine at 0.4 mM increased the protein level of NQO1 by approximately 3.5-fold compared to vehicle (0.1% DMSO) treatment, while the other treatments did not have similar effects (Fig. 4D). Thus, we observed that the protein levels of Nrf2, HO-1 and NQO1 generally matched their mRNA expression levels.
Figure 4.
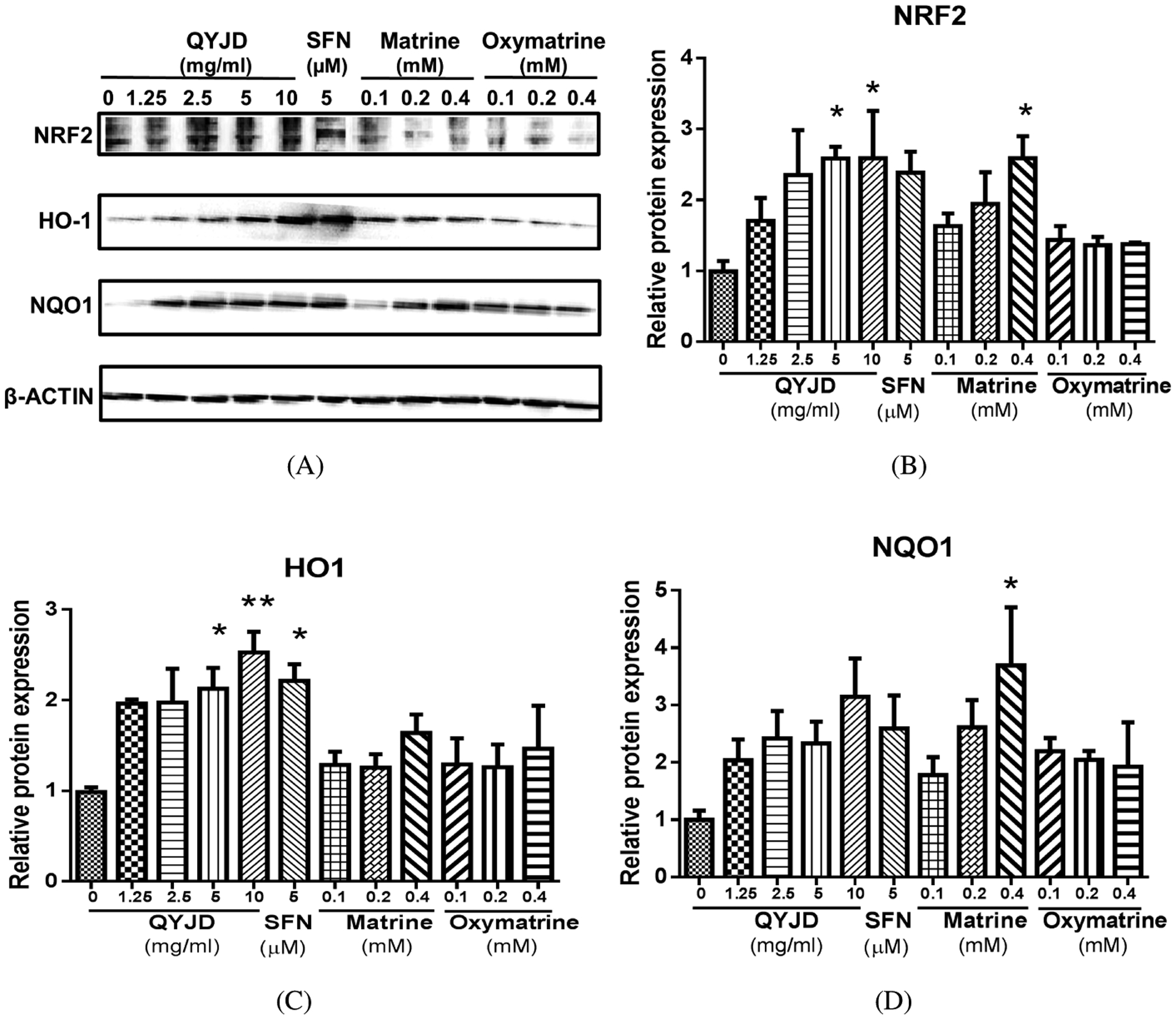
Protein expression of NRF2 and its target enzymes in HT29 cells treated with QYJD, matrine and oxymatrine. (A) Representative images of Western blots from three independent experiments. Sulforaphane (SFN, 5 μM) was used as a positive control for activating NRF2. β-ACTIN served as a loading control. (B–D) Relative protein levels of Nrf2, HO-1 and NQO1 induced by QYJD, matrine and oxymatrine in HT29 cells. All experiments were performed three times. *P < 0:05, one-way ANOVA; compared to the vehicle-treated group.
QYJD Inhibited DSS-Induced Colitis in Mice through Activation of Nrf2 Signaling Pathways
Mice were fed QYJD by oral gavage three weeks prior to DSS administration to test the protective effects of QYJD against DSS-induced colitis. The experimental design of the animal study is shown in Fig. 5A. At the end of the study, the DAI and colon length were measured for each mouse. As shown in Fig. 5B, the average colon length in the DSS group was significantly shorter than in the control group, and QYJD significantly attenuated the colon shortening caused by DSS. End-point DAI is shown in Fig. 5C. A notable increase in DAI was observed in the DSS-treated group, and QYJD administration at 1 g/kg body weight reduced the DAI. However, the DAI in the other two QYJD administration groups was not significantly different than the DSS-treated group. Colon epithelial cells from all groups were used to determine the mRNA expression of Nrf2 and its downstream genes. As shown in Fig. 5D, QYJD administration increased mRNA expression of Nrf2 and HO-1, which could potentially contribute to the protective role of QYJD against DSS-induced colitis in mice.
Discussion
Patients with IBD and inflammatory disorders are at high risk of developing CRC. Understanding the etiology of these diseases is essential for improving the currently available strategies to treat IBD and, more importantly, to prevent CRC. Nrf2, a transcription factor with anti-inflammation and anti-oxidative stress activities, protects intestinal integrity through regulation of proinflammatory cytokines and induction of phase II detoxifying enzymes (Khor et al., 2006). Elucidation of the molecular mechanisms of TCMs as an alternative for therapy and prevention of CRC has become increasingly important (Guo et al., 2018). Our previous study suggested that QYJD was effective in improving the quality of life of CRC patients and in suppressing tumor growth. Oxidative stress and inflammation are closely associated with mucosal erosions and a variety of gastrointestinal diseases, such as Crohn’s disease and ulcerative colitis (Pravda, 2005; Rezaie et al., 2007). As one of the hallmarks of cancer, inflammation is frequently involved in various types of cancers, such as mammary, prostate and colon cancers. Nrf2, the key to the cellular anti-oxidative response, has been shown to suppress carcinogenesis, especially in the early stages. The anti-oxidant and antitumor properties of TCMs have been widely reported in cultured cells, animal models and humans. To further elucidate the mechanisms, we studied the antiproliferative and anti-oxidant effects of QYJD and its major constituents, matrine and oxymatrine. Mechanistically, our results demonstrated that QYJD and matrine significantly inhibited the proliferation of HT29 cells. Furthermore, the antineoplastic effect of QYJD was shown by its inhibition of anchorage-independent growth of CRC cells. Treatment with QYJD and matrine activated ARE-luciferase activity and Nrf2 and its downstream genes. Western blotting analysis revealed that QYJD increased the protein levels of Nrf2 and HO-1. We also demonstrated the chemopreventive role of QYJD in DSS-induced ulcerative colitis, which is considered a precancerous lesion. The results demonstrated that QYJD could attenuate colon shortening and lead to clinical improvement in mice with DSS-induced acute colitis, indicating the therapeutic potential of QYJD. We further analyzed the molecular mechanism underlying the QYJD-mediated suppression of colonic inflammation. The intestinal epithelium sits at the interface between an organism and its luminal environment and is thus susceptible to oxidative damage induced by luminal oxidants (Carriere et al., 2001). During the preparation of this manuscript, Chen et al. published a paper where they found oxymatrine protected against DSS-induced colitis in mouse by inhibiting the PI3K/Akt signaling pathway (Chen et al., 2017). In 2016, a paper by Liu et al. suggested that oxymatrine synergistically enhanced anticancer activity of oxaliplatin by inhibiting the PI3K/Akt/mTOR signaling pathway in colon cancer HT29 and SW480 cells and in a mouse xenograft model (Liu et al., 2016). In our present study, we show that oxymatrine and matrine exert their anticancer effects by activating the Nrf2/ARE anti-oxidative stress pathway in cells and the QYJD formula could also active the Nrf2/ARE anti-oxidative stress pathway in DSS-induced mouse colitis model. These findings suggest that QYJD is a potential therapeutic for CRC.
Acknowledgments
This work was supported by R01 AT009152 and R01 AT007065 from the National Center for Complementary and Integrative Health (NCCIH). This work was partially supported by the Student Study Aboard Project of GZUCM. We thank Prof. Zhongqiu Liu, of the International Institute for Translational Chinese Medicine, for providing useful advice and suggestions.
References
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A and Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66: 683–691, 2017. [DOI] [PubMed] [Google Scholar]
- Carriere V, Chambaz J and Rousset M. Intestinal responses to xenobiotics. Toxicol. In Vitro 15: 373–378, 2001. [DOI] [PubMed] [Google Scholar]
- Chen C, Yu R, Owuor ED and Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res 23: 605–612, 2000. [DOI] [PubMed] [Google Scholar]
- Chen Q, Duan X, Fan H, Xu M, Tang Q, Zhang L, Shou Z, Liu X, Zuo D, Yang J, Deng S, Dong Y, Wu H, Liu Y and Nan Z. Oxymatrine protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway. Int. Immunopharmacol 53: 149–157, 2017. [DOI] [PubMed] [Google Scholar]
- Cheng D, Wu R, Guo Y and Kong AN. Regulation of Keap1-Nrf2 signaling: The role of epigenetics. Curr. Opin. Toxicol 1: 134–138, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley-Usmar V and Halliwell B. Blood radicals: Reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm. Res 13: 649–662, 1996. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A and Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The early start denver model. Pediatrics 125: e17–23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice A and Montella M. Activation of the Nrf2-ARE signaling pathway: A promising strategy in cancer prevention. Bioessays 28: 169–181, 2006. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu R, Gaspar JM, Sargsyan D, Su ZY, Zhang C, Gao L, Cheng D, Li W, Wang C, Yin R, Fang M, Verzi MP, Hart RP and Kong AN. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 39: 669–680, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia YJ, Li XJ, Li C and Zhao C. Clinical efficacy analysis of treating advanced prostate cancer by yiqi jiedu quyu recipe combined endocrine therapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 33: 448–451, 2013. [PubMed] [Google Scholar]
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS and Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66: 11580–11584, 2006. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y and Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res 67: 546–554, 2007. [DOI] [PubMed] [Google Scholar]
- Kong AN, Zhang C and Su ZY. Targeting epigenetics for cancer prevention by dietary cancer preventive compounds — the case of miRNA. Cancer Prev. Res. (Phila.) 6: 622–624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar S and Doroshow JH. Phase 0 trials: Expediting the development of chemoprevention agents. Cancer Prev. Res. (Phila.) 4: 288–292, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Peng W, Deng J and Wang C. Clinical research on method of strengthening spleen and replenishing Qi and detoxicating and removing blood stasis combined with FOLFOX4 chemotherapy treating advanced colorectal carcinoma. Liaoning J. Trad. Chin. Med 39: 849–851, 2012. [Google Scholar]
- Li H, Tan G, Jiang X, Qiao H, Pan S, Jiang H, Kanwar JR and Sun X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am. J. Chin. Med 38: 1115–1130, 2010. [DOI] [PubMed] [Google Scholar]
- Li XL, Lian F and Liu YH. Effects of Quyu Jiedu Granule on expressions of tumor necrosis factor-alpha and interleukin-6 mRNAs in ovarian granulosa cells of endometriosis rats. Zhong Xi Yi Jie He Xue Bao 6: 960–963, 2008. [DOI] [PubMed] [Google Scholar]
- Lian F, Li XL, Sun ZG, Zhang JW, Liu YH and Ma FM. Effect of Quyu Jiedu granule on microenvironment of ova in patients with endometriosis. Chin. J. Integr. Med 15: 42–46, 2009. [DOI] [PubMed] [Google Scholar]
- Lin W, Wu RT, Wu T, Khor TO, Wang H and Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol 76: 967–973, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yuan X, Tao L, Cheng Z, Dai X, Sheng X and Xue D. Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-kappaB and TGF-beta1 signaling pathways. BMC Complement. Altern. Med 15: 201, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu T, Song Y, Chen H, Pan S and Sun X. Matrine inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol. Pharm. Bull 33: 1740–1745, 2010. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao X, Zhang G, Zhang J, Chai Z and Zhou B. Fuzheng Jiedu Quyu on postoperative colorectal cancer chemotherapy synergistic effect. Chin. J. Surg. Integrated Trad. Western Med 22: 150–156, 2016. [Google Scholar]
- Liu Y, Bi T, Wang Z, Wu G, Qian L, Gao Q and Shen G. Oxymatrine synergistically enhances antitumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway. Apoptosis 21: 1398–1407, 2016. [DOI] [PubMed] [Google Scholar]
- Ma W, Tao L, Zhang W, Zhu Y, Xue D, Zhang J and Liu C. Xia-Yu-Xue decoction inhibits intestinal epithelial cell apoptosis in CCl4-induced liver fibrosis. Cell. Physiol. Biochem 44: 333–344, 2017. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A and Balkwill F. Cancer-related inflammation. Nature 454: 436–444, 2008. [DOI] [PubMed] [Google Scholar]
- Park YH, Kim SU, Lee BK, Kim HS, Song IS, Shin HJ, Han YH, Chang KT, Kim JM, Lee DS, Kim YH, Choi CM, Kim BY and Yu DY. Prx I suppresses K-ras-driven lung tumorigenesis by opposing redox-sensitive ERK/cyclin D1 pathway. Antioxid. Redox Signal 19: 482–496, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravda J Radical induction theory of ulcerative colitis. World J. Gastroenterol 11: 2371–2384, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie A, Parker RD and Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci 52: 2015–2021, 2007. [DOI] [PubMed] [Google Scholar]
- Tanaka T Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflamm 2012: 658–786, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LA, Pletschen L, Arends J, Unger C and Massing U. Marine phospholipids — a promising new dietary approach to tumor-associated weight loss. Support Care Cancer 18: 159–170, 2010. [DOI] [PubMed] [Google Scholar]
- Yu HB, Zhang HF, Li DY, Zhang X, Xue HZ and Zhao SH. Matrine inhibits matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. J. Asian Nat. Prod. Res 13: 242–250, 2011. [DOI] [PubMed] [Google Scholar]
- Yu R, Mandlekar S, Lei W, Fahl WE, Tan TH and Kong AN. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J. Biol. Chem 275: 2322–2327, 2000. [DOI] [PubMed] [Google Scholar]
- Zhang E A clinincal study of QYJD and radiofrequency ablation on life quality in colorectal carcinoma patients. J. New Chin. Med 44: 81–82, 2012. [Google Scholar]
- Zhao H, Zhai X, Chen Z, Wan X, Chen L, Shen F and Ling C. Transarterial chemoembolization combined with Jie-du granule preparation improves the survival outcomes of patients with unresectable hepatocellular carcinoma. Oncotarget 8: 45234–45241, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J A Clinical Observation on the prognostic effects of Quyu Jiedu in combination with 5-fluorouracil in colorectal carcinoma patients. Guide China Med 11: 502–503, 2013. [Google Scholar]
- Zhou J, Li M and Li W. Clinical analysis of Quyu Jiedu Decoction combined with capecitabine metronomic chemotherapy in the treatment of elderly advanced colorectal cancer. J. Colorectal Anal. Surg 69–72, 2016. [Google Scholar]
- Zhao X, Zhou B, Zhang G, Chai Z, Liu X and Liu H. Study on the effect and its mechanism of Fuzheng Jiedu Quyu formula combined with oxaliplatin on proliferation and apoptosis of human colon cancer HT-29 cell. China Pharm 28: 2613–2616, 2017. [Google Scholar]


