Abstract
OBJECTIVE
The endoscopic endonasal approach (EEA) has gained increasing popularity for the resection of suprasellar meningiomas (SSMs). Appropriate case selection is critical in optimizing patient outcome. Long-term outcome data are lacking. The authors systematically identified preoperative factors associated with extent of resection (EOR) and determined the relationship between EOR and long-term recurrence after EEA for SSMs.
METHODS
In this retrospective cohort study, the authors identified preoperative clinical and imaging characteristics associated with EOR and built on the recently published University of California, San Francisco resectability score to propose a score more specific to the EEA. They then examined the relationship between gross-total resection (GTR; 100%), near-total resection (NTR; 95%–99%), and subtotal resection (STR; < 95%) and recurrence or progression with Kaplan-Meier survival analysis.
RESULTS
A total of 51 patients were identified. Radiographic GTR was achieved in 40 of 47 (85%) patients in whom it was the surgical goal. Significant independent risk factors for incomplete resection were prior surgery (OR 25.94, 95% CI < 2.00 to 336.49, p = 0.013); tumor lateral to the optic nerve (OR 13.41, 95% CI 1.82–98.99, p = 0.011); and complete internal carotid artery (ICA) encasement (OR 15.12, 95% CI 1.17–194.08, p = 0.037). Tumor size and optic canal invasion were not significant risk factors after adjustment for other variables. A resectability score based on the multivariable model successfully predicted the likelihood of GTR; a score of 0 had a positive predictive value of 97% for GTR, whereas a score of 2 had a negative predictive value of 87.5% for incomplete resection. After a mean follow-up of 40.6 ± 32.4 months (mean ± SD), recurrence was 2.7% after GTR (1 patient with atypical histology), 44.4% after NTR, and 80% after STR (p < 0.0001). Vision was stable or improved in 93.5% and improved in 67.4% of patients with a preoperative deficit. There were 5 (9.8%) postoperative CSF leaks, of which 4 were managed with lumbar drains and 1 required a reoperation.
CONCLUSIONS
The EEA is a safe and effective approach to SSMs, with favorable visual outcomes in well-selected cases. The combination of postoperative MRI-based EOR with direct endoscopic inspection can be used in lieu of Simpson grade to predict recurrence. GTR dramatically reduces recurrence and can be achieved regardless of tumor size, proximity or encasement of the anterior cerebral artery, or medial optic canal invasion. Risk factors for incomplete resection include prior surgery, tumor lateral to the optic nerve, and complete ICA encasement.
Keywords: meningioma, suprasellar, tuberculum, planum sphenoidale, endoscopic endonasal approach, transsphenoidal, skull base
Suprasellar meningiomas (SSMs), arising from the tuberculum sellae (TS) or planum sphenoidale (PS), often present with visual impairment. The goals of surgery are vision preservation or improvement with maximal safe resection, ideally leading to cure or long-term tumor control. SSMs sit adjacent to the optic nerves and chiasm, internal carotid artery (ICA) and anterior cerebral artery (ACA), and pituitary gland, making them technically challenging to remove. A variety of transcranial approaches (TCAs) are effective and were long the standard of care.1-5 However, endoscopic endonasal approaches (EEAs) offer several potential advantages including direct access to the tumor’s meningeal origin, adjacent invaded bone, and blood supply, as well as visualization of the medial optic canal, medial paraclinoid ICA, and diaphragma sellae, with minimal retraction of the frontal lobes and optic apparatus. With experience and technical advances, EEAs can achieve comparable rates of gross-total resection (GTR) with better postoperative visual outcomes compared to TCAs.6-11 Using closure techniques such as the nasoseptal flap and button or gasket seal, rates of postoperative CSF leakage have been significantly reduced compared with early reports.12-14
Although EEA has gained wider acceptance, the criteria for selecting EEA and its long-term results are less well understood.15-18 A recent systematic review and consensus statement suggested that an EEA is likely to be the preferred surgical approach for small- to medium-sized TS meningiomas with inferomedial optic canal invasion.6 Several series have identified criteria that may prevent GTR of SSMs including tumor size,19,20 vascular encasement,19-22 optic canal invasion,20 and short or limited operative experience.23 Other exclusively EEA series have suggested that optic canal invasion19,21 and tumor size21 may not predict incomplete resection. The recently published University of California, San Francisco (UCSF) grading scale by Magill et al.20 is the most comprehensive to date but was developed from a combined series of EEA and TCA surgeries and does not consider several factors and scoring cutoffs that may be important to the EEA specifically.
In order to identify the most critical factors for EEA case selection, we applied the UCSF scale and added several additional criteria that may impact extent of resection (EOR) to our large series of exclusively EEA cases. We could therefore establish which criteria were most predictive of incomplete resection and create a grading scale specific to the EEA approach. We then assessed the relationship between GTR and tumor recurrence or progression to investigate the efficacy of the EEA at tumor control with longer follow-up than previously reported.
Methods
Study Design, Setting, and Participants
This is a retrospective cohort study based on a prospectively collected consecutive database of all endonasal endoscopic surgeries done by the senior author (T.H.S.) at Weill Cornell Medicine. Patients were included if they were ≥ 18 years old and had undergone EEA resection of an SSM (those arising from the TS and PS, in accordance with a recent consensus statement6) between January 2008 and June 2019. We have previously described a classification and management algorithm for anterior skull base tumors that has been in place at our institution since 2008.24 Tumors with > 50% of their rostrocaudal diameter in front of the anterior wall of the sphenoid sinus were managed as olfactory groove meningiomas, generally through an eyebrow incision.24 Meningiomas arising from, or predominantly limited to, the cavernous sinus or anterior or posterior clinoids were also excluded. The surgeon noted prospectively at the time of surgery if the goal was GTR or subtotal resection (STR) and, after surgery, if a small fragment of tumor adherent to a critical neurovascular structure was left behind. These data were used to downgrade radiographic GTRs to near-total resections (NTRs) as discussed below.
The study was approved by the Weill Cornell Medicine Institutional Review Board, and the manuscript follows STROBE guidelines.25
Surgical Technique
Details of the EEA for SSMs have been described in detail elsewhere.23,26 In brief, the superior turbinate on the left side is generally removed to make room for the endoscope. In some circumstances, the inferior or posterior aspect of the middle turbinate is also removed if required to allow adequate instrument maneuverability. The rostrum of the sphenoid sinus is drilled flat to enlarge the working space and allow the nasoseptal flap to cover the defect while minimizing dead space. The posterior ethmoids are opened to provide sufficient lateral exposure. The bone opening is expanded to include the medial opticocarotid recesses and medial optic canals. The PS bone is removed to the most anterior extent of the tumor.
Tumors are internally decompressed using suction or a radiofrequency monopolar ball electrode (Elliquence). The anterosuperior margin of the tumor is dissected free above the planum, where there is the least chance of encountering a cortical blood vessel. Extracapsular dissection is then performed over the top of the tumor—sometimes a 30° endoscope is needed—back to the optic chiasm and then laterally to remove tumor margins from the optic nerves and medial optic canals. The superior intercavernous sinus is cauterized and cut to define the inferior extent of the tumor, and the stalk is identified. Finally, the posterior and inferior midline aspects of the tumor are removed off the stalk and mammillary bodies, typically by using a 0° scope. Any intrasellar component is resected with careful attention to maintaining a plane between tumor and normal pituitary gland.
Closure was performed according to our previously published protocol.14 All intradural nonsellar defects with high-how CSF leaks are preferentially closed first with an inlay of Duraform (Natus Medical), followed by the gasket-seal closure when feasible. The gasket seal involves an onlay of fascia lata (or more recently AlloMax acellular dermal matrix; Davol) held in place with MEDPOR (Porex).12,27 In cases in which there was an inadequate rim of bone or lack of a single plane in which to wedge the MEDPOR for a gasket seal, a button approach was used in which two layers of fascia lata or AlloMax were sutured together in the middle for an inlay-onlay composite.28 The gasket seal or button was then covered with a pedicled, vascularized nasoseptal flap,29 which is held in place with dural sealant. In most cases, a lumbar drain is placed at the beginning of the operation and used to introduce intrathecal fluorescein30 and for intermittent drainage for approximately 24 hours after surgery.
Outcomes
The primary outcome was EOR. Tumor volume was estimated on pre- and postoperative MRI (0.523*A*B*C) and radiographic EOR classified as GTR (100%), NTR (95%–99%), or STR (< 95%) by a board-certified neuroradiologist. We did not use the Simpson grading system for several reasons. First, recent literature has questioned the relevance of a distinction between grades 1, 2, and 3, particularly with respect to tumors located in the skull base.31-33 The original Simpson grading system was developed in 1957 and seems less relevant in the modern era of MRI scans, microsurgery, and stereotactic radiosurgery (sometimes administered to residual tumor before it has a chance to recur). Moreover, it is unclear if one can ever determine the actual Simpson grade of a meningioma removed endonasally. Although the bone and dural attachment are always removed, rendering most tumors in theory grade 1, the lack of adequate lateral visualization along the skull base around the peripheral attachment of the dura mater raises the possibility of residual tumor tail that cannot be adequately inspected. Hence, any Simpson grading following an EEA is unreliable.
Our hypothesis is that EEA in appropriately chosen and performed cases will result in the equivalence of a Simpson grade 1, which will be reflected in appropriately low recurrence rates for patients in whom GTR is achieved with adequate postoperative follow-up. Moreover, given that microscopic inspection of the tumor bed can sometimes reveal small fragments of tumor that the surgeon believes cannot be safely resected because these fragments are adherent to either the optic nerves or carotid artery, which are below the resolution of an MRI scan, some patients with a radiographic GTR need their EOR to be classified as NTR (99% removal).
Tumor progression (after NTR or STR) and recurrence (after GTR) were determined by neuroradiology interpretation of follow-up MRI showing > 25% increase in volume. Vision was classified as improved, stable, or worsened. Formal visual helds were not available on enough patients to include them in this study. Only patients with documented preoperative vision deficits were considered to have potential for improvement. Additional outcomes were CSF leak, new postoperative pituitary dysfunction, return to operating room, and other complications.
Preoperative Clinical Variables
Demographic and clinical characteristics included age, sex, obesity (BMI ≥ 30 kg/m2), prior surgery, preoperative vision impairment, and WHO pathological tumor grade.
Preoperative Radiographic Scoring
We first applied the UCSF scoring system, a 3-part score based on tumor size, optic canal invasion, and arterial encasement.20 The tumor size (diameter) score assigned 0 points for tumors ≤ 17 mm and 1 point for those > 17 mm. The canal invasion score assigned 0 points for no canal invasion, 1 point for unilateral invasion, and 2 points for bilateral invasion. Finally, the artery score assigned 0 points if the tumor does not touch, or simply abuts, the medial wall of the ICA or the anterior wall of the ACA, 1 point if < 180° around the artery, and 2 points if ≥ 180° but not completely around the artery.
We then tested additional radiographic variables that we hypothesized may be more specifically relevant to the EEA derived from the literature and our clinical experience. Based on our experience that ACA encasement is easier to manage than ICA encasement, we separately assigned an ACA and an ICA encasement score and added a possible score of 3 for complete encasement (360° encasement confirmed in > 1 MRI plane).
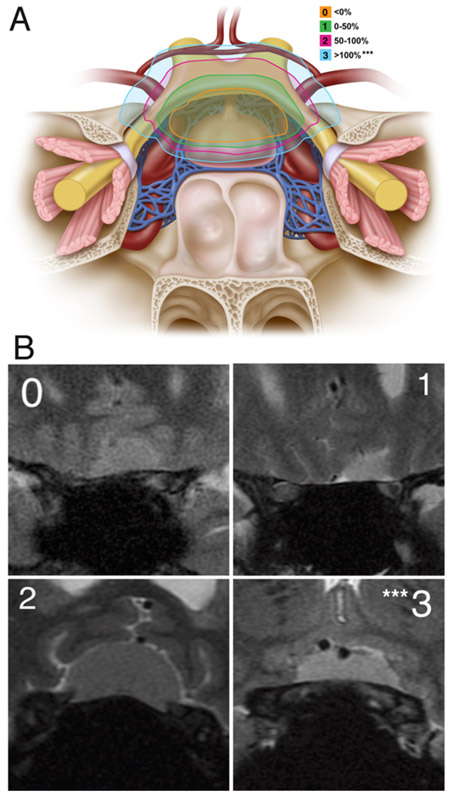
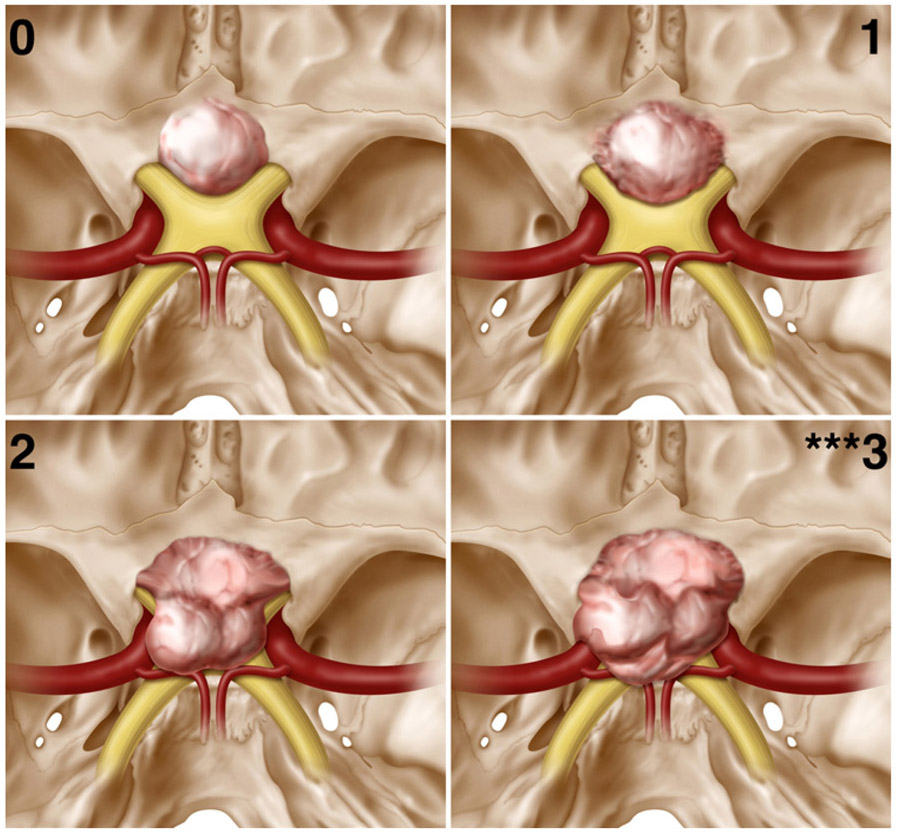
We then created a new “optic nerve laterality score” based on the maximal lateral extension of the tumor on the anterior skull base relative to the optic nerve on either side (Figs. 1 and 2). We assigned 0 points if the tumor was medial to the nerve, 1 point if it extended laterally < 50% over the nerve, 2 points if it extended ≥ 50% but < 100% laterally over the nerve, and 3 points if it extended past the lateral margin (≥ 100%) of the nerve. This score was assigned regardless of whether the tumor was superior or inferior to the nerve in its extension within or over the canal.
FIG. 1.
Optic nerve laterality score. A: The optic nerve laterality score was determined based on the maximal lateral extension of the tumor on the anterior skull base relative to the optic nerve on either side. We assigned a score of 0 if the maximal lateral extension on either side was medial to the optic nerve; 1 if lateral < 50%; 2 if lateral ≥ 50% but < 100%; and 3 if it was completely (≥ 100%) lateral to the optic nerve. B: The optic nerve laterality score was measured on coronal MRI. The coronal image demonstrates lateral extension above the optic nerve at the bony edge of the optic canal on the anterior skull base. ***Optic nerve laterality score of 3 was associated with a significantly increased risk of not achieving GTR. Panel A: Copyright Matthew Holt. Published with permission.
FIG. 2.
Optic nerve laterality scores (superior view). An intracranial, superior-to-inferior view of SSMs in each category of the optic nerve laterality score. Scores were assigned as described in Fig. 1. ***Optic nerve laterality score of 3 was associated with a significantly increased risk of not achieving GTR. Copyright Matthew Holt. Published with permission.
We also scored anterior extension based on the maximal extension of the tumor on the skull base in the sagittal plane anterior to the limbus sphenoid, as well as maximal tumor diameter in any plane. Two authors (B.E.Y. and M.M.G.) independently rated preoperative images for the above criteria and the senior author (T.H.S.) arbitrated any disputes.
Statistical Analysis
All patients, including those in whom STR was anticipated, were included in an analysis to identify predictors of incomplete resection. Although we did not anticipate GTR in all cases, the goal was always maximal resection of as much tumor as the approach would allow, so residual tumor in these cases is informative of our scale. Continuous variables are expressed as the mean ± standard deviation. Univariate analyses were performed with Fisher’s exact test for categorical data and Mann-Whitney U-test for continuous data. For multilevel scores, each increment was tested by univariate analysis. Significant factors in multivariable analysis were assigned 1 point each in the resectability score. Kaplan-Meier survival analysis was performed to assess the relationship between EOR and tumor recurrence or progression (IBM SPSS Statistics, version 25; IBM Corp.).
Results
Extent of Resection
Fifty-one patients who underwent EEA for SSMs were identified and included in the analysis. Radiographic GTR was achieved in 40 (78%) of 51 total patients and in 40 (85%) of 47 patients in whom it was the surgical goal (Table 1). Six patients had a radiographic NTR, and 3 patients with radiographic GTR were reclassified as NTR based on intraoperative microscopic residual, for a total of 9/51 (17.6%) NTRs (mean EOR 97.2% ± 1.9%, range 95%–99%). There were 4 STRs anticipated preoperatively. The primary surgical goal was maximal resection as well as decompression of the chiasm or separation of tumor from the optic apparatus and/or pituitary gland in preparation for radiation in the event of progression. No patients received immediate postoperative radiation or craniotomy. All were followed for progression. There was 1 unanticipated STR for a total of 5/51 (9.8%) STRs (mean EOR 77.4% ± 16.8%, range 49%–94%). The most common locations where residual tumors could be identified were either in the supraclinoid region lateral to the optic nerve on the PS (n = 7) or in the cavernous sinus with complete encasement of the carotid artery (n = 3).
TABLE 1.
Outcomes in 51 patients with SSMs
| Outcome | Value (%) |
|---|---|
| Radiographic EOR* | |
| GTR (100%) | 40/47 (85.1) |
| NTR (95–99%) | 6/47 (12.8) |
| STR (<95%) | 1/47 (2.1) |
| Progression or recurrence | 9/51 (17.6) |
| Recurrence after GTR | 1/37 (2.7) |
| Progression after NTR | 4/9 (44.4)† |
| Progression after STR | 4/5 (80.0)‡ |
| Management | |
| Radiation | 4 |
| Craniotomy | 3 |
| Monitoring | 2 |
| Median follow-up in mos, range | 35.0, 0–132 |
| Postop visual outcome | |
| Improved (preop deficit) | 31/46 (67.4)§ |
| Stable (preop deficit) | 12/46 (26.1)§ |
| Worse (all pts) | 3/51 (5.9) |
| Adverse events | |
| CSF leak | 5 (9.8) |
| Infection | 6 (11.8) |
| Long-term pituitary dysfunction¶ | |
| Anterior | 2/49 (4.1) |
| Posterior | 0/49 (0) |
| Vascular injury | 1 (2) |
pts = patients.
Radiographic EOR among patients in whom GTR was the surgical goal.
For purposes of the analysis identifying predictors of EOR, NTR includes 3 cases in which there was a radiographic GTR but the surgeon prospectively noted a small intraoperative residual (6 + 3 = 9).
For purposes of the analysis identifying predictors of EOR, STR includes 4 patients in whom GTR was not the surgical goal (1 + 4 = 5).
Among patients with a preoperative visual deficit and postoperative follow-up.
Two patients had preoperative pituitary dysfunction in multiple axes, one of whom experienced improvement postoperatively.
Significant risk factors for not achieving GTR in univariate analysis are presented in Table 2. These include prior surgery (OR 13.13, 95% CI 2.22–77.48, p = 0.003), tumor completely lateral to the optic nerve (laterality score of 3; OR 12.91, 95% CI 3.02–55.18, p < 0.0001), unilateral or bilateral canal invasion (UCSF canal score of 1 or 2 vs 0; OR 4.86, 95% CI 1.31–18.05, p = 0.017), and complete encasement of the ICA (Weill Cornell modified ICA score of 3; OR 14.4, 95% CI 1.44–143.71, p = 0.017). Many of these variables coexisted and it should be noted that of the 8 patients who had only canal invasion, all achieved a GTR.
TABLE 2.
Univariate analysis of risk factors for incomplete resection (STR or NTR—i.e., non-GTR) and calculation of the Weill Cornell SSM resectability score
| Variable | No. of Pts (%)* | Mean % EOR (± SD) | No. w/ GTR†‡ | OR (non-GTR)‡ | 95% CI | p Value§ |
|---|---|---|---|---|---|---|
| Total pts | 51 | |||||
| Clinical variables | ||||||
| Age >60 yrs | 22 (43.1) | 96.7 ± 7.4 | 13 (59.1%) | 3.32 | 0.92–12.01 | 0.06 |
| Sex, female | 36 (70.6) | 98.4 ± 5 | 27 (75.0%) | 1.5 | 0.40–5.57 | 0.389 |
| Obesity | 21 (41.2) | 96.8 ± 7.6 | 14 (66.7%) | 1.64 | 0.48–5.68 | 0.318 |
| Prior surgery | 8 (15.7) | 93.7 ± 9.7 | 2 (25.0%) | 13.13 | 2.22–77.48 | 0.003 |
| WHO pathological tumor grade | ||||||
| Grade I | 49 (96) | 97.3 ± 8.8 | 36 (73.5%) | 0.36 | 0.02–6.2 | 0.478 |
| Grade II or III | 2 (4.0) | 97.2 ± 4 | 1 (50%) | Ref | ||
| Peritumoral edema | 3 (5.9) | 99.6 ± 0.7 | 2 (66.7%) | 1.34 | 0.11–16.13 | 0.627 |
| Preop vision impairment | 47 (92.2) | 97.1 ± 8.6 | 33 (70.2%) | NA¶ | NA¶ | NA¶ |
| Radiographic variables | ||||||
| Max tumor diam >2 cm | 21 (41.2) | 95.9 ± 11.8 | 14 (66.7%) | 1.9 | 0.5–7.2 | 0.265 |
| Anterior extension >10 mm | 18 (35.3) | 97.8 ± 7 | 12 (66.7%) | 1.56 | 0.44–5.52 | 0.352 |
| UCSF tumor diam score | ||||||
| 1, <17 mm | 22 (43.1) | 97.7 ± 6.4 | 16 (72.7%) | Ref | ||
| 2, ≥17 mm | 29 (56.9) | 97 ± 10 | 21 (72.4%) | 1.02 | 0.29–3.51 | 0.617 |
| UCSF canal invasion score | ||||||
| 0, none | 32 (62.7) | 97.4 ± 9.7 | 27 (84.4%) | Ref | ||
| 1, unilat | 10 (19.6) | 95.4 ± 9 | 4 (40%) | 6.1 | 1.4–27.2 | 0.018 |
| 2, bilat | 9 (17.6) | 99.1 ± 1.7 | 6 (66.7%) | 1.41 | 0.3–6.62 | 0.692 |
| 1 or 2, any canal invasion | 19 (37.2) | 97.3 ± 8.6 | 10 (52.6%) | 4.86 | 1.31–18.05 | 0.017 |
| WC ICA encasement score | ||||||
| 0, none | 9 (17.6) | 99.6 ± 1.3 | 8 (88.9%) | Ref | ||
| 1, <50% | 17 (33.3) | 98 ± 7.1 | 13 (76.5%) | 0.73 | 0.19–2.82 | 0.749 |
| 2, ≥50% | 20 (39.2) | 99.2 ± 1.8 | 15 (75%) | 0.82 | 0.23–2.91 | 1 |
| 3, complete–100% | 5 (9.8) | 83.5 ± 20.9 | 1 (20.0%) | 14.4 | 1.44–143.71 | 0.017 |
| WC ACA score | ||||||
| 0, none | 27 (52.9) | 96.4 ± 10.4 | 19 (70.4%) | Ref | ||
| 1, <50% | 13 (25.5) | 99.6 ± 1.4 | 12 (92.3%) | 0.16 | 0.02–1.37 | 0.082 |
| 2, ≥50% | 6 (11.8) | 94.8 ± 12 | 4 (66.7%) | 1.38 | 0.22–8.49 | 0.661 |
| 3, complete—100% | 5 (9.8) | 99.3 ± 0.9 | 2 (40.0%) | 4.77 | 0.71–32.33 | 0.12 |
| WC optic nerve laterality score | ||||||
| 0, medial | 7 (13.7) | 99.4 ± 1.5 | 6 (85.7%) | Ref | ||
| 1, <50% | 12 (23.5) | 99.5 ± 1.6 | 11 (91.7%) | 0.18 | 0.02–1.56 | 0.142 |
| 2, 50–99% | 16 (31.4) | 99.9 ± 0.3 | 14 (87.5%) | 0.27 | 0.05–1.41 | 0.176 |
| 3, ≥100% | 16 (31.4) | 92.2 ± 14.3 | 6 (37.5%) | 12.91 | 3.02–55.18 | <0.0001 |
| Continuous variables | Total | GTR | Non-GTR | p Value** | ||
| Age in yrs, mean ± SD | 57 (range 21–84) | 53.85 ± 14.84 | 62.02 ± 18.59 | 0.069 | ||
| BMI in kg/m2, mean ± SD | 29.3 ± 7.1 | 28.95 ± 7.53 | 30.23 ± 5.88 | 0.416 | ||
| Tumor diam in mm, mean ± SD | 22.5 ± 7.6 | 21.8 ± 6.7 | 24.4 ± 9.7 | 0.216 |
Diam = diameter; max = maximal; NA = not applicable; Ref = reference value; WC = Weill Cornell.
Percentages calculated by column.
Percentages calculated by row.
For purposes of analysis, GTR refers to those patients who received a radiographic and surgical GTR. Patients with a radiographic STR (<95%) or NTR (95% –99%) and/or those in whom the surgeon prospectively noted leaving a small amount of residual were classified as non-GTR. The OR is for non-GTR relative to GTR.
Fisher’s exact test; boldface type indicates significance (p < 0.05).
All patients with non-GTR had vision impairment.
Kruskal-Wallis test.
In multivariable analysis (Table 3), significant independent risk factors were prior surgery (OR 25.94, 95% CI < 2.00 to 336.49, p = 0.013), tumor completely lateral to the optic nerve (i.e., optic laterality score of 3; OR 13.41, 95% CI 1.82–98.99, p = 0.011), and complete ICA encasement (i.e., ICA encasement score of 3; OR 15.12, 95% CI 1.17–194.08, p = 0.037). Each significant variable was assigned 1 point in the new resectability score for a maximum possible score of 3. Canal invasion approached but did not reach statistical significance (OR 6.41, 95% CI 0.87–47.23, p = 0.068). Figure 3 demonstrates the numbers of GTR and incomplete resection at each level of the score and the ability of the score to predict EOR with high confidence. A score of 0 had a positive predictive value of 97% for GTR, whereas a score of 2 had a negative predictive value of 87.5%.
TABLE 3.
Multivariable analysis and resectability score in patients with SSMs
| Variable | OR* | 95% CI |
p Value† |
Score Points |
|---|---|---|---|---|
| Prior surgery | 25.94 | 2–336.49 | 0.013 | 1 |
| Unilat or bilat canal invasion | ||||
| UCSF canal invasion score 1, 2 (vs 0) | 6.41 | 0.87–47.23 | 0.068 | 0 |
| Complete ICA encasement | ||||
| WC ICA encasement score 3 (vs 0, 1, 2) | 15.12 | 1.17–194.08 | 0.037 | 1 |
| Extension >100% lateral to optic nerve | ||||
| WC optic nerve laterality score 3 (vs 0, 1, 2) | 13.41 | 1.82–98.99 | 0.011 | 1 |
The OR is for non-GTR relative to GTR.
Multivariable logistic regression; boldface type indicates significance (p < 0.05).
FIG. 3.
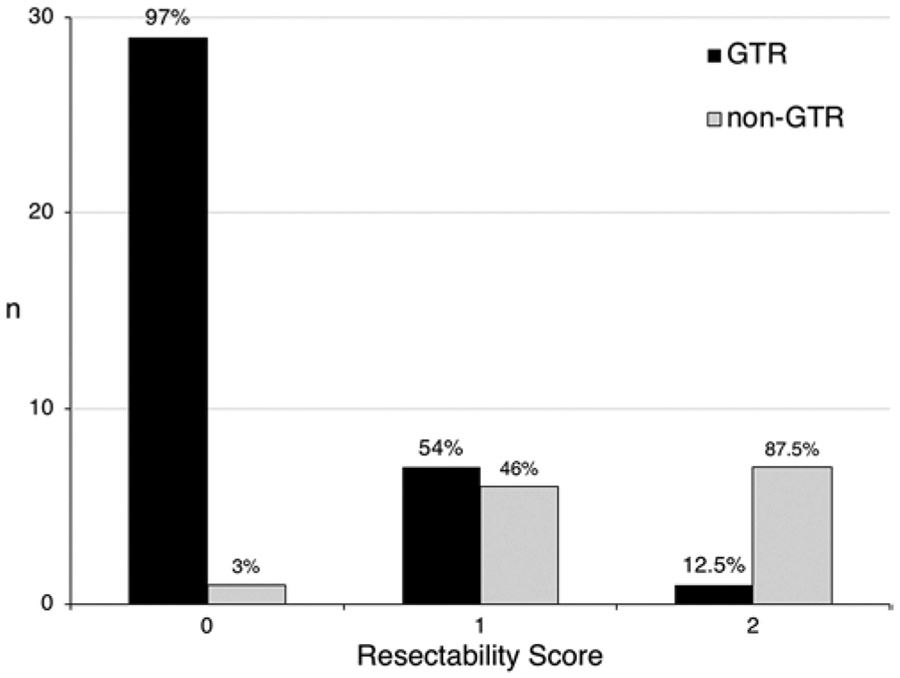
EOR by the Weill Cornell resectability score. The number of patients in whom GTR and non-GTR was achieved is stratified by the Weill Cornell resectability score. A score of 0 had a positive predictive value of 97% for GTR, whereas a score of 2 had a negative predictive value of 87.5%.
Recurrence and Progression
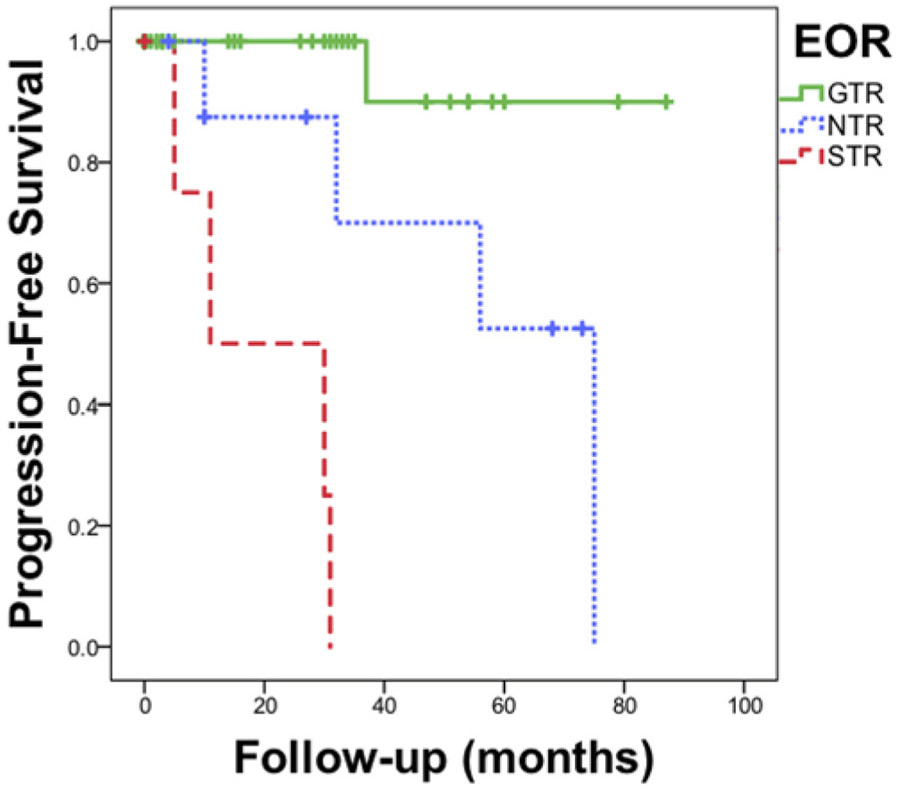
After a mean follow-up of 40.6 ± 32.4 months, recurrence was 2.7% after GTR, 44.4% after NTR, and 80% after STR. GTR was strongly associated with a lower rate of recurrence or progression (HR 0.057, 95% CI 0.01–0.46, p < 0.0001) (Fig. 4). The one recurrence after GTR occurred 38 months after surgery, and was located just below the optic nerve, where there was no canal invasion on preoperative MRI. The patient underwent a supraorbital craniotomy for GTR without complication. Pathological investigation revealed that the tumor had transformed into an atypical meningioma (grade 2). Four of 9 patients with NTR had progression at the site of the residual; 2 were managed with craniotomy and 2 with fractionated external-beam radiation therapy. Four of 5 patients with STR had progression; 2 were managed with fractionated external-beam radiation therapy and 2 continued to be monitored without deficit.
FIG. 4.
Progression-free survival by EOR. Progression-free survival after radiographic and surgical GTR (100%), NTR (95%–99% radiographic resection and/or small residual tumor noted intraoperatively), and STR (< 95%). Hash marks represent censored data due to the end of the follow-up period for each patient. Log-rank test, p < 0.0001. Figure is available in color online only.
No patients received upfront adjuvant treatment. In all cases with residual tumor, decompression of critical structures was achieved and patients were monitored for progression with serial imaging. Recurrence and progression were managed on an individualized basis considering factors such as location, symptoms, age and comorbidity, and prior treatments and recurrence preceding EEA.
Visual Outcomes
Of 46 patients with preoperative visual deficits and postoperative follow-up, vision was stable or improved in 43 (93.5%) and improved in 31 (67.4%). Four patients did not have preoperative deficits and 1 international patient was excluded due to early loss to follow-up after discharge. Of the 51 patients in the series, 3 (5.9%) had postoperative declines. Univariate and multivariable analysis found no independent risk factors for lack of vision improvement.
Complications
Five patients had postoperative CSF leaks (9.8%), which occurred between postoperative days 2 and 12; 4 cases were successfully managed with additional lumbar drainage and antibiotics. One patient, who was too obese for upfront spinal drain placement, experienced a CSF leak on postoperative day 4 and was taken back to the operating room for cutdown lumbar drain placement. His leak continued on postoperative day 5 and he was taken back to the operating room to replace the gasket seal, which appeared to have dislodged during an episode of vomiting.
Three other patients returned to the operating room in the immediate postoperative period for visual loss; two improved with repositioning of the graft and the third improved with hypervolemic, hypertensive therapy.
Two patients (4%) had long-term anterior pituitary dysfunction (1 adrenocorticotropic hormone [ACTH] and 1 ACTH and thyroid). There were no cases of permanent diabetes insipidus. There was 1 vascular injury (2%) to the A1 segment of the ACA and 1 delayed subdural hematoma, which was drained 47 days after surgery. Postoperatively, lumbar drains were removed after a median of 2 days (IQR 1–3 days).
Discussion
In this paper we present a simple grading scheme for determining resectability of PS and TS meningiomas via an EEA. The most critical factors are the extent of the tumor lateral to the optic nerve, complete encasement of the ICA, and prior surgery. The presence of two of these risk factors portends an 87.5% chance of incomplete resection, whereas GTR was achieved in 97% of patients with none of these specific factors. By comparison, the UCSF cumulative score includes variables that were not significantly associated with incomplete resection and does not offer a simple way of predicting the likelihood of GTR. Contrary to what has been previously published, the absolute size of the tumor, medial optic canal involvement, ACA or partial ICA encasement, and brain edema were not predictive of resectability. Moreover, we demonstrate that for these meningiomas, recurrence rates can be projected based on the combination of radiographic EOR and microscopic inspection. Because Simpson grading is difficult to apply to cases treated with an EEA, given the inability to accurately assess the extent of the possible lateral or anterior margins of the dural tail from below, the combination of radiographic GTR and absence of microscopic residual can serve as a reasonable substitute for a Simpson grade 1, particularly because the EEA by design removes the involved bone and dura during the approach.
Rationale for the EEA for PS and TS Meningiomas
Overall outcomes in our series were favorable and further support the use of EEA to manage PS and TS meningiomas. For cases in which GTR was the surgical goal, we report an 85% (40/47) radiographic GTR and a 79% (37/47) microscopic rate of GTR, which compares favorably with the published EEA rates, ranging from 50% to 90% (Table 4).6-11 More importantly, the GTR rates are competitive with TCAs, which have been shown to achieve GTR in 84.1% (range 40%–90%) of cases.48,49 The real advantage of EEAs over TCAs is the visual outcome (Table 4), which has been demonstrated in numerous systematic literature reviews,6-11 and our data support this concept. We report long-term visual deterioration rates of only 6%, which also compare favorably with TCAs that result in visual deterioration in 14.2%.49
TABLE 4.
Literature review of the EEA for SSMs
| Authors & Year | No. of Pts |
Mean Tumor Vol (cm3) |
Mean Max Diam (cm) |
EOR | Visual Outcome |
CSF Leak |
Postop Endocrine Abnormality |
Other Complications |
Recurrence After GTR; Progression After NTR or STR |
Follow-Up Period (mos) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kitano et al., 200734 | 16 | 7.5 | NA | GTR: NA (mean EOR 98.5%) | (+): 13/16 (81%); (−): 6/16 (38%)* | 2/16 (13%) | 0 (0%) | 2 (13%) asymptomatic perforating artery infarct | NA | ≥3 |
| de Divitiis et al., 200835 | 7 | NA | NA | GTR: 6/7 (85.7%) | (+): 5/7 (71%); (−): 0/7 (0%) | 2/7 (29%) | 1 (14%) permanent DI | 1 (14%) IVH, death | NA | Range 3 wks–20 mos |
| Fatemi et al., 200936 | 14 | NA | 2.5 | GTR: 7/14 (50%); NTR: 3/14 (21%); STR: 4/14 (29%) | (+): 9/11 (82%); (−): 1/14 (7%) | 4/14 (29%) | 5 (36%); 1 new permanent adrenal insufficiency, 2 delayed hyponatremia, 2 transient DI | 2 (14%) revision for excessively large fat graft; 2 (14%) IMA embolization for delayed epistaxis | R: 0/7 (0%); P: 1/7 (14%) | Mean 32.3; median 27.5; range 6–65 |
| Wang et al., 201037 | 12 | 7.7 | 3.1 | GTR: 11/12 (92%) | (+): 11/12 (92%); (−): 0/12 (0%) | 1/12 (8%) | 1 (8%) transient DI | NR | NA | Mean 25.2; median 28; range 6–60 |
| Van Gompel et al., 201138 | 13 | NA | 2.4 | GTR: 7/13 (54%) | (+): 8/12 (67%); (−): 0/12 (0%) | 0/13 (0%) | NA | 1 (8%) ACA injury stroke, RLE hemiparesis | NA | Mean 13; median 8; range 0–65 |
| Bohman et al., 201239 | 5 | NA | 2.0 | GTR: 4/5 (80%) | (+): 4/5 (80%); (−): 0/5 (0%) | 1/5 (20%) | 2 (40%) transient DI | NR | 0 | Mean 7.8; median 6.9; range 2.2–17.0 |
| Ogawa & Tominaga, 201240 | 19 | 2.2 | 0.4 | GTR: 15/19 (79%) | (+): 14/19 (74%); (−): 2/19 (11%) | 1/19 (5%) | 0 (0%) | NR | R: 1/15 (6.7%) | Mean 35.8; range 6–59 |
| Chowdhury et al., 201241 | 6 | NA | 3.5 | GTR: 5/6 (83%) | (+): 2/6 (33%); (−): 1/6 (17%) | 1/6 (17%) | 2 (33%) transient hyponatremia | NR | R: 0/5 (0%); P: 0/1 (0%) | Mean 7; median 7; range 2–12 |
| Gadgil et al., 201342 | 5 | 6.3 | 2.0 | GTR: 4/5 (80%) | (+): 2/2 (100%); (−): 0/5 (0%) | 1/5 (20%), meningitis | 3/5 (60%) transient DI | NR | R: 0/4 (0%); P: NA | Mean 15; range 3–27 |
| Ottenhausen et al., 201423 | 20 | 12.0 | NA | GTR: 16/20 (80%) | (+): 14/17 (82%); (−): 0/20 (0%) | 2/20 (10%); 0/12 (0%) post-NSF | 2/20 (10%) transient DI | 1 (5%) death | R: 0/16 (0%); P: 2/4 (50%) | Mean 51.5; range 5–96 |
| Khan et al., 201443 | 17 | 5.1 | 2.3 | GTR: 11/17 (65%) | (+): 9/14 (65%); (−): 0/14 (0%) | 2/17 (12%) | 3/17 (18%); 1 permanent hypopituitarism, 2 transient DI | NR | R: 0/11 (0%); P: 1/6 (16.7%) | Mean 9.4; median 6; range 3–26 |
| Koutourousiou et al., 201419 | 75 | NA | 2.3 | GTR: 57/75 (76%) | (+): 48/56 (86%); (−): 2/75 (3%) | 19/75 (25%); 10/62 (16%) post-NSF | 5/75 (6.5%); 1 permanent DI, 4 SIADH | 1 (1%) infarct (Heubner) | R: 1/57 (1.8%); P: 3/18 (16.7%) | Mean 29; median 19; range 1–98 |
| Ceylan et al., 201544 | 23 | NA | 2.5 | GTR: 17/23 (74%) | (+): 14/20 (70%); (−): 0/23 (0%) | 2/23 (9%) | 2/23 (9%); 1 permanent DI, 1 transient DI | NR | NA | NA; range 2–70 |
| Hayhurst et al., 201645 | 9 | NA | 2.5 | GTR: 6/9 (67%); NTR: 1/9 (11%); STR: 2/9 (22%) | (+): 0/9 (0%); (−): 0/9 (0%) | 0/9 (0%) | NA | 1 (11%) infarct (ACA It frontal lobe) | NA | NA |
| Linsler et al., 201722 | 6 | 2.1 ±0.8 | NA | GTR: 5/6 (83%) | (+): 2/6 (33%); (−): 1/6 (17%) | 0/6 (0%) | 0/6 (0%) | NR | R: 1/5 (20%); P: 0/1 (0%) | Mean 15.3; range 3–60 |
| Bernat et al., 201846 | 20† | NA | NA | GTR: 15/20 (75%) | (+): NA; (−): NA | NA | NA | NA | R: 1/15 (6.7%); P: NA | Mean 38 |
| Song et al., 201847 | 44 | 5.8 ±3.4 | 2.5 | GTR: 37/44 (84%) | (+): 43/44 (98%); (−): 1/44 (2%) | 1/44 (2%) | 3/44 (7%) transient DI | NR | R: 5/37 (14%); P: 1/7 (14%) | Median 27; range 0–54 |
| Elshazly et al., 201821 | 25 | 5.3 | NA | GTR 19/25 (76%); NTR: 3/25 (12%); STR: 3/25 (12%) | (+): 15/17 (88%); (−): 4/16 (25%) | 2/25 (8%) | 3/25 (12%) transient DI | 1 (4%) SAH, hydrocephalus, EVD | R: 0/19 (0%); P: 1/6 (17%) | Mean 21; range 1–53 |
| Magill et al., 201820 | 44 | NA | NA | NA | NA | NA | NA | NA | NA | Mean 45.7; median 29; range 0–174 |
| Present study | 51 | 4.9 ± 4.4 | 2.3 ± 0.8 | Radiographic—GTR: 40/47 (85%); NTR: 6/47 (13%); STR: 1/47 (2%). Microscopic–GTR: 37/47 (79%); NTR: 9/47 (19%); STR: 1/47 (2%) | (+): 31/47 (65.9); (−): 3/51(5.9) | 5/51 (10%) | 2/49 (4%) anterior dysfunction; 3/49 transient DI | 1 vascular injury; 1 delayed SDH | R: 1/37 (2.7%); P: 8/14 (57%) | Mean 40.6; median 35.9; range 0–132 |
DI = diabetes insipidus; EVD = external ventricular drain; IMA = internal maxillary artery; IVH = intraventricular hemorrhage; NA = not available; NR = not reported; NSF = nasoseptal flap; P = progression; R = recurrence; RLE = right lower extremity; SAH = subarachnoid hemorrhage; SDH = subdural hematoma; SIADH = syndrome of inappropriate antidiuretic hormone; + = visual improvement; − = visual deterioration.
Study reported objective visual testing and outcomes as improvement or deterioration in at least 1 eye.
However, the last piece of the puzzle has always been long-term outcome data and the inability for surgeons who perform the EEA to reliably report the Simpson grade of their patients. If recurrence rates are significantly higher following EEA than following transcranial surgery, absent radiation, then the visual outcome advantages may not be justified. Although Simpson grading has always been the gold standard in determining EOR for meningiomas, the scale was developed in 1957 before EOR could be determined with postoperative MRI scans and before microscopic inspection of the tumor bed was possible.50 It is well documented that a surgeon’s estimate of EOR is not as accurate as postoperative MRI scans and it is intuitive that the microscope would be able to visualize tumor below the resolution of the naked eye or even loupes.51 Likewise, after the introduction of stereotactic radiation to the treatment of meningiomas we can infer that a partially resected tumor that responds to radiation may never recur, rendering the EOR irrelevant in this situation and potentially not worth the increased risk that a radical resection might incur. For these reasons, the utility of the Simpson grading at predicting recurrence has been questioned for skull base meningiomas.31-33
In this paper we demonstrate that EEA in well-selected cases, in which a radiographic GTR is achieved with no residual microscopic disease, results in a durable response with a very low rate of recurrence. Thus, the key decision that must be made by the operating surgeon is whether a particular tumor is amenable to EEA and if GTR can be achieved with a high degree of reliability. For this reason, we have developed a grading scale that can be used to choose cases based on preoperative imaging and historical data.
Predictors of STR
In the early days of EEA for meningiomas, several criteria were discussed that might lead to STR including tumor size, brain edema, presence of a “cortical cuff,” and proximity to intracranial vasculature.52 As surgeons have become more adept, none of these factors are still considered absolute contraindications to EEA, and in fact in this paper we show that they are not reliable predictors of STR.
In validating the UCSF score, Magill et al.20 reported that unilateral canal invasion and, to a greater extent, bilateral invasion appear to limit GTR. However, this was based on a combined series of patients who underwent EEA and TCA, so it was not clear which approach was more apt to leave tumor in this location. In our experience, the medial canal is actually easier to decompress via an EEA than through a craniotomy. Our results support that the midline EEA offers equal access to unilateral or bilateral invasion,53 and neither is independently predictive of incomplete resection. Figure 5A and B demonstrates an example of significant bilateral canal invasion in which GTR was achieved. All TS meningiomas probably have some degree of optic canal invasion,54 and other experienced centers have also reported that canal invasion does not limit the EOR.19,21 By comparison, the TCA typically approaches the tumor from one side, limiting visualization of the ipsilateral medial optic canal.6,7,46
FIG. 5.
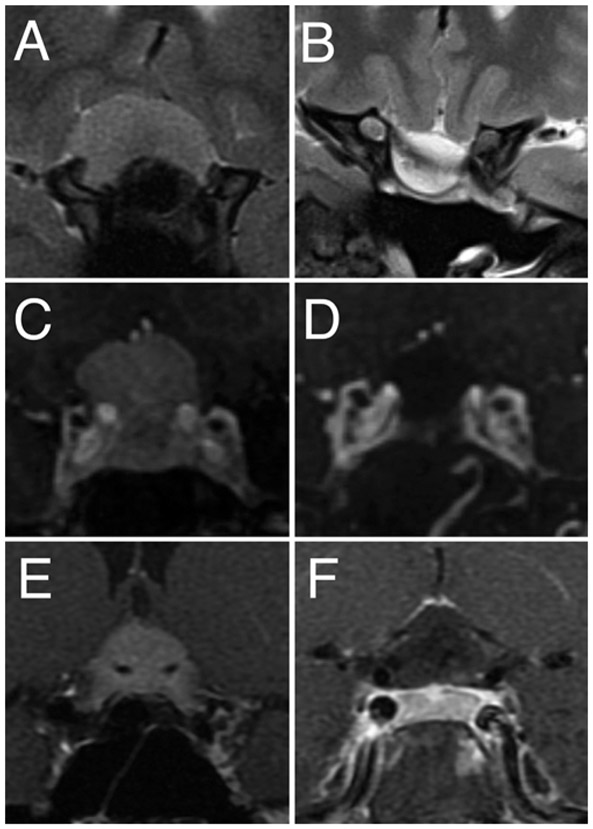
Examples of GTR with bilateral optic canal invasion, incomplete ICA encasement, and complete ACA encasement. A and B: Preoperative coronal T2-weighted MR image (A) demonstrates significant bilateral optical canal invasion and a Weill Cornell optic nerve laterality score of 2, and postoperative T2-weighted MR image (B) reveals GTR. C and D: Preoperative coronal T1-weighted MR image with contrast (C) demonstrates incomplete (> 50% but < 99%) intracavernous ICA encasement (Weill Cornell ICA score of 2), and postoperative T1-weighted MR image with contrast (D) reveals GTR. E and F: Preoperative coronal T1-weighted MR image with contrast (E) demonstrates complete encasement of the bilateral ACAs (Weill Cornell ACA score of 3), and postoperative coronal T1-weighted MR image with contrast (F) demonstrates GTR.
As we show in this manuscript, the relationship between the tumor and the optic nerve is critically important, but only if the tumor extends lateral to the optic nerve, because EEA generally provides limited visualization and access to this area (Fig. 2). Magill et al.20 found that lateral tumor diameter was not associated with EOR, but we found that specifically looking at the relationship to the optic nerve was important with the EEA. Opening the medial optic canal allows for tumor removal within the canal and directly above the nerve, but the optic nerves may limit visualization on the far side of the nerve, just as the medial nerve is difficult to access through a TCA. The anterior clinoid has previously been described as a likely location for residual tumor,46 and we generally prefer TCA for tumors with significant extension over the clinoid.24
The relationship between the tumor and the surrounding vasculature has also been discussed as a potential factor limiting resectability. This idea was first conceived as a requirement for a “cortical cuff” to encircle the tumor as a safety buffer between the tumor and the arteries.55 Other series have suggested that cavernous sinus invasion, partial encasement of the ICA, or lateral extension beyond the ICA limit GTR.20,22,40,46 Koutourousiou et al.19 found vascular encasement (degree and vessel unspecified) to be strongly associated with incomplete resection. We previously demonstrated that the lack of a cortical cuff is not predictive of STR.52 In this study, we further refine the limitation posed by neighboring vasculature and demonstrate that ACA encasement is less important than ICA encasement and that only complete 360° ICA encasement is a true limitation to achieving GTR. The UCSF score does not differentiate between ICA and ACA encasement or partial and complete encasement. The ACA encasement or lesser degrees of ICA involvement captured in the UCSF scale do not preclude complete resection with the EEA (Fig. 5C-F).20 We were able to achieve GTR in the majority of cases involving surrounding vasculature so long as there was not complete encasement of the ICA.
The last predictor, prior surgery, was also independently associated with incomplete resection in our series. Prior surgery, as well as subsequent radiation, can lead to scarring and loss of arachnoid planes, making tumors more difficult to resect. This factor becomes critically important if one is choosing between radiation and surgery for an asymptomatic but enlarging tumor recurrence, because reoperation may be less successful than a first-time operation, which may encourage the use of radiation in these cases. Prior surgery was not included in the UCSF score.
Adverse Events
Our CSF leak rate of 9.8% (5/51) is reasonable for this high-risk population and is in the lower end of the 0%–29% rate reported in the recent literature (Table 4). Of note, 4 of 5 leaks were controlled with lumbar drainage. In the one patient who required reoperation, morbid obesity prevented initial placement of the lumbar drain. Although the use of lumbar drains remains controversial in this population, we continue to advocate their use in these high-risk patients, particularly in light of our own published data as well as randomized studies showing efficacy for patients with high-risk leaks.56,57
Limitations of the Study
As with any single-surgeon retrospective series, there are several limitations to our study. Case selection inherently represents institutional bias.24 There also remains the possibility of confounding by unmeasured variables. Our follow-up, albeit on average longer than in most EEA meningioma series, may be inadequate to reveal the actual rate of 10-year recurrence-free survival for these slow-growing tumors. Longer follow-up in larger series of patients will be required to validate our results. Finally, findings may not be generalizable outside of our center.
Conclusions
The EEA is a safe and effective approach to SSMs and has favorable visual outcomes in well-selected cases. The combination of radiographic GTR with direct endoscopic inspection dramatically reduces the chance of recurrence and can be used in lieu of Simpson grade to predict recurrence. Risk factors for incomplete resection include prior surgery, tumor lateral to the optic nerve, and complete ICA encasement. A simple, 3-item resectability score based on these findings can be used to predict patients in whom GTR can be achieved.
ABBREVIATIONS
- ACA
anterior cerebral artery
- EEA
endoscopic endonasal approach
- EOR
extent of resection
- GTR
gross-total resection
- ICA
internal carotid artery
- NTR
near-total resection
- PS
planum sphenoidale
- SSM
suprasellar meningioma
- STR
subtotal resection
- TCA
transcranial approach
- TS
tuberculum sellae
- UCSF
University of California, San Francisco
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Mathiesen T, Kihlström L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59(3):570–576. [DOI] [PubMed] [Google Scholar]
- 2.Ganna A, Dehdashti AR, Karabatsou K, Gentili F. Frontobasal interhemispheric approach for tuberculum sellae meningiomas; long-term visual outcome. Br J Neurosurg. 2009;23(4):422–430. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud M, Nader R, Al-Mefty O. Optic canal involvement in tuberculum sellae meningiomas: influence on approach, recurrence, and visual recovery. Neurosurgery. 2010;67(3)(Suppl Operative):ons108–ons119. [DOI] [PubMed] [Google Scholar]
- 4.Nanda A, Ambekar S, Javalkar V, Sharma M. Technical nuances in the management of tuberculum sellae and diaphragma sellae meningiomas. Neurosurg Focus. 2013;35(6):E7. [DOI] [PubMed] [Google Scholar]
- 5.Karsy M, Raheja A, Eli I, et al. Clinical outcomes with transcranial resection of the tuberculum sellae meningioma. World Neurosurg. 2017;108:748–755. [DOI] [PubMed] [Google Scholar]
- 6.Wang EW, Gardner PA, Zanation AM. International consensus statement on endoscopic skull-base surgery: executive summary. Int Forum Allergy Rhinol. 2019;9(S3):S127–S144. [DOI] [PubMed] [Google Scholar]
- 7.Graffeo CS, Dietrich AR, Grobelny B, et al. A panoramic view of the skull base: systematic review of open and endoscopic endonasal approaches to four tumors. Pituitary. 2014;17(4):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin M, Kondo K, Saito N. Current status of endoscopic endonasal surgery for skull base meningiomas: review of the literature. Neurol Med Chir (Tokyo). 2015;55(9):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turel MK, Tsermoulas G, Reddy D, et al. Endonasal endoscopic transsphenoidal excision of tuberculum sellae meningiomas: a systematic review. J Neurosurg Sci. 2016;60(4):463–475. [PubMed] [Google Scholar]
- 10.Clark AJ, Jahangiri A, Garcia RM, et al. Endoscopic surgery for tuberculum sellae meningiomas: a systematic review and meta-analysis. Neurosurg Rev. 2013;36(3):349–359. [DOI] [PubMed] [Google Scholar]
- 11.Muskens IS, Briceno V, Ouwehand TL, et al. The endoscopic endonasal approach is not superior to the microscopic transcranial approach for anterior skull base meningiomas—a meta-analysis. Acta Neurochir (Wien). 2018;160(1):59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80(5):563–568. [DOI] [PubMed] [Google Scholar]
- 13.McCoul ED, Anand VK, Singh A, et al. Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg. 2014;81(1):136–143. [DOI] [PubMed] [Google Scholar]
- 14.Patel KS, Komotar RJ, Szentirmai O, et al. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg. 2013;119(3):661–668. [DOI] [PubMed] [Google Scholar]
- 15.Makarenko S, Carreras EM, Akagami R. Craniotomy for perisellar meningiomas: comparison of simple (appropriate for endoscopic approach) versus complex anatomy and surgical outcomes. J Neurosurg. 2017;126(4):1191–1200. [DOI] [PubMed] [Google Scholar]
- 16.Bander ED, Singh H, Ogilvie CB, et al. Endoscopic endonasal versus transcranial approach to tuberculum sellae and planum sphenoidale meningiomas in a similar cohort of patients. J Neurosurg. 2018;128(1):40–48. [DOI] [PubMed] [Google Scholar]
- 17.Mortazavi MM, Brito da Silva H, Ferreira M Jr, et al. Planum sphenoidale and tuberculum sellae meningiomas: operative nuances of a modern surgical technique with outcome and proposal of a new classification system. World Neurosurg. 2016;86:270–286. [DOI] [PubMed] [Google Scholar]
- 18.Kshettry VR, Elshazly K, Evans JJ. Endoscopic transnasal surgery for planum and tuberculum sella meningiomas: decision-making, technique and outcomes. CNS Oncol. 2016;5(4):211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutourousiou M, Fernandez-Miranda JC, Stefko ST, et al. Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients. J Neurosurg. 2014;120(6):1326–1339. [DOI] [PubMed] [Google Scholar]
- 20.Magill ST, Morshed RA, Lucas CG, et al. Tuberculum sellae meningiomas: grading scale to assess surgical outcomes using the transcranial versus transsphenoidal approach. Neurosurg Focus. 2018;44(4):E9. [DOI] [PubMed] [Google Scholar]
- 21.Elshazly K, Kshettry VR, Farrell CJ, et al. Clinical outcome after endoscopic endonasal resection of tuberculum sella meningiomas. Oper Neurosurg (Hagerstown). 2018;14(5):494–502. [DOI] [PubMed] [Google Scholar]
- 22.Linsler S, Fischer G, Skliarenko V, et al. Endoscopic assisted supraorbital keyhole approach or endoscopic endonasal approach in cases of tuberculum sellae meningioma: which surgical route should be favored? World Neurosurg. 2017;104:601–611. [DOI] [PubMed] [Google Scholar]
- 23.Ottenhausen M, Banu MA, Placantonakis DG, et al. Endoscopic endonasal resection of suprasellar meningiomas: the importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg. 2014:82(3–4):442–449. [DOI] [PubMed] [Google Scholar]
- 24.Ottenhausen M, Rumalla K, Alalade AF, et al. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg Focus. 2018;44(4):E7. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 26.Kulwin C, Schwartz TH, Cohen-Gadol AA. Endoscopic extended transsphenoidal resection of tuberculum sellae meningiomas: nuances of neurosurgical technique. Neurosurg Focus. 2013;35(6):E6. [DOI] [PubMed] [Google Scholar]
- 27.Leng LZ, Brown S, Anand VK, Schwartz TH. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62(5)(suppl 2):E342–E343. [DOI] [PubMed] [Google Scholar]
- 28.Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120(5):876–880. [DOI] [PubMed] [Google Scholar]
- 29.Nyquist GG, Anand VK, Singh A, Schwartz TH. Janus flap: bilateral nasoseptal flaps for anterior skull base reconstruction. Otolaryngol Head Neck Surg. 2010;142(3):327–331. [DOI] [PubMed] [Google Scholar]
- 30.Placantonakis DG, Tabaee A, Anand VK, et al. Safety of low-dose intrathecal fluorescein in endoscopic cranial base surgery. Neurosurgery. 2007;61(3)(suppl):161–166. [DOI] [PubMed] [Google Scholar]
- 31.Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012;117(1):121–128. [DOI] [PubMed] [Google Scholar]
- 32.Otero-Rodriguez A, Tabernero MD, Munoz-Martin MC, et al. Re-evaluating Simpson grade I, II, and III resections in neurosurgical treatment of World Health Organization grade I meningiomas. World Neurosurg. 2016;96:483–488. [DOI] [PubMed] [Google Scholar]
- 33.Sughrue ME, Kane AJ, Shangari G, et al. The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg. 2010;113(5):1029–1035. [DOI] [PubMed] [Google Scholar]
- 34.Kitano M, Taneda M, Nakao Y. Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: results of the extended transsphenoidal and transcranial approaches. J Neurosurg. 2007;107(2):337–346. [DOI] [PubMed] [Google Scholar]
- 35.de Divitiis E, Esposito F, Cappabianca P, et al. Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery. 2008;62(3):556–563. [DOI] [PubMed] [Google Scholar]
- 36.Fatemi N, Dusick JR, de Paiva Neto MA, et al. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64(5)(suppl 2):269–286. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Lu XJ, Ji WY, et al. Visual outcome after extended endoscopic endonasal transsphenoidal surgery for tuberculum sellae meningiomas. World Neurosurg. 2010;73(6):694–700. [DOI] [PubMed] [Google Scholar]
- 38.Van Gompel JJ, Frank G, Pasquini E, et al. Expanded endonasal endoscopic resection of anterior fossa meningiomas: report of 13 cases and meta-analysis of the literature. Neurosurg Focus. 2011;30(5):E15. [DOI] [PubMed] [Google Scholar]
- 39.Bohman L-E, Stein SC, Newman JG, et al. Endoscopic versus open resection of tuberculum sellae meningiomas: a decision analysis. ORL J Otorhinolaryngol Relat Spec. 2012;74(5):255–263. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa Y, Tominaga T. Extended transsphenoidal approach for tuberculum sellae meningioma—what are the optimum and critical indications? Acta Neurochir (Wien). 2012;154(4):621–626. [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury FH, Haque MR, Goel AH, Kawsar KA. Endoscopic endonasal extended transsphenoidal removal of tuberculum sellae meningioma (TSM): an experience of six cases. Br J Neurosurg. 2012;26(5):692–699. [DOI] [PubMed] [Google Scholar]
- 42.Gadgil N, Thomas JG, Takashima M, Yoshor D. Endoscopic resection of tuberculum sellae meningiomas. J Neurol Surg B Skull Base. 2013;74(4):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan OH, Krischek B, Holliman D, et al. Pure endoscopic expanded endonasal approach for olfactory groove and tuberculum sellae meningiomas. J Clin Neurosci. 2014;21(6):927–933. [DOI] [PubMed] [Google Scholar]
- 44.Ceylan S, Anik I, Koc K, Cabuk B. Extended endoscopic transsphenoidal approach infrachiasmatic corridor. Neurosurg Rev. 2015;38(1):137–147. [DOI] [PubMed] [Google Scholar]
- 45.Hayhurst C, Sughrue ME, Gore PA, et al. Results with expanded endonasal resection of skull base meningiomas technical nuances and approach selection based on an early experience. Turk Neurosurg. 2016;26(5):662–670. [DOI] [PubMed] [Google Scholar]
- 46.Bernat AL, Priola SM, Elsawy A, et al. Recurrence of anterior skull base meningiomas after endoscopic endonasal resection: 10 years’ experience in a series of 52 endoscopic and transcranial cases. World Neurosurg. 2018;120:e107–e113. [DOI] [PubMed] [Google Scholar]
- 47.Song SW, Kim YH, Kim JW, et al. Outcomes after transcranial and endoscopic endonasal approach for tuberculum meningiomas—a retrospective comparison. World Neurosurg 2018;104:e434–e455. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz TH, Morgenstern PF, Anand VK. Lessons learned in the evolution of endoscopic skull base surgery. J Neurosurg. 2019;130(2):337–346. [DOI] [PubMed] [Google Scholar]
- 49.Komotar RJ, Starke RM, Raper DMS, et al. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012;77(5-6):713–724. [DOI] [PubMed] [Google Scholar]
- 50.Simpson D The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alhilali LM, Little AS, Yuen KCJ, et al. Early postoperative MRI and detection of residual adenoma after transsphenoidal pituitary surgery. J Neurosurg. Published online February 7, 2020. doi: 10.3171/2019.11.JNS191845 [DOI] [PubMed] [Google Scholar]
- 52.Khan OH, Anand VK, Schwartz TH. Endoscopic endonasal resection of skull base meningiomas: the significance of a “cortical cuff” and brain edema compared with careful case selection and surgical experience in predicting morbidity and extent of resection. Neurosurg Focus. 2014;37(4):E7. [DOI] [PubMed] [Google Scholar]
- 53.Attia M, Kandasamy J, Jakimovski D, et al. The importance and timing of optic canal exploration and decompression during endoscopic endonasal resection of tuberculum sella and planum sphenoidale meningiomas. Neurosurgery. 2012;71(1)(Suppl Operative):58–67. [DOI] [PubMed] [Google Scholar]
- 54.Nimmannitya P, Goto T, Terakawa Y, et al. Characteristic of optic canal invasion in 31 consecutive cases with tuberculum sellae meningioma. Neurosurg Rev. 2016;39(4):691–697. [DOI] [PubMed] [Google Scholar]
- 55.Kassam AB, Prevedello DM, Carrau RL, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011;114(6):1544–1568. [DOI] [PubMed] [Google Scholar]
- 56.Cohen S, Jones SH, Dhandapani S, et al. Lumbar drains decrease the risk of postoperative cerebrospinal fluid leak following endonasal endoscopic surgery for suprasellar meningiomas in patients with high body mass index. Oper Neurosurg (Hagerstown). 2018;14(1):66–71. [DOI] [PubMed] [Google Scholar]
- 57.Zwagerman NT, Wang EW, Shin SS, et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. 2019;131(4):1172–1178. [DOI] [PubMed] [Google Scholar]