Abstract
Nuclear NADPH oxidase-4 (Nox4) is a key component of metabolic reprogramming and is often overexpressed in renal cell carcinoma (RCC). However, its prognostic role in RCC remains unclear. Here we examined the significance of nuclear Nox4 on disease progression and development of drug resistance in advanced RCC.
We analyzed human RCC tissue from multiple regions in the primary index tumor, cancer-associated normal adjacent parenchyma, intravascular tumor in locally advanced cancer patients. We found that the higher nuclear Nox4 expression was significantly associated with progression and death. These findings were consistent after controlling for other competing clinical variables. In contrast, patients with lower nuclear Nox4, even in higher stage RCC had better prognosis. We identified a subset of patients with high nuclear Nox4 who had rapid disease progression or died within 6 months of surgery. In addition, higher nuclear Nox4 level correlated with resistance to targeted therapy and immunotherapy. Western blotting performed on fresh human RCC tissue as well as cell-lines revealed increased nuclear Nox4 expression.
Our data support an important prognostic role of Nox4 mediated regulation of RCC independent of other competing variables. Nox4 localizes to the nucleus in high-grade, high-stage RCC. Higher nuclear Nox4 has prognostic significance for disease progression, poor survival and development of drug resistance in RCC.
Keywords: NADPH oxidase-4, Nuclear localization, Renal cell carcinoma, Renal intravascular tumor extension, Tumor thrombus
Introduction
In 2018, there were approximately 403,262 new cases of renal cell carcinoma (RCC) and 175,098 related deaths worldwide. [1] Localized RCC can be surgically resected; however, 30% of patients present with metastatic disease, which is notoriously resistant to targeted therapies. This poor response is thought to be related to metabolic reprogramming in cancer cells via alterations in transcription, translation, post-translational events, epigenetic modulation, and activation of compensatory signaling pathways. [2, 3]
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-4 (Nox4) family members are reactive oxygen species (ROS)-generating enzymes that regulate redox-sensitive signaling pathways, which affect the likelihood of developing RCC and the potential for progression and metastasis. [4–7] We recently demonstrated that Nox4 functions as a checkpoint in metabolic reprogramming of cancer cells in RCC and couples energy metabolism with drug resistance in these cells. [8] To date, studies of Nox4 have focused on elucidating its molecular mechanisms and role in biological outcomes using established RCC cell lines in vitro and in vivo. [3, 6–11] However, little is understood about the prognostic role of Nox4 in human RCC. Nox4 has been reported to be variably present in the endoplasmic reticulum, mitochondria, cytoskeleton, plasma membrane and nucleus in different cancer cell types. [12] There is emerging data on the pivotal role played by functionally active, nuclear Nox4 in modulating nuclear signal transduction in liver cancer cells. [13] However, despite presence of nuclear Nox4 in RCC, there is no data defining its prognostic significance. In this context, we undertook a large-scale approach to evaluate the distribution of nuclear Nox4 in human RCC specimens and its prognostic and therapeutic implications.
We have developed tissue microarrays (TMAs) of RCC for immunohistochemical studies to understand better the relationship between expression of Nox4 and RCC. [8] Approximately 15% of patients with RCC have intravascular tumor extension. [14, 15] These tumors represent locally advanced disease with increased propensity for progression and metastases. Therefore, these patients provide us a unique opportunity to study the biological mechanism along with progression of disease. We have developed a dedicated TMA with a high density of RCC patients with intravascular tumor extension (pT3a, pT3b, pT3c disease). These tumors represent solid tumor extension into the intravascular space and are referred to as “tumor thrombus” or “renal intravascular tumor extension” (RITE). For clarity and consistency, all patients with tumor thrombus are referred to as RITE in this manuscript. This study investigated the relationship between expression and nuclear localization of Nox4 and its prognostic significance in human RCC tissue, including RCC with RITE and RCC cell lines as well as its role in development of drug resistance (targeted therapy or immunotherapy).
Material and Methods
Patient Selection and Creation of Tissue Microarrays
The study protocol was approved by the University of Texas Health institutional review board (IRB# HSC20160214H). The need for informed consent was waived because all patients were identified retrospectively by medical chart review. This manuscript conforms to the ethical guidelines for human research. We identified 350 patients who had RCC with (n=85) or without (n=265) RITE and underwent extirpative resection between January 2013 and June 2016.
Fifty patients with RCC and Renal Intravascular Tumor Extension (RITE) were matched 1:1 for age, sex, ethnicity, Fuhrman grade, total tumor volume, body mass index, and comorbid diabetes/hypertension with 50 patients who had RCC without RITE (Table 1).
Table 1.
Patient demographics and clinical characteristics at baseline
| RCC without RITE (n=50) | RCC with RITE (n=50) | Total | |
|---|---|---|---|
| Age*, y, mean (range) | 55.6 (52.26–58.94) | 60.70 (56.55–64.84) | 57.48 (55.03–59.93) |
| Sex*, n (%) | |||
| Male | 30 (60) | 34 (68) | 64 (64) |
| Female | 20 (40) | 16 (32) | 36 (36) |
| BMI*, mean (range) | 32.53 (29.73–35.33) | 29.13 (26.94–31.33) | 31.19 (29.28–33.10) |
| Ethnicity*, n (%) | |||
| Hispanic/Latino | 35 (71.43) | 33 (68.75) | 68 (70.1) |
| Non-Hispanic | 14 (28.57) | 15 (31.25) | 29 (29.9) |
| Diabetes, n (%) | |||
| Yes | 24 (48) | 18 (36) | 42 (42) |
| No | 26 (52) | 32 (64) | 58 (58) |
| Hypertension, n (%) | |||
| Yes | 41 (82) | 32 (64) | 73 (73) |
| No | 9 (18) | 18 (36) | 27 (27) |
| Type of RCC*, n (%) | |||
| Clear cell | 44 (88) | 40 (80) | 84 (84) |
| Non-clear cell | 6 (12) | 10 (20) | 16 (16) |
| Fuhrman grade*, n (%) | |||
| 1 | 2 (4) | 0 (0) | 2 (2.02) |
| 2 | 15 (30) | 11 (22.45) | 26 (26.26) |
| 3 | 21 (42) | 21 (42.86) | 42 (42.42) |
| 4 | 12 (24) | 17 (34.69) | 29 (29.29) |
| T stage, n (%) | |||
| 1 | 9 (18) | 0 (0) | 9 (9) |
| 2 | 11 (22) | 0 (0) | 11 (11) |
| 3 | 30 (60) | 48 (96) | 78 (78) |
| 4 | 0 (0) | 2 (4) | 2 (2) |
| N stage, n (%) | |||
| 1 | 1 (2.17) | 8 (16.67) | 9 (9.57) |
| 0 | 45 (97.83) | 40 (83.33) | 85 (90.43) |
| M stage, n (%) | |||
| 1 | 3 (7.32) | 9 (20) | 12 (13.95) |
| 0 | 38 (92.68) | 36 (80) | 74 (86.05) |
| Tumor volume*, mm3, mean (range) | 411.78 (265.95–557.61) | 664.13 (446.87–881.39) | 525.94 (399.13–652.75) |
Matched variables. BMI, body mass index; RCC, renal cell carcinoma; RITE, renal intravascular tumor extension
Two pathologists (GG, AN) reviewed the hematoxylin-eosin-stained sections cut from formalin-fixed, paraffin-embedded tissue blocks for all patients and identified tumor-rich regions for creation of TMAs that included index RITE or non-RITE primary tumors, cancer-associated normal adjacent parenchyma (CANA), and intravascular tumors (IVTs) (Figure 1).
Figure 1.
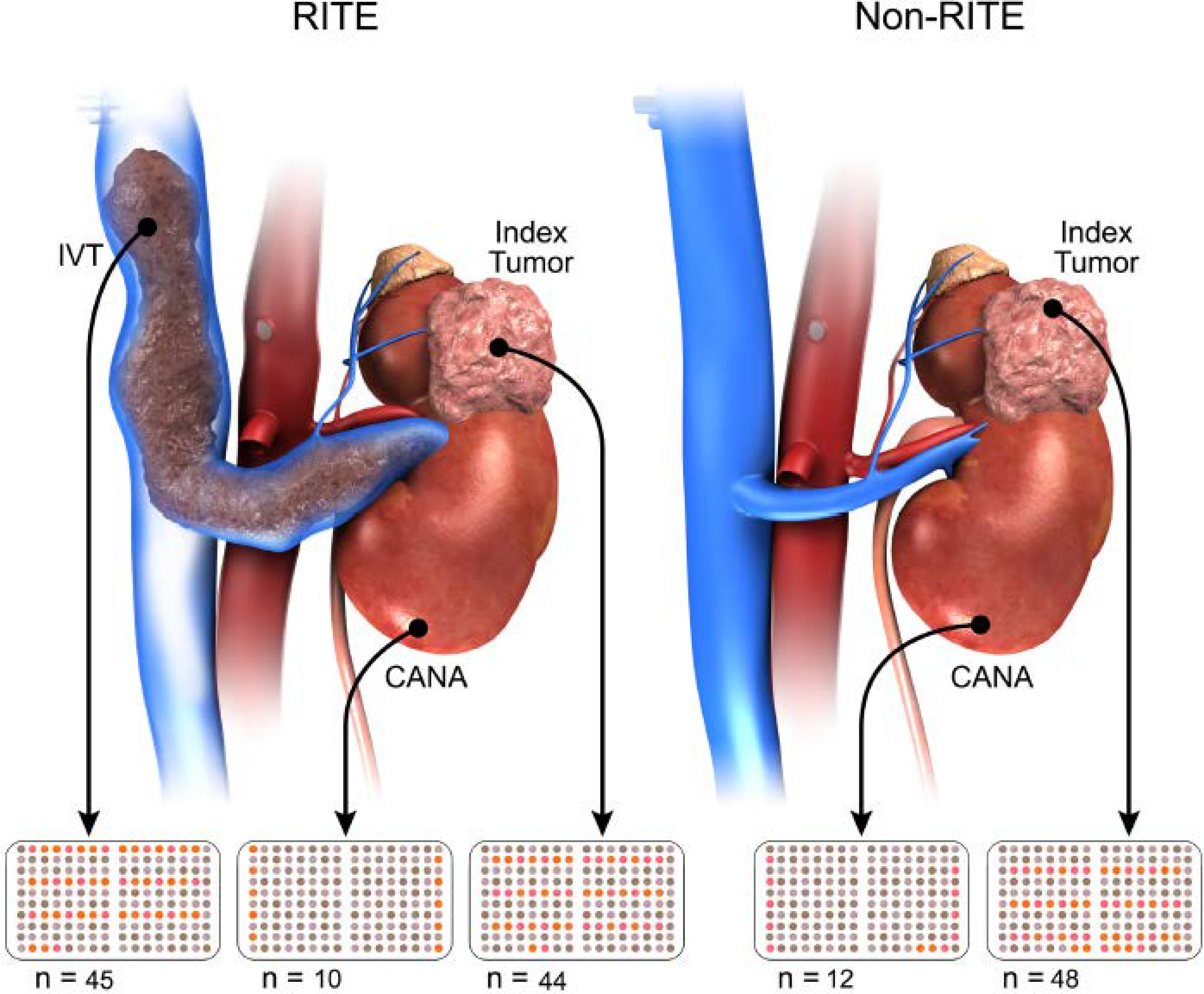
Creation of Tissue Microarrays utilizing different regions of RCC tissue CANA, cancer-associated normal adjacent parenchyma; IVT, intravascular tumor; RITE, renal intravascular tumor extension.
The final analyses were based on 159 tissue samples from 100 patients (44 index tumors with RITE, 48 index tumors without RITE, 10 tumors with RITE and CANA, 12 tumors without RITE and CANA, and 45 IVTs).
The index tumor, CANA, and IVT specimens were extracted from the archived formalin-fixed, paraffin-embedded tissue. The TMAs were constructed as previously described. [16] Tissue cores with a diameter of 2 mm were punched from each specimen in triplicate and arrayed into a recipient paraffin block. The template used to produce the TMAs was designed using randomized samples within the template (Figure 1) to avoid artifacts caused by technical problems. Orientation markers were added to the template for correct alignment. Histologic follow-up of the tissue content was performed by an experienced pathologist (AN).
Immunohistochemistry Protocol
The 159 tissue microarray slides were incubated for 15 minutes in an oven at 60°C, deparaffinized, placed in boiling 0.01 mol/L citrate buffer (Poly Scientific R&D Corp, Bay Shore, NY, USA) for antigen retrieval, and incubated in 3% H2O2 to block endogenous peroxidase activity. An M.O.M. kit (Vector Laboratories Inc., Burlingame, CA, USA) was used for staining according to the manufacturer’s instructions. The slides were incubated for 30 minutes with anti-Nox4 (LS-c313066; LifeSpan Biosciences, Inc., Seattle, WA, USA) at a dilution of 1:250, flooded with Vector NovaRED solution (Vector Laboratories) as per the manufacturer’s instructions, counterstained with hematoxylin, dehydrated, coverslipped with Permount mounting medium (Thermo Fisher Scientific, Waltham, MA, USA), and air-dried overnight. Secondary antibody alone was used on off cuts of tissue microarray slides as a negative control.
Evaluation of Immunohistochemistry staining pattern
Nuclear Localization of Nox4
Nox4-stained renal cell carcinoma (RCC) nuclei were evaluated for roundness, elongation, and size. Nox4 nuclear staining was categorized according to the threshold intensity as strongly positive, moderate, weak, or negative as per the protocol described below.
Slides stained with anti-Nox4 were scanned using the Aperio ScanScope® CS system (Leica Microsystems GmbH, Wetzlar, Germany) with a 20× objective lens. The histologic parameters were evaluated using the Aperio ImageScope software (version 12.3.2.8013. v11.2.0.780; Leica Microsystems). Individual nuclei and nuclear localization of Nox4 were identified using the nuclear localization algorithm built into the ImageScope software. To distinguish between true nuclei and staining artifact, the default algorithm settings were changed as follows: minimum nuclear roundness, 0.5; minimum nuclear elongation, 0.5; minimum nuclear size, 40 μm2; and maximum nuclear size, 500 μm2.
Nuclear localization of Nox4 was categorized based on the threshold intensity (red, green, and blue values) as strongly positive (0–162), moderate (162–188), weak (188–230), or negative (230–250). The immunohistochemistry results were further evaluated semi-quantitatively by assigning an H-score to the tumor samples. [17] The H-score was used to quantify a net aggregate score for assessment of the nuclear staining pattern. The nuclear staining intensity (0, 1+, 2+, or 3+) was determined for each cancer cell. The H-score is the weighted sum of the individual percentages of cells stained at each intensity level. We calculated the percentage of cells at each staining intensity level and assigned the H-score using the following formula:
H-score = [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)].
Evaluation of Nuclear Nox4 in human RCC cell lines and fresh RCC tissue
Cell Culture
A normal human renal epithelial cell line (HK-2; American Type Culture Collection, Manassas, VA) was grown in Dulbecco’s modified Eagle’s medium (DMEM)-F12 (Gibco Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1× antibiotic. Five different RCC cell lines (786-O, A498, RCC-4, RCC-10, and UOK-262) were maintained in DMEM with 2 mM L-glutamine that was modified to contain 1.0 mM sodium pyruvate, 1 mM nonessential amino acids, and 1.5 g/l sodium bicarbonate supplemented with 10% fetal bovine serum and 100 units/m of penicillin/streptomycin at 37°C and 5% CO2.
Nuclear Fractionation
Cells were lysed in low salt lysis buffer (20 mM HEPES, 5 mM NaCl, 5 mM MgCl2, 1 mM EDTA and 10% NP40 supplemented with protease inhibitors). After centrifugation, the supernatant (cytoplasmic fraction) was removed. The pellet (nuclei) was then lysed with high salt buffer (0.4 M NaCl, 1 mM ethylenediaminetetraacetic acid, and 20 mM HEPES supplemented with protease inhibitors). After centrifugation, the nuclear lysate (supernatant) was removed for determination of the protein concentration.
Nuclear Protein Extraction from Human Frozen Tissue
Approximately 40–80 mg of human frozen tissue was ground in 1 ml of buffer A (10 mM HEPES-KOH, pH 7.9, 1.5 mmol/l MgCl2, 10 mmol/l KCl, and 1 mmol/l dithiothreitol) containing 0.1% NP-40 (Sigma-Aldrich, St. Louis, MO) and protease inhibitors (Boehringer Mannheim, Indianapolis, IN), followed by centrifugation at 15,000 rpm for 5 min at 4°C. The pellet was resuspended in 50 μl of buffer B (20 mmol/l HEPES-KOH, pH 7.9, 25% glycerol, 420 mmol NaCl, 1.5 mmol MgCl2, and 0.2 mmol EDTA) and incubated on ice for 30 min for high salt extraction. The samples were centrifuged at 15,000 rpm for 30 min at 4°C, and the supernatant was transferred to a prechilled tube. The total protein concentration was measured using the bicinchoninic acid method according to the manufacturer’s protocol (Pierce Biotechnology, Rockford, IL).
Western blot analysis
For Western blotting, about 30–50 μg of lysate was electrophoresed by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The proteins separated from the gel were electro-transferred onto a nitrocellulose membrane. The membrane was subjected to a 60-min blocking step as recommended by the manufacturers of the primary antibodies, i.e., Nox4 1:500 (NB110–58849; Novus Biologicals, Centennial, CO) and CREB-2 1:5000 (SC-200; Santa Cruz Biotechnology, Dallas, TX). The two primary antibodies were probed at 4°C overnight. The membrane was then washed and incubated for 1 h with the appropriate horseradish peroxidase-conjugated anti-rabbit secondary antibody (NA934–1ML; GE Healthcare Life Sciences, Chicago, IL). Finally, the membrane was washed and developed to visualize the protein bands using enhanced chemiluminescence reagent (GE Healthcare UK Limited, Little Chalfont, UK) or SuperSignal West Pico Luminol/Enhancer solution (Thermo Scientific, Rockford, IL).
Survival
Local recurrence was defined as recurrence of cancer in the surgical bed or ipsilateral renal fossa. Metastasis was defined as any spread of disease to/in the viscera or bone. Progression of disease was classified as attenuated (not until at least 6 months after surgery) or rapid (progression or death within 6 months of surgery). [18] Resistance to targeted therapy was defined as failure to respond to a first targeted therapy and a need to switch to a newer one. This was based on treating physician determination. Time to development of resistance to targeted therapy was defined as the interval between the date of surgery and the date on which the newer targeted therapy was started or the date of final follow-up.
Statistical Analysis
Differences in Nox4 expression between the primary tumor, CANA, and RITE specimens were assessed using the chi-squared test or two-samples t-test. Overall survival (OS), progression-free survival (PFS), and time to development of resistance to targeted therapy stratified by Nox4 staining intensity were estimated as the time from nephrectomy to the event of interest or last follow-up using the Kaplan-Meier method and were compared with the log-rank test.
To further evaluate the biological gradient of Nox4 on survival, patients were stratified into risk tertiles based on their Nox4 H-scores, their OS and PFS were estimated using the Kaplan-Meier method and compared with the log rank test. Next, we utilized maximally selected rank test statistics [19] to find an optimal cut-off point for H-score value [Nox4high or Nox4low] to divide the cohort into two groups to conduct survival analyses. This method is better than using ROC (receiver operating characteristic curve) to find the cutoff with the greatest discriminant power, as there is no need to transform the time variables. [19] Maximally selected rank statistics (MaxStat package in R statistical software) was used to identify an optimal cut-off point for survival based on log-rank scores. Kaplan-Meier survival estimates for OS and PFS based on these cut- off points were calculated, and high and low Nox4 distributions were compared using log-rank tests.
A Cox regression model was used to investigate the relationship between Nox4 staining intensity and patient survival in multivariate analyses. The proportional hazards assumption was assessed for the univariate and multivariable ratio models. All tests were two-tailed. The statistical analyses were performed using StataCorp 2013 software (Release 13; StataCorp LP, College Station, TX, USA). A p-value <0.05 was considered statistically significant.
Results
Immunohistochemistry Staining Patterns
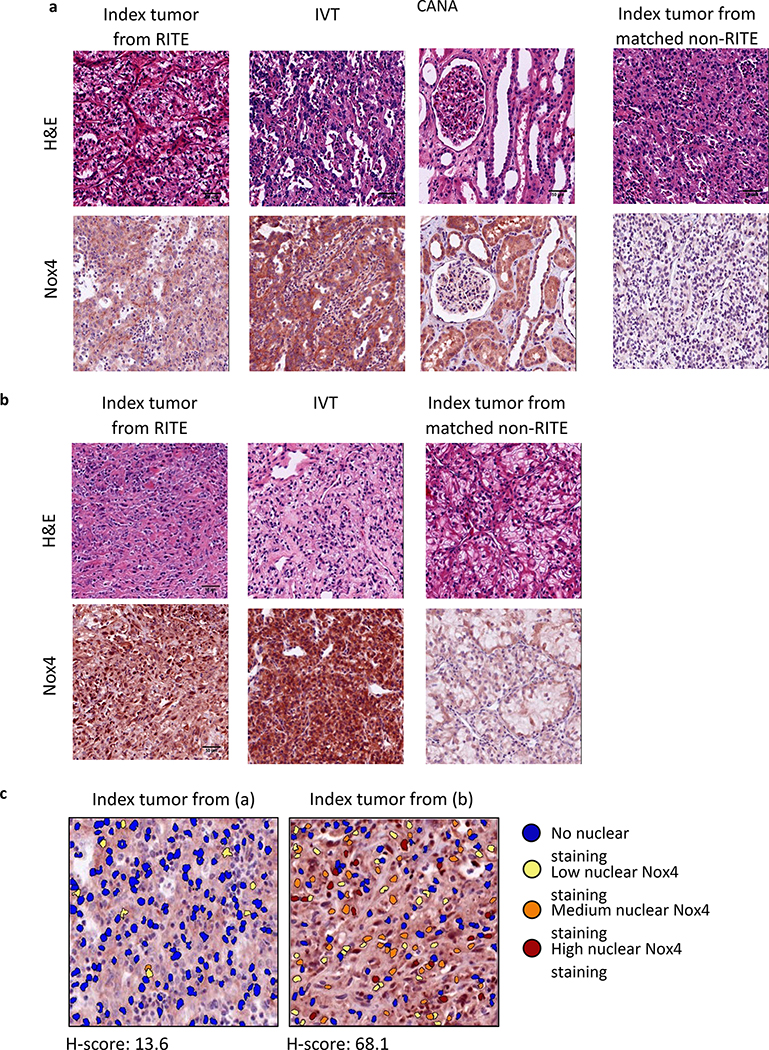
Using a Nox4-specific antibody, we stained multiple TMAs containing samples from patients with RITE (Renal Intravascular Tumor Extension or Tumor Thrombus) and clinically matched samples from patients without RITE. These samples were from the primary index tumor, intravascular tumor (IVT), cancer-associated normal adjacent parenchyma (CANA), and the matched non-RITE index tumor (Figure 1).
Many of the TMA samples contained cells with Nox4 staining that was predominantly localized to the nucleus (Figure 2a and 2b). By utilizing nuclear localization algorithm, we gave each individual nucleus an intensity score (H- score) for Nox4 nuclear staining, as shown in Figure 2c.
Figure 2.
Staining of NoX4 in 50 patients with RITE and clinically matched samples (n=50) reveal different Nox4 localization. a. Representative set of tissues collected and stained with Nox4 on the TMA. Each RITE patient present on the TMA includes samples taken from Index tumor, IVT, as well as the Index tumor from a clinically matched non-RITE patient. A subset also includes CANA. The index tumor from RITE exhibits non-nuclear localized Nox4 staining corresponding to a low H-score (13.6). b. Representative image of an index tumor that exhibits nuclear localization of Nox4 staining corresponding to a high H-score (68.1). The IVT from this patient and the clinically matched non-RITE index tumor are included for comparison. c. Visual representation of algorithmic quantification of nuclear localization of Nox4 staining in the Index tumor from 2a and 2b. Colored circles represent individual nuclei and different colors represent the intensity of Nox4 stain localizing with the nucleus. CANA, cancer-associated normal adjacent parenchyma; IVT, intravascular tumor; RITE, renal intravascular tumor extension.
Correlation of Nox4 with Survival Outcome
Overall survival (OS), progression-free survival (PFS), metastases, and local recurrence outcomes were stratified by nuclear Nox4 staining (H-score) as shown in table 2. Patients with a higher H-score (and by definition a higher percentage of nuclear Nox4 staining) had worse OS (p<0.05). There was an increased trend towards poor PFS and higher rates of metastasis and local recurrence with a higher H-score.
Table 2.
NADPH oxidase-4 staining according to disease progression status
| Deceased (n=48) | Alive (n=70) | Progression (n=85) | No progression (n=62) | Metastases (n=99) | No metastases (n=58) | Local recurrence (n=25) | No local recurrence (n=126) | |
|---|---|---|---|---|---|---|---|---|
| H-score* | 39.73 (34.73, 44.73) | 30.29 (26.04, 34.53)** | 33.98 (30.23, 37.73) | 29.8 (24.95, 34.65) | 35.61 (32.01, 39.22) | 28.95 (24.06, 33.84) | 36.84 (31.1, 42.58) | 31.64 (28.35, 34.93) |
| Percentage of nuclear staining*** | 23.44 (21.02, 25.86) | 18.58 (16.45, 20.71) | 20.13 (18.25, 22.01) | 18.21 (15.79, 20.63) | 21.18 (19.37, 23) | 17.76 (15.33, 20.2) | 22.37 (19.3, 25.43) | 19.01 (17.36, 20.65) |
| (3+) Percent nuclei | 4.91 (3.83, 5.99) | 3.41 (2.58, 4.24) | 4.15 (3.41, 4.9. ) | 3.44 (2.5, 4.37) | 4.3 (3.59, 5.01) | 3.3 (2.34, 4.27) | 4.1 (2.92, 5.28) | 3.79 (3.15, 4.44) |
| (2+) Percent nuclei | 6.48 (5.64, 7.31) | 4.89 (4.15, 5.62) | 5.54 (4.93, 6.16) | 4.72 (3.88, 5.57) | 5.83 (5.23, 6.44) | 4.57 (3.72, 5.43) | 6.27 (5.29, 7.25) | 5.05 (4.49, 5.61) |
| (1+) Percent nuclei | 12.06 (10.81, 13.31) | 10.28 (9.22, 11.35) | 10.44 (9.53, 11.34) | 10.05 (8.91, 11.2) | 11.05 (10.16, 11.95) | 9.89 (8.7, 11.07) | 12 (10.23, 13.76) | 10.17 (9.37, 10.96) |
H-score (intensity weighted percentage of nuclear staining) = (3+) percent nuclei ×3 +(2+) percent nuclei ×2 +(1+) percent nuclei
mean with 95% confidence interval. Numbers marked in bold indicate numbers in two groups that are significant different (p<0.05)
percentage of nuclear staining = (3+) percent nuclei+(2+) percent nuclei+(1+) percent nuclei
To evaluate the biological- gradient (dose-response relationship) of Nox4 status on survival, we divided the entire cohort into tertiles based on the H-score and performed survival analysis with the log-rank test. We found statistically significant trend in survival curves for both PFS [(p-value =0.013) and OS (p-value=0.026); figures 3a and 3b)] in patients based on their H-score status.
Figure 3a.
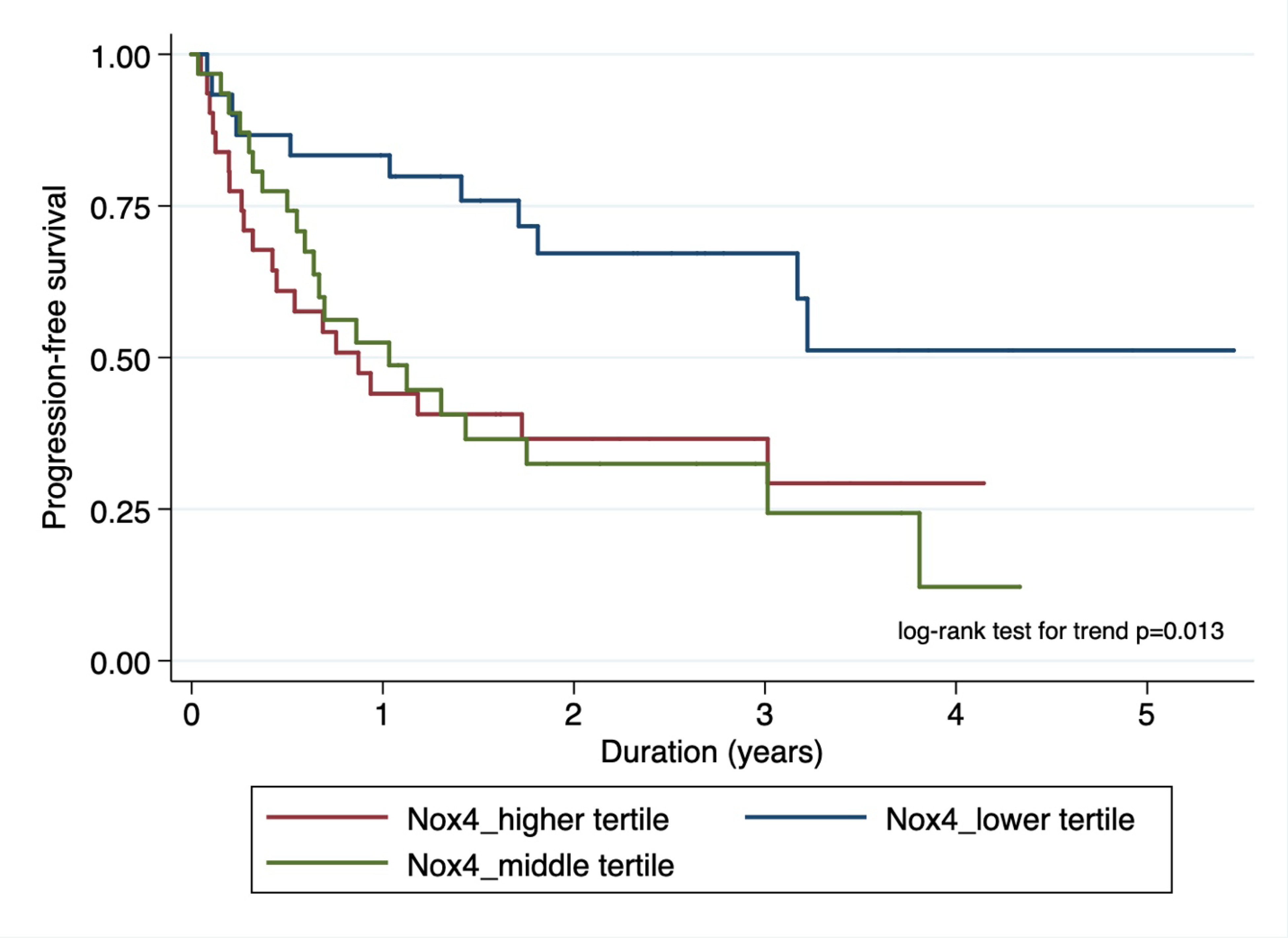
Kaplan-Meier plot showing association of nuclear Nox4 stratified by risk tertiles with progression-free survival.
Figure 3b.
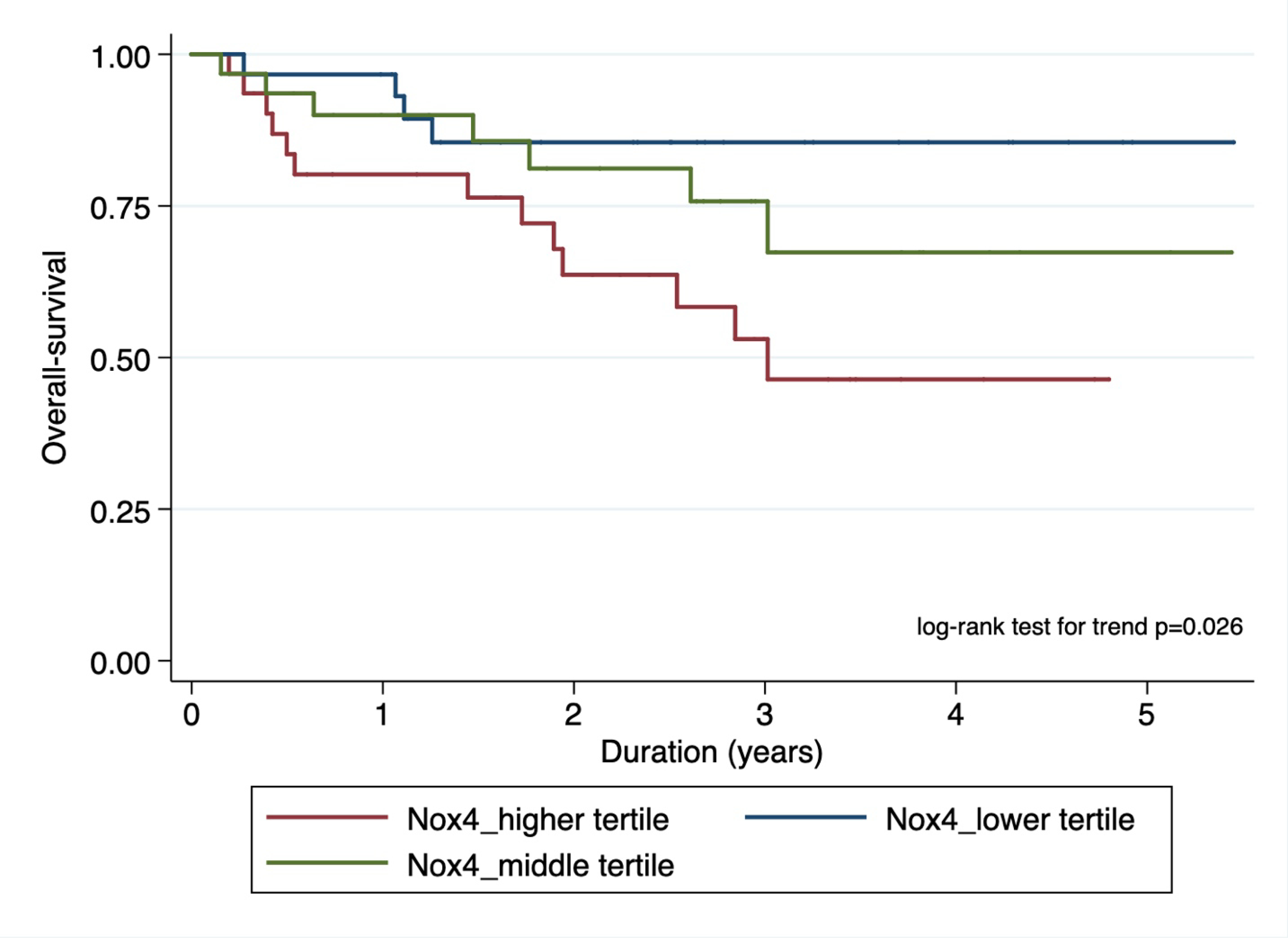
Kaplan-Meier plot showing association of nuclear Nox4 stratified by risk tertiles with overall survival.
In order to find an optimal cut- off point for H-score value (Nox4high or Nox4low), we utilized maximally selected rank test statistics and divided the entire cohort into two groups to conduct survival analyses (figures 4a and 4b showing maximally selected test). Based on these cut-off values for H-score (Cut-off values for PFS: 26.13; OS: 37.38), we performed survival analysis with the log-rank test for PFS and OS stratified as Nox4high or Nox4low. We observed significant differences in PFS (HR 2.72, 95% CI 1.50 – 4.92; p < 0.001, Figure 5a) and OS (HR 2.88, 95% CI 1.29 – 6.42; p=0.007, Figure 5b)
Figure 4a.
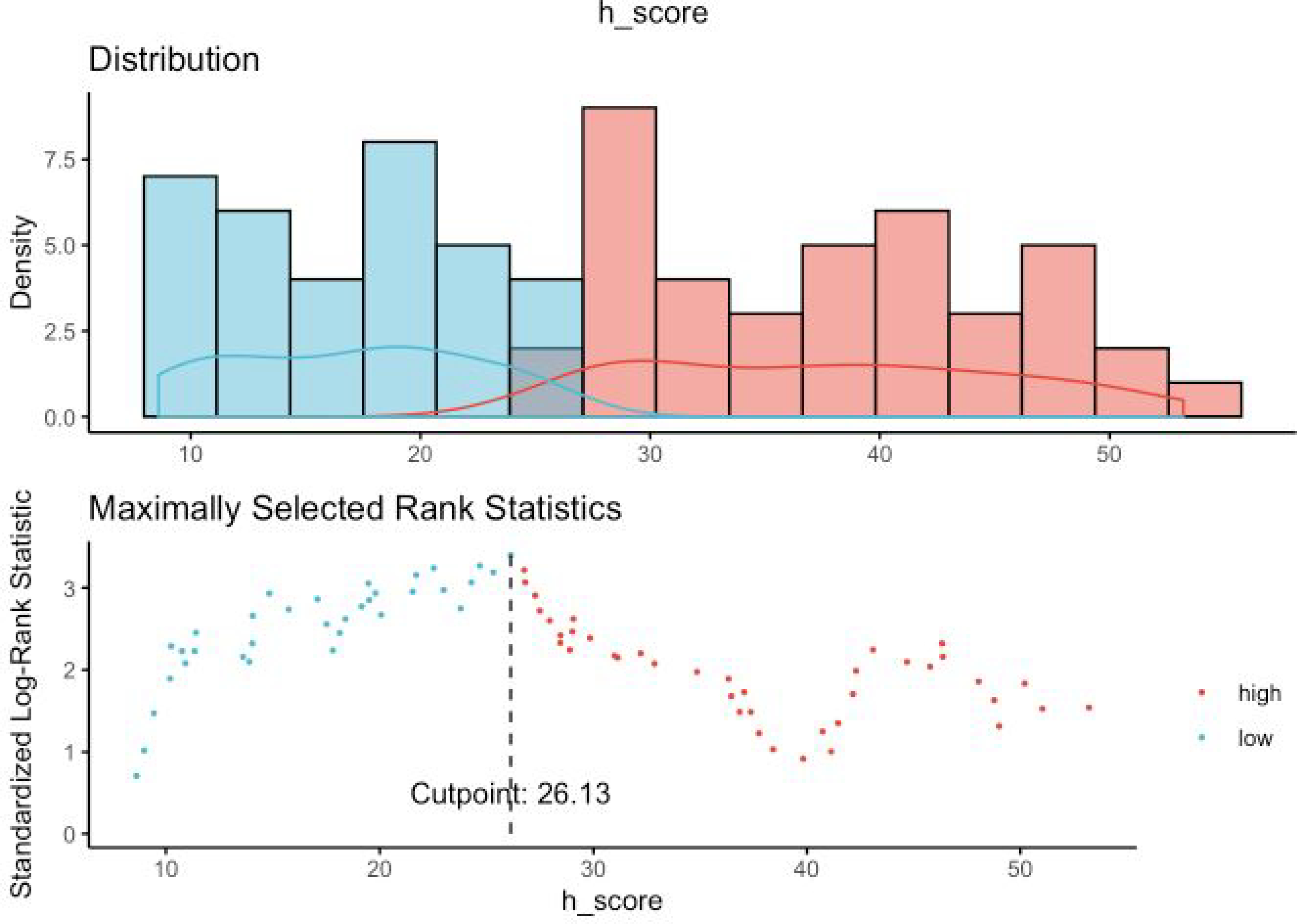
Maximally selected rank statistics for PFS (Progression-free survival) and Nuclear Nox4 H-score. Maximally selected rank statistics were used to identify the optimal discriminator value for the PFS/Nuclear Nox4 H-score. For every potential cutoff point, the absolute value of the standardized log-rank statistic was computed. The cutoff point that provided the best separation of the survival outcome into two groups, where the standardized statistics reached their maximum, was selected as the cutoff point.
Figure 4b.
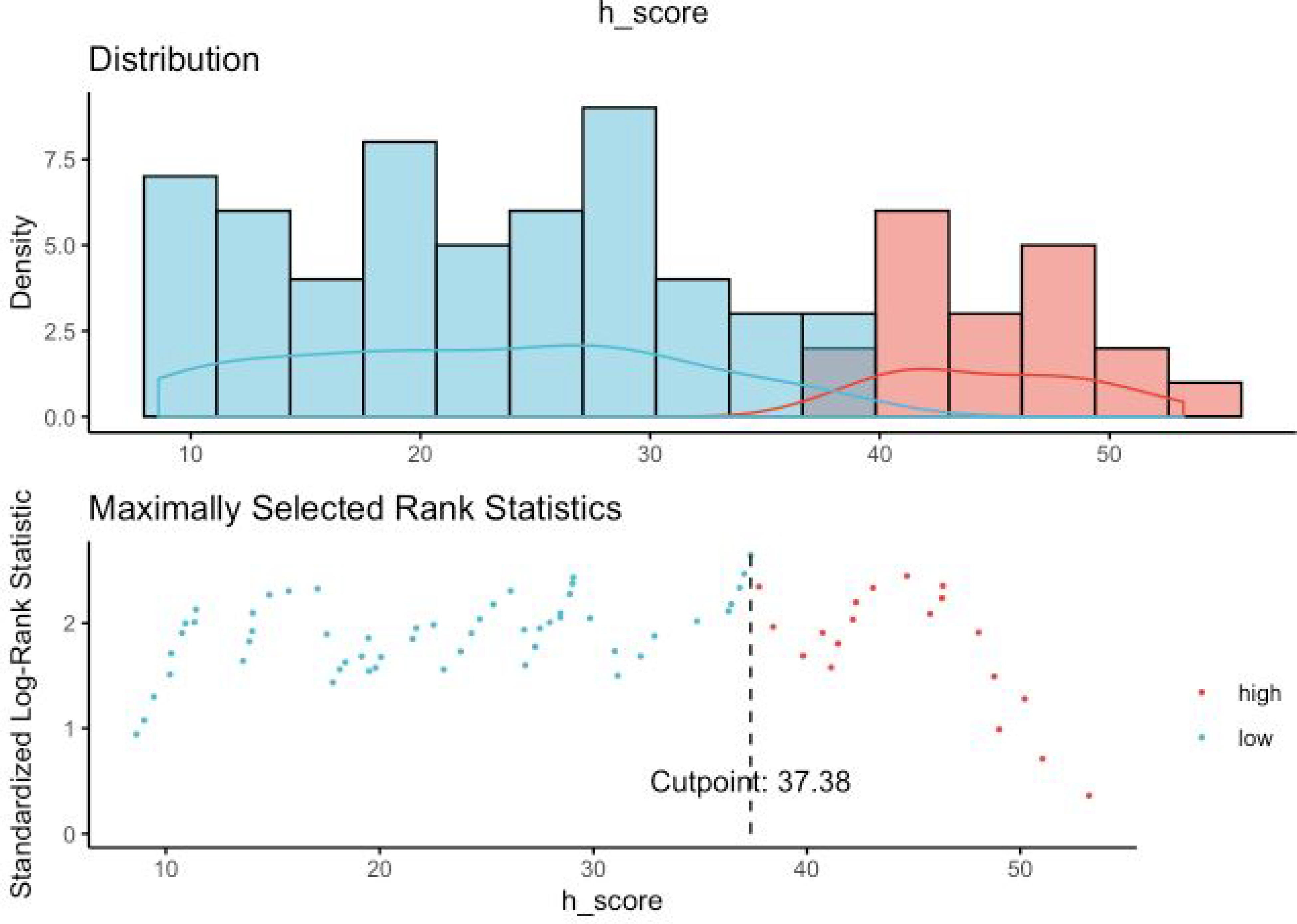
Maximally selected rank statistics for OS (overall survival) and Nuclear Nox4 H- score. Maximally selected rank statistics were used to identify the optimal discriminator value for the OS/Nuclear Nox4 H-score. For every potential cutoff point, the absolute value of the standardized log-rank statistic was computed. The cutoff point that provided the best separation of the survival outcome into two groups, where the standardized statistics reached their maximum, was selected as the cutoff point.
Figure 5a.
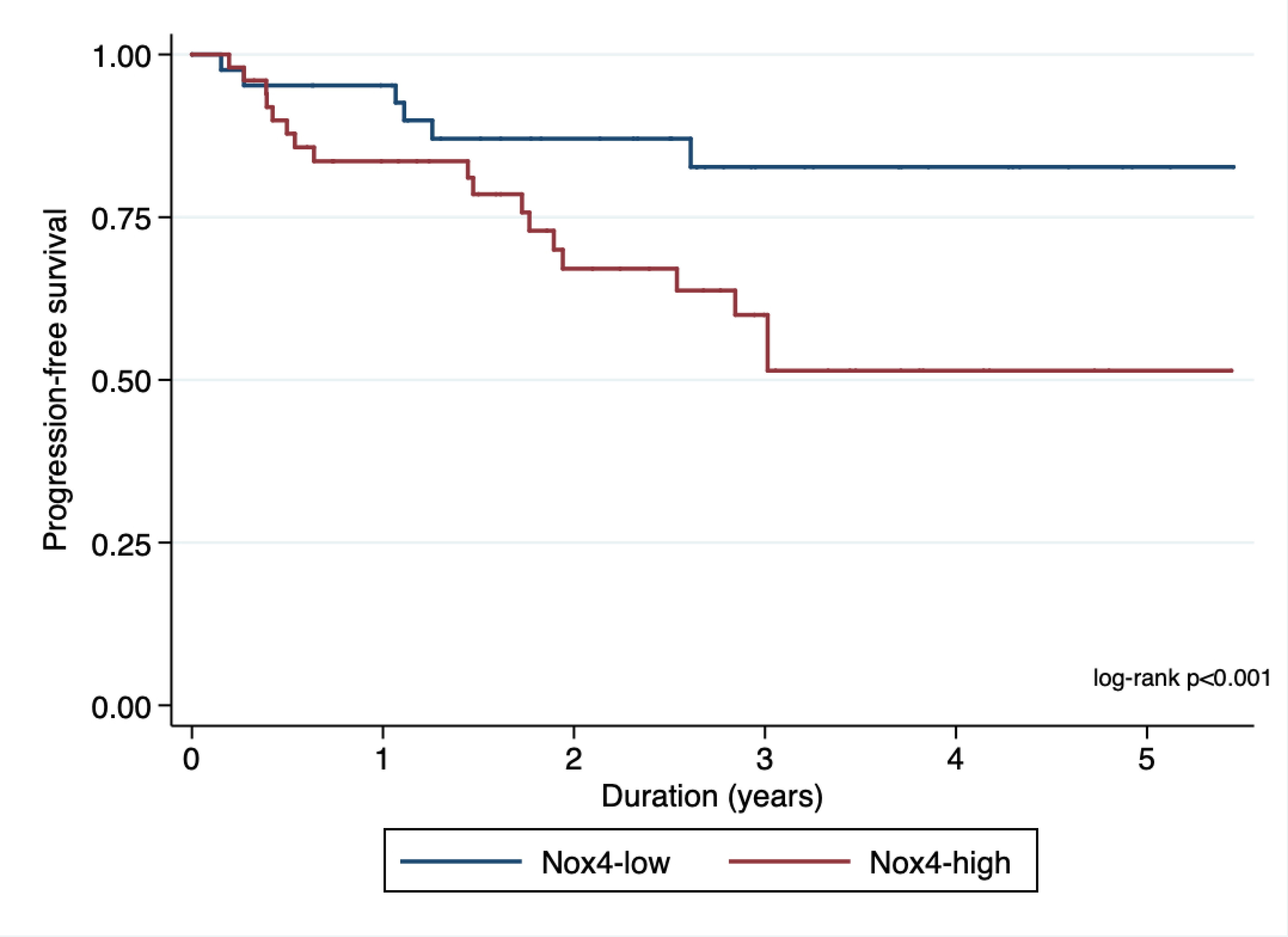
Kaplan-Meier plot showing association of nuclear Nox4 with progression-free survival.
Figure 5b.
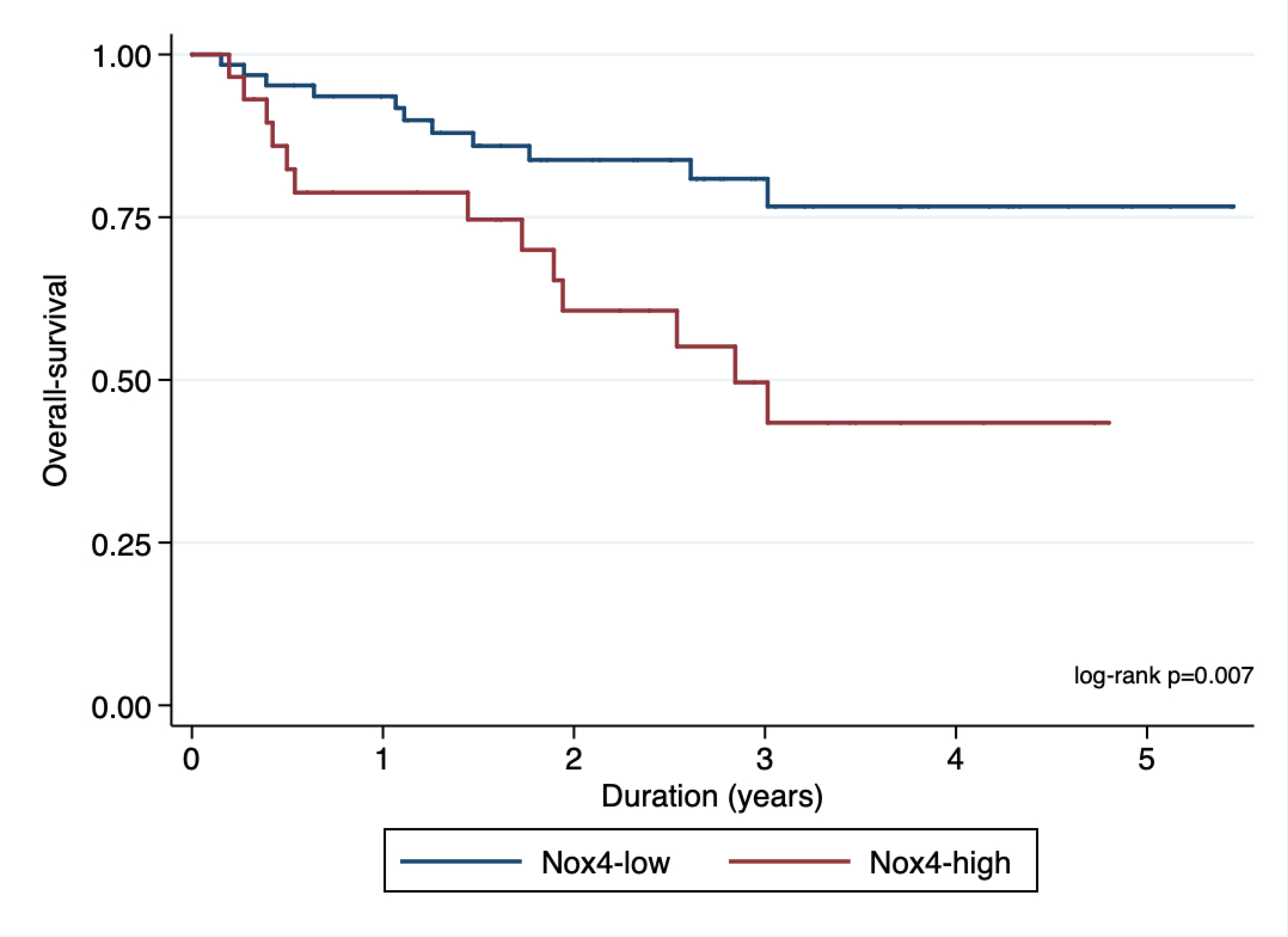
Kaplan-Meier plot showing association of nuclear Nox4 with overall survival.
Multivariate analysis revealed similar trends for PFS (Nox4high vs Nox4low: HR 2.50, 95% CI 1.25–5.01, p=0.01) and OS (Nox4high vs Nox4low: HR 3.23, 95% CI 1.21–8.58, p=0.019) when relevant clinicopathologic variables such as age, sex, RITE status, histology and TNM staging were included (Table 3a).
Table 3a.
Univariate and multivariate analyses of nuclear Nox4 status according to clinical outcome
| Outcome = Progression* | ||||
| Univariate | Multivariate | |||
| Hazard Ratio (95%CI) | P Value | Hazard Ratio (95%CI) | P Value | |
| Age | 1.04 (1, 1.07) | 0.03 | 0.99 (0.97, 1.02) | 0.58 |
| Sex | 1.36 (0.56, 3.29) | 0.49 | 1.33 (0.65, 2.73) | 0.42 |
| NOX4 | 2.72 (1.50, 4.92) | <0.001 | 2.50 (1.25, 5.01) | 0.01 |
| RITE^^ | 3.8 (1.56, 9.25) | 0.003 | 2.06 (0.94, 4.49) | 0.07 |
| Histology^ | 1.9 (0.75, 4.81) | 0.17 | 1.15 (0.28, 4.75) | 0.85 |
| T stage | 3.1 (1.19, 8.1) | 0.021 | 2.11 (0.89, 4.98) | 0.09 |
| N stage | 3.95 (1.56, 9.99) | 0.004 | 1.72 (0.66, 4.51) | 0.26 |
| M stage | 1.14 (0.33, 3.93) | 0.83 | 1.34 (0.5, 3.58) | 0.56 |
| Outcome=Death* | ||||
| Univariate | Multivariate | |||
| Hazard Ratio with 95%CI | P Value | Hazard Ratio with 95%CI | P Value | |
| Age | 1.02 (1, 1.04) | 0.12 | 1.02 (0.98, 1.06) | 0.42 |
| Sex | 1.22 (0.68, 2.17) | 0.51 | 2.06 (0.68, 6.3) | 0.20 |
| NOX4 | 2.88 (1.29, 6.42) | 0.007 | 3.23 (1.21, 8.58) | 0.019 |
| RITE^^ | 3.61 (1.99, 6.57) | <0.001 | 2.60 (0.63, 10.81) | 0.18 |
| Histology^ | 0.91 (0.43, 1.95) | 0.81 | 0.69 (0.26, 1.88) | 0.46 |
| T stage | 2.63 (1.47, 4.69) | 0.001 | 3.31 (0.68, 16.05) | 0.13 |
| N stage | 3.74 (1.77, 7.89) | 0.001 | 2.78 (0.86, 9) | 0.08 |
| M stage | 2.96 (1.46, 6.01) | 0.003 | 0.45 (0.11, 1.94) | 0.28 |
Maximum selected rank statistic was used to select the optimal cutoff points for progression and death.
RITE- Renal Intravascular Tumor Extension. Non- RITE is the reference
Clear-cell renal cell carcinoma is the reference.
CI, confidence interval
We further stratified the clinical progression of RCC based on the clinical definition by Turajlic et al [18] into attenuated (not until at least 6 months after surgery) or rapid (progression or death within 6 months of surgery) and stratified survival according to Nox4 nuclear staining (Nox4high vs Nox4low). As shown in Table 3b, there were significantly more patients with elevated Nox4 nuclear staining in both the attenuated progression group (Nox4high vs Nox4low: 68.97% vs 31.03%) and the rapid progression group (Nox4high vs Nox4low: 69.57% vs 30.43%) than in the group that did not progress (Nox4high vs Nox4low: 35% vs 65%).
Table 3b.
Correlation of clinical progression with NADPH oxidase-4 level
| ALL patients | ||||
| No Progression (n=40) | Attenuated Progression (n=29) | Rapid progression (n=23) | Total (n=92) | |
| Nox4low | 26 (65%) | 9 (31.03%) | 7 (30.43%) | 42 (45.65%) |
| Nox4high | 14 (35%) | 20 (68.97%) | 16 (69.57%) | 50 (54.35%) |
| RITE* patients | ||||
| No Progression (n=9) | Progression (n=35) | Total (n=44) | ||
| Nox4low | 6 (66.67%) | 11 (31.43%) | 17 (38.64%) | |
| Nox4high | 3 (33.33%) | 24 (68.57%) | 27 (61.36%) | |
P=0.005 calculated by ANOVA test
p=0.053 calculated by chi-square test
renal intravascular tumor extension
Next, we examined the pattern of Nox4 nuclear expression in patients with RITE. Most patients with disease progression (either attenuated or rapid) had higher nuclear Nox4 (Table 3b). Given that IVT is clonally related to its index tumor in 95% of cases [20], we evaluated PFS in patients with RITE by stratifying only the index tumor as Nox4high or Nox4low regardless of the Nox4 level in the IVT. Patients who had Nox4high in the index lesion showed rapid disease progression regardless of the Nox4 level in the IVT (Figure 6).
Figure 6.
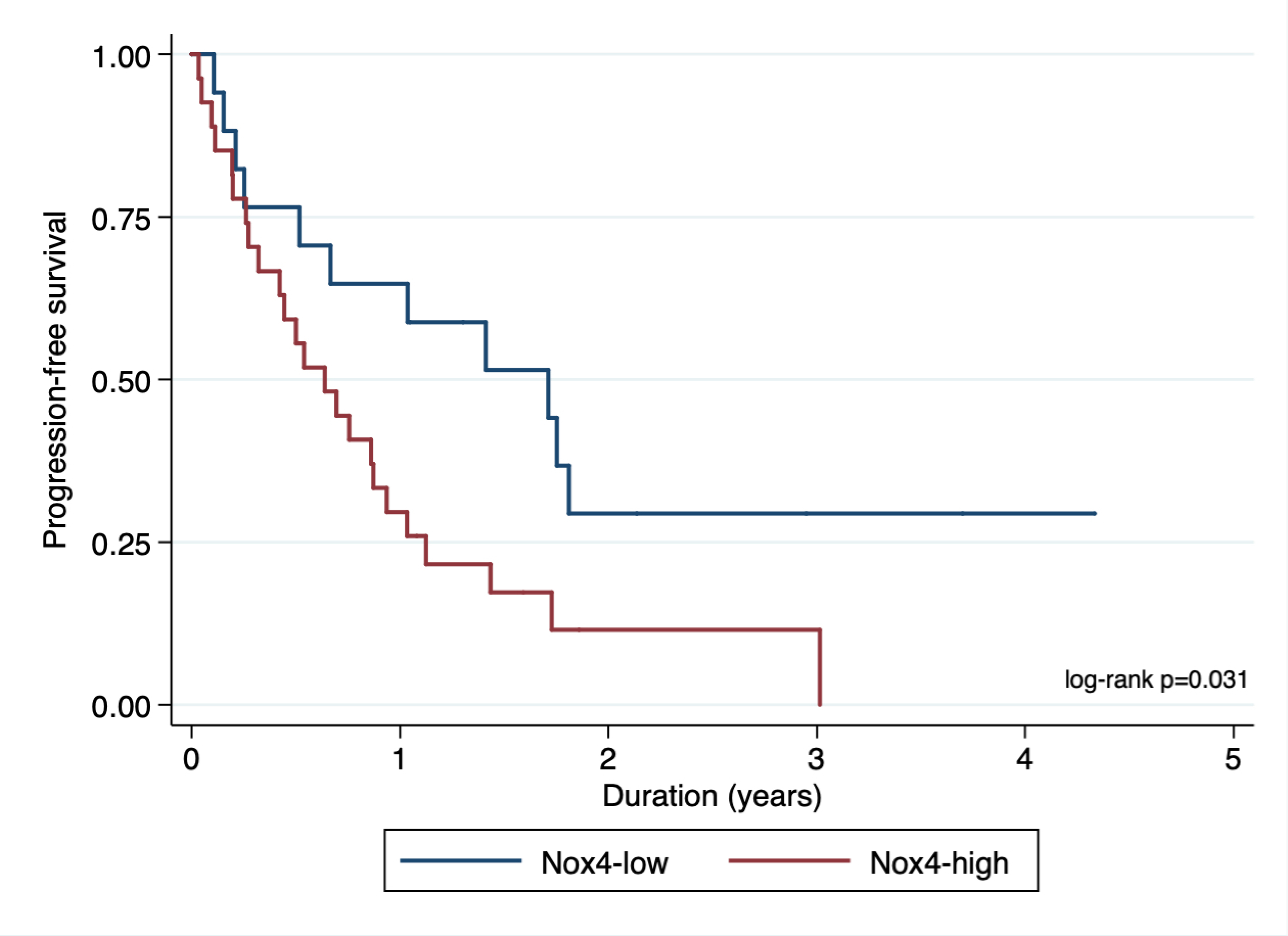
Progression-free survival in patients with RITE when stratified according to the Nox4 level in the index tumor. Nox4high: Nox4 ≥ 26.13 in RITE index tumor tissue. Nox4low: Nox4 <26.13 in RITE index tumor tissue. Hazard ratio 2.23, 95% confidence interval (1.07–4.63), p=0.031. RITE, renal intravascular tumor extension.
We found a significant correlation between higher nuclear Nox4 expression and development of resistance to targeted therapy (Nox4high vs Nox4low; HR 4.25, 95% CI 1.35–13.37; p=0.013; Figures 7a and 7b) We also observed that as patient failed multiple targeted therapy, their relative risk of having higher nuclear Nox4 increased.
Figure 7a.

CONSORT diagram showing resistance to targeted therapy
Figure 7b.
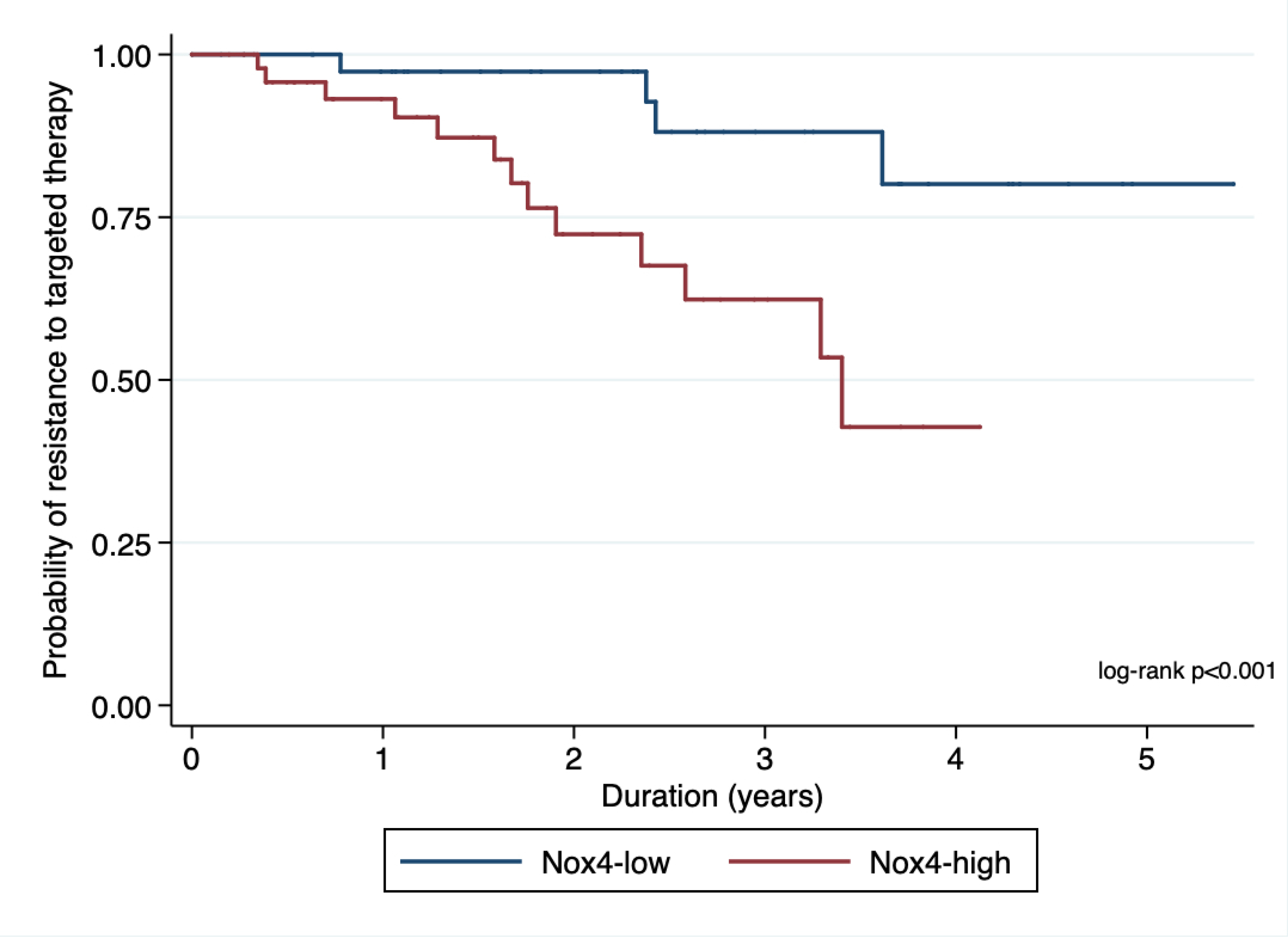
Kaplan-Meier plot showing association of nuclear Nox4 with resistance to targeted therapy.
Increased Expression of Nox4 in Nuclear Fractions of Human RCC and Cell Lines
We examined the nuclear protein expression of Nox4 in a panel consisting of fresh human RCC primary tissue, CANA, and IVTs using immunoblotting. Western blot analyses of nuclear tissue lysates demonstrated markedly higher Nox4 protein levels in the primary tumor and IVT than in CANA (Figure 8).
Figure 8.
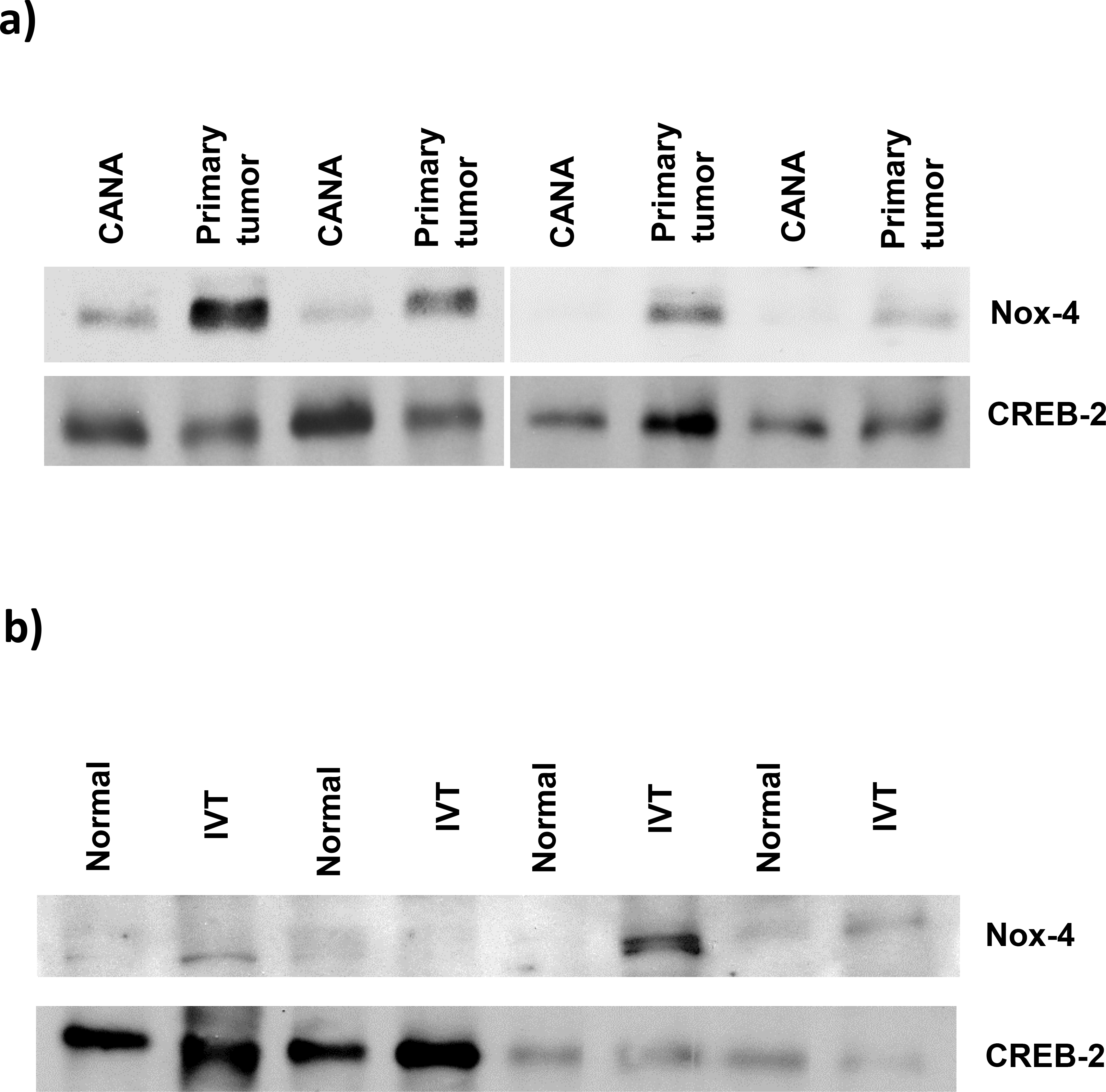
Nuclear expression of Nox4 in fresh human renal cell carcinoma tissue. (a) The nuclear protein fraction was isolated from the RCC primary index tumor and cancer-associated normal adjacent parenchyma (CANA). Nuclear proteins were immunoblotted for Nox4 levels. CREB-2 immunoblot was included as a loading control. (b) Nuclear protein isolated from an intravascular tumor (IVT) and matched cancer-associated normal adjacent parenchyma (CANA) was immunoblotted for Nox4 protein levels. CREB-2 immunoblot was included as a loading control.
Next, we examined nuclear Nox4 protein levels in a panel of established RCC cell lines (786-O, A498, RCC4, RCC-10, and UOK-262). The Nox4 protein levels were higher in the nuclear fractions of all cultured cell lines than those in a normal renal epithelial (HK-2) cell line (Figure 9).
Figure 9.
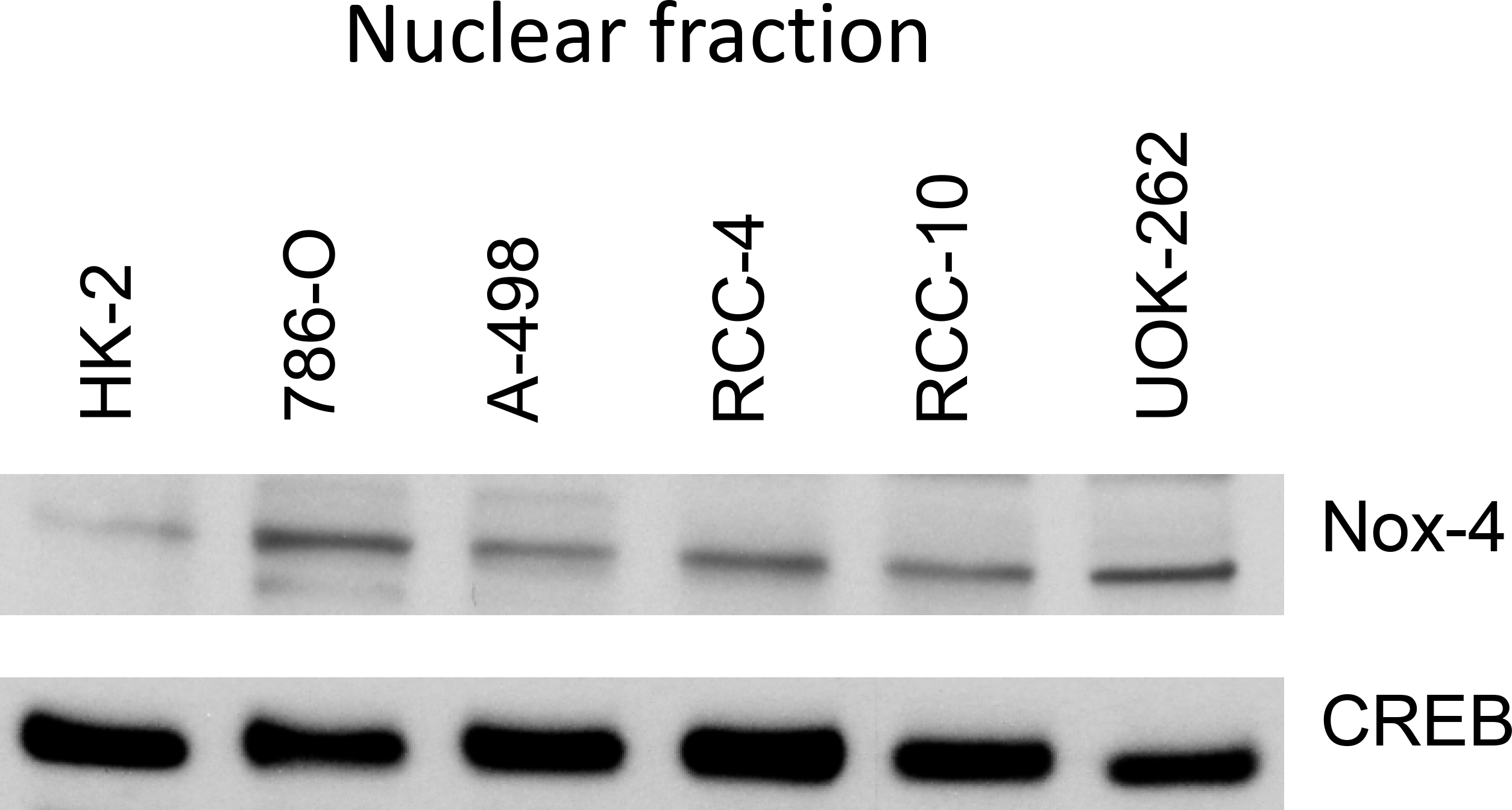
Nuclear Nox4 protein levels in renal cell carcinoma lines relative to that in a normal renal epithelial (HK-2) cell line. CREB was included as a loading control.
Discussion
There is an urgent unmet need for developing novel prognostic biomarkers to ensure adequate risk stratification and predict development of resistance to targeted therapy and immunotherapy in patients with RCC. In this context, we have studied the clinical relevance of nuclear Nox4 staining patterns in human RCC and found nuclear Nox4 to be upregulated at the translational level. Furthermore, results from survival analyses suggest that nuclear Nox4 expression may be an independent adverse prognostic marker in patients with high-grade/high-stage RCC. In addition, our analysis indicates that high nuclear Nox4 expression is an important driver of resistance to targeted therapy. We confirmed our clinical data by building TMAs of high-grade RCC and demonstrated high in-vitro expression of nuclear Nox4 in RCC cell lines and fresh human RCC tissue.
Our group has previously confirmed elevated expression and activity of Nox4 in RCC cell lines and in fresh human tissue collected at the time of surgery. [7–9] We have also demonstrated that Nox4 is an energetic sensor that can serve as a checkpoint for coupling mitochondrial energy metabolism to drug resistance in RCC cells. [8] In the present study, higher nuclear Nox4 activity seen in high-grade, high-stage RCC further underscores the pivotal role played by Nox4 in the pathogenesis of RCC. Our data suggest that a high level of nuclear Nox4 activity is a necessary component of the metabolic program that enables progression of high-stage, high-grade RCC, in part through drug resistance (Figure 7a and 7b). Our analysis of patients who progressed on targeted therapy indicates that high nuclear Nox4 expression is an important driver of resistance to targeted therapy. In contrast, patients with low nuclear Nox4 expression, even those with high-stage, high-grade RCC, are responsive to targeted therapy and have better PFS and OS (Figures 5a and 5b).
These observations suggest fundamental differences in the molecular underpinnings of high and low nuclear Nox4 levels in RCC. When evaluating the prognostic impact of nuclear Nox4 in the RITE and non-RITE groups, we found that patients with Nox4high were more likely to have rapid disease progression and that this effect was more pronounced in those with RITE. To test the clinical relevance of this finding further, we stratified patients with RITE according to the Nox4 status of their primary lesion regardless of the level of tumor extension into the inferior vena cava. As shown in Figure 6, the level of Nox4 in the index tumor was the primary driver of progression. Subcellular localization is important for the signaling functions of Nox4; hence our effort to determine the timing of nuclear localization of Nox4 (in the index tumor as an early event and in the IVT as a later event).
There are emerging data in melanoma [21] and in pancreatic [22], thyroid [23], and ovarian [24] cancers indicating that elevated Nox4 expression can induce oxidative DNA damage via the increased intracellular ROS generated by Nox4. Therefore, targeted therapies are being developed to exploit the increased expression of Nox4 in prostate, colon, rectal, and pancreatic cancers. [25, 26 ] Our data, build upon these in-vitro studies, further provides clinically translational evidence suggesting that subcellular translocation of NOX4 from the cytoplasm to the nucleus plays an important role in cancer progression in RCC.
In RCC, there is evidence suggesting that Nox4 is directly responsible for expression of hypoxia-inducible factor (HIF)1α [11],HIF-2α [27] and is critical to maintaining its activity even in Von Hippel-Lindau-deficient RCC. Given that loss of the Von Hippel-Lindau tumor suppressor gene requires two hits, Nox4 conceivably has a role in the loss of function of this or other genes mediating progression or metastasis of RCC. There is emerging evidence to suggest that complex interactions in the Nox4/ROS/HIF/VEGF pathway may be involved in modulation and development of resistance to targeted therapy and immunotherapy. [28] Our clinicopathologic survival data on development of resistance to targeted therapy supported by immunohistochemical staining of human RCC tissue corroborate these findings.
In order to avoid statistical pitfall of inferring a causal relationship between presence of nuclear Nox4 and poor prognosis in RCC patients, we studied the biological gradient (“dose-response relationship”, figures 3a and 3b). In addition, to control other significant confounding variables, we performed multivariate analysis. We performed a robust statistical analysis to identify optimal discriminator value for OS and PFS/Nuclear Nox4 H-score. For every potential cutoff point, the absolute value of the standardized log-rank statistic was computed. The cutoff point that provided the best separation of the survival outcome into two groups, where the standardized statistics reached their maximum, was utilized to stratify survival analyses. Our results consistently showed that high nuclear Nox4 seems to define a subgroup of patients with RCC who have a poor prognosis. Importantly, the prognostic role of Nox4 was evident in our study independent of age, sex, RITE status, histology and TNM-staging, underscoring the value of nuclear Nox4 as a potential biomarker.
The molecular role of Nox4 in RCC is being elucidated. Nox4 regulates HIF-2α activity and mitochondrial reprogramming and directly modulates production of H2O2 in response to hypoxia. H2O2 causes single-strand breaks and DNA damage in a concentration-dependent manner. If this is coupled with increased replication fork stress, such injury could result in more somatic copy number alterations (SCNA). [29] In the current model of RCC, distinct evolutionary trajectories are described, i.e., linear, branched, and punctuated. [18, 20] The punctuated trajectory is characterized by low intra-tumoral heterogeneity with elevated SCNA, which appears to be associated with rapid disease progression. It is plausible that the high nuclear Nox4 seen in our data was a driver for higher intranuclear ROS, especially H2O2, which leads to DNA damage, resulting in multiple SCNA and poor OS and PFS. We identified a subset of patients with high nuclear Nox4 who had rapid disease progression or died within 6 months of surgery (Table 3b). These patients would be ideal candidates for a study of survival outcomes using adjuvant targeted therapy that includes a Nox4 inhibitor in the future.
We recognize the limitations of this study. Most important, the inferences from our results are confounded by the selection bias of the retrospective non-randomized design over a prolonged time period. Robust statistical methods for clinical correlation and corroborating evidence from Western Blot test was utilized for bias reduction. We also acknowledge small sample size for evaluating development of drug resistance. Further future studies with large sample size are required to confirm our findings. Our results highlight the pivotal role of nuclear Nox4 in prognosis and development of targeted therapy resistance in RCC patients. Given that there is an urgent unmet need for developing strategies to ensure adequate risk stratification and predict development of resistance to targeted and immunotherapy, these results further contribute toward development of a novel prognostic biomarker. We acknowledge that the findings of this study will require external validation.
Conclusions
This large-scale study in RCC human tissue and cell lines provides evidence that nuclear expression and compartmentalization of Nox4 may be a prognostic biomarker. Our results indicate that nuclear localization of Nox4 constitutes a major molecular abnormality in high-grade/high-stage RCC with a considerable prognostic impact, including development of drug resistance. Subcellular localization of Nox4 may be important for its signaling functions. Mechanistic studies are required to elucidate the pathophysiologic effects of Nox4 on intracellular redox signaling and DNA damage. High nuclear Nox4 staining in index lesions may be a marker of an aggressive RCC phenotype.
Acknowledgements
The manuscript was copy-edited by Susan Albrecht at MedSurgBio Ltd.
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. We believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare. All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors. All authors have read the journal’s policy on disclosure of potential conflicts of interest. There are no conflict of interest by any authors.
Financial support
This project was supported by a grant from the Littenberg Foundation to DK. The content is solely the responsibility of the authors and does not necessarily represent the views of the Littenberg Foundation.
Tissue Microarray (TMA) construction as well as Aperio imaging was performed using the UT Health San Antonio Mays Cancer Center Genomics Shared Resource Facility (NCI P30 CA054174) that also receives institutional support.
Abbreviations:
- Nox4
Nuclear nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-4
- RCC
Renal cell carcinoma
- ROS
reactive oxygen species
- TMAs
Tissue microarrays
- RITE
Renal intravascular tumor extension
- IVT
Intravascular tumor
- CANA
Cancer-associated normal adjacent parenchyma
- OS
Overall survival
- PFS
Progression-free survival
- SCNA
Somatic copy number alterations
Footnotes
Conflicts of interest: The authors have declared that no conflict of interest exists
Data sharing
Data are available for bona fide researchers who request them from the authors.
References:
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Courtney KD, Bezwada D, Mashimo T, et al. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell metabolism. 2018;28:793–800.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wettersten HI, Aboud OA, Lara PN Jr., et al. Metabolic reprogramming in clear cell renal cell carcinoma. Nature reviews Nephrology. 2017;13:410–9. [DOI] [PubMed] [Google Scholar]
- [4].Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cellular and molecular life sciences : CMLS. 2012;69:2435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Landry WD, Cotter TG. ROS signalling, NADPH oxidases and cancer. Biochemical Society transactions. 2014;42:934–8. [DOI] [PubMed] [Google Scholar]
- [6].Roy K, Wu Y, Meitzler JL, et al. NADPH oxidases and cancer. Clinical science (London, England : 1979). 2015;128:863–75. [DOI] [PubMed] [Google Scholar]
- [7].Block K, Gorin Y, Hoover P, et al. NAD(P)H oxidases regulate HIF-2alpha protein expression. The Journal of biological chemistry. 2007;282:8019–26. [DOI] [PubMed] [Google Scholar]
- [8].Shanmugasundaram K, Nayak BK, Friedrichs WE, et al. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nature communications. 2017;8:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Block K, Gorin Y, New DD, et al. The NADPH oxidase subunit p22phox inhibits the function of the tumor suppressor protein tuberin. Am J Pathol. 2010;176:2447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gregg JL, Turner RM 2nd, Chang G, et al. NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2alpha. Cancer Res. 2014;74:3501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fitzgerald JP, Nayak B, Shanmugasundaram K, et al. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS One. 2012;7:e30712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eun HS, Chun K, Song IS, et al. High nuclear NADPH oxidase 4 expression levels are correlated with cancer development and poor prognosis in hepatocellular carcinoma. Pathology. 2019;51:579–85. [DOI] [PubMed] [Google Scholar]
- [14].Libertino JA, Zinman L, Watkins E Jr. Long-term results of resection of renal cell cancer with extension into inferior vena cava. J Urol. 1987;137:21–4. [DOI] [PubMed] [Google Scholar]
- [15].Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:390–5. [DOI] [PubMed] [Google Scholar]
- [16].Kampf C, Olsson I, Ryberg U, et al. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. Journal of visualized experiments : JoVE. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McCarty KS Jr., Szabo E, Flowers JL, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–8s. [PubMed] [Google Scholar]
- [18].Turajlic S, Xu H, Litchfield K, et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell. 2018;173:581–94.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wright MN, Dankowski T, Ziegler A. Unbiased split variable selection for random survival forests using maximally selected rank statistics. Stat Med. 2017;36:1272–84. [DOI] [PubMed] [Google Scholar]
- [20].Turajlic S, Xu H, Litchfield K, et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell. 2018;173:595–610.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamaura M, Mitsushita J, Furuta S, et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–54. [DOI] [PubMed] [Google Scholar]
- [22].Vaquero EC, Edderkaoui M, Pandol SJ, et al. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. The Journal of biological chemistry. 2004;279:34643–54. [DOI] [PubMed] [Google Scholar]
- [23].Tang P, Dang H, Huang J, et al. NADPH oxidase NOX4 is a glycolytic regulator through mROS-HIF1alpha axis in thyroid carcinomas. Scientific reports. 2018;8:15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xia C, Meng Q, Liu LZ, et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. [DOI] [PubMed] [Google Scholar]
- [25].Sampson N, Brunner E, Weber A, et al. Inhibition of Nox4-dependent ROS signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int J Cancer. 2018;143:383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tang CT, Gao YJ, Ge ZZ. NOX4, a new genetic target for anti-cancer therapy in digestive system cancer. Journal of digestive diseases. 2018;19:578–85. [DOI] [PubMed] [Google Scholar]
- [27].Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Seebacher NA, Richardson DR, Jansson PJ. Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics. British journal of pharmacology. 2015;172:2557–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ye B, Hou N, Xiao L, et al. Dynamic monitoring of oxidative DNA double-strand break and repair in cardiomyocytes. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2016;25:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]