Abstract
Exposure to endocrine disrupting chemicals (EDCs) is ubiquitous. EDC exposure, especially during critical periods of development like the prenatal window, may interfere with the body’s endocrine system, which can affect growth and developmental outcomes such as puberty. Most studies have examined one EDC at a time in relation to disease; however, humans are exposed to many EDCs. By studying mixtures, the human experience can be more closely replicated. We investigated the association of prenatal exposure to persistent EDCs (poly- and perfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs)) as mixtures with early menarche among female offspring in a nested case-control study within the Avon Longitudinal Study of Parents and Children (ALSPAC) recruited in the United Kingdom in 1991–1992. Concentrations of 52 EDCs were quantified in maternal serum samples collected during pregnancy. Daughter’s age at menarche was ascertained through mailed questionnaires sent annually. We used repeated holdout weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR) to examine the association between prenatal exposure to multiple EDCs and early menarche (<11.5 (n = 218) vs. ≥11.5 years (n = 230)) for each chemical class separately (PFAS, PCBs, and OCPs) and for all three classes combined. Models adjusted for maternal age at menarche, maternal education, parity, pre-pregnancy body mass index, maternal age, prenatal smoking, and gestational week at sample collection. Mixture models showed null associations between prenatal exposure to EDC mixtures and early menarche. Using WQS regression, the odds ratio for early menarche for a one-decile increase in chemical concentrations for all three classes combined was 0.89 (95% CI: 0.76, 1.05); using BKMR, the odds ratio when all exposures were at the 60th percentile compared to the median was 0.98 (95% CI: 0.91, 1.05). Results suggest the overall effect of prenatal exposure to persistent EDC mixtures is not associated with early menarche.
Keywords: Puberty, Menarche, Poly- and perfluoroalkyl substances, Polychlorinated biphenyls, Organochlorine pesticides, ALSPAC
1. Introduction
Puberty is a critical phase of development and growth. The timing and patterning of milestones during puberty can offer insight into general health and earlier exposures, while possibly forecasting later health (Golub et al., 2008; Biro et al., 2001), like breast cancer (Kelsey et al., 1993). Menarche, which is a girl’s first menstrual period, has been a frequently utilized marker of pubertal development because of its clearly observable occurrence and accurate recall even years later (Cairns et al., 2011; Cooper et al., 2006; Must et al., 2002).
On average, age at menarche has trended younger since the end of the 19th century (Wyshak and Frisch, 1982; Zacharias and Wurtman, 1969) and earlier occurrence of secondary sexual characteristics has also been observed (Rubin et al., 2009). Current estimates of mean age at menarche (12.4 years) are close to one year younger than the mean age at menarche of women born in the 1920s (13.3 years); further, decreases in average age at menarche have been seen across races and ethnicities in the United States (McDowell et al., 2007). There are a number of factors potentially contributing to this trend of altered pubertal timing and patterning, including improvements in nutrition, a higher prevalence of childhood obesity, and exposure to endocrine disrupting chemicals (EDCs) (Herman-Giddens et al., 1997; Blanck et al., 2000; Biro et al., 2012; Buck Louis et al., 2008).
The National Institute of Environmental Health Sciences (NIEHS) defines an EDC as a chemical that may interfere with the body’s endocrine system and produce adverse developmental, immune, neurological, and reproductive effects in humans (National Institute of Env, 2019). Environmentally persistent EDCs, such as poly- and perfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs), are often very resistant to degradation, likely to bioaccumulate in living organisms, and have been used throughout the 20th and 21st centuries for a variety of purposes (Agency for Toxic Substanc, 2000; Agency for Toxic Substanc, 2002; Agency for Toxic Substanc, 2009). Many countries have banned or severely limited the production, handling, and disposal of several PCBs and OCPs and certain PFAS. While exposure appears to have declined in the general population, nearly every human has detectable concentrations of some of these chemicals (Schoeters et al., 2017; Centers for Disease Contr, 2017). Further, biologically persistent EDCs are able to cross the placental barrier, leading to potential fetal exposure (Tang et al., 2018; Sala et al., 2001; Kezios et al., 2012; Inoue et al., 2004). Exposure to EDCs during critical windows of vulnerability, such as the prenatal period, can lead to increased risks of disease and disability across the lifespan (The American College of O, 2013). EDCs in maternal and cord sera are strongly correlated with each other (Kim et al., 2011). EDC concentrations tend to be higher in maternal serum than cord serum, and characteristics such as parity potentially influence the transfer of EDCs from mother to fetus (Zhang et al., 2018; Patayová et al., 2013). Maternal age, pre-pregnancy body mass index (BMI), and parity are often predictive of maternal serum concentrations of EDCs (Zhang et al., 2018; Workman et al., 2019).
EDCs may affect synthesis, binding, bioavailability, and metabolism of steroid and thyroid hormones (Diamanti-Kandarakis et al., 2009). EDCs may interfere with pubertal development and reproductive function through actions at various levels, including changes to neuroendocrine signaling, the hypothalamic-pituitary axis, the gonads, and peripheral target organs such as breasts, hair follicles, and genitals (Mouritsen et al., 2010). Prior epidemiologic studies of prenatal exposure to persistent EDCs and age at menarche have shown mixed results. Previous examinations of prenatal PFAS exposure and age at menarche have shown no association (Christensen et al., 2011), earlier menarche (Ernst et al., 2019), and later menarche (Kristensen et al., 2013). Prenatal PCB exposure was not associated with age at menarche in previous studies (Blanck et al., 2000; Kristensen et al., 2016; Leijs et al., 2008), though some observed weak associations with early menarche (Gladen et al., 2000; Vasiliu et al., 2004). Previous studies of prenatal OCP exposure and age at menarche have shown both null results (Kristensen et al., 2016; Namulanda et al., 2016) and associations with earlier menarche, namely for dichlor-odiphenyldichloroethylene (DDE) (Gladen et al., 2000; Vasiliu et al., 2004).
Most studies to date have examined one EDC at a time in relation to health outcomes, and this may have led to inconsistent results in the association between prenatal exposure to EDCs and growth and developmental outcomes in offspring. Because humans are exposed to many EDCs, the human experience can be more closely replicated by studying combined exposures, or “mixtures” (National Institute of Env, 2018). In this context, NIEHS defines an environmental mixture as a combination of three or more independent chemicals or chemical groups (National Institute of Env, 2017).
While there have been a number of studies examining prenatal exposure to persistent EDCs and age at menarche, none have examined persistent EDCs as a mixture. Our aim was to investigate the association of maternal gestational concentrations of 52 persistent EDCs (PFAS, PCBs, and OCPs) and prospectively collected age at menarche data in a nested case-control study of a population-based birth cohort.
2. Materials and methods
2.1. Study population
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a continuing prospective birth cohort following 14,541 pregnancies. Pregnant women from three health districts in the former county of Avon, Great Britain were enrolled in ALSPAC. To be enrolled, women needed to have an expected delivery date between April 1,1991 and December 31, 1992. ALSPAC collected information on parents and children through clinic visits, interviews, and mailed questionnaires. Details on study recruitment and methods have previously been described (Boyd et al., 2013; Fraser et al., 2013). A nested case-control study (N = 448) was conducted to investigate associations of prenatal concentrations of EDCs and early menarche among the daughters (Fig. S1). The nested case-control study design has previously been described in detail (Christensen et al., 2011). Briefly, from the original base population of 14,062 live births, cases and controls were selected from singleton daughters who had completed at least two (out of five possible) puberty staging questionnaires at 8, 9, 10, 11, or 13 years old. A cut-off of 11.5 years was selected to define ‘early’ menarche. To be eligible, cases had to complete at least two questionnaires, with one needing to be completed after menarche. Controls had to return the 13-year old questionnaire to establish that menarche had not occurred before the cutoff of 11.5 years.
The study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bris.ac.uk/alspac/researchers/our-data/). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee, the Local Research Ethics Committees, and the Centers for Disease Control and Prevention (CDC) Institutional Review Board. Consent for biological samples was collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
2.2. Exposure assessment
Maternal fasting blood samples were collected from mothers during pregnancy at median 15 (interquartile range (IQR): 10e28) weeks gestation. Samples were collected in 1991–1992, and processed and frozen at −80 °C for later analysis. Maternal serum samples were stored at the University of Bristol until they were transferred under controlled conditions and analyzed at the CDC National Center for Environmental Health (Atlanta, GA). Laboratory analyses included low- and high-concentration pooled quality control materials, standards, reagent blanks, and study samples. Concentrations below the limit of detection (LOD) were imputed by dividing the LOD by the square root of 2 prior to statistical analysis. EDCs detected in greater than 75% of mothers were included in the main analyses.
2.2.1. Poly- and perfluoroalkyl substances
Eight PFAS were quantified (Table S1) in serum via on-line solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry (Kuklenyik et al., 2005). LODs were 0.082 (PFNA), 0.10 ng/mL (FOSA, PFHxS, PFOA), 0.174 (MeFOSAA), and 0.20 ng/mL (EtFOSAA, PFOS, PFDA). Coefficients of variation (CVs) were generally below 10%.
2.2.2. Organochlorine pesticides and polychlorinated biphenyls
Nine OCPs and 35 PCBs were measured (Table S1) in serum using gas chromatography isotope dilution high resolution mass spectrometry (Sjödin et al., 2004). PCB congeners 138 and 158 could not be separated and were quantified as a summed concentration hereafter referred to as PCB138. Similarly, PCB congeners 196 and 203 could not be separated and were quantified as a summed concentration hereafter referred to as PCB196. LODs for PCBs and OCPs are dependent on the size of the sample available, thus an individual LOD was reported for each individual result rather than an overall LOD. CVs were generally below 10%.
2.3. Outcome assessment
A ‘Growing and Changing’ questionnaire was used to collect information on pubertal development. The questionnaire was mailed annually to participants between the ages of 8–17 years (1999–2008), except in the year 2003 due to funding constraints. The parent or child reported menarche status. If it had occurred, month and year of occurrence was reported and used to calculate age at menarche. Age at menarche was reported quite consistently across questionnaires: correlation between adjacent questionnaires was high (r = 0.93) and 258 (58%) of girls reported the same age at menarche in each response and another 142 (32%) never reported ages differing by more than one year.
2.4. Covariates
Covariate information was collected by clinical staff or through self-report on questionnaires completed by the mother during or immediately after pregnancy. Covariates under consideration include: gestational age at biological sample collection (weeks), maternal age at delivery (years), maternal pre-pregnancy BMI (kg/m2), maternal race ethnicity (white/nonwhite), maternal education (defined as < ordinary level (O-level: required and completed at 16 years of age), O-level, or > O-level), parity (nulliparous/multiparous), smoking during pregnancy (any/none), hours of physical activity (enough to work up a sweat) per week during pregnancy (>0 h/0 h), and maternal age at menarche (years). Breastfeeding the index child (yes/no and duration) was considered as an effect modifier.
2.5. Statistical analyses
Descriptive analyses were conducted to compare mother-daughter dyad characteristics by case-control status using chi-square tests. Wilcoxon rank sum tests were utilized to compare median EDC concentrations by case-control status.
The chemical concentrations under study were modeled as natural log-transformed continuous variables. Per the nested case-control study design, age at menarche was dichotomized as early (<11.5 years; cases) versus not early menarche (≥11.5 years; controls) (Christensen et al., 2011). Confounding was evaluated using previous knowledge which we assessed using a directed acyclic graph (DAG) and by taking into consideration the associations between persistent EDCs with maternal characteristics. All models were adjusted for maternal age at menarche, maternal education, parity, pre-pregnancy body mass index, maternal age at delivery, prenatal smoking, and gestational week at sample collection and included all participants with complete data on relevant exposures and covariates (Fig. S2).
First, we ran single-chemical logistic regression models to examine independent associations between each chemical and early menarche. Next, we ran multi-chemical logistic regression models to examine associations between each chemical in a class (e.g., PFAS) and early menarche, independent of other chemicals in the class (e.g., adjusting for other chemicals in the class). Sensitivity analyses were conducted comparing the odds of early menarche among those with versus without detectable concentrations.
Bayesian kernel machine regression (BKMR) was used to visualize the exposure-response function and verify assumptions using the R package bkmr (Bobb et al., 2018; Bobb et al., 2015; Coull et al., 2015). In the case of no identification of non-linearity and or interaction within the mixture through BKMR, weighted quantile sum (WQS) regression was used to estimate associations of maternal EDC mixtures with early menarche using the R package gWQS (Renzetti et al., 2021). Mixtures under study included each chemical class separately (PFAS, PCBs, and OCPs) and all three chemicals classes combined.
WQS regression allows for the creation of a weighted linear index of correlated predictors that are weighted by their strength of association with the outcome of interest (Carrico et al., 2015). Specifically, the equation seeks to calculate the weights of c set of correlated variables:
The sum term is the index for c items, scored into quantiles (denoted qi), and weights are signified by the sum of wi. Each wi is constrained between 0 and 1. All covariates are represented by z’ϕ. Before analysis, the data are randomly split into two datasets: a training dataset (40%) and a validation dataset (60%). Bootstrap samples (n = 100) are selected using the training dataset, and the strength of the associations for each c item is determined by the beta coefficient (Carrico et al., 2015). The index is calculated based on the average wis across all bootstrap samples and is readily interpretable as an estimation of the total mixture effect (Carrico et al., 2015; Christensen et al., 2013; Czarnota et al., 2015; Gennings et al., 2010). To improve the stability of the estimates of weights across training and validation data partitions, repeated holdout validation was applied; this approach combines cross-validation and bootstrap resampling (Tanner et al., 2019). A distribution of results was generated by repeating WQS regression 100 times on data split randomly into training (40%) and validation (60%) sets and the mean was taken as the final estimate.
Bayesian kernel machine regression (BKMR) was used as a complementary mixture method to WQS regression. BKMR is a flexible semi-parametric technique that models the combined effects of different chemicals, while also allowing for nonlinearity and interactions among chemicals (Gibson et al., 2019). This approach allows for the examination of independent effects of mixture members, interactions among them, and the overall mixture effect. We used hierarchical variable selection to obtain group importance scores (posterior inclusion probabilities (PIPs)) for pre-defined mutually exclusive groups of variables. Additionally, we estimated the importance of a chemical given that the group that contained the chemical was important (conditional PIPs) (Bobb et al., 2018; Bobb et al., 2015; Coull et al., 2015). Within BKMR, we standardized all continuous variables to improve computational efficiency. SAS software 9.4 (Cary, NC) was used for descriptive analyses. R software 3.5.0 (Vienna, Austria) was used for WQS regression and BKMR analyses.
3. Results
3.1. Descriptive statistics
The study sample consisted of predominantly white mothers (>97%) who achieved secondary levels of education or higher (81.9%) (Table 1). About half of mothers were nulliparous (49.6%) and most were 25 years or older (79.3%). Some mothers smoked during pregnancy (18.5%) and the majority were physically active during pregnancy (≥1 h per week) (65.5%). Mothers of cases were more likely to be non-white (3.3% among case mothers versus <2.2% among control mothers) and to have experienced early menarche (between 8 and 11 years) themselves (32.5% versus 15.2%). Additionally, case mothers were more likely to enter pregnancy at an overweight or obese BMI (≥25 kg/m2) (29.4% versus 15.1%).
Table 1.
Characteristics of the Avon Longitudinal Study of Parents and Children (ALSPAC) nested case-control study population (N = 448 mother-daughter dyads).
| Characteristica,b | Menarche <11.5 years Cases (n = 218) | Menarche >11.5 years Controls (n = 230) |
|---|---|---|
| n (%) | n (%) | |
| Maternal race | ||
| White | 205(96.7)e | 218(−)ce |
| Non-white | 7(3.3)e | <5(−)ce |
| Maternal educationd | ||
| < O-level | 43(21.0) | 32(15.2) |
| O-level | 67(32.7) | 73(34.8) |
| >O-level | 95(46.3) | 105(50.0) |
| Maternal age at menarche, years | ||
| 8–11 | 63(32.5)e | 30(15.2)e |
| >12 | 131(67.5)e | 168(84.8)e |
| Maternal pre-pregnancy BMI, kg/m2 | ||
| <25 (under/normal weight) | 139(70.6)e | 174(84.9)e |
| >25 (overweight/obese) | 58(29.4)e | 31(15.1)e |
| Prenatal smoking | ||
| Any | 45(21.4) | 34(15.7) |
| None | 165(78.6) | 183(84.3) |
| Physical activity | ||
| Any | 123(66.5) | 129(64.5) |
| None | 62(33.5) | 71(35.5) |
| Maternal age at delivery, years | ||
| <25 | 44(20.4) | 48(21.0) |
| 25–29 | 83(38.4) | 81(35.4) |
| >30 | 89(41.2) | 100(43.7) |
| Child birth order | ||
| First born | 110(53.9) | 98(45.6) |
| Second born or later | 94(46.1) | 117(54.4) |
| Child birth weight, g | ||
| <2500 | 7(3.3) | 10(4.4) |
| >2500 | 208(96.7) | 215(95.6) |
| Breastfeeding | ||
| Any | 164(80.8) | 174(80.2) |
| None | 39(19.2) | 43(19.8) |
| Gestational age at sample, weeks | ||
| <20 | 147(67.4) | 150(65.2) |
| >20 | 71(32.6) | 80(34.8) |
Abbreviations: g, grams; kg/m2, kilograms per meter-squared.
Compared using chi-square tests (or Fisher’s exact test, where appropriate).
Percentages are among mothers with non-missing data for each characteristic. Data were missing on maternal race (n = 17, 3.8%), maternal education (n = 33, 7.4%), maternal age at menarche (n = 56, 12.5%), maternal pre-pregnancy BMI (n = 46, 10.3%), prenatal smoking (n = 21, 4.7%), physical activity (n = 63, 14.1%), maternal age at delivery (n = 3, 0.7%), child birth order (n = 29, 6.5%), child birth weight (n = 8, 1.8%), and breastfeeding (n = 28, 6.3%). Gestational age at sample data were complete (n = 0, 0.0%).
Counts and percents suppressed due to small cell sizes.
< O-level = none, Certificate of Secondary Education, and vocational education, which are equivalent to no diploma or a GED in the United States. O-levels (ordinary levels) are required and completed at the age of 16. >O-level = A-levels (advanced levels) completed at 18, which are optional, but required to get into university; and a university degree.
Cases and controls are significantly different (p < 0.05).
There were associations between maternal characteristics and concentrations of EDCs (PFOA, PCB153, and p,p’-DDE were selected as representative EDCs) (Table S2). Higher maternal education was associated with higher PCB153 and p,p’-DDE concentrations. Older maternal age was also associated with higher PCB153 and p,p’-DDE concentrations. Mothers carrying their first-born child had higher concentrations of PFOA.
Of the 52 chemicals measured, 31 chemicals were detected in greater than 75% of mothers. Certain OCPs were very rarely detected (<2% of samples > LOD) (e.g., o,p′-DDT and Mirex) and certain PCBs were also rarely detected (e.g., PCB128 and PCB151) (Table 2). The majority of PFAS were detected in most samples, except for PFDA (<3% of samples > LOD).
Table 2.
Serum concentrations of persistent endocrine disrupting chemicals among mothers of the Avon Longitudinal Study of Parents and Children (ALSPAC) during pregnancy by age at menarche of their daughters (N = 448 mother-daughter dyads).
| Menarche <11.5 years Cases | Menarche >11.5 years Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Median | Q3 | % <LODa | Q1 | Median | Q3 | % <LODa | |
| Per- and polyfluoroalkyl substances (PFAS) (ng/mL) | ||||||||
| PFOA | 2.9 | 3.85 | 5.0 | 0.0% | 2.7 | 3.6 | 4.7 | 0.0% |
| PFOS | 15.4 | 19.5 | 24.8 | 0.0% | 14.6 | 20.0 | 24.9 | 0.0% |
| PFHxS | 1.3 | 1.7 | 2.2 | 0.0% | 1.2 | 1.6 | 2.2 | 0.4% |
| PFNA | 0.41 | 0.57 | 0.66 | 0.5% | 0.41 | 0.49 | 0.66 | 0.0% |
| FOSA | <LOD | 0.1 | 0.3 | 29.8% | <LOD | 0.2 | 0.3 | 31.3% |
| MeFOSAA | 0.26 | 0.35 | 0.61 | 17.9% | 0.26 | 0.35 | 0.70 | 11.3% |
| EtFOSAA | 0.4 | 0.7 | 1.0 | 2.8% | 0.4 | 0.6 | 0.9 | 2.2% |
| PFDA | <LOD | <LOD | <LOD | 97.7% | <LOD | <LOD | <LOD | 97.0% |
| Polychlorinated biphenyls (PCBs) (ng/g lipid) | ||||||||
| PCB28 | 3.6 | 5.6 | 8.4 | 7.8% | 3.5 | 5.2 | 8.1 | 9.6% |
| PCB44 | <LOD | 1.8 | 4.0 | 29.8% | <LOD | 2.0 | 3.9 | 30.9% |
| PCB49 | <LOD | <LOD | 1.8 | 60.6% | <LOD | <LOD | 1.9 | 56.1% |
| PCB52 | <LOD | 3.1 | 7.7 | 30.3% | <LOD | 3.4 | 7.2 | 30.0% |
| PCB66 | <LOD | 1.6 | 2.6 | 29.4% | <LOD | 1.6 | 2.5 | 31.3% |
| PCB74 | 8.4 | 11.1 | 15.1 | 0.5% | 8.6 | 11.1 | 15.2 | 0.0% |
| PCB87 | <LOD | <LOD | 1.5 | 60.6% | <LOD | <LOD | 1.8 | 58.7% |
| PCB99 | 6.6 | 9.4 | 11.9 | 0.9% | 7.2 | 9.3 | 12.2 | 0.9% |
| PCB101 | <LOD | 2.2 | 5.1 | 33.0% | <LOD | 2.2 | 5.9 | 27.8% |
| PCB105 | 2.0 | 3.0 | 4.1 | 6.4% | 2.0 | 2.8 | 3.9 | 8.3% |
| PCB110 | <LOD | <LOD | 2.3 | 54.1% | <LOD | <LOD | 2.9 | 53.0% |
| PCB118 | 10.7 | 15.2 | 20.7 | 0.0% | 10.9 | 14.8 | 20.4 | 0.0% |
| PCB128 | <LOD | <LOD | <LOD | 87.2% | <LOD | <LOD | <LOD | 91.7% |
| PCB138b | 30.2 | 40.5 | 52.5 | 0.5% | 30.9 | 43.5 | 54.3 | 0.0% |
| PCB146 | 4.6 | 5.9 | 8.1 | 2.8% | 4.6 | 6.0 | 8.1 | 2.2% |
| PCB149 | <LOD | <LOD | 1.7 | 63.8% | <LOD | <LOD | 2.0 | 58.3% |
| PCB151 | <LOD | <LOD | <LOD | 80.3% | <LOD | <LOD | <LOD | 78.7% |
| PCB153 | 48.1 | 62.1 | 85.5 | 0.0% | 48.7 | 68.2 | 86.0 | 0.0% |
| PCB156 | 4.6 | 6.0 | 8.1 | 1.4% | 5.0 | 6.6 | 8.5 | 2.2% |
| PCB157 | <LOD | 1.3 | 1.8 | 34.4% | <LOD | 1.4 | 2.0 | 33.5% |
| PCB167 | 0.5 | 2.0 | 2.9 | 24.8% | <LOD | 2.1 | 2.8 | 27.4% |
| PCB170 | 14.0 | 18.1 | 24.6 | 0.0% | 14.7 | 19.8 | 25.8 | 0.0% |
| PCB172 | 1.1 | 1.9 | 2.7 | 21.6% | <LOD | 2.0 | 2.7 | 24.4% |
| PCB177 | 2.3 | 3.0 | 4.2 | 8.7% | 2.4 | 3.1 | 4.1 | 9.1% |
| PCB178 | 1.8 | 2.7 | 3.6 | 15.6% | 1.9 | 2.8 | 3.9 | 13.0% |
| PCB180 | 31.6 | 44.0 | 59.3 | 0.0% | 36.0 | 47.1 | 61.8 | 0.0% |
| PCB183 | 4.6 | 6.1 | 8.0 | 2.3% | 4.7 | 6.3 | 8.2 | 4.4% |
| PCB187 | 8.4 | 10.9 | 15.8 | 0.9% | 9.0 | 11.5 | 14.9 | 1.3% |
| PCB189 | <LOD | <LOD | <LOD | 79.4% | <LOD | <LOD | 1.0 | 69.6% |
| PCB194 | 5.3 | 7.3 | 10.0 | 3.7% | 5.7 | 7.8 | 10.8 | 3.0% |
| PCB195 | 1.3 | 2.1 | 2.9 | 20.6% | 1.6 | 2.3 | 3.0 | 17.4% |
| PCB196b | 5.3 | 7.4 | 10.0 | 2.3% | 6.0 | 7.9 | 10.7 | 1.7% |
| PCB199 | 3.7 | 5.1 | 7.5 | 2.8% | 4.2 | 5.7 | 8.0 | 2.6% |
| PCB206 | 1.6 | 2.2 | 3.0 | 10.6% | 1.8 | 2.5 | 3.3 | 10.0% |
| PCB209 | <LOD | 1.4 | 1.9 | 30.7% | <LOD | 1.6 | 2.1 | 24.8% |
| Organochlorine pesticides (OCPs) (ng/g lipid) | ||||||||
| HCB | 37.4 | 50.7 | 63.7 | 0.0% | 38.3 | 50.0 | 63.4 | 0.0% |
| ß-HCH | 33.5 | 45.3 | 59.9 | 1.8% | 37.3 | 47.5 | 63.2 | 1.7% |
| g-HCH | <LOD | <LOD | <LOD | 80.7% | <LOD | <LOD | <LOD | 77.4% |
| Oxychlordane | <LOD | <LOD | 3.5 | 74.3% | <LOD | <LOD | 4.6 | 69.6% |
| Trans-nonachlor | <LOD | <LOD | 4.7 | 66.5% | <LOD | <LOD | 4.6 | 67.4% |
| p,p‘-DDE | 184 | 314 | 522 | 0.5% | 200 | 310 | 484 | 0.0% |
| o,p‘-DDT | <LOD | <LOD | <LOD | 98.6% | <LOD | <LOD | <LOD | 98.3% |
| p,p‘-DDT | 7.4 | 11.3 | 16.5 | 10.6% | 8.0 | 10.5 | 16.1 | 12.2% |
| Mirex | <LOD | <LOD | <LOD | 100.0% | <LOD | <LOD | <LOD | 98.7% |
Abbreviations: Q1, quartile 1; Q3, quartile 3; LOD, limit of detection; ng/mL, nanogram per milliliter; ng/g lipid, nanogram per gram lipid.
The LODs for PFAS were 0.082 ng/mL for PFNA, 0.10 ng/mL for PFOA, PFHxS, and FOSA, 0.174 ng/mL for MeFOSAA, and 0.20 ng/mL for PFOS, EtFOSAA, and PFDA. LODs of OCPs and PCBs are dependent on the sample size and blanks, thus, an individual LOD is reported for each individual result rather than an overall LOD.
PCB congeners 138 and 158 could not be separated and were quantified as a summed concentration hereafter referred to as PCB138. Similarly, PCB congeners 196 and 203 could not be separated and were quantified as a summed concentration hereafter referred to as PCB196.
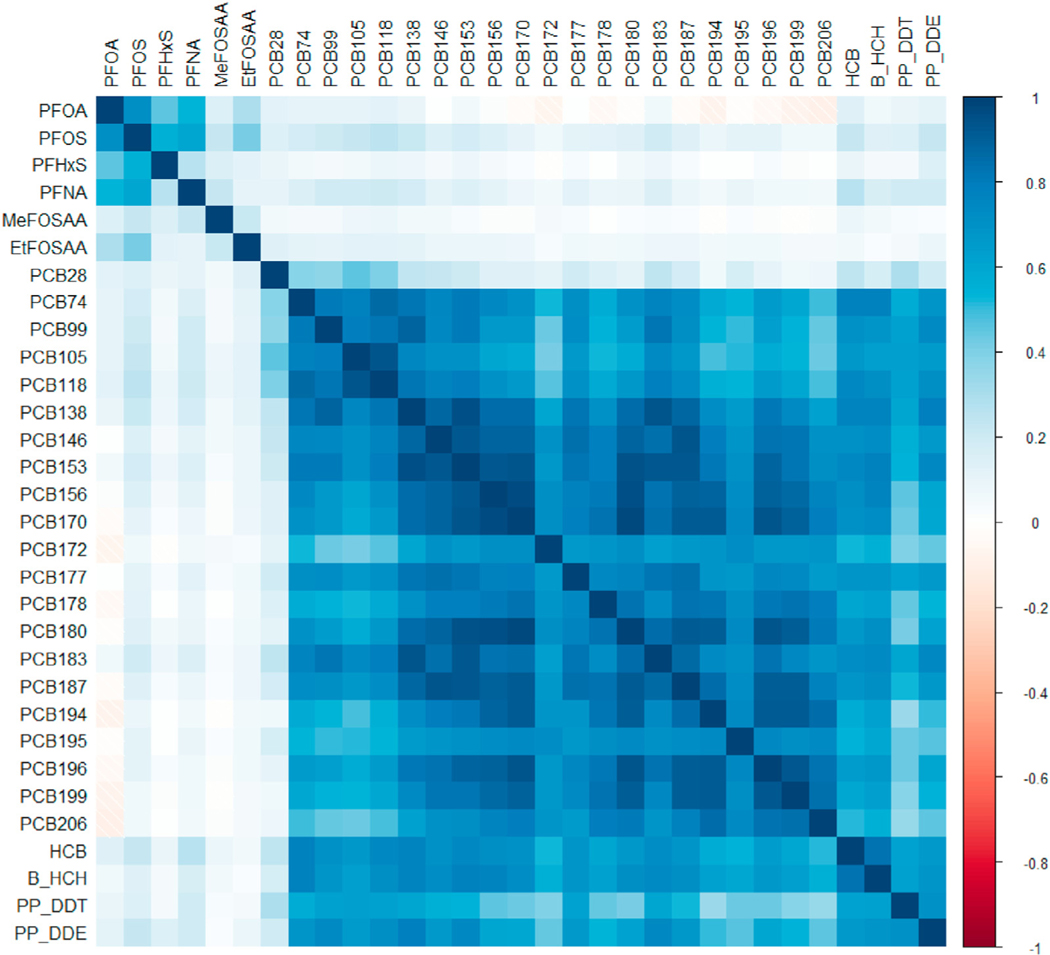
Correlation among the 31 chemicals was high (Fig. 1). Overall, PCBs and OCPs showed high inter-class correlation, while PFAS were less correlated with PCBs and OCPs. Among PCBs, there was strong intra-class correlation (up to rSpearman = 0.98 between PCB170 and PCB180). Correlation within OCPs was also strong (as high as rSpearman = 0.82 between HCB and β-HCH). PFAS exhibited lower intra-class correlation but were still positively correlated with some strong correlations (up to rSpearman = 0.72 between PFOA and PFOS).
Fig. 1.
Correlation heatmap of serum concentrations of persistent endocrine disrupting chemicals in women during pregnancy in the Avon Longitudinal Study of Parents and Children (N = 448). Spearman correlation coefficients presented for untransformed distributions of per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs). PCB and OCP concentrations were lipid-adjusted.
3.2. Single- and multi-chemical models
Few differences were observed in chemical concentration by case-control status. PCB180, which was detected in all samples, was higher among controls than cases (median 47.1 versus 44.0 ng/g lipid) (Table 3). Similarly, PCB170 was also higher among controls than cases (19.8 versus 18.1 ng/g lipid). No differences were observed by case-control status among PFAS or OCPs. In adjusted single-chemical models, no PFAS or OCPs were associated with early menarche (Table 3). PCB180, PCB196, and PCB206 were inversely associated with early menarche. For example, the odds ratio for early menarche for 10% higher PCB180 was 0.93 (95% CI: 0.87, 1.00).
Table 3.
Adjusteda associations of maternal serum concentrations of persistent endocrine disrupting chemicals with early age at menarche (<11.5 years) of the Avon Longitudinal Study of Parents and Children (ALSPAC) nested case-control study, modeled as a mixture using weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR), as well as in single- and multi-chemical models using logistic regression.
| Mixture ORb (95% CI) | Average Weightc,d | Mixture ORe (95% CI) | PIP | Single-chemical OR (95% CI)f | Multi-chemical OR (95% CI)g | |
|---|---|---|---|---|---|---|
| WQS regression | BKMR | |||||
| Per- and polyfluoroalkyl substances (PFAS) (n = 331) | 1.09 (0.98, 1.21) | 1.03 (0.93, 1.13) | ||||
| PFOA | 0.19 | 0.48 | 1.02 (0.96, 1.09) | 1.04 (0.95, 1.13) | ||
| PFOS | 0.07 | 0.44 | 1.01 (0.94, 1.07) | 0.93 (0.82, 1.04) | ||
| PFHxS | 0.13 | 0.52 | 1.01 (0.97,1.05) | 1.02 (0.98, 1.07) | ||
| PFNA | 0.18 | 0.30 | 1.00 (0.95,1.05) | 1.03 (0.96, 1.10) | ||
| MeFOSAA | 0.02 | 0.51 | 0.98 (0.96, 1.01) | 0.97 (0.94, 1.00) | ||
| EtFOSAA | 0.40 | 0.49 | 1.03 (0.99, 1.07) | 1.05 (1.01, 1.10) | ||
| Polychlorinated biphenyls (PCBs) (n = 292) | 0.88 (0.78, 1.00) | 0.92 (0.77, 1.10) | ||||
| PCB28 | 0.03 | 0.63 | 1.01 (0.97, 1.04) | 1.00 (0.95, 1.05) | ||
| PCB74 | 0.05 | 0.52 | 0.97 (0.92, 1.02) | 0.98 (0.86, 1.12) | ||
| PCB99 | 0.09 | 0.37 | 0.99 (0.94, 1.04) | 0.94 (0.75, 1.17) | ||
| PCB105 | 0.02 | 0.46 | 1.00 (0.94, 1.06) | 1.13 (0.89, 1.45) | ||
| PCB118 | 0.02 | 0.44 | 0.99 (0.95, 1.04) | 0.89 (0.66, 1.21) | ||
| PCB138h | 0.03 | 0.41 | 0.97 (0.92, 1.02) | 1.00 (0.68, 1.46) | ||
| PCB146 | 0.00 | 0.42 | 1.00 (0.95,1.05) | 0.99 (0.85, 1.17) | ||
| PCB153 | 0.00 | 0.50 | 0.96 (0.90, 1.02) | 1.29 (0.71, 2.37) | ||
| PCB156 | 0.05 | 0.49 | 0.96 (0.91, 1.01) | 1.01 (0.88, 1.15) | ||
| PCB170 | 0.01 | 0.43 | 0.94 (0.87, 1.00) | 1.01 (0.68, 1.51) | ||
| PCB172 | 0.01 | 0.48 | 1.02 (0.97, 1.06) | 1.15 (1.05, 1.26) | ||
| PCB177 | 0.02 | 0.48 | 0.98 (0.93, 1.02) | 0.89 (0.80, 0.99) | ||
| PCB178 | 0.02 | 0.35 | 0.97 (0.92, 1.02) | 1.01 (0.92, 1.11) | ||
| PCB180 | 0.04 | 0.48 | 0.93 (0.87, 1.00) | 0.69 (0.40, 1.18) | ||
| PCB183 | 0.05 | 0.54 | 0.97 (0.92, 1.03) | 0.92 (0.74, 1.15) | ||
| PCB187 | 0.00 | 0.56 | 0.99 (0.93, 1.04) | 1.37 (1.08, 1.75) | ||
| PCB194 | 0.04 | 0.39 | 0.97 (0.93, 1.02) | 1.02 (0.94, 1.11) | ||
| PCB195 | 0.26 | 0.51 | 0.96 (0.91, 1.01) | 0.94 (0.86, 1.03) | ||
| PCB196h | 0.04 | 0.45 | 0.92 (0.86, 0.99) | 1.04 (0.79, 1.38) | ||
| PCB199 | 0.03 | 0.39 | 0.96 (0.91, 1.01) | 0.97 (0.86, 1.09) | ||
| PCB206 | 0.18 | 0.63 | 0.92 (0.87, 0.98) | 0.85 (0.73, 0.98) | ||
| Organochlorine pesticides (OCPs) (n = 302) | 0.95 (0.85, 1.05) | 0.97 (0.91, 1.04) | ||||
| HCB | 0.12 | 0.30 | 0.97 (0.92, 1.03) | 1.01 (0.93, 1.09) | ||
| β-HCH | 0.50 | 0.67 | 0.96 (0.92, 1.00) | 0.96 (0.91, 1.02) | ||
| p,p‘-DDE | 0.26 | 0.36 | 0.98 (0.94, 1.01) | 0.98 (0.93, 1.03) | ||
| p,p‘-DDT | 0.12 | 0.33 | 0.98 (0.93, 1.03) | 1.02 (0.95, 1.10) | ||
| Overall mixture (PFAS, PCBs, OCPs) (n = 284) | 0.89 (0.76, 1.05) | 0.98 (0.91, 1.05) | ||||
| PFAS | 0.49i | |||||
| PFOA | 0.02 | 0.13 | ||||
| PFOS | 0.03 | 0.08 | ||||
| PFHxS | 0.03 | 0.15 | ||||
| PFNA | 0.03 | 0.11 | ||||
| MeFOSAA | 0.19 | 0.18 | ||||
| EtFOSAA | 0.02 | 0.36 | ||||
| PCBs | 0.53i | |||||
| PCB28 | 0.02 | 0.19 | ||||
| PCB74 | 0.03 | 0.04 | ||||
| PCB99 | 0.05 | 0.04 | ||||
| PCB105 | 0.01 | 0.01 | ||||
| PCB118 | 0.01 | 0.2 | ||||
| PCB138h | 0.01 | 0.03 | ||||
| PCB146 | 0.00 | 0.02 | ||||
| PCB153 | 0.00 | 0.03 | ||||
| PCB156 | 0.03 | 0.07 | ||||
| PCB170 | 0.00 | 0.05 | ||||
| PCB172 | 0.01 | 0.01 | ||||
| PCB177 | 0.01 | 0.04 | ||||
| PCB178 | 0.01 | 0.04 | ||||
| PCB180 | 0.02 | 0.04 | ||||
| PCB183 | 0.01 | 0.03 | ||||
| PCB187 | 0.00 | 0.03 | ||||
| PCB194 | 0.04 | 0.07 | ||||
| PCB195 | 0.17 | 0.05 | ||||
| PCB196h | 0.02 | 0.05 | ||||
| PCB199 | 0.02 | 0.05 | ||||
| PCB206 | 0.12 | 0.10 | ||||
| OCPs | 0.66i | |||||
| HCB | 0.01 | 0.16 | ||||
| β-HCH | 0.05 | 0.55 | ||||
| P,P’-DDE | 0.02 | 0.20 | ||||
| P,P’-DDT | 0.03 | 0.10 | ||||
Abbreviations: OR, odds ratio; CI, confidence interval; WQS, weighted quantile sum; BKMR, Bayesian kernel machine regression; PIP, posterior inclusion probability.
Adjusted for maternal age at menarche, education, parity, pre-pregnancy body mass index, maternal age at delivery, prenatal smoking, and gestational week at sample collection.
The odds ratio for early menarche for one-unit higher of the WQS index (representing a one-decile increase in chemical concentrations).
Weights greater than 1/number of chemicals in the mixture are considered significant contributors to the overall mixture effect.
Weights may not add to 1 due to rounding.
The odds ratio (and 95% credible interval) comparing the outcome when all exposures are at the 60th percentile to the median; estimates for other quantiles compared to the median can be seen in Fig. 2.
Single-chemical logistic regression models were run to examine independent associations between each chemical and early menarche. Odds ratios represent a change of 10% higher chemical concentrations.
Multi-chemical logistic regression models were run to examine associations between each chemical in a class (e.g., PFAS) and early menarche, independent of other chemicals in the class (e.g., adjusting for other chemicals in the class). Odds ratios represent a change of 10% higher chemical concentrations.
PCB congeners 138 and 158 could not be separated and were quantified as a summed concentration hereafter referred to as PCB138. Similarly, PCB congeners 196 and 203 could not be separated and were quantified as a summed concentration hereafter referred to as PCB196.
Italicized numbers indicate group PIPs; all other PIPs in the overall mixture (PFAS, PCBs, OCPs) are conditional PIPs (representing the importance of a chemical given that the group that contained the chemical was important).
In the multi-chemical PFAS model, 10% higher EtFOSAA was associated with 5% higher odds of early menarche (OR: 1.05, 95% CI: 1.01, 1.10) when adjusting for all other PFAS (Table 3). In the multi-chemical PCB model, PCB172 and PCB187 were associated with higher odds of early menarche, while PCB177 and PCB206 were associated with lower odds of early menarche when all PCBs were in the model. With 21 chemicals in the multi-chemical PCB model, some estimates were highly imprecise, exhibiting very wide confidence intervals. Null associations were observed for all OCPs in the multi-chemical OCP model, though β-HCH appeared somewhat protective (OR: 0.96, 95% CI: 0.91, 1.02).
3.3. Weighted quantile sum regression
Weighted quantile sum regression models showed null associations between the indices for mixtures (PFAS, PCBs, OCPs, and all three classes combined) and early menarche (Table 3). The odds ratio for early menarche for one-unit higher of the WQS index (representing a one-decile increase in chemical concentrations) for all three classes combined was 0.89 (95% CI: 0.76, 1.05). When examining classes on their own, the PFAS mixture, driven by EtFOSAA (weight: 0.40), was weakly associated with early menarche (OR: 1.09, 95% CI: 0.98, 1.21), while the PCB mixture, driven by PCB206 (weight: 0.18), was weakly inversely associated with early menarche (OR: 0.88, 95% CI: 0.78, 1.00). Being breastfed did not modify the association of prenatal exposure to persistent EDC mixtures and early menarche.
3.4. Bayesian kernel machine regression
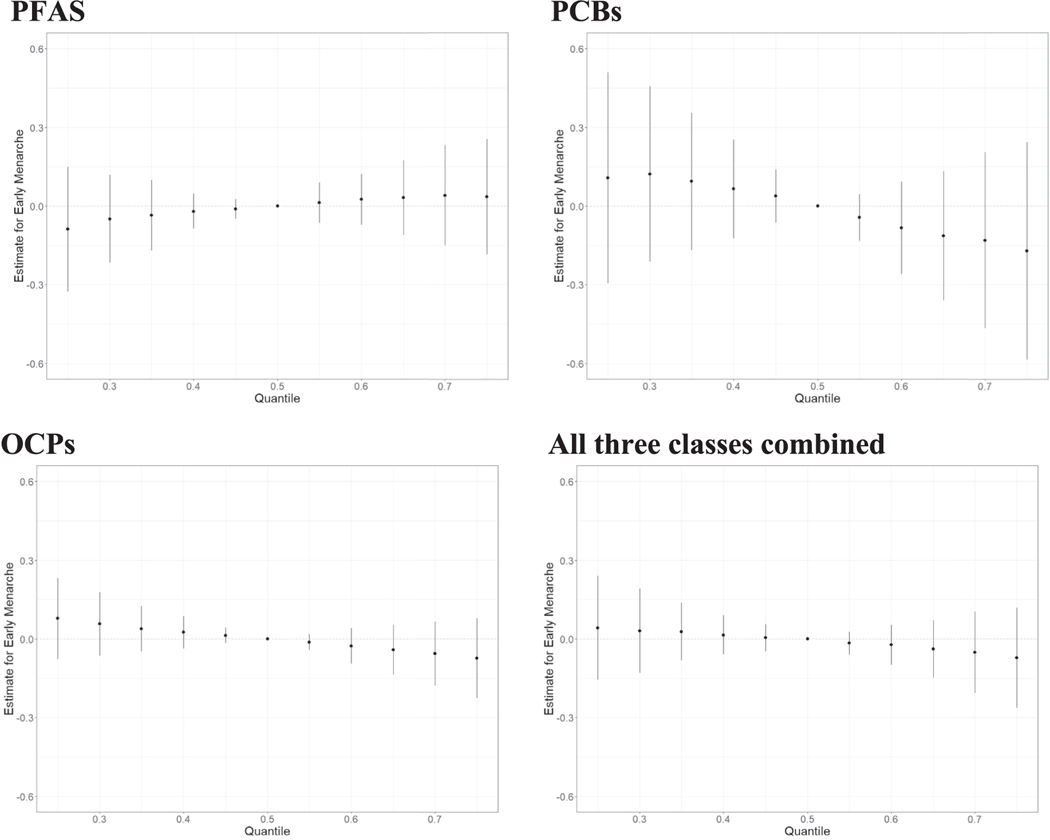
In the BKMR model for all three classes combined, the independent chemical associations all appear fairly linear (Fig. S3). Some chemicals had slightly positive associations (PFHxS, EtFO-SAA), some appeared to have negative associations (MeFOSAA, PCB206, β-HCH), but most showed no association with early menarche. We observed no interaction among mixture members (Fig. S4). We did not find an overall mixture effect for any of the mixtures; the overall effect of the PFAS mixture was in the positive direction while all other mixtures (PCBs, OCPs, and all three classes combined) were in the inverse direction (Fig. 2, Table 3). PIPs, which indicate the importance of a chemical to the mixture, are reported in Table 3.
Fig. 2.
Overall effect of the mixture on early menarche status (estimates and 95% credible intervals), comparing the outcome when all exposures are at a particular quantile to the median using Bayesian kernel machine regression for four different mixtures: PFAS, PCBs, OCPs, and all three classes combined (PFAS, PCBs, and OCPs). The model adjusted for maternal age at menarche, education, parity, pre-pregnancy body mass index, maternal age at delivery, prenatal smoking, and gestational week at sample collection. All chemical concentrations were natural log-transformed and standardized; PCB and OCP concentrations were lipid-adjusted.
3.5. Sensitivity analyses
We conducted a sensitivity analysis to explore differences in early menarche status among those with detectable concentrations versus those with non-detectable concentrations (Table S3). We found that daughters born to women with detectable concentrations of PCB189 were less likely to experience early menarche (OR: 0.45, 95% CI: 0.26, 0.78) than those with non-detectable concentrations. Some other differences were seen for other chemicals: those with MeFOSAA, PCB177, PCB178, and PCB206 concentrations above the LOD were less likely to experience early menarche, while those with detectable FOSA concentrations were more likely to experience early menarche, but these estimates were imprecise.
4. Discussion
In this study, we examined the association of prenatal exposure to multiple PFAS, PCBs, and OCPs (as individual classes and collectively) and early menarche (<11.5 years) among British girls, and mostly observed null associations. We employed WQS regression and BKMR to accomplish this, and results from these two methods were largely in agreement. This study responds to a recent call for research to evaluate the combined effects of exposure to EDCs on pubertal timing (Seltenrich, 2019).
The results from single-chemical logistic regression, WQS regression, and BKMR models were quite similar. First, almost all models suggested that higher prenatal exposure to persistent EDCs was not associated with early menarche, though effect sizes varied. Associations were further away from the null in WQS regression models than in BKMR. WQS regression assumes that all associations are in the same direction; if this assumption is not met, results can be biased away from the null (Keil et al., 2020). This potential for bias could explain the differences in magnitude between WQS regression and BKMR. Second, models were generally in agreement on the most important contributors in each class; for PFAS, EtFO-SAA; for PCBs, PCB206; and for OCPs, β-HCH was consistently the most important component of the mixture.
Previous studies in this ALSPAC population examined prenatal exposure to single EDCs and early menarche, specifically for PFAS (Christensen et al., 2011) and OCPs (Namulanda et al., 2016). Neither study found an effect of prenatal exposure to EDCs on early menarche, as we confirmed here when examining mixtures of these chemicals using WQS and BKMR. The null findings from ALSPAC are in agreement with many studies published for these EDCs under the single-chemical paradigm (Blanck et al., 2000; Christensen et al., 2011; Kristensen et al., 2013; Leijs et al., 2008; Gladen et al., 2000; Vasiliu et al., 2004; Namulanda et al., 2016), though two previous studies found an association between DDE and early menarche (Gladen et al., 2000; Vasiliu et al., 2004). The DDE and early menarche association was not replicated in our study in single-chemical analyses nor was DDE identified as an important component in mixture analyses using WQS and BKMR. Within ALSPAC, this is the first study to report on the association of PCBs and early menarche, and we found that certain PCBs, including PCB180, decreased the odds of early menarche in single-chemical models. In mixture models, associations of PCBs and early menarche were null though in the inverse direction.
While there has been previous work on the topic of persistent EDCs (modeled as single chemicals) and early menarche, there is motivation for a study using a mixtures approach. Because it is thought that several EDCs can operate through a common mechanism to affect an outcome, it seems reasonable that individual EDCs could act together at lower concentrations than the concentration that would be required for each chemical to achieve the same outcome on its own (Darbre, 2015). This idea has been shown in in vitro and in vivo studies where mixtures of EDCs are able to produce significant effects, even when each individual EDC is present at concentrations below the no-observed-effects levels (Silva et al., 2002; Rajapakse et al., 2002; Kortenkamp, 2008; Al-Gubory, 2014). For example, a mixture effect was observed in an epidemiological study of breast cancer using a novel biomarker of combined EDC exposure. A positive association was seen between the mixture and breast cancer, yet individual EDCs showed no associations with breast cancer (Pastor-Barriuso et al., 2016). These results suggest that the numerous studies of single EDCs with null findings may have considerably underestimated the risks of exposure to EDCs (Kortenkamp, 2007; Lazarevic et al., 2019), which is why we have re-analyzed data within this ALSPAC nested case-control study of early menarche using mixture methods.
Twelve persistent organic pollutants including PCBs, DDT, and HCB were banned or limited globally in a 2004 treaty at the Stockholm Convention on Persistent Organic Pollutants (POPs), and HCH was one of nine pollutants added in a 2009 amendment (The Stockholm Convention., 2008). In the years since, global monitoring of POPs has increased (Hung et al., 2016; Magulova and Priceputu, 2016). Human PCB and OCP concentrations have decreased among the general population in recent decades and concentrations among ALSPAC mothers (1990–1992) were higher than was last seen when the National Health and Nutrition Examination Survey (NHANES) measured these chemicals in Americans (2003–2004) (Patterson et al., 2009). PFAS concentrations were higher in Americans than ALSPAC mothers the first time NHANES examined PFAS (1999–2000), but Americans’ PFAS concentrations have since dropped below concentrations of ALSPAC mothers (Centers for Disease Contr, 2017). Like PCBs and OCPs, there has been a downward trend for a number of PFAS (Kato et al., 2011); however, newer PFAS formulations have replaced them. Moreover, there have been changes in age at menarche over time and place. In the United States, median age at menarche decreased from 1995 (12.1 years) to 2013–2017 (11.9) (Martinez, 2020). The same downward trend is seen in the United Kingdom, though age at menarche has been consistently been slightly older in the United Kingdom compared to the United States (McDowell et al., 2007; Morris et al., 2011).
Our study is strengthened by its prospective design within a population-based birth cohort. Further, the frequent and thorough longitudinal data collection over a long follow up period allows us to examine exposures during pregnancy with distal outcomes such as pubertal development. Thirdly, we have utilized reliable biomarker indicators of exposure to over 50 persistent EDCs, allowing us to examine some less commonly studied chemicals as part of chemical mixtures. Further, our study is enriched by the extensive covariate data available within ALSPAC. Lastly, our mixtures approach using multiple complementary methods (WQS regression, BKMR) allowed us to better replicate the human experience of exposure to multiple chemicals. Strengths of these mixture methods include their robustness to multicollinearity due to correlated exposures, dimensionality reduction, and ability to estimate mixture health effects while identifying important mixture components.
This study also has some limitations. The size of this sub-sample (n = 448) of ALSPAC is modest. Due to the study design, we are unable to examine the association between prenatal mixtures of persistent EDCs and late age at menarche; there are a limited number of girls in the study with both measured maternal EDC concentrations and menarche at or after 14 years old (n = 24). We are unable to account for persistent EDC exposure during childhood, which could also influence pubertal development, though there is evidence to suggest that prenatal exposure to persistent EDCs affects pubertal development independent of childhood exposures (Blanck et al., 2000). Additionally, there may be residual confounding by unknown and unmeasured (e.g., health-seeking behaviors) or poorly measured covariates (e.g., SES). Approaches to mixture analyses that involve regressing the outcome on several correlated exposures simultaneously can in some cases amplify rather than reduce confounding bias (“co-exposure amplification bias”) (Weisskopf et al., 2018). As recommended, we controlled for variables (to the extent that they were known and measured) that introduce confounding to the association of prenatal EDC exposure and early menarche. Further, due to the large number of variables (many with some missing data) used in mixture analyses, we were missing data on roughly one-third of the sub-sample (Fig. S2). We compared mother-daughter characteristics for those in the analytic dataset used for mixture analyses (n = 284) to those in the nested case-control study (n = 448) and to the population from which the case-control study was drawn (n = 3913) (Table S4). Characteristics were similar across subsets; while we saw a higher proportion of mothers with an earlier maternal age at menarche and overweight/obese pre-pregnancy BMI in the nested case-control study and analytic data compared to those enrolled at puberty, this was expected due to the relation of these factors with case status. There is the potential for misclassification of daughter’s age at menarche because it was self-reported annually between the ages of 8 and 17, though this is unlikely to be a concern given the close proximity of the event to the time of reporting and generally good recall of this outcome (Cairns et al., 2011; Cooper et al., 2006; Must et al., 2002). Lastly, the sub-sample used in the present study differed from the original base population of ALSPAC in a few ways. Mothers in our sub-sample were more highly educated and older than mothers in the original ALSPAC cohort. These differences are somewhat expected given that to be included in this sub-sample, children still had to be involved in the study during puberty. Largely, nonparticipation and loss to follow-up are more common among the less healthy and less advantaged (Wilhelmsen et al., 1976; Strandhagen et al., 2010; Strandberg et al., 1995; Knudsen et al., 2010; Heilbrun et al., 1982; Goldberg et al., 2001; Barchielli and Balzi, 2002).
5. Conclusions
We found no association between prenatal exposure to mixtures of persistent EDCs and early menarche status. This study fills a knowledge gap relating to prenatal exposure to mixtures of EDCs and puberty and comes closer to replicating the human experience by accounting for low-level exposure to many chemicals.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Funding
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and they will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This work was specifically funded by the Centers for Disease Control and Prevention (AY5350).
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2021.116705.
References
- Agency for Toxic Substances and Disease Registry (ATSDR), 2000. Toxicological Profile for Polychlorinated Biphenyls (PCBs). Atlanta, GA. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=142&tid=26. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2002. Toxicological Profile for DDT, DDE, and DDD. Department of Health and Human Services PHS, Atlanta, GA. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2009. Toxicological Profile for Perfluoroalkyls. (Draft for Public Comment). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Al-Gubory KH, 2014. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 29 (1), 17–31. [DOI] [PubMed] [Google Scholar]
- Barchielli A, Balzi D, 2002. Nine-year follow-up of a survey on smoking habits in Florence (Italy): higher mortality among non-responders. Int. J. Epidemiol 31 (5), 1038–1042. [DOI] [PubMed] [Google Scholar]
- Biro FM, McMahon RP, Striegel-Moore R, et al. , 2001. Impact of timing of pubertal maturation on growth in black and white female adolescents: the national heart, lung, and blood Institute growth and health study. J. Pediatr 138 (5), 636–643. [DOI] [PubMed] [Google Scholar]
- Biro FM, Greenspan LC, Galvez MP, 2012. Puberty in girls of the 21st century. J. Pediatr. Adolesc. Gynecol 25 (5), 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, et al. , 2000. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 11 (6), 641–647. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16 (3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, et al. , 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health : Glob. Access Sci. Sour 17 (1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, et al. , 2013. Cohort profile: the ‘children of the 90s’– the index offspring of the Avon longitudinal study of parents and children. Int. J. Epidemiol 42 (1), 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE Jr., Marcus M, et al. , 2008. Environmental factors and puberty timing: expert panel research needs. Pediatrics 121 (Suppl. 3), S192–S207. [DOI] [PubMed] [Google Scholar]
- Cairns BJ, Liu B, Clennell S, et al. , 2011. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med. Res. Methodol 11, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, et al. , 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat 20 (1), 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics (CDC), 2017. National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, et al. , 2011. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ. Int 37 (1), 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KLY, Carrico CK, Sanyal AJ, et al. , 2013. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int. J. Hyg Environ. Health 216 (6), 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Blell M, Hardy R, et al. , 2006. Validity of age at menarche self-reported in adulthood. J. Epidemiol. Community Health 60 (11), 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull B, Bobb J, Wellenius G, et al. , 2015. Part 1. Statistical learning methods for the effects of multiple air pollution constituents, 183 Pt 1–2 Res. Rep 5–50. [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Colt JS, et al. , 2015. Analysis of environmental chemical mixtures and non-hodgkin lymphoma risk in the NCI-SEER NHL study. Environ. Health Perspect 123 (10), 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, 2015. How could endocrine disrupters affect human health? Endocrine Disruption and Human Health. Elsevier, pp. 27–45. [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. , 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev 30 (4), 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, et al. , 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish national birth cohort. Environ. Health Perspect 127 (1), 17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, et al. , 2013. Cohort profile: the Avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol 42 (1), 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C, Sabo R, Carney E, 2010. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology 21 (Suppl. 4), S77–S84. [DOI] [PubMed] [Google Scholar]
- Gibson EA, Nunez Y, Abuawad A, et al. , 2019. An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environ. Health : Glob. Access Sci. Sour 18 (1), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, Ragan NB, Rogan WJ, 2000. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J. Pediatr 136 (4), 490–496. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Chastang JF, Leclerc A, et al. , 2001. Socioeconomic, demographic, occupational, and health factors associated with participation in a long-term epidemiologic survey: a prospective study of the French GAZEL cohort and its target population. Am. Jo. Epidemiol 154 (4), 373–384. [DOI] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PM, et al. , 2008. Public health implications of altered puberty timing. Pediatrics 121 (Suppl. 3), S218–S230. [DOI] [PubMed] [Google Scholar]
- Heilbrun LK, Nomura A, Stemmermann GN, 1982. The effects of nonresponse in a prospective study of cancer. Am. Jo. Epidemiol 116 (2), 353–363. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, et al. , 1997. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99 (4), 505–512. [DOI] [PubMed] [Google Scholar]
- Hung H, Katsoyiannis AA, Guardans R, 2016. Ten years of global monitoring under the Stockholm convention on persistent organic pollutants (POPs): trends, sources and transport modelling. Environ. Pollut 217, 1–3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, et al. , 2004. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ. Health Perspect 112 (11), 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, et al. , 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol 45 (19), 8037–8045. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, et al. , 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect 128 (4), 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM, 1993. Reproductive factors and breast cancer. Epidemiol. Rev 15 (1), 36–47. [DOI] [PubMed] [Google Scholar]
- Kezios KL, Liu X, Cirillio PM, et al. , 2012. Prenatal polychlorinated biphenyl exposure is associated with decreased gestational length but not birth weight: archived samples from the Child Health and Development Studies pregnancy cohort. Environ. Health : Glob. Access Sci. Sour 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Lee KT, Kang CS, et al. , 2011. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ. Pollut 159 (1), 169–174. [DOI] [PubMed] [Google Scholar]
- Knudsen AK, Hotopf M, Skogen JC, et al. , 2010. The health status of non-participants in a population-based health study: the Hordaland Health Study. Am. Jo. Epidemiol 172 (11), 1306–1314. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, 2007. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect 115 (Suppl. 1), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A, 2008. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int. J. Androl 31 (2), 233–240. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, et al. , 2013. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Human Reprod. (Oxford, England) 28 (12), 3337–3348. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, et al. , 2016. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ. Int 92–93, 366–372. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM, 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal. Chem 77 (18), 6085–6091. [DOI] [PubMed] [Google Scholar]
- Lazarevic N, Barnett AG, Sly PD, et al. , 2019. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: a review of existing approaches and new alternatives. Environ. Health Perspect 127 (2), 26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, Olie K, et al. , 2008. Delayed initiation of breast development in girls with higher prenatal dioxin exposure; a longitudinal cohort study. Chemosphere 73 (6), 999–1004. [DOI] [PubMed] [Google Scholar]
- Magulova K, Priceputu A, 2016. Global monitoring plan for persistent organic pollutants (POPs) under the Stockholm Convention: triggering, streamlining and catalyzing global POPs monitoring. Environ. Pollut 217, 82–84. [DOI] [PubMed] [Google Scholar]
- Martinez GM, 2020. Trends and patterns in menarche in the United States: 1995 through 2013–2017. Natl. Health Stat. Rep (146), 1–12. [PubMed] [Google Scholar]
- McDowell MA, Brody DJ, Hughes JP, 2007. Has age at menarche changed? Results from the national health and nutrition examination survey (NHANES) 1999–2004. official publication of the Society for Adolescent Medicine J. Adolesc. Health 40 (3), 227–231. [DOI] [PubMed] [Google Scholar]
- Morris DH, Jones ME, Schoemaker MJ, et al. , 2011. Secular trends in age at menarche in women in the UK born 1908–93: results from the Breakthrough Generations Study. Paediatr. Perinat. Epidemiol 25 (4), 394–400. [DOI] [PubMed] [Google Scholar]
- Mouritsen A, Aksglaede L, Sørensen K, et al. , 2010. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int. J. Androl 33 (2), 346–359. [DOI] [PubMed] [Google Scholar]
- Must A, Phillips SM, Naumova EN, et al. , 2002. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am. Jo. Epidemiol 155 (7), 672–679. [DOI] [PubMed] [Google Scholar]
- Namulanda G, Maisonet M, Taylor E, et al. , 2016. In utero exposure to organochlorine pesticides and early menarche in the Avon Longitudinal Study of Parents and Children. Environ. Int 94, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences, 2017. Powering Research through Innovative Methods for Mixtures in Epidemiology (PRIME) (R01) RFA-ES-17–001. https://grants.nih.gov/grants/guide/rfa-files/RFA-ES-17-001.html. [DOI] [PMC free article] [PubMed]
- National Institute of Environmental Health Sciences, 2018. 2018–2023 Strategic Plan: Advancing Environmental Health Science, Improving Health 2.0. Department of Health and Human Services, Durham, NC. [Google Scholar]
- National Institute of Environmental Health Sciences, 2019. Endocrine Disruptors. North Carolina, Research Triangle Park. https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm. (Accessed 25 November 2019). [Google Scholar]
- Pastor-Barriuso R, Fernandez MF, Castano-Vinyals G, et al. , 2016. Total effective xenoestrogen burden in serum samples and risk for breast cancer in a population-based multicase-control study in Spain. Environ. Health Perspect 124 (10), 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patayová H, Wimmerová S, Lancz K, et al. , 2013. Anthropometric, socioeconomic, and maternal health determinants of placental transfer of organochlorine compounds. Environ. Sci. Pollut. Control Ser 20 (12), 8557–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J, Donald G, Wong L-Y, Turner WE, et al. , 2009. Levels in the US population of those persistent organic pollutants (2003 2004) included in the Stockholm Convention or in other long-range transboundary air pollution agreements. Environ. Sci. Technol 43 (4), 1211–1218. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A, 2002. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ. Health Perspect 110 (9), 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Curtin PC, Just AC, Bello G, Gennings C, 2021. gWQS: an R package for linear and generalized weighted quantile sum (WQS) regression. The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/gWQS/vignettes/gwqs-vignette.html. (Accessed 10 February 2021). [Google Scholar]
- Rubin C, Maisonet M, Kieszak S, et al. , 2009. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr. Perinat. Epidemiol 23 (5), 492–504. [DOI] [PubMed] [Google Scholar]
- Sala M, Ribas-Fito N, Cardo E, et al. , 2001. Levels of hexachlorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere 43 (4–7), 895–901. [DOI] [PubMed] [Google Scholar]
- Schoeters G, Govarts E, Bruckers L, et al. , 2017. Three cycles of human biomonitoring in Flanders - time trends observed in the flemish environment and health study. Int. J. Hyg Environ. Health 220 (2 Pt A), 36–45. [DOI] [PubMed] [Google Scholar]
- Seltenrich N, 2019. Chemical exposures and pubertal timing: new evidence in a complex area. Environ. Health Perspect 127 (7), 74003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A, 2002. Something from “nothing”–eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol 36 (8), 1751–1756. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, et al. , 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem 76 (7), 1921–1927. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Salomaa VV, Vanhanen HT, et al. , 1995. Mortality in participants and non-participants of a multifactorial prevention study of cardiovascular diseases: a 28 year follow up of the Helsinki Businessmen Study. Br. Heart J 74 (4), 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandhagen E, Berg C, Lissner L, et al. , 2010. Selection bias in a population survey with registry linkage: potential effect on socioeconomic gradient in cardiovascular risk. Eur. J. Epidemiol 25 (3), 163–172. [DOI] [PubMed] [Google Scholar]
- Tang M, Yin S, Zhang J, et al. , 2018. Prenatal exposure to polychlorinated biphenyl and umbilical cord hormones and birth outcomes in an island population. Environ. Pollut 237, 581–591. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Bornehag CG, Gennings C, 2019. Repeated holdout validation for weighted quantile sum regression. MethodsX 6, 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists, 2013. Exposure to toxic environmental agents. Fertil. Steril 100 (4), 931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Stockholm Convention, 2008. The 1 2 Initial POPs under the Stockholm Convention. Switzerland. [Google Scholar]
- Vasiliu O, Muttineni J, Karmaus W, 2004. In utero exposure to organochlorines and age at menarche. Human Reprod. (Oxford, England) 19 (7), 1506–1512. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Seals RM, Webster TF, 2018. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ. Health Perspect 126 (4), 047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen L, Ljungberg S, Wedel H, et al. , 1976. A comparison between participants and non-participants in a primary preventive trial. J. Chron. Dis 29 (5), 331–339. [DOI] [PubMed] [Google Scholar]
- Workman CE, Becker AB, Azad MB, et al. , 2019. Associations between concentrations of perfluoroalkyl substances in human plasma and maternal, infant, and home characteristics in Winnipeg, Canada. Environ. Pollut 249, 758–766. [DOI] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE, 1982. Evidence for a secular trend in age of menarche. N. Engl. J. Med 306 (17), 1033–1035. [DOI] [PubMed] [Google Scholar]
- Zacharias L, Wurtman RJ, 1969. Age at menarche. Genetic and environmentalinfluences. N. Engl. J. Med 280 (16), 868–875. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu X, Lei B, et al. , 2018. Transplacental transfer characteristics of organochlorine pesticides in paired maternal and cord sera, and placentas and possible influencing factors. Environ. Pollut 233, 446–454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.