Abstract
Objectives:
To compare forced-eruption times for palatally impacted canines treated with and without the ostectomy-decortication technique and to assess the influence of palatally impacted canine pretreatment position and angle on forced-eruption time.
Materials and Methods:
The sample was composed of 118 patient-subjects with 151 palatally impacted canines treated with the ostectomy-decortication technique (n = 72) and without (n = 79). The orthopantomogram radiographs (OPGs) were analyzed for palatally impacted canine angle and horizontal and vertical position. Recovery time was measured from the start of forced eruption until the canine was within ±1 mm of final dental arch position.
Results:
The time of forced canine eruption with ostectomy-decortication technique was significantly shorter than without (6.6 vs 21.0 months). Pretreatment canine position significantly increased forced-eruption time in the ostectomy-decortication group but not in the control sample.
Conclusions:
Forced-eruption time of palatally impacted canines using the ostectomy-decortication technique was 3.2 times more rapid than without. Forced-eruption time increased significantly as a function of pretreatment palatally impacted canine position severity in the ostectomy-decortication group but not in the control.
Keywords: Orthodontics, Palatally impacted canine, Forced eruption
INTRODUCTION
Time needed to forcibly erupt palatally impacted canines presents a particularly vexing multidisciplinary clinical problem because active orthodontic treatment time is usually extended. Conditions cited in the literature influencing the duration of canine forced-eruption time are technique of surgery (open or closed) and pretreatment position of the palatally impacted canine. Forced-eruption time is often reported as overall orthodontic treatment time1–13 rather than time until the impacted canine is aligned in the dental arch.1,6,9,14,15 Hence, the clinical time needed to forcibly erupt palatally impacted canines remains ill-defined and lacks consensus.
Open and closed surgery techniques were recently compared in a systematic review and meta-analysis16 of data from three publications.10,15,17 A mean difference between open and closed techniques of 2.14 months was reported, with no clear delineation of palatally impacted canine forced-eruption times.
Advocates of alveolar decortication surgical procedures in combination with orthodontic therapy purport reduced palatally impacted canine forced-eruption times.18 The ostectomy-decortication technique for palatally impacted canine exposure has been described as follows19 (Figure 1A–F):
Figure 1.
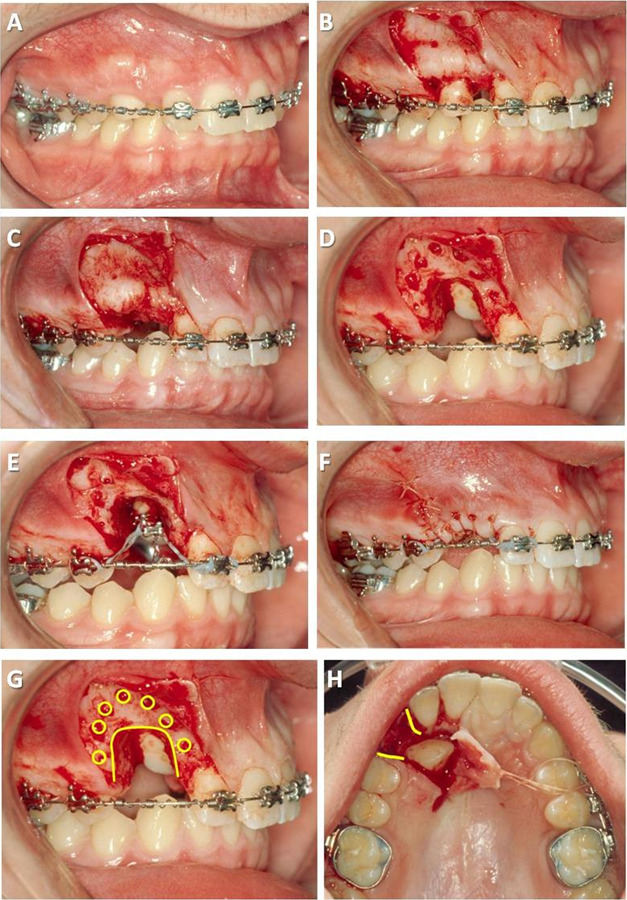
Ostectomy-decortication technique: (A) presurgery, (B) full-thickness flap, (C) extracted primary canine, (D) ostectomy-decortication, (E) bracket placement and traction forces, (F) flap closure over surgical exposure, (G) cortical bone penetrations (decortication) surrounding palatally impacted canine (circles in G), and (H) removal of bone (ostectomy) between palatally impacted canine crown and future occlusal position (curved lines in G and H).
full-thickness flaps to uncover the clinical crown and to place the orthodontic bracket as ideally as possible;
ostectomy between the first premolar and lateral incisor, clearing a pathway from the impacted canine crown to the final archwire position but leaving about 1.5 mm of bone interproximally;
intramarrow penetrations over the root prominence of the impacted tooth facing in the direction of movement;
bracket placement and application of power-chain traction forces used directionally as needed to steer clear of obstructions in the path of eruption; and
full-thickness flaps returned to their original position and sutured.
The unique features of the ostectomy-decortication technique include cortical bone penetrations (selective decortication) surrounding the impacted canine and removal of bone (ostectomy) between the impacted canine crown and future occlusal position (Figure 1G,H).
Fischer20 reported six cases of bilateral palatally impacted canines treated in a split-mouth design with randomly assigned surgical exposure on one side and selective alveolar decortication (without ostectomy) on the other; the treatment duration was 28% to 33% more rapid for the corticotomy-assisted technique. No significant differences were observed in canine periodontal status between these two techniques.
Forcibly erupting palatally impacted canines using the ostectomy-corticotomy technique has not been reported. The primary aims of this study were twofold: (1) to compare the duration of forced eruption recovery for palatally impacted canines with and without the ostectomy-corticotomy technique and (2) to assess the influence of palatally impacted canine pretreatment position on forced-eruption time. The null hypotheses tested were (1) no difference as a function of technique (ie, with and without ostectomy-corticotomy assistance) and (2) no difference as a function of the pretreatment position of the palatally impacted canine.
MATERIALS AND METHODS
Sample
This retrospective cohort study compared two samples totaling 118 patients treated for 151 palatally impacted canines using a forced orthodontic eruption strategy with and without application of an ostectomy-corticotomy assistance technique. The ostectomy-corticotomy (experimental) sample was composed of 57 patients with 72 palatally impacted canines. Ostecotomy procedure and exposure allowed optimal bracket positioning on the impacted canine in a high percentage of cases. The sample treated without the ostectomy-corticotomy technique (control) included 61 patients with 79 impacted canines; records of 30 patients were retrieved from the European University College orthodontic clinic in Dubai, United Arab Emirates, and 31 patient records were retrieved from the private practices of Drs PJ and Roelien Stapelberg, Nelpruit, South Africa. In the control group, the open technique included surgical exposure of the palatally impacted canine crown by removing the overlying bone and/or soft tissue directly, bonding the bracket, and applying immediate forced-eruption traction with power chain. The closed technique involved raising a full mucoperiosteal flap, exposing the canine crown, and bonding an attachment followed by flap replacement; orthodontic traction with power chain was applied immediately until the canine erupted into the oral cavity. Limited access to the palatally impacted canine crown at the time of surgical exposure initially required bracket repositioning in many cases of open or closed technique.
Criteria for subject selection included the following: (1) palatally impacted canine diagnosed at pretreatment; (2) surgical exposure of the palatally impacted canine with a bonded attachment device affixed to the palatally impacted canine during surgery and orthodontic force immediately applied using elastic chain traction; (3) fixed, comprehensive, nonextraction orthodontic treatment; (4) at least 12 years of age at the time of palatally impacted canine surgery; (5) successful forced eruption of impacted canine; and (6) availability of records at pre- and postorthodontic canine forced eruption.
The primary target variable was palatally impacted canine forced-eruption time, which was the difference between surgery date and date the recovered canine was tied into the orthodontic arch wire, assuming a vertical and facial-lingual position similar to the adjacent teeth. Independent study variables used from the orthopantomogram radiograph (OPG) were pretreatment impacted canine angle, horizontal and vertical positions.
Procedures
Approval of the Institutional Review Board at European University College was obtained to conduct this research project. All pretreatment OPGs were taken in the natural head position. Analogue OPGs were scanned at 600 dpi with a millimeter ruler embedded in the corner of each scan to normalize measurements. Digital OPGs were taken at 1200 dpi. Patient age at time of surgery and date of surgery were recorded. ImageJ software was used for all measurements and has been shown to be reliable when measuring study casts, photographs,21–24 and OPGs.25,26 ImageJ software, a Java-based image-processing program, is a freeware distributed by the National Institutes of Health (Bethesda, Md).
Validity testing included measurement comparisons of the maxillary central incisor tooth width of 10 patient-subjects between digital OPGs taken at 1200 dpi and actual study casts. Although mesial-distal measurements of maxillary central incisors magnify on OPG,27 the digital OPGs taken at 1200 dpi averaged 1.27% larger. Consistency between measurement techniques, digital OPGs, and study casts was demonstrated by intraclass correlation (ie, 0.980; 95% confidence interval: 0.968–0.992). To test for intraoperator reliability, the pretreatment OPGs of six patients were randomly selected, and the palatally impacted canines were remeasured for angular, vertical, and horizontal position using ImageJ software. Measurements were repeated for five separate weeks, and no significant differences (P > .05) were found between means using paired t-tests.
Each digital panoramic radiograph was measured using image J as follows (Figure 2):
Figure 2.
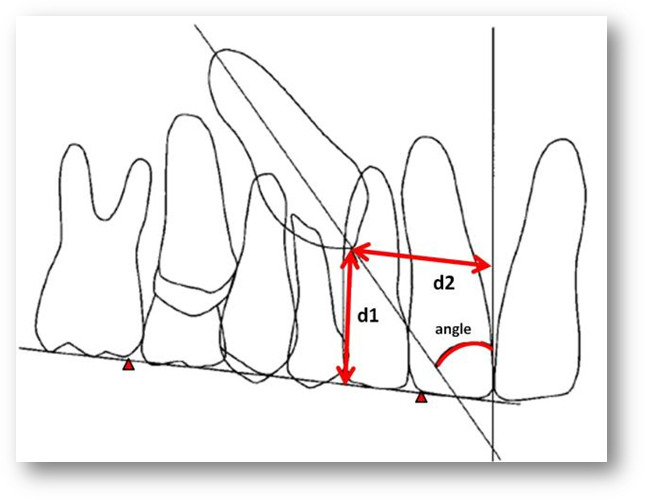
Position of palatally impacted canine was measured by angle of the canine long axis to OPG midline (angle), vertical distance from canine cusp tip to OPG occlusal plane (d1), and horizontal distance from canine cusp tip to OPG midline (d2).
Angle: Angle created by the long axis of the palatally impacted canine to the midline constructed on the OPG13,27,28
Horizontal: Linear horizontal distance of the palatally impacted canine cusp tip to the midline constructed on the OPG
Vertical: Linear vertical distance of the palatally impacted canine cusp tip to the occlusal plane constructed on the OPG using the mesial-buccal cusp of the first molar and the incisal distal margin of the maxillary central incisor12,15,27
Data from the experimental and control groups were subgrouped from least deviated (subgroup 1) to most deviated (subgroup 3) according to impaction condition (Table 1).
Table 1.
Subgroupings of the Impacted Caninesa
| Subgroup |
|||
| 1 |
2 |
3 |
|
| Angle, ° | 0 to 28.4 | >28.4 to <35 | >35 |
| Vertical distance, mm | 0 to 9.7 | >9.7 to <11.9 | >11.9 |
| Horizontal distance, mm | >9.25 | >6.33 to <9.25 | 0 to 6.33 |
Based on the ostectomy-decortication sample, three subgroups equal in size were created representing the position of the pretreatment maxillary palatally impacted canines in angle to midline, vertical distance of incisal tip to occlusal plane, and horizontal distance of incisal tip to midline. For example, the experimental group angle ranged from 6.7° to 60.7°, and the range was divided into three equal samples (n = 24). Subgroups 1 and 3 had the least and most deviated or clinically difficult canine positions, respectively. Canine angle to OPG midline reference line was reported in degrees, and vertical and horizontal position was reported in millimeters.
Forced-eruption time was measured in days from date of surgery to date of near final canine position tied into the archwire. Forced-eruption time was judged completed when facial/buccal photographs verified the canine was within ±1 mm of adjacent teeth facial-lingually and slot position vertically.
Statistical Analysis
Sample size was calculated for the number of patient-subjects necessary to achieve 80% power with an alpha of .05 on forced-eruption times between the three subgroups, using Cohen f value to indicate the size of the effect: three-group power calculation for Cohen f value of .5 (very large effect size) = sample size 13.9, Cohen f value of .4 (large effect size) = 21.1. Data were collected and stored in Excel and later transformed for use with the Statistical Package for Social Sciences (SPSS) software, version 15.0.1, for analysis. Parametric testing of continuous data was performed unless the assumption of variance equality was not verified. When variances were unequal, intergroup differences in forced-eruption time were compared using nonparametric Mann-Whitney U-test for two groups and Kruskal-Wallis H-test with Bonferroni post hoc for subgroups of three or more. The .05 probability level of significance was used for all testing purposes.
RESULTS
There were no significant differences (P > .05) between control and experimental sample demographics (Table 2). No differences were found within samples in the comparisons between right and left sides for forced-eruption time and canine position; therefore, the right and left sides were combined.
Table 2.
Sample Characteristicsa
| Control |
Experimental |
P |
|
| Age, y | 14.5 ± 2.8 | 15.3 ± 2.3 | NS |
| Patients, n | 61 | 57 | NS |
| Total, n (%) | 79 (100) | 72 (100) | NS |
| Right, n (%) | 47 (59.5) | 44 (61.1) | NS |
| Left, n (%) | 32 (40.5) | 28 (38.9) | NS |
| Bilateral, n (%) | 18 (29.5) | 15 (26.3) | NS |
Comparison of control and experimental samples by age (in years, ± standard deviations), patient number, total number of palatally impacted canines, and right, left, and bilateral (number and percentages). Note there were no significant differences (P < .05) between the two samples for any of the characteristics. P indicates probability significance; NS, not significant.
Forced-Eruption Time as a Function of Technique
During parametric testing of forced-eruption time by technique, unequal variances were demonstrated by Levene's test. Therefore, Mann-Whitney U nonparametric testing was used. A significantly greater forced-eruption time for control than experimental groups was demonstrated: 629 ± 307.2 days or 21.0 months vs 198.4 ± 100.2 days or 6.6 months (P = .000), respectively.
Forced-Eruption Time as a Function of Canine Position
Using Mann-Whitney U nonparametric testing, statistically homogeneous pretreatment subgroup samples were established in order to judge fairly the influence of pretreatment canine position on forced-eruption time. To achieve pretreatment impacted canine angle homogeneity, two patients with the highest canine angle were removed from the control sample (n = 77), while the experimental group (n = 72) remained constant. For horizontal position, the control sample was reduced by nine patients with highest horizontal distances and one experimental patient with lowest horizontal distance; pretreatment horizontal position homogeneity was achieved with experimental (n = 71) and control (n = 70) groups. For vertical position, statistically homogeneous pretreatment groups were achieved by removing five control participants with the highest vertical distances: experimental (n = 72) and control (n = 74) groups (Table 3).
Table 3.
Adjustments to Achieve Homogeneity Between Groupsa
| Experimental |
Control |
P |
|||||||||
| n |
χ |
SD |
Min |
Max |
n |
χ |
SD |
Min |
Max |
||
| Angle, ° | 72 | 30.3 | 12.8 | 6.7 | 60.7 | 77 | 34.1 | 14.5 | 1.4 | 60.9 | .065 |
| Horizontal | 71 | 7.9 | 3.3 | 1.5 | 16.8 | 70 | 9.0 | 4.2 | .1 | 17.4 | .064 |
| Vertical | 72 | 10.8 | 2.5 | 2.9 | 16.9 | 74 | 11.8 | 2.6 | 6.9 | 17.0 | .055 |
Impacted canine angle and horizontal and vertical position means were adjusted to establish statistically insignificant (P > .05) pretreatment means by removing the highest values from control patients. This was done to fairly judge differences in forced eruption duration as a function of canine position between the experimental and control groups. n indicates sample size; χ, mean; SD, standard deviation; min, minimum; max, maximum; P, probability significance.
The experimental sample was reorganized into three equal subgroups per independent study variable (ie, angle, horizontal, and vertical position). Subgroup 1 represented mild impacted and subgroup 3 represented severe impacted (or most difficult to clinically resolve). The control group was then reorganized into three equal or nearly equal subgroups based on the three impacted canine positions.
Kruskal-Wallis H-tests with Bonferroni post hoc tests were used to determine differences in forced-eruption time as a function of impacted canine position subgroup. In the control sample, no differences were found in forced-eruption time as a function of pretreatment palatally impacted canine position. For the experimental group, treatment duration was significantly longer when pretreatment impacted canine angle to midline was highest (284.9 vs 166.3 and 144.1 days, P = .000), when the horizontal position was closest to the midline (248.5 and 224.0 vs 122.7 days, P = .000), and when it was farthest vertically from the occlusal plane (246.0 vs 149.0 and 200.3 days, P = .002). Lastly, the three pretreatment position variables were then categorized by angle-horizontal-vertical to create three equal subgroups: subgroup 1 = low-angle + low-vertical + high-horizontal, subgroup 3 = high-angle + high vertical + low horizontal, and so on. Treatment duration for subgroup 3 (289.5 days) demonstrated significantly greater forced-eruption time than subgroup 1 (124.2 days, P < .000) but not subgroup 2 (181.6 days, P > .05; Table 4; Figure 3)
Table 4.
Forced-Eruption Time Descriptive Statisticsa
| n |
Subgroup 1 |
Subgroup 2 |
Subgroup 3 |
P |
||||
| χ |
SD |
χ |
SD |
χ |
SD |
|||
| Control | ||||||||
| Angle | 26 | 588.4 | 300.2 | 636.5 | 243.1 | 651.7 | 362.7 | NS |
| Horizontal | 23 | 578.5 | 306.8 | 610.7 | 300.0 | 765.4 | 308.4 | NS |
| Vertical | 25 | 527.7 | 311.4 | 715.3 | 331.3 | 654.0 | 303.8 | NS |
| Experimental | ||||||||
| Angle | 24 | 144.1 | 61.5 | 166.3 | 79.6 | 284.9 | 94.3 | 3 > 1 = 2, P = .000 |
| Horizontal | 24 | 122.7 | 51.1 | 224.0 | 97.1 | 248.5 | 98.2 | 3 = 2 > 1, P = .000 |
| Vertical | 24 | 149.0 | 79.6 | 200.3 | 98.4 | 246.0 | 100.2 | 3 > 1, P = .002 |
| Ang-hor-ver | 24 | 124.2 | 48.9 | 181.6 | 68.1 | 289.5 | 96.1 | 3 > 2 > 1, P < .000 |
Forced-eruption time descriptive statistics (in days) for palatally impacted canines (ie, descriptive statistics for equal or near-equal subgroups per category of angle to midline, horizontal distance from midline, and vertical distance from occlusal plane). Results of Kruskal-Wallis H-test demonstrated significantly greater treatment duration for the most difficult category (subgroup 3) in all three independent variables in only the experimental group. n indicates sample size; χ, mean; SD, standard deviation; P, probability significance.
Figure 3.
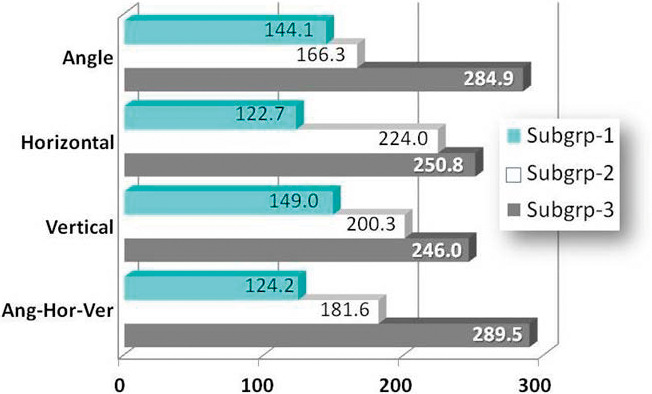
Forced-eruption times in the experimental group compared by palatally impacted canine position (angle, horizontal, and vertical) as a function of severity (subgrp) demonstrated significantly greater forced-eruption times for the most severe position (subgrp 3).
DISCUSSION
The time required to forcibly erupt palatally impacted canines into arch alignment was dependent on technique: the canine forced-eruption time was 3.2 times more rapid with the ostectomy-decortication technique (6.6 months) compared with control (21.0 months). To illustrate the differences in forced-eruption times between the two techniques, the most severely displaced palatally impacted canine by combined angle-horizontal-vertical positions was compared between the experimental and control groups (ie, 62.7° vs 62.9°, 2.4 mm vs 2.6 mm horizontal, and 15.8 vs 16.0 mm vertical, respectively). The experimental forced-eruption time was 2.4 times more rapid (ie, 13.6 months vs 31.9 months; Figure 4).
Figure 4.
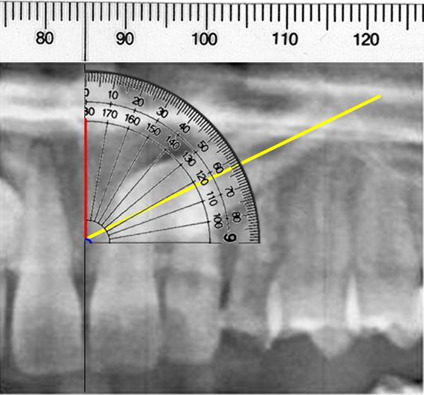
Representation of severe palatally impacted canine positions with 63° angle, 3 mm horizontal to midline, and 13.5 mm vertical to occlusal plane illustrated with portion of OPG and diagrammatic overlays. Forced-eruption times for two patients with these nearly identical severe palatally impacted canine conditions were 407 days (13.6 months) for experimental and 956 days (31.9 months) for control—a difference of 2.4 times.
Three previous studies6,9,13 reported canine forced-eruption time using similar definitions as the present study. Control group canine forced eruption in the present study (21.0 months) was similar to the 18.0 months reported by Schubert and Baumert,9 the average of 18.5 months reported by Iramaneerat et al.,6 and the average of 26.3 months reported by Fleming et al.13 for unilateral impacted canines.
The second main finding was that pretreatment palatally impacted canine position influenced forced-eruption time in the experimental group but not in the control. When palatally impacted canines were categorized by angle-horizontal-vertical pretreatment positions, differences in forced-eruption times increased 45.9% from subgroup 1 (124.2 days) to subgroup 2 (181.6 days), 59.3% from subgroup 2 to subgroup 3 (289.5 days), and 2.3 times between subgroup 1 and subgroup 3. Angle severity had a greater influence over the mild and moderate subgroups than did severe horizontal and vertical pretreatment positions (Table 3; Figure 3).
Previous scholarly literature is conflicted on the influence of pretreatment position of the palatally impacted canine on forced-eruption time. Some investigations reported that pretreatment canine position influenced palatally impacted canine forced-eruption times,12,13,28,29 while other investigations reported no differences.1,14,30 The results of the control group in the present study were consistent with the latter. Factors such as bony and soft tissue resistance were eliminated by ostectomy-corticotomy, but variations in these very same factors likely mollified the influence of pretreatment canine position on control forced-eruption time.
Formal periodontal assessment of patients in the present study after forced canine eruption would have provided value-added information. The scholarly literature indicated that the periodontal condition of treated impacted canines was almost always affected. However, only slight differences were found between previously impacted teeth and contralateral canine teeth.13,31–33 Evren et al.34 reported statistically different pocket depths and gingival and bone levels for orthodontically treated palatally impacted canines compared with contralateral untreated canines, but the differences were clinically insignificant or only marginally significant. Others reported no significant differences in posttreatment periodontal status after forcibly erupting palatally impacted canines.10,14
In the present study, the experimental group was treated by one orthodontist and one periodontal surgeon, whereas two surgeons and multiple orthodontists treated some of the control group. Multiple clinicians were a confounding factor, but the impact on the results was deemed small. Another potential confounding factor was that the force applied to the palatally impacted canine was not calibrated in this retrospective study. Applied traction forces were limited by the properties of the elastic and/or power chains used, and as such, traction force was likely not a strong confounding influence on the results.
Caution must be taken with any retrospective cohort research because of the possibility of bias. Subgroups were resized in the present study to create pretreatment homogeneity. The steps taken were systematic: the control group started with a greater number of palatally impacted canines (79 vs 72); therefore, the control group was reduced. No other subject features were considered when homogeneity was established.
CONCLUSIONS
Palatally impacted canine forced-eruption time averaged 6.6 months using the ostectomy-decortication technique compared with 21.0 months using open-closed surgical exposure techniques (ie, 3.2 times more rapid), and the null hypothesis was rejected.
The pretreatment position of palatally impacted canines influenced the forced-eruption time in the ostectomy-decortication sample but not the control group, and the null hypothesis was rejected.
Using the ostectomy-decortication technique, it took more than twice as much time to forcibly erupt palatally impacted canines that were identified on the pretreatment OPG as being severely vs mildly positioned.
REFERENCES
- 1.Becker A, Chaushu S. Success rate and duration of orthodontic treatment for adult patients with palatally impacted maxillary canines. Am J Orthod Dentofacial Orthop. 2003;124:509–514. doi: 10.1016/s0889-5406(03)00578-x. [DOI] [PubMed] [Google Scholar]
- 2.Becker A, Zogakis I, Luchian I, Chaushu S. Surgical exposure of impacted canines: open or closed surgery? Semin Orthod. 2016;22:27–33. [Google Scholar]
- 3.Bishara SE. Impacted maxillary canines: a review. Am J Orthod Dentofacial Orthop. 1992;101:159–171. doi: 10.1016/0889-5406(92)70008-X. [DOI] [PubMed] [Google Scholar]
- 4.Cooke J, Wang H-L. Canine impactions: incidence and management. Int J Periodontics Restorative Dent. 2006;26:483–491. [PubMed] [Google Scholar]
- 5.Chaushu S, Becker A, Zeltser R, Branski S, Vasker N, Chaushu G. Patients' perception of recovery after exposure of impacted teeth: a comparison of closed- versus open-eruption techniques. J Oral Maxillofac Surg. 2005;63:323–329. doi: 10.1016/j.joms.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Iramaneerat S, Cunningham SJ, Horrocks EN. The effect of two alternative methods of canine exposure upon subsequent duration of orthodontic treatment. Int J Paediatr Dent. 1998;8:123–129. doi: 10.1046/j.1365-263x.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 7.McDonald F, Yap W. The surgical exposure and application of direct traction of unerupted teeth. Am J Orthod. 1982;89:331–340. doi: 10.1016/0002-9416(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 8.Pearson MH, Robinson SN, Reed R, Birnie DJ, Zaki GA. Management of palatally impacted canines: the findings of a collaborative study. Eur J Orthod. 1997;19:511–515. doi: 10.1093/ejo/19.5.511. [DOI] [PubMed] [Google Scholar]
- 9.Schubert M, Baumert U. Alignment of impacted maxillary canines: critical analysis of eruption path and treatment time. J Orofac Orthop. 2009;70:200–212. doi: 10.1007/s00056-009-0901-3. [DOI] [PubMed] [Google Scholar]
- 10.Smailiene D, Kavaliauskiene A, Pacauskiene I, Zasciurinskiene E, Bjerklin K. Palatally impacted maxillary canines: choice of surgical orthodontic treatment method does not influence post-treatment periodontal status. A controlled prospective study. Eur J Orthod. 2013;35:803–810. doi: 10.1093/ejo/cjs102. [DOI] [PubMed] [Google Scholar]
- 11.Spuntarelli M, Cecchetti F, Arcuri L, et al. Combined orthodontic-surgical approach in the treatment of impacted maxillary canines: three clinical cases. Oral Implantol. 2015;53:63–67. doi: 10.11138/orl/2015.8.2.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart JA, Heo G, Glover K, Williamson PC, Lam EWN, Major PW. Factors that relate to treatment duration for patients with palatally impacted maxillary canines. Am J Orthod Dentofacial Orthop. 2001;119:216–225. doi: 10.1067/mod.2001.110989. [DOI] [PubMed] [Google Scholar]
- 13.Fleming PS, Scott P, Heidari N, DiBiase AT. Influence of radiographic position of ectopic canines on the duration of orthodontic treatment. Angle Orthod. 2009;79:442–446. doi: 10.2319/042708-238.1. [DOI] [PubMed] [Google Scholar]
- 14.Zuccati G, Ghobadlu J, Nieri M, Clausera C. Factors associated with the duration of forced eruption of impacted maxillary canines: a retrospective study. Am J Orthod Dentofacial Orthop. 2006;130:349–356. doi: 10.1016/j.ajodo.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Parkin NA, Milner RS, Deery C, et al. Periodontal health of palatally displaced canines treated with open or closed surgical technique: a multicenter, randomized controlled trial. Am J Orthod Dentofacial Orthop. 2013;144:176–184. doi: 10.1016/j.ajodo.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Cassina C, Papageorgiou SN, Eliades T. Open versus closed surgical exposure for permanent impacted canines: a systematic review and meta-analyses. Eur J Orthod. 2018;40:1–10. doi: 10.1093/ejo/cjx047. [DOI] [PubMed] [Google Scholar]
- 17.Marzouk W, ElMostehy R, Al-Qurashi A. Combined surgical and orthodontic treatment of impacted maxillary canines. Saudi Dent J. 1997;9:90–98. [Google Scholar]
- 18.Wilcko WM, Wilcko MT, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 19.Wilcko WM, Wilcko MT, Marquez MG, Ferguson DJ. The contribution of periodontics to orthodontic therapy. In: Dibart S, editor. Practical Advanced Periodontal Surgery. Ames, Ia: Blackwell; 2007. pp. 28–42. [Google Scholar]
- 20.Fischer TJ. Orthodontic treatment acceleration with corticotomy assisted exposure of palatally impacted canines. Angle Orthod. 2007;77:417–420. doi: 10.2319/0003-3219(2007)077[0417:OTAWCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Almasoud N, Bearn D. Little's irregularity index: photographic assessment vs study model assessment. Am J Orthod Dentofacial Orthop. 2010;138:787–794. doi: 10.1016/j.ajodo.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Kerne S, Etienne D. Root coverage assessment: validity and reproducibility of an image analysis system. Clin Periodontol. 2007;34:969–976. doi: 10.1111/j.1600-051X.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 23.Tran AM, Rugh JD. Reliability and validity of a computer-based Little irregularity index. Am J Orthod Dentofacial Orthop. 2003;123:349–351. doi: 10.1067/mod.2003.76. [DOI] [PubMed] [Google Scholar]
- 24.Makki L, Ferguson DJ, Stapelberg R. Measuring irregularity index: comparing study cast caliper method with 2D dimensional ImageJ photogrammetry and 3D STL image measurement. APOS Trends Orthod. 2017;7:260–266. [Google Scholar]
- 25.Bulut E, Sahin B, Muuuglali M, Bekciollu B. Comparison of the planimetry and point-counting methods for the assessment of the size of the mandible cysts on orthopantomograms. Med Oral Patol Oral Cir Bucal. 2012;17:e442–e446. doi: 10.4317/medoral.17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rejebian GP. A statistical correlation of individual tooth size distortions on the orthopantomographic radiograph. Am J Orthod. 1979;75:525–534. doi: 10.1016/0002-9416(79)90071-x. [DOI] [PubMed] [Google Scholar]
- 27.Ericson S, Kurol J. Radiographic examination of ectopically erupting maxillary canines. Am J Orthod Dentofacial Orthop. 1987;91:483–492. doi: 10.1016/0889-5406(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 28.Motamedi MHK, Tabatabaie FA, Navi F, Shafeie HA, Fard BK, Hayati ZH. Assessment of radiographic factors affecting surgical exposure and orthodontic alignment of impacted canines of the palate: a 15-year retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:772–775. doi: 10.1016/j.tripleo.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Power SM, Short MB. An investigation into the response of palatally displaced canines to the removal of deciduous canines and an assessment of factors contributing to favourable eruption. Br J Orthod. 1993;20:215–223. doi: 10.1179/bjo.20.3.215. [DOI] [PubMed] [Google Scholar]
- 30.Grande T, Stolze A, Goldbecher H, Kahl-Nieke B. The displaced maxillary canine: a retrospective study. J Orofac Orthop. 2006;67:441–449. doi: 10.1007/s00056-006-0616-7. [DOI] [PubMed] [Google Scholar]
- 31.Oz AZ, Ciger S. Health of periodontal tissues and resorption status after orthodontic treatment of impacted maxillary canines. Niger J Clin Pract. 2018;21:301–305. doi: 10.4103/njcp.njcp_419_16. [DOI] [PubMed] [Google Scholar]
- 32.Crescini A, Nieri M, Buti J, Baccetti T, Pini Prato GP. Orthodontic and periodontal outcomes of treated impacted maxillary canines. Angle Orthod. 2007;77:571–577. doi: 10.2319/080406-318.1. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Lagares D, Hita-Iglesias P, Azcarate-Velazquez F, et al. What are the histologic effects of surgical and orthodontic treatment on the gingiva of palatal impacted canines? J Oral Maxillfac Surg. 2015;73:2273–2281. doi: 10.1016/j.joms.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Evren AD, Nevzatoglu S, Arun T, Acar A. Periodontal status of ectopic canines after orthodontic treatment. Angle Orthod. 2014;84:18–23. doi: 10.2319/041513-290.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


